Artificial intelligence use in abdominal wall reconstruction: a systematic review
Abstract
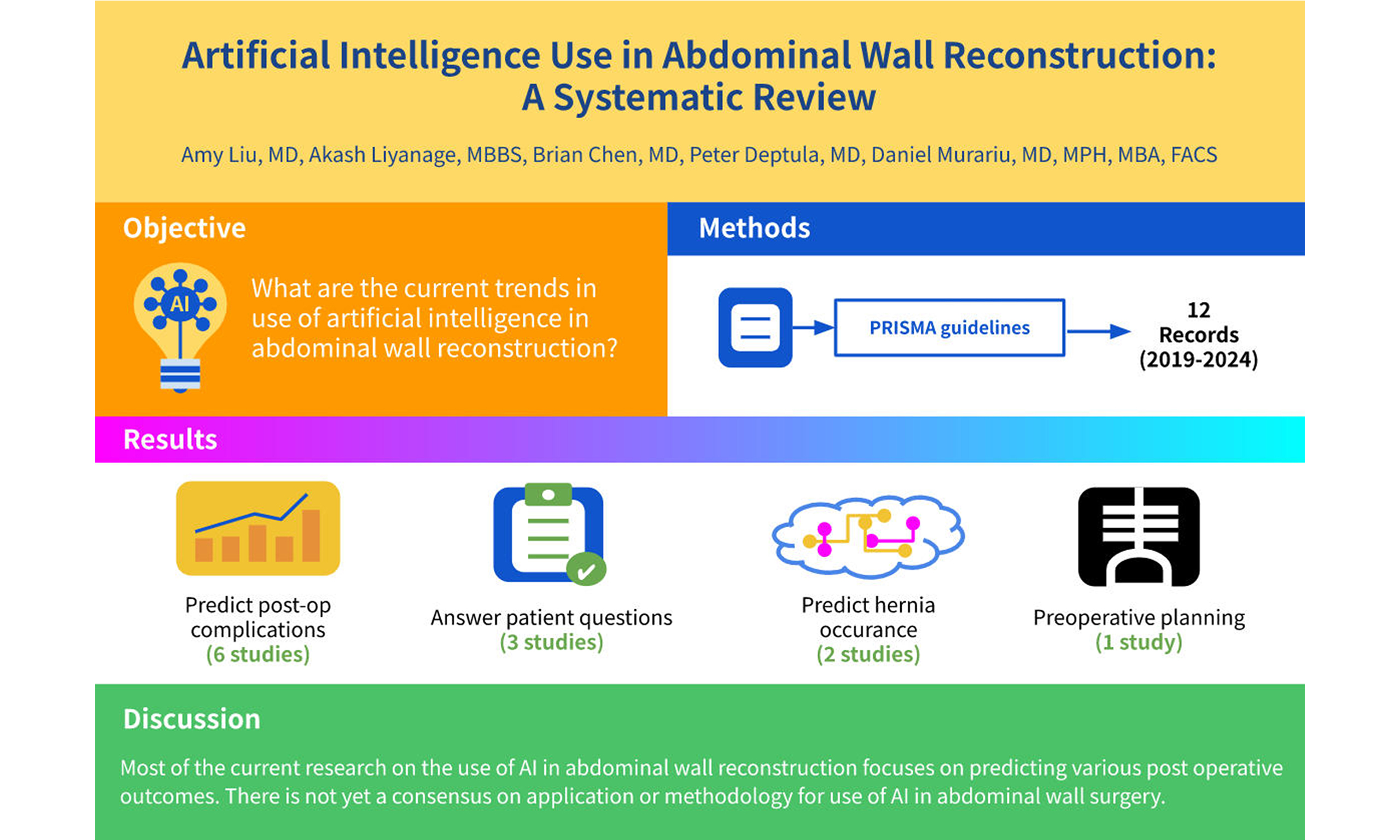
Aim: The use of artificial intelligence (AI) in medicine has grown significantly in recent years. This systematic review aims to highlight current trends in the application of AI specifically in abdominal wall reconstruction, which represents one of many medical fields utilizing AI technology.
