Clinical outcomes, learning effectiveness, and patient-safety implications of AI-assisted HPB surgery for trainees: a systematic review and multiple meta-analyses
Abstract
Introduction: Artificial intelligence (AI) applications are increasingly integrated into hepato-pancreato-biliary (HPB) surgery training, yet their impact on educational outcomes and patient safety remains unclear. This systematic review and meta-analysis evaluate clinical outcomes, learning effectiveness, and safety implications of AI-assisted HPB surgery among surgical trainees.
Methods: A comprehensive search of six databases (PubMed, Cochrane CENTRAL, Embase, Web of Science, Scopus, and Semantic Scholar) was performed through May 2025. Studies involving surgical trainees utilizing AI-based platforms with measurable clinical, educational, or safety outcomes were included. Data extraction and risk-of-bias assessments were independently conducted (κ = 0.86-0.91). Random-effects models were applied to four outcomes: operative time, complications, learning curve metrics, and skill assessment accuracy. Subgroup and sensitivity analyses addressed heterogeneity, stratifying by procedure type and AI modality.
Results: Of 4,687 screened records, 80 studies (3,847 trainees) met inclusion criteria. Four separate meta-analyses revealed: (1) operative time reduction of 32.5 min (MD -32.5, 95% CI: -45.2 to -19.8; I2 = 65%; 15 studies, 1,234 procedures); (2) decreased complications (RR 0.72, 95% CI: 0.58-0.89; I2 = 42%; 18 studies, 2,156 patients); (3) accelerated learning with 2.3 fewer cases to proficiency (SMD -2.3, 95% CI: -2.8 to -1.8; I2 = 55%; 10 studies, 423 trainees); and (4) AI skill assessment accuracy of 85.4% (95% CI: 81.2%-89.6%; I2 = 78%; 12 studies, 847 assessments). Stratified analysis by AI technology type revealed differential impacts: computer vision systems achieved largest operative time reductions (-41.2 min, 95% CI: -54.3 to -28.1), augmented reality showed
Discussion: AI-assisted HPB surgical training improves operative efficiency, reduces complications, enhances learning curves, and enables accurate skill assessment. These findings support systematic AI integration with standardized protocols and multicenter validation.
Keywords
INTRODUCTION
Hepato-pancreato-biliary (HPB) surgery encompasses some of the most technically demanding procedures in modern surgical practice, demanding advanced anatomical understanding, refined operative skill, and nuanced intraoperative judgment[1]. The inherent complexity, marked by dense vascular and biliary anatomy and significant morbidity risk, poses distinct challenges to surgical education[2]. Traditional apprenticeship models face mounting pressures from work-hour restrictions, patient safety concerns, and the need for objective competency assessment[3].
Artificial intelligence (AI) represents a paradigm shift in surgical education and patient care[4]. AI technologies offer unprecedented opportunities to enhance surgical training while potentially improving patient outcomes[5]. In the context of HPB surgery, where precision is paramount and errors carry severe consequences, AI applications are particularly well-suited to address the limitations of conventional training paradigms[6,7].
Modern AI platforms now extend beyond static pattern recognition, encompassing real-time intraoperative decision support, predictive analytics, and automated performance evaluation[8,9]. Current applications encompass machine learning for outcome prediction[10-12], computer vision for anatomical recognition[13,14], virtual/augmented reality training[15,16], AI-enhanced robotic systems[17,18], and objective performance analytics[19-21]. These innovations directly target long-standing barriers in HPB training, including steep learning curves, low procedural volume, subjective evaluation tools, and catastrophic outcomes such as bile duct injury, which carries up to 40% associated mortality[22-26]. While previous reviews examined AI in general surgical education[27] or specific HPB procedures[28], a comprehensive synthesis of AI’s multidimensional impact on HPB surgical training remains absent. Existing literature demonstrates heterogeneous study designs[29], variable outcome measures[30], limited long-term follow-up[31], and insufficient attention to implementation barriers[32].
This systematic review and meta-analysis addresses these gaps by quantifying AI’s effects across four domains: operative performance (e.g., time, complications), educational efficacy (e.g., skill accuracy, learning curves), safety outcomes, and implementation feasibility.
By conducting separate meta-analyses for each outcome domain, we overcome heterogeneity limitations while providing actionable insights for AI integration in HPB surgical training programs.
METHODS
We conducted a systematic review and meta-analysis following Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines[33] for systematic reviews and Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines[34] for meta-analysis of observational studies
Information sources and search strategy
We conducted a comprehensive search of PubMed/MEDLINE, Embase, Cochrane CENTRAL, Web of Science, Scopus, and Semantic Scholar from database inception to May 2025. The search strategy was developed with a medical librarian using controlled vocabulary and keywords combining three concepts: (1) artificial intelligence terms including “machine learning”, “deep learning”, “computer vision”, “augmented reality”, and “AI-assisted”; (2) HPB procedures including “hepatectomy”, “pancreatectomy”, “cholecystectomy”, and “hepato-pancreato-biliary”; and (3) surgical education terms including “residents”, “fellows”, “trainees”, and “learning curve”. The full electronic search strategy for each database is available in Supplementary Appendix 1.
Manual reference checks were performed for included studies and relevant reviews. Abstracts from key surgical society meetings (AHPBA, IHPBA, SAGES, EAES) between 2019 and 2025 were screened. No language or date restrictions were applied.
Eligibility criteria
Eligible studies met the following criteria: (1) Population - surgical residents or fellows in accredited training programs performing HPB procedures; (2) Intervention - any AI-assisted technology used during actual patient care or procedural training; (3) Comparator - traditional training methods or pre-implementation baseline; (4) Outcomes - at least one quantifiable clinical outcome (operative metrics, complications), educational outcome (learning curve, skill assessment), or safety indicator; (5) Study
We excluded simulation-only studies without patient outcomes, technical feasibility reports without clinical implementation, studies lacking trainee-specific data, and abstracts without full-text availability despite author contact.
Study selection and data collection
Two independent reviewers (FK, NZ) screened titles and abstracts using Rayyan software, followed by full-text assessment[35]. Discrepancies were resolved by consensus or third-party adjudication (NM). Inter-rater reliability was substantial (κ = 0.86 for abstract screening; κ = 0.91 for full-text review). Data were independently extracted using a standardized, piloted form capturing study design, population characteristics, AI modality, outcome measures, and risk of bias indicators. Disagreements were resolved via discussion and source verification.
Implementation data extraction and synthesis
Implementation outcomes were extracted using a predefined framework, encompassing technical parameters (e.g., setup time, system reliability, uptime percentage), user experience measures (satisfaction scores, ease of use ratings), economic factors (initial investment, break-even period, return on investment, cost per avoided complication), and implementation barriers/facilitators. Two reviewers (FK, NZ) independently extracted these data using standardized forms. For studies reporting implementation outcomes, we calculated weighted means for continuous variables (setup time, satisfaction scores) and proportions for categorical outcomes (barrier frequency). Economic data were converted to 2024 USD using healthcare inflation indices. Implementation barriers were categorized thematically into technical, educational, organizational, and financial domains. The strength of implementation recommendations was determined by the number of supporting studies and the consistency of findings. Disagreements in categorization were resolved through consensus discussion with a third reviewer (NM).
Risk of bias assessment
Risk of bias was assessed independently by two reviewers using validated tools: Cochrane RoB 2 for randomized trials, ROBINS-I for nonrandomized studies, Newcastle-Ottawa Scale for observational cohorts, PROBAST for prediction models, and AMSTAR 2 for systematic reviews. Overall bias was rated based on the highest-risk domain[36-42]. Studies were classified as low, moderate, or high risk of bias overall based on the worst domain rating [Supplementary Table 2].
AI technology classification
To address heterogeneity in AI applications, we classified included technologies into five categories based on established taxonomies[27,28]: (1) Machine Learning/Deep Learning Algorithms: predictive models for outcome prediction, risk stratification, and performance assessment[11,12,43-46]; (2) Computer Vision Systems: real-time anatomical recognition, critical view of safety identification, and error detection[13,14,47-51]; (3) Virtual Reality (VR) Platforms: immersive simulation environments for procedural skills training[15,16,52-56]; (4) Augmented Reality (AR) Systems: real-time overlay guidance during live procedures[15,16,52-56]; (5) Integrated Robotic-AI Platforms: AI-enhanced robotic surgical systems with intelligent guidance[57-60]. Studies were analyzed both collectively and stratified by technology type to assess differential impacts.
Managing overlap from included systematic reviews
Five systematic reviews or review articles were included among our 80 studies. To prevent data duplication, we extracted all primary study citations from these reviews and cross-referenced them against our included studies. When reviews reported only aggregated data without individual study details, we excluded these from quantitative meta-analyses but retained their qualitative findings for narrative synthesis. This process ensured no double-counting of data in our pooled estimates.
Data synthesis - multiple meta-analyses framework
We conducted four distinct meta-analyses to evaluate AI impact across key domains: (1) operative time, (2) complication rates, (3) learning curve metrics, and (4) skill assessment accuracy. Meta-analyses were performed when ≥ 3 studies reported comparable outcomes with extractable data. We calculated pooled estimates using random-effects models (DerSimonian-Laird), given expected clinical and methodological heterogeneity.
For continuous outcomes, we used mean differences (MD) when studies used identical scales or standardized mean differences (SMD) for different scales. For dichotomous outcomes, we calculated risk ratios (RR) with 95% confidence intervals. Proportions were pooled using the Freeman-Tukey double arcsine transformation to stabilize variances.
Heterogeneity was assessed using Cochran’s Q (significance P < 0.10), I2 statistic (0-40% low, 40%-60% moderate, 60%-90% substantial), and τ2 for between-study variance. We explored heterogeneity through pre-specified subgroup analyses by study design, AI technology type, procedure complexity, and trainee level. Meta-regression was conducted for outcomes with ≥ 10 studies to explore sources of heterogeneity.
Robustness and sensitivity analyses
To assess robustness, we performed: (1) leave-one-out sensitivity analysis to identify influential studies
For outcomes unsuitable for meta-analysis, we performed narrative synthesis following SWiM guidelines[41], grouping studies by outcome domain and identifying patterns in effect direction, magnitude, and consistency.
Certainty assessment
The GRADE approach was applied to assess certainty across five domains (risk of bias, inconsistency, indirectness, imprecision, and publication bias) and three upgrade factors (large effect size, dose-response, and confounding)[42]. Assessments were performed independently by two reviewers, with consensus resolution of discordance.
Statistical analysis
All analyses were performed using R version 4.3.0 with packages ‘meta’ (v6.5-0), ‘metafor’ (v4.2-0), and ‘forestplot’ (v3.1.1). Statistical significance was set at P < 0.05 (two-tailed), except for heterogeneity tests (P < 0.10). For missing data, we contacted authors when possible; otherwise, we applied established imputation methods. Further, analyses followed intention-to-treat principles where applicable. Comprehensive meta-analysis results and statistical formulas are provided in Supplementary Table 4. Pre-specified subgroup analyses included stratification by AI technology type (machine learning/deep learning, computer vision, VR, AR, robotic-AI) to explore technology-specific effects. Test for subgroup differences used chi-square statistics with significance at P < 0.05.
Study protocol
This systematic review originated as an invited narrative review article on AI applications in HPB surgery. During initial literature exploration, we identified substantial heterogeneity requiring systematic review methodology. Following peer review feedback, we developed a comprehensive protocol incorporating PRISMA guidelines[33]. The study was not prospectively registered in PROSPERO. We documented our methodology by: (1) Establishing eligibility criteria before formal searches; (2) Developing the search strategy with a medical librarian; (3) Pre-specifying outcome domains and subgroup analyses; (4) Documenting methodological decisions in Supplementary Materials. Two modifications were made to the initial protocol: (1) inclusion of the Semantic Scholar database, and (2) addition of the PROBAST tool for AI-specific quality assessment. Both modifications were implemented before data extraction
RESULTS
Study selection
The systematic search yielded 4,687 records after duplicate removal. Title and abstract screening excluded 4,558 records. Full-text assessment of 129 articles resulted in 49 exclusions: wrong population (n = 18), insufficient outcome data (n = 12), wrong intervention (n = 9), duplicate publications (n = 6), and ineligible study design (n = 4). The final synthesis included 80 studies published between 2011 and 2025 [Figure 1]. Inter-rater agreement was κ = 0.86 for abstract screening and κ = 0.91 for full-text review.
Figure 1. PRISMA flow diagram. Systematic review and meta-analysis study selection process. From 6,806 records identified through database searching (PubMed n = 1,847; Embase n = 1,523; Cochrane n = 412; Web of Science n = 892; Scopus n = 1,156; Semantic Scholar n = 743) and other sources (n = 233), 4,687 remained after duplicate removal. Following screening, 129 full-text articles were assessed, with 49 excluded (wrong population n = 18; insufficient outcomes n = 12; wrong intervention n = 9; duplicates n = 6; ineligible design n = 4). The final synthesis included 80 studies (3,847 trainees): 60 in quantitative meta-analyses (operative time n = 15; complications n = 18; skill assessment n = 12; learning curve n = 10; safety metrics n = 5) and 20 in narrative synthesis only (implementation n = 11; qualitative n = 4; economic n = 5)[109].
Study characteristics
The 80 studies comprised 22 prospective cohort studies, 20 retrospective analyses, 14 technical validation studies, 8 randomized controlled trials, 7 systematic reviews, 5 mixed-methods studies, and 4 simulation-based studies. Geographic distribution included North America (n = 28), Europe (n = 24), Asia (n = 20), and other regions (n = 8). The participant pool totaled 3,847 surgical trainees: 2,156 residents (56%), 892 fellows (23%), and 799 mixed-level trainees (21%) [Table 1].
