Update on neurodegenerative markers in isolated rapid eye movement sleep behavior disorder
Abstract
α-synucleinopathy, as represented by Parkinson’s disease and dementia with Lewy bodies, is a group of common neurodegenerative diseases that have insidious onset and irreversible progression. Therefore, identifying markers related to neurodegenerative progression during the prodromal stage is crucial for the early diagnosis and effective treatment of α-synucleinopathy. A distinct parasomnia, isolated rapid eye movement sleep behavior disorder (iRBD), is confirmed as the most specific prodromal phase of α-synucleinopathy, with most of the patients with iRBD eventually converting into a subtype of α-synucleinopathy. Throughout the disease progression of α-synucleinopathy, an array of multidimensional markers, such as clinical, neuroimaging, and neuropathological markers, have been increasingly discovered and validated. These markers may hold potential diagnostic, disease-monitoring, and prognostic values for iRBD. In this review, we summarized the current evidence on the neurodegenerative markers associated with iRBD and discussed their significant role in the diagnosis, monitoring, and prediction of phenoconversion into α-synucleinopathy in iRBD. A better understanding of these markers will facilitate their implementation in risk stratification, population selection for future clinical trials, and monitoring of response to the disease-modifying strategies in iRBD.
Keywords
INTRODUCTION
Rapid eye movement (REM) sleep behavior disorder (RBD) is a distinct parasomnia characterized by repetitive dream enactment behaviors (e.g., talking, shouting, punching, or even kicking) and the excessive muscle tone during REM sleep (i.e., REM sleep without atonia). Isolated RBD (iRBD), defined as RBD not secondary to other neurologic diseases or known factors such as substance use, is the most common form, affecting nearly 1% of individuals aged 60 years or older[1]. The pathogenesis of iRBD involves brainstem substrates, specifically the sublaterodorsal tegmental nucleus (SLD) and ventral medullary (vM) neurons. In normal REM sleep, SLD neurons stimulate vM neurons, which suppress spinal motoneurons to induce REM atonia. Following the aggregation of misfolded α-synuclein proteins in these brain areas, the dysfunction of these cells leads to REM sleep without atonia and dream enactment behaviors in patients with iRBD[2]. Eventually, pathology spreads rostrally toward the substantia nigra pars compacta (SNpc) and corticolimbic circuitry, causing motor and neuropsychiatric manifestations of α-synucleinopathy. Thus, iRBD is recognized as the most specific prodromal stage for α-synucleinopathy. A meta-analysis showed that more than 95% of patients with iRBD will progress to a certain subtype of α-synucleinopathy within 14 years from diagnosis, with nearly 50% converted to Parkinson’s disease (PD), 30% to dementia with Lewy bodies (DLB), and less frequently to multiple system atrophy (MSA)[3]. For this reason, iRBD provides a valuable time window for identifying high-risk individuals, enabling the development and validation of future neuroprotective strategies.
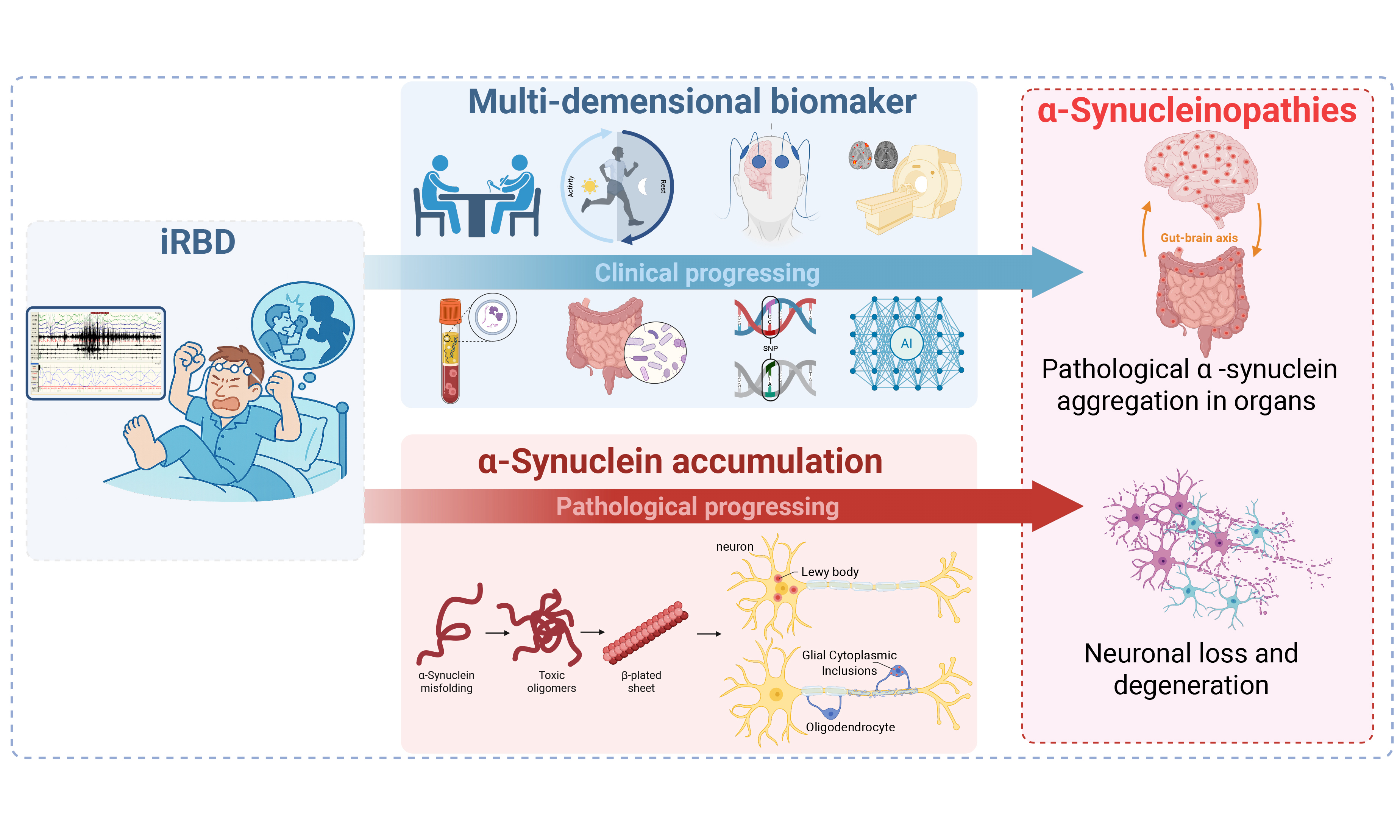
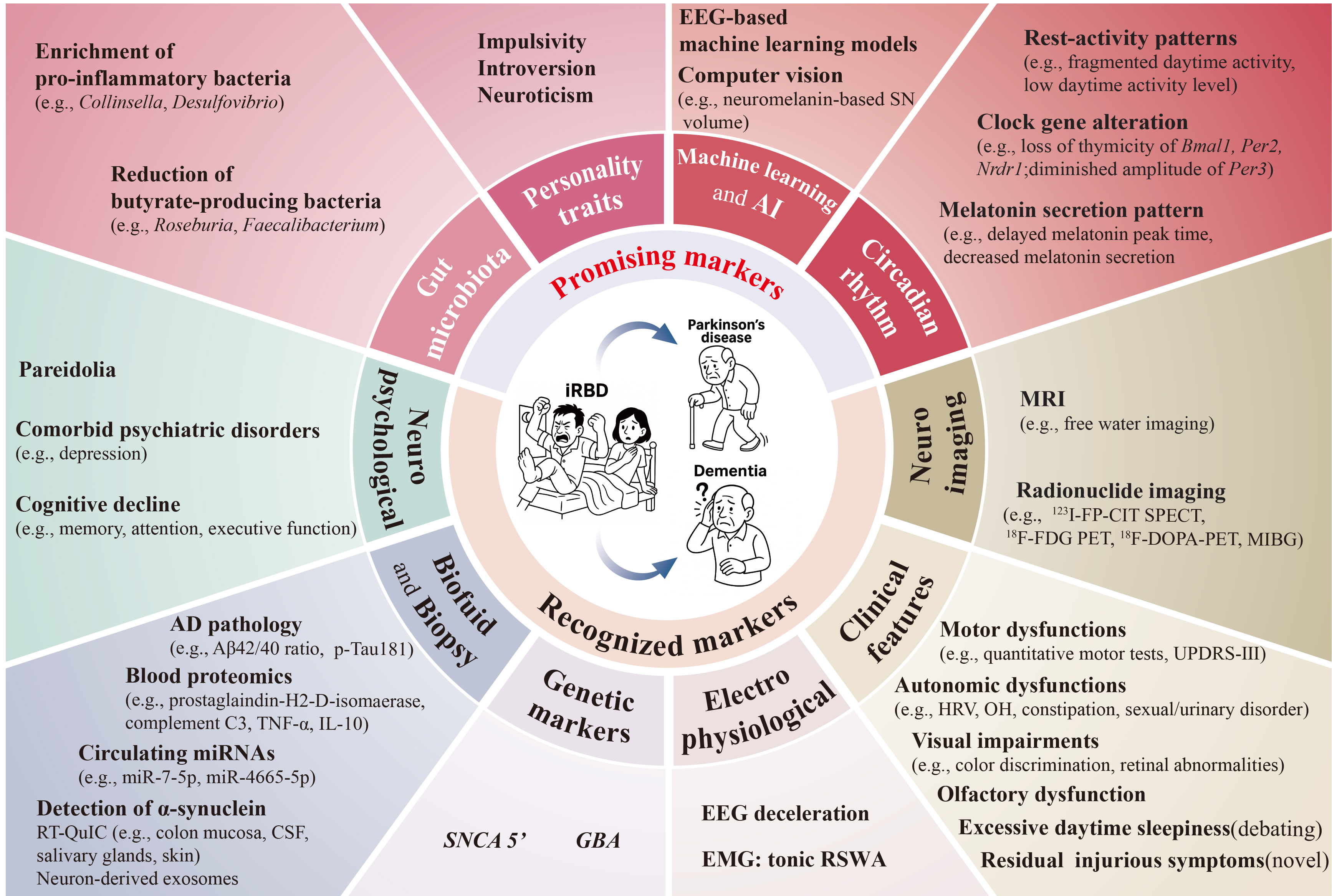
Identifying reliable markers that are closely associated with the progression of neurodegeneration in iRBD is crucial for the early diagnosis of α-synucleinopathies and the evaluation of neuroprotective efficacy. As previously summarized in a comprehensive review, ideal markers should have good sensitivity, specificity, and reproducibility, as well as a combination of affordability and convenience[4]. Based on the current evidence, we systematically searched PubMed, EMBASE, Scopus, and Web of Science from inception to June 2025 (please refer to the Supplementary Materials for detailed search strategy and PRISMA flowchart of the literature reviewed). In this review, we summarized and updated the profiles of neurodegenerative markers in iRBD [Figure 1], with a specific focus on the novel biomarkers that are promising in helping the early diagnosis of neurodegeneration and disease monitoring.