Methods: A systematic review was conducted following the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines. Electronic databases including PubMed, Google Scholar, EBSCO, Ovid, and the Cochrane Library were searched for studies published between 2000 and 2024 that evaluated AI applications in abdominal wall reconstruction.
Results: A total of 142 publications were identified, of which 12 met the inclusion criteria and were included in this review. All included studies were published between 2019 and 2024. Among these, 2 studies investigated AI models for predicting hernia occurrence and the need for abdominal wall reconstruction; 1 study focused on AI for preoperative planning; 6 articles examined AI-based prediction of postoperative complications; and 3 publications explored the use of AI to answer patient questions.
Conclusion: Current research on AI in abdominal wall reconstruction primarily focuses on predicting postoperative outcomes and minimizing complications. However, there is no established consensus regarding the optimal applications or methodologies for integrating AI in this surgical field.
Keywords
INTRODUCTION
Ventral hernias develop in up to 15% of patients after laparotomy[1]. Closing these hernias can be difficult in the presence of large fascial defects or loss of domain - common sequelae of prolonged use of temporary abdominal closure. Such situations often require complex abdominal wall reconstruction. Without well-perfused tissue coverage, complications such as mesh exposure, hernia recurrence, and even enteric fistulae may arise[2]. Recurrent treatment failures and multiple reoperations impose significant financial burdens on healthcare systems and impact patients’ quality of life[3,4].
Over the last decade, artificial intelligence (AI) has increasingly permeated everyday life[5] and has been adopted across various medical disciplines[6-9]. Data play a key role in evidence-based medicine; however, the growing volume of data generated through electronic medical records often exceeds the capacity of traditional analytical methods to fully harness its potential[10]. In plastic and reconstructive surgery, AI has demonstrated value in assessing burn severity and treatment recommendations, wound and flap monitoring, and surgical planning[11-13]. Applying machine learning to monitor wound healing and predict recovery trajectories could significantly enhance clinical decision making in abdominal wall reconstruction[10,12,13]. Despite these advances, clinical integration of AI in surgical fields remains in its infancy compared to other specialties[14,15].
This systematic literature review aims to highlight current trends in the use of AI in abdominal wall reconstruction.
METHODS
A systematic literature search was conducted in accordance with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines[16]. The electronic databases PubMed, Google Scholar, EBSCO, Ovid, and Cochrane Library were searched for studies published between 2000 and 2024 that evaluated the use of AI in abdominal wall reconstruction. The search strategy utilized Boolean operators, Medical Subject Headings (MeSH), and synonymous terms. Keywords included: abdominal wall reconstruction, hernia, component separation, Rives-Stoppa, diastasis, abdominal wall defect, AI, machine learning, and large language models. Results were limited to studies published in or translated into English and involving adult patient populations. No restrictions were applied regarding the country of publication or patient gender. Reference lists of relevant articles were manually screened to identify additional eligible studies.
After removing duplicates, titles and abstracts were screened to select studies for full-text evaluation. Included publications had to report on the application of AI or machine learning specifically in the context of abdominal wall reconstruction. Letters to the editor, editorials, review articles, and conference proceedings were excluded. Studies focusing on groin hernias without addressing ventral or incisional hernias were also excluded. Full texts of the remaining articles were then assessed for eligibility.
