Electrocatalytic CO2 reduction to CO enabled by Ni-Nx high activity sites formed by three-dimensional porous foams
Abstract
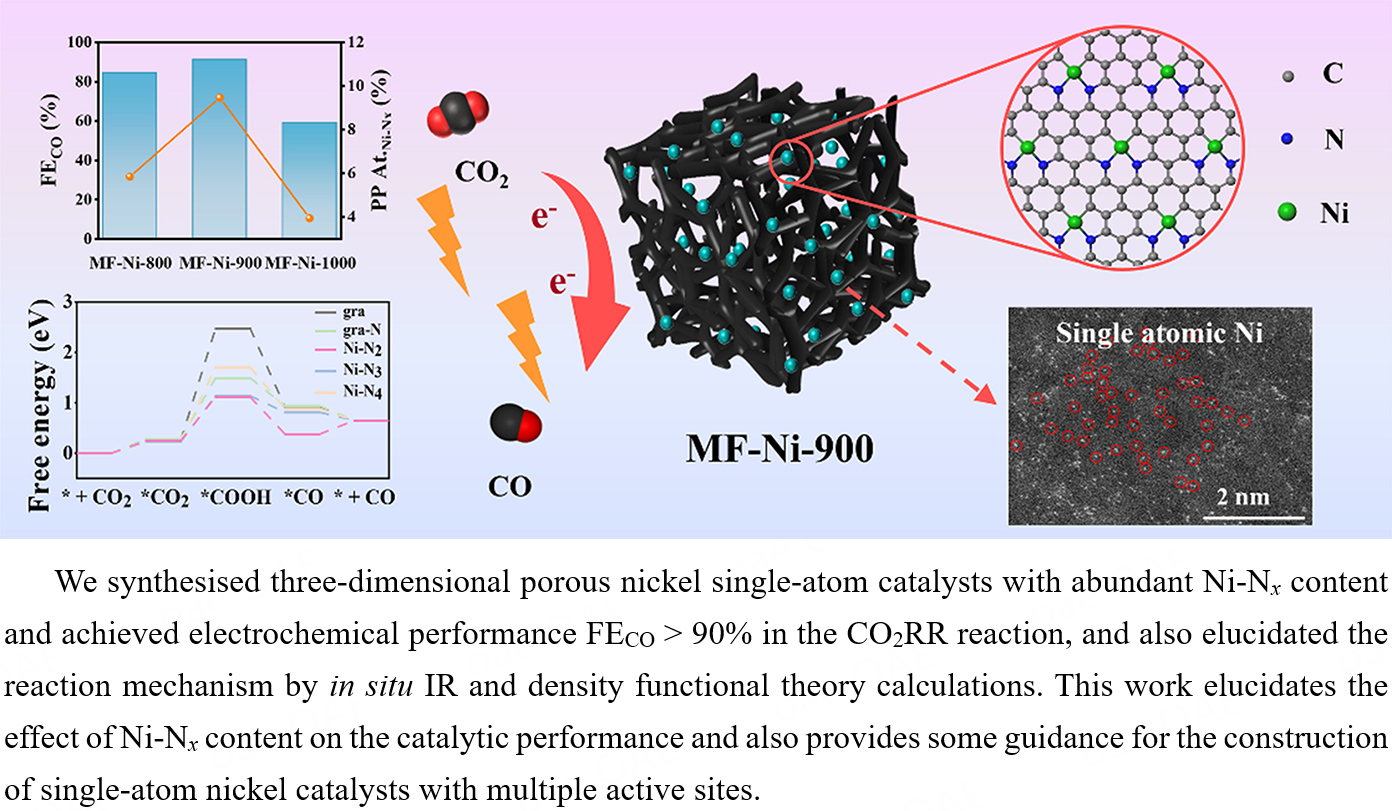
Single-atom catalysts (SACs) are widely used in carbon dioxide reduction reaction
Keywords
INTRODUCTION
The utilization of renewable energy sources for the electrocatalytic carbon dioxide reduction reaction
Metal-nitrogen-carbon (M-N-C) catalysts demonstrate remarkable efficacy in CO2RR due to their distinctive chemical structure[8-10]. In comparison with conventional M-N-C materials, M-N-C catalysts with a porous structure exhibit larger specific surface area and an increased number of active sites[11,12]. Liu et al. developed porous carbon carriers, which resulted in a 5-fold increase in active site exposure. This structure was shown to significantly enhance catalytic performance[13]. Pan et al. prepared atomically dispersed
Melamine foam (MF) is a highly cross-linked thermosetting organic porous plastic with high mass transfer rates and nitrogen content. The pristine framework of this material possesses a high specific surface area and through pores, which facilitate the diffusion of reactants (carbon dioxide) and desorption of products. Furthermore, the pyrolysis of MF results in the formation of N-doped carbon in the form of graphene-like structures. This process enhances the electrical conductivity of the material and prevents agglomeration into particles by anchoring the nickel single-atom with nitrogen atoms. Herein, in this paper we synthesized Ni SACs by MF pyrolysis. The goal was to form Ni-Nx structures with highly active sites to improve CO2RR selectivity. The X-ray photoelectron spectroscopy (XPS) test results showed a peak content of Ni-Nx sites in the material (9.47 at.%) at a calcination temperature of 900 °C, a value significantly higher than other calcination temperatures. The Faradaic efficiency of CO (FECO) at -0.66 V vs. reversible hydrogen electrode (RHE) could reach 91% and maintain the basic performance (FE > 80%) after 35 h of testing. This finding indicated that Ni-Nx was the primary active site of CO2RR and its content exhibited a direct correlation with catalytic activity. In situ infrared spectroscopy (IR) and the density functional theory (DFT) calculations indicated that high-temperature calcination might promote the stable coordination of Ni with N, modulate the electronic state of Ni, and enhance the adsorption of the reaction intermediate *CO. The results of this experiment demonstrate the potential of a novel approach to the construction of porous catalysts with a binding effect.
EXPERIMENTAL
Materials
Nickel nitrate hexahydrate [Ni(NO3)2·6H2O; 98%] from Sigma-Aldrich; potassium bicarbonate (KHCO3; AR) from Shanghai Rhawn Chemical Technology Company; MF from Taobao; liquid nitrogen from Harbin Daoli District Mingying Cryogenic Materials Supply Department; anhydrous ethanol (C2H5OH; AR) from Tianjin Fuyu Fine Chemical Company; Nafion 117 perfluorinated resin solution (~5% in a mixture of lower aliphatic alcohols and water) from Shanghai Rhawn Chemical Technology Company. The cation exchange membrane employed in this experiment was the Nafion 117 membrane, provided by DuPont. All reagents were provided in a ready-to-use format, requiring no further purification. All solutions were prepared with ultrapure water (specific conductivity ≥ 18.2 MΩ·cm-1).
Synthesis of MF-900
MF was impregnated in an equal volume of ultrapure water, frozen with liquid nitrogen and then dried in a vacuum freeze dryer at -80 °C for 48 h. It was removed into a tube furnace, sealed, and purged with flowing argon for 30 min to allow the air to escape completely. The tube furnace was heated to 550 °C and held for two h, then heated again to 900 °C and held for two h. After this, the furnace was allowed to cool naturally to room temperature, after which the sample was removed and designated MF-900.