Figure 1. Neurodegenerative biomarkers in iRBD. The lower half shows recognized markers including clinical and neuropsychological features, neuroimaging, electrophysiological, genetic, biofluid, and biopsy-based biomarkers. The upper half summarizes the promising markers suggested in recent studies, concerning features related to personality traits, circadian rhythm, gut microbiota, and those derived from artificial intelligence and machine learning algorithms. AD: Alzheimer’s disease; AI: artificial intelligence; CSF: cerebrospinal Fluid; iRBD: isolated rapid eye movement sleep behavior disorder; MRI: magnetic resonance imaging; MIBG: metaiodobenzylguanidin; PET: positron emission tomography; RBD: rapid eye movement sleep behavior disorder; RT-QuIC: real-time quaking-induced conversion; SN: substantia nigra; SPECT: single photon emission computed tomography; UPDRS-III: unified Parkinson’s disease rating scale part III.
CLINICAL MARKERS
Motor dysfunction is the core clinical feature of PD, and in its prodromal stage, iRBD, subtle motor dysfunction has already emerged. A large-sample, multicenter study demonstrated that motor function assessed by the Unified Parkinson’s Disease Rating Scale Part III (UPDRS-III) and quantitative motor tests was a strong predictor of iRBD phenotype conversion, as patients with iRBD exhibiting motor dysfunction had a threefold increased risk of developing PD[5]. More recent studies utilizing wearable devices or computer-based detection algorithms have incorporated objective measures such as gait, balance, eye movements, and bradykinesia to evaluate iRBD patients[6,7]. The results revealed that the sensitivity and specificity for identifying iRBD reached up to 80%. The assessment of these motor dysfunction markers is relatively simple and convenient, making them a promising diagnostic marker for widespread use in clinical practice and neuroprotective trials. However, whether and to what extent these digital motor markers could forecast the phenoconversion in iRBD are pending further prospective evidence.
Olfactory reduction or loss is considered one of the earliest features of PD and is already present in the majority of iRBD patients. A multicenter study reported that olfactory deficit is associated with a higher rate of phenoconversion to overt dementia and parkinsonism in patients with iRBD, and its predictive ability surpassed that of all other non-motor symptoms with a hazard ratio (HR) of 2.62[5]. Another study reported that olfactory deficit was closely associated with reduced dopamine transporter binding in the amygdala, and the progressive deterioration of visuospatial abilities and verbal memory, suggesting that olfactory deficit may predict the future cognitive decline in iRBD[8]. In a recent study, researchers revealed that olfactory deficit was also associated with α-synuclein deposition in skin biopsies[9]. Moreover, prospective data show that olfactory reduction does not worsen over time[10]; thus, it may be best suited for baseline prognosis but not for phenoconversion subtype prediction or treatment response monitoring. Nonetheless, when hyposmia was combined with imaging indices of the dopamine transporter, its relative risk for PD prediction was as high as 17.47[11], which highlights the potential that hyposmia can be used as an adjunct prognostic biomarker for risk stratification.
Visual impairments, especially color discrimination deficit, are common in patients with α-synucleinopathy. Previous studies have confirmed that significant impairments in color discrimination are already present in patients with iRBD[12]. A prospective study found that color discrimination deficits in iRBD patients are strongly associated with a higher risk of phenoconversion[13]. This suggests that color discrimination impairments in iRBD patients are strong predictors of progression to α-synucleinopathy.
Thinning of the retinal ganglion cell complex is reported to be associated with dopaminergic loss in the substantia nigra and visual impairments in PD patients[14]. In iRBD patients, this thinning is related to olfactory loss and reduced striatal dopamine transporter levels[15]. In addition, melanopsin retinal ganglion cell (mRGC)-mediated pupillary light reflex (PLR) abnormalities have been documented in several neurodegenerative disorders including PD. As measured by chromatic pupillometry and compared to controls, patients with iRBD demonstrated larger pupil diameter and decreased rod-transient PLR amplitude, and the latter was associated with REM atonia index and phosphorated α-syncuclein deposition in the skin[16]. This evidence suggests that indices measured by optical coherence tomography and chromatic pupillometry may serve as potential diagnostic and prognostic markers in iRBD.
Autonomic dysfunction symptoms, such as orthostatic hypotension, sexual dysfunction, urinary disorders, and constipation, can occur in up to 94% of iRBD patients[17]. Previous evidence has suggested that autonomic symptoms were associated with a faster phenoconversion of iRBD[18]. For example, a multicenter study reported that constipation and erectile dysfunction could predict phenoconverison to parkinsonism or dementia with an HR of 1.67 and 2.13, respectively[5]. Moreover, studies have suggested that orthostatic hypotension and iRBD collectively reflected a ‘malignant’ phenotype of PD with early cognitive impairment and postural instability[19]. However, the predicted value of orthostatic hypotension in iRBD was not supported by the multicenter study[5]. In addition, autonomic cardiac/vascular dysfunction is prevalent across the α-synucleinopathies, including their prodrome stage of iRBD. For example, heart rate variability (HRV) demonstrates robust diagnostic utility for identifying iRBD [area under the curve (AUC) = 0.80, P ≤ 0.001]. Crucially, central cardiac autonomic outflow remains intact in iRBD compared to healthy controls, indicating that cardiovascular dysfunction in iRBD originates from peripheral rather than central autonomic degeneration[20]. A newly published cohort study revealed that the combination of motor symptoms (high MDS-UPDRS Part III scores) and cardiovagal dysfunction (abnormal HRV optimally predicts phenoconversion in iRBD (AUC = 0.77). When further combined with RBD symptom duration, the predictive accuracy elevated to 0.89[21]. This indicates that cardiovascular autonomic impairment is not only highly prevalent in iRBD but also potentially holds significant predictive value for neurodegeneration. Extended longitudinal research is imperative to validate HRV metrics as standalone biomarkers.
Excessive daytime sleepiness (EDS) is one of the most common non-motor symptoms of PD, affecting approximately 50% of PD patients. However, the predictive value of EDS for phenoconversion risk remains to be clarified. Earlier longitudinal studies have shown that the prevalence of EDS increased from 4.1% at baseline to 40.8% after 8 years of follow-up, indicating that it tends to increase as PD progresses. A cohort study in Hong Kong reported that EDS in iRBD was significantly associated with an increased risk of neurodegenerative diseases (HR: 2.56), particularly PD (HR: 3.55)[22]. However, these findings were not replicated in the Montreal cohort and the Spanish cohort; the Epworth Sleepiness Scale scores were similar between those who underwent phenoconversion and those remaining disease-free (6.7 ± 4.4 vs. 7.1 ± 4.7, P = 0.70)[23]. Additionally, a later multicenter study with additional cohorts did not support the predictive value of EDS in the phenoconversion of iRBD, despite heterogeneity in the measurement of EDS[5]. Thus, whether EDS is a reliable predictor of impending neurodegeneration in iRBD remains a subject of debate. Future studies should utilize objective measurement and extend the follow-up period to further confirm the association between EDS and neurodegeneration in iRBD.
NEUROPSYCHOLOGICAL MARKERS
Cognitive decline is common in iRBD patients, with more than one-third of iRBD patients exhibiting mild cognitive impairment (MCI). Furthermore, iRBD patients with MCI have a higher risk of phenoconversion to neurodegenerative disorders compared to their counterparts with normal cognition[5]. Mounting evidence has shown that iRBD patients exhibit impairments in various cognitive domains, including memory, attention, and executive function[24,25]. The pattern and severity of cognitive deficits in iRBD patients may predict the subtype of α-synucleinopathy[26]. In a longitudinal study, significant deficits in attention and executive function (e.g., Trail Making Test-Part B) were observed in iRBD patients up to six years before a diagnosis of DLB, while abnormalities in verbal episodic memory and verbal fluency emerged 2-4 years before DLB diagnosis[24]. Attention and executive function, verbal fluency, and verbal memory were identified as the top predictors of DLB (AUC = 0.90-0.97)[26]. A recent prospective study showed that visuospatial dysfunction, as reflected by the MoCA posterior subscores, is the strongest predictor of iRBD progression to dementia (HR: 5.48, 95% CI 1.67-17.98). This study suggested that visuospatial dysfunction might have the potential as both a prognostic marker and an intervention target in neuroprotective trials[27]. Therefore, cognitive dysfunction seems to be an early prognostic marker for α-synucleinopathies, particularly for DLB. It also has the potential to serve as a marker for monitoring prognosis and evaluating the efficacy of neuroprotective interventions.
Visual hallucinations (VH) and pareidolia are complex neuropsychiatric phenomena that are commonly observed in patients with α-synucleinopathy. VH represent a core diagnostic feature of DLB, often manifesting in the early clinical stages[28]. In contrast, VH typically occur in later stages of PD and are associated with the status of iRBD[29], as well as more severe cognitive impairment and motor dysfunction[30]. However, VH remain under-investigated in iRBD, likely due to a lack of recognition and reporting of this symptom. On the other hand, pareidolia, a complex misperception characterized by the perception of specific meaningful objects (e.g., faces of humans or animals) in random or ambiguous visual patterns, has been shown to be closely associated with the severity of VH[31]. Using the pareidolia test, which contains a series of blurred pictures to provoke the pareidolic responses, it is estimated that over 70% of patients with DLB presented with pareidolia, and it could effectively discriminate dementia with DLB from Alzheimer’s disease with high diagnostic accuracy (AUC = 0.98)[31]. Some researchers propose that pareidolia and VH exist on a continuum of symptoms, where pareidolia represents a subclinical manifestation or susceptibility to VH[32]. In recent years, an increasing number of studies have investigated the clinical significance of pareidolia in iRBD, and the data show that more than half of the iRBD patients exhibit pareidolic responses, significantly exceeding the prevalence observed in healthy controls (53.5% vs. 21.7%). Multivariate analysis revealed that pareidolia in iRBD patients is associated with cognitive decline[33]. Another cohort study further reported that pareidolia in iRBD is associated with visuospatial abilities, attention/executive function, and memory. Moreover, within 1.6 years of follow-up, 19% of iRBD patients with pareidolia, compared to only 8% of those without, had phenoconverted[34]. These findings may suggest that pareidolia in iRBD serves as a valuable predictive marker for the future development of DLB.