RESULTS
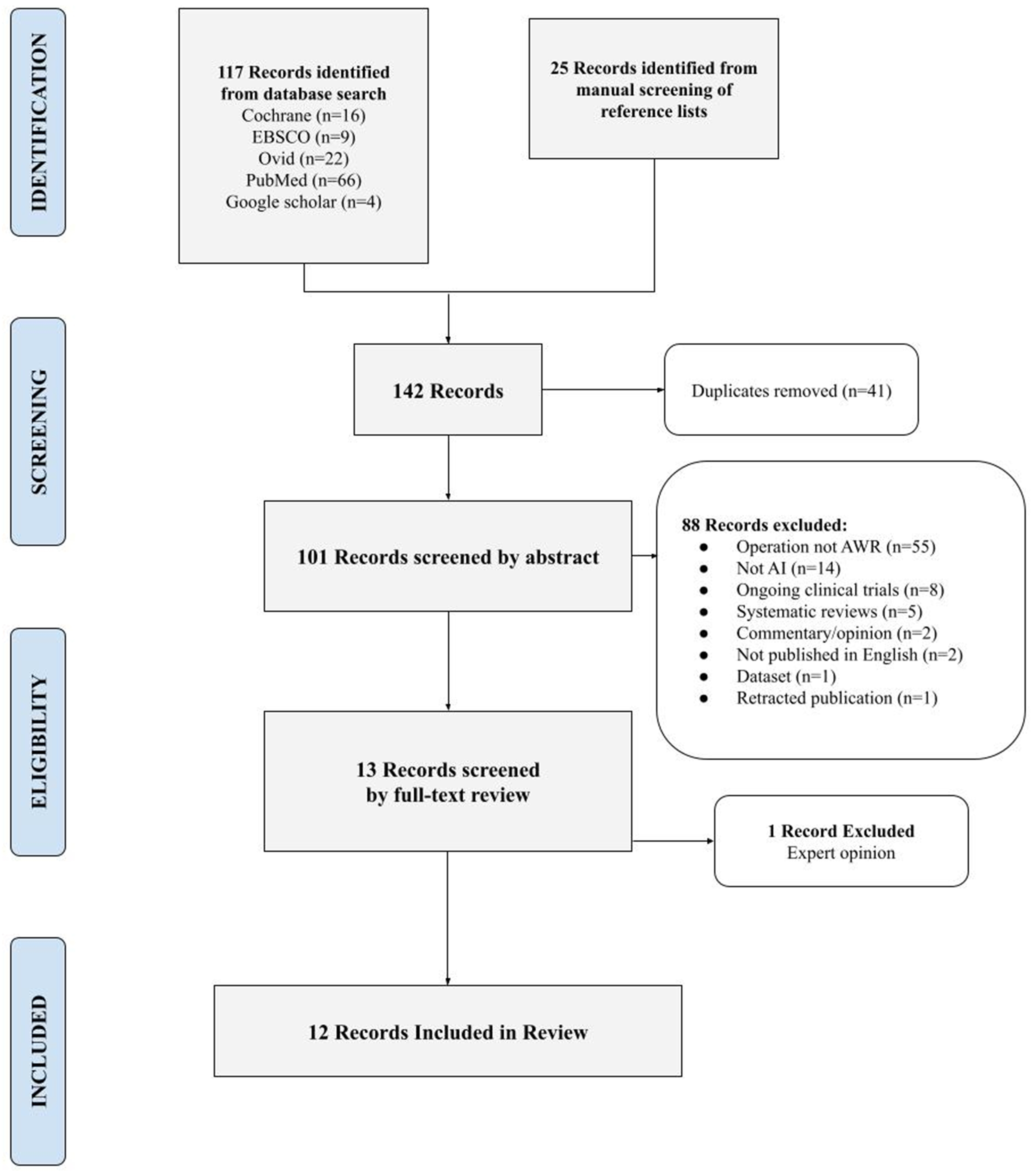
A total of 142 publications were identified through database searches and manual screening of reference lists [Figure 1]. After removing duplicates, 102 articles remained for abstract screening. Of these, 55 focused on surgical procedures other than complex abdominal wall reconstruction and were excluded from the review; among these, 14 addressed inguinal hernias exclusively, and 2 concerned breast reconstruction. Fourteen studies did not involve the use of AI and were therefore excluded. Thirteen articles met the inclusion criteria for full-text review. Of these, 12 were included in the final review, while one was an expert opinion and was excluded. All included studies were published between 2019 and 2024.
Figure 1. PRISMA diagram: search strategy. PRISMA: Preferred reporting items for systematic reviews and meta-analyses.
AI was most commonly applied to predict postoperative complications, with six publications addressing this application. The second most frequent use of AI in abdominal wall surgery was for answering patient questions. Two studies evaluated AI models for predicting hernia occurrence and the need for abdominal wall reconstruction. Only one of the 12 publications discussed AI use for preoperative planning [Table 1].