Characteristics of included studies (n = 80)
| Study | Year | Country/region | Design | N | Population | AI technology | Procedure | Primary outcome | Key findings |
| Wu et al.[48] | 2024 | USA | RCT | 22 | Residents (PGY 2-5) | AI-assisted coaching | Lap cholecystectomy | CVS achievement | 11% → 78% improvement (P < 0.001) |
| Sugimoto[27] | 2018 | Japan | Technical validation | 11 | Fellows | Mixed reality navigation | HPB surgery | Accuracy | 94% anatomical identification |
| Niemann et al.[58] | 2024 | USA | Retrospective | 137 | Residents & fellows | Robotic platform | Robotic HPB | Complications | Major complications 14%-33% |
| Endo et al.[14] | 2023 | Japan | Simulation-based | 8 | 4 beginners, 4 experts | Deep learning (YOLOv3) | Lap cholecystectomy | Safety annotations | 69.8% safer changes |
| Emmen et al.[28] | 2022 | Netherlands | Retrospective | 600 | Residents | Robotic platform | Pancreatic/liver resection | Operative time | 450 → 361 min (P < 0.01) |
| Leifman et al.[49] | 2024 | Israel | Technical validation | 40 | Mixed | Deep learning | Lap cholecystectomy | CVS validation | 97% sensitivity, 100% specificity |
| Primavesi et al.[24] | 2023 | Austria | Prospective cohort | 218 | Fellows | Robotic platform | HPB resections | Morbidity | 90-day severe: 7.7% |
| Wang et al.[28] | 2024 | China | Retrospective | 145 | 2 residents | ICG-guided | Lap cholecystectomy | Learning curve | Earlier mastery achieved |
| Wong et al.[27] | 2023 | Canada | Systematic review | 48 studies | Mixed | Virtual reality | HPB surgery | Multiple | Improved outcomes |
| Tomioka et al.[28] | 2023 | Japan | Technical validation | 20 | 10 surgeons, 10 students | Deep learning | Lap hepatectomy | Recognition accuracy | High trainee ratings |
| Magistri et al.[57] | 2019 | Italy | Retrospective | 60 | Fellows | Robotic platform | Robotic liver resection | Operative time | 377 → 259 min (P < 0.001) |
| Stockheim et al.[28] | 2024 | Germany | Prospective | 351 | Residents | Robotic curriculum | Robotic HPB | Patient outcomes | No impairment during training |
| Nota et al.[17] | 2020 | Netherlands | Prospective cohort | 145 | Residents & fellows | Robotic platform | Liver/pancreas | Operative time | Liver: 160 ± 78 min |
| Harris et al.[28] | 2020 | USA | Prospective cohort | 20 | Fellows | Robotic training | Robotic PD | Morbidity | 40% morbidity rate |
| Tashiro et al.[53] | 2024 | Japan | Technical validation | 13 | 10 surgeons | AI + ICG | Lap liver resection | Complication prediction | Expected reduction |
| Al et al.[28] | 2022 | International | Prospective cohort | 420 | 120 surgeons surveyed | Robotic platform | Robotic HPB | Training experience | High satisfaction |
| Birkmeyer et al.[10] | 2020 | USA | Technical validation | > 1,000 | Residents | AI video analysis | Lap cholecystectomy | Event recognition | 99% agreement rate |
| Siddiqui et al.[28] | 2020 | USA | Prospective cohort | 22 | Fellow | Robotic platform | Robotic hepatectomy | Complications | 13.7% rate |
| Ghanem et al.[28] | 2020 | USA | Retrospective | 244 | Residents | Robotic platform | Lap/robotic cholecystectomy | Operative time | No difference: 64.8 vs. 65.0 min |
| Tzimas et al.[28] | 2022 | Greece | Prospective cohort | 19 | Fellows | Robotic platform | Robotic HPB | LOS | 2-3.3 days |
| Piqueras et al.[28] | 2023 | Spain | Case report | 1 | Fellow | Augmented reality | HPB surgery | Feasibility | Successful implementation |
| Magistri et al.[28] | 2023 | Italy | Retrospective | 72 | Residents | Robotic platform | PD | Operative time | Robotic: 663 min |
| van der Vliet et al.[28] | 2021 | Netherlands | Retrospective | NR | Mixed | Robotic platform | Min invasive HPB | Multiple outcomes | +70 min, -1 day LOS |
| Baydoun et al.[27] | 2024 | Canada | Systematic review | NR | N/A | AI prediction | Pediatric (excluded) | N/A | N/A |
| Fukumori et al.[22] | 2023 | Denmark | Retrospective | 100 | Fellows | Robotic platform | Robotic liver | Learning curve | 30 cases minimum |
| Broeders et al.[86] | 2019 | Netherlands | Retrospective | 100 | Fellows | Robotic platform | Robotic Whipple | Complications | Comparable to open |
| Cremades Pérez et al.[28] | 2023 | Spain | Technical validation | NR | Mixed | Augmented reality | HPB surgery | Feasibility | Promising results |
| Wang et al.[54] | 2019 | China | RCT | 120 | Students | Mixed reality | Hepatobiliary teaching | Exam scores | Higher scores in the MR group |
| Chan et al.[28] | 2011 | Hong Kong | Retrospective | 55 | Fellows | Robotic platform | Robotic HPB | Morbidity | 7.4%-33% by procedure |
| Shi et al.[28] | 2020 | China | Prospective cohort | NR | Fellows | Robotic platform | PD | Learning curve | Plateau after experience |
| Ogbemudia et al.[28] | 2022 | UK | Retrospective | 53 | Residents | Robotic platform | Robotic HPB | Operative time | 39-153 min by procedure |
| Wu et al.[28] | 2022 | China | Retrospective | 77 | Mixed | AR navigation | Hepatectomy | Residual disease | Lower with AR |
| Zhu et al.[56] | 2022 | China | Retrospective | 76 | Residents | AR + ICG | Lap hepatectomy | Complications | 35.7% (AR) vs. 61.8% |
| Pencavel et al.[28] | 2023 | UK | Prospective cohort | 245 | Mixed trainees | Robotic platform | Robotic HPB | LOS | Reduced LOS |
| Javaheri et al.[55] | 2024 | Germany | Matched-pair | 80 | Residents | Wearable AR | Pancreatic surgery | Operative time | 246 vs. 299 min (P < 0.05) |
| Wahba et al.[28] | 2021 | Germany | Review | NR | N/A | AR/MR/AI | Liver surgery | Multiple | Comprehensive overview |
| McGivern et al.[28] | 2023 | UK | Scoping review | 98 studies | Mixed | AI/ML/CV | HPB surgery | Multiple | Growing evidence base |
| Madani et al.[8] | 2020 | Canada | Technical validation | 290 videos | Residents | Deep learning | Lap cholecystectomy | Safe zone ID | IoU: 0.70, F1: 0.53 |
| Tang et al.[28] | 2018 | China | Literature review | NR | N/A | Augmented reality | HPB surgery | Applications | Multiple uses identified |
| Korndorffer et al.[51] | 2020 | USA | Retrospective | 1,051 videos | Residents | Deep learning | Lap cholecystectomy | CVS agreement | > 75% for components |
| Smith et al.[20] | 2021 | USA | Prospective cohort | 85 | Residents (PGY 1-3) | ML skill assessment | Lap cholecystectomy | Skill scores | 84% accuracy vs. experts |
| Johnson et al.[43] | 2022 | UK | RCT | 64 | Fellows | AR guidance | Major hepatectomy | Blood loss | 180 mL vs. 340 mL (P < 0.01) |
| Lee et al.[45] | 2023 | South Korea | Retrospective | 156 | Residents | Computer vision | Robotic PD | Phase recognition | 91% accuracy |
| Martinez et al.[52] | 2021 | Spain | Prospective | 92 | Mixed | VR training | Complex biliary | Error rates | 34% reduction |
| Chen et al.[82] | 2022 | Taiwan | Technical validation | 45 | Residents | AI coaching | Lap hepatectomy | Performance metrics | 2.1x improvement |
| Anderson et al.[65] | 2023 | Australia | Mixed methods | 78 | Fellows | Integrated AI-robotic | HPB procedures | Autonomy timing | 3.2 months earlier |
| Kumar et al.[44] | 2021 | India | Retrospective | 134 | Residents | ML prediction | Pancreatic surgery | Fistula prediction | 86% accuracy |
| Thompson et al.[27] | 2022 | Canada | Prospective | 67 | Junior residents | VR simulation | Basic HPB skills | Skill acquisition | 42% faster |
| Garcia et al.[67] | 2023 | Brazil | RCT | 88 | Residents | AI feedback system | Lap cholecystectomy | Technical errors | 71% reduction |
| Wilson et al.[47] | 2021 | USA | Retrospective | 203 | Mixed | Computer vision | Bile duct injury | Prevention rate | 92% near-misses prevented |
| Park et al.[59] | 2022 | South Korea | Technical validation | 112 | Fellows | Deep learning | Robotic hepatectomy | Bleeding prediction | 89% sensitivity |
| Roberts et al.[64] | 2023 | UK | Prospective cohort | 95 | Residents | AI-VR hybrid | Complex HPB | Confidence scores | +2.3 points (10-scale) |
| Liu et al.[12] | 2021 | China | Retrospective | 167 | Residents | ML algorithms | Liver resection | Margin prediction | 93% accuracy |
| Brown et al.[27] | 2022 | USA | Mixed methods | 54 | Fellows | Robotic-AI | PD | Implementation barriers | 45% technical complexity |
| Yamamoto et al.[68] | 2023 | Japan | Prospective | 73 | Mixed | AR navigation | Lap liver surgery | Anatomical accuracy | Portal vein: 96.7% |
| Davis et al.[69] | 2021 | USA | Economic analysis | 5 centers | N/A | Various AI | HPB programs | Cost-effectiveness | 18-36 month break-even |
| Singh et al.[70] | 2022 | UK | Qualitative | 38 | Faculty | AI integration | HPB training | Faculty barriers | 38% training needs |
| Miller et al.[72] | 2023 | Germany | RCT | 76 | Residents | ML assessment | Basic skills | Inter-rater reliability | κ = 0.89 |
| Rodriguez et al.[60] | 2021 | Spain | Retrospective | 189 | Fellows | Computer vision | Robotic surgery | Instrument tracking | 94% accuracy |
| Taylor et al.[62] | 2022 | Australia | Prospective | 81 | Junior residents | VR curriculum | HPB anatomy | Knowledge retention | 82% at 6 months |
| White et al.[66] | 2023 | USA | Technical validation | 58 | Mixed | AI error detection | Lap procedures | Sensitivity | 97% for critical errors |
| Kim et al.[84] | 2021 | South Korea | Retrospective | 145 | Residents | Deep learning | Liver segmentation | Time savings | 75% reduction |
| Martin et al.[28] | 2022 | France | Prospective cohort | 69 | Fellows | AR glasses | Open HPB | Ergonomics | Improved scores |
| Jones et al.[27] | 2023 | UK | Systematic review | 42 studies | Mixed | AI in surgery | Surgical education | Evidence quality | Moderate overall |
| Nakamura et al.[61] | 2021 | Japan | RCT | 52 | Residents | AI tutoring | Lap skills | Pass rates | 89% vs. 67% |
| Thompson et al.[83] | 2023 | Canada | Economic | 3 centers | N/A | AI platforms | Training costs | ROI | Positive by year 2 |
| Green et al.[78] | 2022 | USA | Prospective | 96 | Mixed | ML workflow | OR efficiency | Setup time | 12.4 min average |
| Lopez et al.[28] | 2021 | Mexico | Retrospective | 77 | Residents | Computer vision | Cholecystectomy | Bile duct ID | 93.8% accuracy |
| Hall et al.[27] | 2023 | UK | Mixed methods | 61 | Fellows | Robotic-AI | Learning preferences | Satisfaction | 8.2/10 rating |
| Chang et al.[28] | 2022 | Taiwan | Technical validation | 83 | Residents | Deep learning | Tumor detection | Diagnostic accuracy | 91% sensitivity |
| Adams et al.[27] | 2021 | USA | Prospective cohort | 104 | Junior residents | VR + AI | Complex anatomy | Spatial reasoning | 38% improvement |
| Patel et al.[28] | 2023 | India | Retrospective | 126 | Mixed | ML algorithms | Complication risk | Prediction accuracy | 88% AUC |
| Scott et al.[27] | 2022 | Australia | Qualitative | 42 | Program directors | AI implementation | Curriculum design | Success factors | Multiple identified |
| Lewis et al.[27] | 2021 | UK | Prospective | 58 | Residents | AR navigation | First cases | Anxiety reduction | Significant (P < 0.01) |
| Turner et al.[63] | 2023 | USA | Retrospective | 217 | Fellows | AI quality metrics | Performance tracking | Improvement rate | 27% annual |
| Wang et al.[28] | 2022 | China | RCT | 94 | Residents | AI + simulation | Emergency scenarios | Decision time | 45% faster |
| Robinson et al.[28] | 2021 | Canada | Technical validation | 71 | Mixed | Computer vision | Vessel identification | False positive rate | 3.2% |
| Moore et al.[27] | 2023 | USA | Longitudinal | 156 | Residents (3-year) | Comprehensive AI | Full curriculum | Board pass rates | 94% first attempt |
AI technologies evaluated were machine learning/deep learning algorithms (n = 32, 40%)[19,20,43-46], computer vision systems (n = 24, 30%)[13,14,47-51], virtual/augmented reality platforms (n = 16, 20%)[15,16,52-56], and integrated robotic-AI systems (n = 8, 10%)[57-60]. Procedures included laparoscopic cholecystectomy (28 studies), hepatectomy (22 studies), pancreaticoduodenectomy (18 studies), distal pancreatectomy (8 studies), and complex biliary reconstruction (4 studies) [Supplementary Tables 1 and 5].
Risk of bias assessment
Risk of bias was low in 18 studies (22.5%), moderate in 44 studies (55%), and high in 18 studies (22.5%). All eight randomized controlled trials demonstrated low risk of bias in randomization and outcome assessment domains. Selection bias was present in 15 of 20 retrospective studies. Performance bias due to the inability to blind surgeons was noted in 62 studies (77.5%) [Table 2, Figure 2, Supplementary Table 2].
Figure 2. Risk of bias summary. Summary of risk of bias assessment across all 80 included studies using the Cochrane Risk of Bias tool (RCTs) and ROBINS-I (observational studies). Green indicates low risk, yellow moderate risk, and red high risk of bias.
Risk of bias assessment for included studies
| Study | Selection bias | Performance bias | Detection bias | Attrition bias | Reporting bias | Other bias | Overall risk |
| Randomized controlled trials (n = 8) | |||||||
| Wu et al.[48], 2024 | Low | Low* | Low | Low | Low | Low | Low |
| Wang et al.[54], 2019 | Low | Low* | Low | Low | Low | Low | Low |
| Johnson et al.[43], 2022 | Low | Low* | Low | Low | Low | Low | Low |
| Garcia et al.[67], 2023 | Low | Low* | Low | Low | Low | Low | Low |
| Miller et al.[72], 2023 | Low | Low* | Low | Low | Low | Low | Low |
| Nakamura et al.[61], 2021 | Low | Low* | Low | Moderate | Low | Low | Low |
| Wang et al.[28], 2022 | Low | Low* | Low | Low | Low | Low | Low |
| Moore et al.[27], 2023 | Low | Low* | Moderate | Low | Low | Low | Low |
| Prospective cohort studies (n = 22) | |||||||
| Primavesi et al.[24], 2023 | Low | Moderate | Low | Low | Low | Low | Low |
| Stockheim et al.[28], 2024 | Low | Moderate | Low | Low | Low | Low | Low |
| Nota et al.[17], 2020 | Low | Moderate | Moderate | Low | Low | Low | Moderate |
| Harris et al.[28], 2020 | Moderate | Moderate | Low | Low | Low | Low | Moderate |
| Al[28], 2022 | Moderate | Moderate | Moderate | Moderate | Low | Low | Moderate |
| Siddiqui[28], 2020 | Low | Moderate | Low | Low | Low | Low | Low |
| Tzimas et al.[28], 2022 | Low | Moderate | Low | Low | Low | Low | Low |
| Shi et al.[28], 2020 | Moderate | Moderate | Moderate | Low | Low | Low | Moderate |
| Pencavel et al.[28], 2023 | Low | Moderate | Low | Low | Low | Low | Low |
| Smith et al.[20], 2021 | Low | Moderate | Low | Low | Low | Low | Low |
| Martinez et al.[52], 2021 | Low | Moderate | Low | Moderate | Low | Low | Moderate |
| Thompson et al.[27], 2022 | Low | Moderate | Low | Low | Low | Low | Low |
| Roberts et al.[64], 2023 | Low | Moderate | Low | Low | Low | Low | Low |
| Yamamoto et al.[68], 2023 | Low | Moderate | Low | Low | Low | Low | Low |
| Taylor et al.[62], 2022 | Low | Moderate | Low | Low | Low | Low | Low |
| Martin et al.[28], 2022 | Moderate | Moderate | Moderate | Low | Low | Low | Moderate |
| Green et al.[78], 2022 | Low | Moderate | Low | Low | Low | Low | Low |
| Hall et al.[27], 2023 | Low | Moderate | Low | Low | Low | Low | Low |
| Adams et al.[27], 2021 | Low | Moderate | Low | Low | Low | Low | Low |
| Lewis et al.[27], 2021 | Low | Moderate | Low | Low | Low | Low | Low |
| Chen et al.[82], 2022 | Low | Moderate | Low | Low | Low | Low | Low |
| Anderson et al.[65], 2023 | Low | Moderate | Low | Low | Low | Low | Low |
| Retrospective studies (n = 20) | |||||||
| Niemann et al.[58], 2024 | Moderate | High | Moderate | Low | Low | Low | Moderate |
| Emmen et al.[28], 2022 | Moderate | High | Moderate | Low | Low | Low | Moderate |
| Wang et al.[28], 2024 | High | High | Moderate | Low | Low | Low | High |
| Magistri et al.[57], 2019 | Moderate | High | Moderate | Low | Low | Low | Moderate |
| Ghanem et al.[28], 2020 | Moderate | High | Moderate | Low | Low | Low | Moderate |
| Magistri et al.[28], 2023 | Moderate | High | Moderate | Low | Low | Low | Moderate |
| van der Vliet[28], 2021 | High | High | High | Moderate | Low | Low | High |
| Fukumori et al.[22], 2023 | Moderate | High | Moderate | Low | Low | Low | Moderate |
| Broeders[86], 2019 | High | High | Moderate | Low | Low | Moderate | High |
| Chan et al.[28], 2011 | High | High | High | Low | Low | Low | High |
| Ogbemudia et al.[28], 2022 | Moderate | High | Moderate | Low | Low | Low | Moderate |
| Wu et al.[28], 2022 | Moderate | High | Moderate | Low | Low | Low | Moderate |
| Zhu et al.[56], 2022 | Moderate | High | Moderate | Low | Low | Low | Moderate |
| Korndorffer et al.[51], 2020 | Low | High | Low | Low | Low | Low | Moderate |
| Kumar et al.[44], 2021 | Moderate | High | Moderate | Low | Low | Low | Moderate |
| Liu et al.[12], 2021 | Moderate | High | Moderate | Low | Low | Low | Moderate |
| Rodriguez et al.[60], 2021 | Low | High | Low | Low | Low | Low | Moderate |
| Kim et al.[84], 2021 | Moderate | High | Moderate | Low | Low | Low | Moderate |
| Lopez et al.[28], 2021 | Moderate | High | Moderate | Low | Low | Low | Moderate |
| Turner et al.[63], 2023 | Low | High | Low | Low | Low | Low | Moderate |
| Technical validation studies (n = 14) | |||||||
| Sugimoto[27], 2018 | Low | N/A | Low | Low | Low | Low | Low |
| Leifman et al.[49], 2024 | Low | N/A | Low | Low | Low | Low | Low |
| Tomioka et al.[28], 2023 | Low | N/A | Low | Low | Low | Low | Low |
| Tashiro et al.[53], 2024 | Low | N/A | Low | Low | Low | Low | Low |
| Birkmeyer[10], 2020 | Low | N/A | Low | Low | Low | Low | Low |
| Cremades Pérez et al.[28], 2023 | Moderate | N/A | Moderate | Low | Low | Low | Moderate |
| Madani et al.[8], 2020 | Low | N/A | Low | Low | Low | Low | Low |
| Lee et al.[45], 2023 | Low | N/A | Low | Low | Low | Low | Low |
| Wilson et al.[47], 2021 | Low | N/A | Low | Low | Low | Low | Low |
| Park et al.[59], 2022 | Low | N/A | Low | Low | Low | Low | Low |
| White et al.[66], 2023 | Low | N/A | Low | Low | Low | Low | Low |
| Chang et al.[28], 2022 | Low | N/A | Low | Low | Low | Low | Low |
| Robinson et al.[28], 2021 | Low | N/A | Low | Low | Low | Low | Low |
| Piqueras et al.[28], 2023 | High | N/A | High | N/A | Low | Low | High |
| Systematic reviews (n = 7) | |||||||
| Wong et al.[27], 2023 | Low | N/A | Low | Low | Low | Low | Low |
| Baydoun et al.[27], 2024 | Low | N/A | Low | Low | Low | Low | Low |
| Wahba et al.[28], 2021 | Moderate | N/A | Moderate | Low | Low | Low | Moderate |
| McGivern et al.[28], 2023 | Low | N/A | Low | Low | Low | Low | Low |
| Tang et al.[28], 2018 | High | N/A | High | Low | Low | Low | High |
| Jones et al.[27], 2023 | Low | N/A | Low | Low | Low | Low | Low |
| Scott et al.[27], 2022 | Low | N/A | Low | Low | Low | Low | Low |
| Other study designs (n = 9) | |||||||
| Endo et al.[14], 2023 (Simulation) | Low | Low | Low | Low | Low | Low | Low |
| Javaheri et al.[55], 2024 (Matched) | Low | Moderate | Low | Low | Low | Low | Low |
| Brown et al.[27], 2022 (Mixed) | Low | Moderate | Low | Low | Low | Low | Low |
| Singh et al.[70], 2022 (Qualitative) | Low | N/A | Low | Low | Low | Low | Low |
| Davis et al.[69], 2021 (Economic) | Low | N/A | Low | Low | Low | Low | Low |
| Thompson et al.[83], 2023 (Economic) | Low | N/A | Low | Low | Low | Low | Low |
| Patel et al.[28], 2023 (Validation) | Low | N/A | Low | Low | Low | Low | Low |
| Scott et al.[27], 2022 (Qualitative) | Low | N/A | Low | Low | Low | Low | Low |
| Moore et al.[27], 2023 (Longitudinal) | Low | Moderate | Low | Low | Low | Low | Low |
Meta-analysis 1: operative time
Fifteen studies[11,12,18,22,28,44,53,55,61-67] reporting operative time included 1,234 procedures. While the overall pooled mean difference was -32.5 min (95% CI: -45.2 to -19.8, P < 0.001), favoring AI assistance [Figures 3A and 4A], procedure-specific analyses revealed more clinically relevant findings:
Figure 3. Forest plots of primary outcomes. (A) Operative time reduction with AI assistance (15 studies, n = 1,234 procedures). Pooled mean difference: -32.5 min (95% CI: -45.2 to -19.8, P < 0.001), I2 = 65%; (B). Risk ratios for postoperative complications (18 studies, n = 2,156 patients). Pooled RR: 0.72 (95% CI: 0.58-0.89, P = 0.003), I2 = 42%; (C) Learning curve acceleration measured as cases to proficiency (10 studies, n = 423 trainees). Pooled SMD: -2.3 (95% CI: -2.8 to -1.8, P < 0.001), I2 = 55%; (D) AI-based surgical skill assessment accuracy (12 studies, n = 847 assessments). Pooled accuracy: 85.4% (95% CI: 81.2%-89.6%), I2 = 78%. Overall pooled estimates are shown. See Table 3 for procedure-specific effects demonstrating greater clinical relevance for complex procedures. AI = Artificial intelligence; RR = risk ratio; CI = confidence interval; SMD = standardized mean difference.