Synthesis of MF-Ni-800, MF-Ni-900, and MF-Ni-1000
Weigh 0.0012 g of nickel nitrate hexahydrate in a beaker. Subsequently, 4.5 mL of ultrapure water was pipetted into the beaker and stirred with ultrasound until complete dissolution was achieved. Then, 0.05 g of MF was weighed and impregnated in the solution. The material was frozen with liquid nitrogen and subjected to vacuum drying at -80 °C for a period of 48 h. Subsequently, the material was removed and placed in a tube furnace, which was sealed and purged with flowing argon gas for 30 min to remove air. The tube furnace was initially heated to 550 °C and maintained at this temperature for a period of two h. Subsequently, the material was heated to 800, 900, or 1,000 °C and maintained at this temperature for a further two h. The temperature was reduced to ambient levels. The resulting materials were designated
Preparation of working electrode
MF was affixed to the working electrode via a special working electrode clamp.
Electrochemical test
The CHI760 workstation from Shanghai Chenhua Instrument Company., Ltd. or the Squidstat Plus electrochemical workstation from Admiral, US, was used for the test.
Principles of electrochemical test
Converting the electrode potential relative to the Ag/AgCl electrode to the electrode potential relative to the standard hydrogen electrode: ERHE = EAg/AgCl + 0.197 + 0.0592 × pH where EAg/AgCl is the potential at the time of the test (V), and pH is the saturated 0.5 M KHCO3 solution.
Faradaic efficiency can be calculated by FE = ZnF/Q where Z denotes the number of electrons transferred (in the reduction of CO2 to form CO, Z = 2), n represents the number of moles of the product (mol), F is Faradaic constant (96,485 C/mol), and Q is the charge (C).
The Tafel curve is closely related to the kinetic rate and activity, which is calculated as: η = a + blogj where η denotes overpotential (V), a indicates the intercept (V), b denotes the slope (mV/dec), and j signifies the current density (mA/cm3).
In order to reflect the intrinsic activity of the catalyst, the transition frequency (TOF) is calculated for the catalyst by
Characterization
Calcination of samples using tube furnace of Hefei Kejing Material Technology Co. The vacuum freeze dryer of Ningbo Xinzhi Freeze Drying Equipment Company was used to freeze dry the samples. Scanning electron microscope (SEM; EM-30plus) and high-resolution transmission electron microscope (HRTEM; Talos F200s) were used to observe the morphology of the materials. Spherical aberration corrected transmission electron microscope (AC-TEM; JEOL JEM-ARM200F) was used to determine the presence of single atoms. Spectroscopy was tested by X-ray diffraction (XRD; HZL10004) and Raman spectroscopy (Raman; Thermo Fisher DXR2). N2 absorption/desorption isotherms were recorded on the Quantachrome station. XPS was performed using a ThermoFisher Scientific ESCALAB 250 Xi+ spectrometer. An Agilent 8890 gas chromatograph was used to test the gaseous products, and FL97Plus was used to test the liquid products. In situ IR was detected using a Fourier-transform infrared spectroscopy (FTIR) spectrometer (WQF-530A) with a customized ATR accessory and a liquid nitrogen-cooled Mercury Cadmium Telluride (MCT) detector.
Density functional theory calculations details
The DFT was implemented in the Vienna ab initio simulation package (VASP) to calculate this work[23,24]. The projector augmented-wave (PAW) was carried out to handle the interactions between the core and the valence electrons[25,26]. The Perdew-Burke-Ernzerhof (PBE) functional was utilized along with the generalized gradient approximation (GGA) to model the exchange-correlation energy[27] with the plane-wave cutoff energy of 450 eV. In all calculations, the convergence criterion of energy and force was set to be 10-6 eV and 0.02 eV·Å-1, respectively. For Brillouin zone sampling, the Γ-centered k-point grid of 3 × 3 × 1 was employed during structure optimization and static self-consistent calculations. The catalytic substrate was modeled by a 5 × 5 graphene supercell, of which the thickness of the vacuum layer was 20 Å in the z-direction to avoid interaction between periodic images. In addition, van der Waals (vdW) interaction was considered by Grimme’s DFT-D3 with Becke-Johnson (BJ) damping[28,29]. To describe the process of electrochemical CO2RR, the computational hydrogen electrode (CHE) model proposed firstly by Nørskov et al. was referred to obtain the free energy profile[30].
The Gibbs free energy change (ΔG) of each elementary step was defined as ΔG = ΔE + ΔEZPE - TΔS + ∫CpdT where ΔE and ΔEZPE represent the change of total and zero-point energy of the system, respectively. The ΔS is the contribution of entropy, and the Cp is heat capacity. T is the temperature is set to 298.15 K.