Included studies
| Title | Authors | Publication year | Use of AI |
| Use of the KSVM-based system for the definition, validation and identification of the incisional hernia recurrence risk factors | Licari et al.[26] | 2019 | Predict complications |
| Neural network model to detect long-term skin and soft tissue infection after hernia repair | O’Brien et al.[28] | 2020 | Predict complications |
| Biomechanics applied to incisional hernia repair - considering the critical and the gained resistance towards impacts related to pressure | Kallinowski et al.[22] | 2021 | Preoperative planning |
| Development and validation of image-based deep learning models to predict surgical complexity and complications in abdominal wall reconstruction | Elhage et al.[25] | 2021 | Predict complications |
| Novel machine learning approach for the prediction of hernia recurrence, surgical complication, and 30-day readmission after abdominal wall reconstruction | Hassan et al.[29] | 2022 | Predict complications |
| Preoperative computed tomography morphological features indicative of incisional hernia formation after abdominal surgery | McAuliffe et al.[21] | 2022 | Predict occurrence |
| Predicting rare outcomes in abdominal wall reconstruction using image-based deep learning models | Ayuso et al.[27] | 2023 | Predict complications |
| Optimal computed tomography-based biomarkers for prediction of incisional hernia formation | Talwar et al.[17] | 2024 | Predict occurrence |
| Exploring the potential of ChatGPT-4 in responding to common questions about abdominoplasty: an AI-based case study of a plastic surgery consultation | Li et al.[35] | 2024 | Answer patient questions |
| How appropriate are recommendations of online chat-based artificial intelligence (ChatGPT) to common questions on ventral hernia repair? | Lima et al.[37] | 2024 | Answer patient questions |
| Deep learning model utilizing clinical data alone outperforms image-based model for hernia recurrence following abdominal wall reconstruction with long-term follow up | Wilson et al.[30] | 2024 | Predict complications |
| Can AI answer my questions? Utilizing artificial intelligence in the perioperative assessment for abdominoplasty patients | Lim et al.[36] | 2024 | Answer patient questions |
DISCUSSION
Predicting hernia occurrence
Two studies have evaluated the use of AI to predict incisional hernia. Talwar et al. aimed to establish a predictive framework using advanced image analysis and machine learning by analyzing CT scans from 212 adult patients who underwent elective colorectal surgery between 2005 and 2016[17]. The images were analyzed for 279 features, including abdominopelvic volume, body wall skeletal musculature volume, and pelvic volume pressure ratio. The authors identified optimal predictive biomarkers for incisional hernia: volumes and ratios of visceral adipose tissue (VAT), inner and outer aspects of the body wall skeletal musculature. Machine learning models were then trained on these CT-based biomarkers to predict hernia occurrence, using ensemble boosting, random forest, and support vector machine (SVM) algorithms.
Ensemble boosting combines multiple algorithms to enhance predictive performance, often outperforming individual models[18]. It involves stacking decision trees or neural networks to improve accuracy. The random forest method uses multiple decision trees run in parallel, each built on random data subsets, with final predictions derived from aggregating individual tree outputs[19]. SVM algorithms are widely used for handling both linear and non-linear classification tasks[20]. Among these, ensemble boosting was the best-performing classifier.
A second study also assessed CT imaging to predict incisional hernia following colorectal surgery[21]. Using an SVM algorithm, this study evaluated the predictive accuracy of various features and found that widening of the rectus complex, increased visceral volume, and body wall skeletal muscle atrophy were all indicative of hernia risk. Both studies highlight increased intra-abdominal pressure - as measured by 3D imaging metrics such as abdominopelvic VAT volume, abdominopelvic inner abdominal musculature (IAM) volume, and the pelvic VAT to outer abdominal musculature (OAM) volume ratio - as key contributors to the pathophysiology of incisional hernia. The authors propose future clinical translation of these findings into a point-of-care tool for preoperative hernia risk assessment.