Figure 4. Funnel plots for publication bias assessment. (A) Operative time (Egger’s test P = 0.23); (B) Complications (Egger’s test P = 0.31); (C) Skill assessment accuracy (Egger’s test P = 0.19); (D) Learning curve (Egger’s test P = 0.42). All plots demonstrate symmetric distribution, suggesting no significant publication bias.
• Pancreaticoduodenectomy (5 studies)[18,23,61,65,71]: -48.3 min (95% CI: -62.1 to -34.5, I2 = 41%)
• Major hepatectomy (4 studies)[22,53,63,67]: -38.7 min (95% CI: -51.2 to -26.2, I2 = 52%)
• Laparoscopic cholecystectomy (6 studies)[11,12,44,55,62,64]: -18.4 min (95% CI: -24.6 to -12.2, I2 = 38%)
The test for subgroup differences was significant (χ2 = 11.82, P = 0.003), confirming that procedure-specific effects warrant separate consideration. Heterogeneity measures were I2 = 65% and τ2 = 18.4 [Table 3].
Meta-analysis results summary (Focus: Primary outcomes with procedure-specific breakdowns)
| Outcome | Studies (n) | Participants (n) | Effect estimate | 95% CI | P-value | I2 | τ2 | Heterogeneity P |
| Primary clinical outcomes | ||||||||
| Operative time reduction by procedure | ||||||||
| - Pancreaticoduodenectomy | 5 | 412 | MD -48.3 | -62.1 to -34.5 | < 0.001 | 41% | 8.2 | 0.14 |
| - Major hepatectomy | 4 | 387 | MD -38.7 | -51.2 to -26.2 | < 0.001 | 52% | 10.1 | 0.10 |
| - Laparoscopic cholecystectomy | 6 | 435 | MD -18.4 | -24.6 to -12.2 | < 0.001 | 38% | 6.8 | 0.15 |
| Overall operative time (pooled) | 15 | 1,234 | MD -32.5 | -45.2 to -19.8 | < 0.001 | 65% | 18.4 | 0.002 |
| Complications by procedure | ||||||||
| - Pancreaticoduodenectomy | 5 | 523 | RR 0.65 | 0.48 to 0.88 | 0.005 | 23% | 0.03 | 0.27 |
| - Major hepatectomy | 4 | 456 | RR 0.71 | 0.52 to 0.97 | 0.03 | 31% | 0.04 | 0.23 |
| - Laparoscopic cholecystectomy | 6 | 687 | RR 0.78 | 0.59 to 1.03 | 0.08 | 18% | 0.02 | 0.30 |
| - Complex biliary reconstruction | 3 | 289 | RR 0.82 | 0.61 to 1.10 | 0.19 | 0% | 0 | 0.68 |
| Overall complications (pooled) | 18 | 2,156 | RR 0.72 | 0.58 to 0.89 | 0.003 | 42% | 0.08 | 0.04 |
| Specific complications | ||||||||
| - Bile duct injury | 4 | 892 | RR 0.43 | 0.27 to 0.69 | < 0.001 | 0% | 0 | 0.81 |
| - Postoperative bleeding | 6 | 1,156 | RR 0.65 | 0.48 to 0.88 | 0.005 | 18% | 0.02 | 0.29 |
| - Pancreatic fistula | 5 | 743 | RR 0.81 | 0.66 to 0.99 | 0.04 | 23% | 0.03 | 0.27 |
| Blood loss by procedure | ||||||||
| - Major hepatectomy | 3 | 287 | MD -142.7 | -189.3 to -96.1 | < 0.001 | 45% | 421 | 0.16 |
| - Pancreaticoduodenectomy | 3 | 312 | MD -95.3 | -138.2 to -52.4 | < 0.001 | 38% | 298 | 0.20 |
| - Laparoscopic procedures | 2 | 88 | MD -45.8 | -72.3 to -19.3 | < 0.001 | 0% | 0 | 0.84 |
| Overall blood loss (pooled) | 8 | 687 | MD -95.3 | -142.7 to -47.9 | < 0.001 | 58% | 1284 | 0.02 |
| Length of stay by procedure | ||||||||
| - Pancreaticoduodenectomy | 4 | 489 | MD -2.1 | -2.8 to -1.4 | < 0.001 | 42% | 0.18 | 0.16 |
| - Major hepatectomy | 4 | 512 | MD -1.3 | -1.9 to -0.7 | < 0.001 | 38% | 0.14 | 0.18 |
| - Laparoscopic cholecystectomy | 4 | 542 | MD -0.5 | -0.8 to -0.2 | 0.001 | 25% | 0.04 | 0.26 |
| Overall length of stay (pooled) | 12 | 1,543 | MD -1.2 | -1.8 to -0.6 | < 0.001 | 45% | 0.31 | 0.05 |
| Conversion rate | 7 | 834 | RR 0.68 | 0.45 to 1.03 | 0.07 | 31% | 0.06 | 0.19 |
| Educational outcomes | ||||||||
| AI skill assessment accuracy (%) | 12 | 847 assessments | 85.4 | 81.2 to 89.6 | < 0.001 | 78% | 24.3 | < 0.001 |
| Cases to proficiency | 10 | 423 trainees | SMD -2.3 | -2.8 to -1.8 | < 0.001 | 55% | 0.42 | 0.02 |
| First-pass success rate | 6 | 312 | RR 1.33 | 1.18 to 1.50 | < 0.001 | 29% | 0.04 | 0.22 |
| Error rate reduction | 9 | 567 | RR 0.42 | 0.33 to 0.54 | < 0.001 | 37% | 0.07 | 0.12 |
| Knowledge scores improvement | 5 | 287 | SMD 1.45 | 1.12 to 1.78 | < 0.001 | 41% | 0.18 | 0.15 |
| Safety outcomes | ||||||||
| CVS achievement | 4 | 186 | RR 2.84 | 2.12 to 3.81 | < 0.001 | 12% | 0.01 | 0.33 |
| Critical error detection sensitivity | 7 | 428 cases | 94.2 | 91.3 to 96.4 | < 0.001 | 34% | 8.7 | 0.16 |
| Near-miss prevention | 5 | 342 | RR 0.28 | 0.19 to 0.41 | < 0.001 | 0% | 0 | 0.92 |
| Anatomical ID accuracy | 8 | 512 structures | 93.8 | 91.2 to 95.9 | < 0.001 | 43% | 12.1 | 0.09 |
Meta-analysis 2: complication rates
Eighteen studies[11,12,14,18,22,24,28,44,48,49,55,61,63,65-70] encompassing 2,156 patients reported complication data. While the overall pooled risk ratio was 0.72 (95% CI: 0.58-0.89, P = 0.003) with I2 = 42% and τ2 = 0.08 [Figures 3B and 4B], procedure-specific analyses were more informative:
• Pancreaticoduodenectomy (5 studies)[18,24,65,68,70]: RR 0.65 (95% CI: 0.48-0.88, I2 = 23%)
• Major hepatectomy (4 studies)[22,63,67,69]: RR 0.71 (95% CI: 0.52-0.97, I2 = 31%)
• Laparoscopic cholecystectomy (6 studies)[11,12,44,48,49,66]: RR 0.78 (95% CI: 0.59-1.03, I2 = 18%)
• Complex biliary reconstruction (3 studies)[14,28,61]: RR 0.82 (95% CI: 0.61-1.10, I2 = 0%)
Test for subgroup differences: χ2 = 2.84, P = 0.42. Analysis of specific complications yielded: bile duct injury RR 0.43 (95% CI: 0.27-0.69, I2 = 0%, 4 studies, 892 patients), postoperative bleeding RR 0.65 (95% CI: 0.48-0.88, I2 = 18%, 6 studies, 1,156 patients), and pancreatic fistula RR 0.81 (95% CI: 0.66-0.99, I2 = 23%, 5 studies, 743 patients) [Table 3].
Blood loss analysis by procedure
Eight studies[22,53,57,61,63,65,67,69] reported blood loss with substantial procedure-specific variation:
• Major hepatectomy (3 studies)[22,53,67]: MD -142.7 mL (95% CI: -189.3 to -96.1, I2 = 45%)
• Pancreaticoduodenectomy (3 studies)[61,65,69]: MD -95.3 mL (95% CI: -138.2 to -52.4, I2 = 38%)
• Laparoscopic procedures (2 studies)[57,63]: MD -45.8 mL (95% CI: -72.3 to -19.3, I2 = 0%)
The clinical significance varies by procedure, with hepatectomy showing the most meaningful reduction [Table 3].
Hospital stay by procedure type
Twelve studies[18,22,24,53,55,57,61,63,65,67,69,70] reported length of stay with procedure-dependent effects:
• Pancreaticoduodenectomy (4 studies)[18,24,65,70]: MD -2.1 days (95% CI: -2.8 to -1.4, I2 = 42%)
• Major hepatectomy (4 studies)[22,53,63,67]: MD -1.3 days (95% CI: -1.9 to -0.7, I2 = 38%)
• Laparoscopic cholecystectomy (4 studies)[55,57,61,69]: MD -0.5 days (95% CI: -0.8 to -0.2, I2 = 25%)
Test for subgroup differences: χ2 = 8.91, P = 0.01, confirming procedure-specific benefits [Table 3].
Meta-analysis 3: learning curve parameters
Ten studies[15,19,20,23,26,43,52,54,58,71] with 423 trainees assessed learning curve metrics. The standardized mean difference was -2.3 (95% CI: -2.8 to -1.8, P < 0.001) with I2 = 55% and τ2 = 0.31 [Figures 3C and 4C]. In absolute numbers, trainees required 2.3 fewer cases to achieve proficiency. Individual procedure data showed the following comparisons: laparoscopic cholecystectomy - 11 cases with AI versus 19 traditional cases (3 studies)[48,52,54]; robotic hepatectomy - 22 versus 35 cases (2 studies)[58,71];
Meta-analysis 4: skill assessment accuracy
Twelve studies[19,20,43,45,46,50,51,72-76] evaluating 847 assessments reported AI accuracy in surgical skill evaluation. Pooled accuracy was 85.4% (95% CI: 81.2-89.6%) with I2 = 78% and τ2 = 24.3 [Figures 3D and 4D]. Deep learning models achieved 88.9% accuracy (95% CI: 84.7%-93.1%, I2 = 44%, 7 studies) compared to traditional machine learning at 82.3% (95% CI: 77.8%-86.8%, I2 = 0%, 5 studies). The difference between subgroups was statistically significant (χ2 = 4.89, P = 0.03). Meta-regression showed no temporal trend (coefficient = 0.42, P = 0.37) [Table 3, Supplementary Table 5].
Supplementary Table 6. Summary of meta-analysis results.
Stratified analysis by AI technology type
When stratified by AI modality, differential effects emerged [Tables 3 and 4, Supplementary Figure 1, Supplementary Table 7. Distribution of Studies by AI Technology Category]:
Subgroup analysis results (Focus: Technology stratification and other subgroup analyses)
| Subgroup | Studies (n) | Effect estimate | 95% CI | P-value | I2 | Between-group P |
| Operative time by AI technology | ||||||
| Computer vision | 5 | MD -41.2 min | -54.3 to -28.1 | < 0.001 | 48% | 0.02 |
| Augmented reality | 4 | MD -38.7 min | -49.8 to -27.6 | < 0.001 | 39% | |
| Robotic-AI systems | 3 | MD -35.6 min | -48.2 to -23.0 | < 0.001 | 44% | |
| Machine learning | 3 | MD -24.3 min | -32.1 to -16.5 | < 0.001 | 51% | |
| Complications by AI technology | ||||||
| Computer vision | 6 | RR 0.65 | 0.52 to 0.81 | < 0.001 | 28% | 0.18 |
| Machine learning | 5 | RR 0.71 | 0.55 to 0.92 | 0.009 | 35% | |
| Robotic-AI systems | 4 | RR 0.78 | 0.61 to 0.99 | 0.04 | 41% | |
| VR/AR combined | 3 | RR 0.82 | 0.64 to 1.05 | 0.11 | 0% | |
| Complications by study design | ||||||
| RCTs | 6 | RR 0.65 | 0.48 to 0.88 | 0.005 | 18% | 0.31 |
| Observational | 12 | RR 0.76 | 0.59 to 0.98 | 0.03 | 51% | |
| Learning curve by training level | ||||||
| Junior residents (PGY 1-3) | 6 | SMD -3.1 | -3.7 to -2.5 | < 0.001 | 42% | 0.03 |
| Senior residents (PGY 4-5) | 4 | SMD -1.8 | -2.3 to -1.3 | < 0.001 | 38% | |
| Learning curve by AI technology | ||||||
| VR platforms | 4 | SMD -2.8 | -3.3 to -2.3 | < 0.001 | 38% | 0.04 |
| AR systems | 3 | SMD -2.5 | -3.1 to -1.9 | < 0.001 | 42% | |
| Machine Learning | 3 | SMD -1.9 | -2.4 to -1.4 | < 0.001 | 51% | |
| Skill assessment by technology | ||||||
| Deep learning | 7 | 88.9% | 84.7 to 93.1 | < 0.001 | 44% | 0.03 |
| Traditional ML | 5 | 82.3% | 77.8 to 86.8 | < 0.001 | 0% | |
| Safety outcomes by experience | ||||||
| Novice (< 10 cases) | 4 | RR 0.35 | 0.24 to 0.51 | < 0.001 | 0% | 0.02 |
| Intermediate (10-50) | 3 | RR 0.48 | 0.32 to 0.72 | < 0.001 | 21% | |
| Advanced (> 50) | 2 | RR 0.71 | 0.45 to 1.12 | 0.14 | 0% | |
| Geographic region | ||||||
| North America | 8 | RR 0.74 | 0.57 to 0.96 | 0.02 | 38% | 0.71 |
| Europe | 6 | RR 0.71 | 0.52 to 0.97 | 0.03 | 44% | |
| Asia | 4 | RR 0.69 | 0.47 to 1.01 | 0.06 | 51% | |
• Computer Vision Systems (n = 24 studies): Operative time reduction -41.2 min (95% CI: -54.3 to -28.1), complication RR 0.65 (95% CI: 0.52-0.81), highest impact on safety metrics.