The limiting potential (UL) was adopted as a catalytic activity indicator to evaluate the CO2RR performance of catalysts, which was obtained by UL = -ΔGmax/e where ΔGmax presents the maximum ΔG of the elementary step corresponding to the potential determining step (PDS) in the reaction pathway.
RESULTS AND DISCUSSION
Synthesis and morphology characterizations
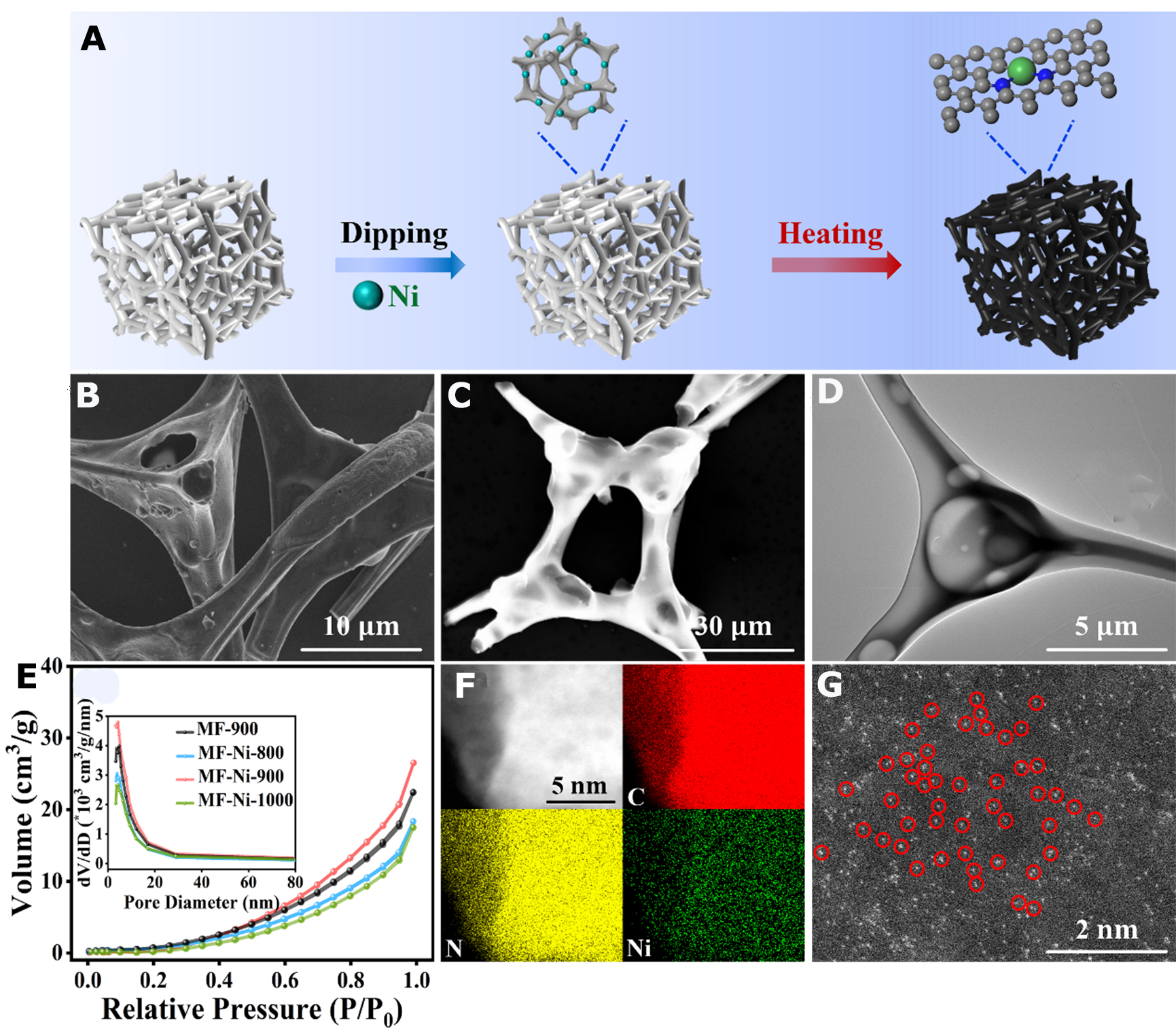
The synthesis process was illustrated in Figure 1A. MF was impregnated with an equal volume of nickel nitrate solution. The materials were freeze-dried and subsequently carbonized in a tube furnace at temperatures of 800, 900, and 1,000 °C with an atmosphere of argon. The carbonation products were collected and designated MF-Ni-800, MF-Ni-900, and MF-Ni-1000, respectively. Additionally, pure MF was obtained at 900 °C using the same synthesis method and was named MF-900. The porous network structure of MF allowed for a homogeneous dispersion of Ni atoms. Subsequent carbonization at different temperatures formed a Ni SAC with multiple active sites. It was a lightweight black foam that shrank in equal proportions [Supplementary Figure 1 and Supplementary Table 1].
Figure 1. (A) Schematic diagram of catalyst synthesis; (B-D) SEM images (B-C), HR-TEM image (D) of MF-Ni-900; (E) N2 adsorption and desorption curves and pore size distributions; (F) EDS images of MF-Ni-900; (G) AC-TEM image of MF-Ni-900. SEM: Scanning electron microscope; HR-TEM: high-resolution transmission electron microscope; MF: melamine foam; EDS: energy dispersive spectroscopy; AC-TEM: spherical aberration corrected transmission electron microscope.
Scanning electron microscopy (SEM) showed that MF-Ni-900 had a skeletal morphology of cross-linked structures [Figure 1B and C]. This suggested that MF could be used as a suitable support material to effectively confine nickel atoms within its porous structure, thus providing more active sites for CO2RR. In Figure 1D and Supplementary Figure 2, it could be seen that a large number of pore-like structures suspected to be mesopores appeared at the cross-linked skeleton structure, which was in agreement with the results of later Brunauer-Emmett-Teller (BET) tests. Furthermore, the enhanced degree of skeleton shrinkage of MF-Ni-900 led to a denser material structure and a thinner conductive skeleton in comparison to that of MF-900 [Supplementary Figure 2]. Consequently, this resulted in a reduction in the path length for electron transport, thereby lowering the resistance and thus favoring electron transport.
The pore structure of the samples was determined by N2 adsorption [Figure 1E]. The specific surface areas of MF-900, MF-Ni-800, MF-Ni-900, and MF-Ni-1000 were 17.2, 13.3, 20.7, and 12.2 m2/g, respectively. It could be observed that the specific surface area increased following the loading of Ni single atoms, which might be attributed to the fact that the anchoring of single atoms was typically dependent on defects in the carbon carrier (e.g., vacancies, edge sites or heteroatom doping sites). The majority of these strategies necessitated high-temperature calcination to facilitate the anchoring of atomic positions. The process of monoatomic anchoring during high-temperature treatment resulted in the formation of mesopores, thereby increasing the specific surface area. Furthermore, it was observed that the specific surface area exhibited variation when the Ni SAC was subjected to different carbonization temperatures. The increase in specific surface area as the carbonization temperature rises from 800 to 900 °C might be attributed to the complete decomposition of impurities blocking the ultra-microporous at 900 °C. As the carbonation temperature was increased to 1,000 °C, the specific surface area of the material was observed to decrease. This was attributed to the fact that the mechanical properties of MF were weakened by the elevated carbonization temperature, resulting in a collapse of the pore structure. The pore distribution, as calculated by the Barret-Joyner-Halenda (BJH) method, indicated that the pore size of the catalysts was predominantly distributed within the mesoporous range [Figure 1E]. The mesopores (2-50 nm) possessed a pore size that was conducive to the physical adsorption of CO2 molecules. This process engendered a localized high concentration of gas-phase enrichment in the pore channels by means of capillary action. This was highly conducive to the transport, activation, and reduction of CO2, which enhanced an increase in the concentration of CO2 in the surrounding area.
Furthermore, energy dispersive spectroscopy (EDS) revealed the presence of Ni within MF-Ni-900 [Figure 1F]. The uniform dispersion of Ni atoms in MF-Ni-900 could be unambiguously demonstrated by means of spherical aberration corrected transmission electron microscopy (AC-TEM) [Figure 1G].