Preoperative planning
Only one study has evaluated the use of AI in preoperative planning for complex abdominal wall reconstruction. The authors utilized abdominal CT scans taken both at rest and during Valsalva maneuvers to generate models of tissue elasticity, mesh placement, and fixation point distribution. They then applied algorithms based on non-rigid B-spline registration combined with AI to integrate these results for surgical planning. This preoperative planning model was applied to 96 patients, resulting in reduced postoperative pain and no recurrences after one year[22]. The authors also described their calculation of a gained resistance to impacts related to pressure (GRIP) value, considering mesh type, fixation method, positioning, overlap, native tissue elasticity, and peritoneal closure.
Non-rigid B-spline is a statistical deformation model commonly used in medical image processing[23]. It allows flexible matching of two images and has been incorporated into radiation oncology for image matching during respiratory motion[24]. Evidently, non-rigid B-splines have supported clinical practice with excellent outcomes in several hundred cases, demonstrating favorable three-year results.
Elhage et al. used deep learning models (DLMs) to analyze preoperative imaging with the goal of predicting operative and postoperative outcomes[25]. They assessed the ability of DLMs to predict the need for advanced surgical techniques, such as component separation. When evaluating CT images, the DLMs outperformed expert surgeons in predicting surgical complexity and the necessity for component separation, achieving an accuracy of 81.3%, sensitivity of 88.9%, and specificity of 73.5%. In comparison, the surgeon group demonstrated 65% accuracy, 53.3% sensitivity, and 76.7% specificity. The authors conclude that further refinement of this technology can improve preoperative planning and enhance the informed consent process in surgical care.
Predicting postoperative complications
Licari et al. investigated the use of a kernel support vector machine (KSVM)-based intelligent data analysis technique to identify and validate known risk factors for hernia recurrence[26]. Their machine learning approach focused on analyzing and mining data to confirm established risk factors such as age, sex, urgency of surgery, surgical technique, ASA class, BMI, comorbidities, mesh type and size, and postoperative infections. Their relapse classification model achieved an accuracy of 86.67%, sensitivity of 86.25%, and specificity of 87.14%.
In addition to predicting surgical complexity using DLMs, Elhage et al. developed models to predict surgical site infection and pulmonary complications. The model for surgical site infection achieved 85.2% accuracy, 78.5% sensitivity, and 87.4% specificity. However, their model for pulmonary complications was less effective, with 77.5% accuracy, 26.9% sensitivity, and 84.0% specificity. Notably, they used conventional
Ayuso et al. also assessed hernia recurrence using CT imaging-based DLMs[27]. They compared a conventional DLM trained on both positive and negative outcomes with models based on a generative adversarial network anomaly (GANomaly) framework, which is trained only on negative outcomes and detects positive cases as anomalies. Their findings showed that the conventional method had higher specificity, while the GANomaly framework demonstrated greater sensitivity for detecting mesh infection or pulmonary failure. Specifically, conventional DLM predicted mesh failure with 96% specificity but only 25% sensitivity, compared to 78% specificity and 68% sensitivity for the GANomaly framework. Similarly, for pulmonary failure, conventional DLM achieved 92% specificity vs. 67% for GANomaly, but only 27% sensitivity vs. 73%. The authors suggested that because GANomaly is trained only on negative cases (no mesh infection or no pulmonary failure), it is less affected by the limited number of positive outcomes. However, they concluded that the conventional DLM framework should not be discarded, given its superior specificity, but rather used alongside the GANomaly method.
While AI has proven useful in imaging evaluation and prediction, several studies have also incorporated clinical data into predictive models[28-30]. Several risk assessment tools already exist, such as the Ventral Hernia Risk Score, Hernia Wound Risk Assessment Tool, and NSQIP Surgical Risk Calculator[31-33]. However, these rely on linear regression models that assume risk factors behave in a linear and additive manner[34]. O’Brien et al. applied a machine learning model to predict surgical site infection using clinical data extracted from medical records[28], achieving accurate discrimination between infection and non-infection. Hassan et al. demonstrated that machine learning algorithms based on preoperative clinical data - such as patient comorbidities and medical history - effectively predicted recurrence, surgical site infections, and readmission[29]. Wilson et al. evaluated whether adding clinical data to DLMs improved the prediction of hernia recurrence[30]. Their results showed that models trained solely on clinical data (including age, sex, BMI, diabetes status, and tobacco use history) outperformed those trained on images only or on images combined with clinical data. This finding is notable, given that prior studies predicting initial hernia occurrence primarily relied on imaging data alone[17,21].