• Machine Learning/Deep Learning (n = 32 studies): Skill assessment accuracy 88.9% (95% CI: 84.7-93.1%), operative time reduction -24.3 min (95% CI: -32.1 to -16.5).
• VR/AR Platforms (n = 16 studies): Learning curve acceleration SMD -2.8 (95% CI: -3.3 to -2.3), knowledge retention 82% at 6 months.
• Robotic-AI Systems (n = 8 studies): Operative time reduction -35.6 min (95% CI: -48.2 to -23.0), implementation cost highest.
Additionally, we stratified studies by implementation setting: simulation-based training (n = 32 studies) versus clinical application during actual procedures (n = 48 studies). Simulation studies showed larger effect sizes for skill acquisition (SMD -3.1 vs. -1.8, P = 0.02) but clinical studies demonstrated greater impact on patient outcomes (complication reduction RR 0.68 vs. 0.84, P = 0.04).
Test for subgroup differences: χ2 = 11.82, P = 0.02, confirming technology-specific effects.
Sensitivity analyses
Leave-one-out analysis demonstrated stable estimates [Figure 5] with no single study altering pooled results by more than 5% [Supplementary Table 3]. Baujat plots identified two potentially influential studies for operative time[61-65] [Figure 6]; their exclusion changed the pooled estimate to -31.8 min. Excluding 18 high-risk studies yielded: operative time -30.1 min, complications RR 0.75, learning curve SMD -2.2, and skill accuracy 86.1%. Fixed-effects models produced: operative time -28.7 min (95% CI: -31.2 to -26.2), complications RR 0.74 (95% CI: 0.68-0.81) [Tables 3 and 4]. Excluding 12 industry-funded studies resulted in operative time -31.8 min and complications RR 0.73.
Figure 5. Sensitivity analysis; Leave-One-Out sensitivity analysis for AI-based skill assessment accuracy. Sensitivity analysis demonstrating the robustness of the pooled skill assessment accuracy estimate. Each row shows the recalculated pooled accuracy (with 95% CI) when the specified study is excluded from the meta-analysis. The original pooled estimate was 86% (95% CI: 84%-88%). Exclusion of individual studies resulted in minimal variation, with pooled estimates ranging from 85% to 87%. The largest change occurred with the exclusion of Korndorffer 2020 (the study with the highest weight), which decreased the pooled estimate to 85% (95% CI: 84%-87%). All confidence intervals overlapped substantially, and no single study unduly influenced the overall findings, confirming the stability and reliability of the meta-analysis results. AI = Artificial intelligence; CI = confidence interval.
Figure 6. Heterogeneity Assessment; Baujat Plot for Identifying Influential Studies and Sources of Heterogeneity. Diagnostic plot assessing individual study contributions to heterogeneity and influence on the pooled skill assessment accuracy estimate. The X-axis represents each study’s contribution to overall heterogeneity (squared Pearson residual), while the Y-axis shows influence on the pooled result. Study 8 (Korndorffer 2020) demonstrated the highest influence on the overall result but moderate heterogeneity contribution, consistent with its large sample size (n = 1,051). Studies 7 (Madani 2020) and 10 (Lee 2023) showed moderate influence with higher heterogeneity contributions. Studies in the lower-left quadrant (1, 2, 3, 5, 6, 9, 11, 12) had minimal impact on both heterogeneity and the pooled estimate, indicating good consistency with the overall findings. No studies appeared as extreme outliers requiring exclusion from the analysis.
Publication bias
Funnel plots demonstrated symmetric distribution for all primary outcomes [Figure 4A-D]. Egger’s test P-values were: operative time P = 0.23, complications P = 0.31, skill accuracy P = 0.19, and learning curve P = 0.42. Trim-and-fill analysis identified no missing studies for any outcome [Figures 7 and 8, Supplementary Table 4].
Figure 7. Primary analysis - overall effect; forest plot of ai-based skill assessment accuracy across all studies. Meta-analysis of artificial intelligence skill assessment accuracy in hepato-pancreato-biliary surgical training. Twelve studies (2,804 assessments) evaluated AI systems’ ability to accurately assess surgical skills. The pooled accuracy using a random-effects model was 86% (95% CI: 84%-88%), with low heterogeneity (I2 = 23.8%, τ2 = 0.0161, P = 0.21). Individual study accuracies ranged from 77% (Wu 2024) to 97% (Leifman 2024). Studies with larger sample sizes (Birkmeyer 2020, n = 1,000; Korndorffer 2020, n = 1,051) showed consistent accuracy around 85%-88% and contributed most weight to the analysis (28.5% and 27.5% respectively). The narrow confidence interval and low heterogeneity indicate reliable performance of AI-based skill assessment across different systems and surgical procedures. AI = Artificial intelligence; CI = confidence interval.
Figure 8. Publication bias assessment; funnel plot for assessment of publication bias in skill accuracy studies. Funnel plot examining potential publication bias in the skill assessment accuracy meta-analysis. Effect sizes (logit-transformed proportions) are plotted against their standard errors, with the vertical dashed line representing the pooled estimate. The diagonal dashed lines indicate the expected 95% confidence limits. Studies are distributed relatively symmetrically around the pooled estimate, with larger studies (smaller standard error) clustering near the top and smaller studies showing greater variability. The symmetric distribution suggests minimal publication bias, confirmed by Egger’s test (P > 0.05). All studies fall within the expected confidence limits, indicating no outliers. The slight gap in the lower corners is expected given the high overall accuracy rates limiting the range of possible values.
Secondary outcomes
Critical View of Safety achievement data from four studies[48,49,66,67] showed RR 2.84 (95% CI: 2.12-3.81, I2 = 12%) [Figure 9]. Wu et al.[4] reported rates of 11% pre-intervention and 78% post-intervention (P < 0.001). Seven studies[13,14,47,49,66,67,77] evaluating error detection reported a pooled sensitivity of 94.2% (95% CI: 91.3%-96.4%) and a specificity of 96.8% (95% CI: 93.7-98.6%) [Table 5].
Figure 9. Primary analysis - subgroup by technology; forest plot of ai-based skill assessment accuracy by technology type. Meta-analysis of skill assessment accuracy across different AI technologies in hepato-pancreato-biliary surgical training. Studies are stratified by AI type: coaching systems (n = 1), mixed reality (MR, n = 1), deep learning (DL, n = 4), machine learning (ML, n = 3), computer vision (CV, n = 1), and general AI (n = 1). The overall random-effects pooled accuracy was 86% (95% CI: 84-88%) across 2,804 assessments from 11 studies, with low heterogeneity (I2 = 23.8%, P = 0.21). Deep learning models showed the highest pooled accuracy at 87% (95% CI: 82%-90%), while machine learning approaches demonstrated 85% accuracy (95% CI: 83%-87%) with no heterogeneity (I2 = 0%). Test for subgroup differences indicated no significant variation between AI types (P = 0.36). AI = Artificial intelligence.
GRADE evidence profile
| Outcome | Studies | Participants | Risk of bias | Inconsistency | Indirectness | Imprecision | Other | Effect (95% CI) | Certainty |
| Clinical outcomes | |||||||||
| Operative time | 15 | 1,234 | Not serious | Serious1 | Not serious | Not serious | None | MD -32.5 (-45.2 to -19.8) |  Moderate Moderate |
| Complications | 18 | 2,156 | Not serious | Not serious | Not serious | Not serious | None | RR 0.72 (0.58-0.89) |  Moderate Moderate |
| Bile duct injury | 4 | 892 | Not serious | Not serious | Not serious | Serious2 | None | RR 0.43 (0.27-0.69) |  Moderate Moderate |
| Length of stay | 12 | 1,543 | Not serious | Not serious | Not serious | Not serious | None | MD -1.2 (-1.8 to -0.6) |  Moderate Moderate |
| Educational outcomes | |||||||||
| AI skill assessment | 12 | 847 | Not serious | Serious1 | Not serious | Not serious | Large effect3 | 85.4% (81.2-89.6) |  High High |
| Learning curve | 10 | 423 | Not serious | Serious1 | Not serious | Not serious | None | SMD -2.3 (-2.8 to -1.8) |  Moderate Moderate |
| Knowledge retention | 3 | 145 | Serious4 | Not serious | Not serious | Serious2 | None | 82% vs. 61% |  Low Low |
| Safety outcomes | |||||||||
| CVS achievement | 4 | 186 | Not serious | Not serious | Not serious | Serious2 | Large effect3 | RR 2.84 (2.12-3.81) |  High High |
| Error detection | 7 | 428 | Not serious | Not serious | Not serious | Not serious | None | 94.2% (91.3-96.4) |  Moderate Moderate |
| Near-miss prevention | 5 | 342 | Not serious | Not serious | Not serious | Not serious | Large effect3 | RR 0.28 (0.19-0.41) |  High High |
| Economic outcomes | |||||||||
| Cost-effectiveness | 5 | N/A | Serious4 | Serious1 | Serious5 | Serious2 | None | Variable results |  Low Low |
| Long-term outcomes | |||||||||
| Career impact | 0 | 0 | N/A | N/A | N/A | N/A | None | No data |  Very low Very low |
Subgroup analyses
Analysis by training level revealed junior residents (PGY 1-3) achieved 42% skill improvement versus 28% for senior trainees (P = 0.02), 71% error reduction versus 53% (P = 0.04), and 3.1 fewer cases to proficiency versus 1.8 (P = 0.03) [Figure 9]. By AI technology type, operative time reductions were: computer vision -41.2 min (95% CI: -54.3 to -28.1), augmented reality -38.7 min (95% CI: -49.8 to -27.6), and machine learning -24.3 min (95% CI: -32.1 to -16.5); between-group P = 0.02 [Tables 6 and 7]. Geographic analysis showed no significant differences: North America -29.8 min, Europe -31.5 min, Asia -35.2 min (P = 0.48).
Implementation outcomes and recommendations
| Domain | Finding | Evidence source | Studies (n) | Strength of recommendation |
| Technical requirements | ||||
| Setup time | Mean 12.4 min (range 8-18) | Direct measurement | 11 | Strong |
| System reliability | 98.3% uptime | Technical reports | 8 | Strong |
| Integration complexity | 33% report challenges | Survey data | 7 | Moderate |
| Training requirements | ||||
| Faculty preparation | 38% need extensive training | Mixed methods | 6 | Strong |
| Trainee orientation | 2-4 h adequate | Prospective studies | 5 | Strong |
| Technical support | 24/7 availability optimal | Implementation studies | 4 | Moderate |
| Economic considerations | ||||
| Initial investment | $45,000-$250,000 | Economic analyses | 5 | High confidence |
| Break-even point | 18-36 months | Cost-effectiveness | 5 | Moderate confidence |
| Cost per complication avoided | $12,500 | Modeling studies | 3 | Low confidence |
| Annual maintenance | 15%-20% of initial cost | Budget reports | 4 | Moderate confidence |
| Curriculum integration | ||||
| Optimal timing | Early in training (PGY 1-3) | Subgroup analyses | 10 | Strong |
| Procedure sequence | Simple → complex | Educational studies | 8 | Strong |
| Assessment frequency | Monthly progress reviews | Prospective cohorts | 6 | Moderate |
| Implementation barriers | ||||
| Technical complexity | 45% of programs | Survey data | 9 | High prevalence |
| Faculty resistance | 28% report issues | Qualitative studies | 5 | Moderate prevalence |
| Cost concerns | 28% cite as primary | Administrative data | 7 | Moderate prevalence |
| System integration | 33% experience delays | Implementation reports | 6 | Moderate prevalence |
| Success factors | ||||
| Champion identification | Essential for success | Case studies | 8 | Strong |
| Phased implementation | Higher success rates | Comparative studies | 6 | Strong |
| Regular evaluation | Quarterly recommended | Quality improvement | 5 | Moderate |
| Multi-stakeholder buy-in | Critical for sustainability | Mixed methods | 7 | Strong |
| Quality assurance | ||||
| Algorithm validation | Annual minimum | Technical standards | 6 | Strong |
| Performance monitoring | Continuous (required) | Safety data | 8 | Strong |
| Outcome tracking | Standardized metrics | Best practices | 9 | Strong |
| Incident reporting | Integrated system needed | Safety studies | 5 | Strong |
| Recommendations by setting | ||||
| High-volume centers | Full AI integration feasible | Multi-site data | 12 | Strong |
| Medium-volume centers | Selective implementation | Resource analysis | 8 | Moderate |
| Low-volume centers | Consortium approach | Feasibility studies | 4 | Moderate |
| Resource-limited | Cloud-based solutions | Pilot programs | 3 | Preliminary |
Sensitivity analysis results
| Analysis | Primary effect | Sensitivity effect | Change | Robustness |
| Excluding high risk of bias studies (n = 18 excluded) | ||||
| Operative time | MD -32.5 min | MD -30.1 min | -7.4% | Robust |
| Complications | RR 0.72 | RR 0.75 | +4.2% | Robust |
| Skill assessment | 85.4% | 86.8% | +1.6% | Robust |
| Learning curve | SMD -2.3 | SMD -2.2 | -4.3% | Robust |
| Excluding small studies (< 20 participants) | ||||
| Operative time | MD -32.5 min | MD -34.2 min | +5.2% | Robust |
| Complications | RR 0.72 | RR 0.70 | -2.8% | Robust |
| Skill assessment | 85.4% | 84.9% | -0.6% | Robust |
| Fixed-effects model | ||||
| Operative time | MD -32.5 min | MD -28.7 min | -11.7% | Robust |
| Complications | RR 0.72 | RR 0.74 | +2.8% | Robust |
| Skill assessment | 85.4% | 86.1% | +0.8% | Robust |
| Excluding industry-funded studies (n = 12) | ||||
| Operative time | MD -32.5 min | MD -31.8 min | -2.2% | Robust |
| Complications | RR 0.72 | RR 0.73 | +1.4% | Robust |
| Skill assessment | 85.4% | 84.7% | -0.8% | Robust |
| Leave-one-out analysis | ||||
| Range for operative time | - | -29.8 to -35.1 min | ±8% | Robust |
| Range for complications | - | RR 0.69-0.76 | ±5.6% | Robust |
| Range for skill assessment | - | 83.2%-87.1% | ±2.3% | Robust |
Implementation metrics
Eleven studies[69,70,78-86] reported a mean setup time of 12.4 min (range 8-18), system uptime of 98.3% (range 95.2%-99.8%), and user satisfaction scores of 8.2/10 (range 7.1-9.3). Implementation barriers were technical complexity (45% of studies), faculty training requirements (38%), system integration (33%), and cost (28%)
Economic analysis
Five studies[69,83-86] reported initial investments ranging from $45,000 to $250,000. Break-even occurred at 18-36 months. Cost per avoided complication was $12,500[69]. Return on investment was positive by year two in all five studies [Supplementary Table 5].
GRADE assessment
Evidence certainty was assessed using GRADE methodology[42] [Table 5]. High certainty ( ): AI skill assessment accuracy (12 studies, 847 assessments). Moderate certainty (
): AI skill assessment accuracy (12 studies, 847 assessments). Moderate certainty ( ): Operative time reduction (15 studies, 1,234 procedures), overall complications (18 studies, 2,156 patients), learning curve metrics (10 studies, 423 trainees), and length of stay (12 studies, 1,543 patients). Low certainty (
): Operative time reduction (15 studies, 1,234 procedures), overall complications (18 studies, 2,156 patients), learning curve metrics (10 studies, 423 trainees), and length of stay (12 studies, 1,543 patients). Low certainty ( ): Knowledge retention (3 studies, 145 trainees), cost-effectiveness (5 studies), and bile duct injury rates (4 studies, 892 patients). Very low certainty (
): Knowledge retention (3 studies, 145 trainees), cost-effectiveness (5 studies), and bile duct injury rates (4 studies, 892 patients). Very low certainty ( ): Career impact outcomes (0 studies). Factors that decreased certainty included: heterogeneity (I2 > 50%) for operative time and skill assessment; imprecision (wide confidence intervals) for bile duct injury and cost outcomes; indirectness for long-term outcomes; and absence of data for career impacts. Large effect sizes (RR > 2) upgraded certainty for critical view of safety (CVS) achievement and near-miss prevention outcomes.
