Progress of construction of metal-zeolite catalysts for propane dehydrogenation
Abstract
The dehydrogenation of propane into propene is a crucial value-added process for producing raw chemicals from light hydrocarbons, such as shale gas. However, it faces challenges such as low propene yield and significant catalyst deactivation. In recent years, zeolite supported subnanometer or nanoparticles catalysts have attracted considerable attention and made remarkable progress due to their superior activity and exceptionally high thermal stability. In this review, we present an overview of design ideas of metal-containing zeolite catalysts used for propane dehydrogenation (PDH) reactions, with an emphasis on developments over the past ten years. A comprehensive summary is provided, encompassing the methodologies for preparing zeolite-supported metal catalysts, regulating the active site, modifying the pore structure and acid properties of zeolites, as well as their catalytic performances in PDH. The analyses emphasize the role and mechanism of metal-metal and/or metal-zeolite interactions in adjusting the structure of active sites, stabilizing metal species, enhancing catalytic performance and facilitating propylene production. Finally, the future directions of catalyst design for alkane dehydrogenation are envisioned.
Keywords
INTRODUCTION
Light olefins are among the most essential raw materials, playing a pivotal role in producing chemicals such as methyl tert-butyl ether, alkylates that are further utilized in co-polymerization processes, and high-octane gasoline additive production[1-4]. Among them, propylene is the most important as it can be further processed into valuable chemicals such as polypropylene, acrylonitrile, and propylene oxide[5-9]. Therefore, as the demand for propylene in the industry continues to rise, propylene production technology has garnered significant attention from scientists and researchers. Compared to other production routes of propylene, such as methanol-to-olefins, Fischer-Tropsch synthesis, and the steaming or catalytic cracking of naphtha, propane dehydrogenation (PDH) generally exhibits high selectivity to propylene[9,10]. It is predicted that the processing capacity of PDH will increase from the current approximately 7,000,000 to 30,000,000 t/a by 2030 in China[11].
To date, there are two primary commercial technologies for PDH process: one is Oleflex process, which employs PtSn/Al2O3-based catalysts; the other is Catofin process with CrOx/Al2O3-based catalysts[12-16]. However, the reaction is greatly endothermic in nature, requiring a high quantity of heat. This results in the aggregation and sintering of metal particles at elevated temperatures, which in turn diminishes the commercial catalytic efficacy and leads to poor reaction stability. On the other hand, consideration should also be given to developing non-toxic and environmentally friendly catalyst components to replace those prone to toxicity, such as the toxic effects of hexavalent chromium (Cr6+) species on Cr-based catalysts produced during operation[17]. Based on the analyses on thermodynamic and reaction mechanism of light alkanes dehydrogenation processes, Wang et al. summarized the catalytic behaviors of noble/non-noble metal catalysts, especially the environment-friendly catalysts (VOx-, Ni- and Sn-based, etc.), and also compared the characteristics of commercial and novel dehydrogenation catalysts[7,18].
However, these alternatives typically utilize metal oxide supports, which result in the mobility and loss of active species during the reaction. As a result, many studies are now focusing on zeolite support. The intricate network of zeolite channels and micropores enables exerting robust confinement effects, thereby impeding the growth of metal particles within a predefined size range during light alkanes dehydrogenation at high temperatures[19,20]. Furthermore, zeolite encapsulated with metal nanoparticles catalysts currently gives high activity, stability, and selectivity[21,22]. Previously published reviews have provided broad coverage of the synthesis methodologies, characterization techniques and catalytic applications of zeolite supported metal nanoparticles catalysts[23,24]. The progress in the synthesis of metal-zeolite, particularly in incorporating and tailoring active metal sites within zeolites, is emphasized, along with advanced characterization techniques for identifying the location, distribution, and coordination environment of metal species in zeolites. However, there is still an urgent need for systematic investigation into the role of metal-zeolite catalysts in PDH reaction, particularly concerning the structure-reactivity relationships.
In accordance with the reaction mechanism of PDH, this review summarizes the methodologies for preparing zeolite-supported metal catalysts, especially focusing on remarkable progress over the past ten years. The preparation method plays a crucial role in determining the catalytic behavior of PDH catalysts. Therefore, this review systematically summarized the development on the preparation methods, involving incipient wetness impregnation (IWI), ion-exchange, one-pot synthesis, atomic layer deposition (ALD), and recrystallization. Furthermore, the regulation strategies on the structure and location of active sites in zeolite are comparatively summarized in terms of alloying metals with promoters, anchoring metals via creating silanol groups nest, and creating hierarchical pores and optimizing the pore structure of the zeolite support. Finally, the challenges in the catalyst design for improving the catalytic stability and activity in the alkane dehydrogenation are discussed.
CATALYTIC MECHANISMS OF PDH
Reaction route of PDH
The PDH reaction is an endothermic process, as given in
and the molecular numbers increase during the reaction. Therefore, to facilitate the reaction equilibrium sifting to more propylene, a high temperature (450-700 °C) and a moderately low partial pressure are necessary, as shown in Figure 1. However, such conditions may cause the main side reactions, which include cracking and deep dehydrogenation of propane, as, respectively, given in
Figure 1. Thermodynamic equilibrium conversion of C3H8 to C3H6 as a function of temperature under different pressures, as calculated with HSC 6.0 software.
Furthermore, propylene typically exhibits higher reactivity than propane, resulting in the occurrence of side reactions, such as isomerization, deep dehydrogenation and C–C bond cracking, as depicted in Scheme 1[25].
Scheme 1. Reaction path network for PDH (all elementary steps are considered reversible). Reprinted with permission[25]. Copyright 2020, American Chemical Society. PDH: Propane dehydrogenation.
Reaction mechanism on metal-based catalysts
Mechanism on Pt-based catalysts
Pt-based catalysts have been widely utilized in PDH because of their ability to effectively activate C–H bonds[26]. The ensuing discussion will take Pt as an illustrative example. The analogous mechanism is observed in other precious metals, including Au, Pd, etc.[27]. The dehydrogenation reaction over Pt-based catalysts is typically regarded as following the reverse Horiuti-Polanyi mechanism, which involves two sequential β-H elimination steps in propane, culminating in the concerted liberation of H2 and C3H6 from active catalytic sites. Quite a large amount of theoretical and experimental studies have been carried out to explain the mechanism of alkane dehydrogenation over Pt-based catalysts[25,28-32]. Based on the previously performed standard density functional theory (DFT) calculations that have delivered a detailed description of the reaction path comprising isomerization, deep dehydrogenation and dehydrogenation to propylene, Lian et al. proposed the presence of two stages of PDH[28]. These are fast deactivation and steady state. The main precursor of coke is generated in the fast deactivation period. As shown in Figure 2A and B, the reaction intermediates formed during the dehydrogenation process did not accumulate on the catalyst surfaces but were reacted quickly and consumed. The results indicated that the surface reactions were unlikely to represent the rate-determining step (RDS).
Figure 2. (A) Surface reactions network for PDH; (B) Formation mechanism of Carbon species in PDH. Reprinted with permission[28]. Copyright 2018, American Chemical Society. PDH: Propane dehydrogenation.
Indeed, the Horiuti-Polanyi mechanism still works for certain dehydrogenation processes, involving three consecutive dehydrogenation steps, which are then followed by the selective hydrogenation of alkyne intermediates to produce alkenes [Figure 3][33,34]. It has been verified that the reaction mechanism is dependent on the particle size and coordination environment of Pt[35]. Based on the Nudged Elastic Band calculations, the reaction on Pt species in small clusters, two-atom clusters and single-atom Pt sites follows the non-reverse Horiuti-Polanyi mechanism during dehydrogenation, whereas reverse Horiuti-Polanyi mechanism occurred on the remaining bigger Pt clusters[36]. However, the size-dependent structure sensitivity remains elusive in PDH. In the following chapters, the influence of particle size will also be illustrated by catalysts performance.
Figure 3. (A) Non-reverse Horiuti-Polanyi mechanism for PDH; (B) Reverse Horiuti-Polanyi mechanism for PDH. (Blue represents Si, red represents O, and gray represents Pt). Reprinted with permission[36]. Copyright 2024, Wiley-VCH. PDH: Propane dehydrogenation.
Mechanism on non-noble metal-based catalysts
Pt-based catalyst serves as typical ones in large-scale PDH processes, but their application in industry is limited as a result of high cost of Pt sources. Therefore, non-noble metal catalysts as an alternative candidate have received much more attention from researchers. The CrOx- and VOx-based catalysts have been used in commercial Catofin processes and FBD-4, respectively. The V5+ species can be reduced into the V4+ and V3+ species during the course of the reactions. A close relationship has been found between the propene formation rate and the concentration of V3+ ions[37]. Therefore, the V3+ ions are recognized as the active sites for PDH among the V5+, V4+ and V3+ ions[38]. The VOx/Al2O3 exhibited an initial C3H8 conversion of 82%, declining significantly to 31% after 7 h of reaction in PDH at 600 °C[39]. The propene selectivity was only 65% and decreased to 55% within 7 h. Similarly, the Cr3+ species are obtained from the reduction of Cr6+ species[40]. The C–H bond of C3H8 molecules is initiated by the V and Cr species with low valence state. However, this causes low propene selectivity and quick catalyst deactivation. Other metal oxides, including ZrO2 and TiO2, have also been developed for PDH. Different from above mentioned fact that the cleavage of C–H bond occurred on the metal ions with low valence states, the oxygen vacancies and (Ovac) coordinatively unsaturated metal sites (Mcus) around Ovac play an important role in activating the C–H bonds[41-47]. The C–H bond can be activated via the dynamic H adsorbates on tetracoordinated Mcus[Metal atoms surrounding oxygen vacancies (Ovac)] sites, resulting in the formation of intermediates of C3H7*. The desorption of H2 is simultaneously promoted, thus giving the high propene selectivity and low deactivation. In this way, the TiOx catalyst modified with Ni to form the Ni@TiOx showed the C3H6 selectivity of 94% and C3H8 conversion of 40%, along with an exceptional stability under industrial PDH conditions (600 °C, WHSVC3H8 of 3 h-1, C3H8/H2 = 1/1)[41].
In PDH reactions, the activation of the C–H bond is considered to be the RDS that usually determines the catalyst activity[48,49]. Metal-based catalysts have been proven to be favorable in the activation of C–H bonds. The development of catalysts for C3H8 dehydrogenation is still faced with the following primary challenges: (1) eliminating the sintering of metal particles under high temperatures; (2) promoting the activation of C–H bonds; (3) explicating the nature and role of the metals. The C–H cleavage is highly dependent upon the micro-environment that surrounds the regulatory metal. The subsequent Sections will focus on addressing and tuning the physicochemical micro-environment around metal for constructing high stability and dehydrogenation activity catalysts.
CATALYST PREPARATION
Methods
IWI
IWI is considered a general, flexible and easy method for preparing the zeolite-supported subnanometric metal clusters[50,51]. Generally, the procedures are as follows: (1) preparation of a metal compound solution with a given concentration and volume equal to the pore volume of the support; (2) addition of a certain amount of support into the above solution; (3) drying of the mixtures. The obtained solid is subjected to treatment under a specific atmosphere (oxidative and/or reductive) at elevated temperatures, resulting in the conversion of the metal salt into metal or oxide. It should be noted that the process of continuous evaporation of the used solvent is usually accompanied by metal precursor diffusion into the zeolite pores as a result of capillary force[52]. Theoretically, in this way, micropores of the zeolite could be completely filled with metal precursor, achieving a very high metal loading. Therefore, uniformity of the distribution and dispersion degree is highly dependent on the metal ion amount[14].
The introduction of Pt species on zeolites via impregnation often leads to metal aggregation and catalyst deactivation[53,54]. To address poor metal dispersion, Ponomaryov et al. developed an impregnation-calcination-washing (ICW) method using NaCl additive, yielding (NaSn)(Pt)w catalyst with C3H8 conversion of 36%, C3H6 selectivity of 96%, and a deactivation constant of 0.009 h-1 at 570 °C with a weight hourly space velocity (WHSV) of 28 h-1[55]. The coexistence of NaCl in the preparation aids in diluting SnCl2, thereby enhancing Pt dispersion[56]. In contrast, deposition-precipitation (DP) confines metals within zeolite channels. A Pt@Sn/D-ERB-1 catalyst[57] prepared via DP method formed Pt nanoparticles in 5 nm, achieving C3H8 conversion over 60% and C3H6 selectivity over 95% at 575 °C. The SnO2 shell in this catalyst effectively modified the edge and corner of Pt nanoparticles, thereby inhibiting the production of by-products and coke. Bimetallic impregnation further reduces particle size[58]. Wang et al. prepared ultra-small PtCo bimetallic nanoparticles (1-2.5 nm) loaded on dealuminated nano-Beta zeolite, while obtaining Pt particles in 10 nm without the presence of Co promoters[59]. Under CO2 atmosphere, the C3H8 conversion of ~52%, C3H6 selectivity of ~92%, and C3H6 yield of ~48% were achieved using the PtCo-SiBEA catalyst, whereas much lower C3H8 conversion of 3.6% was acquired on the Pt-SiBEA catalyst. Besides, rare-earth elements (REEs), such as La and Y, were incorporated with Pt into mesoporous MFI zeolites via impregnation to strengthen metal-support interactions (MSI), stabilizing ultrafine Pt nanoparticles[60]. The PtLa/mz-deGa catalyst achieved initial propane conversion of 40% (near equilibrium) with minimal deactivation over 30 days (at 580 °C under pure propane flow with WHSV of 11 h-1). The silanol nests stabilize single-atom rare earth elements via coordination bonds and critically govern alloy formation. Furthermore, silanol density of the support modulates the chemical state of PtSn species, triggering a transition from atomically dispersed Ptδ+-Ox-Sn sites to PtSn alloy as the silanol density decreases[61].
Recent research has demonstrated a zeolite-confined Rh-In catalyst (In/Rh@S-1) for PDH, where the stabilization of Rh single atoms (SAs) is achieved through the dynamic migration of In species into silanol groups (Si-OH) under reaction conditions[22]. The Rh@S-1 catalyst exhibited high C–H bond activation activity but underwent rapid deactivation due to Rh cluster formation and non-selective propane cracking. Introducing In via impregnation or physical mixing with In2O3 facilitated In migration into S-1 pores during induction periods, forming RhIn4 active sites anchored via In–O bonds. This configuration suppressed Rh aggregation and carbon deposition, achieving propane conversion of 63% and propylene selectivity of 98% with exceptional stability (> 1,200 h at 600 °C) and no detectable deactivation over 5,500 h under pure propane at 550 °C. In situ XAS and CO-IR analyses revealed that reduced In3+ species migrated into S-1 channels, coordinating with Rh to form electron-rich RhIn clusters stabilized by the zeolite framework.
Ion exchange
The ion exchange (IE) method serves as one of the earliest techniques for introducing metal ions into zeolites. The process typically involves the exchange of one metal ion between the dilute solution and ion-exchange sites within the zeolite. The exchanged metal amount can be easily controlled by changing the concentration in solution, exchange time, and temperature, which determine the degree of exchange[62-64].
The IE method offers more flexibility as it allows for the use of many metals without ligands, so it is widely used for metal-zeolite catalysts[65-67]. Liu et al. prepared PtZn@Zn-4 catalyst by ion-exchanging zincosilicate zeolite (Si/Zn = 84) with Pt-Zn species[66]. Notably, it exhibited remarkable performance on PDH with a propane conversion of ~30% and a propene selectivity of ~99% under pure propane flow at 550 °C for at least 168 h without significant deactivation. The Pt-X catalyst, prepared via IE method with low platinum loading (0.3 wt%), exhibited better PDH performance (C3H8 conversion of 36.9% and C3H6 selectivity of 30.4% at 550 °C) than Pt-USY catalyst which showed C3H8 conversion of 31.2% and C3H6 selectivity of 7.3% at the same conditions[67]. It is speculated that, on zeolite-X, Pt species anchored at pore mouths collaborate with weak acid sites and promote the formation of C3H6, while USY-encapsulated Pt species synergize with strong acid sites to produce aromatics due to its localization in the pore channels. However, the inherent inefficiency of the ion-exchange method necessitates sacrificial precursor, as evidenced by the preparation of Pt-X catalyst where 40% of Pt remains unutilized. Metal IE saturation is commonly observed in solution-based IE processes, whereas the solid-state ion exchange (SSIE) method demonstrates enhanced IE capacity. Zn/H-MFI with distinct Zn/Al ratios, which was prepared via SSIE by ZnCl2, showed different activity in PDH[65]. At lower Zn/Al ratios (0.06-0.52), Zn is present as [ZnCl]+ (Lewis acidic) and [ZnCl(HCl)]+, whereas the selectivity of C3H6 increased from ~55% to ~90%, with the increase of Zn loading. At higher Zn/Al ratios (0.79 and 0.94), the presence of ZnAl2O4 and/or ZnAl2O4-xCl2x was noted; however, no additional enhancement in the dehydrogenation rates was observed. It is likely due to the poor dehydrogenation activity on ZnAl2O4. These indicated that the active Zn2+ cations are formed in lower Zn/Al ratios even though Zn is only present as a Lewis acidic site, which may change the framework Al distribution through cation-exchanging.
Prior to identifying IE sites (within zeolite channels or on framework surfaces), the exchange mechanism should be clarified through surface charge analysis. Zeta potential (ζ) measurements reveal negatively charged surface groups in zeolites, indicating that NH4+ removal primarily follows an IE mechanism rather than electrostatic adsorption. This is further supported by the insensitivity of zeta potential to NH3/NH4+ concentration variations, as fixed structural charges dominate the exchange process[68,69]. Furthermore, the interaction between metal catalysts and support materials in dehydrogenation of cyclohexanone to phenol can be rationalized by their surface charge compatibility, as demonstrated by zeta potential studies. Metals with positive ζ values such as Pd, Ru, Re, and Rh bind effectively to negatively charged supports such as mesoporous carbon, silica, or H-Y zeolite. Conversely, Pt exhibits optimal activity on positively charged
One-pot synthesis
Although many efforts have been devoted to the uniform metal clusters via the abovementioned methods, it is still a challenge to obtain the highly uniform and dispersed metal clusters into the zeolite. In recent years, a new method has been developed through the addition of one or more metal species into the zeolite synthesis precursor gel before the crystallization process, denoted as one-pot synthesis. Although this methodology was first articulated in 1962 by Weisz et al. in their seminal work[71], it was employed to synthesize catalysts for alkane dehydrogenation reactions up until the last decade[72-74]. This approach can facilitate the formation of a zeolite with encapsulated metal species of controllable size, which range from SAs to large nanoparticles. Furthermore, a uniform distribution of metal nanoparticles within the zeolite matrix is usually obtained because the zeolite structure assembles around the metal precursors. However, during the crystallization process of zeolites under hydrothermal conditions, the high surface energy of metal clusters results in severe aggregation within the zeolite precursors. Thus, it is a challenge to build a protective approach to balance the rates of metal aggregation or fast precipitation of ultra-small metal clusters in the synthesis gel and zeolite nucleation during the thermal crystallization processes.
Otto et al. synthesized various Al-containing zeolites with the confinement of monodisperse AuPd, AuPt, and PdPt nanoparticles through ligand-stabilized methods[75-81]. The confining nanometer voids of zeolite restricted metal clusters mobility during thermal treatment, which effectively enhanced the catalytic stability in oxidative dehydrogenation of alkanols. Based on this, Sun et al. synthesized silicalite-1 (S-1) zeolite encapsulated with subnanometer Pt-Zn nanoclusters through one-pot synthesis method by adding Pt and Zn precursors coordinated with ethylenediamine into the synthesis gel [Figure 4][21]. The H2-pretreated PtZn@S-1-H (H represents the direct reduction with H2 flow without calcination process) catalyst exhibited high C3H6 selectivity (over 99%) with a WHSVC3H8 of 3.6-54 h-1 and specific activity of C3H6 formation (up to 65.5 molC3H6·gPt-1·h-1, WHSV = 108 h-1) at 550 °C, and over 40% C3H8 conversion was maintained even after 216.7 h on stream (WHSV = 3.6 h-1) in PDH. In contrast, the C3H8 conversion over Pt@S-1-H and Pt@S-1-C (C represents conventional calcination/reduction process) decreased sharply to 19.8% and 2% within 48.3 and 5.6 h on stream, respectively. Besides, PtZn@S-1 with ultra-small PtZn bimetallic nanoclusters confined within S-1 zeolite was synthesized by ligand-stabilized methods[82]. Within 60 h reaction at 550 °C, no obvious large PtZn bimetallic particles were observed and the rate of C3H6 formation only decreased from 28.2 to 26.7 mol·gPt-1·h-1. In contrast, the C3H6 formation rate decreased from 21.5 to 17.9 mol·gPt-1·h-1 after
Figure 4. Scheme of synthesizing S1 zeolite with encapsulation of subnanometer PtZn cluster. Reprinted with permission[21]. Copyright 2020, Wiley-VCH.
Similarly, Pt and PtSn nanoclusters were confined in sinusoidal channels of silicalite-1 for Pt@MFI and PtSn@MFI samples, respectively, via one-pot synthesis[83,84]. It is found that a portion of reduced Sn(II) species promotes the Pt dispersion by forming the PtSn alloys [Figure 5], resulting in enhanced stability. The K-PtSn@MFI sample after being reduced at 600 °C for 22 h displayed a high specific activity of
Figure 5. Structural evolutions of Pt and Sn species over K-PtSn@MFI during reduction with hydrogen. Reprinted with permission[83]. Copyright 2020, Springer Nature.