Chat functions and patient education
The utility of AI chatbots for patient counseling and education has also been a topic of investigation. Li et al. evaluated ChatGPT’s ability to answer common patient questions regarding abdominoplasty[35]. They provided the chatbot with a list of questions from the American Society of Plastic Surgeons related to abdominoplasty. Although the chatbot could answer frequently asked questions, some responses were outdated and lacked personalized recommendations. Lim et al. extended this work by comparing four popular chatbots - OpenAI’s ChatGPT, Anthropic’s Claude, Google Gemini, and Bing CoPilot[36]. They found that while all platforms delivered instant and easily accessible responses, there was variability in language quality and supplementary information; similarly, none could offer personalized advice. Lima et al. also assessed the appropriateness of ChatGPT’s responses to questions about ventral hernia repair[37]. While chatbots may serve as valuable initial tools for patient education, they should not replace surgical consultations.
Alternative uses and future directions of AI
The application of AI has been rapidly expanding across medical specialties, with the most frequent use in diagnostic fields[38]. Machine learning, AI, and large language models have been used to tailor cardiac treatments and assist in clinical decision making. DLMs have been applied in precision medicine to predict the risk of coronary artery disease by analyzing risk factors such as smoking, diabetes, hypertriglyceridemia, and correlating these with electrocardiogram (EKG) or echocardiography findings[9]. Additionally, integrating histopathology, genetic risk factors, and imaging modalities - such as cardiac MRI, echocardiography, and coronary angiography - has improved prediction of hospital admissions due to cardiac complications, including acute decompensated heart failure or stroke[8].
Radiology remains the specialty with the highest volume of AI-related publications[38]. AI has proven useful in enhancing radiographic diagnoses of conditions such as appendicitis, fractures, and pneumothorax, thereby facilitating faster emergency care. Studies report high accuracy in detecting stroke, intracranial hemorrhage, and suspicious breast lesions[6]. Beyond diagnosis, AI has been creatively applied to workflow management by tracking imaging processing times and alerting providers to delays, thus expediting patient care[7].
Minimally invasive surgical techniques hold promise for generating abundant new data. Most laparoscopic and robot-assisted surgical platforms already feature recording capabilities, and AI has shown effectiveness in image processing[6,7,17,22,25,27,39]. With the ongoing digitization of medicine, intraoperative DLM-based assistance tools may soon become common. Such models could also shape the future of abdominal wall reconstruction surgery by integrating patient data - including age, BMI, tobacco use, occupation, and comorbidities - to predict hernia recurrence risk and identify potential postoperative complications.
Machine learning can further assist by analyzing patient imaging to assess muscle weakness, hernias, fat distribution, and other anatomical factors influencing surgical outcomes. AI systems may enable augmented reality via 3D simulations, helping surgeons prepare for procedures and providing real-time anatomical feedback tailored to the individual patient. Moreover, personalized treatment plans informed by AI can enhance postoperative care quality following hernia surgery by offering customized recommendations for nutrition, physical therapy, and recovery.
Limitations
This review included only publications written in English. Although efforts were made to minimize bias, this restriction may have led to the exclusion of relevant information, introducing some inherent bias. The use of AI in surgical specialties is an evolving field, as reflected by the recent publication dates of most included studies - all published within the last 5 years.
In conclusion, AI has significant potential to enhance both the efficacy and efficiency of medical practice. While machine learning models offer numerous possibilities to support clinical decision making, there is currently no consensus on optimal methodologies or applications. The diversity of models and variability in frameworks used across the included studies highlight this lack of standardization. Presently, AI applications in abdominal wall reconstruction primarily focus on predicting postoperative outcomes; however, there is considerable potential to expand its utility across all stages of reconstructive care.