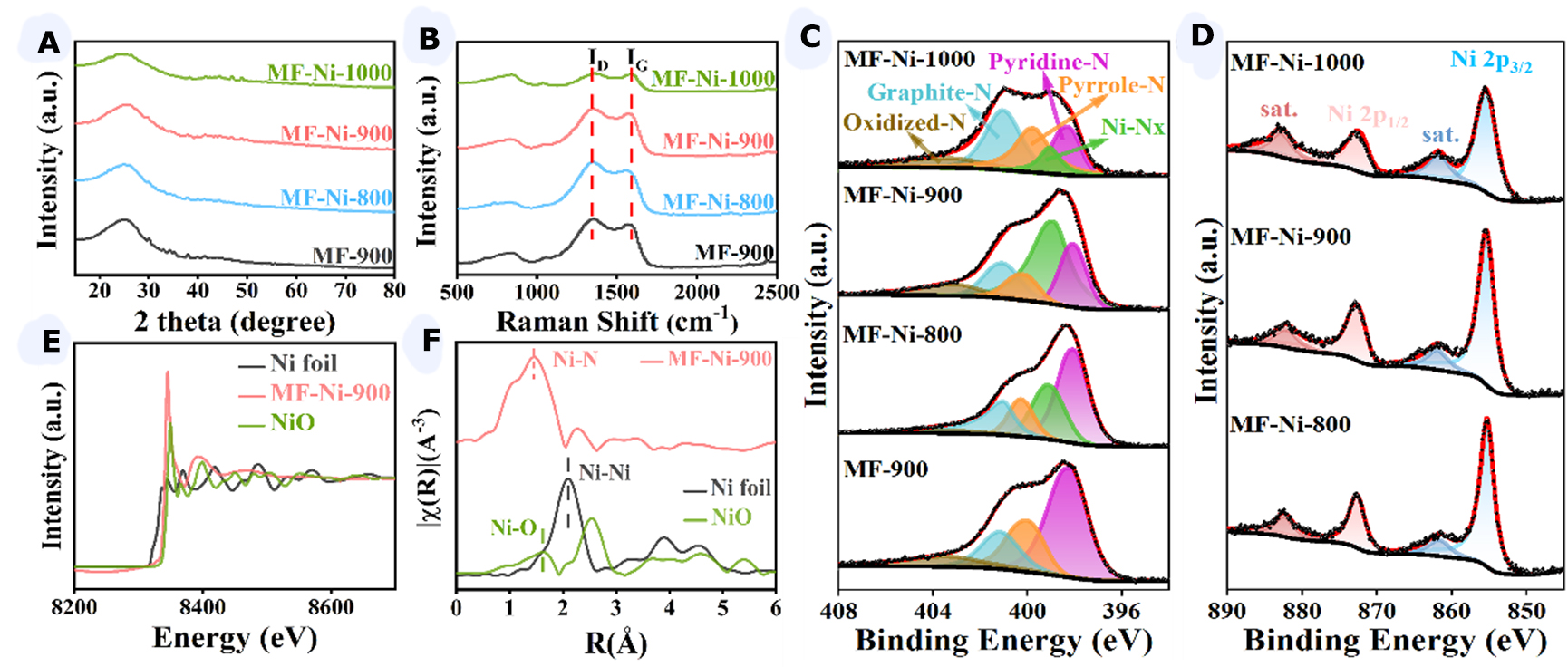
The crystal structure of the catalyst was characterized by XRD, as illustrated in Figure 2A. The XRD image showed peaks similar to those of amorphous carbon, with no visible metallic nickel particles or nickel compound peaks observed. This indicated a low nickel content on the carbon carrier, suggesting that the nickel was loaded on MF in monoatomic form. The broad peak observed at 2θ = 25° could be attributed to the (002) crystal plane of graphitic carbon. In contrast, the characteristic peak of the graphitic carbon (002) crystalline facet of MF-Ni-1000 was shifted to 2θ = 25°, indicating the formation of a more ordered carbon structure at 1,000 °C. Furthermore, the XRD plot of MF-Ni-1000 exhibited an additional diffraction peak at 44°, which correlated with the (100) crystal plane of graphitic carbon. This indicated that the graphitization of the material might be intensified at elevated carbonization temperatures, resulting in the formation of ordered structures.
Figure 2. (A-C) XRD image (A), Raman image (B), N 1s spectrum of XPS image (C) of MF-900, MF-Ni-800, MF-Ni-900, and MF-Ni-1000; (D) Ni 2p spectrum of XPS image of MF-Ni-800, MF-Ni-900, and MF-Ni-1000; (E) Ni K-edge XANES spectra; (F) Fourier transform EXAFS spectra for MF-Ni-900, Ni foil, and NiO. XRD: X-ray diffraction; XPS: X-ray photoelectron spectroscopy; MF: melamine foam; XANES: X-ray absorption near edge structure; EXAFS: extended X-ray absorption fine structure.
Subsequently, the material was subjected to Raman spectroscopy analysis [Figure 2B]. Two broad peaks were observed at 1,350 cm-1 (D band) and 1,580 cm-1 (G band) for the catalysts, which corresponded to amorphous carbon (D band) and sp2 hybridization of graphitic carbon (G band), respectively. The graphitization coefficient (ID/IG) of the catalysts was above one, suggesting the presence of a highly defective local structure. As the carbonization temperature increased from 800 to 1,000 °C, the graphitization coefficient decreased from 1.17 to 1.04, indicating that the degree of graphitization increased at high carbonization temperatures. In contrast, the graphitization coefficient increased from 1.06 to 1.09 after loading monatomic nickel at the same carbonization temperature. This indicated a modification in the degree of graphitization and structure of MF-Ni-900 upon the addition of monatomic nickel, resulting in an increase in the number of defects present and thus providing a greater number of active sites.
The surface valence states between Ni single atoms and doped N were investigated by XPS. Supplementary Figure 3 illustrated the complete XPS spectrum of the catalysts, which revealed the presence of sodium and oxygen in addition to the characteristic elements carbon, nitrogen, and nickel [Supplementary Table 2]. The presence of O might be attributed to the incorporation of formaldehyde during the synthesis of melamine formaldehyde resin or partial oxygen adsorption on the surface of the sample. The presence of Na was attributable to the addition of sodium hydroxide to adjust the pH of the solution during the synthesis of melamine-formaldehyde resin.
In the C 1s spectrum of XPS [Supplementary Figure 4], a peak with a binding energy of 284.8 eV was observed, which was typically associated with C-C bonds. Additionally, two peaks were present at 286.2 and 289.8 eV, which were attributed to C-O-C and O-C=O bonds, respectively[31]. To ascertain whether there was an interaction between nitrogen and nickel atoms in the catalyst, we investigated the N 1s spectrum of XPS [Figure 2C] and compared it with the spectrum of MF-900. By fitting the N 1s spectra, four characteristic peaks were identified at 398.1, 400, 401.1, and 403.2 eV, which correspond to pyridine-N, pyrrole-N, graphite-N, and oxidized-N, respectively. The introduction of nickel atoms gave rise to the emergence of a novel component at 399.1 eV, accompanied by a reduction in the intensity of the pyridine-N spectral line[32]. This indicated a transition from the pyridine-N to the Ni-Nx state. In the N 1s spectra
Figure 2D illustrated the binding energies of Ni 2p3/2 and Ni 2p1/2, which were observed to be centered at 855.4 and 872.7 eV, respectively. The positions of the spectral lines of Ni 2p were similar to those of
The post-reaction SEM, XRD and XPS measurements were conducted to ascertain whether there were alterations on catalysts before and after the reaction. As illustrated in Supplementary Figure 5A, the SEM image revealed that the melamine skeleton exhibited no signs of cracking or pitting following the reaction. This finding suggested that it was well-stabilized, making it suitable for use as a carbon carrier for the loading of single atoms. Furthermore, the presence of flakes or lumps on the surface of the skeleton might be attributable to carbonate precipitation without thorough washing. As demonstrated in Supplementary Figure 5B, the post-reaction XRD plot displayed peaks and shapes consistent with those of amorphous carbon as observed in the pre-reaction XRD plot. Furthermore, no peaks indicative of Ni particles were evident. This finding suggested that the reacted Ni active sites did not agglomerate and persist as individual atoms, thereby further substantiating the catalyst's stability. In order to ascertain whether a change in the valence state of the Ni atoms had occurred, post-reaction XPS tests were performed. The N 1s plot following peak splitting [Supplementary Figure 5C] demonstrated that the quantity of pyridine N decreased whilst the quantity of pyrrole N increased, and the quantity of other types of N remained almost unchanged. This phenomenon might be attributed to the hydrogenation of pyridine N to form a pyrrole N structure by the addition of H in a reducing reaction environment. Furthermore, the coordination structure of the active site remained intact during the reaction, indicating that the Ni-N bond was not broken and its catalytic property was retained. The Ni 2p spectra were fitted as shown in Supplementary Figure 5D. The half-peak widths of Ni 2p3/2 and Ni 2p1/2 were consistent with the pre-reaction values and did not show peaks of Ni particles. This finding excluded the possibility of Ni reduction or oxidation and provided further evidence that Ni existed as a single atom.