Figure 6. Scheme of the formation of Pt/Sn-ZSM-5 using the ultrafast crystallization method. Reprinted with permission[86]. Copyright 2020, Wiley-VCH.
This method can also be applied to zeolite with encapsulation of non-noble metal. The ultra-small CoO clusters encapsulated in S-1 zeolite (CoO@S-1) were manufactured in this way[87]. This catalyst exhibited an attractive C3H6 formation rate of 13.66 mmolC3H6·gcat-1·h-1 with a selectivity of approximately 92% at 600 °C in PDH (WHSVC3H8 = 4.5 h-1). Interestingly, it can remain stable for 120 h through five consecutive regeneration cycles. This is attributed to the enhancement of C3H8 adsorption and stabilization of detached H* species from C3H8 on CoO clusters. Similarly, single-site Fe ions confined into an MFI-type framework were synthesized through rapid mixing of a ferric precursor and mineralizing agent at high crystallization temperature[88]. This catalyst achieved C3H6 selectivity > 95% and total olefins selectivity > 99% at 600 °C and WHSV molC3H8·molFe-1·h-1 = 900 in PDH. It had an excellent coke tolerance with a low deactivation rate constant (0.01 h-1). The isolated Fe ions within the framework resulted in the distortion of the tetrahedral framework, and formed elliptical 10-MR ring. It exhibited a shorter semi-axis than that observed in traditional MFI zeolites and proved that it is beneficial for selective dehydrogenation of C3H8 to C3H6. This resulted in a reduction in shape selectivity for aromatic species, limiting the potential for coke formation.
Besides, the nanoparticles are confined within the other topological zeolite, such as MWW, which is formed accompanied by the transformation from 2D (stacked two-dimensional) to 3D (stacked three-dimensional) structure during the calcination process[89]. The Pt coordinated with ligand penetrated into the interlayer space between 2D layers of MWW which can be swollen by surfactants. The subsequent calcination treatment resulted in the removal of the template and made Pt trapped within MWW zeolite [Figure 7]. Pt@MWW with a Pt particle size of 1-2 nm showed a better catalytic performance [turnover frequency (TOF) of 0.12 s-1] and stability than MWW zeolite with impregnated Pt. Following five reaction-regeneration cycles, the Pt@MCM-22 catalyst retains approximately 90% of its initial activity, whereas the other impregnated sample (Pt/MCM-22) has lost over 40% of its initial activity in the C3H8 dehydrogenation reaction at 550 °C (WHSV = 2.7 h-1).
Figure 7. Scheme of the synthesis of Pt@MCM-22. Reprinted with permission[89]. Copyright 2016, Springer Nature.
ALD
ALD has emerged as a promising technique in recent years, offering precise control of the metal structure at the atomic level[90-93]. Pt deposition as an example, during an ALD process, two gaseous precursors are alternately pulsed, where each reacts with the surface functional groups generated during the previous deposition cycle [Figure 8]. The metal structures and size can be tuned by designing the repeated number of deposition cycles and precursor materials.
Figure 8. Schematic illustration of Pt deposition on zeolite using MeCpPtMe3 and O3 as Pt precursor and carrier gas, respectively, by film ALD. Reprinted with permission[94]. Copyright 2021, Wiley-VCH. ALD: Atomic layer deposition.
Typically, an ALD process is costly due to the frequent necessity of numerous cycles to attain the metal loading of approximately 1%. Therefore, variable surface modifications of zeolites were used to improve metal loadings[95]. The modification of TDMA Sn (tetrakis(dimethylamido)tin)/SnO2 as nucleation sites showed a potential for enhancing the Pt content via ALD, yielding up to 40 times higher than that obtained via ALD with 20 cycles in the absence of TDMA-Sn/SnO2 (1.42 wpt% in Pt/TDMASn/S-1 vs. 0.034 wpt% in Pt/S-1). The initial conversion of C3H8 was 36.5% on Pt/TDMASn/S-1, remarkably higher than that on Pt/SnO2/S-1 (9.28%) and Pt/S-1(1.14%) at 550 °C and WHSVC3H8 of 5.4 h-1 in PDH reaction. More importantly, S-1 with chemical modification could greatly enhance the anchored metal amount in ALD process. For example, the Lewis active [ZnOH]+ sites were formed on the hollow porous structure of HPS-1, which was favorable for promoting the dispersion of Pt through the ALD process. Pt/Zn-HPS-1 showed higher initial C3H8 conversion (34.3%) and high C3H6 selectivity (nearly 99%) at 550 °C with WHSV = 0.5 h-1 in PDH[96]. In contrast, the sample synthesized by IM method (Pt/S-1) gave initial C3H8 conversion of ~2%.
Despite its atomic-level precision, ALD encounters substantial barriers in industrial adoption, including but not limited to[97,98]: (a) requiring high reactivity and sufficient thermal stability of the precursor under the deposition temperature; (b) involving costly precursor and deposition conditions; (c) an unacceptably low growth rate so that it causes difficulties in batch production; (d) hard to achieve targeted phase purity, crystallographic orientations, or optimal crystallinity. Currently, the application of ALD in constructing PDH catalysts remains limited.
Recrystallization
Encapsulating metal through a process of dissolution-recrystallization has been proven efficient on preparing catalyst with high resistance to metal sintering at high temperatures[99,100]. That is, the catalysts were subjected to hydrothermal treatment in certain types of alkaline solutions, such as tetrapropylammonium hydroxide (TPAOH), etc. The resulting metal@zeolite composites exhibited a yolk-shell structure, wherein the metal particles were confined. Despite the fact that this method has been demonstrated to be one of the most effective and straightforward techniques for encapsulating metal nanoparticles into zeolite, the average size of the metal nanoparticles is not reduced to below 5 nm, even for catalysts with a metal content of less than 1 wt%[101,102]. However, Chen et al. successfully synthesized the multi-hollow Silicalite-1 structure through a recrystallization process[103]. This structure not only enabled the confinement of highly dispersed single or bimetallic nanoparticles [Figure 9], but also facilitated an increase in the Pt content up to 1.67 wt%. The scanning transmission electron microscopy (STEM) images of the Pt@S-1 verified that the Pt nanoparticles had a diameter of approximately 2.9 nm; the Pt particle size was 29.1 nm for the Pt/S-1(IM). Propane conversion was about 55% over the Pt@S-1, while the Pt/S-1 gave the initial conversion of propane of only 19% (WHSV = 6 h-1, 600 °C).
Figure 9. Schemes of the formation of hollow or multi-hollow silicalite-1 zeolite. Reprinted with permission[103]. Copyright 2021, Elsevier.
Similarly, the core-shell structure serves to impede the accumulation of carbon. Kubota et al. prepared core-shell structured S-1 crystals confined with Co nanoparticles (CS-S-Co) with Co nanoparticles in the external S-1 layers [Figure 10][104]. The weight loss of carbon in the period from 300 to 800 °C by Thermogravimetric analysis showed that the mass loss of CS-S-Co (~0.2%) was much smaller than S-Co (~2.5%). Therefore, CS-S-Co catalyst showed superior activity in the PDH reaction, achieving a C3H6 yield of 47.1% at 600 °C.
Figure 10. Scheme of formation of core-shell-structured catalyst. Reprinted with permission[104]. Copyright 2021, Royal Society of Chemistry.
Pre-treatment
The pre-treatment conditions had a great impact on the catalysts, such as zeolite surface area, the structure of the metallic phase and the MSI. All these factors can affect the performance of PDH[84,105-107]. The redispersion of metal particles in zeolites with the assistance of reduced gaseous treatment has been previously documented in the research[108]. However, the positions of atoms and/or particles at the atomic level have not been fully elucidated in those prior works. With the development of characterization techniques, Liu et al. elucidated the structural dynamic evolutions of subnanometric platinum species with the redox treatments at high temperatures[109]. It was found that a portion of the platinum species present at the surface or subsurface of the material was able to diffuse into the internal voids of the MFI zeolite during the calcination process conducted in air at 650 °C. Following an oxidation-reduction treatment, external platinum nanoparticles on the surface will migrate into the internal surface and subsequently be stabilized within the sinusoidal channels [Figure 11]. The pre-treatment conditions had significant influence on the catalytic performance of catalysts. When the as-prepared sample K-PtSn@MFI was reduced with hydrogen in the reactor at 600 °C for 1 h directly before the catalytic test, a low initial selectivity to C3H6 (~50%) was obtained. In contrast, in the case of one calcination-reduction cycle (at 650 °C), the initial selectivity to C3H6 reached about 95% (reaction condition: 600 °C, WHSV= 1.7 h-1).
Figure 11. Scheme of Pt species evolutions during high-temperature oxidation and reduction treatments. Reprinted with permission[109]. Copyright 2020, Elsevier.
The pre-reduction temperature and time duration affect the location and chemical states of metal species in catalysts. The pre-reduction process would induce the framework metal ions to migrate out of the framework, leading to the formation of extra-framework metal species. The pre-reduction in hydrogen atmosphere at different temperatures is employed to adjust the Co-species and silica interaction, thereby influencing the C3H6 selectivity in PDH[110]. In order to study the intrinsic properties of these Co catalysts, the C3H8 conversion was kept at about 10%. The unreduced Co@MCM-41 comprising the framework Co2+ exhibited excellent interaction with the support featuring a specific reaction rate at 4.0 × 10-3 s-1, as well as a C3H6 selectivity of 91% in PDH. However, the reduction at 650-700 °C resulted in the migration of Co species to the extra-framework and subsequently the formation of small-sized metallic Co0 nanoclusters with a moderate interaction with silica. This resulted in two- to three-fold reaction rates (12.2 × 10-3 s-1) and high C3H6 selectivity (95.1%) at the same conditions.
In addition, alteration of the treatment atmosphere has a non-negligible effect on the metal valence state and location in zeolites. Various Co species over S-1 are achieved through the modulation of heat-treatment atmosphere. In comparison with different treatment atmospheres, the TOF of Co-S-1-Ar for PDH is over nine times higher than that of Co/S-1-Air (41.9 vs. 4.6 h-1) within 600 min at 500 °C and WHSV= 21.6 h-1 in PDH[111]. In situ FT-IR analysis revealed that Co2+ species are incorporated into the S-1 framework through the replacement of the silanol nests of S-1 in the Ar atmosphere. This process is driven by Ar, which causes the Co precursors to reach the silanol nests in a highly dispersed state. The precursors were further anchored by silanol nests and incorporated into the S-1 framework in ion species form during calcination at high temperatures in atmospheres free from oxygen, for example, H2 and vacuum. A novel strategy has been developed for the production and stabilization of sub-nanometre, even atomically dispersed PtSn clusters within S-1 crystals. This was achieved by changing the calcination atmosphere from air to Ar[112]. The PtSn@Silicalite-1 gave uniform PtSn clusters (~0.7 nm) through Ar calcination atmosphere, compared to
Figure 12. Scheme of tailoring ultra-small bimetallic PtSn clusters within Silicalite-1 crystals and their catalytic performances for PDH. Reprinted with permission[112]. Copyright 2024, Elsevier. PDH: Propane dehydrogenation.
ENGINEERING THE STRUCTURE OF ACTIVE SITES
Promoters
Pt alloys
In section “Mechanism on Pt-based catalysts” concerning the PDH mechanisms on Pt-based catalysts, it has been stated that the C–C bond breakage can result in the decrease of propylene selectivity and increase in carbon deposition, representing undesirable side reactions. However, C–C bonds exhibit a more favorable cleavage energy (~246 kJ·mol-1) compared to C–H bonds (~363 kJ·mol-1), from the thermodynamic perspective, and thus results in the generation of by-products (CH4 and C2H4) and coke deposition on catalysts. Theoretical research shows that modifying Pt with oxyphilic metals promotes the C–H bond activation over C–C scission, due to the fact that smaller HOMO-LUMO gap of oxyphilic metal species compared to pure Pt enables a superior overlap with the C–H anti-bonding orbitals.[113] Moreover, large Pt particles with exposed (111) surface favors the C–C bond breakage and leads to the side reactions, such as cracking and over-dehydrogenation.[114] Consequently, it is necessary to modify the geometric and electronic structures of the platinum surface by incorporating promoters such as: Sn[57,109,115-133], Zn[82,115,134-142], Fe[143-147], Ga[148,149], In[150-152], Cu[153-155],Co[59,155], Bi[156],Ce[143] and La, Y[60], etc., which effectively facilitate the formation of PtM alloys.
These Pt-based catalysts for alkane dehydrogenation are summarized in Table 1. Wei et al. made a comparison of PtZn@S-1, PtCu@S-1 and PtSn@S-1 samples (platinum loading ~0.17 wt%)[115]. PtSn@S-1 showed C3H8 conversion of 45% and C3H6 selectivity of 99% at 550 °C in PDH, which approached the thermodynamic equilibrium. In contrast, Pt@S-1 gave a lower C3H6 selectivity of 88% at a C3H8 conversion of 41% with WHSV of 3.6 h-1. Both the C3H8 conversion and C3H6 selectivity increased in the order of Pt@S-1 < PtCu@S-1 < PtZn@S-1 < PtSn@S-1. The average coordination number (CN) of Pt–O bonds on PtCu@S-1, PtZn@S-1 and PtSn@S-1 was 2.7, 2.0, and 1.7, respectively, considerably lower than 6 of PtO2. The average CN of Pt-M (M for Pt or promoters) bonds was 2.3, 2.9 and 3.2, respectively, also lower than 12 that represents the saturated Pt–Pt bonds. It is deduced that the introduction of a second metal could decrease the CN of Pt–Pt bonds, thereby promoting the dispersion of Pt species.
Comparison of the catalytic performance for PDH over various Pt-based catalysts
| Catalyst | Temp. (oC) | WHSVa (h-1) | Feed composition | Conv. (%) | Sel. (%) | Operation time (h) | Specific activityb (s-1) | kdc (h-1) | Ref. |
| PtSn/ZSM-5 | 600 | 4.0 | C3H8/H2 = 17/14 | 42.2-30.9 | 85.7-78.6 | 7 | 0.34 | 0.07 | [117] |
| PtSn/ZSM-5 | 600 | 4.0 | C3H8/H2 = 17/14 | 42.2-30.9 | 85.7-78.5 | 7 | 0.34 | 0.07 | [118] |
| PtSn/SAPO-34/ZSM-5 | 600 | 4.0 | C3H8/H2 = 17/14 | 47.7-40.9 | 92.2-85.9 | 10 | 0.42 | 0.05 | [119] |
| Pt–Sn/ZSM-5 | 590 | 3 | C3H8/H2 = 4/1 | 33.1 | 44.7 | 6 | 0.21 | 0.023 | [122] |
| PtSn1.00/ZSM-5(0.8) | 570 | 7.07 | C3H8/H2/N2 = 1/1/8 | 46 | 98 | 72 | 0.942 | 0.0175 | [123] |
| Pt/Sn-silicalite-1 | 600 | 3.2 | Pure C3H8 | 42 | 90-95 | 70 | 0.33 | 0.012 | [124] |
| PtSn@Silicalite-1 | 600 | 760 | C3H8/N2= 3/7 | 49.4 | 98.5 | 64 | 0.002 | 0.0001 | [112] |
| Pt/Sn-ZSM-5 | 600 | 5.2 | C3H8/N2 = 1/5.5 | 45.0 | ~99.0 | 25 | 1.60 | 0.043 | [86] |
| Pt0.2Sn1.4@S-1 | 550 | 3.6 | C3H8/N2 = 1/3 | 45-35 | 97-99 | 300 | 1.16 | 0.001 | [115] |
| Pt/Sn2.00-beta | 550 | 140 | C3H8/H2/He = 1/1/8 | 51.1-46.1 | 95.4-98.6 | 24 | 16.45 | 0.0083 | [125] |
| 0.3Pt/0.5Sn-Si-beta | 550 | 1 | C3H8/N2 = 1/19 | 27.5-25.2 | 99.1-99.9 | 24 | 0.13 | 0.007 | [126] |
| Pt@Sn-beta | 500 | 0.19 | C3H8/N2 = 1/19 | 47 | > 99 | 300 | 0.0255 | 0.001 | [127] |
| Pt-Sn/SBA-15 | 590 | 3 | C3H8/H2 = 4/1 | 11.0-6.0 | 80 | 6 | 0.08 | 0.110 | [122] |
| Pt/0.5Sn-SBA-15 | 580 | 8.25 | C3H8/Ar = 7/3 | 43.8-38.2 | 97.2-97.3 | 6 | 0.42 | 0.0386 | [128] |
| Pt-Na/Sn-ZSM-5 | 590 | 3 | C3H8/H2 = 4/1 | 41.7 | 95.3 | 24 | 0.29 | 0.012 | [129] |
| PtSnNa/Ce-ZSM-5 | 590 | 3 | C3H8/H2 = 4/1 | 43.5 | 84.5 | 7 | 0.29 | 0.017 | [130] |
| PtSnK/ZSM-5 | 590 | 3.0 | C3H8/H2 = 4/1 | 34.4-31.8 | 92.3-94.1 | 7.83 | 0.25 | 0.015 | [131] |
| K-PtSn@MFI | 600 | 1.7 | C3H8/N2 = 1/3.2 | 71-42 | 88-95 | 65 | 0.31 | 0.019 | [84] |
| K-PtSn@MFI-H2 | 600 | 29.5 | C3H8/He = 24/76 | 38.7-31.9 | 97 | 25 | 3.5 | 0.012 | [83] |
| (3NaSn)(Pt)ww | 570 | 28 | Pure C3H8 | 36 | 96 | 25 | 2.84 | 0.009 | [55] |
| PtSnNa/SUZ-4 | 590 | 3 | C3H8/H2 = 1/3 | 24.7-20.2 | 90-94.7 | 9 | 0.164 | 0.029 | [132] |
| 0.5Pt2Sn-1Na/SUZ-4 | 590 | 3 | C3H8/H2 = 1/3 | 24.6-20.6 | 81.3-89.7 | 10 | 0.14 | 0.0229 | [133] |
| PtZn4@S-1-H | 550 | 3.6 | C3H8/N2 = 1/3 | 47.7-40.4 | 93.2-99.2 | 216.7 | 0.25 | 0.001 | [21] |
| PtZn4@S-1-H | 550 | 108 | C3H8/N2 = 1/3 | 21.2-11.8 | 98.3-98.6 | 10.33 | 3.55 | 0.072 | [21] |
| 0.3Pt0.5Zn@S-1 | 550 | 6.5 | C3H8/N2 =11/19 | 45-42 | 99.0-99.9 | 60 | 1.53 | 0.002 | [82] |
| 0.3Pt1Zn@Beta | 550 | 4.7 | C3H8/N2 = 1/4 | 36.8 | 99.3 | 180 | 2.3 | 0.004 | [136] |
| PtZn@S-1-Fin | 600 | 12 | Pure C3H8 | 48.3-44.9 | 97.1-96.9 | 80 | 1.51 | 0.0017 | [137] |
| PtNa/Zn-ZSM-5 | 590 | 3 | C3H8/H2= 4/1 | 40.6-37.6 | 93-97 | 10 | 0.279 | 0.013 | [138] |
| ZnPt/HZSM-5 | 525 | 0.24 | C3H8/N2 = 1/19 | 55.9-43 | 84.7-93.2 | 65 | 0.14 | 0.008 | [139] |
| Pt0.0219%Zn2%@S-1 | 550 | 8 | C3H8/N2 = 14.8/44.4 | 33.9-24.3 | 99.5-99.4 | 6.4 | 18.4 | 0.073 | [140] |
| Pt0.2Zn0.8@S-1 | 550 | 3.6 | C3H8/N2 = 1/3 | 43-34 | 98-99 | 30 | 1.05 | 0.012 | [115] |
| PtZn/0.02TS-170 | 600 | 12 | C3H8/H2/N2 = 1/1/4 | 54.4-20.5 | 98.2-96.3 | 96.8 | 1.579 | 0.0158 | [141] |
| 0.7Pt0.7Zn/MZ | 580 | 13 | Pure C3H8 | 35-15 | 98-92 | 600 | 0.816 | 0.001 | [135] |
| 0.1Pt-2Zn/Si-beta | 550 | 2.4 | C3H8/He = 1/9 | 65-37 | 98-99 | 150 | 1.891 | 0.008 | [142] |
| PtFe@Pt/SBA-15 | 600 | 3.48 | C3H8/H2/N2 = 26/26/48 | 35 | 85 | 2 | 0.169 | 0.083 | [144] |
| Pt/Fe-3 | 580 | 17 | Pure C3H8 | 39.2-36.5 | 90.2-98.2 | 74 | 7.508 | 0.0015 | [145] |
| PtSn/Fe-S-1 | 580 | 0.055 | C3H8/Ar = 1/4 | 52.0 | 95.0 | 50 | 0.21 | 0.049 | [146] |
| FePt/Sn-SBA-15 | 580 | 0.055 | C3H8/Ar= 1/4 | 56.8 | 95.2 | 35 | - | 0.015 | [147] |
| PtGa@MFI | 600 | 5.9 | C3H8/H2/N2 = 1/1/2 | 22.5 | 98 | 20 | 0.357 | 0.02 | [149] |
| PtGa/MFI | 600 | 5.9 | C3H8/H2/N2 = 1/1/2 | 24 | 98 | 20 | 0.322 | 0.06 | [149] |
| Pt/Ga-MFI | 600 | 5.9 | C3H8/H2/N2 = 1/1/2 | 14 | 95 | 20 | 0.193 | 0.03 | [149] |
| 1.5Ga0.1Pt@S-1 | 600 | 0.65 | C3H8/N2 = 1/19 | 45.9-41.6 | 92-95 | 24 | 1.111 | 0.0068 | [148] |
| PtIn@S-1 | 580 | 4.7 | C3H8/H2/Ar= 2/2/1 | 31.4 | 96.0 | 870 | 2.06 | 0.000028 | [150] |
| 0.5Pt0.5InK@S-1 | 550 | 6.75 | C3H8/N2 = 1/3 | 41.8-39.2 | 95.7-99.5 | 20 | 1.02 | 0.0025 | [151] |
| 0.5Pt0.5Ce/beta-557 | 550 | 1.18 | C3H8/H2/Ar= 1/1/38 | 71.1 | 81.2 | 20 | 0.199 | 0.141 | [143] |
| Pt0.2Cu0.8@S-1 | 550 | 3.6 | C3H8/N2 = 1/3 | 44-25 | 96-99 | 22 | 1.13 | 0.038 | [115] |
| Cu0.6Pt0.1@S-1 | 610 | 1.3 | Pure C3H8 | 46 | 93.2 | - | 0.12 | 0.68 | [154] |
| 0.2Pt0.3Co0.5CuK@S-1 | 550 | 5.4 | C3H8/N2 = 1/3 | 44.4-41.9 | 90.5-98.7 | 93 | 7.51 | 0.0011 | [155] |
| 0.1Pt0.4CuK@S-1 | 500 | 5.4 | C3H8/N2 = 1/3 | 24.2-24.8 | 97.6-98.2 | 73 | 1.77 | 0.00032 | [153] |
| PtLa/meso-MFI | 580 | 11 | Pure C3H8 | 41 | 98 | 720 | 0.55 | 0.003 | [60] |
| PtY/meso-MFI | 580 | 11 | Pure C3H8 | 41 | 94 | 240 | 0.55 | 0.006 | [60] |
It has been widely demonstrated that promoters can suppress unwanted side reactions, inhibiting coke deposition, and stabilizing active compounds. Recently, Luo et al. reported that ultra-stable PtIn clusters encapsulated within silicalite-1 efficiently caused the olefin molecules to desorb from the surface, improving the catalytic lifetime[150]. The mean lifetime of PtIn@S-1 was calculated to be 37,317 h, with a high C3H6 selectivity over 97% at 580 °C and WHSVC3H8 of 4.7 h-1.