DECLARATIONS
Authors’ contributions
Made substantial contributions to the conception and design of the study and performed data analysis and interpretation: Liu A, Liyanage A, Murariu D
Performed data acquisition, as well as providing administrative, technical, and material support: Chen B, Deptula P
Drafted the manuscript and made revisions: Liu A, Liyanage A, Chen B, Deptula P, Murariu D
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Goodenough CJ, Ko TC, Kao LS, et al. Development and validation of a risk stratification score for ventral incisional hernia after abdominal surgery: hernia expectation rates in intra-abdominal surgery (the HERNIA Project). J Am Coll Surg. 2015;220:405-13.
2. Marxen T, Faulkner HR, Losken A. Evaluating the effect of socioeconomic status on complex abdominal wall reconstruction outcomes. Ann Plast Surg. 2022;89:670-4.
3. Poulose BK, Shelton J, Phillips S, et al. Epidemiology and cost of ventral hernia repair: making the case for hernia research. Hernia. 2012;16:179-83.
4. van Ramshorst GH, Eker HH, Hop WC, Jeekel J, Lange JF. Impact of incisional hernia on health-related quality of life and body image: a prospective cohort study. Am J Surg. 2012;204:144-50.
6. Yordanova MZ. The applications of artificial intelligence in radiology: opportunities and challenges. Eur J Med Health Sci. 2024;6:11-4.
7. Buijs E, Maggioni E, Mazziotta F, Lega F, Carrafiello G. Clinical impact of AI in radiology department management: a systematic review. Radiol Med. 2024;129:1656-66.
8. Singh M, Kumar A, Khanna NN, et al. Artificial intelligence for cardiovascular disease risk assessment in personalised framework: a scoping review. EClinicalMedicine. 2024;73:102660.
9. Panjiyar BK, Davydov G, Nashat H, et al. A systematic review: do the use of machine learning, deep learning, and artificial intelligence improve patient outcomes in acute myocardial ischemia compared to clinician-only approaches? Cureus. 2023;15:e43003.
10. Qin F, Gu J. Artificial intelligence in plastic surgery: current developments and future perspectives. Plast Aesthet Res. 2023;10:3.
11. Catania LJ. Foundations of artificial intelligence in healthcare and bioscience: a user friendly guide for IT professionals, healthcare providers, researchers, and clinicians. Academic Press; 2020. Available from: https://www.google.com/books/edition/Foundations_of_Artificial_Intelligence_i/2Mr2DwAAQBAJ?hl=zh-CN&gbpv=0. [Last accessed on 12 Aug 2025].
12. Anisuzzaman DM, Wang C, Rostami B, Gopalakrishnan S, Niezgoda J, Yu Z. Image-based artificial intelligence in wound assessment: a systematic review. Adv Wound Care. 2022;11:687-709.
13. Huang RW, Tsai TY, Hsieh YH, et al. Reliability of postoperative free flap monitoring with a novel prediction model based on supervised machine learning. Plast Reconstr Surg. 2023;152:943e-52e.
14. Popa SL, Ismaiel A, Brata VD, et al. Artificial intelligence and medical specialties: support or substitution? Med Pharm Rep. 2024;97:409-18.
15. Haug CJ, Drazen JM. Artificial intelligence and machine learning in clinical medicine, 2023. N Engl J Med. 2023;388:1201-8.
16. PRISMA Statement. Available from: https://www.prisma-statement.org/. [Last accessed on 12 Aug 2025].
17. Talwar AA, Desai AA, McAuliffe PB, et al. Optimal computed tomography-based biomarkers for prediction of incisional hernia formation. Hernia. 2024;28:17-24.
18. Opitz D, Maclin R. Popular ensemble methods: an empirical study. J Artif Intell Res. 1999;11:169-98. Available from: https://www.d.umn.edu/~rmaclin/publications/opitz-jair99.pdf. [Last accessed on 12 Aug 2025].