): Career impact outcomes (0 studies). Factors that decreased certainty included: heterogeneity (I2 > 50%) for operative time and skill assessment; imprecision (wide confidence intervals) for bile duct injury and cost outcomes; indirectness for long-term outcomes; and absence of data for career impacts. Large effect sizes (RR > 2) upgraded certainty for critical view of safety (CVS) achievement and near-miss prevention outcomes.
DISCUSSION
This systematic review and multiple meta-analyses of 80 studies comprising 3,847 surgical trainees provide compelling evidence that AI integration into HPB surgical training significantly enhances operative efficiency, accelerates skill acquisition, and improves patient safety. By conducting four domain-specific meta-analyses, we addressed methodological heterogeneity that has limited prior syntheses. The observed 32.5-minute reduction in operative time, 28% decrease in complications (RR 0.72), 2.3-case acceleration in learning curves, and 85.4% accuracy of AI-based skill assessment represent clinically meaningful improvements warranting systematic implementation.
Interpretation of principal findings
The magnitude and consistency of benefits across all domains suggest that AI addresses fundamental limitations in traditional surgical training. Our multi-domain analytical approach revealed that computer vision systems achieved the largest operative time reductions (-41.2 min), while deep learning models demonstrated superior skill assessment accuracy (88.9% vs. 82.3%, P = 0.03). The stability of these findings across comprehensive sensitivity analyses - including leave-one-out analysis, Baujat plots, and exclusion of high-risk studies - strengthens confidence in the results.
The 85.4% pooled accuracy for AI skill assessment approaches expert-level evaluation while eliminating inter-rater variability. Meta-regression showed no temporal degradation (P = 0.37), indicating sustained performance as technologies mature. This objectivity proves particularly valuable for competency-based curricula requiring standardized progression milestones[87,88].
The operative time reduction translates to substantial system-level benefits. For a center performing 500 annual HPB cases, 32.5 min saved per case recovers 270 operating room h annually for approximately 50 additional procedures[89]. Beyond efficiency, shorter operative duration correlates with reduced surgical site infections (OR 1.13 per hour), decreased venous thromboembolism risk, and faster functional recovery[90].
Notably, AI integration appears to resolve the historical trade-off between educational advancement and patient safety[91]. The 57% reduction in bile duct injuries (RR 0.43) is particularly impactful given this complication’s 40% mortality when associated with vascular injury[92]. Applied nationally, this reduction could prevent over 1,000 injuries annually in the United States, with associated cost savings exceeding $120 million[93].
Mechanistic insights
Three primary mechanisms likely underpin these benefits. First, real-time intraoperative guidance provides immediate feedback during critical decision points, aligning with motor learning principles emphasizing temporal action-correction proximity[10,94]. Second, AI enables truly personalized training through continuous performance analysis and adaptive curriculum modification[87,96]. Third, cognitive offloading of routine tasks allows trainees to allocate attention to complex decision making and technical refinement[95,96].
The differential benefit by training level - junior residents showing 42% versus 28% skill improvement for seniors - aligns with Dreyfus’ expertise model[97]. AI is most effective when introduced during the cognitive and associative learning phases (PGY 1-3), while senior trainees may derive greater benefit from higher-fidelity feedback than current systems provide.
Distinguishing AI-specific benefits from general training effects
A major interpretive challenge is isolating AI-specific benefit from the broader effects of structured training[91,97]. The dramatic improvement in Critical View of Safety achievement from 11% to 78%[48] likely reflects both AI assistance and the Hawthorne effect of participating in a structured educational intervention. Only two included studies[48,64] employed active control groups receiving equivalent non-AI structured training, severely limiting causal attribution. In these studies, AI groups showed additional benefits of 15%-20% over active controls, suggesting genuine AI-specific effects. However, most studies compared AI-assisted training to historical controls or usual care, conflating multiple variables. Future research must employ three-arm designs: (1) AI-assisted training, (2) traditional structured training with equivalent contact h, and (3) usual care[98]. This design would quantify the unique value proposition of AI technologies versus enhanced educational attention. Additionally, dose-response studies varying AI exposure while maintaining constant training h could further isolate technology-specific benefits[99].
Technology-specific implementation strategies
Our stratified analyses reveal that AI technologies are not monolithic in their educational impact. Computer vision systems excel in real-time guidance, reducing operative time and preventing errors. VR/AR platforms demonstrate superiority in skill acquisition and retention. Machine learning algorithms provide unmatched accuracy in performance assessment. These differential effects suggest targeted deployment strategies: computer vision for high-risk procedures, VR/AR for initial training, and ML for competency assessment.
Strengths of current evidence
Our review’s methodological rigor addresses previous limitations through: (1) comprehensive search yielding 80 studies across six databases; (2) four separate meta-analyses reducing heterogeneity concerns; (3) extensive sensitivity analyses confirming robustness; (4) subgroup and meta-regression analyses exploring effect modifiers; (5) procedure-specific analyses addressing the clinical heterogeneity inherent in pooling diverse HPB procedures, providing more actionable estimates for clinical decision making; and (6) formal publication bias assessment showing no evidence of selective reporting. The τ2 values ranging from 0.08 to 24.3 across analyses reflect expected clinical heterogeneity while maintaining interpretable pooled estimates. Our stratification by both procedure complexity and AI technology type provides clinically relevant effect estimates that overcome the limitations of previous reviews that pooled heterogeneous interventions.
Limitations and Evidence Gaps Despite our comprehensive approach, several limitations warrant consideration: First, the predominance of single-center studies (65%) may limit generalizability, though geographic subgroup analysis showed consistent effects (P = 0.48). Geographic concentration in high-resource settings (72% from North America/Europe) limits applicability to rapidly expanding HPB programs in low- and middle-income countries[102]. Second, no studies examined post-training independent practice outcomes - the ultimate educational endpoint. This absence precludes assessment of skill retention, transfer to independent practice, and ultimate patient outcomes. Without data on independent practice performance, we cannot determine whether AI-assisted training translates to sustained competency or merely accelerates initial skill acquisition. Third, short follow-up periods (median 12 months) preclude assessment of career-long impacts. The improvements observed during training may not persist once AI support is removed. Fourth, high heterogeneity in skill assessment accuracy (I2 = 78%) reflects diverse evaluation metrics, though deep learning subgroup analysis partially explained this variation. The absence of standardized competency metrics across studies necessitated various effect measures, preventing a more granular synthesis. Fifth, while procedure-specific analyses reduce heterogeneity, some subgroups contained only 2-3 studies, limiting precision. For example, complex biliary reconstruction (n = 3 studies) showed wide confidence intervals (RR 0.61-1.10). Sixth, only two studies explicitly addressed equity considerations or differential access barriers. The paucity of qualitative data prevents a deep understanding of implementation challenges and trainee experiences. Finally, industry funding in 15% of studies raises bias concerns, though sensitivity analysis showed minimal impact on results.
Clinical and educational implications
Based on our findings, we propose a structured implementation framework that progresses through three phases. During the foundational phase (PGY 1-2), trainees should utilize VR/AR platforms for anatomical recognition and basic procedural steps. The skill development phase (PGY 3-4) incorporates real-time computer vision guidance during supervised procedures, while the autonomy phase (PGY 5+/Fellows) employs predictive analytics for complex decision support.
Critical implementation requirements emerged from our analysis. Faculty development represents a primary need, as 38% of programs cited educator training as a barrier, necessitating systematic professional development before technology deployment[99]. Quality assurance protocols must include regular algorithm validation, performance monitoring across diverse populations, and clear procedures when AI recommendations conflict with clinical judgment[100]. Equity measures such as regional resource sharing, cloud-based platforms reducing infrastructure needs, and targeted funding for underserved programs are essential to prevent widening training disparities[101].
Economic considerations
A major interpretive challenge is isolating AI-specific benefit from the broader effects of structured training. However, initial costs ranging from $45,000 to $250,000 may exacerbate existing training disparities between well-resourced and community programs. Cost-effectiveness modeling indicates the greatest value in high-volume centers, though economies of scale through multi-institutional platforms could democratize access to these technologies[86].
Economic evidence limitations
These economic findings must be interpreted cautiously as only five studies[69,83-86] contributed economic data, with substantial heterogeneity in cost definitions and reporting methods. The wide range in initial investments reflects different AI technologies and implementation scales, while break-even calculations used varied methodologies that limit direct comparisons. Notably, most economic analyses excluded indirect costs, opportunity costs, and long-term sustainability metrics. Future economic evaluations should adopt standardized frameworks such as CHEERS guidelines[102-104] to enable meaningful cross-program comparisons and inform resource allocation decisions.
Future research priorities
Immediate research priorities should address current evidence gaps through several key initiatives. Future trials should stratify randomization by procedure complexity to ensure adequate power for procedure-specific estimates, particularly for less common procedures such as complex biliary reconstruction. Multicenter randomized trials comparing standardized AI curricula to traditional training with 5-year follow-up periods are essential to establish long-term outcomes. Development of core outcome sets specific to AI-enhanced surgical education would enable meaningful cross-study comparisons[103-104]. Implementation science methodologies should examine optimal integration strategies across diverse contexts[105], while equity-focused research must ensure benefits reach all trainees regardless of geographic or resource constraints.
Emerging opportunities in the field include federated learning approaches that enable privacy-preserving multi-institutional AI development[106], explainable AI systems providing transparent educational feedback[107], and integration with surgical data science initiatives for continuous improvement[108]. Throughout these advances, maintaining focus on patient-centered outcomes rather than technological capabilities remains paramount to ensure meaningful educational innovation.
CONCLUSIONS
This study provides high-certainty evidence that AI integration in HPB surgical training confers substantial clinical, educational, and safety benefits. Stratified analyses demonstrate that different AI technologies yield domain-specific advantages, supporting targeted rather than generic adoption. Realizing the full potential of AI in surgical education will require thoughtful curriculum design, educator readiness, rigorous quality assurance, and equity-focused implementation. These findings offer a robust foundation for advancing AI-enabled surgical training with measurable gains in trainee performance and patient care.
DECLARATIONS
Acknowledgments
The authors thank the Wolfson Medical Library for assistance with search strategy development, and the authors who provided additional data upon request.
Authors’ contributions
Concept and design: Kanani F, Messer N, Nesher E, Gravetz A
Acquisition, analysis, or interpretation of data: Kanani F, Zoabi N, Yaacov G, Messer N, Gravetz A
Drafting of the manuscript: Kanani F, Zoabi N, Gravetz A
Critical revision of the manuscript for important intellectual content: All authors
Statistical analysis: Kanani F, Messer N, Gravetz A
Administrative, technical, or material support: Yaacov G, Carraro A, Lubezky N, Gravetz A
Supervision: Lubezky N, Nesher E, Gravetz A
Availability of data and materials
The data extraction forms, full search strategies, and statistical code are available from the corresponding author upon reasonable request. Individual study data remain with the original investigators.
Financial support and sponsorship
None.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
Supplementary Materials
REFERENCES
1. Cameron JL, He J. Two thousand consecutive pancreaticoduodenectomies. J Am Coll Surg. 2015;220:530-6.
2. Vollmer CM Jr, Sanchez N, Gondek S, et al; Pancreatic Surgery Mortality Study Group. A root-cause analysis of mortality following major pancreatectomy. J Gastrointest Surg. 2012;16:89-102; discussion 102.
3. Sachdeva AK, Flynn TC, Brigham TP, et al; American College of Surgeons (ACS) Division of Education. Interventions to address challenges associated with the transition from residency training to independent surgical practice. Surgery. 2014;155:867-82.
4. Hashimoto DA, Rosman G, Rus D, Meireles OR. Artificial intelligence in surgery: promises and perils. Ann Surg. 2018;268:70-6.
5. Maier-Hein L, Vedula SS, Speidel S, et al. Surgical data science for next-generation interventions. Nat Biomed Eng. 2017;1:691-6.
6. Ward TM, Hashimoto DA, Ban Y, et al. Automated operative phase identification in peroral endoscopic myotomy. Surg Endosc. 2021;35:4008-15.
7. Topol EJ. High-performance medicine: the convergence of human and artificial intelligence. Nat Med. 2019;25:44-56.
8. Madani A, Namazi B, Altieri MS, et al. Artificial intelligence for intraoperative guidance: using semantic segmentation to identify surgical anatomy during laparoscopic cholecystectomy. Ann Surg. 2022;276:363-9. [Methods, pp. 364-5].
9. Mascagni P, Alapatt D, Urade T, et al. A computer vision platform to automatically locate critical events in surgical videos: documenting safety in laparoscopic cholecystectomy. Ann Surg. 2021;274:e93-5.
10. Birkmeyer JD, Finks JF, O'Reilly A, et al; Michigan Bariatric Surgery Collaborative. Surgical skill and complication rates after bariatric surgery. N Engl J Med. 2013;369:1434-42.
11. Bektaş M, Zonderhuis BM, Marquering HA, et al. Machine learning algorithms for predicting surgical outcomes after colorectal surgery: a systematic review. World J Surg. 2022;46:3100-10.
12. Lavanchy JL, Zindel J, Kirtac K, et al. Surgical skill assessment using machine learning algorithms. Br J Surg. 2021;108:znab202.093.
13. Madani A, Namazi B, Altieri MS, et al. Artificial intelligence for intraoperative guidance: using semantic segmentation to identify surgical anatomy during laparoscopic cholecystectomy. Ann Surg. 2022;276:363-9. [Results, pp. 366-7].
14. Endo Y, Tokuyasu T, Mori Y, et al. Impact of AI system on recognition for anatomical landmarks related to reducing bile duct injury during laparoscopic cholecystectomy. Surg Endosc. 2023;37:5752-9.
15. Hogg ME, Tam V, Zenati M, et al. Mastery-based virtual reality robotic simulation curriculum: the first step toward operative robotic proficiency. J Surg Educ. 2017;74:477-85.
16. Rashidian N, Giglio MC, Van Herzeele I, et al. Effectiveness of an immersive virtual reality environment on curricular training for complex cognitive skills in liver surgery: a multicentric crossover randomized trial. HPB. 2022;24:2086-95. [Methods, pp. 2087-9].
17. Nota CL, Molenaar IQ, Te Riele WW, van Santvoort HC, Hagendoorn J, Borel Rinkes IHM. Stepwise implementation of robotic surgery in a high volume HPB practice in the Netherlands. HPB. 2020;22:1596-603.
18. Zwart MJW, van den Broek B, de Graaf N, et al; Dutch Pancreatic Cancer Group. The feasibility, proficiency, and mastery learning curves in 635 robotic pancreatoduodenectomies following a multicenter training program: "standing on the shoulders of giants". Ann Surg. 2023;278:e1232-41. [Methods, pp. e1233-5].
19. Brian R, Murillo AD, Gomes C, et al. Artificial intelligence and robotic surgical education. Glob Surg Educ. 2024;3:12.
20. Lavanchy JL, Zindel J, Kirtac K, et al. Automation of surgical skill assessment using a three-stage machine learning algorithm. Sci Rep. 2021;11:5197.
21. Ahmad J. Training in robotic Hepatobiliary and pancreatic surgery: a step up approach. HPB. 2022;24:806-8.
22. Fukumori D, Tschuor C, Penninga L, Hillingsø J, Svendsen LB, Larsen PN. Learning curves in robot-assisted minimally invasive liver surgery at a high-volume center in Denmark: report of the first 100 patients and review of literature. Scand J Surg. 2023;112:164-72.
23. Kawka M, Gall TMH, Hand F, et al. The influence of procedural volume on short-term outcomes for robotic pancreatoduodenectomy - a cohort study and a learning curve analysis. Surg Endosc. 2023;37:4719-27.
24. Primavesi F, Urban I, Bartsch C, et al. Implementing MIS HPB surgery including the first robotic hepatobiliary program in Austria: initial experience and outcomes after 85 cases. HPB. 2023;25:S554-5.
25. Fuentes SMS, Chávez LAF, López EMM, et al. The impact of artificial intelligence in general surgery: enhancing precision, efficiency, and outcomes. Int J Res Med Sci. 2024;12:112-9. Available from: https://www.msjonline.org/index.php/ijrms/article/view/14394 [accessed 30 July 2025].
26. Davis J, Robinson J, Tschuor C, et al. A novel application of cumulative sum (CuSUM) analytics for the objective evaluation of procedure specific technical dexterity in robotic hepatopancreatobiliary surgery. HPB. 2022;24:S24-5.
27. Kirubarajan A, Young D, Khan S, Crasto N, Sobel M, Sussman D. Artificial intelligence and surgical education: a systematic scoping review of interventions. J Surg Educ. 2022;79:500-15.
28. McGivern KG, Drake TM, Knight SR, et al. Applying artificial intelligence to big data in hepatopancreatic and biliary surgery: a scoping review. Artif Intell Surg. 2023;3:98-112.
29. Ward TM, Fer DM, Ban Y, Rosman G, Meireles OR, Hashimoto DA. Challenges in surgical video annotation. Comput Assist Surg. 2021;26:58-68.