Electrochemical test in H-cell
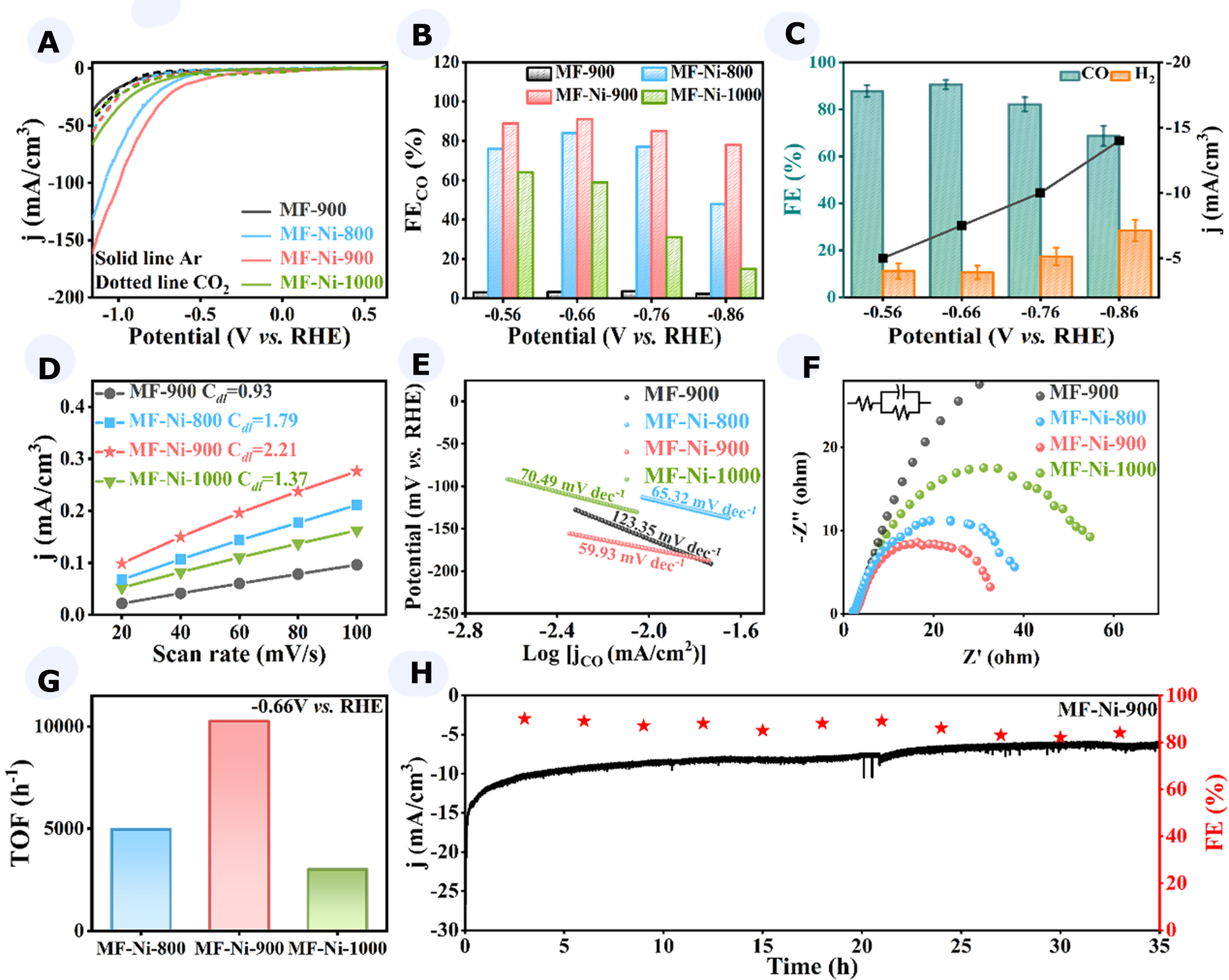
The activity of the catalysts for electrocatalytic CO2RR was investigated in an H-type electrolytic cell, with the electrodes separated by the Nafion 117 membrane. Figure 3A demonstrated that the catalysts exhibited considerable cathodic current densities in CO2-saturated electrolytes at potentials ranging from 0.54 to
Figure 3. (A) Linear sweep voltammetry (LSV) image of catalysts in Ar-saturated or CO2-saturated 0.5 M KHCO3 electrolytes; (B) FECO image of catalysts at -0.56 to -0.86 V vs. RHE potentials; (C) CO Faradaic efficiency error bars and current density image of MF-Ni-900; (D-G) ECSA linear image (D), Tafel image (E), EIS image (F), TOF image (G) of MF-900, MF-Ni-800, MF-Ni-900, and MF-Ni-1000; (H) 35 h long-term stability of MF-Ni-900. FECO: Faradaic efficiency of CO; RHE: reversible hydrogen electrode; MF: melamine foam; ECSA: electrochemically active surface area; EIS: electrochemical impedance spectroscopy; TOF: transition frequency.
To provide further evidence of the CO2RR performance of MF-Ni-900, the reduction products and corresponding FE of the catalyst at varying potentials were subjected to quantitative analysis via online gas chromatography (GC), as illustrated in Figure 3B. At -0.66 V vs. RHE, MF-Ni-900 exhibited a current of
To ascertain the reasons for the excellent CO2RR performance, a test was conducted into the electrochemical active surface area (ECSA) of the catalysts. This was accomplished by assessing the ECSA through the quantification of the double-layer capacitance (Cdl) at varying scan rates on the cyclic voltammetry curves [Supplementary Figure 8]. As illustrated in Figure 3D, MF-Ni-900 exhibited the highest Cdl of 2.21 mF·cm-3. This finding indicated that the Ni-Nx content in MF-Ni-900 was proportional to Cdl, thereby substantiating the validity of our hypothesis derived from XPS analysis. Furthermore, for electrochemical reactions, an enlarged active surface area of the electrode signified a magnified effective area on the electrode where the reaction could occur, resulting in an augmented reaction rate and current density, which was more conducive to the CO2RR.