PtZn bimetallic nanoparticles supported on Beta zeolite were synthesized by one-pot method[136]. It is found that skeletal Zn atoms easily migrated out of the zeolite framework and reduced to metal Zn, resulting in the formation of Pt1Zn1 alloy [Figure 13] in PtZn@Beta catalyst. The agglomeration of metallic Pt nanoparticles occurred in PtZn/Beta (prepared by the impregnation method). The obtained PtZn@Beta catalyst showed the initial C3H8 conversion of 36.8% and C3H6 selectivity of 99.3% and extremely low deactivation rate constant (0.004 h-1) past 24 h with WHSVC3H8 of 4.7 h-1 at 550 °C in PDH. Zhou et al. prepared an S-1 zeolite encapsulated with highly dispersed platinum clusters alloyed with copper[153]. PtCuK@S-1 exhibited a C3H8 conversion of 24.8% with a C3H6 selectivity of 98.2% in PDH (at 500 °C). The specific activity of C3H6 formation was up to 32 mol·gpt-1·h-1. No apparent deactivation was observed even beyond 73 h of operation, resulting in an ultra-low deactivation rate constant of 0.00032 h-1. The copper promoted the dispersion of platinum and provided electrons to platinum. Furthermore, CoCu-modified platinum species were confined in S-1 zeolite by one-pot method[155]. This held true that introduction of bimetallic additive of Co, resulting in prolonged the lifetime in PDH. It is found that no apparent deactivation was observed on 0.2Pt0.3Co0.5CuK@S-1 after 93 h of operation. It showed an ultra-low deactivation rate constant of
Figure 13. (A-D) Cs-corrected HAADF-STEM image and corresponding line intensity profile of PtZn@Beta; (E-H) HAADF-STEM image and corresponding line intensity profile of PtZn/Beta. The red represents Pt and blue represents Zn. Reprinted with permission[136]. Copyright 2023, Elsevier. HAADF-STEM: High-angle annular dark-field scanning transmission electron microscopy.
In addition, certain alloy systems generating Lewis acid sites (LAS) exhibit distinct promotional effects on PDH reactivity, differing fundamentally from conventional geometric or electronic modulation mechanisms of promoters[96]. For example, formation of PtZn alloys in PtZn/Zn-4 induced the electron transfer from Zn species to Pt species, enriching Pt with electrons and creating stronger LAS[66]. Despite having more LAS than PtZn/Zn-4, PtCu/Zn-4 and PtGa/Zn-4 showed suppressed PDH activity. The PtZn/Zn-4 showed a propene selectivity of 99%, with an initial propane conversion of 40.5% at 600 °C, while the PtCu/Zn-4 and PtGa/Zn-4 showed an initial propane conversion of ~37%. This result indicated that the LAS requires precise regulation rather than maximization.
Non-noble metal alloy
Other non-noble bimetallic catalytic systems might serve as candidates to replace the Pt-based bimetallic catalysts for PDH.
Highly dispersed and stable NiSn subnanoclusters were synthesized in S-1 zeolite via the one-pot method[157]. The as-prepared catalyst showed excellent PDH performance with high C3H6 formation rate (about 12.3 mmol·gcat-1·h-1) and C3H6 selectivity (over 88%) at 500 °C. Notably, no loss of Sn was observed even after three regeneration cycles. It was found that the Ni sites, embedded in the geminal silanols of the zeolite framework, played a crucial role in anchoring the Sn species and preventing the loss and sintering of active sites. This caused the C-H activation and C3H6 desorption with the cooperative action of robust NiSn subnanoclusters.
MSI
Hydroxyl groups (mainly including isolated hydroxyls and/or hydroxyl nests) of zeolite materials can provide functional groups for grafting metal sites or anchoring metal compounds. There are two-step procedures forming active single Pt active sites through the above anchoring strategy with hydroxyl group: (1) a non-noble metal is introduced; (2) single noble metal atom is linked with non-noble metal[158]. It is proposed that the non-noble metal can interact with framework Si-OHs through Si–O–M bond formation. The noble metal can strongly interact and be further stabilized by non-noble metal[159]. In this Section, the most common auxiliary grafting metal or oxide sites, such as Zn[158], Ge[160], Fe[161,162], Ce[163], Sn[164], CeOx[165] and InOx[166], as well as ZnO[167], are discussed.
Qi et al. synthesized zeolite encapsulated with isolated Pt atoms, which was anchored by Zn atoms located in nest silanols of dealuminated zeolite [Figure 14][158]. It was found that Pt was auto-reduced to the Pt0 state, whereas Zn had an average oxidized state near +1 when treated in He at 550 °C according to in situ XAS characterization. At the same time, it was a hypothesis that the single Pt atom for Pt-Zn DeAlBEA sample with the Pt-Zn CN of 4-6, where Zn species were grafted on the zeolite framework in the form of ≡Si–O–Zn. This catalyst showed the high specific activity of 6.8 molC3H6·molPt-1·s-1 and high C3H6 selectivity (> 99.4%), and a high stability for 160 h at 550 °C and WHSV = 216 h-1.
Figure 14. Scheme of (≡Si–O–Zn)4-6Pt species formation process. Reprinted with permission[158]. Copyright 2021, American Chemical Society.
Very recently, the subnanometric Pt clusters located in the Ge-UTL zeolite were synergistically confined through the interaction with the Ge ions in special d4r building units[160]. The Pt-Ge-d4r@UTL exhibited a high C3H8 conversion (> 54%), high C3H6 selectivity (> 99%) and an ultra-high lifetime (> 1,520 h) at 500 °C and WHSVC3H8 of 0.75 h-1 in PDH due to the formation of stable Pt–O–Ge bonds. Additionally, the PtFe2 structure (Pt–O–Fe) embedded in the MFI framework, where iron occupied the T4 site of MFI, has also been showing the ow deactivation rate constant of kd < 0.012 h-1[162].
Besides, dealuminated zeolite BEA (DeAlBEA) is an attractive support with more created Si-OHs for anchoring the Pt species by forming the structure of [≡SiO)n-mMn+-(OH)m]. The Sn-DeAlBEA was prepared by incorporating Sn(IV) cations into the BEA framework through the wetness impregnation method[168]. This Sn-DeAlBEA supported with Pt exhibited high selectivity to C3H6 (> 97%) for PDH at 550 °C and WHSV of 864 h-1. IR spectroscopy of adsorbed CO as a probe molecule confirmed that Al plays an important role in the dispersion of Pt supported on DeAlBEA [Figure 15]. At the Pt/Al ratios < 0.013, Pt-Sn-DeAlBEA contained both Ptδ+ species (νCO = 2,136 cm-1) and Pt-Sn species (νCO = 2,068 cm-1). As the Pt/Al ratio increases (≥ 0.013), Pt clusters (νCO = 2,089 cm-1) are formed. With a Pt/Al ratio ≥ 0.026, only obvious Ptδ+ species and metallic Pt clusters are present.
Figure 15. CO-IR spectra of Pt-DeAlBEA and Pt-Sn-DeAlBEA catalysts. Reprinted with permission[168]. Copyright 2024, American Chemical Society. CO-IR: CO-infrared; DeAlBEA: dealuminated zeolite BEA.
Metal oxide, such as amorphous CeOx islands, can also play a role in anchoring Pt SA[165]. Partially embedded Pt species in CeOx islands were obtained after reduction treatment with H2 due to the affinity of CeOx with Pt. Pt/Ce-SiBeta, which contained 4.55 wt% Ce and 0.72 wt% Pt loadings, showed a high C3H6 specific activity of 74.4 s-1 and a low deactivation rate constant of 0.08 h-1 at 550 °C in PDH. More importantly, catalytic activity could be recovered after simple calcination-reduction regeneration.
Similarly, the functional ZnOx (x= 0-2) islands were formed by introducing Zn species into dealuminated beta zeolite, and would be transformed to Pt1Zn1 alloy when Pt was incorporated[167]. The catalyst (Pt/ZnOx-SiBeta) showed over 59% C3H8 conversion with high C3H6 selectivity of 99% and no apparent deactivation even after 100 h of reaction in PDH.
Additives
Additives usually serve as modifiers for the whole catalyst, rather than a building part for the catalysts. The optimal surface acidity of the zeolite is beneficial for the improvement of catalytic performances in light alkanes dehydrogenation reaction, while the excessive acidity of zeolite typically leads to reduced propylene selectivity[169,170]. The addition of alkali metal ions has been demonstrated to facilitate the reduction of catalyst acidity and the dispersion of the metals[171,172]. For example, the addition of MgO, which could reduce the acidity of the catalysts and facilitated the dispersion of Ga2O3, enhanced the selectivity to
Furthermore, additives play a critical role in immobilizing specific metals, such as zinc (Zn). A recent study suggested that the loss of zinc could be effectively mitigated without compromising the PDH performance due to the robust interaction between magnesium ions and zinc oxides[174]. The ZnO/S-1 catalyst synthesized via impregnation showed a high activity of 0.57 mmol·g-1·min-1 at 550 °C. The deactivation rate constant for Mg-modified ZnO/S-1, calculated for the second and twentieth cycles, was in excess of three times less than that of the not containing Mg system (0.0076 vs. 0.0234 h-1). Besides, the production of [ZnH]+ can be regulated by modulating the concentration of [ZnOH]+ species through the introduction of specific additives[175]. The Cu-ZnO@S-1, synthesized via a one-pot method, was comprised exclusively of isolated [ZnOH]+ sites, which showed a C3H8 conversion of 43% with a C3H6 selectivity of higher than 88%, nearly reaching the thermodynamic equilibrium. Specifically, this catalyst achieved an STYC3H6 of
Supports modification
Creating nest silanol defects
The silanol group sites can be utilized to anchor the metal clusters for prolonging the catalytic lifetime in PDH[127]. The (Si-O-Zn)4-Pt-SP [self-pillared silicalite-1 zeolite nanosheets (SP-S-1)] catalyst was kept stable after 12 days of operation[159]. Additionally, the C3H6 selectivity remained at approximately 100% for PDH at 550 °C, WHSV = 1,350 h-1. They assumed that the catalyst can keep stable because the majority of Pt centers are located on the external surface of the zeolite. On these Pt centers, the coke precursor has already been desorbed before the formation of coke [Figure 16].
Figure 16. Proposed PDH mechanism on PtZn/SP-S-1 catalyst. Reprinted with permission[159]. Copyright 2022, American Chemical Society. PDH: Propane dehydrogenation.
The removal of aluminum from the zeolite framework can improve selectivity for propylene in the PDH process due to the elimination of unfavorable Brønsted acid sites (BAS). At the same time, it would lead to the formation of silanol nests. The silanol nests are capable of interacting and anchoring metal precursors. For example, Zn species occupied the vacant T-atom sites of dealuminated β zeolite via silanol anchoring, generating ZnO nanoclusters as active PDH phases[176]. The 10 wt% Zn/dealuminated β-zeolite catalyst achieved propane conversion of 53% and propylene selectivity of 93% at 600 °C. Similarly, cobalt oxide (CoOx) supported on Si-Beta zeolite exhibited the high activity in PDH (propene selectivity of 92% and TOF of 0.01 s-1)[177]. More recently, Li et al. synthesized prototype zeolite (PZ) as a support for anchoring PtSn nanoclusters[61]. The PtSn/PZ catalyst showed an exceptional C3H6 selectivity of over 99% at 550 °C with a WHSV of 3.6 h-1 and a high apparent rate coefficient of 565 molC3H6·gPt-1·h-1·bar-1 (WHSV = 108 h-1). In another paper, defective porous S-1 (DPS-1) supported with uniformly distributed ZnO nanoclusters was prepared through the one-pot method[178]. As the results shown, the S-1@PtZn@DPS-1 displayed high C3H8 conversion over 40% and high C3H6 selectivity over 97% in PDH.
Pore structures
The microporous dimensions of zeolites limit the diffusion of PDH reactants[179-181]. Mesoporous zeolites supported with metals give terrible stability due to the weak interaction between the zeolite and active metal sites, resulting in the constraint on the industrial applications for PDH. Hierarchical zeolites possessing mesoporous porosity enhance hydrothermal stability owing to fast transport of reactants[182]. Wang et al. synthesized a series of Pt-Sn/ZSM-5 catalysts, in which Pt-Sn nanoparticles were encapsulated in the hierarchical ZSM-5[123]. The N2 adsorption/desorption isotherms of this zeolite showed the representative isotherm of type IV and the H3-type hysteresis loop [Figure 17], indicating the formation of hierarchical structure. The Pt-Sn/ZSM-5(0.8) exhibited the high C3H8 conversion over 46% and the high C3H6 selectivity over 98% at 570 °C. Additionally, it had excellent cycling stability, the deactivation rate constant of approximately 0.017 s-1. Besides, the impact of zeolites topology on the dehydrogenation performance has been investigated in PDH[183,184]. For example, Gounder et al. deduced that the cracking-to-dehydrogenation ratios rates of the C3H8 dehydrogenations were different between zeolites with different channel structures. Specifically, it involves H-MFI, H-FER and H-MOR[183].
Figure 17. Nitrogen adsorption and desorption isotherms and pore size distribution (inset) of hierarchical ZSM-5 samples. Reprinted with permission[123]. Copyright 2022, Elsevier.
Acid properties
The silicoaluminate zeolite, possessing relatively strong Brønsted (B) acid properties, can easily induce deep cracking reactions of propane. The incorporation of In+ species in Pt/In-ZSM-5 catalyst neutralized the BAS, suppressed side reactions, modulated the Pt electronic states, and subsequently enhanced the dehydrogenation selectivity of propane[185]. This Pt/In-ZSM-5 catalyst exhibited propane conversion closing to thermodynamic equilibrium data with minimal deactivation, as evidenced by a slight decrease of C3H8 conversion from 36.2% to 34.7% over 70 h. Gong et al. synthesized ZnO-modified zeolites (ZSM-5 and Y) by the ALD method using Et2Zn and H2O as gaseous precursors[186]. It was found that the incorporation of ZnO species could effectively eliminate the BAS and increase the number of strong LAS of zeolites. Introduction of ZnO species led to a considerable improvement in the activities of ZSM-5 and Y zeolites in PDH at 550 °C. Notably, the BAS was completely diminished on ZnO/Y zeolite, resulting in a propylene selectivity of 80%. Choi et al. hydrothermally synthesized Ga-MFI catalysts through MPS (3-mercaptopropyl-trimethoxysilane)-assisted methods, in which process MPS altered Ga incorporation mechanisms and reduced BAS while enhancing LAS[187]. The MPS-modified catalyst exhibited superior PDH performance and superior resistance to coke formation compared with Cr2O3/Al2O3. At the same initial
Besides, in other catalyst systems, researchers have discovered that the presence of acid centers can also facilitate PDH reactions. High-silica Ga-Beta (Si/Ga = 177) exhibited notable dehydrogenation performance due to its large microporosity and BAS. The Ga-Beta catalyst showed high propane conversion of 54.3% and propene yield of 31.5%, while the Na/Ga-Beta catalyst (without BAS) displayed the propane conversion of 0.8%[188]. Furthermore, the presence of Lewis-Brønsted acid pairs in Ga/H-ZSM-5 favored the PDH, where enhanced LAS promoted the C–H bond activation and BAS lowered hydrogen elimination barriers[189].
DEACTIVATION MECHANISMS OF PDH
As discussed in Section “CATALYTIC MECHANISMS OF PROPANE DEHYDROGENATION”, the PDH process usually operates under harsh conditions, such as high temperature and low partial pressure, which may cause numerous side reactions including deep dehydrogenation, hydrolysis, isomerization, and coke formation. Therefore, the deactivation of PDH catalyst primarily originates from two factors, i.e., coke formation and sintering of active sites. The latter involves aggregation of active metal particles into larger clusters, thus triggering side reactions and irreversible catalyst deactivation.
Coke formation
The deactivation of PDH catalysts due to coke deposition mainly occurs through two pathways: the poisoning of active site and the blockage of zeolite pores leading to reactant diffusion limitations. In the Pt-based catalyst, coordinatively saturated Pt sites exhibit specific selectivity for propene, whereas coordinatively unsaturated Pt centers drive side reactions (C-C cracking, deep dehydrogenation) that thermodynamically favor coke deposition. The impact of coke accumulation critically depends on the catalytic system and deposition location, but not the absolute amount of coke[190]. Coke deposits exhibit a random distribution across catalysts, encompassing both metallic active sites and support matrices. Although coke on metallic sites exhibits higher removability through oxidation-reduction treatments compared to support-deposited coke, catalyst deactivation is mainly attributed to coke on metallic sites[191]. Furthermore, DFT analyses elucidated the interaction between coke and metallic sites, revealing that carbon adsorption elevates dehydrogenation energy barriers. The co-adsorption of carbonaceous species triggers a downward shift in the d-band center of Pt through electronic modulation, which reduces the adsorption strength of Pt toward hydrogen species and consequently inhibits the activation of C–H bonds[192].
Due to the structure-sensitive nature of PDH reaction, the catalyst requires control of active site architectures, in which rational design of coordination geometries and electronic states proves critical. Concurrently, modulation of support acidity and migration of coke are also viable strategies for deactivation inhibition.
Sintering of active sites
In the Pt-based catalyst, the size of Pt cluster critically dictates dehydrogenation pathways, but nanoparticle thermal instability during PDH and regeneration induces irreversible sintering and deactivation. Sintering mechanisms involve[193]: (1) Ostwald ripening mechanism, which usually occurs in systems with distinct sizes. Surface energy gradients drive mobile species migration from smaller to larger particles, resulting in progressive metal particle growth; (2) Particle migration and coalescence mechanism, which describes the merging process of nanoparticles with comparable sizes, driven by Brownian motion.
However, the mechanisms governing sintering are still controversially discussed. Strategies for mitigating particle sintering focus on enhancing the interaction between the support and metal or constructing metal-zeolites catalysts, as we discussed above.
SUMMARY AND PERSPECTIVES
Over the past decade, metal-zeolite catalysts have achieved remarkable achievements in the field of PDH. This article summarizes the design ideas of metal-containing zeolite catalysts used for PDH, with a focus on the preparation and modification of catalysts based on the dehydrogenation mechanism, and their impact on the structure and catalytic behavior.
It has been widely established that the activation of the first C–H bond in C3H8 plays an important role in the dehydrogenation reaction, making metal-based catalysts essential for the efficient activation of the C–H bond and the constrained C-C cleavage. The development of metal-zeolite systems, which utilize the high specific surface area, microporous structure of molecular size, and extensive pore system of zeolite, has proven to be effective in enhancing catalytic efficiency and suppressing the sintering of metal species, ultimately improving stability. Therefore, numerous studies have been conducted on the introduction of metal species onto zeolite using various preparation processes, including IWI, ion-exchange, one-pot synthesis, ALD, and recrystallization methods, which has a vital influence on the crystal size, location and interaction of metal on zeolite.