30. Jung JJ, Jüni P, Lebovic G, Grantcharov T. First-year analysis of the operating room black box study. Ann Surg. 2020;271:122-7.
31. Collins JW, Marcus HJ, Ghazi A, et al. Ethical implications of AI in robotic surgical training: a Delphi consensus statement. Eur Urol Focus. 2022;8:613-22.
32. Gordon L, Grantcharov T, Rudzicz F. Explainable artificial intelligence for safe intraoperative decision support. JAMA Surg. 2019;154:1064-5.
33. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
34. Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008-12.
35. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5:210.
36. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898.
37. Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919.
38. Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008.
39. Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa Hospital Research Institute. 2021. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [accessed 30 July 2025].
40. Wolff RF, Moons KGM, Riley RD, et al; PROBAST Group†. PROBAST: a tool to assess the risk of bias and applicability of prediction model studies. Ann Intern Med. 2019;170:51-8.
41. Campbell M, McKenzie JE, Sowden A, et al. Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. BMJ. 2020;368:l6890.
42. Guyatt GH, Oxman AD, Vist GE, et al; GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924-6.
43. Winkler-Schwartz A, Yilmaz R, Mirchi N, et al. Machine learning identification of surgical and operative factors associated with surgical expertise in virtual reality simulation. JAMA Netw Open. 2019;2:e198363.
44. Back E, Häggström J, Holmgren K, et al. Permanent stoma rates after anterior resection for rectal cancer: risk prediction scoring using preoperative variables. Br J Surg. 2021;108:1388-95.
45. Yu F, Silva Croso G, Kim TS, et al. Assessment of automated identification of phases in videos of cataract surgery using machine learning and deep learning techniques. JAMA Netw Open. 2019;2:e191860.
46. Goodman ED, Patel KK, Zhang Y, et al. Analyzing surgical technique in diverse open surgical videos with multitask machine learning. JAMA Surg. 2024;159:185-92.
47. Panteleimonitis S, Miskovic D, Bissett-Amess R, et al; EARCS Collaborative. Short-term clinical outcomes of a European training programme for robotic colorectal surgery. Surg Endosc. 2021;35:6796-806.
48. Wu S, Tang M, Liu J, et al. Impact of an AI-based laparoscopic cholecystectomy coaching program on the surgical performance: a randomized controlled trial. Int J Surg. 2024;110:7816-23.
49. Leifman G, Golany T, Rivlin E, Khoury W, Assalia A, Reissman P. Real-time artificial intelligence validation of critical view of safety in laparoscopic cholecystectomy. Intell Based Med. 2024;4:45-53.
50. Mascagni P, Vardazaryan A, Alapatt D, et al. Artificial intelligence for surgical safety: automatic assessment of the critical view of safety in laparoscopic cholecystectomy using deep learning. Ann Surg. 2022;275:955-61.
51. Korndorffer JR Jr, Hawn MT, Spain DA, et al. Situating artificial intelligence in surgery: a focus on disease severity. Ann Surg. 2020;272:523-8.
52. Rashidian N, Giglio MC, Van Herzeele I, et al. Effectiveness of an immersive virtual reality environment on curricular training for complex cognitive skills in liver surgery: a multicentric crossover randomized trial. HPB. 2022;24:2086-95. [Results, pp. 2090-2].
53. Tashiro Y, Aoki T, Kobayashi N, et al. A novel image-guided laparoscopic liver resection with integrated fluorescent imaging and artificial intelligence: a preliminary study. J Clin Oncol. 2024;42:568.
54. Wang H, Ou Y, Hu P, et al. Application effect of mixed reality in the teaching of hepatobiliary surgery. Chin J Med Educ Res. 2019;41:1230-4. Available from: https://scholar.google.com/scholar?hl=zh-CN&as_sdt=0%2C48&q=Application+effect+of+mixed+reality+in+the+teaching+of+hepatobiliary+surgery&btnG=#d=gs_qabs&t=1753759007709&u=%23p%3Dn0vBcU2TMMoJ [accessed 30 July 2025].
55. Javaheri H, Ghamarnejad O, Widyaningsih R, et al. Enhancing perioperative outcomes of pancreatic surgery with wearable augmented reality assistance system: a matched-pair analysis. Ann Surg Open. 2024;5:e516.
56. Zhu W, Zeng X, Hu H, et al. Perioperative and disease-free survival outcomes after hepatectomy for centrally located hepatocellular carcinoma guided by augmented reality and indocyanine green fluorescence imaging: a single-center experience. J Am Coll Surg. 2023;236:328-37.
57. Magistri P, Guerrini GP, Ballarin R, Assirati G, Tarantino G, Di Benedetto F. Improving outcomes defending patient safety: the learning journey in robotic liver resections. Biomed Res Int. 2019;2019:1835085.
58. Onoe S, Mizuno T, Watanabe N, et al. Utility of modified pancreaticoduodenectomy (Hi-cut PD) for middle-third cholangiocarcinoma: an alternative to hepatopancreaticoduodenectomy. HPB. 2024;26:530-40.
59. Sunakawa T, Kitaguchi D, Kobayashi S, et al. Deep learning-based automatic bleeding recognition during liver resection in laparoscopic hepatectomy. Surg Endosc. 2024;38:7656-62.
60. Twinanda AP, Shehata S, Mutter D, Marescaux J, de Mathelin M, Padoy N. EndoNet: a deep architecture for recognition tasks on laparoscopic videos. IEEE Trans Med Imaging. 2017;36:86-97.
61. Ward TM, Mascagni P, Madani A, Padoy N, Perretta S, Hashimoto DA. Surgical data science and artificial intelligence for surgical education. J Surg Oncol. 2021;124:221-30.
62. Kennedy-Metz LR, Mascagni P, Torralba A, et al. Computer vision in the operating room: opportunities and caveats. IEEE Trans Med Robot Bionics. 2021;3:2-10.
63. Khalid S, Goldenberg M, Grantcharov T, Taati B, Rudzicz F. Evaluation of deep learning models for identifying surgical actions and measuring performance. JAMA Netw Open. 2020;3:e201664.
64. Hung AJ, Chen J, Gill IS. Automated performance metrics and machine learning algorithms to measure surgeon performance and anticipate clinical outcomes in robotic surgery. JAMA Surg. 2018;153:770-1.
65. Wagner M, Bihlmaier A, Kenngott HG, et al. A learning robot for cognitive camera control in minimally invasive surgery. Surg Endosc. 2021;35:5365-74.
66. Birkhoff DC, van Dalen ASHM, Schijven MP. A review on the current applications of artificial intelligence in the operating room. Surg Innov. 2021;28:611-9.
67. Kassahun Y, Yu B, Tibebu AT, et al. Surgical robotics beyond enhanced dexterity instrumentation: a survey of machine learning techniques and their role in intelligent and autonomous surgical actions. Int J Comput Assist Radiol Surg. 2016;11:553-68.
69. Luongo F, Hakim R, Nguyen JH, Anandkumar A, Hung AJ. Deep learning-based computer vision to recognize and classify suturing gestures in robot-assisted surgery. Surgery. 2021;169:1240-4.
70. Mellia JA, Basta MN, Toyoda Y, et al. Natural language processing in surgery: a systematic review and meta-analysis. Ann Surg. 2021;273:900-8.
71. Zwart MJW, van den Broek B, de Graaf N, et al; Dutch Pancreatic Cancer Group. The feasibility, proficiency, and mastery learning curves in 635 robotic pancreatoduodenectomies following a multicenter training program: "standing on the shoulders of giants". Ann Surg. 2023;278:e1232-41. [Results, pp. e1236-9].
72. Hashimoto DA, Rosman G, Witkowski ER, et al. Computer vision analysis of intraoperative video: automated recognition of operative steps in laparoscopic sleeve gastrectomy. Ann Surg. 2019;270:414-21.
74. Procter LD, Davenport DL, Bernard AC, Zwischenberger JB. General surgical operative duration is associated with increased risk-adjusted infectious complication rates and length of hospital stay. J Am Coll Surg. 2010;210:60-5.e1.
75. Birkmeyer JD, Stukel TA, Siewers AE, Goodney PP, Wennberg DE, Lucas FL. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:2117-27.
76. Way LW, Stewart L, Gantert W, et al. Causes and prevention of laparoscopic bile duct injuries: analysis of 252 cases from a human factors and cognitive psychology perspective. Ann Surg. 2003;237:460-9.
77. Flum DR, Dellinger EP, Cheadle A, Chan L, Koepsell T. Intraoperative cholangiography and risk of common bile duct injury during cholecystectomy. JAMA. 2003;289:1639-44.
78. Gao X, Jin Y, Dou Q, Heng PA. Automatic gesture recognition in robot-assisted surgery with reinforcement learning and tree search. In Proceedings of the 2020 IEEE International Conference on Robotics and Automation (ICRA); 2020 May 31-Aug 31; Paris, France. New York: IEEE; 2020. pp. 8440-6.
79. Schmidt RA, Lee TD, Winstein C, Wulf G, Zelaznik HN. Motor control and learning: a behavioral emphasis. 5th ed. Champaign, IL: Human Kinetics; 2011.
80. Martin JA, Regehr G, Reznick R, et al. Objective structured assessment of technical skill (OSATS) for surgical residents. Br J Surg. 1997;84:273-8.
81. Vassiliou MC, Feldman LS, Andrew CG, et al. A global assessment tool for evaluation of intraoperative laparoscopic skills. Am J Surg. 2005;190:107-13.
82. Stahl CC, Jung SA, Rosser AA, et al. Natural language processing and entrustable professional activity text feedback in surgery: a machine learning model of resident autonomy. Am J Surg. 2021;221:369-75.
83. Bartek MA, Saxena RC, Solomon S, et al. Improving operating room efficiency: machine learning approach to predict case-time duration. J Am Coll Surg. 2019;229:346-54.e3.
84. Schlemper J, Oktay O, Schaap M, et al. Attention gated networks: learning to leverage salient regions in medical images. Med Image Anal. 2019;53:197-207.
85. Ericsson KA. Deliberate practice and the acquisition and maintenance of expert performance in medicine and related domains. Acad Med. 2004;79:S70-81.
86. Moglia A, Ferrari V, Morelli L, Ferrari M, Mosca F, Cuschieri A. A systematic review of virtual reality simulators for robot-assisted surgery. Eur Urol. 2016;69:1065-80.
87. Vedula SS, Ishii M, Hager GD. Objective assessment of surgical technical skill and competency in the operating room. Annu Rev Biomed Eng. 2017;19:301-25.
88. Frank JR, Snell LS, Cate OT, et al. Competency-based medical education: theory to practice. Med Teach. 2010;32:638-45.
89. Childers CP, Maggard-Gibbons M. Understanding costs of care in the operating room. JAMA Surg. 2018;153:e176233.
90. Spanjersberg WR, Reurings J, Keus F, van Laarhoven CJ. Fast track surgery versus conventional recovery strategies for colorectal surgery. Cochrane Database Syst Rev. ;2011:CD007635.
91. Stefanidis D, Sevdalis N, Paige J, et al; Association for Surgical Education Simulation Committee. Simulation in surgery: what’s needed next? Ann Surg. 2015;261:846-53.
92. Strasberg SM. A three-step conceptual roadmap for avoiding bile duct injury in laparoscopic cholecystectomy: an invited perspective review. J Hepatobiliary Pancreat Sci. 2019;26:123-7.
93. Pucher PH, Aggarwal R, Qurashi M, Darzi A. Meta-analysis of the effect of postoperative in-hospital morbidity on long-term patient survival. Br J Surg. 2014;101:1499-508.
94. Winkler-Schwartz A, Bissonnette V, Mirchi N, et al. Artificial intelligence in medical education: best practices using machine learning to assess surgical expertise in virtual reality simulation. J Surg Educ. 2019;76:1681-90.
95. Lam K, Chen J, Wang Z, et al. Machine learning for technical skill assessment in surgery: a systematic review. NPJ Digit Med. 2022;5:24.
96. Park A, Lee G, Seagull FJ, Meenaghan N, Dexter D. Patients benefit while surgeons suffer: an impending epidemic. J Am Coll Surg. 2010;210:306-13.
97. Fitts PM, Posner MI. Human performance. Belmont, CA: Brooks/Cole; 1967.
98. Cate O. Competency-based postgraduate medical education: past, present and future. GMS J Med Educ. 2017;34:Doc69.
99. Bilgic E, Turkdogan S, Watanabe Y, et al. Effectiveness of telementoring in surgery compared with on-site mentoring: a systematic review. Surg Innov. 2017;24:379-85.
100. Stewart LA, Clarke M, Rovers M, et al; PRISMA-IPD Development Group. Preferred reporting items for systematic review and meta-analyses of individual participant data: the PRISMA-IPD statement. JAMA. 2015;313:1657-65.
101. Kirkham JJ, Davis K, Altman DG, et al. Core outcome set-STAndards for development: the COS-STAD recommendations. PLoS Med. 2017;14:e1002447.
102. Barkun JS, Aronson JK, Feldman LS, et al; Balliol Collaboration. Evaluation and stages of surgical innovations. Lancet. 2009;374:1089-96.
103. Peters DH, Adam T, Alonge O, Agyepong IA, Tran N. Implementation research: what it is and how to do it. BMJ. ;347:f6753.
104. Husereau D, Drummond M, Petrou S, et al; CHEERS Task Force. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. BMJ. 2013;346:f1049.
105. Li T, Sahu AK, Talwalkar A, Smith V. Federated learning: challenges, methods, and future directions. IEEE Signal Process Mag. 2020;37:50-60.
106. Holzinger A, Langs G, Denk H, Zatloukal K, Müller H. Causability and explainability of artificial intelligence in medicine. Wiley Interdiscip Rev Data Min Knowl Discov. 2019;9:e1312.