The Tafel curves of the catalysts were plotted [Figure 3E]. The Tafel slope (b) of MF-Ni-900 was
In order to ascertain the true catalytic capacity of the catalyst, an inductively coupled plasma test (ICP) was performed to calculate the TOF of the catalyst. As illustrated in Figure 3G, the maximum turnover frequency of MF-Ni-900 at -0.66 V vs. RHE reached 10,266 h-1, a value that was considerably higher than the TOF values of MF-Ni-800 (4,976 h-1) and MF-Ni-1000 (3,007 h-1). This finding suggested that MF-Ni-900 exhibited superior intrinsic activity and could convert reactants more rapidly under identical conditions. The cathodic energy efficiency (CEE) for CO generation was 74.7% at -0.66 V vs. RHE, which was comparable to the CEE values reported for the most recently developed Ni-based monoatomic catalysts. The durability of the catalyst was a further crucial factor in the assessment of electrocatalytic performance. As illustrated in Figure 3H, the current density remained largely unaltered after 35 h, and the FECO exhibited only a modest decline and sustained a high value (FECO > 80%). The electrochemical performance of
The ATR-SEIRAS
In order to detect the reaction mechanism of CO2RR, in situ attenuated total reflectance surface-enhanced infrared absorption spectroscopy (ATR-SEIRAS) was conducted to monitor the catalytic reaction route and intermediates. We designed a three-electrode reaction device similar to an H-type electrolytic cell and tested it in CO2-saturated 0.5 M KHCO3 aqueous solution. The experimental evidence indicated that *CO and
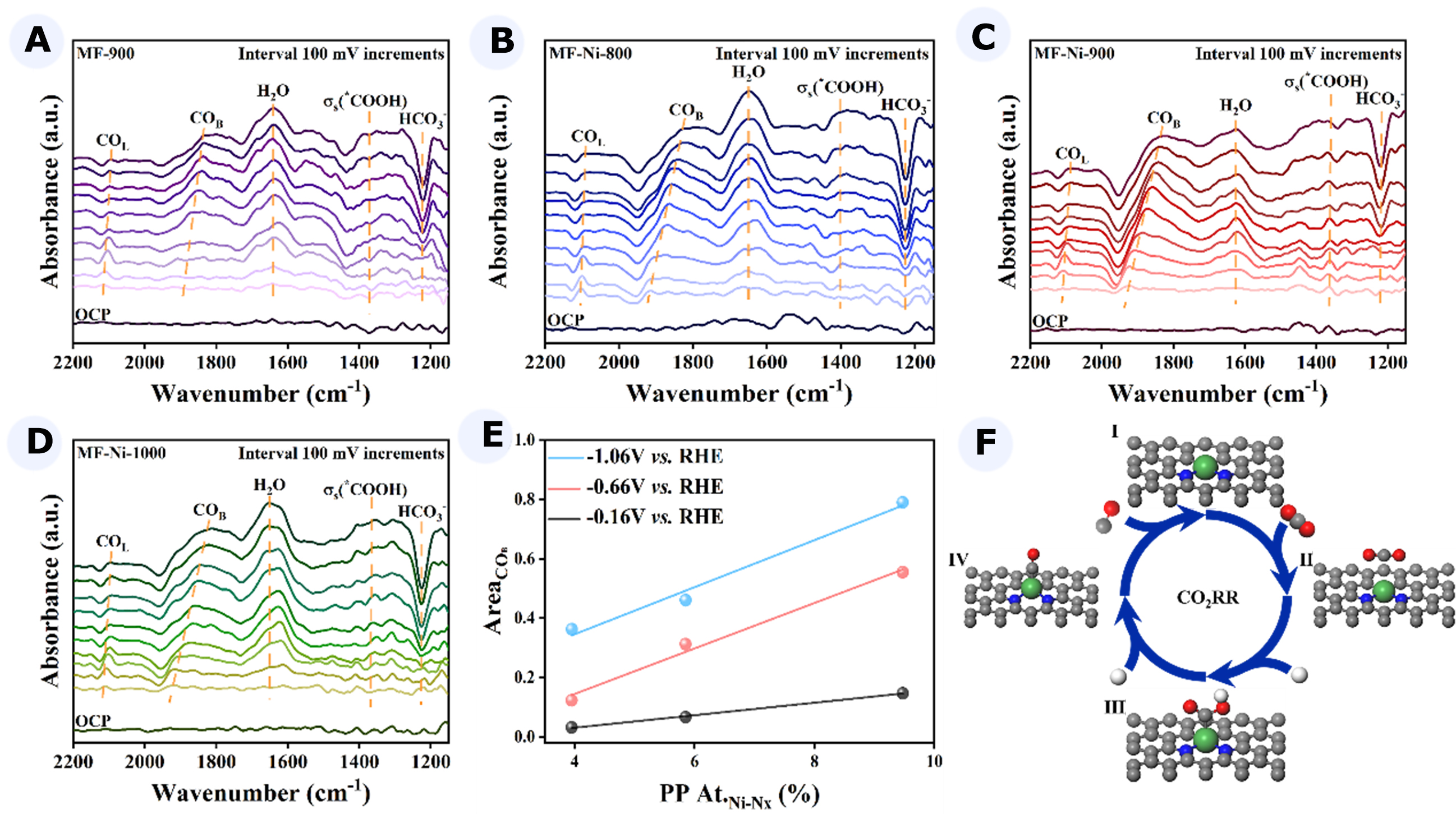
Figure 4. (A-D) At -0.16 to -1.06 V vs. RHE potentials, in situ IR images of MF-900 (A), MF-Ni-800 (B), MF-Ni-900 (C), and MF-Ni-1000 (D); (E) Linear relationship between COB peak area and Ni-Nx content; (F) Mechanism diagram of CO2 to CO. RHE: Reversible hydrogen electrode; IR: infrared spectroscopy; MF: melamine foam; COB: CO adsorption.
In situ IR revealed the presence of an HCO3- stretching peak at approximately 1,210 cm-1, which was attributed to the adsorption of electrolyte on the catalyst surface. It was observed that the depletion of
The positive peak observed at approximately 1,400 cm-1 was attributed to the stretching of the C-O bond in the bidentate functional group, *COOH. The intensity of the *COOH signal demonstrated a gradual increase with increasing potential from -0.16 to -1.06 V vs. RHE. It was noteworthy that the *COOH peak intensity of MF-Ni-900 was more pronounced than that observed on the other catalysts and appeared earlier in the potential. This finding suggested that the Ni-Nx active site exerted a favorable effect on the adsorption and activation of H2O and CO2 molecules, thereby facilitating the generation and adsorption of *COOH intermediates. Moreover, the positive peak observed at 1,630 cm-1 was indicative of the bending of the
The peaks at 1,930 and 2,118 cm-1 in the in situ IR spectrum were indicative of the bridging CO adsorption (COB) species and the telescoping vibrations of linear CO (COL), respectively. MF-Ni-900 exhibited the earliest and most pronounced appearance of COB peaks, suggesting that it displayed greater selectivity for
To further substantiate the assertion that Ni-Nx was the active site, a linear fit was conducted on the peak area and Ni-Nx content of COB [Figure 4E]. It could be observed that there was a strong linear relationship between the peak area of COB and the Ni-Nx content at all the potentials that were tested. It could thus be concluded that Ni-Nx was the active center of the catalysts and that COB was the key intermediate in the CO2RR.
The results of in situ IR indicated that the adsorption and activation of CO2 and water molecules on Ni-Nx active sites promoted the formation of *COOH, which accelerated the conversion of CO2 to CO. A mechanism for the CO2RR was therefore proposed as follows: first, CO2 was adsorbed on the catalyst, resulting in the initial electron transfer to form a *CO2- intermediate; subsequently, *CO2- protonated to form *COOH, with the H+ derived from H2O. Finally, *COOH was dehydrated to form a *CO intermediate on the surface and then desorbed to form the target product [Figure 4F].