Additionally, there has been a growing number of studies examining the comorbidity of psychiatric disorders such as depression and anxiety with iRBD. A recent study found that 9% of patients with depression also had iRBD[35], which is significantly higher than the prevalence of iRBD in the general population (~ 1%). Furthermore, compared with patients with depression only, patients with comorbid depression and iRBD showed significant abnormalities in prodromal markers, including color vision and olfaction deficit, as well as a higher probability for prodromal PD as defined by the MDS research criteria[36]. This suggests that the comorbidity of depression and iRBD may represent a distinct subtype of depression, with a higher future risk of developing α-synucleinopathies[35]. Additionally, the authors conducted a family-based case-control study and found that patients with comorbid depression and iRBD, but not patients with depression alone, had a significant familial susceptibility to α-synucleinopathy spectrum features, including iRBD, overt neurodegenerative disorders, and related prodromal markers[37]. These findings further highlight the viewpoint that depression is heterogeneous concerning its relationship with neurodegeneration, with iRBD signaling a subtype of depression with underlying neurodegenerative pathology. Nonetheless, it is possible that depression itself might also correlate with an elevated risk of neurodegeneration, potentially through the shared mechanisms such as oxidative stress and neuroinflammation, and further research is needed to uncover the underlying associations.
ELECTROPHYSIOLOGICAL MARKERS
Changes in electrophysiological features, such as those observed in electroencephalography (EEG), might signal the progress of neurodegeneration. For example, iRBD patients have been found to exhibit attenuated dominant alpha rhythms in occipital, parietal, and temporal regions, coupled with elevated delta/theta power in frontal and central lobes, as compared with healthy subjects[38]. Specifically, iRBD patients with MCI show characteristic EEG slowing in posterior cortical regions, which has emerged as a biomarker indicating elevated risk of cognitive decline[39]. Prospective studies have further demonstrated that specific EEG alterations, notably enhanced alpha phase synchronization, decreased delta amplitude correlations, and EEG slowing, are strongly associated with neurodegenerative progression in iRBD during longitudinal follow-up[40,41]. In the future, large-scale cohort studies should be conducted to evaluate the predictive capacity of these EEG signatures for phenoconversion in iRBD.
REM sleep without atonia (RSWA) is the most prominent electrophysiological feature of iRBD. Liu et al. reported that the severity of RSWA increased in the prodromal stage of iRBD, revealing that baseline electromyographic (EMG) activity during REM sleep predicts the development of neurodegenerative diseases over time in patients with iRBD[42]. Specifically, tonic EMG activity (AUC = 0.68), but not phasic EMG activity, may serve as a stable biomarker for predicting the phenoconversion to PD in patients with iRBD, with an adjusted HR of 2.76[43]. Similarly, another recent prospective study also confirmed that tonic RSWA is the strongest biomarker for predicting phenoconversion among all sleep electrophysiological parameters (AUC = 0.80; 73.7% sensitivity; 75.6% specificity)[44]. These findings suggested that tonic EMG activities during REM sleep might be a promising marker for the prediction of phenoconversion in iRBD.
NEUROIMAGING MARKERS
Over the past decade, neuroimaging in PD has advanced rapidly, especially using PET and SPECT imaging to detect early damage to the nigrostriatal dopaminergic pathway and changes in dopamine transport in the basal ganglia. Similarly, PET and SPECT studies have confirmed that nigrostriatal dopaminergic pathways were already damaged in iRBD patients[45]. 123I-FP-CIT SPECT has shown that reduced dopamine uptake in the striatum and putamen can predict clinical phenoconversion in iRBD, especially when combined with clinical markers such as cognitive impairment and autonomic dysfunction[46]. Therefore, 123I-FP-CIT SPECT might hold the promise as a strong biomarker for phenoconversion and a prognostic biomarker for neuroprotective treatment trials.
Motor dysfunction in parkinsonism arises from dopaminergic deficiency in the striatum due to neurodegeneration of the nigrostriatal pathway. 18F-AV-133 vesicular monoamine transporter type 2 (VMAT2) PET quantifies the severity of this degeneration and was suggested to be a sensitive measure to monitor neurodegenerative progression of PD[47]. Cross-sectional studies revealed significantly reduced VMAT2 levels in the caudate and putamen of iRBD patients, indicating nigrostriatal degeneration[48]. However, longitudinal research demonstrated no significant difference in the annual change of VMAT2 decline between iRBD subjects and healthy controls over a 26-month follow-up period[49]. Thus, the potential predictive ability of AV-133 PET in iRBD phenoconversion requires further investigation.
Other studies have used PET and SPECT to assess glucose metabolism and cerebral perfusion, suggesting that abnormal hippocampal perfusion at baseline can predict progression to PD or DLB in iRBD patients[50]. Recent research identified a novel brain perfusion pattern in iRBD, as monitored by 99mTc-HMPAO SPECT imaging, and it may serve as a potential biomarker for predicting the progression of iRBD to DLB[51]. The PD-related pattern (PDRP) is a glucose metabolic pattern of PD measured by 18F-FDG PET, which is characterized by relatively increased glucose metabolism in the pallidum, putamen, thalamus, cerebellum, pons, and sensorimotor cortex, along with relatively decreased metabolism in the bilateral frontal and parieto-occipital cortices[52]. In several longitudinal imaging studies, the expression of PDRP in iRBD patients increases with disease progression and is also associated with prodromal features of PD, suggesting its potential as a biomarker for short-term phenoconversion[53,54]. Furthermore, abnormal functional connections were identified at baseline in both PDRP and dopaminergic networks, and the rate of network progression is closely related to the timing of phenoconversion[55]. These findings indicate that alterations in the PDRP network could serve as a potential tool for monitoring treatment outcomes in patients with iRBD.
Previous studies have confirmed that activated microglia play a role in the pathological progression of PD. However, the role of activated microglia in iRBD patients and how they affect disease progression remain unclear. Stær et al. performed 11C-(R)-PK11195-PET scans on 15 iRBD patients at baseline and 18F-DOPA-PET scans after three years of follow-up to assess changes in nigrostriatal function. They found that iRBD patients with increased 11C-(R)-PK11195 binding at baseline had more significant declines in dopamine uptake, suggesting that microglial activation has a detrimental effect in the prodromal phase of α-synucleinopathy[56]. Further large-scale prospective studies are needed to confirm these findings.
In recent years, metaiodobenzylguanidine (MIBG) has been used to assess cardiac sympathetic function as a biomarker for α-synucleinopathy. In iRBD patients, cardiac sympathetic dysfunction begins before nigrostriatal degeneration. A recent prospective study showed that reduced cardiac MIBG uptake can predict phenoconversion to DLB in iRBD patients several years later (sensitivity: 100%, specificity:
Although these imaging biomarkers show good predictive value, the relatively low availability of SPECT and PET in hospitals and their associated radiation exposure limit their use. Therefore, MRI biomarkers may serve as an alternative. Structural MRI studies have shown reduced deep and cortical gray matter in iRBD patients, as well as white matter changes commonly seen in neurodegenerative diseases. Functional MRI studies have shown altered functional connectivity in the basal ganglia, corticostriatal, and cortico-cortical networks in iRBD patients[58]. Additionally, quantitative susceptibility mapping and free water imaging have similar diagnostic capabilities for iRBD and PD, and their combination improves the ability to detect early PD[59]. Specifically, free-water values in the substantia nigra (SN) were significantly higher in patients with iRBD compared to healthy controls, albeit lower than in PD patients. Furthermore, the longitudinal free-water imaging analyses showed a significant Group × Time interaction in SN[60]. A recent study also demonstrated that free-water values in the dorsoposterior putamen progressively increased and correlated with the clinical disease progression[61]. These findings indicated that free-water imaging may have potential utility in monitoring disease progression during the phenoconverison of iRBD.
Neuromelanin (NM) is a metabolite of catecholaminergic neurotransmitters, which is predominantly localized within norepinephrine-producing neurons of the locus coeruleus (LC) and dopamine-synthesizing neurons in the SN. NM-sensitive MRI utilizes the magnetization transfer effect to visualize these nuclei. Previous evidence supports its potential as a high-value biomarker for prodromal α-synucleinopathy. NM-sensitive MRI achieves high diagnostic accuracy in detecting iRBD, whether targeting the SN (AUC=0.76) or LC (sensitivity 82.5%, specificity 81%)[62,63]. In limited prospective studies, French researchers have documented progressive decay of NM-MRI signal intensity correlating with α-synucleinopathy progression. This finding facilitated the development of a predictive model, indicating 23.1% SN volumetric loss at the time of PD diagnosis[64]. While these advances enhance therapeutic monitoring in disease-modifying trials, large-scale cohort studies remain imperative to validate NM-MRI's reliability in forecasting iRBD phenoconversion trajectories.
In addition, hyperechogenicity of the SN, as visualized by transcranial sonography (TCS), occurs in most PD patients. Therefore, in recent years, several studies have investigated the echogenicity of the SN in iRBD patients and its association with the risk of future neurodegenerative conversion. A cross-sectional study conducted in 2010 demonstrated that the iRBD group (0.20 ± 0.13 cm2) exhibited a significantly larger area of SN echogenicity compared to the control group (0.06 ± 0.06 cm2)[65]. However, another five-year prospective study found no differences in the mean size of the SN or the percentage of patients with SN hyperechogenicity between the first and second examination[66]. This finding suggests that TCS may not be suitable for monitoring the degenerative progression in iRBD.