In addition to the preparation process, the microenvironment of metal active sites can also be adjusted through the coexistence of promoters or additives, thereby optimizing the geometric/electronic state of metal. It has been found that promoters or additives can enhance dehydrogenation activity and inhibit metal sintering by promoting metal dispersion, donating electrons through alloy structure, and anchoring metal SAs through Si-O-M structures located on the zeolite framework and/or grafted on zeolite defect sites. It is noteworthy that the construction strategies of zeolite, which involves the creation of silanol defects, modification of acid properties and adjustment of pore structure, are highly effective. This ultimately enhances the selectivity of dehydrogenation reactions and suppresses the formation of carbon deposits.
While existing research has significantly enhanced the stability of precious metal-zeolite catalysts used in PDH, sintering and reconstruction under high temperature conditions remain unavoidable, significantly impacting catalyst regeneration. Achieving the regeneration and reuse of metal catalysts remains a crucial issue that must be carefully considered and addressed during industrial application.
It has been proven that the strategy of encapsulating and stabilizing ultrasmall metal species using microporous zeolites is highly effective, especially after introducing a hierarchical pore structure. However, to date, the aforementioned research has only been expanded to hydrocarbon feedstocks such as n-hexane dehydrogenation by utilizing ultraporous zeolites, which include 24-ring pores as carriers[194]. This demonstrates the immense potential of zeolite-supported metals in dehydrogenating longer carbon chain hydrocarbons. Currently, Pt-Sn/Al2O3 remains the primary catalyst for long-chain alkane dehydrogenation. However, exploring the use of zeolites encapsulated with sub-nanometer metals as the innovative catalyst for direct dehydrogenation of long-chain alkanes holds promise. Given that long-chain alkenes can be further transformed into various chemicals such as detergents, plasticizers, and surfactants, their potential for widespread application and further development in the polymer industry is significant[195].
DECLARATIONS
Authors’ contributions
Prepared and revised the manuscript: Li, Q.; Li, S.
Revised the manuscript: Wang, J.; Wang, S.; Fan, W.; Dong, M.
Availability of data and materials
Not applicable.
Financial support and sponsorship
The authors are grateful to the financial supports of the National Key R&D Program of China (2020YFA0210902), National Natural Science Foundation of China (22272195; U22A20431), Strategic Priority Research Program of the Chinese Academy of Sciences (XDB1190303), Natural Science Foundation of Shanxi Province of China (202303021212378; 202203021224009; 202303021222277), Innovation foundation of Institute of Coal Chemistry, Chinese Academy of Sciences (SCJC-DT-2023-06), Youth Innovation Promotion Association CAS (2021172), Project funded by China Postdoctoral Science Foundation (2022TQ0351; 2023M743640), Technical Support Talent Program of the Chinese Academy of Sciences (YJSZC2023001).
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Chernyak, S. A.; Corda, M.; Dath, J. P.; Ordomsky, V. V.; Khodakov, A. Y. Light olefin synthesis from a diversity of renewable and fossil feedstocks: state-of the-art and outlook. Chem. Soc. Rev. 2022, 51, 7994-8044.
2. Nawaz, Z. Light alkane dehydrogenation to light olefin technologies: a comprehensive review. Rev. Chem. Eng. 2015, 31, 413-36.
3. Mériaudeau, P.; Thangaraj, A.; Dutel, J. F.; Naccache, C. Studies on PtxSny Bimetallics in NaY. II. Further characterization and catalytic properties in the dehydrogenation and hydrogenolysis of propane. J. Catal. 1997, 167, 180-6.
4. Zhang, Y.; Yao, W.; Fang, H.; Hu, A.; Huang, Z. Catalytic alkane dehydrogenations. Sci. Bull. 2015, 60, 1316-31.
5. Feng, Z.; Liu, X.; Wang, Y.; Meng, C. Recent advances on gallium-modified ZSM-5 for conversion of light hydrocarbons. Molecules 2021, 26, 2234.
6. Shi, L.; Wang, Y.; Yan, B.; Song, W.; Shao, D.; Lu, A. H. Progress in selective oxidative dehydrogenation of light alkanes to olefins promoted by boron nitride catalysts. Chem. Commun. 2018, 54, 10936-46.
7. Li, C.; Wang, G. Dehydrogenation of light alkanes to mono-olefins. Chem. Soc. Rev. 2021, 50, 4359-81.
8. Chen, S.; Chang, X.; Sun, G.; et al. Propane dehydrogenation: catalyst development, new chemistry, and emerging technologies. Chem. Soc. Rev. 2021, 50, 3315-54.
9. Dai, Y.; Gao, X.; Wang, Q.; Wan, X.; Zhou, C.; Yang, Y. Recent progress in heterogeneous metal and metal oxide catalysts for direct dehydrogenation of ethane and propane. Chem. Soc. Rev. 2021, 50, 5590-630.
10. Farshi, A.; Shaiyegh, F.; Burogerdi, S. H.; Dehgan, A. FCC process role in propylene demands. Petrol. Sci. Technol. 2011, 29, 875-85.
11. Monai, M.; Gambino, M.; Wannakao, S.; Weckhuysen, B. M. Propane to olefins tandem catalysis: a selective route towards light olefins production. Chem. Soc. Rev. 2021, 50, 11503-29.
12. Sinfelt, J. Catalytic hydrogenolysis and dehydrogenation over copper-nickel alloys. J. Catal. 1972, 24, 283-96.
13. Dry, M. E.; Hoogendoorn, J. C. Technology of the Fischer-Tropsch process. Catal. Rev. 1981, 23, 265-78.
14. Besoukhanova, C.; Guidot, J.; Barthomeuf, D.; Breysse, M.; Bernard, J. R. Platinum–zeolite interactions in alkaline L zeolites. Correlations between catalytic activity and platinum state. J. Chem. Soc. Faraday. Trans. 1. 1981, 77, 1595-604.
16. Lieske, H.; Litez, G.; Spindler, H.; Völter, J. Reactions of platinum in oxygen- and hydrogen-treated Pt/γ-Al2O3 catalysts I. Temperature-programmed reduction, adsorption, and redispersion of platinum. J. Catal. 1983, 81, 8-16.
17. Melnikov, D. P.; Novikov, A. A.; Glotov, A. P.; et al. Dehydrogenation of light alkanes (a review). Pet. Chem. 2022, 62, 1027-46.
18. Wang, G.; Zhu, X.; Li, C. Recent progress in commercial and novel catalysts for catalytic dehydrogenation of light alkanes. Chem. Rec. 2020, 20, 604-16.
19. Ni, L.; Khare, R.; Bermejo-Deval, R.; et al. Highly active and selective sites for propane dehydrogenation in zeolite Ga-BEA. J. Am. Chem. Soc. 2022, 144, 12347-56.
20. Long, J.; Tian, S.; Wei, S.; et al. Direct dehydrogenation of propane over Co@silicalite-1 zeolite: steaming-induced restructuring of Co2+ active sites. Appl. Surf. Sci. 2023, 614, 156238.
21. Sun, Q.; Wang, N.; Fan, Q.; et al. Subnanometer bimetallic platinum-zinc clusters in zeolites for propane dehydrogenation. Angew. Chem. Int. Ed. Engl. 2020, 59, 19450-9.
22. Zeng, L.; Cheng, K.; Sun, F.; et al. Stable anchoring of single rhodium atoms by indium in zeolite alkane dehydrogenation catalysts. Science 2024, 383, 998-1004.
23. Chai, Y.; Shang, W.; Li, W.; et al. Noble metal particles confined in zeolites: synthesis, characterization, and applications. Adv. Sci. 2019, 6, 1900299.
24. Zhang, Q.; Gao, S.; Yu, J. Metal sites in zeolites: synthesis, characterization, and catalysis. Chem. Rev. 2023, 123, 6039-106.
25. Xiao, L.; Shan, Y.; Sui, Z.; et al. Beyond the reverse Horiuti–Polanyi mechanism in propane dehydrogenation over Pt catalysts. ACS. Catal. 2020, 10, 14887-902.
26. Liu, S.; Zhang, B.; Liu, G. Metal-based catalysts for the non-oxidative dehydrogenation of light alkanes to light olefins. React. Chem. Eng. 2021, 6, 9-26.
27. Li, S.; Yan, H.; Liu, Y.; et al. Rational screening of transition metal single-atom-doped ZSM-5 zeolite catalyst for naphtha cracking from microkinetic analysis. Chem. Eng. J. 2022, 445, 136670.
28. Lian, Z.; Ali, S.; Liu, T.; Si, C.; Li, B.; Su, D. S. Revealing the Janus character of the coke precursor in the propane direct dehydrogenation on Pt catalysts from a kMC simulation. ACS. Catal. 2018, 8, 4694-704.
29. Saerens, S.; Sabbe, M. K.; Galvita, V. V.; Redekop, E. A.; Reyniers, M.; Marin, G. B. The positive role of hydrogen on the dehydrogenation of propane on Pt(111). ACS. Catal. 2017, 7, 7495-508.
30. Yang, M. L.; Zhu, Y. A.; Fan, C.; Sui, Z. J.; Chen, D.; Zhou, X. G. DFT study of propane dehydrogenation on Pt catalyst: effects of step sites. Phys. Chem. Chem. Phys. 2011, 13, 3257-67.
31. Hauser, A. W.; Gomes, J.; Bajdich, M.; Head-Gordon, M.; Bell, A. T. Subnanometer-sized Pt/Sn alloy cluster catalysts for the dehydrogenation of linear alkanes. Phys. Chem. Chem. Phys. 2013, 15, 20727-34.
32. Huang, Y. A.; Cheng, G.; Lei, M.; et al. Decoding the kinetic complexity of Pt-catalyzed n-butane dehydrogenation by machine learning and microkinetic analysis. ACS. Catal. 2024, 14, 7978-95.
33. Yang, B.; Gong, X. Q.; Wang, H. F.; Cao, X. M.; Rooney, J. J.; Hu, P. Evidence to challenge the universality of the Horiuti-Polanyi mechanism for hydrogenation in heterogeneous catalysis: origin and trend of the preference of a non-Horiuti-Polanyi mechanism. J. Am. Chem. Soc. 2013, 135, 15244-50.
34. Lee, I.; Zaera, F. Infrared spectroscopy characterization of the chemistry of C4 hydrocarbons on Pt(111) single-crystal surfaces. J. Phys. Chem. C. 2007, 111, 10062-72.
35. Vajda, S.; Pellin, M. J.; Greeley, J. P.; et al. Subnanometre platinum clusters as highly active and selective catalysts for the oxidative dehydrogenation of propane. Nat. Mater. 2009, 8, 213-6.
36. Kumar, P.; Srivastava, V. C. Ethane and propane dehydrogenation on small platinum clusters supported on silica: an ab initio molecular dynamics and DFT study. Chempluschem 2024, 89, e202300347.
37. Liu, G.; Zeng, L.; Zhao, Z.; Tian, H.; Wu, T.; Gong, J. Platinum-modified ZnO/Al2O3 for propane dehydrogenation: minimized platinum usage and improved catalytic stability. ACS. Catal. 2016, 6, 2158-62.
38. Zhao, Z. J.; Wu, T.; Xiong, C.; et al. Hydroxyl-mediated non-oxidative propane dehydrogenation over VOx/γ-Al2O3 catalysts with improved stability. Angew. Chem. Int. Ed. Engl. 2018, 57, 6791-5.
39. Bai, P.; Yang, M.; Chen, X.; et al. Modulation of surface chemistry by boron modification to achieve a superior VOX/Al2O3 catalyst in propane dehydrogenation. Catal. Today. 2022, 402, 248-58.
40. Shee, D.; Sayari, A. Light alkane dehydrogenation over mesoporous Cr2O3/Al2O3 catalysts. Appl. Catal. A. Gen. 2010, 389, 155-64.
41. Chen, S.; Xu, Y.; Chang, X.; et al. Defective TiOx overlayers catalyze propane dehydrogenation promoted by base metals. Science 2024, 385, 295-300.
42. Xie, Z.; Yu, T.; Song, W.; et al. Highly active nanosized anatase TiO2-x oxide catalysts in situ formed through reduction and ostwald ripening processes for propane dehydrogenation. ACS. Catal. 2020, 10, 14678-93.
43. Li, C.; Guo, X.; Shang, Q.; et al. Defective TiO2 for propane dehydrogenation. Ind. Eng. Chem. Res. 2020, 59, 4377-87.
44. Xu, Y.; Chen, S.; Chang, X.; et al. Ultrathin TiOx nanosheets rich in tetracoordinated Ti sites for propane dehydrogenation. ACS. Catal. 2023, 13, 6104-13.
45. Otroshchenko, T.; Kondratenko, V. A.; Rodemerck, U.; Linke, D.; Kondratenko, E. V. ZrO2-based unconventional catalysts for non-oxidative propane dehydrogenation: factors determining catalytic activity. J. Catal. 2017, 348, 282-90.
46. Zhang, Y.; Zhao, Y.; Otroshchenko, T.; et al. The effect of phase composition and crystallite size on activity and selectivity of ZrO2 in non-oxidative propane dehydrogenation. J. Catal. 2019, 371, 313-24.
47. Zhang, Y.; Zhao, Y.; Otroshchenko, T.; et al. Control of coordinatively unsaturated Zr sites in ZrO2 for efficient C-H bond activation. Nat. Commun. 2018, 9, 3794.
48. Chen, K.; Bell, A. T.; Iglesia, E. The relationship between the electronic and redox properties of dispersed metal oxides and their turnover rates in oxidative dehydrogenation reactions. J. Catal. 2002, 209, 35-42.
49. Latimer, A. A.; Kulkarni, A. R.; Aljama, H.; et al. Understanding trends in C-H bond activation in heterogeneous catalysis. Nat. Mater. 2017, 16, 225-9.
50. Konnov, S. V.; Bruter, D. V.; Pavlov, V. S.; Ivanova, I. I. State-of-the-art strategies for the synthesis of zeolite-encapsulated subnanometric metal clusters. Inorg. Chem. Front. 2024, 11, 3669-706.
51. Buchmeiser, M. R. Recent advances in the synthesis of supported metathesis catalysts. New. J. Chem. 2004, 28, 549.
52. Martínez, C.; Corma, A. Inorganic molecular sieves: preparation, modification and industrial application in catalytic processes. Coord. Chem. Rev. 2011, 255, 1558-80.
53. Centi, G.; Trifiro, F. Catalytic behavior of V-containing zeolites in the transformation of propane in the presence of oxygen. Appl. Catal. A. Gen. 1996, 143, 3-16.
54. Cola PL, Gläser R, Weitkamp J. Non-oxidative propane dehydrogenation over Pt–Zn-containing zeolites. Appl. Catal. A. Gen. 2006, 306, 85-97.
55. Ponomaryov, A. B.; Smirnov, A. V.; Pisarenko, E. V.; Shostakovsky, M. V. PtSn/MFI catalysts for propane dehydrogenation prepared by an impregnation–calcination–washing method. Appl. Catal. A. Gen. 2024, 673, 119588.
56. Ponomaryov, A. B.; Smirnov, A. V.; Pisarenko, E. V.; Shostakovsky, M. V. Enhanced Pt dispersion and catalytic properties of NaCl-promoted Pt/MFI zeolite catalysts for propane dehydrogenation. Micropor. Mesopor. Mat. 2022, 339, 112010.
57. Liu, S.; Wu, G.; Gong, J.; et al. Synthesis gold and jade type core shell structure Pt@Sn in deboronated MWW zeolite and its good performance for light alkane dehydrogenation. Chem. Eng. J. 2023, 476, 146410.
58. Zhang, B.; Song, M.; Xu, M.; Liu, G. Recent advances in metal−zeolite catalysts for direct propane dehydrogenation. Energy. Fuels. 2023, 37, 19419-32.
59. Wang, H.; Zhang, X.; Su, Z.; Chen, T. Dealuminated Beta stabilized bimetallic PtCo nanoparticles for oxidative dehydrogenation of propane with CO2. Fuel 2024, 358, 130248.
60. Ryoo, R.; Kim, J.; Jo, C.; et al. Rare-earth-platinum alloy nanoparticles in mesoporous zeolite for catalysis. Nature 2020, 585, 221-4.
61. Li, J.; Zhang, Q.; He, G.; et al. Silanol-stabilized atomically dispersed Ptδ+-Ox-Sn active sites in protozeolite for propane dehydrogenation. J. Am. Chem. Soc. 2024, 146, 24358-67.
62. Ćurković, L.; Cerjan-Stefanović, Š.; Filipan, T. Metal ion exchange by natural and modified zeolites. Water. Res. 1997, 31, 1379-82.
63. Yuna, Z. Review of the natural, modified, and synthetic zeolites for heavy metals removal from wastewater. Environm. Eng. Sci. 2016, 33, 443-54.
64. Wang, S.; Peng, Y. Natural zeolites as effective adsorbents in water and wastewater treatment. Chem. Eng. J. 2010, 156, 11-24.
65. Nozik, D.; Tinga, F. M. P.; Bell, A. T. Propane dehydrogenation and cracking over Zn/H-MFI prepared by solid-state ion exchange of ZnCl2. ACS. Catal. 2021, 11, 14489-506.
66. Liu, H.; Liu, L.; Qin, Q.; et al. Comparative study of PtM (M=Cu, Zn, Ga, Mn, Fe, In, Ce) bimetals on zincosilicate for propane dehydrogenation reaction. Chemistry 2024, 30, e202402764.
67. Katranas, T. K.; Triantafyllidis, K. S.; Vlessidis, A. G.; Evmiridis, N. P. Propane reactions over faujasite structure zeolites type-X and USY: effect of zeolite silica over alumina ratio, strength of acidity and kind of exchanged metal ion. Catal. Lett. 2007, 118, 79-85.
68. Englert, A.; Rubio, J. Characterization and environmental application of a Chilean natural zeolite. Int. J. Miner. Process. 2005, 75, 21-9.
69. Alshameri, A.; Ibrahim, A.; Assabri, A. M.; Lei, X.; Wang, H.; Yan, C. The investigation into the ammonium removal performance of Yemeni natural zeolite: modification, ion exchange mechanism, and thermodynamics. Powder. Technol. 2014, 258, 20-31.
70. Agrawal, S.; Mantri, K.; Sharma, V.; Jasra, R. V.; Munshi, P. Catalytic dehydrogenation of cyclohexanone to phenol over the Ru, Rh, Pd and Pt surfaces in sub-critical water. Catal. Lett. 2022, 152, 2119-30.
71. Weisz, P. Catalysis by crystalline aluminosilicates II. Molecular-shape selective reactions. J. Catal. 1962, 1, 307-12.
72. Ou, Z.; Li, Y.; Wu, W.; et al. Encapsulating subnanometric metal clusters in zeolites for catalysis and their challenges. Chem. Eng. J. 2022, 430, 132925.
73. Sun, Q.; Wang, N.; Yu, J. Advances in catalytic applications of zeolite-supported metal catalysts. Adv. Mater. 2021, 33, e2104442.
74. Qu, Z.; Sun, Q. Advances in zeolite-supported metal catalysts for propane dehydrogenation. Inorg. Chem. Front. 2022, 9, 3095-115.
75. Otto, T.; Zones, S. I.; Iglesia, E. Synthetic strategies for the encapsulation of nanoparticles of Ni, Co, and Fe oxides within crystalline microporous aluminosilicates. Micropor. Mesopor. Mat. 2018, 270, 10-23.
76. Otto, T.; Zones, S. I.; Hong, Y.; Iglesia, E. Synthesis of highly dispersed cobalt oxide clusters encapsulated within LTA zeolites. J. Catal. 2017, 356, 173-85.
77. Otto, T.; Ramallo-López, J. M.; Giovanetti, L. J.; Requejo, F. G.; Zones, S. I.; Iglesia, E. Synthesis of stable monodisperse AuPd, AuPt, and PdPt bimetallic clusters encapsulated within LTA-zeolites. J. Catal. 2016, 342, 125-37.
78. Goel, S.; Zones, S. I.; Iglesia, E. Encapsulation of metal clusters within MFI via interzeolite transformations and direct hydrothermal syntheses and catalytic consequences of their confinement. J. Am. Chem. Soc. 2014, 136, 15280-90.
79. Wu, Z.; Goel, S.; Choi, M.; Iglesia, E. Hydrothermal synthesis of LTA-encapsulated metal clusters and consequences for catalyst stability, reactivity, and selectivity. J. Catal. 2014, 311, 458-68.
80. Choi, M.; Wu, Z.; Iglesia, E. Mercaptosilane-assisted synthesis of metal clusters within zeolites and catalytic consequences of encapsulation. J. Am. Chem. Soc. 2010, 132, 9129-37.
81. Goel, S.; Wu, Z.; Zones, S. I.; Iglesia, E. Synthesis and catalytic properties of metal clusters encapsulated within small-pore (SOD, GIS, ANA) zeolites. J. Am. Chem. Soc. 2012, 134, 17688-95.
82. Wang, Y.; Hu, Z.; Lv, X.; Chen, L.; Yuan, Z. Ultrasmall PtZn bimetallic nanoclusters encapsulated in silicalite-1 zeolite with superior performance for propane dehydrogenation. J. Catal. 2020, 385, 61-9.
83. Liu, L.; Lopez-haro, M.; Lopes, C. W.; et al. Structural modulation and direct measurement of subnanometric bimetallic PtSn clusters confined in zeolites. Nat. Catal. 2020, 3, 628-38.