Density functional theory calculations
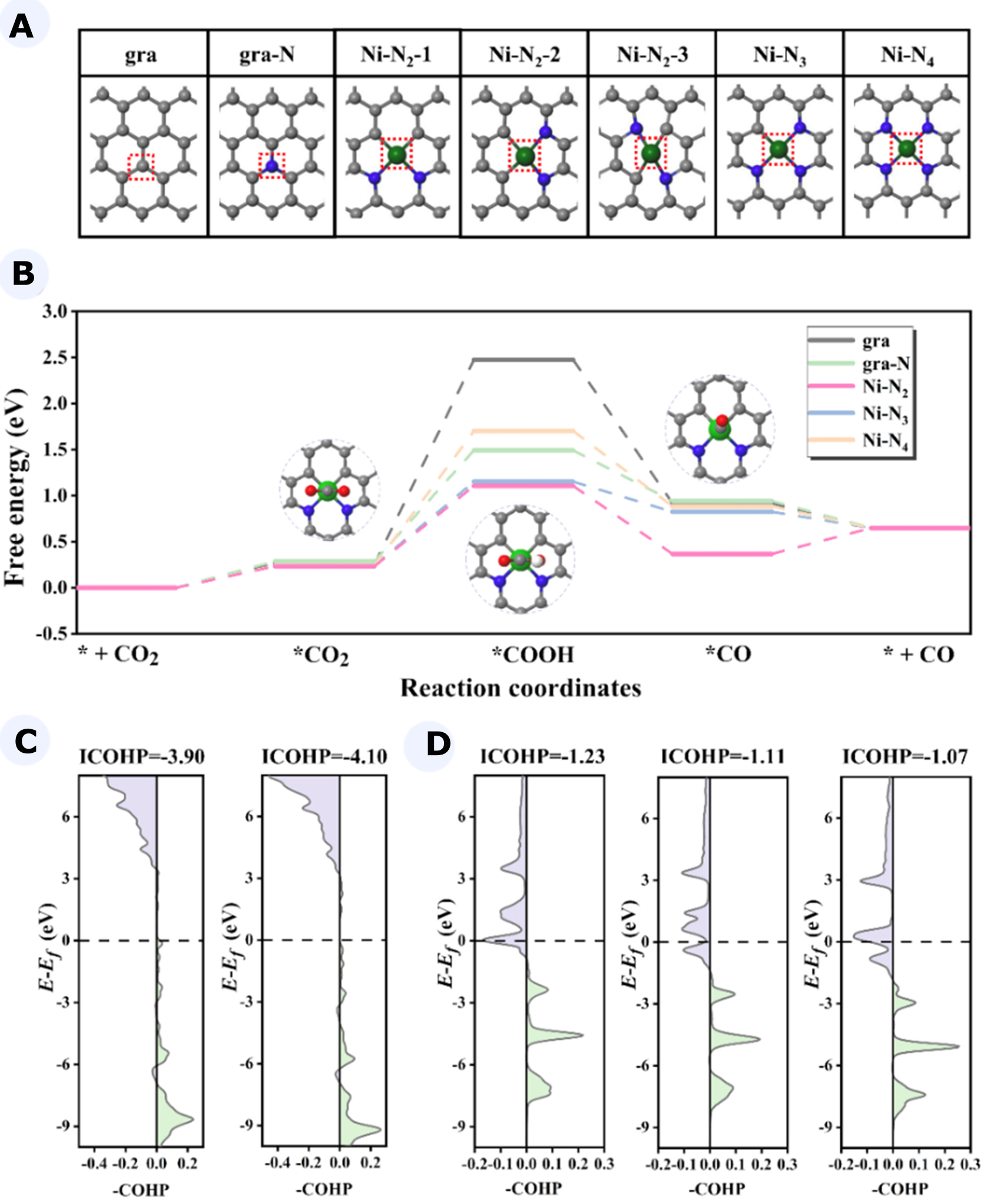
In order to gain further insight into the electrocatalytic CO2RR behavior of Ni-Nx, we adopted DFT calculations in this work. Previous studies had concluded that Ni-N2, Ni-N3, and Ni-N4 were stable coordination structures of Ni at different N concentrations in synthetic processes[36]. Therefore, the active sites on the Ni-Nx surface that we considered include gra, gra-N, Ni-N2, Ni-N3, and Ni-N4 [Figure 5A]. Particularly, it could be found that Ni-N2 had three possible initial configurations (Ni-N2-1, Ni-N2-2, and Ni-N2-3), of which Ni-N2-1 had the lowest energy with superior stability. Thus, Ni-N2-1 was selected for subsequent Ni-N2 related studies.
Figure 5. (A) The optimized structures of different active sites on the Ni-Nx surface; (B) The Gibbs free energy profile of CO2RR to CO on gra, gra-N, Ni-N2, Ni-N3, and Ni-N4. The COHP for COOH adsorbed on active site of (C) gra and gra-N, (D) Ni-N2, Ni-N3, and Ni-N4. CO2RR: Carbon dioxide reduction reaction; COHP: crystal orbital Hamilton populations.
The calculated Gibbs free energy profile of CO2RR to CO on the catalysts (gra, gra-N, Ni-N2, Ni-N3, and
The crystal orbital Hamilton populations (COHP) between *COOH (one of the intermediates in PDS) and the active sites were then calculated [Figure 5C and D] to reveal the activity origin of CO2RR on these catalysts. It was evident that the bonding states of *COOH and active sites on catalysts occurred below the Fermi level. Therefore, the bonding states were filled with electrons, which led to a strong interaction between the active sites and *COOH. The performance of Ni-Nx was governed by the energy change of
In conclusion, the results of DFT calculations indicated that Ni-N2 served as the active site that played a significant role in Ni-Nx.
CONCLUSIONS
In conclusion, the successful synthesis of Ni single-atom foam catalysts with multiple active sites was achieved by means of an impregnation-carbonation approach. The findings demonstrated that the highly cross-linked structure of MF provided a substantial amount of space for the escape of the product, which not only enhanced the dispersion of the active site but also facilitated electron transport. The content of
DECLARATIONS
Acknowledgments
We would like to acknowledge the technical support from Analysis and Testing Center of Northeast Forestry University.
Authors’ contributions
Made substantial contributions to the conception and design of the study, performed data analysis and interpretation, and wrote original draft: Wei, A.; Xu, Y.
Conducted the investigation of the study and performed data acquisition: Wang, J.; Shi, L.
Revised the manuscript and provided administrative, technical, and material support: Liu, S.; Wu, Y.; Wang, W.
Availability of data and materials
The detailed experimental methods and data are available from Supplementary Materials.
Financial support and sponsorship
This work was financially supported by the National Natural Science Foundation of China (22208048, 22172044, and 22478067), the National Key R&D Program of Heilongjiang Province, China (2022ZX02C23), the Natural Science Foundation of Heilongjiang Province (YQ2022B005), the Young Elite Scientists Sponsorship Program by CAST (YESS20210262), the Fundamental Research Funds for the Central Universities (2572023CT10).
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
Supplementary Materials
REFERENCES
1. Chen, P. C.; Chen, C.; Yang, Y.; et al. Chemical and structural evolution of AgCu catalysts in electrochemical CO2 reduction. J. Am. Chem. Soc. 2023, 145, 10116-25.