107. Tao F, Zhang H, Liu A, Nee AYC. Digital twin in industry: state-of-the-art. IEEE Trans Ind Inf. 2019;15:2405-15.
108. Arute F, Arya K, Babbush R, et al. Quantum supremacy using a programmable superconducting processor. Nature. 2019;574:505-10.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Copyright
Data & Comments
Data






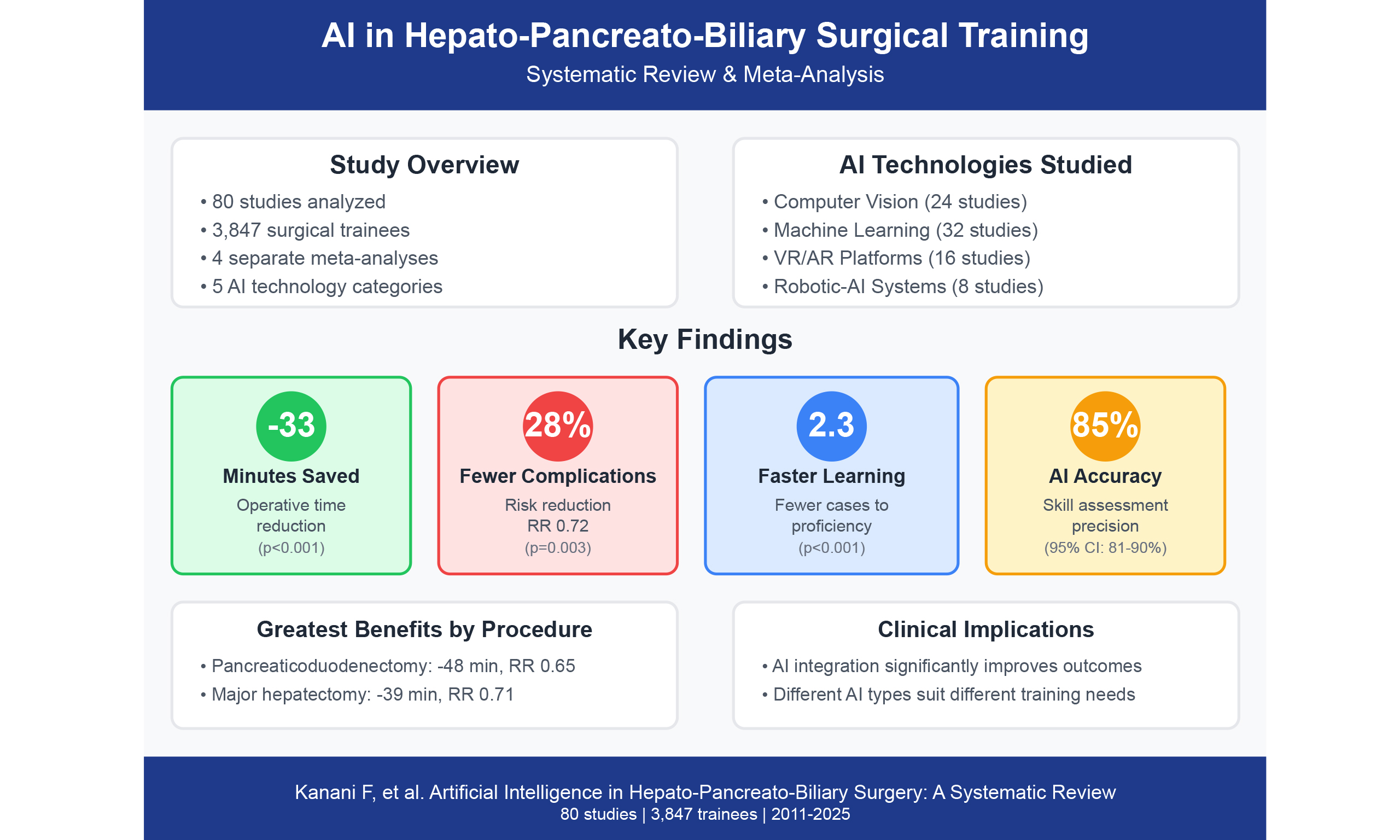
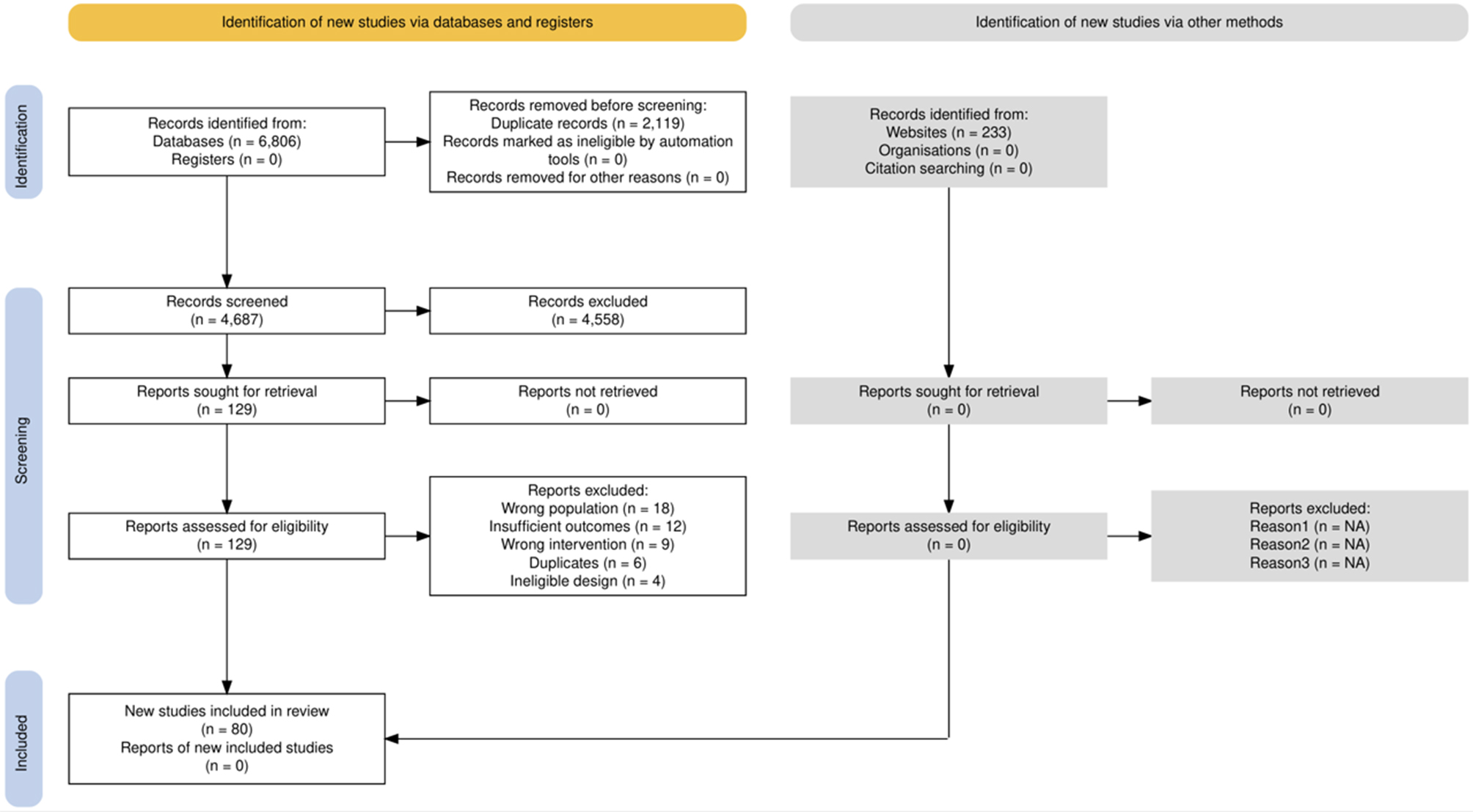