84. Liu, L.; Lopez-Haro, M.; Lopes, C. W.; et al. Regioselective generation and reactivity control of subnanometric platinum clusters in zeolites for high-temperature catalysis. Nat. Mater. 2019, 18, 866-73.
85. Dou, X.; Li, W.; Zhang, K.; et al. Size-dependent structural features of subnanometer PtSn catalysts encapsulated in zeolite for alkane dehydrogenation. ACS. Catal. 2024, 14, 2859-71.
86. Zhu, J.; Osuga, R.; Ishikawa, R.; et al. Ultrafast encapsulation of metal nanoclusters into MFI zeolite in the course of its crystallization: catalytic application for propane dehydrogenation. Angew. Chem. Int. Ed. Engl. 2020, 59, 19669-74.
87. Song, S.; Li, J.; Wu, Z.; et al. In situ encapsulated subnanometric CoO clusters within silicalite-1 zeolite for efficient propane dehydrogenation. AIChE. J. 2022, 68, e17451.
88. Xu, G.; Zhang, X.; Dong, Z.; et al. Ferric single-site catalyst confined in a zeolite framework for propane dehydrogenation. Angew. Chem. Int. Ed. Engl. 2023, 62, e202305915.
89. Liu, L.; Díaz, U.; Arenal, R.; Agostini, G.; Concepción, P.; Corma, A. Generation of subnanometric platinum with high stability during transformation of a 2D zeolite into 3D. Nat. Mater. 2017, 16, 132-8.
90. Feng, H.; Elam, J. W.; Libera, J. A.; Setthapun, W.; Stair, P. C. Palladium catalysts synthesized by atomic layer deposition for methanol decomposition. Chem. Mater. 2010, 22, 3133-42.
91. Christensen, S. T.; Feng, H.; Libera, J. L.; et al. Supported Ru-Pt bimetallic nanoparticle catalysts prepared by atomic layer deposition. Nano. Lett. 2010, 10, 3047-51.
92. Feng, H.; Libera, J. A.; Stair, P. C.; Miller, J. T.; Elam, J. W. Subnanometer palladium particles synthesized by atomic layer deposition. ACS. Catal. 2011, 1, 665-73.
93. Feng, H.; Lu, J.; Stair, P. C.; Elam, J. W. Alumina over-coating on Pd nanoparticle catalysts by atomic layer deposition: enhanced stability and reactivity. Catal. Lett. 2011, 141, 512-7.
94. Xu, D.; Yin, J.; Gao, Y.; Zhu, D.; Wang, S. Atomic-scale designing of zeolite based catalysts by atomic layer deposition. Chemphyschem 2021, 22, 1287-301.
95. Zhang, B.; Wu, Z.; Xing, S.; et al. Small-molecule modification provides Pt nucleation sites for enhanced propane dehydrogenation performance. J. Phys. Chem. C. 2023, 127, 5754-62.
96. Yang, F.; Zhang, J.; Chen, J.; et al. Promoting propane dehydrogenation over Zn/hollow porous silicalite-1 catalysts via modulating the electronic structures of Pt. Fuel 2024, 364, 131163.
97. Putkonen, M.; Sajavaara, T.; Niinistö, L.; Keinonen, J. Analysis of ALD-processed thin films by ion-beam techniques. Anal. Bioanal. Chem. 2005, 382, 1791-9.
98. Emslie, D. J.; Chadha, P.; Price, J. S. Metal ALD and pulsed CVD: fundamental reactions and links with solution chemistry. Coord. Chem. Rev. 2013, 257, 3282-96.
99. Li, S.; Burel, L.; Aquino, C.; et al. Ultimate size control of encapsulated gold nanoparticles. Chem. Commun. 2013, 49, 8507-9.
100. Li, S.; Boucheron, T.; Tuel, A.; Farrusseng, D.; Meunier, F. Size-selective hydrogenation at the subnanometer scale over platinum nanoparticles encapsulated in silicalite-1 single crystal hollow shells. Chem. Commun. 2014, 50, 1824-6.
101. Pagis, C.; Morgado, P. A. R.; Farrusseng, D.; Bats, N.; Tuel, A. Hollow zeolite structures: an overview of synthesis methods. Chem. Mater. 2016, 28, 5205-23.
102. Dai, C.; Zhang, A.; Liu, M.; Guo, X.; Song, C. Hollow ZSM-5 with silicon-rich surface, double shells, and functionalized interior with metallic nanoparticles and carbon nanotubes. Adv. Funct. Mater. 2015, 25, 7479-87.
103. Chen, Y.; Zhu, X.; Wang, X.; Su, Y. A reliable protocol for fast and facile constructing multi-hollow silicalite-1 and encapsulating metal nanoparticles within the hierarchical zeolite. Chem. Eng. J. 2021, 419, 129641.
104. Kubota, S.; Sumi, T.; Kitamura, H.; Miyake, K.; Uchida, Y.; Nishiyama, N. Promoted propane dehydrogenation over Co confined within core–shell silicalite-1 zeolite crystals. Catal. Sci. Technol. 2024, 14, 1201-8.
105. Zhao, D.; Tian, X.; Doronkin, D. E.; et al. In situ formation of ZnOx species for efficient propane dehydrogenation. Nature 2021, 599, 234-8.
106. Otto, T.; Zones, S. I.; Iglesia, E. Challenges and strategies in the encapsulation and stabilization of monodisperse Au clusters within zeolites. J. Catal. 2016, 339, 195-208.
107. Bai, L.; Zhou, Y.; Zhang, Y.; Liu, H.; Sheng, X.; Duan, Y. Effect of calcination atmosphere on the catalytic properties of PtSnNaMg/ZSM-5 for propane dehydrogenation. Catal. Commun. 2009, 10, 2013-7.
108. Jones, J.; Xiong, H.; DeLaRiva, A. T.; et al. Thermally stable single-atom platinum-on-ceria catalysts via atom trapping. Science 2016, 353, 150-4.
109. Liu, L.; Lopez-Haro, M.; Lopes, C. W.; et al. Atomic-level understanding on the evolution behavior of subnanometric Pt and Sn species during high-temperature treatments for generation of dense PtSn clusters in zeolites. J. Catal. 2020, 391, 11-24.
110. Gao, Y.; Peng, L.; Long, J.; Wu, Y.; Dai, Y.; Yang, Y. Hydrogen pre–reduction determined Co–silica interaction and performance of cobalt catalysts for propane dehydrogenation. Micropor. Mesopor. Mat. 2021, 323, 111187.
111. Ren, Z.; He, Y.; Yang, M.; et al. The investigation into the different Co species over Silicalite-1 via modulating heat-treatment atmosphere for propane dehydrogenation. Mol. Catal. 2022, 530, 112580.
112. Wu, B.; Shi, Y.; Zhang, J.; et al. Stabilizing ultra-small bimetallic PtSn clusters within S-1 crystals for effective propane dehydrogenation with low Pt loading. Chem. Eng. J. 2024, 498, 155205.
113. Hauser, A. W.; Horn, P. R.; Head-Gordon, M.; Bell, A. T. A systematic study on Pt based, subnanometer-sized alloy cluster catalysts for alkane dehydrogenation: effects of intermetallic interaction. Phys. Chem. Chem. Phys. 2016, 18, 10906-17.
114. Tait, S. L.; Dohnálek, Z.; Campbell, C. T.; Kay, B. D. n-alkanes on Pt(111) and on C(0001)Pt(111): chain length dependence of kinetic desorption parameters. J. Chem. Phys. 2006, 125, 234308.
115. Wei, X.; Cheng, J.; Li, Y.; et al. Bimetallic clusters confined inside silicalite-1 for stable propane dehydrogenation. Nano. Res. 2023, 16, 10881-9.
116. He, Y.; Deng, H.; Zhang, Y.; et al. Boosting propane dehydrogenation over Sn stabilizing dispersed Ptδ+ confined in Silicalite-1 at low temperature. Fuel 2023, 352, 129044.
117. Razavian, M.; Fatemi, S.; Komasi, M. Seed-assisted OSDA-free synthesis of ZSM-5 zeolite and its application in dehydrogenation of propane. Mater. Res. Bull. 2015, 65, 253-9.
118. Razavian, M.; Fatemi, S. Synthesis and evaluation of seed-directed hierarchical ZSM-5 catalytic supports: inductive influence of various seeds and aluminosilicate gels on the physicochemical properties and catalytic dehydrogenative behavior. Mater. Chem. Phys. 2015, 165, 55-65.
119. Razavian, M.; Fatemi, S. Synthesis and application of ZSM-5/SAPO-34 and SAPO-34/ZSM-5 composite systems for propylene yield enhancement in propane dehydrogenation process. Micropor. Mesopor. Mat. 2015, 201, 176-89.
120. Nawaz, Z.; Wei, F. Integrated bi-modal fluidized bed reactor for butane dehydrogenation to corresponding butylenes. Chem. Eng. J. 2014, 238, 249-53.
121. Nawaz, Z.; Qing, S.; Jixian, G.; Tang, X.; Wei, F. Effect of Si/Al ratio on performance of Pt–Sn-based catalyst supported on ZSM-5 zeolite for n-butane conversion to light olefins. J. Ind. Eng. Chem. 2010, 16, 57-62.
122. Zhang, Y.; Zhou, Y.; Shi, J.; et al. Comparative study of bimetallic Pt-Sn catalysts supported on different supports for propane dehydrogenation. J. Mol. Catal. A. Chem. 2014, 381, 138-47.
123. Wang, T.; Xu, Z.; Yue, Y.; Wang, T.; Lin, M.; Zhu, H. Bimetallic PtSn nanoparticles confined in hierarchical ZSM-5 for propane dehydrogenation. Chin. J. Chem. Eng. 2022, 41, 384-91.
124. Lezcano-González, I.; Cong, P.; Campbell, E.; et al. Structure-activity relationships in highly active platinum-Tin MFI-type zeolite catalysts for propane dehydrogenation. ChemCatChem 2022, 14, e202101828.
125. Xu, Z.; Yue, Y.; Bao, X.; Xie, Z.; Zhu, H. Propane dehydrogenation over Pt clusters localized at the Sn single-site in zeolite framework. ACS. Catal. 2020, 10, 818-28.
126. Wang, Y.; Hu, Z.; Tian, W.; Gao, L.; Wang, Z.; Yuan, Z. Framework-confined Sn in Si-beta stabilizing ultra-small Pt nanoclusters as direct propane dehydrogenation catalysts with high selectivity and stability. Catal. Sci. Technol. 2019, 9, 6993-7002.
127. Ma, Y.; Chen, X.; Guan, Y.; et al. Skeleton-Sn anchoring isolated Pt site to confine subnanometric clusters within *BEA topology. J. Catal. 2021, 397, 44-57.
128. Li, B.; Xu, Z.; Chu, W.; Luo, S.; Jing, F. Ordered mesoporous Sn-SBA-15 as support for Pt catalyst with enhanced performance in propane dehydrogenation. Chin. J. Catal. 2017, 38, 726-35.
129. Zhang, Y.; Zhou, Y.; Huang, L.; Xue, M.; Zhang, S. Sn-modified ZSM-5 As support for platinum catalyst in propane dehydrogenation. Ind. Eng. Chem. Res. 2011, 50, 7896-902.
130. Zhang, Y.; Xue, M.; Zhou, Y.; et al. Propane dehydrogenation over Ce-containing ZSM-5 supported platinum–tin catalysts: Ce concentration effect and reaction performance analysis. RSC. Adv. 2016, 6, 29410-22.
131. Zhang, Y.; Zhou, Y.; Zhang, S.; et al. Catalytic structure and reaction performance of PtSnK/ZSM-5 catalyst for propane dehydrogenation: influence of impregnation strategy. J. Mater. Sci. 2015, 50, 6457-68.
132. Zhou, H.; Gong, J.; Xu, B.; Yu, L.; Fan, Y. PtSnNa@SUZ-4-catalyzed propane dehydrogenation. Appl. Catal. A. Gen. 2016, 527, 30-5.
133. Zhou, H.; Gong, J.; Xu, B.; et al. PtSnNa/SUZ-4: an efficient catalyst for propane dehydrogenation. Chin. J. Catal. 2017, 38, 529-36.
134. Wang, P.; Yang, M.; Liao, H.; et al. Restructured zeolites anchoring singly dispersed bimetallic platinum and zinc catalysts for propane dehydrogenation. Cell. Rep. Phys. Sci. 2023, 4, 101311.
135. Han, S. W.; Park, H.; Han, J.; et al. PtZn intermetallic compound nanoparticles in mesoporous zeolite exhibiting high catalyst durability for propane dehydrogenation. ACS. Catal. 2021, 11, 9233-41.
136. Zhang, L.; Ma, Y.; Liu, C.; et al. Demetallation and reduction induced ultra-dispersed PtZn alloy confined in zeolite for propane dehydrogenation. Chin. J. Catal. 2023, 55, 241-52.
137. Zhang, B.; Li, G.; Liu, S.; et al. Boosting propane dehydrogenation over PtZn encapsulated in an epitaxial high-crystallized zeolite with a low surface barrier. ACS. Catal. 2022, 12, 1310-4.
138. Zhang, Y.; Zhou, Y.; Huang, L.; et al. Structure and catalytic properties of the Zn-modified ZSM-5 supported platinum catalyst for propane dehydrogenation. Chem. Eng. J. 2015, 270, 352-61.
139. Chen, C.; Sun, M.; Hu, Z.; Ren, J.; Zhang, S.; Yuan, Z. New insight into the enhanced catalytic performance of ZnPt/HZSM-5 catalysts for direct dehydrogenation of propane to propylene. Catal. Sci. Technol. 2019, 9, 1979-88.
140. Qu, Z.; Zhang, T.; Yin, X.; Zhang, J.; Xiong, X.; Sun, Q. Zeolite-encaged ultrasmall Pt-Zn species with trace amount of Pt for efficient propane dehydrogenation. Chem. Res. Chin. Univ. 2023, 39, 870-6.
141. Zhu, X.; Wang, X.; Su, Y. Propane dehydrogenation over PtZn localized at Ti sites on TS-1 zeolite. Catal. Sci. Technol. 2021, 11, 4482-90.
142. Xie, L.; Chai, Y.; Sun, L.; et al. Optimizing zeolite stabilized Pt-Zn catalysts for propane dehydrogenation. J. Energy. Chem. 2021, 57, 92-8.
143. Bian, K.; Zhang, G.; Wang, M.; et al. Promoting propane dehydrogenation over PtFe bimetallic catalysts by optimizing the state of Fe species. Chem. Eng. Sci. 2023, 275, 118748.
144. Cai, W.; Mu, R.; Zha, S.; et al. Subsurface catalysis-mediated selectivity of dehydrogenation reaction. Sci. Adv. 2018, 4, eaar5418.
145. Miao, C.; Liu, M.; Tan, S.; et al. Pt–Sn nanoalloys on Sn-Beta zeolite for efficient propane dehydrogenation. Micropor. Mesopor. Mat. 2023, 361, 112736.
146. Zhou, S.; Liu, S.; Jing, F.; et al. Effects of dopants in PtSn/M-Silicalite-1 on structural property and on catalytic propane dehydrogenation performance. ChemistrySelect 2020, 5, 4175-85.
147. Qiu, Y.; Li, X.; Zhang, Y.; et al. Various metals (Ce, In, La, and Fe) promoted Pt/Sn-SBA-15 as highly stable catalysts for propane dehydrogenation. Ind. Eng. Chem. Res. 2019, 58, 10804-18.
148. Wang, Y.; Suo, Y.; Lv, X.; Wang, Z.; Yuan, Z. Y. Enhanced performances of bimetallic Ga-Pt nanoclusters confined within silicalite-1 zeolite in propane dehydrogenation. J. Colloid. Interface. Sci. 2021, 593, 304-14.
149. Zhang, B.; Zheng, L.; Zhai, Z.; Li, G.; Liu, G. Subsurface-regulated PtGa nanoparticles confined in Silicalite-1 for propane dehydrogenation. ACS. Appl. Mater. Interfaces. 2021, 13, 16259-66.
150. Luo, L.; Zhou, T.; Li, W.; et al. Close intimacy between PtIn clusters and zeolite channels for ultrastability toward propane dehydrogenation. Nano. Lett. , 2024, 7236-43.
151. Zhou, J.; Liu, H.; Xiong, C.; et al. Potassium-promoted Pt–In bimetallic clusters encapsulated in silicalite-1 zeolite for efficient propane dehydrogenation. Chem. Eng. J. 2023, 455, 139794.
152. Li, S.; Li, Q.; Li, B.; et al. Efficient dehydrogenation of propane to propene over PtIn nanoclusters encapsulated in hollow-structured Silicalite-1. ACS. Catal. 2024, 14, 17825-36.
153. Zhou, J.; Zhang, Y.; Liu, H.; et al. Enhanced performance for propane dehydrogenation through Pt clusters alloying with copper in zeolite. Nano. Res. 2023, 16, 6537-43.
154. Zhang, X.; He, N.; Liu, C.; Guo, H. Pt–Cu alloy nanoparticles encapsulated in Silicalite-1 molecular sieve: coke-resistant catalyst for alkane dehydrogenation. Catal. Lett. 2019, 149, 974-84.
155. Zhou, J.; Sun, Q.; Qin, Y.; et al. Bimetallic CoCu-modified Pt species in S-1 zeolite with enhanced stability for propane dehydrogenation. J. Colloid. Interface. Sci. 2024, 663, 94-102.
156. Zhu, X.; Chen, B.; Wang, X. High active and stable structure of PtBi0.5K4/Si-Beta catalyzing propane dehydrogenation. Chem. Eng. J. 2023, 474, 145894.
157. Liu, M.; Sun, X.; Zhang, Y.; et al. Highly dispersed and stable NiSn subnanoclusters encapsulated within Silicalite-1 zeolite for efficient propane dehydrogenation. Fuel 2024, 357, 130069.
158. Qi, L.; Babucci, M.; Zhang, Y.; et al. Propane dehydrogenation catalyzed by isolated Pt atoms in ≡SiOZn-OH nests in dealuminated zeolite beta. J. Am. Chem. Soc. 2021, 143, 21364-78.
159. Qi, L.; Zhang, Y.; Babucci, M.; et al. Dehydrogenation of propane and n-butane catalyzed by isolated PtZn4 sites supported on self-pillared zeolite pentasil nanosheets. ACS. Catal. 2022, 12, 11177-89.
160. Ma, Y.; Song, S.; Liu, C.; et al. Germanium-enriched double-four-membered-ring units inducing zeolite-confined subnanometric Pt clusters for efficient propane dehydrogenation. Nat. Catal. 2023, 6, 506-18.
161. Zhang, L.; Chen, X.; Ma, Y.; et al. Single Pt coordinated with framework Fe in MWW-type ferrisilicate toward efficient propane dehydrogenation. ACS. Catal. 2024, 14, 9431-9.
162. Liu, H.; Zhou, J.; Chen, T.; et al. Isolated Pt species anchored by hierarchical-like heteroatomic Fe-Silicalite-1 catalyze propane dehydrogenation near the thermodynamic limit. ACS. Catal. 2023, 13, 2928-36.
163. Zhang, Y.; Wang, X.; Deng, H.; et al. Synergistic mechanism of isolated Fe3+ and highly dispersed Ptδ+ over zeolite for boosting propane dehydrogenation. AIChE. J. 2023, 69, e18249.
164. Zhang, H.; Zhong, L.; Bin Samsudin, I.; et al. Mg-stabilized subnanometer Rh particles in zeolite Beta as highly efficient catalysts for selective hydrogenation. J. Catal. 2022, 405, 489-98.
165. Wang, H.; Zhang, X.; Su, Z.; Chen, T. Amorphous CeOx islands on dealuminated zeolite beta to stabilize Pt nanoparticles as efficient and antisintering catalysts for propane dehydrogenation. Langmuir 2023, 39, 18366-79.
166. Zhu, C.; Li, W.; Chen, T.; et al. Boosting the stability of subnanometer Pt catalysts by the presence of framework indium(III) sites in zeolite. Angew. Chem. Int. Ed. Engl. 2024, 63, e202409784.
167. Liao, H.; Tao, X.; Wu, L.; Tang, Y.; Wang, P.; Tan, L. Anchoring Pt sites via chemical confining functional ZnOx islands in propane dehydrogenation. Fuel 2024, 369, 131730.
168. Lefton, N. G.; Bell, A. T. Effects of structure on the activity, selectivity, and stability of Pt-Sn-DeAlBEA for propane dehydrogenation. ACS. Catal. 2024, 14, 3986-4000.
169. Liu, H.; Zhou, Y.; Zhang, Y.; Bai, L.; Tang, M. Effect of preparation processes on catalytic performance of PtSnNa/ZSM-5 for propane dehydrogenation. Ind. Eng. Chem. Res. 2009, 48, 5598-603.
170. Grasselli, R. K.; Stern, D. L.; Tsikoyiannis, J. G. Catalytic dehydrogenation (DH) of light paraffins combined with selective hydrogen combustion (SHC). Appl. Catal. A. Gen. 1999, 189, 1-8.
171. Ghashghaee, M.; Karimzadeh, R. Applicability of protolytic mechanism to steady-state heterogeneous dehydrogenation of ethane revisited. Micropor. Mesopor. Mat. 2013, 170, 318-30.