2. Guo, H.; Si, D. H.; Zhu, H. J.; Chen, Z. A.; Cao, R.; Huang, Y. B. Boosting CO2 electroreduction over a covalent organic framework in the presence of oxygen. Angew. Chem. Int. Ed. Engl. 2024, 63, e202319472.
3. Yang, X.; Yang, J.; Zhao, T.; et al. Kinetic insights into the effect of promoters on Co/Al2O3 for Fischer-Tropsch synthesis. Chem. Eng. J. 2022, 445, 136655.
4. Wan, X.; Li, Y.; Chen, Y.; et al. A nonmetallic plasmonic catalyst for photothermal CO2 flow conversion with high activity, selectivity and durability. Nat. Commun. 2024, 15, 1273.
5. Li, S.; Kan, Z.; Wang, H.; et al. Single-atom photo-catalysts: synthesis, characterization, and applications. Nano. Mater. Sci. 2024, 6, 284-304.
6. Wang, J.; Huang, Y. C.; Wang, Y.; et al. Atomically dispersed metal-nitrogen-carbon catalysts with d-orbital electronic configuration-dependent selectivity for electrochemical CO2-to-CO reduction. ACS. Catal. 2023, 13, 2374-85.
7. Zeng, Y.; Zhao, J.; Wang, S.; et al. Unraveling the electronic structure and dynamics of the atomically dispersed iron sites in electrochemical CO2 reduction. J. Am. Chem. Soc. 2023, 145, 15600-10.
8. Akula, S.; Mooste, M.; Kozlova, J.; et al. Transition metal (Fe, Co, Mn, Cu) containing nitrogen-doped porous carbon as efficient oxygen reduction electrocatalysts for anion exchange membrane fuel cells. Chem. Eng. J. 2023, 458, 141468.
9. Murphy, E.; Liu, Y.; Matanovic, I.; et al. Elucidating electrochemical nitrate and nitrite reduction over atomically-dispersed transition metal sites. Nat. Commun. 2023, 14, 4554.
10. Zhao, Z.; Shi, X.; Shen, Z.; et al. Single-atom Fe nanozymes coupling with atomic clusters as superior oxidase mimics for ratiometric fluorescence detection. Chem. Eng. J. 2023, 469, 143923.
11. Liu, H.; Zhu, P.; Yang, D.; et al. Pd-Mn/NC dual single-atomic sites with hollow mesopores for the highly efficient semihydrogenation of phenylacetylene. J. Am. Chem. Soc. 2024, 146, 2132-40.
12. Zhang, D.; Wang, Z.; Liu, F.; et al. Unraveling the pH-dependent oxygen reduction performance on single-atom catalysts: from single- to dual-Sabatier optima. J. Am. Chem. Soc. 2024, 146, 3210-9.
13. Liu, Y.; Li, C.; Loubidi, M.; et al. Increasing exposure of atomically dispersed Ni sites via constructing hierarchically porous supports for enhanced electrochemical CO2 reduction. Chem. Eng. J. 2021, 426, 131414.
14. Pan, F.; Zhang, H.; Liu, K.; et al. Unveiling active sites of CO2 reduction on nitrogen-coordinated and atomically dispersed iron and cobalt catalysts. ACS. Catal. 2018, 8, 3116-22.
15. Wang, X.; Li, X.; Ding, S.; et al. Constructing ample active sites in nitrogen-doped carbon materials for efficient electrocatalytic carbon dioxide reduction. Nano. Energy. 2021, 90, 106541.
16. Bai, J.; Sun, Z.; Zhang, H.; et al. Modulating the local coordination environment of M-Nx single-atom site for enhanced electrocatalytic oxygen reduction. Adv. Funct. Mater. 2025, 35, 2417013.
17. Huang, Z. Y.; Guo, X. S.; Tang, Y.; Ye, J. S.; Liu, H. Y.; Xiao, X. Y. Metalloporphyrin doped macroporous ZIF-8 metal-organic framework derived M-Nx carbon material for oxygen reduction reactions. J. Alloy. Compd. 2023, 947, 169441.
18. Miao, Z.; Li, S.; Priest, C.; Wang, T.; Wu, G.; Li, Q. Effective approaches for designing stable M-Nx/C oxygen-reduction catalysts for proton-exchange-membrane fuel cells. Adv. Mater. 2022, 34, e2200595.
19. Xi, D.; Li, J.; Low, J.; et al. Limiting the uncoordinated N species in M-Nx single-atom catalysts toward electrocatalytic CO2 reduction in broad voltage range. Adv. Mater. 2022, 34, e2104090.
20. Devi, H. R.; Bisen, O. Y.; Chen, Z.; Nanda, K. K. Spatially dispersed one-dimensional carbon architecture on oxide framework for oxygen electrochemistry. Chem. Eng. J. 2022, 433, 133649.
21. Hu, H.; Gao, G. H.; Xiao, B. B.; Zhang, P.; Mi, J. L. The oxygen reduction reaction activity and selectivity of porous-carbon supported transition metals (M-C: M = Mn, Fe, Co, Ni, Cu) electrocatalysts. Diam. Relat. Mater. 2023, 134, 109776.
22. Jiang, T.; Jiang, H.; Wang, W.; Mu, H.; Zhang, Y.; Li, B. Atomically dispersed high-active site density copper electrocatalyst for the reduction of oxygen. Materials 2024, 17, 5030.
23. Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B. Condens. Matter. 1996, 54, 11169-86.
24. Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15-50.
25. Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B. Condens. Matter. 1994, 50, 17953-79.
26. Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B. 1999, 59, 1758-75.
27. Perdew, J. P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865-8.
28. Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104.
29. Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456-65.
30. Nørskov, J. K.; Rossmeisl, J.; Logadottir, A.; et al. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B. 2004, 108, 17886-92.
31. Hou, Y.; Qiu, M.; Kim, M. G.; et al. Atomically dispersed nickel-nitrogen-sulfur species anchored on porous carbon nanosheets for efficient water oxidation. Nat. Commun. 2019, 10, 1392.
32. Yan, C.; Li, H.; Ye, Y.; et al. Coordinatively unsaturated nickel-nitrogen sites towards selective and high-rate CO2 electroreduction. Energy. Environ. Sci. 2018, 11, 1204-10.
33. Feng, Y.; Long, S.; Chen, B.; et al. Inducing electron dissipation of pyridinic N enabled by single Ni-N4 sites for the reduction of aldehydes/ketones with ethanol. ACS. Catal. 2021, 11, 6398-405.
34. Delmo, E. P.; Wang, Y.; Song, Y.; et al. In Situ infrared spectroscopic evidence of enhanced electrochemical CO2 reduction and C-C coupling on oxide-derived copper. J. Am. Chem. Soc. 2024, 146, 1935-45.
35. Deng, B.; Huang, M.; Li, K.; et al. The crystal plane is not the key factor for CO2 -to-methane electrosynthesis on reconstructed Cu2O microparticles. Angew. Chem. Int. Ed. Engl. 2022, 61, e202114080.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Special Topic
Copyright
Data & Comments
Data
















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].