BIOFLUID AND BIOPSY MARKERS
Compared to radionuclide imaging studies, biomarkers obtained directly from peripheral blood vessels may be a more feasible and cost-effective alternative. Studies on neuron-derived exosomes, serum neurofilament light chain (NfL), circulating miRNAs, and blood proteomics have yielded some noteworthy results[4]. A longitudinal study found that the α-synuclein content in neuron-derived exosomes was twice as high in iRBD and PD patients compared to controls and other neurodegenerative diseases, with PD patients having significantly higher levels than iRBD patients[67]. This suggests that α-synuclein in neuron-derived exosomes increases in the prodromal phase of PD and persists with disease progression. Recent studies have found that miRNAs in plasma exosomes can accurately differentiate iRBD patients (AUC = 0.97) from PD patients (AUC = 0.92), with miR-7-5p, miR-4665-5p, miR-5001-3p, and miR-550b-3p being highly associated with phenoconversion of iRBD[68].
NfL, a neuronal cytoskeletal protein released during neuronal damage, is elevated in the plasma of iRBD patients and is significantly associated with a higher risk of phenoconversion[69]. Additionally, dysregulation of miRNAs such as miR-19b may help predict the phenoconversion of iRBD and could serve as a potential prognostic marker[4]. In the latest research, scientists have identified altered expression of miRNAs (hsa-miR-142-3p, hsa-miR-139-5p, and hsa-miR-191-5p) in the platelets of iRBD patients, which may indicate cytoskeleton dysfunction[70]. In earlier studies, similar changes in platelet miRNAs were observed in patients with PD and DLB[71]. This suggests that platelet miRNAs might serve as early biomarkers of α-synucleinopathies in iRBD.
Novel proteomic studies on blood samples have identified several proteins with significantly altered expression levels, further elucidating the protein characteristics and molecular pathways involved in iRBD pathogenesis. In a case-control study with 9 iRBD and 10 control subjects, proteomic analysis revealed that the expression levels of 11 proteins altered significantly in iRBD. In particular, the reduction in serum dopamine β-hydroxylase and vitamin D binding protein GC was consistent with alterations in the norepinephrinergic and dopaminergic systems, respectively. In addition, the altered protein profiles indicated that immune responses, inflammation, complement activation, and coagulation also contribute to the pathophysiology of iRBD[72]. The latest study explored the blood proteomics of PD and iRBD using machine learning methods, and successfully identified all PD patients and accurately classified 79% of iRBD individuals up to 7 years before the onset of motor symptoms by analyzing the expression levels of eight proteins: Granulin precursor, Mannan-binding lectin serine peptidase 2, Endoplasmatic reticulum chaperone BiP, Prostaglandin-H2 D-isomerase, Interceullular adhesion molecule 1, Complement C3, Dickkopf WNT signalling pathway inhibitor 3, and Plasma protease C1 inhibitor[73]. More longitudinal studies are needed to validate whether these biomarkers can be used to predict the risk of phenoconversion in iRBD.
Neuroinflammation is increasingly recognized as a key contributor to α-synucleinopathy pathogenesis, potentially mediating pathophysiological progression from prodromal to clinically manifest stages. Alterations in peripheral immune profiles, particularly dysregulated cytokines and chemokines, have also been reported to be highly associated with α-synucleinopathy. Importantly, a recent prospective cohort study demonstrated that iRBD patients with elevated TNF-α/IL-10 exhibit accelerated neurodegenerative conversion (adjusted HR: 1.07, 95% CI 1.01-1.14). Notably, comorbid MCI elevates phenoconversion risk by 290% (adjusted HR: 4.17, 95% CI 1.47-11.81)[74]. Beyond this, previous studies have demonstrated that co-existing α-synucleinopathy and Alzheimer’s disease (AD) pathologies correlate with accelerated global cognitive decline. Therefore, plasma markers of AD pathology might have a promising ability to predict the risk of future phenoconversion to DLB in iRBD. In a longitudinal study, Delva et al. validated plasma Aβ42/40 ratio (HR: 10.22, 95% CI 2.35-44.5) and pTau181 (HR: 15.46, 95% CI 3.55-67.5) as robust prodromal biomarkers predicting iRBD phenoconversion to DLB[75].
In recent years, the quantification of α-synuclein has become a novel biomarker, providing information on its deposition over time. Tissue studies using antibodies against phosphorylated α-synuclein in colon mucosa, salivary glands, and skin samples have shown general level of sensitivity (ranged from 24% to 89%) and excellent specificity (ranged from 97% to 100%) in iRBD patients[76], with skin biopsies being the most promising predictive technique due to their ease of operation and short processing time[76]. Emerging as a breakthrough methodology, the Seed Amplification Assay (SAA) enables ultrasensitive detection of the seeding activity of misfolded proteins, such as α-synuclein (α-Syn). SAA is a broad term that encompasses various seed amplification-based techniques, including Real-Time Quaking-Induced Conversion (RT-QuIC) and Protein Misfolding Cyclic Amplification (PMCA), while RT-QuIC is optimized for high sensitivity and rapid detection of α-synuclein aggregates in biofluids and tissues. To date, several studies have explored the potential of α-Syn SAA in the diagnosis and prediction of phenoconversion in patients with iRBD[77,78]. The results revealed that, whether in oral mucosal or CSF samples, α-Syn SAA showed significantly positive results in iRBD patients compared to healthy controls. α-Syn SAA demonstrated remarkable diagnostic value for iRBD, with a sensitivity of 63.6% and specificity of 90.3%. Moreover, follow-up results indicated that α-Syn seeding activity could also serve as a novel prodromal biomarker for α-synucleinopathies. Similarly, RT-QuIC has shown exciting results in detecting pathological misfolded α-synuclein in CSF, with a sensitivity and specificity of 90% in iRBD patients, and positive results are associated with an increased risk of phenoconversion[79]. The authors further showed that RT-QuIC had similar high sensitivity, specificity, and consistency in detecting α-synuclein in the skin and CSF, and the positive results were associated with other neurodegenerative biomarkers in patients with iRBD[80]. Therefore, α-Syn SAA and RT-QuIC may represent the future direction of novel biomarker detection systems for predicting iRBD conversion risk.
GENETIC BIOMARKERS
Previous studies using candidate gene approaches have reported that the PARKIN gene, which is associated with familial PD, may also be related to iRBD[81]. However, the LRRK2 and MAPT genes, which are associated with familial PD, have not been found to be related to iRBD[82,83]. A GWAS study in iRBD identified five risk loci closely associated with iRBD: SNCA, GBA, TMEM175, INPP5F, and SCARB2[84]. These findings were in line with the prior studies suggesting the GBA and TMEM175 genes correlated with iRBD[4]. In addition, this GWAS study of iRBD suggested that the 5’-SNCA variants were significantly associated with iRBD, in contrast to the 3’-SNCA variants associated with PD. Another study with fine mapping of the SNCA gene confirmed this finding, and suggested that specific 5’-SNCA variants were associated with the rate of phenoconverison in iRBD[85]. Moreover, these studies suggested that the genetic background of iRBD only partially overlaps with that of PD and DLB, suggesting that iRBD has its own unique genetic basis, which may partially explain why iRBD can convert into different subtypes of neurodegenerative diseases.
Among these, GBA is the gene encoding glucocerebrosidase, and its mutation frequency is higher in PD patients with iRBD than those without[86]. The frequency of GBA mutations is also higher in iRBD patients compared to the general population and is associated with a higher risk of conversion to PD and DLB[87]. In addition to GBA, alterations in SNCA regulation have been closely linked to disease progression. In a recent preliminary study of 78 iRBD patients, 16 patients who progressed to PD showed hypomethylation of the SNCA gene CpG island. This suggests that hypomethylation of the SNCA gene CpG region may be highly associated with iRBD symptom progression and phenoconversion risk[88]. These genetic biomarkers may contribute to heterogeneous phenoconversion of iRBD and risk stratification in neuroprotective trials.
NOVEL MARKERS
In recent years, personality traits, symptomatic treatment responses, neurophysiological features, circadian rhythm disruptions, shift in gut microbiota, and AI-derived prediction models have gradually been recognized as promising predictive markers in iRBD. These markers provide new insights into the pathogenesis of iRBD and offer hope for early diagnosis and the development of treatment strategies.
By using the NEO five-factor inventory, a study suggested that personality traits such as impulsivity, neuroticism, and introversion were associated with an increased risk of iRBD and its phenoconversion[89]. Furthermore, the authors reported that decision-making impulsivity, as reflected by several neuropsychological tests, was significantly elevated in iRBD and drug-naïve PD patients[90], suggesting an important role of impulsivity in the pathophysiology of α-synucleinopathy. Future prospective studies should further validate the predictive value of neurodegenerative conversion in iRBD.
On the side of clinical symptoms of iRBD, approximately one-tenth of iRBD patients still have residual sleep-related injury symptoms even after receiving adequate first-line drug treatment. A cohort study showed that this “treatment-resistant” feature could predict iRBD converting to DLB rather than PD[91], making it a potential clinical marker for predicting the subtype of phenoconversion in iRBD.