172. Wang, Z.; Zhang, B.; Liu, G.; Zhang, X. Thermal stable Pt clusters anchored by K/TiO2–Al2O3 for efficient cycloalkane dehydrogenation. Chin. J. Chem. Eng. 2024, 72, 187-98.
173. Yang, G.; Yan, X.; Chen, Y.; Guo, X.; Lang, W.; Guo, Y. Improved propylene selectivity and superior catalytic performance of Ga-xMg/ZSM-5 catalysts for propane dehydrogenation (PDH) reaction. Appl. Catal. A. Gen. 2022, 643, 118778.
174. Zhao, D.; Guo, K.; Han, S.; et al. Controlling reaction-induced loss of active sites in ZnOx/Silicalite-1 for durable nonoxidative propane dehydrogenation. ACS. Catal. 2022, 12, 4608-17.
175. Song, S.; Yang, K.; Zhang, P.; et al. Silicalite-1 stabilizes Zn-hydride species for efficient propane dehydrogenation. ACS. Catal. 2022, 12, 5997-6006.
176. Chen, C.; Hu, Z.; Ren, J.; Zhang, S.; Wang, Z.; Yuan, Z. ZnO nanoclusters supported on dealuminated zeolite β as a novel catalyst for direct dehydrogenation of propane to propylene. ChemCatChem 2019, 11, 868-77.
177. Chen, C.; Zhang, S.; Wang, Z.; Yuan, Z. Ultrasmall Co confined in the silanols of dealuminated beta zeolite: a highly active and selective catalyst for direct dehydrogenation of propane to propylene. J. Catal. 2020, 383, 77-87.
178. Yang, F.; Zhang, J.; Chen, J.; et al. Boosting propane dehydrogenation of defective S-1 stabilized single-atom Pt and ZnO catalysts via coordination environment regulation. Nano. Res. 2024, 17, 5884-96.
179. Kerstens, D.; Smeyers, B.; Van Waeyenberg, J.; Zhang, Q.; Yu, J.; Sels, B. F. State of the art and perspectives of hierarchical zeolites: practical overview of synthesis methods and use in catalysis. Adv. Mater. 2020, 32, e2004690.
