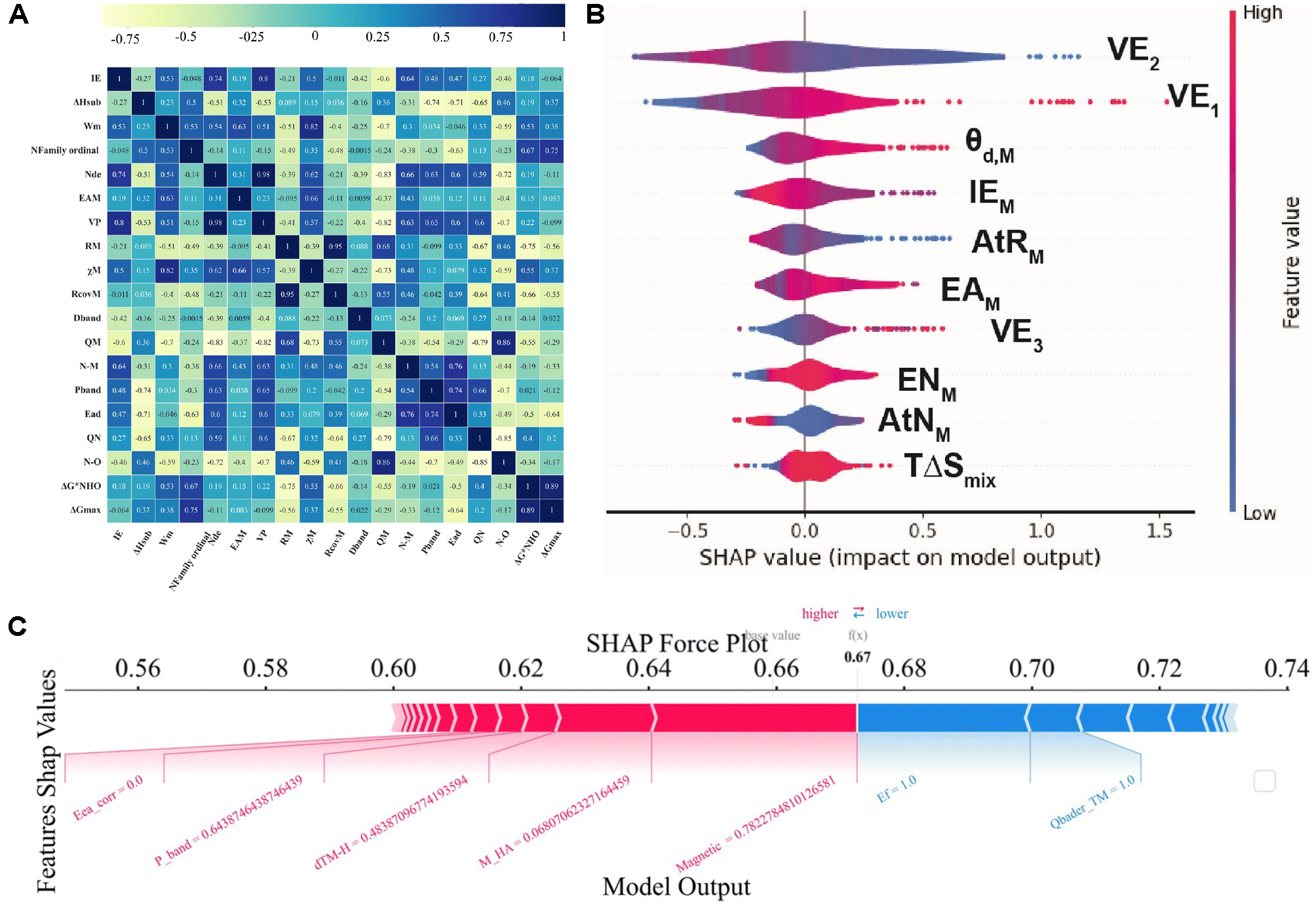
fig4
Figure 4. (A) Pearson correlations. Heatmap of the Pearson coefficient r among all primary features and the target. Color encodes r from -1 (strong negative; yellow) to +1 (strong positive; blue); each cell prints its r value. Adapted with permission from Zhao et al.[36] (© Royal Society of Chemistry 2024); changes made: cropped to only include panel a; (B) SHAP summary (violin) plot. X-axis: SHAP value, feature impact on the model output. Points are individual samples; violin width shows density; features are ordered by mean |SHAP|. Point color encodes the raw feature value. Positive SHAP values shift the prediction upward; negative values shift it downward. Adapted with permission from Tamtaji et al.[6] (© 2024 Elsevier.); changes made: cropped to only include panel c; (C) Local explanation and partial dependence. SHAP force plot for one example: red bars push the prediction higher relative to the model’s expected value; blue bars push it lower. Adapted with permission from Zhao et al.[10] (© Royal Society of Chemistry 2025); changes made: cropped to only include panel e. SHAP: SHapley Additive exPlanations; IE: ionization energy; ΔHsub: enthalpy of sublimation; Wm: work function of the metal; Nfamily ordinal: Family ordinal number; Nde: number of d-electrons; EAM: electron affinity of the metal; VP: valence electron number; RM: atomic radius; χM: electronegativity of the metal; RcovM: covalent radius of the metal; Dband: d-band center; QM: central atom Mulliken charge; N-M: N-metal bond length; Pband: p-band center; Ead: adsorption energy; QN: charge transferred to the N; N-O: bond length of adsorbed NO; ΔG*NHO: Gibbs free-energy change for adsorbed NHO formation; ΔGmax: maximum Gibbs free energy change; TΔSmix: T × ΔSmix (product of temperature and mixing entropy); AtNM: atomic number of the metal; ENM: electronegativity of the metal; VE3: valence electrons in 3rd ring; AtRM: atomic radius of the metal; IEM: ionization energy of the metal; θd,M: number of d electrons; VE1: valence electrons in 1st ring; VE2: valence electrons in 2nd ring; Eca_corr: corrected electron affinity; dTM-H: bond length between transition metal and hydrogen; M_HA: total heteroatom mass; Ef: formation energy; Qbader_TM: bader charge of the transition metal.