21. McAuliffe PB, Desai AA, Talwar AA, et al. Preoperative computed tomography morphological features indicative of incisional hernia formation after abdominal surgery. Ann Surg. 2022;276:616-25.
22. Kallinowski F, Ludwig Y, Löffler T, et al. Biomechanics applied to incisional hernia repair - considering the critical and the gained resistance towards impacts related to pressure. Clin Biomech. 2021;82:105253.
23. Du X, Dang J, Wang Y, Wang S, Lei T. A parallel nonrigid registration algorithm based on B-spline for medical images. Comput Math Methods Med. 2016;2016:7419307.
24. Chun SY, Fessler JA. A simple regularizer for B-spline nonrigid image registration that encourages local invertibility. IEEE J Sel Top Signal Process. 2009;3:159-69.
25. Elhage SA, Deerenberg EB, Ayuso SA, et al. Development and validation of image-based deep learning models to predict surgical complexity and complications in abdominal wall reconstruction. JAMA Surg. 2021;156:933-40.
26. Licari L, Salamone G, Campanella S, et al. Use of the KSVM-based system for the definition, validation and identification of the incisional hernia recurrence risk factors. G Chir. 2019;40:32-8.
27. Ayuso SA, Elhage SA, Zhang Y, et al. Predicting rare outcomes in abdominal wall reconstruction using image-based deep learning models. Surgery. 2023;173:748-55.
28. O’Brien WJ, Ramos RD, Gupta K, Itani KMF. Neural network model to detect long-term skin and soft tissue infection after hernia repair. Surg Infect. 2021;22:668-74.
29. Hassan AM, Lu SC, Asaad M, et al. Novel machine learning approach for the prediction of hernia recurrence, surgical complication, and 30-day readmission after abdominal wall reconstruction. J Am Coll Surg. 2022;234:918-27.
30. Wilson HH, Ma C, Ku D, et al. Deep learning model utilizing clinical data alone outperforms image-based model for hernia recurrence following abdominal wall reconstruction with long-term follow up. Surg Endosc. 2024;38:3984-91.
31. Berger RL, Li LT, Hicks SC, Davila JA, Kao LS, Liang MK. Development and validation of a risk-stratification score for surgical site occurrence and surgical site infection after open ventral hernia repair. J Am Coll Surg. 2013;217:974-82.
32. Nelson JA, Fischer J, Chung CC, et al. Readmission following ventral hernia repair: a model derived from the ACS-NSQIP datasets. Hernia. 2015;19:125-33.
33. Fischer JP, Wink JD, Tuggle CT, Nelson JA, Kovach SJ. Wound risk assessment in ventral hernia repair: generation and internal validation of a risk stratification system using the ACS-NSQIP. Hernia. 2015;19:103-11.
34. Chen JH, Asch SM. Machine learning and prediction in medicine - beyond the peak of inflated expectations. N Engl J Med. 2017;376:2507-9.
35. Li W, Chen J, Chen F, Liang J, Yu H. Exploring the potential of ChatGPT-4 in responding to common questions about abdominoplasty: an AI-based case study of a plastic surgery consultation. Aesthetic Plast Surg. 2024;48:1571-83.
36. Lim B, Seth I, Cuomo R, et al. Can AI answer my questions? Utilizing artificial intelligence in the perioperative assessment for abdominoplasty patients. Aesthetic Plast Surg. 2024;48:4712-24.
37. Lima DL, Nogueira R, Liu J, et al. How appropriate are recommendations of online chat-based artificial intelligence (ChatGPT) to common questions on ventral hernia repair? J Laparoendosc Adv Surg Tech A. 2024;34:365-7.
38. Stewart JE, Rybicki FJ, Dwivedi G. Medical specialties involved in artificial intelligence research: is there a leader. Tasman Med J. 2020;2:20-7. Available from: https://tasmanmedicaljournal.com/2020/02/medical-specialties-involved-in-artificial-intelligence-research-is-there-a-leader/. [Last accessed on 12 Aug 2025].
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Special Issue
Copyright
Data & Comments
Data

















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].