180. Rodaum, C.; Chaipornchalerm, P.; Nunthakitgoson, W.; et al. Highly efficient propane dehydrogenation promoted by reverse water–gas shift reaction on Pt-Zn alloy surfaces. Fuel 2022, 325, 124833.
181. Zhou, S.; Zhou, Y.; Zhang, Y.; Sheng, X.; Zhang, Z. The synthesis of new coke-resistant support and its application in propane dehydrogenation to propene. J. Chem. Technol. Biotechnol. 2016, 91, 1072-81.
182. Feliczak-guzik, A. Hierarchical zeolites: synthesis and catalytic properties. Micropor. Mesopor. Mat. 2018, 259, 33-45.
183. Gounder, R.; Iglesia, E. Catalytic consequences of spatial constraints and acid site location for monomolecular alkane activation on zeolites. J. Am. Chem. Soc. 2009, 131, 1958-71.
184. Bulánek, R.; Novoveská, K. Oxidative dehydrogenation of propane over pentasil ring Co-zeolites. Pol. J. Chem. 2004, 78, 149-58. https://yadda.icm.edu.pl/baztech/element/bwmeta1.element.baztech-article-BUJ1-0023-0153. (accessed 13 Jun 2025)
185. Yuan, Y.; Huang, E.; Hwang, S.; Liu, P.; Chen, J. G. Confining platinum clusters in indium-modified ZSM-5 zeolite to promote propane dehydrogenation. Nat. Commun. 2024, 15, 6529.
186. Gong, T.; Qin, L.; Lu, J.; Feng, H. ZnO modified ZSM-5 and Y zeolites fabricated by atomic layer deposition for propane conversion. Phys. Chem. Chem. Phys. 2016, 18, 601-14.
187. Choi, S.; Kim, W.; So, J.; et al. Propane dehydrogenation catalyzed by gallosilicate MFI zeolites with perturbed acidity. J. Catal. 2017, 345, 113-23.
188. Nakai, M.; Miyake, K.; Inoue, R.; et al. Dehydrogenation of propane over high silica *BEA type gallosilicate (Ga-Beta). Catal. Sci. Technol. 2019, 9, 6234-9.
189. Schreiber, M. W.; Plaisance, C. P.; Baumgärtl, M.; et al. Lewis-Brønsted acid pairs in Ga/H-ZSM-5 to catalyze dehydrogenation of light alkanes. J. Am. Chem. Soc. 2018, 140, 4849-59.