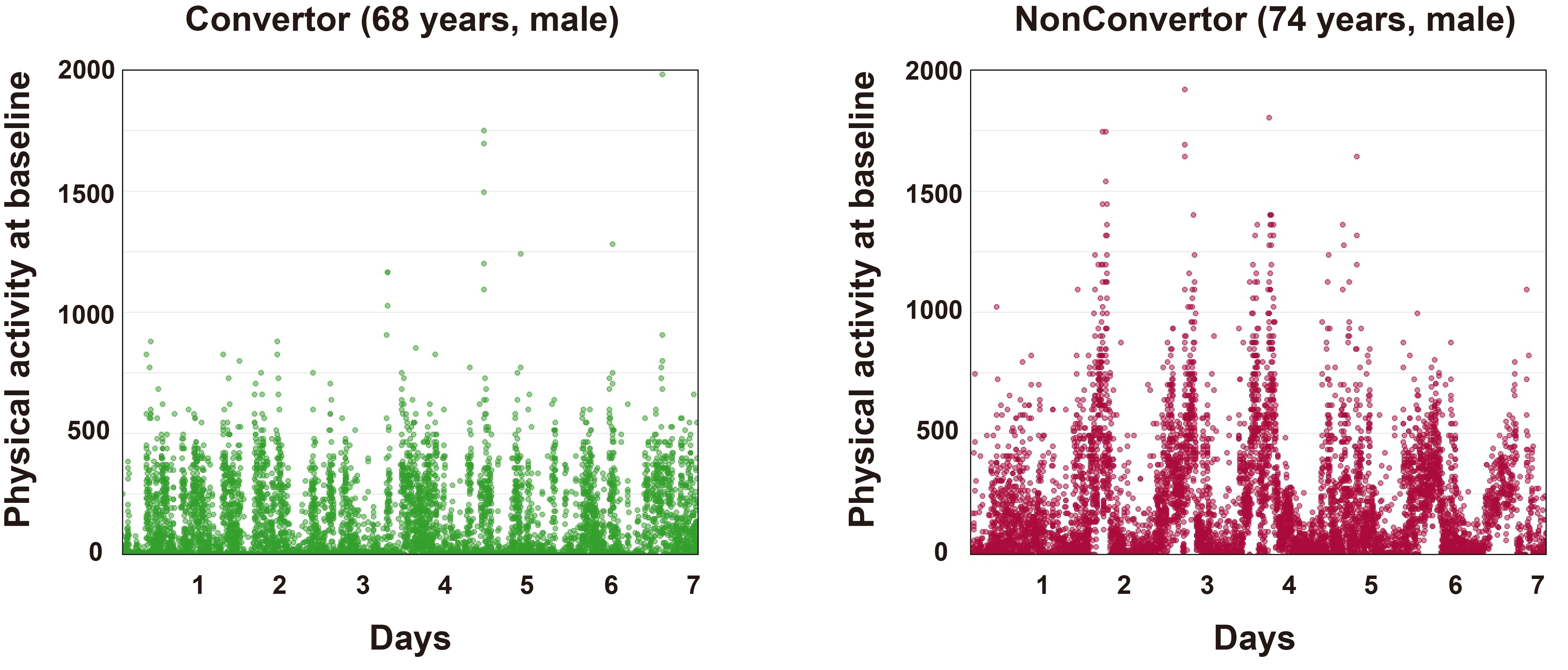
Several studies indicated that changes in physical activity and circadian rhythm disruptions may serve as biomarkers or even risk factors for the development of PD and its prodromal features[92,93]. Studies have shown that disturbances in rest-activity patterns, mainly fragmented daytime activity and low daytime activity levels, may be related to the development of iRBD and could worsen the progression of α-synucleinopathy[94], making rest-activity patterns an important indicator for assessing iRBD risk and progression. Figure 2 demonstrates the 7-day actigraphy-measured physical activity patterns of two participants recruited in our established iRBD cohort, from which we could have a direct impression that the baseline physical activity level is obviously lower in the convertor than in the non-convertor of RBD patients. In addition, melatonin secretion by the pineal gland is an internal marker of circadian rhythm. Preliminary evidence suggested that iRBD patients have a slightly delayed melatonin secretion peak time and significantly reduced secretion levels[95]. Another study demonstrated that iRBD patients did not show circadian rhythmicity for clock genes Per2, Bmal1, and Nr1d1, but the rhythmicity of Per1 remained, and the amplitude of Per3 was diminished[96]. Therefore, future research needs to explore novel treatment strategies aimed at regulating the sleep-wake cycle and clarifying the role of circadian dysregulation in the progression from iRBD to neurodegenerative diseases.
Figure 2. Comparison of baseline physical activity patterns between an iRBD patient who converted to PD (Convertor, 68-year-old male) and a patient who did not convert (Non-converter, 68-year-old male) over a 2-year follow-up period. The converter exhibited lower physical activity at baseline compared to the non-converter. Physical activity was continuously recorded over 7 consecutive days using actigraphy devices. Specifically, the Actiwatch Spectrum Plus (Philips Respironics United Kingdom) was employed, which collects raw activity counts at 100 Hz via 3-axis accelerometers. Data extraction was performed using Philips Actiware version 6, and visualization was generated with the ggplot2 package in R. iRBD: isolated rapid eye movement sleep behavior disorder; PD: Parkinson’s disease.
Recently, iRBD has been proposed as a hallmark of the body-first PD subtype. In this subtype, pathogenic α-synuclein propagates caudal-rostrally from the gut to the brain. Thus, the gut-microbiota-brain axis was increasingly investigated. In patients with iRBD, the overall microbiota profile is highly similar to that seen in early-stage PD[97], with a reduction in butyrate-producing bacteria (e.g., Roseburia and Faecalibacterium) and an enrichment of pro-inflammatory Collinsella and Desulfovibrio[98]. Additionally, the predicted functional profile demonstrated an overall increase in fermentation of fatty acids to lactate and ethanol, along with decreased deazapurine biosynthesis in iRBD and PD. These findings emphasize the potential role of gut microbiota in the pathogenesis of α-synucleinopathy. Therefore, changes in the composition and function of gut microbiota may serve as promising biomarkers for diagnosis and prognosis in iRBD.
Finally, with the development of artificial intelligence (AI) and big data, predictive models based on AI have shown great potential in the study of iRBD, particularly in the early diagnosis of the disease and the prediction of phenotypic conversion. In a longitudinal study conducted by Jeong et al., they developed machine learning models based on the baseline EEG features[99]. The results demonstrated that the model could predict phenoconversion time and its subtype in patients with iRBD (AUC = 0.90)[99]. When deep learning was combined with neuromelanin MRI data, the results showed a significant decrease in neuromelanin in the substantia nigra pars compacta of iRBD and PD patients compared to healthy controls, with the changes in iRBD being intermediate between those in PD and healthy controls[100]. A very recent study reported that AI can accurately identify iRBD through video-polysomnogram (v-PSG) features, achieving an accuracy of 92%, sensitivity of 84%, and specificity of 99%[101]. Whether these AI-derived models hold potential for predicting phenoconversion in iRBD warrants further investigation.
CONCLUSIONS AND FUTURE DIRECTIONS
As a prodromal marker of α-synucleinopathies, iRBD provides a critical window for uncovering the early pathogenic mechanisms of neurodegenerative diseases and developing intervention strategies. Current research has confirmed that iRBD patients exhibit multidimensional abnormalities in motor function, olfaction, vision, autonomic function, cognition, biopsy, fluid, and neuroimaging biomarkers. Meanwhile, biomarkers such as α-synuclein in exosomes, miRNA, and NfL in blood offer new possibilities for dynamic monitoring of disease progression. Genetic studies further reveal the association of variants in genes like GBA and SNCA with the risk of iRBD progression, laying the groundwork for individualized risk stratification. The feasibility and diagnostic properties of these markers were further summarized in Table 1. However, individual markers often have limitations in terms of sensitivity or specificity, and their application may be restricted depending on the clinical context. Therefore, integrating multiple markers shows potential for enhancing the performance of diagnostic and predictive models by capitalizing on complementary information derived from diverse domains. For instance, the combination of dopaminergic system imaging, constipation, and age over 70 years significantly enhances the predictive efficacy of iRBD conversion to PD or DLB[102].
List of the biomarkers of progression of isolated RBD toward α-synucleinopathy
| Category | Availability | Cost | Phenoconversion endpoint | Sensitivity | Specificity | Remarks | |
| Clinical | |||||||
| Motor dysfunction | High | Low | PD | 88-100% | 83%-90%[7,26] | Assessed by UPDRS-III and quantitative motor tests; threefold increased risk of developing PD | |
| Hyposmia | High | Low | Dementia and parkinsonism | 86%-91% | 76%-88%[103] | Strongly predicts α-synucleinopathy (HR: 2.62); a prognostic marker; a prediction or monitoring marker | |
| Visual impairments | High (Low) | Low (Moderate) | α-synucleinopathies | NA | NA | Color discrimination deficit and thinning of the retinal ganglion cell complex (via OCT) are strongly associated with the higher risk of phenoconversion | |
| Autonomic dysfunction | High | Low | Dementia and parkinsonism | NA | NA | Orthostatic hypotension, sexual dysfunction (HR: 2.13), urinary disorders, constipation (HR: 1.67) and HRV | |
| Excessive daytime sleepiness | High | Low | NA | NA | NA | Conflicting results on its predictive value | |
| Neuropsychological | |||||||
| cognitive decline | High | Low | DLB | 82%-100% | 74%-97%[26] | MCI has a higher risk of phenoconversion; attention and executive function, verbal fluency, and verbal memory were identified as the top predictors of DLB (AUC = 0.90-0.97); visuospatial dysfunction is the strongest predictor of iRBD progression to dementia (HR: 5.48). | |
| Visual hallucinations and pareidolia | High | Low | DLB | NA | NA | Pareidolia in iRBD serves as a valuable predictive marker for the future development of DLB | |
| Psychiatric comorbidities | High | Low | α-synucleinopathies | NA | NA | Comorbidity of depression and iRBD has a higher future risk of developing α-synucleinopathies; more research is needed to investigate the association | |
| Electrophysiological markers | |||||||
| EEG | High | Low | α-synucleinopathies | NA | NA | Specific EEG abnormalities (EEG slowing) were identified during wakefulness in iRBD patients who later developed a synucleinopathy. This suggests that EEG features may be a promising marker of neurodegeneration in iRBD patients | |
| EMG | High | Low | α-synucleinopathies | 73.7% | 75.6%[44] | REM sleep without atonia, especially tonic EMG activity (AUC = 0.80; 73.7% sensitivity; 75.6% specificity), may serve as a stable biomarker for predicting the progression of neurodegeneration in iRBD | |
| Neuroimaging | |||||||
| 123I-FP-CIT SPECT | Moderate | Moderate | α-synucleinopathies | 75% | 51%[104] | Reduced dopamine uptake in the striatum and putamen predicts clinical phenoconversion in iRBD | |
| 18F-AV-133 PET | Moderate | Moderate | PD | NA | NA | The potential predictive ability of AV-133 PET in iRBD phenoconversion requires further investigation | |
| 18 F-FDG-PET | Moderate | Moderate | PD | 52.4% | 100%[105,106] | The expression of PDRP in iRBD is associated with prodromal features of PD, suggesting its potential as a biomarker for short-term phenoconversion | |
| 99mTc-HMPAO SPECT | Moderate | Moderate | DLB | NA | NA | Novel brain perfusion patterns in iRBD may serve as a potential biomarker for predicting the progression of iRBD to DLB | |
| 11C-(R)-PK11195-PET | Moderate | Moderate | α-synucleinopathies | NA | NA | Microglial activation has a detrimental effect in the prodromal phase of α-synucleinopathy | |
| 131I-MIBG myocardial scintigraphy | Moderate | Moderate | DLB | 100% | 92.9%[57] | Predicts phenoconversion to DLB in iRBD patients several years later (sensitivity: 100%, specificity: 92.9%) | |
| Structural and functional MRI | Moderate | Moderate | α-synucleinopathies | 52.4% | 100%[107] | Reduced white and gray matter in iRBD; altered functional connectivity in the basal ganglia, corticostriatal, and cortico-cortical networks in RBD patients | |