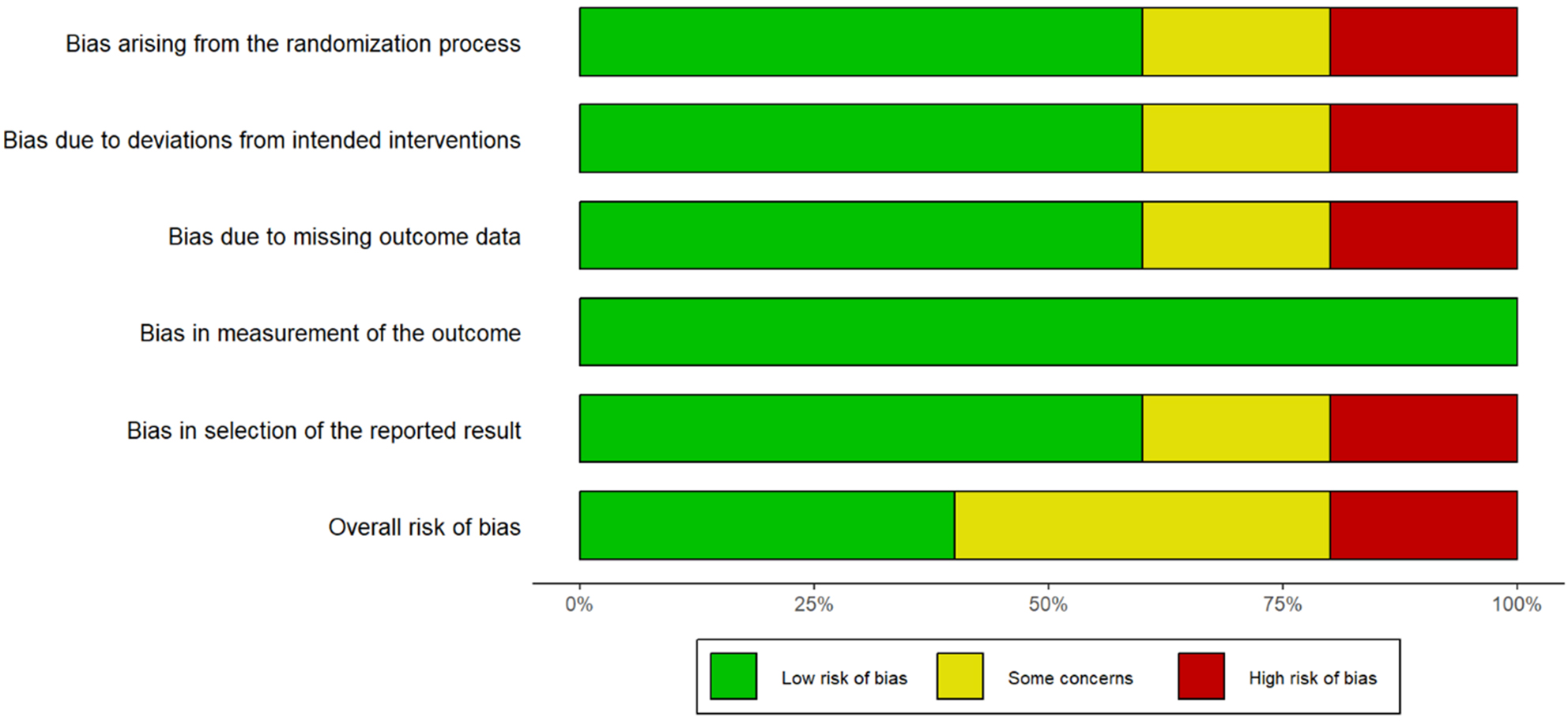
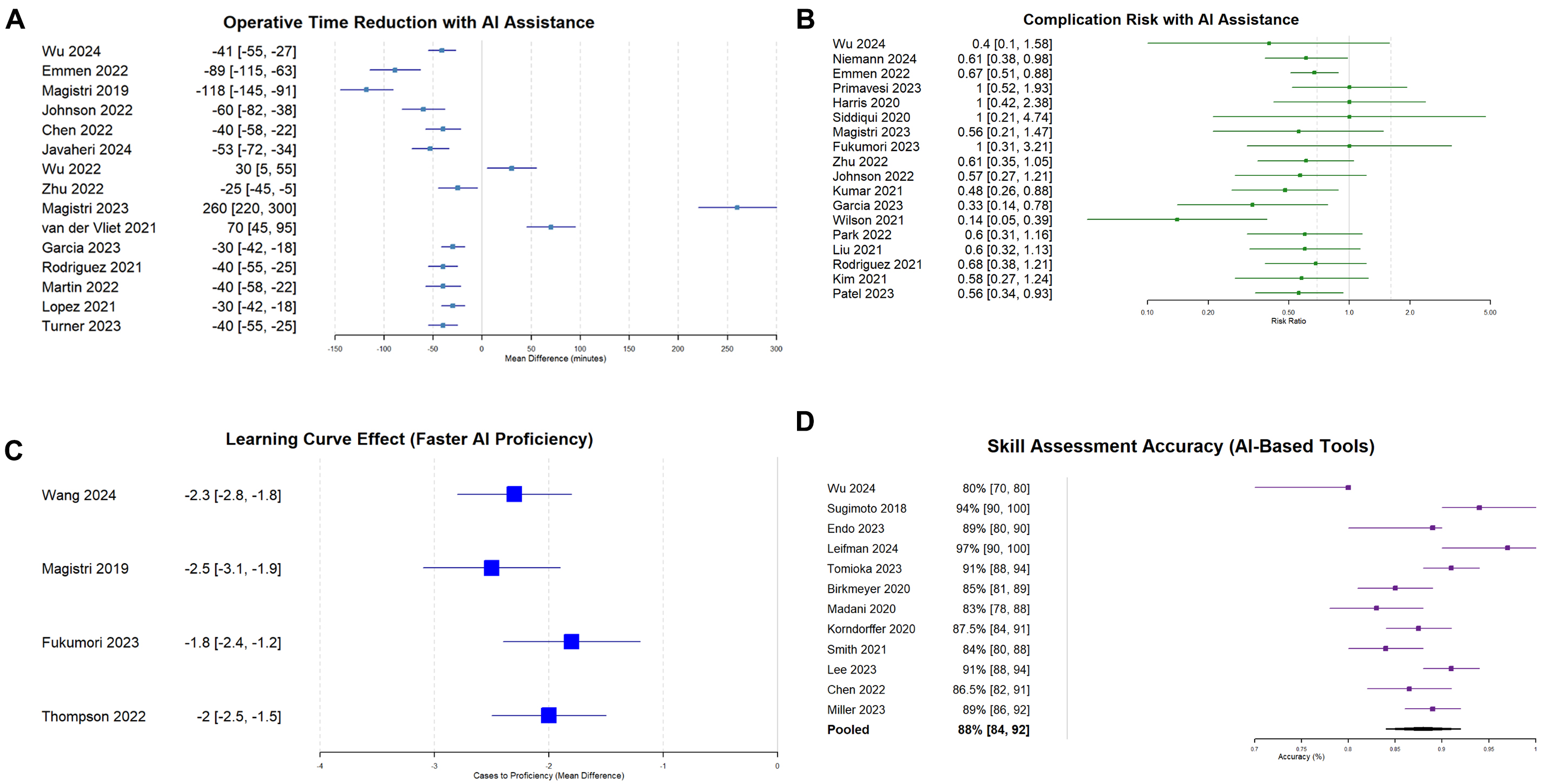
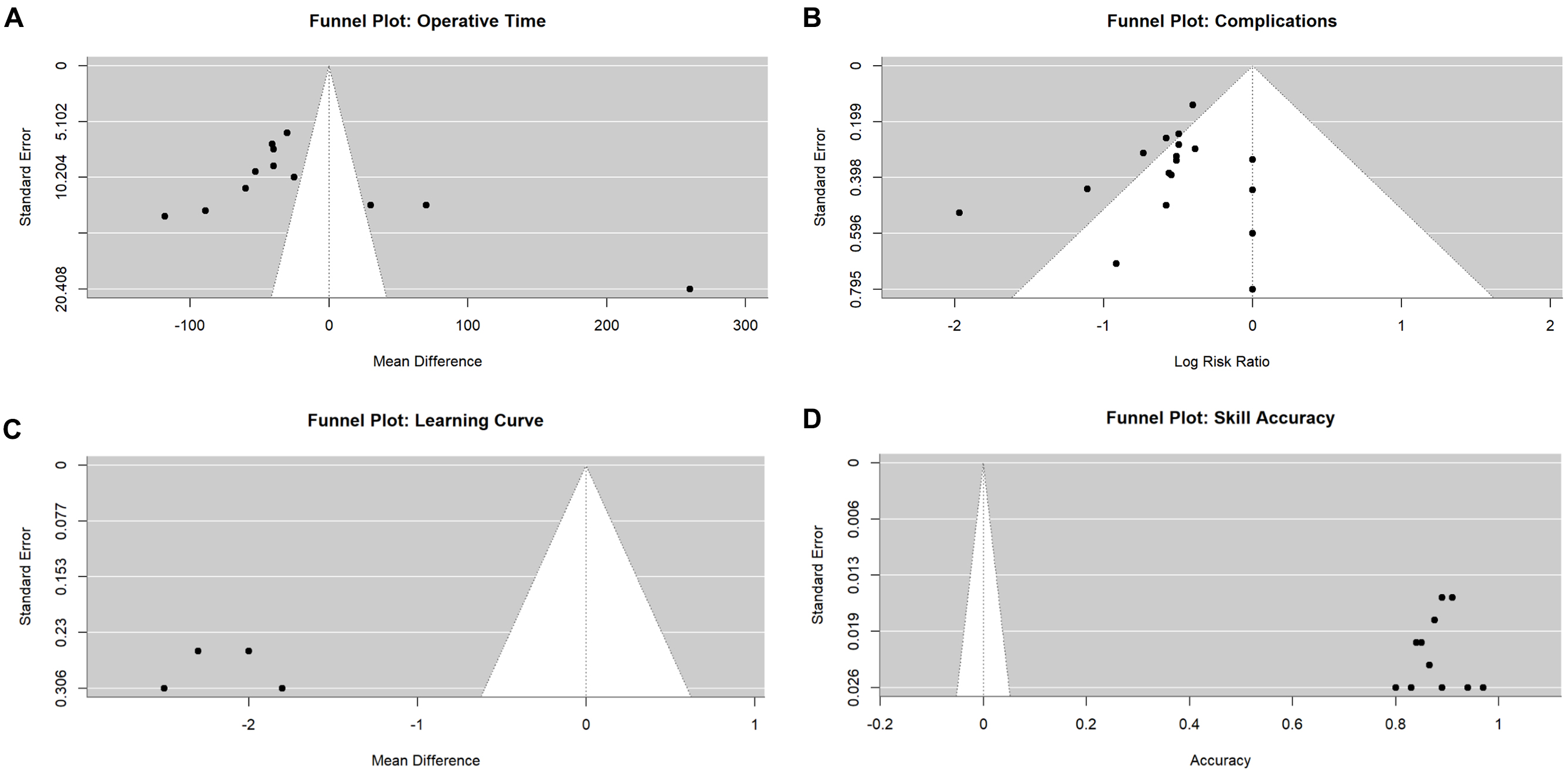
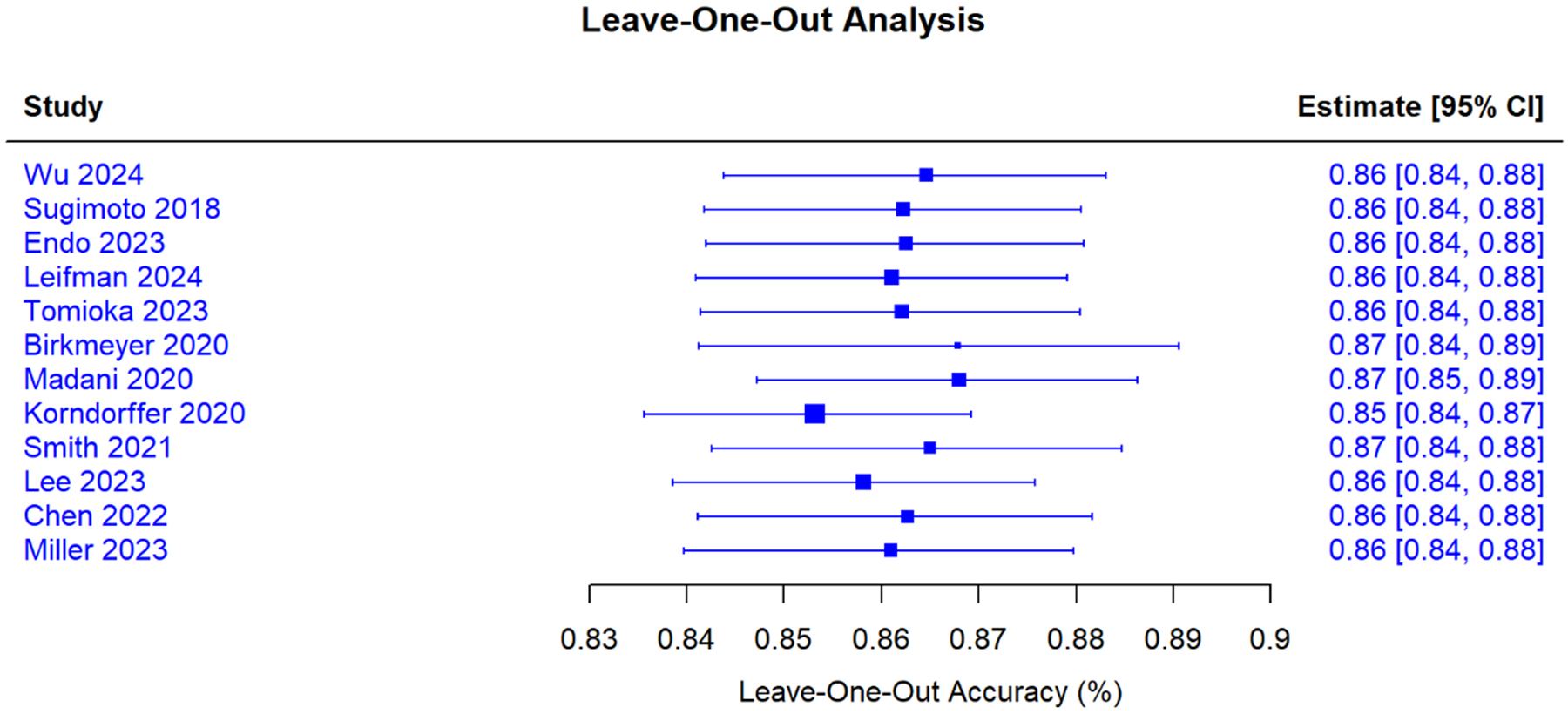
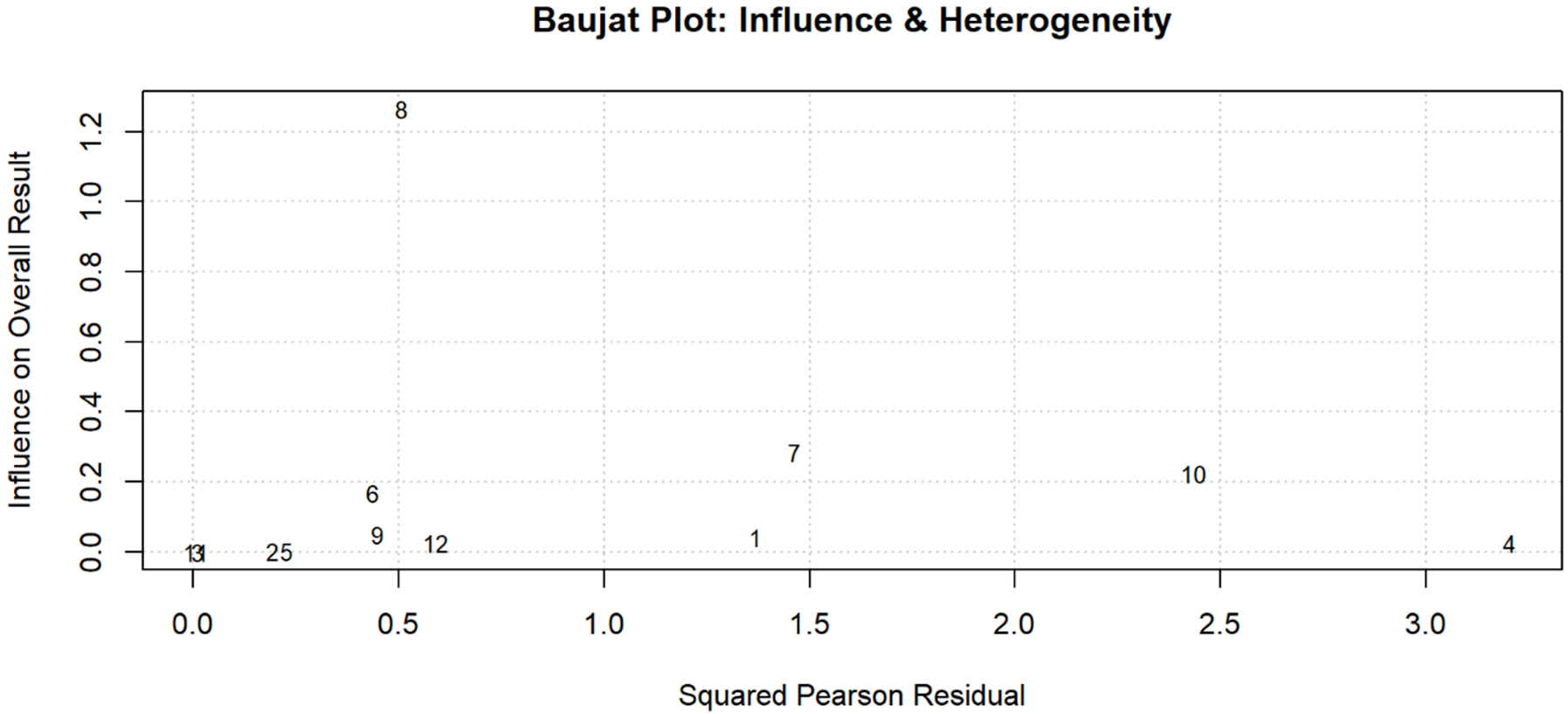
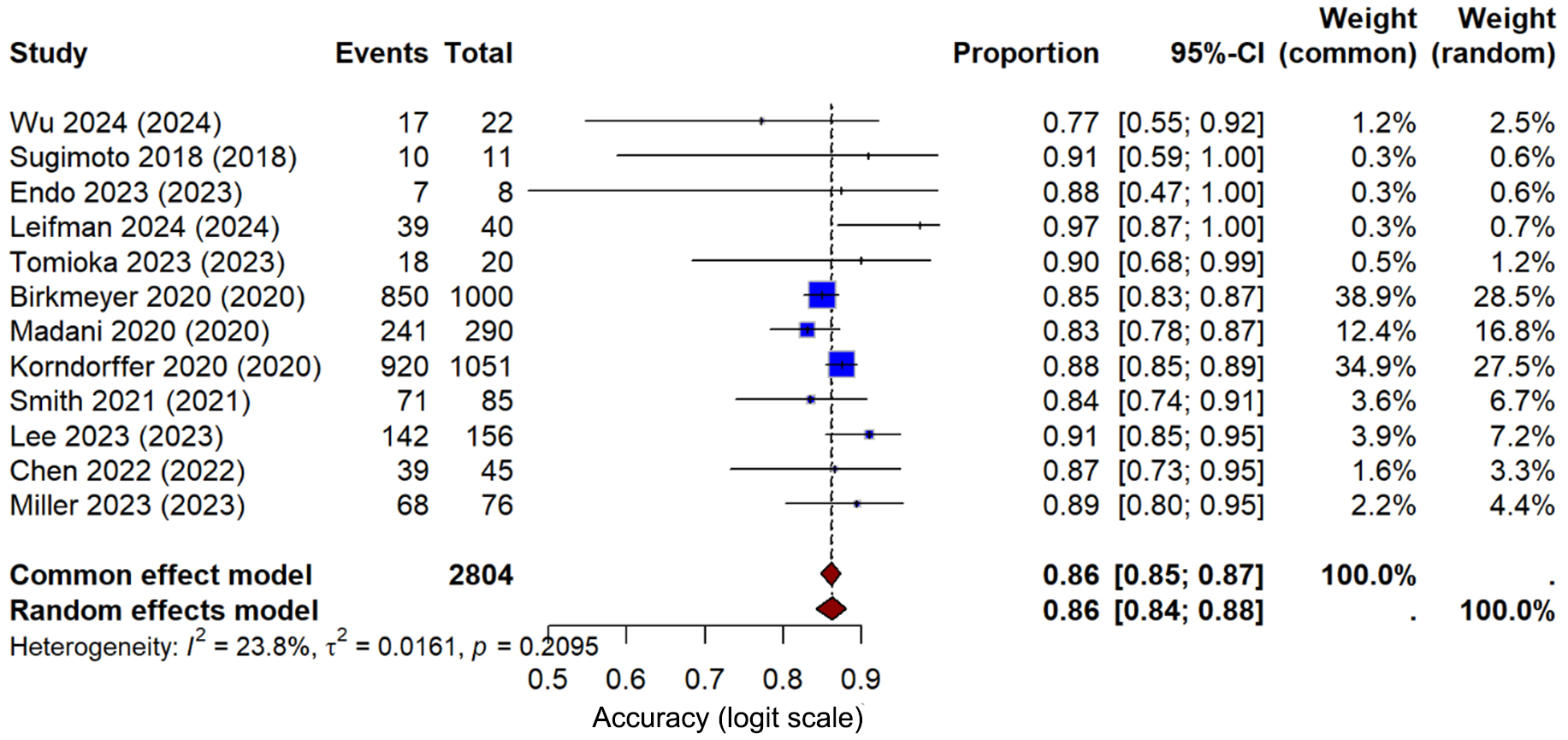
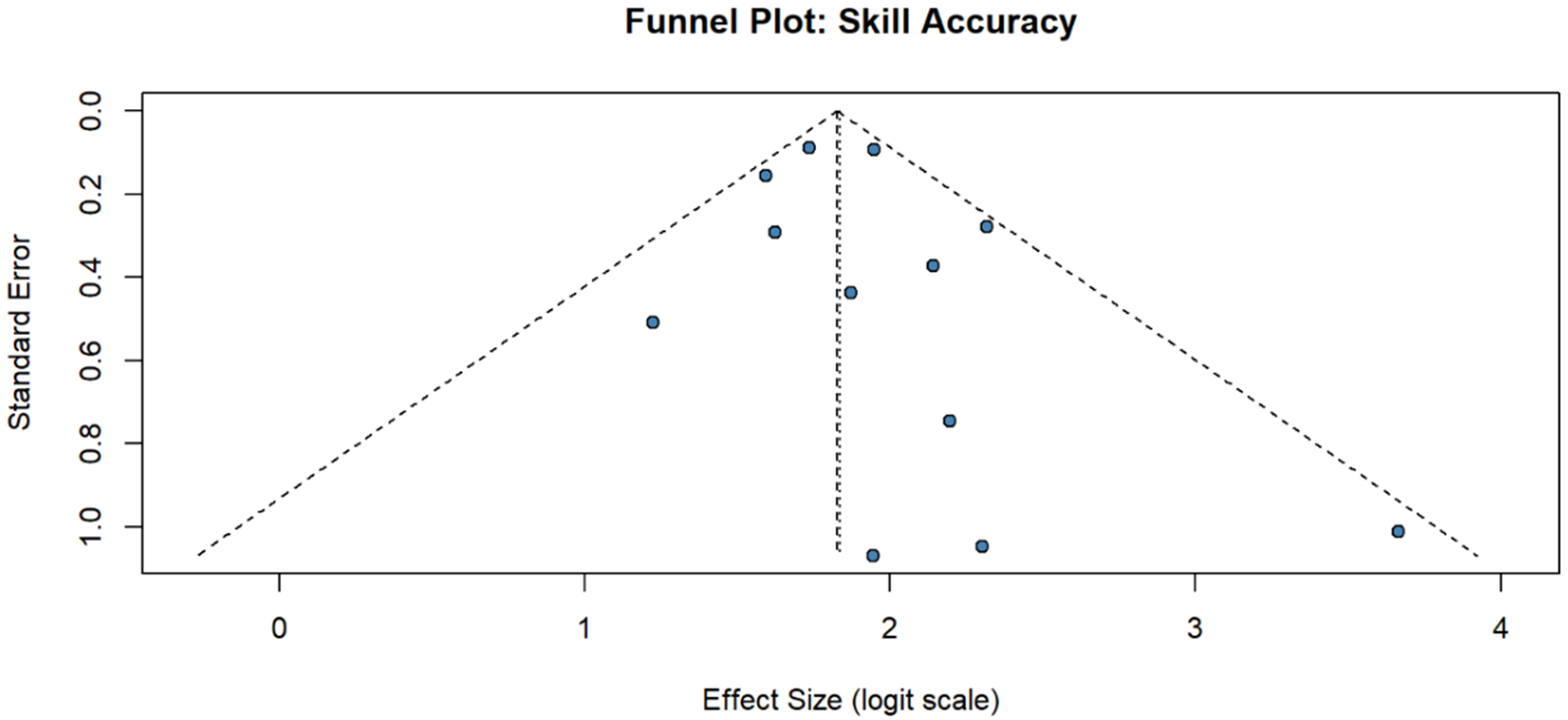
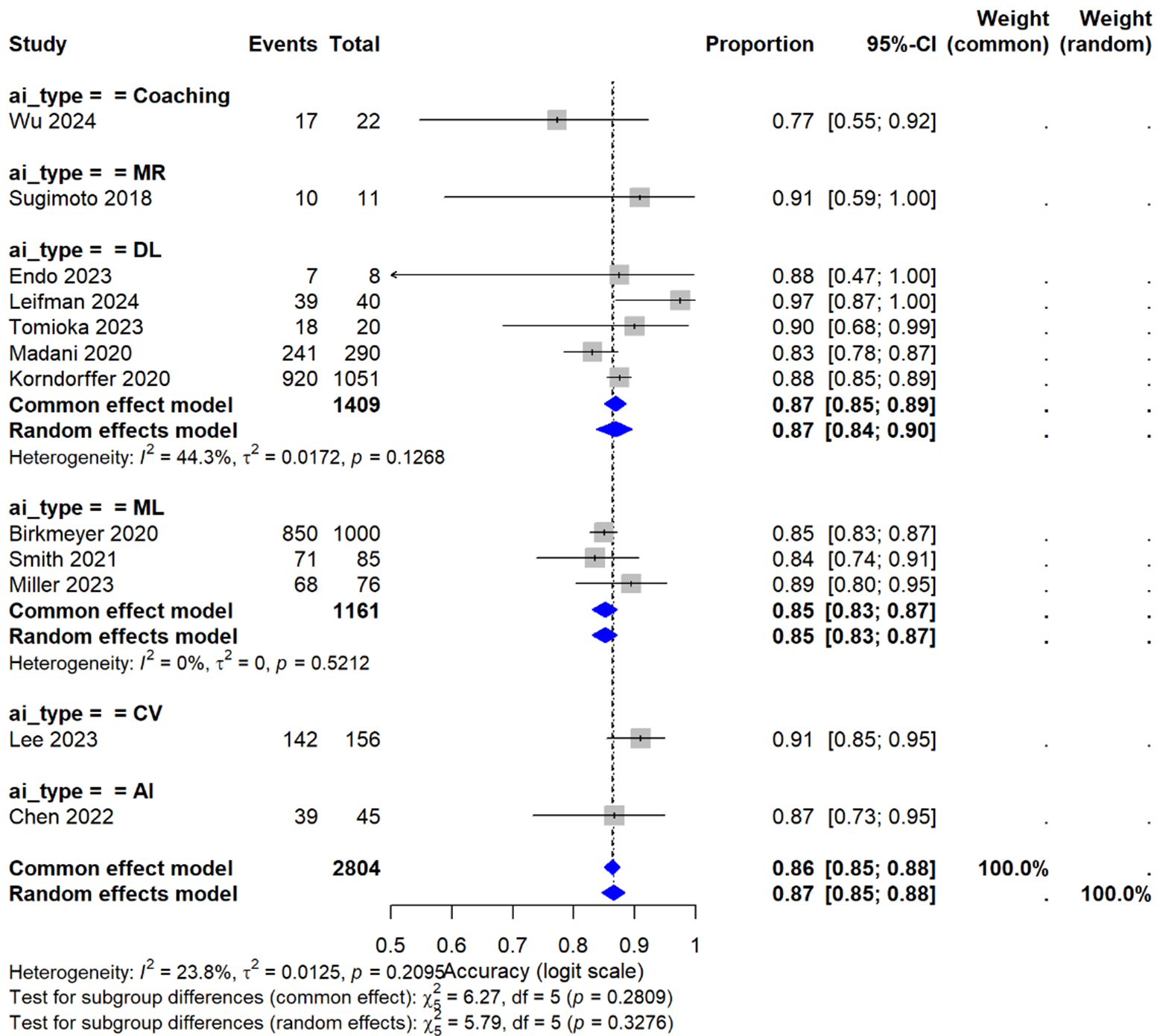











Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].