190. Siddiqi, G.; Sun, P.; Galvita, V.; Bell, A. T. Catalyst performance of novel Pt/Mg(Ga)(Al)O catalysts for alkane dehydrogenation. J. Catal. 2010, 274, 200-6.
191. Li, Q.; Sui, Z.; Zhou, X.; Zhu, Y.; Zhou, J.; Chen, D. Coke formation on Pt–Sn/Al2O3 catalyst in propane dehydrogenation: coke characterization and kinetic study. Top. Catal. 2011, 54, 888-96.
192. Yang, M.; Zhu, J.; Zhu, Y.; et al. Tuning selectivity and stability in propane dehydrogenation by shaping Pt particles: a combined experimental and DFT study. J. Mol. Catal. A. Chem. 2014, 395, 329-36.
193. Wang, L.; Wang, L.; Meng, X.; Xiao, F. S. New strategies for the preparation of sinter-resistant metal-nanoparticle-based catalysts. Adv. Mater. 2019, 31, e1901905.
194. Wang, N.; Zhang, L.; Guan, Y.; Wu, P.; Xu, H. Pt confined in Sn-ECNU-46 zeolite for efficient alkane dehydrogenation. Chin. J. Struct. Chem. 2024, 43, 100248.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Copyright
Data & Comments
Data




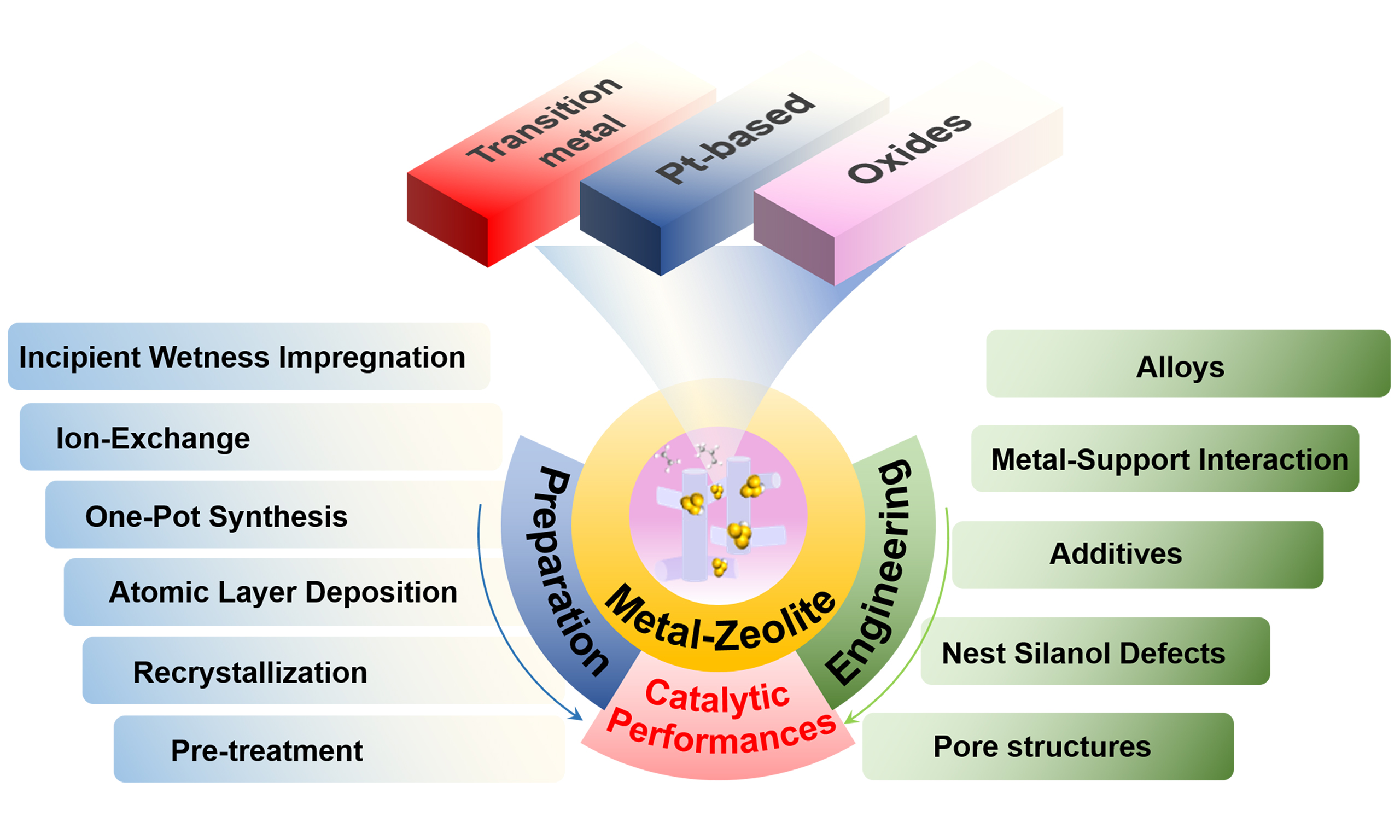
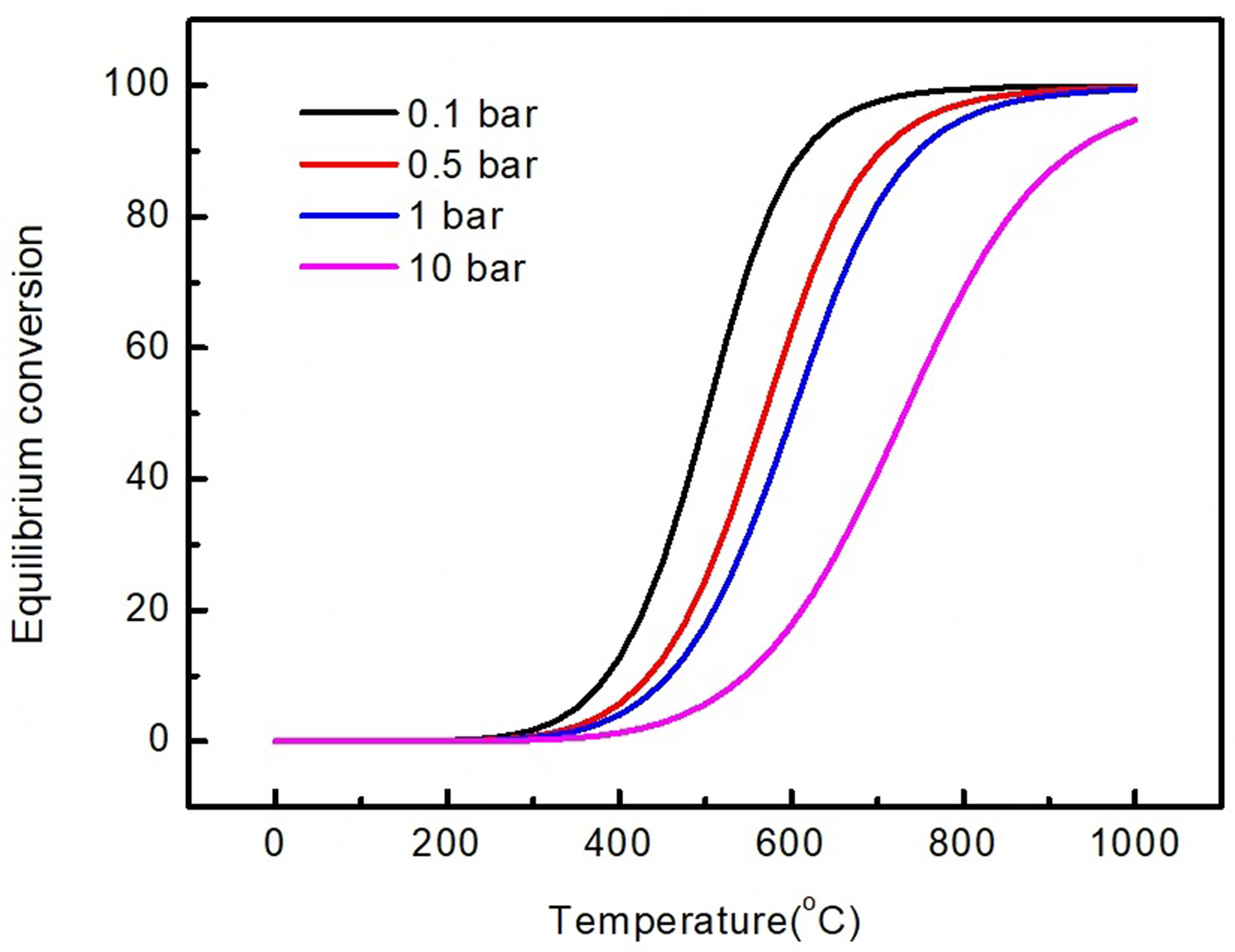
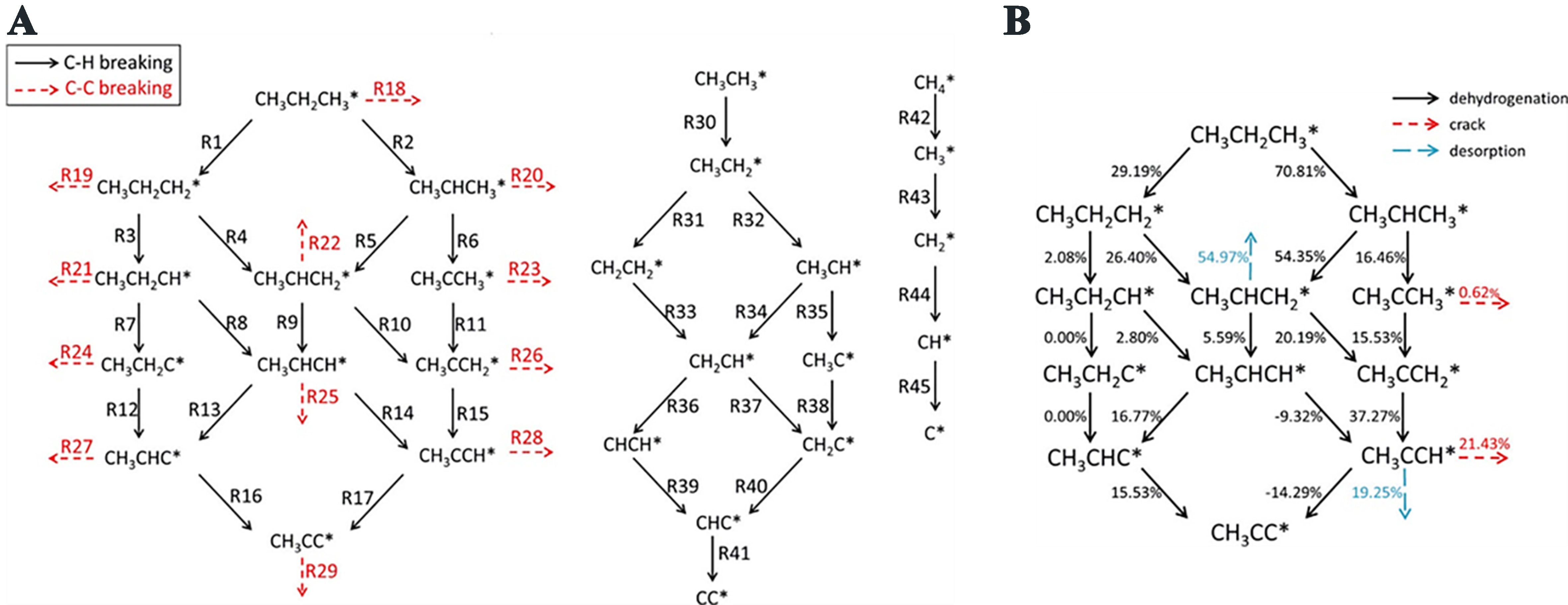
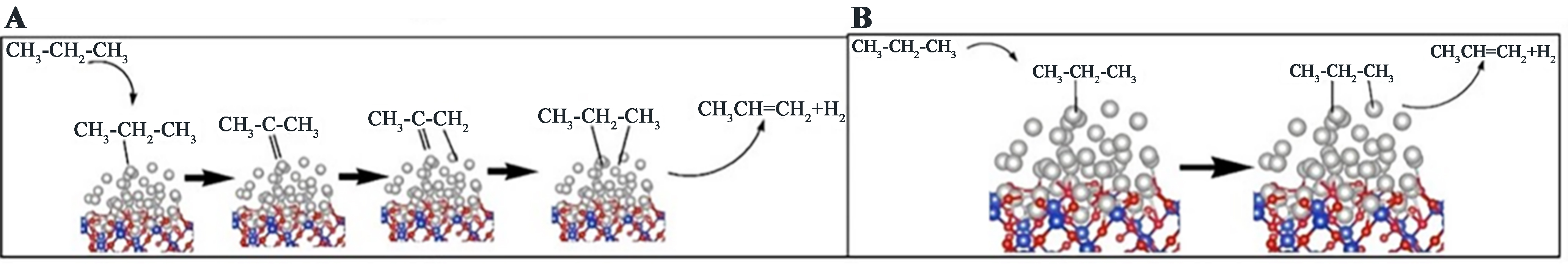
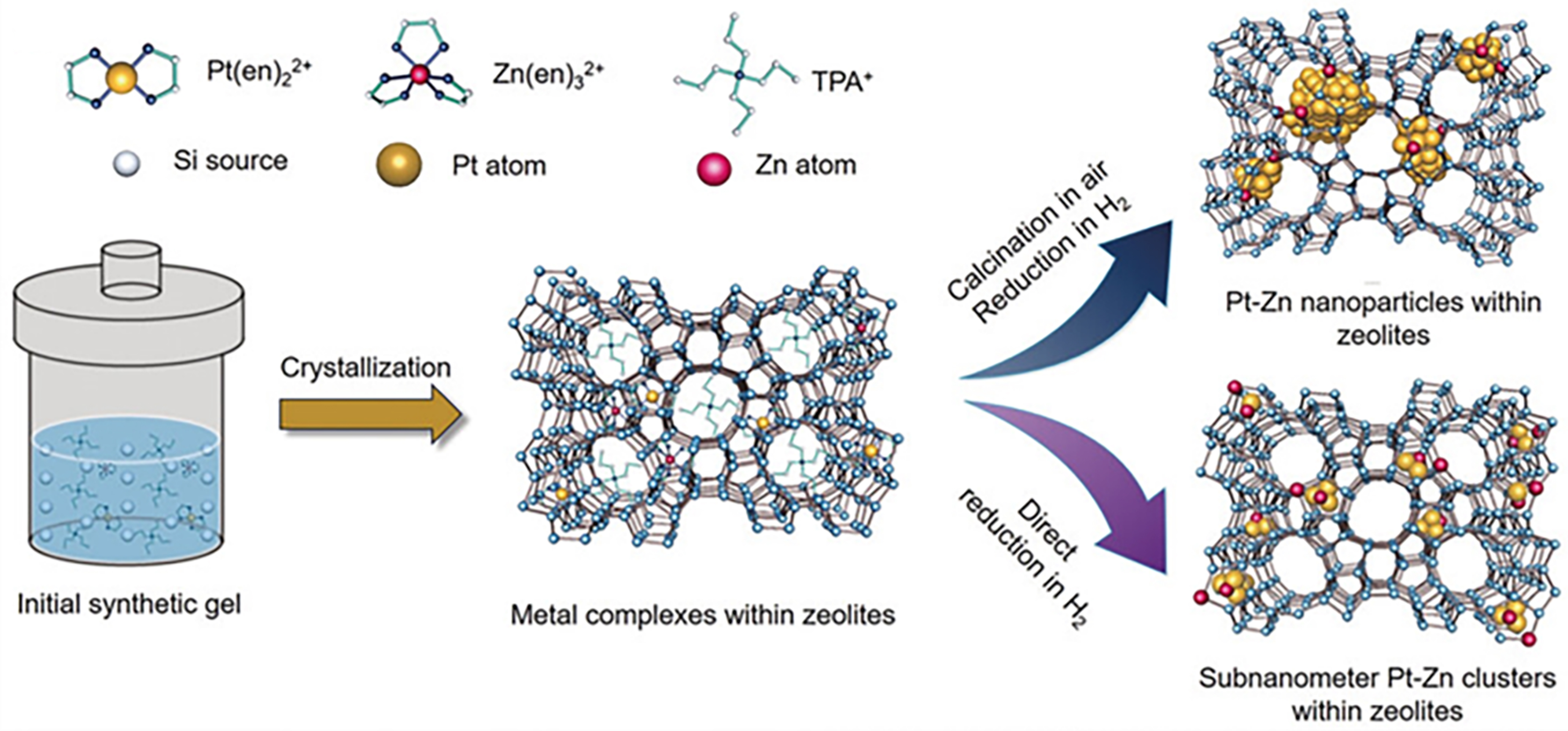
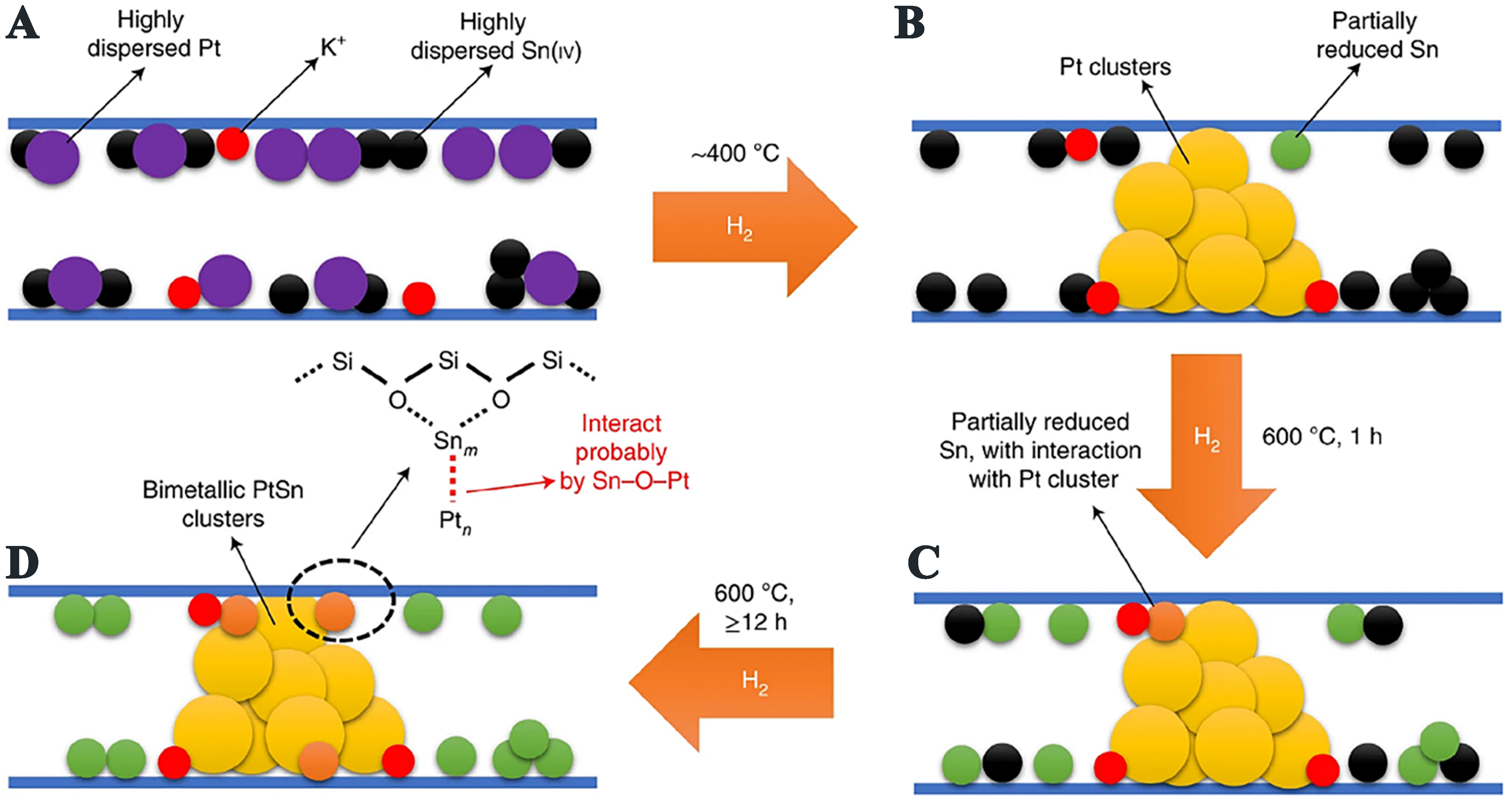
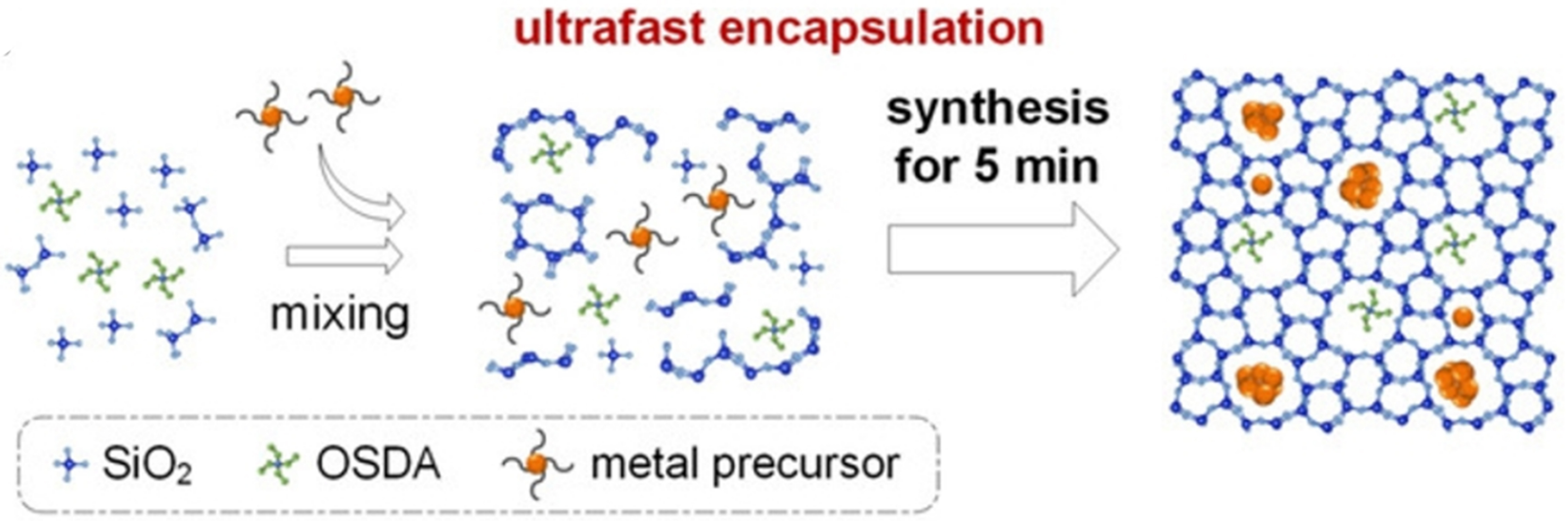
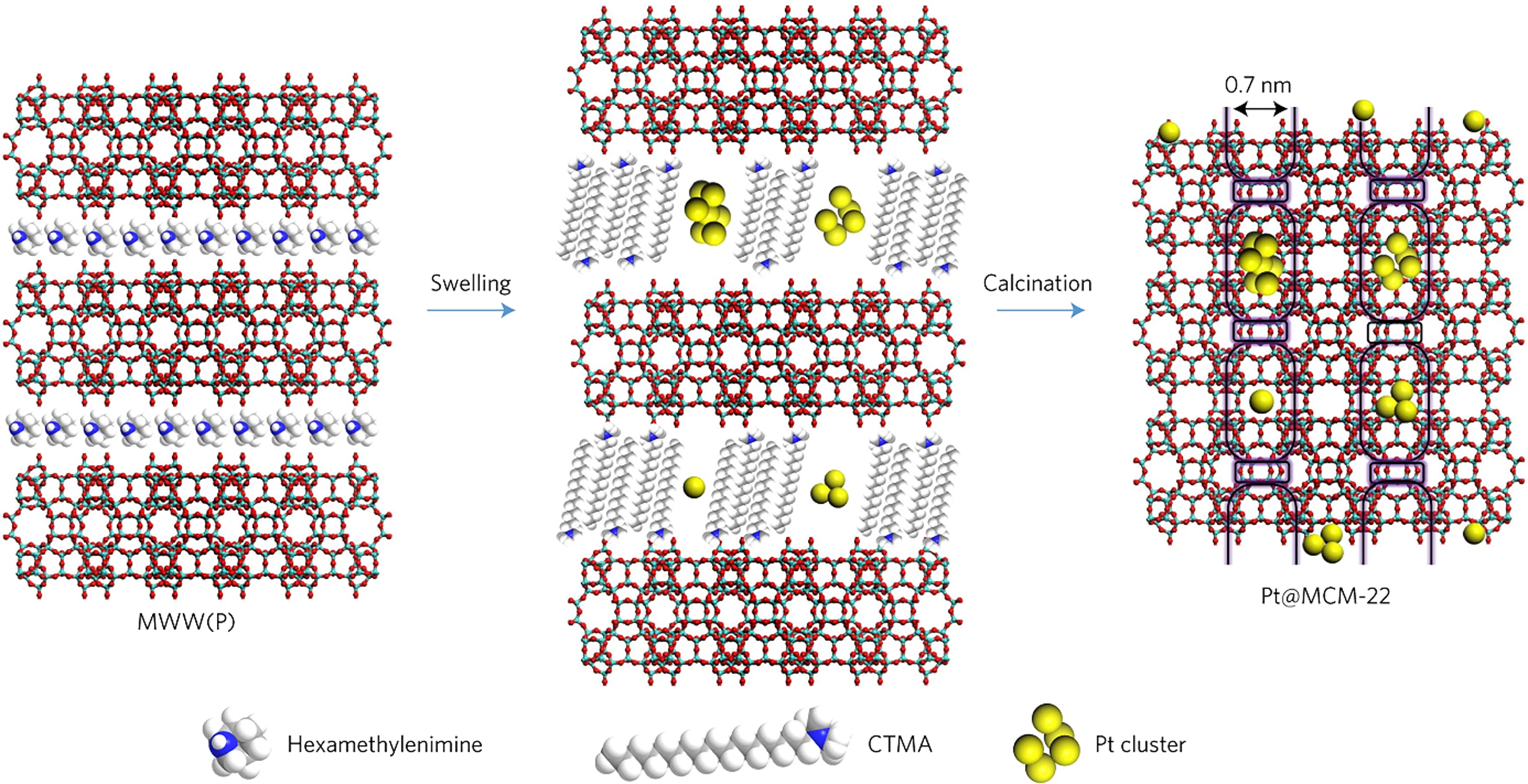
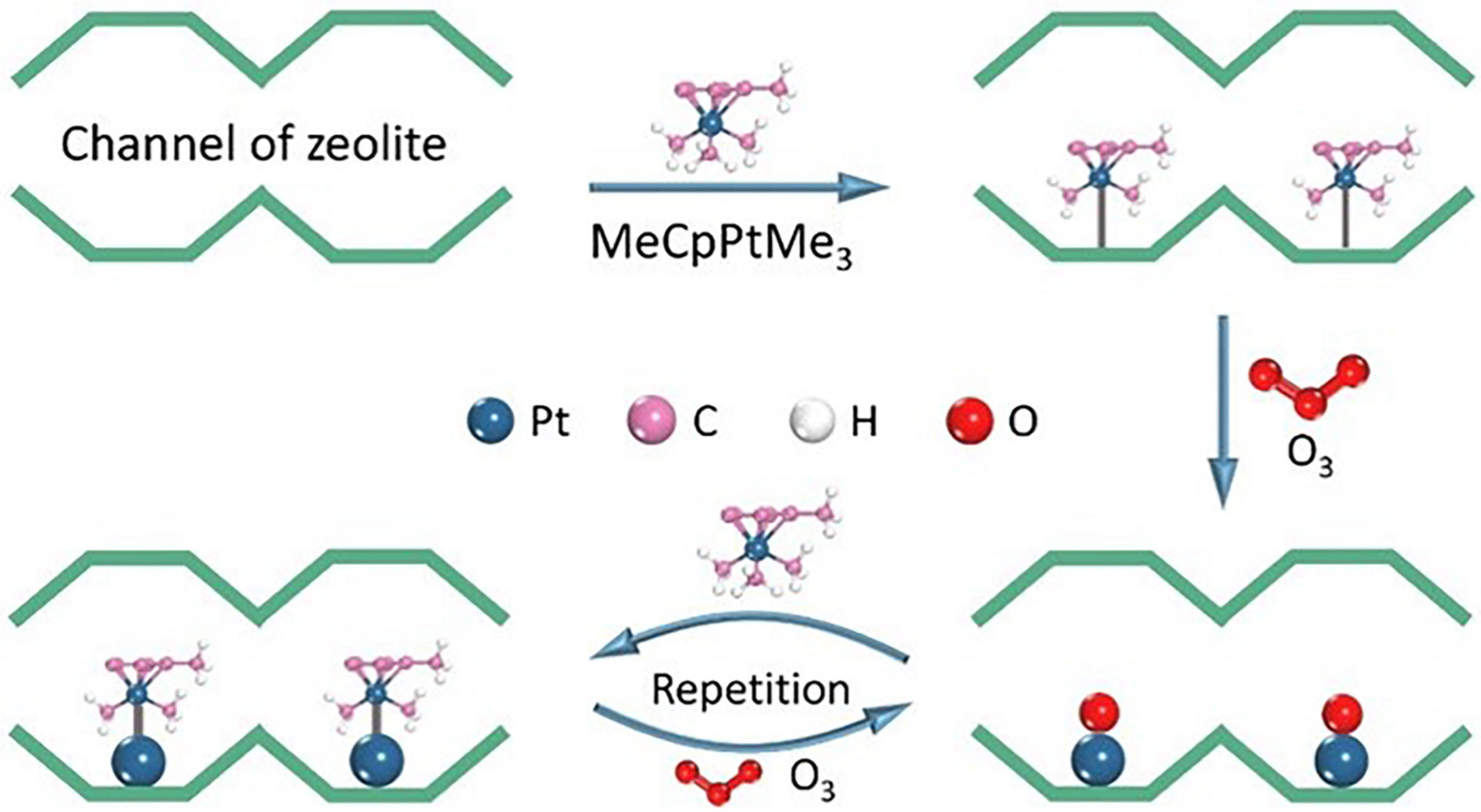
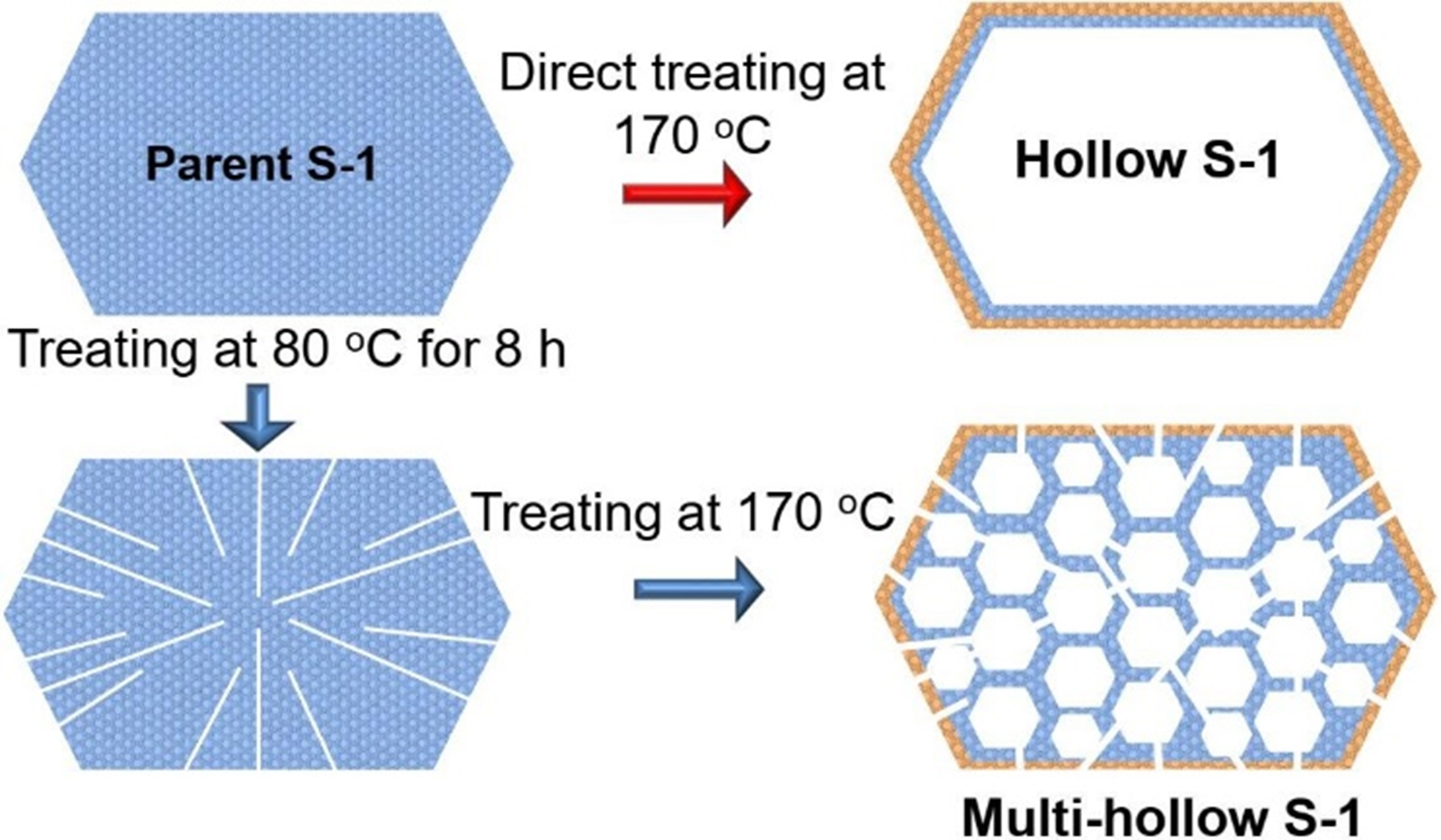
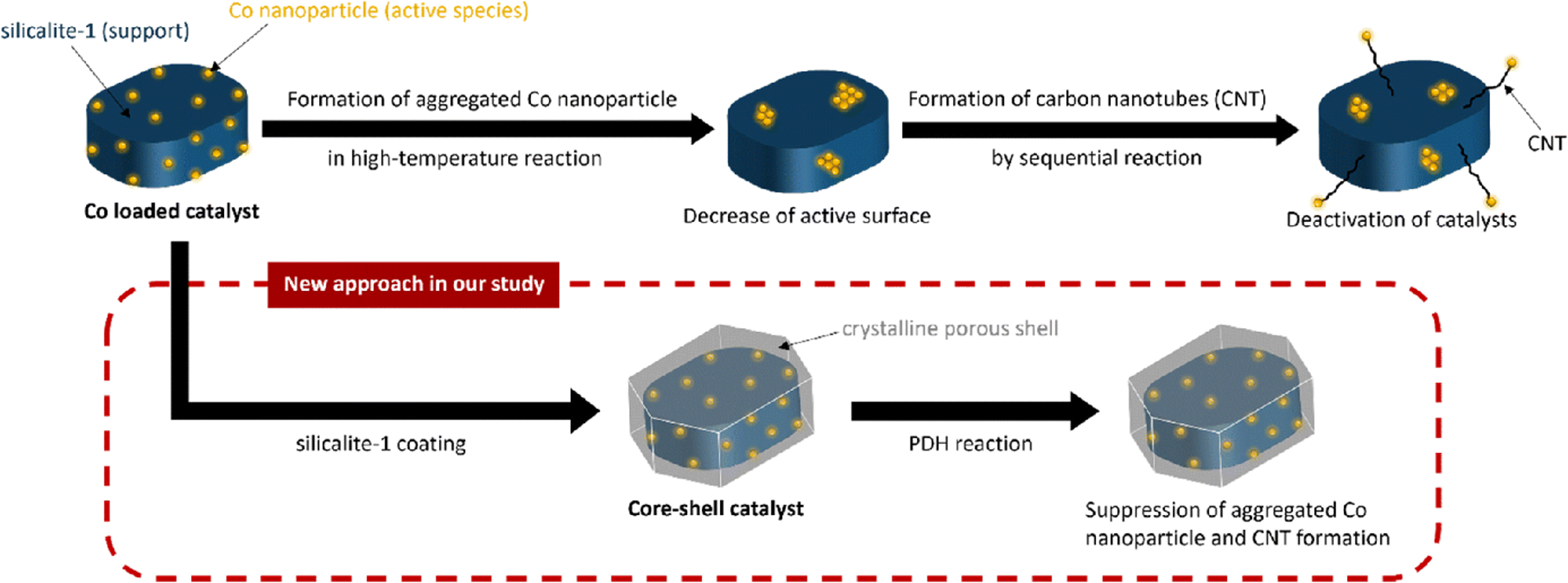
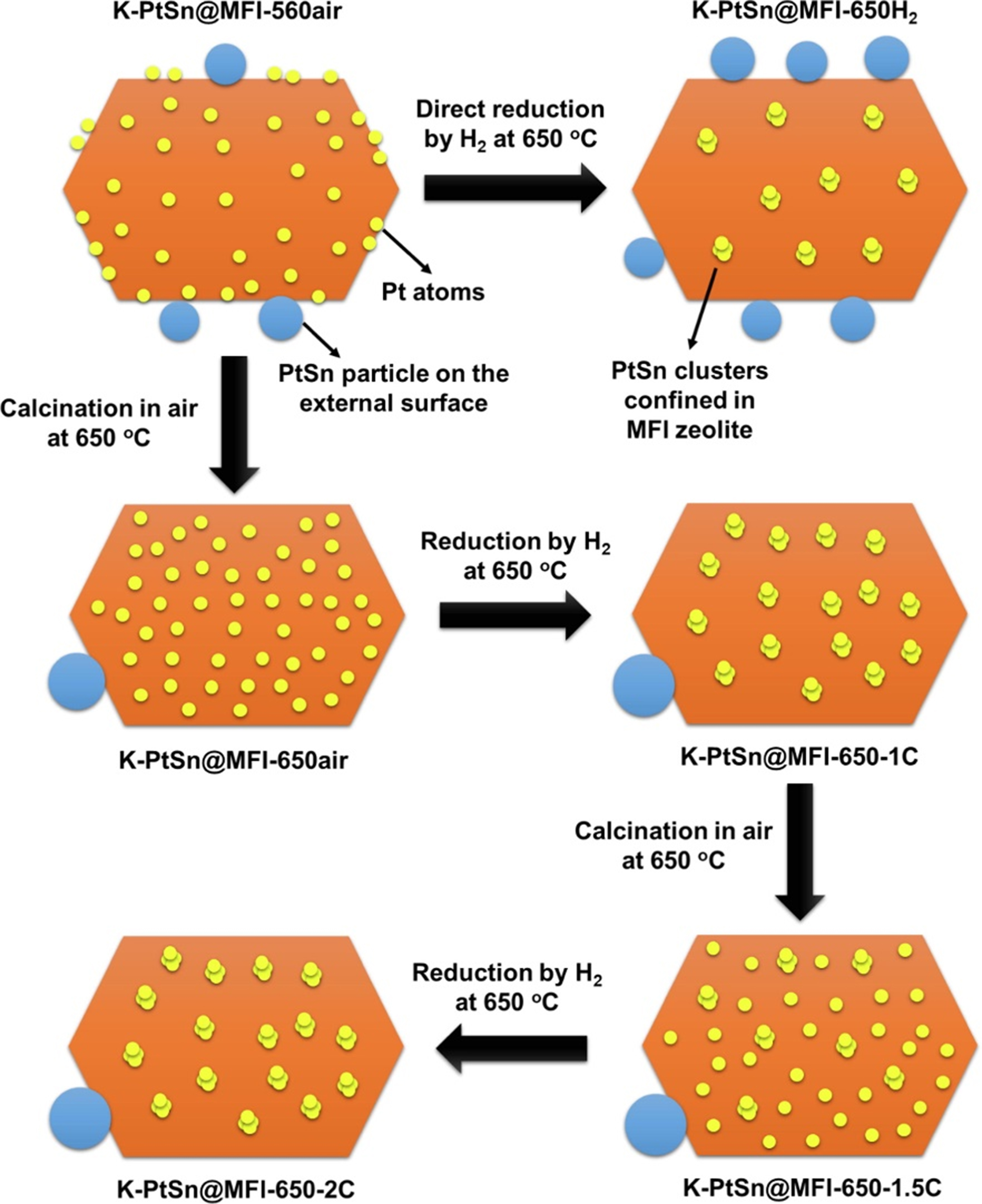
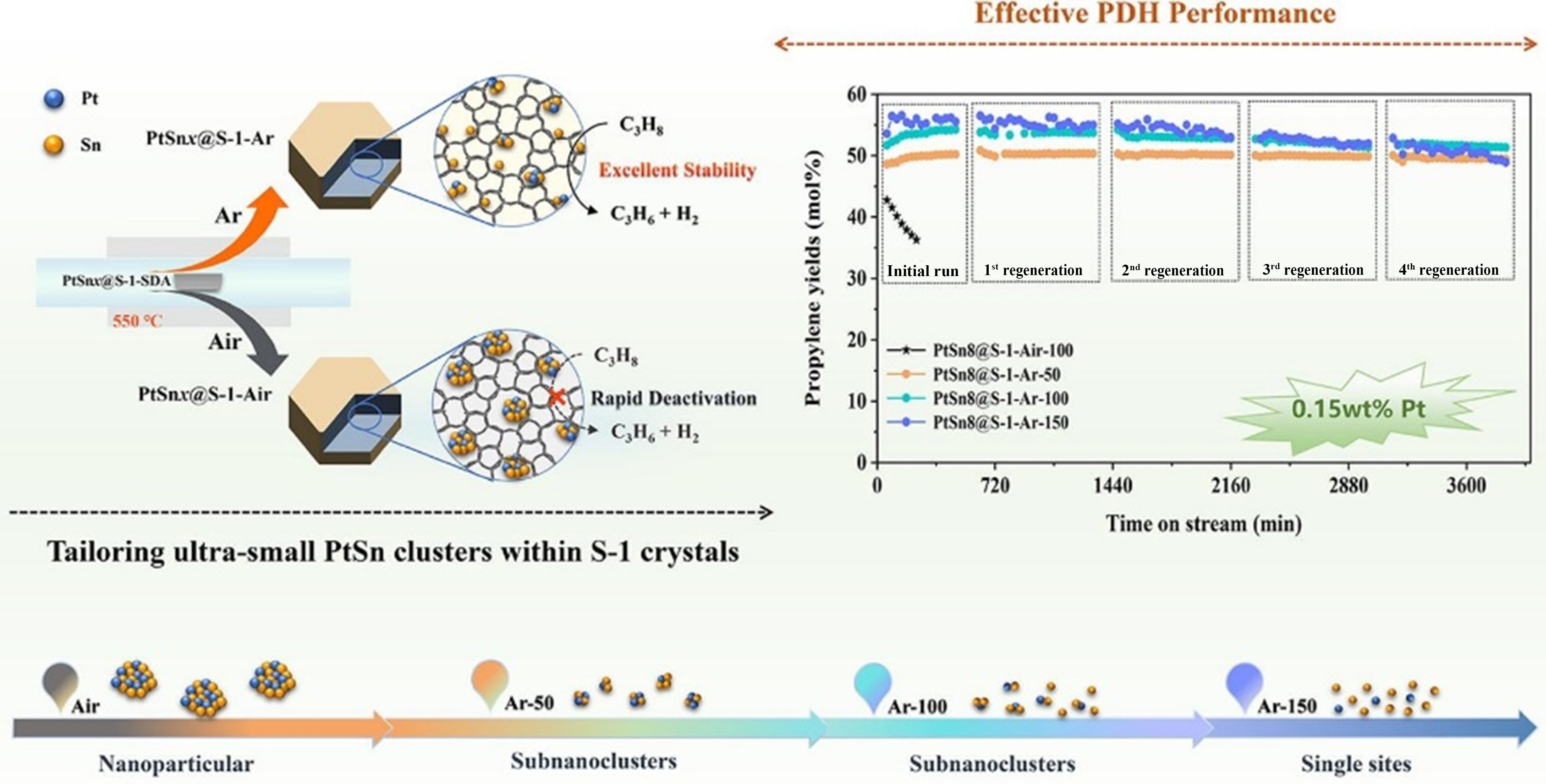
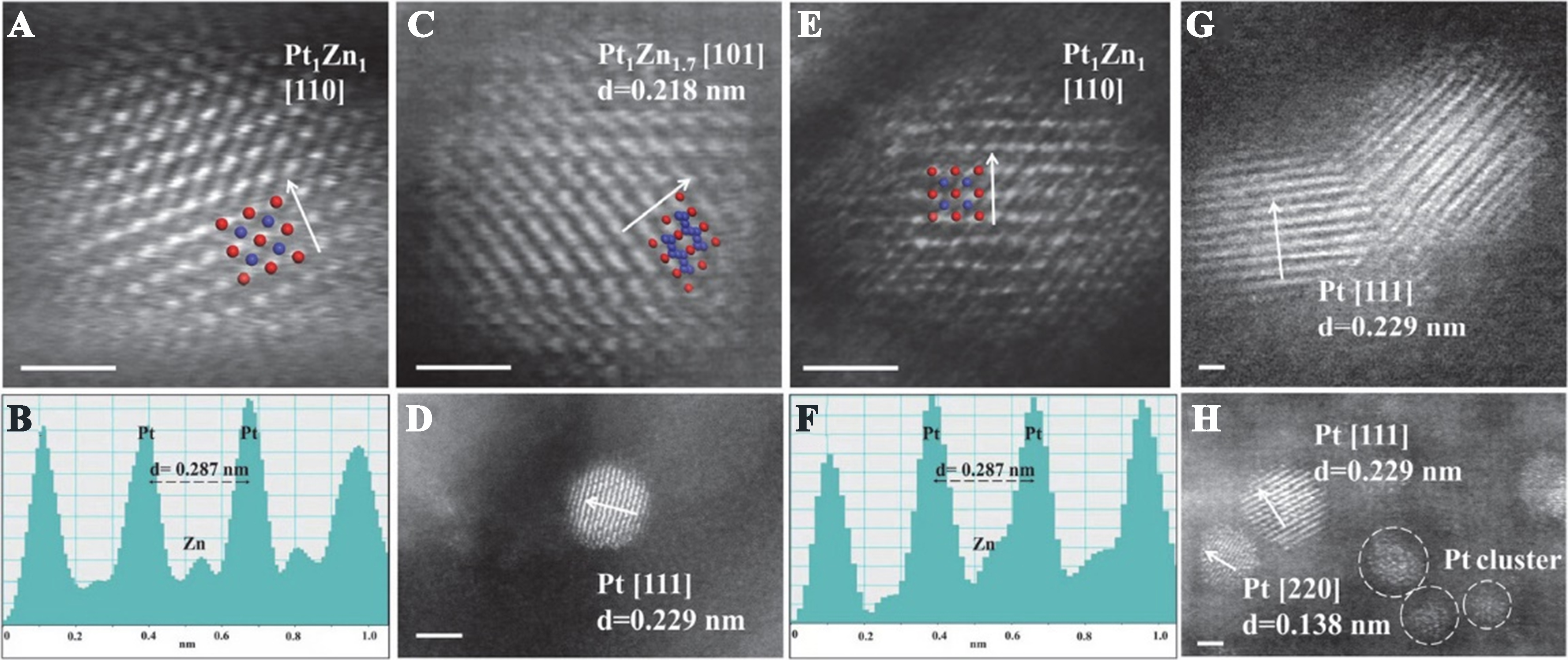
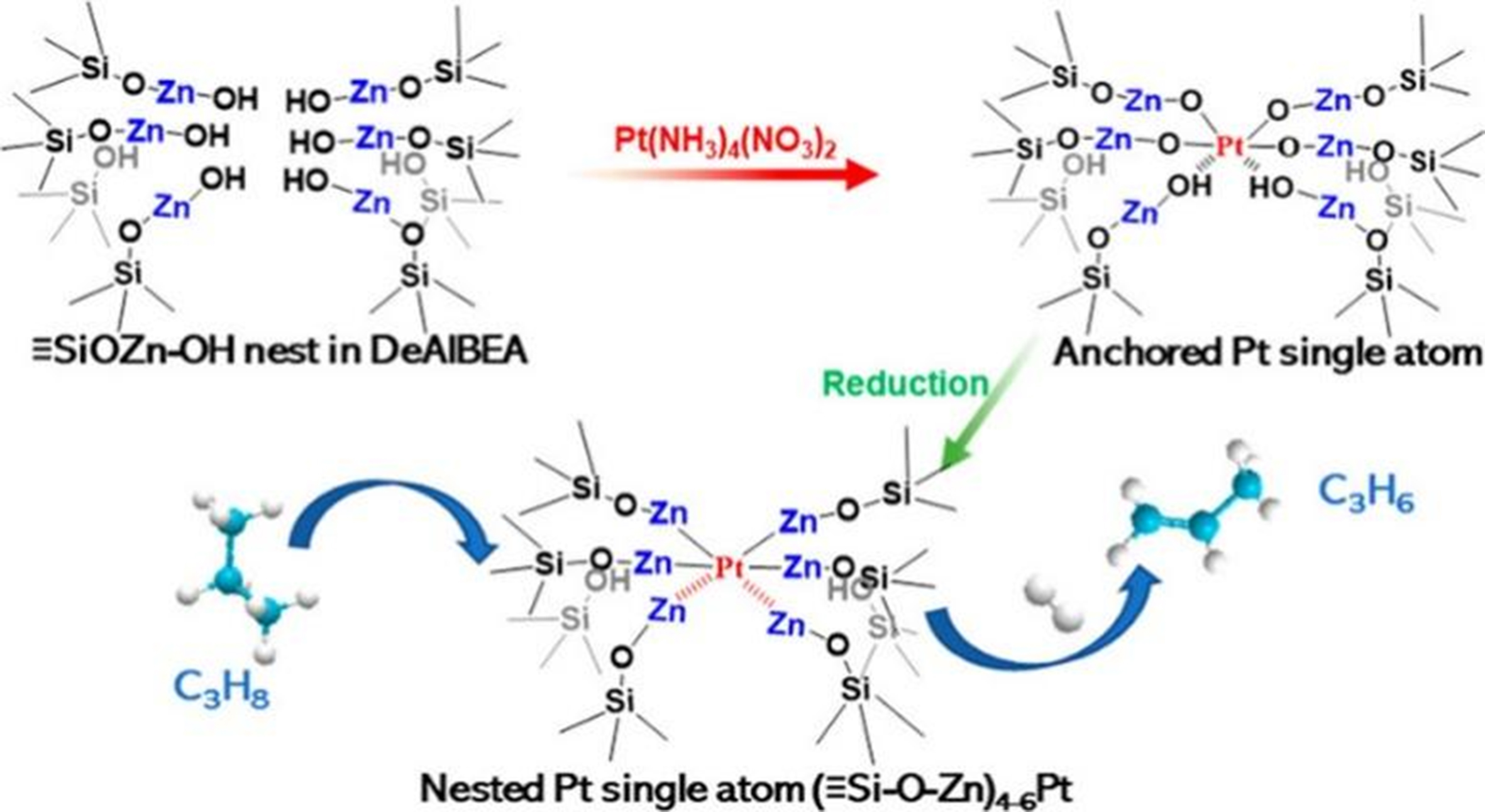
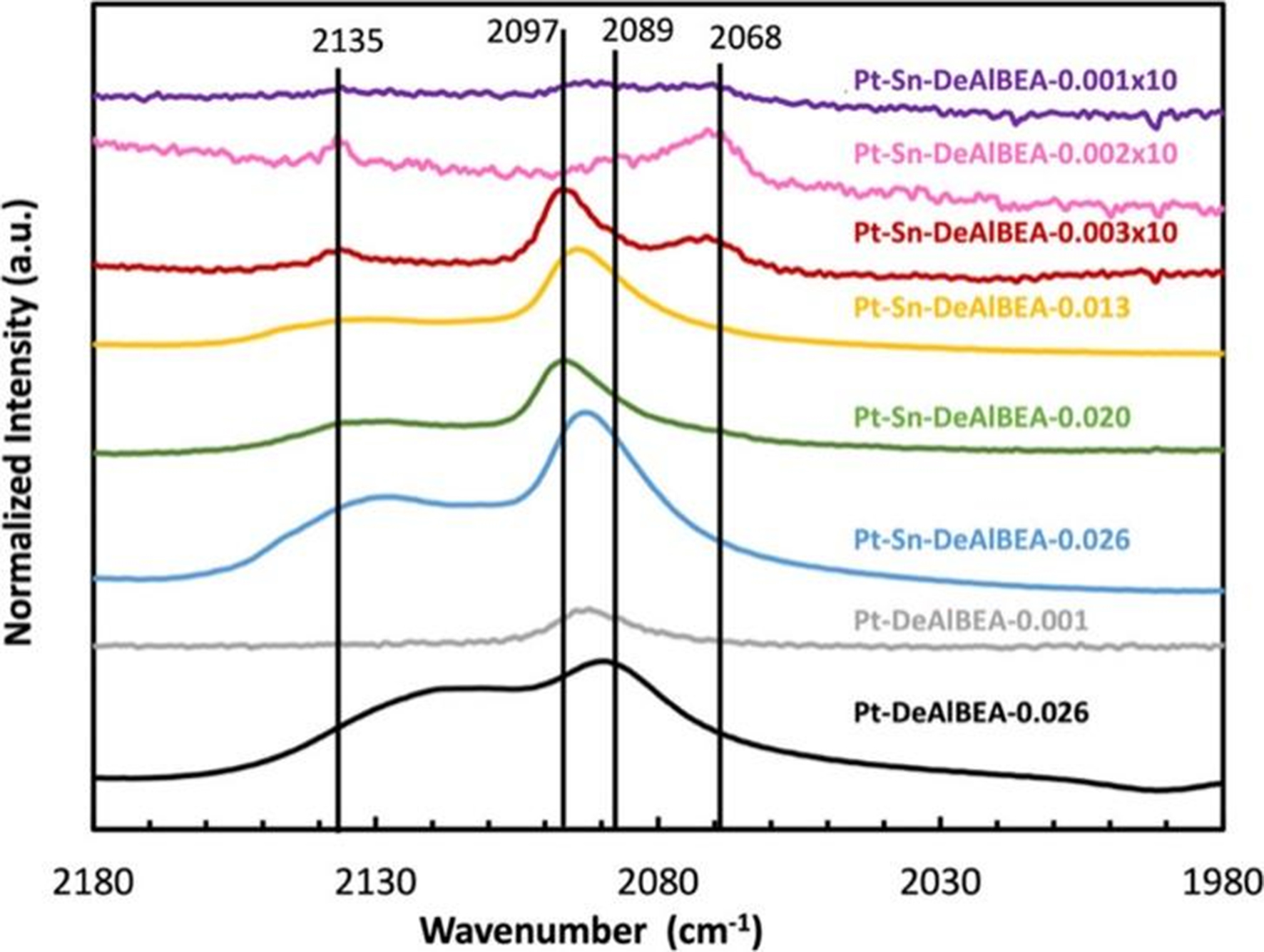
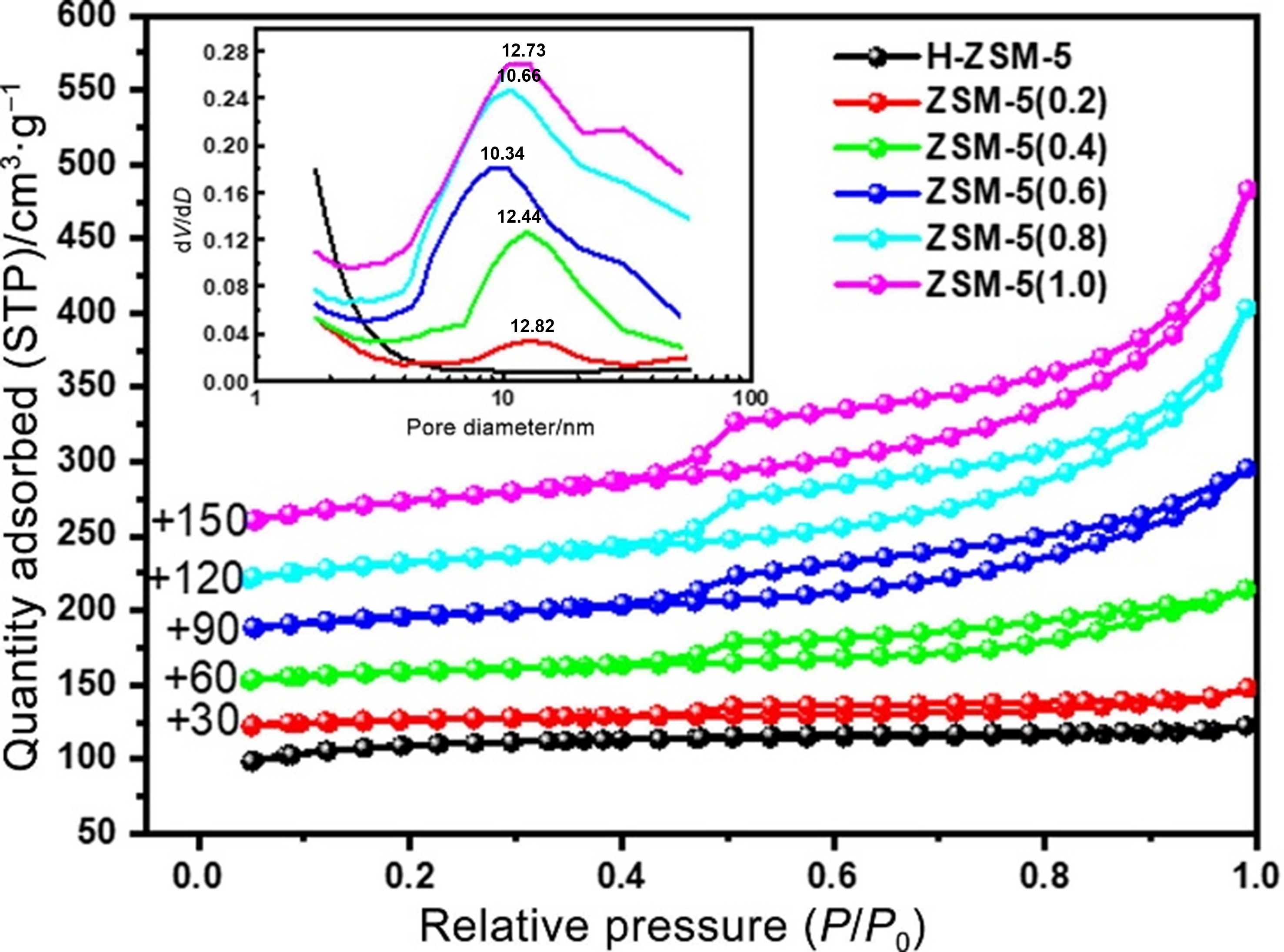












Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].