| Quantitative susceptibility mapping and free water imaging (e.g., substantia nigra, putamen) | Moderate | Moderate | PD | 60-84% | 63-90%[59] | Similar diagnostic capabilities for iRBD and PD, and their combination improves the ability to detect early PD. Specifically, free water increases progressively in the substantia nigra and putamen of iRBD and PD patients, suggesting a potential monitoring effect | |
| NM-sensitive MRI | Moderate | Moderate | α-synucleinopathies | NA | NA | NM-MRI signal intensity correlates with synucleinopathy progression, but the reliability in forecasting phenoconversion needs to be validated | |
| Transcranial sonography | Moderate | Moderate | α-synucleinopathies | NA | NA | Increased echogenicity in iRBD compared to controls, but longitudinal studies show no dynamic monitoring value | |
| Biofluid and biopsy | |||||||
| Neuronal-derived exosomes | Low | High | PD | 95% | 93%[67] | α-synuclein in neuron-derived exosomes increases in the prodromal phase of PD and persists with disease progression | |
| Circulating miRNAs | Moderate | Moderate | PD | NA | NA | miRNAs in plasma exosomes can accurately differentiate iRBD patients (AUC = 0.97) from PD patients (AUC = 0.92), with miR-7-5p, miR-4665-5p, miR-5001-3p, and miR-550b-3p being highly associated with phenoconversion of iRBD. | |
| Neurofilament light chain | Moderate | Moderate | α-synucleinopathies | 75.0% | 83.3%[69] | Elevated in the plasma of iRBD patients and is significantly associated with a higher risk of phenoconversion | |
| Blood proteomics | Moderate | Moderate | PD | NA | NA | Classifies iRBD patients later converting to PD (79% accuracy ≤ 7 years pre-motor symptoms) | |
| Inflammatory factors (e.g., TNF-α/IL-10) | Moderate | Moderate | α-synucleinopathies | NA | NA | Elevated TNF-α/IL-10 in iRBD patients exhibits accelerated neurodegenerative conversion (adjusted HR: 1.07) | |
| Plasma markers of AD pathology (e.g., Aβ42/40 and pTau181) | Moderate | Moderate | DLB | 89% | 63%-73%[75] | Plasma Aβ42/40 ratio (HR: 10.22) and pTau181 (HR: 15.46) as robust prodromal biomarkers predicting iRBD phenoconversion to DLB | |
| Biopsy (e.g., colon mucosa, salivary glands, and skin biopsy) | Low | Moderate | α-synucleinopathies | 24%-89% | 97%-100%[76] | General level of sensitivity (ranged from 24% to 89%) and excellent specificity (ranged from 97% to 100%), with skin biopsies being the most promising predictive technique | |
| RT-QuIC and α-Syn SAA | Low (Moderate) | Moderate | α-synucleinopathies | RT-QuIC: 100% α-Syn SAA: 63.6% | RT-QuIC: 98%[79] α-Syn SAA: 90.3%[78] | Detects misfolded α-synuclein in CSF and skin; α-Syn SAA shows a sensitivity of 63.6% and specificity of 90.3%; RT-QuIC shows a sensitivity and specificity of over 90% in iRBD patients. Both represent the future direction of novel biomarker detection systems for predicting iRBD conversion risk | |
| Genetic | |||||||
| PARKIN | Moderate | Moderate | PD | NA | NA | PARKIN gene, which is associated with familial PD, may also be related to iRBD | |
| GBA and TMEM175 | Moderate | Moderate | PD and DLB | NA | NA | GBA and TMEM175 genes associated with iRBD; GBA mutations are associated with a higher risk of conversion to PD and DLB | |
| SNCA | Moderate | Moderate | α-synucleinopathies | NA | NA | 5’-SNCA variants were associated with the rate of phenoconverison in iRBD; hypomethylation of the SNCA gene CpG region may be highly associated with RBD symptom progression and phenoconversion risk | |
| Novel markers | |||||||
| Personality traits | High | Low | α-synucleinopathies | NA | NA | Impulsivity, neuroticism, and introversion are associated with an increased risk of iRBD and its phenoconversion, but still need to be validated through prospective studies | |
| Residual injury symptoms | High | Low | DLB | NA | NA | This feature probably predicts iRBD converting to DLB rather than PD | |
| Circadian rhythm(e.g., rest-activity patterns and melatonin) | Moderate (Low) | Moderate | α-synucleinopathies | NA | NA | Fragmented daytime activity and low daytime activity levels may be related to the development of RBD and the progression of α-synucleinopathy Slightly delayed melatonin secretion peak time and significantly reduced secretion levels in iRBD patients, melatonin shows its potential as a biomarker for early prediction of neurodegenerative progression in iRBD patients | |
| Gut microbiota | Moderate | Moderate | PD | NA | NA | In iRBD patients, the microbiota profile closely resembles that of early PD, with depletion of butyrate-producing bacteria and enrichment of pro-inflammatory bacteria | |
| AI-derived models | Low | High | α-synucleinopathies | NA | NA | AI-derived models showed their potential for early diagnosis of iRBD and the prediction of phenotypic conversion. For example, EEG-based AI models can be used to predict phenoconversion time and its subtype in patients with iRBD (AUC = 0.90) | |
Nevertheless, current biomarker research still faces numerous challenges: some indicators lack sufficient specificity and require multimodal data integration to enhance diagnostic value; the standardization processes and dynamic monitoring capabilities of novel detection technologies (e.g., RT-QuIC) need further refinement; and the mechanisms of emerging markers such as gut microbiota and rest-activity patterns, as well as their causal relationships with neurodegeneration, require deeper exploration. To address these, future research should adopt multicenter, large-sample longitudinal designs to integrate clinical phenotypes, imaging, biosamples, and genetic data, establishing dynamic predictive models to promote the application of precision medicine in early diagnosis and neuroprotective therapies. It is worth noting that artificial intelligence (AI) holds great promise for discovering biomarkers and predicting disease progression in iRBD. By analyzing complex, multidimensional data, AI can identify subtle patterns and novel predictors, such as EEG and v-PSG features. Additionally, AI models enable precise predictions of phenoconversion risks, supporting early intervention and personalized treatment strategies in iRBD. This transformative approach enhances our ability to combat synucleinopathies through precision medicine. Furthermore, attention should be paid to heterogeneous characteristics within the iRBD population for their potential to indicate the subtype of phenoconversion, providing a basis for developing targeted interventions.
DECLARATIONS
Authors’ contributions
Literature search and screen, data interpretation: Zhang W, Liu A, Peng Y, Yang C
Drafting the manuscript: Zhang W
Screening of the studies, data extraction and visualization: Liu A, Peng Y, Yang C, Feng H
Reviewing and revising this manuscript: Feng H, Liu Y, Zhang J, Ning Y, Wang J
Conception, design of the study and the funding acquisition: Ning Y, Wang J
Being responsible for the overall content as guarantor: Wang J
All authors have reviewed and approved the manuscript for publication.
Availability of data and materials
Not applicable.
Financial support and sponsorship
This study was supported by the National Natural Science Foundation of China (82401742), Guangdong Basic and Applied Basic Research Foundation (2024A1515011349), and Guangzhou Science and Technology Program (2023A03J0829). The study funding bodies had no role in the conception, design, conduct, interpretation, or analysis of the study or in the approval of the publication.
Conflicts of interest
The authors declare that there are no conflicts of interests.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
Supplementary Materials
REFERENCES
1. Lee WJ, Baek SH, Im HJ, et al. REM sleep behavior disorder and its possible prodromes in general population: prevalence, polysomnography findings, and associated factors. Neurology. 2023;101:e2364-75.
2. Stefani A, Antelmi E, Arnaldi D, et al. From mechanisms to future therapy: a synopsis of isolated REM sleep behavior disorder as early synuclein-related disease. Mol Neurodegener. 2025;20:19.
3. Galbiati A, Verga L, Giora E, Zucconi M, Ferini-Strambi L. The risk of neurodegeneration in REM sleep behavior disorder: A systematic review and meta-analysis of longitudinal studies. Sleep Med Rev. 2019;43:37-46.
4. Miglis MG, Adler CH, Antelmi E, et al. Biomarkers of conversion to α-synucleinopathy in isolated rapid-eye-movement sleep behaviour disorder. Lancet Neurol. 2021;20:671-84.
5. Postuma RB, Iranzo A, Hu M, et al. Risk and predictors of dementia and parkinsonism in idiopathic REM sleep behaviour disorder: a multicentre study. Brain. 2019;142:744-59.
6. Ehgoetz Martens KA, Matar E, Hall JM, et al. Subtle gait and balance impairments occur in idiopathic rapid eye movement sleep behavior disorder. Mov Disord. 2019;34:1374-80.
7. Arora S, Baig F, Lo C, et al. Smartphone motor testing to distinguish idiopathic REM sleep behavior disorder, controls, and PD. Neurology. 2018;91:e1528-38.
8. Shin C, Lee JY, Kim YK, et al. Cognitive decline in association with hyposmia in idiopathic rapid eye movement sleep behavior disorder: a prospective 2-year follow-up study. Eur J Neurol. 2019;26:1417-20.
9. Doppler K, Jentschke HM, Schulmeyer L, et al. Dermal phospho-alpha-synuclein deposits confirm REM sleep behaviour disorder as prodromal Parkinson’s disease. Acta Neuropathol. 2017;133:535-45.
10. Iranzo A, Serradell M, Vilaseca I, et al. Longitudinal assessment of olfactory function in idiopathic REM sleep behavior disorder. Parkinsonism Relat Disord. 2013;19:600-4.
11. Jennings D, Siderowf A, Stern M, et al; PARS Investigators. Conversion to Parkinson disease in the PARS hyposmic and dopamine transporter-deficit prodromal cohort. JAMA Neurol. 2017;74:933-40.
12. Postuma RB, Lang AE, Massicotte-Marquez J, Montplaisir J. Potential early markers of Parkinson disease in idiopathic REM sleep behavior disorder. Neurology. 2006;66:845-51.
13. Postuma RB, Gagnon JF, Vendette M, Desjardins C, Montplaisir JY. Olfaction and color vision identify impending neurodegeneration in rapid eye movement sleep behavior disorder. Ann Neurol. 2011;69:811-8.
14. Ahn J, Lee JY, Kim TW, et al. Retinal thinning associates with nigral dopaminergic loss in de novo Parkinson disease. Neurology. 2018;91:e1003-12.
15. Lee JY, Ahn J, Oh S, et al. Retina thickness as a marker of neurodegeneration in prodromal lewy body disease. Mov Disord. 2020;35:349-54.
16. La Morgia C, Romagnoli M, Pizza F, et al. Chromatic pupillometry in isolated rapid eye movement sleep behavior disorder. Mov Disord. 2022;37:205-10.
17. Lee H, Cho YW, Kim HA. The severity and pattern of autonomic dysfunction in idiopathic rapid eye movement sleep behavior disorder. Mov Disord. 2015;30:1843-8.
18. Zitser J, During EH, Chiaro G, Miglis MG. Autonomic impairment as a potential biomarker in idiopathic REM-sleep-behavior disorder. Auton Neurosci. 2019;220:102553.
19. Pilotto A, Romagnolo A, Tuazon JA, et al. Orthostatic hypotension and REM sleep behaviour disorder: impact on clinical outcomes in α-synucleinopathies. J Neurol Neurosurg Psychiatry. 2019;90:1257-63.
20. Dahms C, Guenther A, Schwab M, et al. Dysautonomia in prodromal α-synucleinopathy: peripheral versus central autonomic degeneration. Eur J Neurol. 2016;23:878-90.
21. Taha HB, Zitser J, Miglis MG. Frequency and longitudinal course of autonomic reflex testing abnormalities in isolated rem sleep behavior disorder. Sleep. 2025:zsaf087.
22. Zhou J, Zhang J, Lam SP, et al. Excessive daytime sleepiness predicts neurodegeneration in idiopathic REM sleep behavior disorder. Sleep. 2017;40:zsx041.
23. Postuma RB, Gagnon JF, Pelletier A, Montplaisir JY. Insomnia and somnolence in idiopathic RBD: a prospective cohort study. NPJ Parkinsons Dis. 2017;3:9.
24. Lerche S, Machetanz G, Roeben B, et al. Deterioration of executive dysfunction in elderly with REM sleep behavior disorder (RBD). Neurobiol Aging. 2018;70:242-6.
25. Nagy AV, Leschziner G, Eriksson SH, Lees A, Noyce AJ, Schrag A. Cognitive impairment in REM-sleep behaviour disorder and individuals at risk of Parkinson’s disease. Parkinsonism Relat Disord. 2023;109:105312.
26. Génier Marchand D, Postuma RB, Escudier F, et al. How does dementia with Lewy bodies start? Ann Neurol. 2018;83:1016-26.
27. Wang J, Huang B, Zhou L, et al. Visuospatial dysfunction predicts dementia-first phenoconversion in isolated REM sleep behaviour disorder. J Neurol Neurosurg Psychiatry. 2024;96:76-84.
28. McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB consortium. Neurology. 2017;89:88-100.
29. Arnulf I. Dream imagery, rapid eye movement sleep behavior disorder, and hallucinations: dreaming, visual hallucinations and RBD. Sleep Biol Rhythms. 2013;11:15-20.
30. Williams DR, Lees AJ. Visual hallucinations in the diagnosis of idiopathic Parkinson’s disease: a retrospective autopsy study. Lancet Neurol. 2005;4:605-10.
31. Uchiyama M, Nishio Y, Yokoi K, et al. Pareidolias: complex visual illusions in dementia with Lewy bodies. Brain. 2012;135:2458-69.
32. Ozawa M, Shiraishi T, Murakami H, et al. Structural MRI study of pareidolia and visual hallucinations in drug-naïve Parkinson’s disease. Sci Rep. 2024;14:31293.
33. Sasai-Sakuma T, Nishio Y, Yokoi K, Mori E, Inoue Y. Pareidolias in REM sleep behavior disorder: a possible predictive marker of Lewy body diseases? Sleep. 2017:40.
34. Honeycutt L, Gagnon JF, Pelletier A, De Roy J, Montplaisir JY, Postuma RB. Pareidolias and cognition in isolated REM sleep behavior disorder. Parkinsonism Relat Disord. 2020;75:76-9.
35. Wang J, Chau SWH, Lam SP, et al. Prevalence and correlates of REM sleep behaviour disorder in patients with major depressive disorder: a two-phase study. J Neurol Neurosurg Psychiatry. 2022;93:1010-7.
36. Heinzel S, Berg D, Gasser T, Chen H, Yao C, Postuma RB; MDS task force on the definition of Parkinson’s disease. Update of the MDS research criteria for prodromal Parkinson’s disease. Mov Disord. 2019;34:1464-70.
37. Wang J, Lam SP, Huang B, et al. Familial α-synucleinopathy spectrum features in patients with psychiatric REM sleep behaviour disorder. J Neurol Neurosurg Psychiatry. 2023;94:893-903.
38. Fantini ML, Gagnon JF, Petit D, et al. Slowing of electroencephalogram in rapid eye movement sleep behavior disorder. Ann Neurol. 2003;53:774-80.
39. Rodrigues Brazète J, Montplaisir J, Petit D, et al. Electroencephalogram slowing in rapid eye movement sleep behavior disorder is associated with mild cognitive impairment. Sleep Med. 2013;14:1059-63.
40. Roascio M, Canessa A, Trò R, et al. Phase and amplitude electroencephalography correlations change with disease progression in people with idiopathic rapid eye-movement sleep behavior disorder. Sleep. 2022:45.
41. Brazète J, Gagnon JF, Postuma RB, Bertrand JA, Petit D, Montplaisir J. Electroencephalogram slowing predicts neurodegeneration in rapid eye movement sleep behavior disorder. Neurobiol Aging. 2016;37:74-81.
42. Liu Y, Zhang J, Chau SWH, et al. Evolution of prodromal REM sleep behavior disorder to neurodegeneration: a retrospective longitudinal case-control study. Neurology. 2022;99:e627-37.
43. Liu Y, Zhang J, Lam SP, et al. Electromyography activity level in rapid eye movement sleep predicts neurodegenerative diseases in idiopathic rapid eye movement sleep behavior disorder: a 5-year longitudinal study. Sleep Med. 2019;56:128-34.
44. Singh A, Williams S, Calabrese A, Riha R. Tonic REM sleep muscle activity is the strongest predictor of phenoconversion risk to neurodegenerative disease in isolated REM sleep behaviour disorder. J Sleep Res. 2023;32:e13792.
45. Iranzo A, Valldeoriola F, Lomeña F, et al. Serial dopamine transporter imaging of nigrostriatal function in patients with idiopathic rapid-eye-movement sleep behaviour disorder: a prospective study. Lancet Neurol. 2011;10:797-805.
46. Chahine LM, Brumm MC, Caspell-Garcia C, et al. Dopamine transporter imaging predicts clinically-defined α-synucleinopathy in REM sleep behavior disorder. Ann Clin Transl Neurol. 2021;8:201-12.
47. Hsiao IT, Weng YH, Lin WY, et al. Comparison of 99mTc-TRODAT-1 SPECT and 18 F-AV-133 PET imaging in healthy controls and Parkinson’s disease patients. Nucl Med Biol. 2014;41:322-9.
48. Beauchamp LC, Villemagne VL, Finkelstein DI, et al. Reduced striatal vesicular monoamine transporter 2 in REM sleep behavior disorder: imaging prodromal parkinsonism. Sci Rep. 2020;10:17631.
49. Beauchamp LC, Dore V, Villemagne VL, et al. Using (18)F-AV-133 VMAT2 PET imaging to monitor progressive nigrostriatal degeneration in Parkinson disease. Neurology. 2023;101:e2314-24.
50. Dang-Vu TT, Gagnon JF, Vendette M, Soucy JP, Postuma RB, Montplaisir J. Hippocampal perfusion predicts impending neurodegeneration in REM sleep behavior disorder. Neurology. 2012;79:2302-6.
51. Rahayel S, Postuma R, Baril AA, et al. (99m)Tc-HMPAO SPECT perfusion signatures associated with clinical progression in patients with isolated REM sleep behavior disorder. Neurology. 2024;102:e208015.
52. Peralta C, Biafore F, Depetris TS, Bastianello M. Recent advancement and clinical implications of 18FDG-PET in Parkinson’s disease, atypical Parkinsonisms, and other movement disorders. Curr Neurol Neurosci Rep. 2019;19:56.
53. Holtbernd F, Gagnon JF, Postuma RB, et al. Abnormal metabolic network activity in REM sleep behavior disorder. Neurology. 2014;82:620-7.
54. Shin JH, Lee JY, Kim YK, et al. Parkinson disease-related brain metabolic patterns and neurodegeneration in isolated REM sleep behavior disorder. Neurology. 2021;97:e378-88.
55. Tang CC, Nakano Y, Vo A, et al. Longitudinal network changes and phenoconversion risk in isolated REM sleep behavior disorder. Nat Commun. 2024;15:10797.
56. Stær K, Iranzo A, Stokholm MG, et al. Microglial activation and progression of nigrostriatal dysfunction in isolated REM sleep behavior disorder. Mov Disord. 2024;39:1323-8.
57. Park DG, Kim JY, Kim MS, et al. Neurofilament light chain and cardiac MIBG uptake as predictors for phenoconversion in isolated REM sleep behavior disorder. J Neurol. 2023;270:4393-402.
58. Campabadal A, Segura B, Junque C, Iranzo A. Structural and functional magnetic resonance imaging in isolated REM sleep behavior disorder: a systematic review of studies using neuroimaging software. Sleep Med Rev. 2021;59:101495.
59. Zhang D, Yao J, Sun J, et al. Quantitative susceptibility mapping and free water imaging of substantia nigra in Parkinson’s disease. J Parkinsons Dis. 2022;12:2469-78.
60. Zhou L, Li G, Zhang Y, et al. Increased free water in the substantia nigra in idiopathic REM sleep behaviour disorder. Brain. 2021;144:1488-97.
61. Zhang D, Zhou L, Yao J, et al. Increased free water in the putamen in idiopathic REM sleep behavior disorder. Mov Disord. 2023;38:1645-54.
62. Takahashi H, Kashiwagi N, Arisawa A, et al. Imaging of the nigrostriatal system for evaluating the preclinical phase of Parkinson’s disease development: the utility of neuromelanin, diffusion MRI, and DAT-SPECT. Br J Radiol. 2022;95:20210837.
63. Ehrminger M, Latimier A, Pyatigorskaya N, et al. The coeruleus/subcoeruleus complex in idiopathic rapid eye movement sleep behaviour disorder. Brain. 2016;139:1180-8.
64. Biondetti E, Gaurav R, Yahia-Cherif L, et al. Spatiotemporal changes in substantia nigra neuromelanin content in Parkinson’s disease. Brain. 2020;143:2757-70.
65. Iwanami M, Miyamoto T, Miyamoto M, Hirata K, Takada E. Relevance of substantia nigra hyperechogenicity and reduced odor identification in idiopathic REM sleep behavior disorder. Sleep Med. 2010;11:361-5.
66. Iranzo A, Stockner H, Serradell M, et al. Five-year follow-up of substantia nigra echogenicity in idiopathic REM sleep behavior disorder. Mov Disord. 2014;29:1774-80.
67. Jiang C, Hopfner F, Katsikoudi A, et al. Serum neuronal exosomes predict and differentiate Parkinson’s disease from atypical parkinsonism. J Neurol Neurosurg Psychiatry. 2020;91:720-9.
68. Li Y, Cao Y, Liu W, et al. Candidate biomarkers of EV-microRNA in detecting REM sleep behavior disorder and Parkinson’s disease. NPJ Parkinsons Dis. 2024;10:18.
69. Zhang X, Ma L, Liang D, et al. Neurofilament light protein predicts disease progression in idiopathic REM sleep behavior disorder. J Parkinsons Dis. 2023;13:485-99.
70. Arnaldo L, Mena J, Serradell M, et al. Platelet miRNAs as early biomarkers for progression of idiopathic REM sleep behavior disorder to a synucleinopathy. Sci Rep. 2025;15:12136.
71. Gámez-Valero A, Campdelacreu J, Vilas D, et al. Platelet miRNA biosignature discriminates between dementia with Lewy bodies and Alzheimer’s disease. Biomedicines. 2021;9:1272.
72. Mondello S, Kobeissy F, Mechref Y, et al. Novel biomarker signatures for idiopathic REM sleep behavior disorder: a proteomic and system biology approach. Neurology. 2018;91:e1710-5.
73. Hällqvist J, Bartl M, Dakna M, et al. Plasma proteomics identify biomarkers predicting Parkinson’s disease up to 7 years before symptom onset. Nat Commun. 2024;15:4759.
74. Zhang H, Wang T, Li Y, et al. Plasma immune markers in an idiopathic REM sleep behavior disorder cohort. Parkinsonism Relat Disord. 2020;78:145-50.
75. Delva A, Pelletier A, Somerville E, et al. Plasma pTau181 and amyloid markers predict conversion to dementia in idiopathic REM sleep behaviour disorder. Brain. 2025;148:2049-59.
76. Zitser J, Gibbons C, Miglis MG. The role of tissue biopsy as a biomarker in REM sleep behavior disorder. Sleep Med Rev. 2020;51:101283.
77. Zheng Y, Yu Z, Cai H, et al. Detection of α-synuclein in oral mucosa by seed amplification assay in synucleinopathies and isolated REM sleep behavior disorder. Mov Disord. 2024;39:1300-9.
78. Concha-Marambio L, Weber S, Farris CM, et al. Accurate detection of α-synuclein seeds in cerebrospinal fluid from isolated rapid eye movement sleep behavior disorder and patients with Parkinson’s disease in the DeNovo Parkinson (DeNoPa) cohort. Mov Disord. 2023;38:567-78.
79. Iranzo A, Fairfoul G, Ayudhaya ACN, et al. Detection of α-synuclein in CSF by RT-QuIC in patients with isolated rapid-eye-movement sleep behaviour disorder: a longitudinal observational study. Lancet Neurol. 2021;20:203-12.
80. Iranzo A, Mammana A, Muñoz-Lopetegi A, et al. Misfolded α-synuclein assessment in the skin and CSF by RT-QuIC in isolated rem sleep behavior disorder. Neurology. 2023;100:e1944-54.
81. Kumru H, Santamaria J, Tolosa E, et al. Rapid eye movement sleep behavior disorder in parkinsonism with parkin mutations. Ann Neurol. 2004;56:599-603.
82. Barber TR, Lawton M, Rolinski M, et al. Prodromal Parkinsonism and neurodegenerative risk stratification in REM sleep behavior disorder. Sleep. 2017:40.
83. Li J, Ruskey JA, Arnulf I, et al. Full sequencing and haplotype analysis of MAPT in Parkinson’s disease and rapid eye movement sleep behavior disorder. Mov Disord. 2018;33:1016-20.
84. Krohn L, Heilbron K, Blauwendraat C, et al; 23andMe Research Team. Genome-wide association study of REM sleep behavior disorder identifies polygenic risk and brain expression effects. Nat Commun. 2022;13:7496.
85. Krohn L, Wu RYJ, Heilbron K, et al; 23andMe Research Team. Fine-mapping of snca in rapid eye movement sleep behavior disorder and overt synucleinopathies. Ann Neurol. 2020;87:584-98.
86. Thaler A, Bregman N, Gurevich T, et al. Parkinson’s disease phenotype is influenced by the severity of the mutations in the GBA gene. Parkinsonism Relat Disord. 2018;55:45-9.
87. Honeycutt L, Montplaisir JY, Gagnon JF, et al. Glucocerebrosidase mutations and phenoconversion of REM sleep behavior disorder to parkinsonism and dementia. Parkinsonism Relat Disord. 2019;65:230-3.
88. Li Y, Hao S, Zhang H, et al. Hypomethylation of SNCA in idiopathic REM sleep behavior disorder associated with phenoconversion. Mov Disord. 2021;36:955-62.
89. Zhou L, Chau SWH, Liu Y, et al. Personality profile and its association with conversion to neurodegenerative disorders in idiopathic REM sleep behavior disorder. NPJ Parkinsons Dis. 2022;8:91.
90. Zhou L, Li SX, Chau SW, et al. Altered impulsivity across drug-naïve parkinsonism, isolated rapid eye movement sleep behavior disorder, and their high-risk relatives. Ann Neurol. 2024;95:544-57.
91. Wang J, Liu Y, Chau SWH, et al. Residual injurious symptoms and its association with neurodegenerative outcomes in idiopathic rapid eye movement sleep behavior disorder: a retrospective, longitudinal follow-up study. Mov Disord. 2020;35:2077-85.
92. Leng Y, Musiek ES, Hu K, Cappuccio FP, Yaffe K. Association between circadian rhythms and neurodegenerative diseases. Lancet Neurol. 2019;18:307-18.
93. Hughes KC, Gao X, Molsberry S, Valeri L, Schwarzschild MA, Ascherio A. Physical activity and prodromal features of Parkinson disease. Neurology. 2019;93:e2157-69.
94. Feng H, Chen L, Liu Y, et al. Rest-activity pattern alterations in idiopathic REM sleep behavior disorder. Ann Neurol. 2020;88:817-29.
95. Carpi M, Fernandes M, Risino I, et al. Alteration of circadian sleep-wake rhythm and salivary melatonin secretion in idiopathic/isolated REM sleep behavior disorder: preliminary evidence. Sleep Med. 2024;119:135-8.
96. Weissová K, Škrabalová J, Skálová K, et al. Circadian rhythms of melatonin and peripheral clock gene expression in idiopathic REM sleep behavior disorder. Sleep Med. 2018;52:1-6.
97. Cryan JF, O'Riordan KJ, Sandhu K, Peterson V, Dinan TG. The gut microbiome in neurological disorders. Lancet Neurol. 2020;19:179-94.
98. Huang B, Chau SWH, Liu Y, et al. Gut microbiome dysbiosis across early Parkinson’s disease, REM sleep behavior disorder and their first-degree relatives. Nat Commun. 2023;14:2501.
99. Jeong E, Woo Shin Y, Byun JI, et al. EEG-based machine learning models for the prediction of phenoconversion time and subtype in isolated rapid eye movement sleep behavior disorder. Sleep. 2024;47:zsae031.
100. Gaurav R, Pyatigorskaya N, Biondetti E, et al. Deep learning-based neuromelanin MRI changes of isolated REM sleep behavior disorder. Mov Disord. 2022;37:1064-9.
101. Abdelfattah M, Zhou L, Sum-Ping O, et al. Automated detection of isolated REM sleep behavior disorder using computer vision. Ann Neurol. 2025;97:860-72.
102. Arnaldi D, Chincarini A, Hu MT, et al. Dopaminergic imaging and clinical predictors for phenoconversion of REM sleep behaviour disorder. Brain. 2021;144:278-87.
103. Campabadal A, Segura B, Junque C, et al. Comparing the accuracy and neuroanatomical correlates of the UPSIT-40 and the Sniffin' Sticks test in REM sleep behavior disorder. Parkinsonism Relat Disord. 2019;65:197-202.
104. Iranzo A, Santamaría J, Valldeoriola F, et al. Dopamine transporter imaging deficit predicts early transition to synucleinopathy in idiopathic rapid eye movement sleep behavior disorder. Ann Neurol. 2017;82:419-28.
105. Kogan RV, Janzen A, Meles SK, et al; REMPET Working Group. Four-year follow-up of [18F]fluorodeoxyglucose positron emission tomography-based Parkinson’s disease-related pattern expression in 20 patients with isolated rapid eye movement sleep behavior disorder shows prodromal progression. Mov Disord. 2021;36:230-5.
106. Meles SK, Renken RJ, Janzen A, et al; REMPET Study Group. The metabolic pattern of idiopathic REM sleep behavior disorder reflects early-stage Parkinson disease. J Nucl Med. 2018;59:1437-44.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Copyright
Data & Comments
Data
















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].