Impact of malnutrition on short- and long-term outcomes following laparoscopic liver resection for hepatocellular carcinoma
Abstract
Aim: Laparoscopic liver resection (LLR) is increasingly used in the management of hepatocellular carcinoma (HCC), even among patients traditionally considered high risk due to advanced age or poor nutritional status. Malnutrition, assessed by the prognostic nutritional index (PNI), is known to negatively affect surgical outcomes; however, its impact in the context of LLR remains unclear. We aimed to clarify the effect of malnutrition, defined by the PNI, on short- and long-term outcomes following laparoscopic liver resection for HCC.
Methods: We retrospectively analyzed 121 patients with HCC who underwent primary LLR between 2011 and 2019. Nutritional status was evaluated using the PNI, with a cutoff of < 40 indicating malnutrition. Short-term outcomes were assessed using the textbook outcome (TO), defined as meeting five criteria: no 30-day mortality, R0 resection, no major complications (Clavien–Dindo ≥ III), no unplanned readmission, and no prolonged hospitalization. Long-term outcomes included overall survival (OS) and recurrence-free survival (RFS).
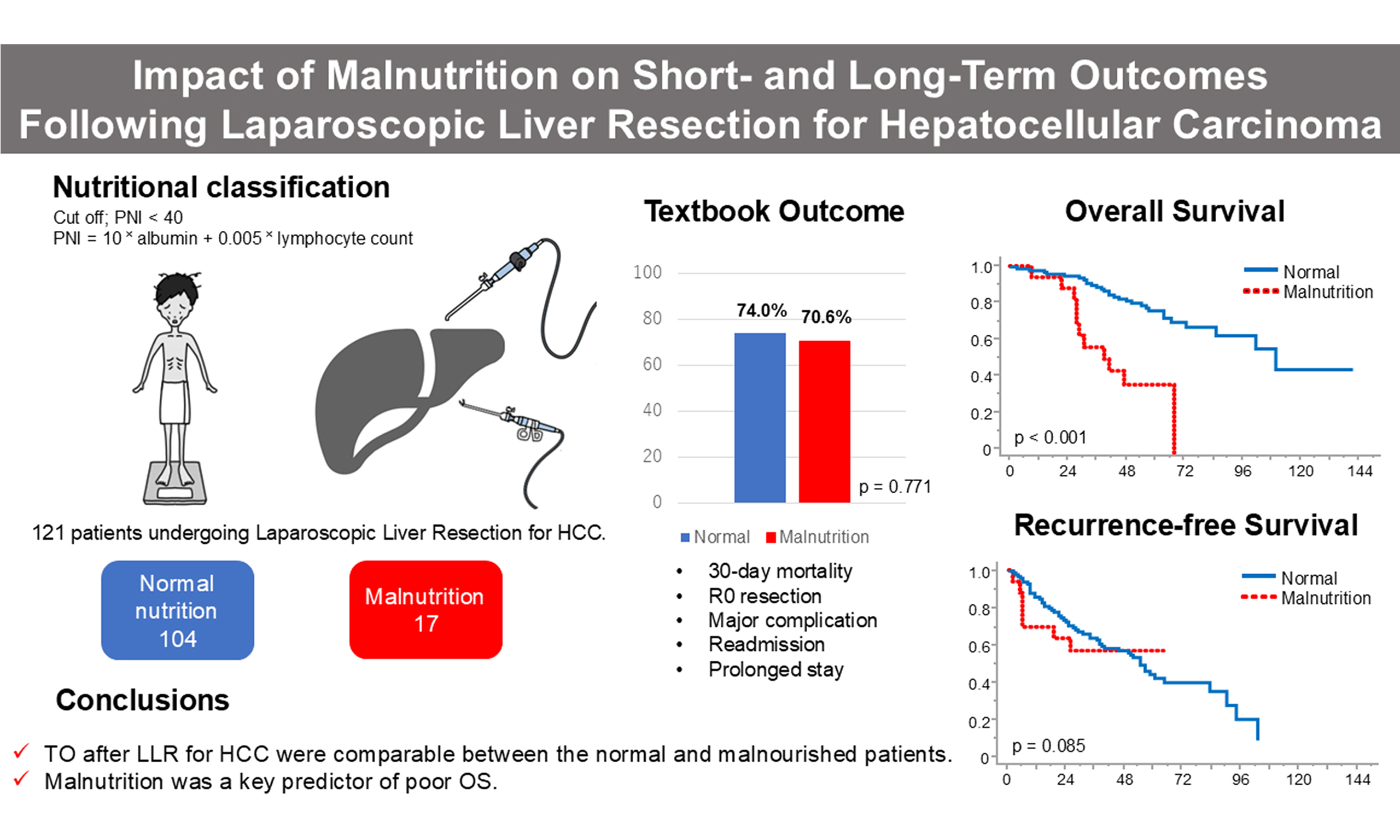
Results: Seventeen patients (14%) were classified as malnourished. TO achievement rates were similar between the malnutrition and normal-nutrition groups (70.6% vs. 74.0%, P = 0.771). No significant differences were observed in individual TO criteria. However, OS was significantly worse in the malnutrition group (median 40 vs. 107 months, P < 0.001), while RFS showed a non-significant trend (P = 0.085). In multivariate analysis, PNI-defined malnutrition was the only independent predictor of poorer OS.
Conclusion: LLR yields acceptable short-term outcomes even in malnourished patients with HCC, as defined by the PNI. However, malnutrition remains a strong independent risk factor for decreased long-term survival. These findings underscore the importance of preoperative nutritional assessment and optimization in surgical candidates with HCC.
Keywords
INTRODUCTION
Hepatocellular carcinoma (HCC) is a major contributor to global cancer mortality, ranking third among all cancers. Despite advances in management, the worldwide 5-year survival rate remains low, at approximately 18%[1,2]. This low survival rate reflects both the aggressive biological behavior of HCC and the fact that many patients are diagnosed at an advanced stage, limiting treatment options. This underscores the critical need for effective treatment strategies tailored to both tumor characteristics and patient condition. While surgical resection remains the most definitive curative approach for HCC[3], recent advances in particle beam therapy and immune checkpoint inhibitors have introduced alternative treatment modalities[4,5]. These non-surgical therapies have shown promise, especially in patients who are ineligible for surgery due to comorbidities or advanced tumor stage, thereby contributing to a more individualized approach to HCC management. Laparoscopic liver resection (LLR) has become widely adopted in recent years and has contributed to expanding surgical indications to populations traditionally considered high risk, such as older patients and those with poor nutritional status[6,7]. Compared to open surgery, LLR is generally associated with less bleeding during the procedure, a shorter hospital stay, and a quicker recovery, making it particularly beneficial for patients with compromised physiological reserves. In this evolving therapeutic landscape, surgeons are increasingly expected to evaluate the patient’s overall condition and decide whether to provide curative treatment through minimally invasive surgery or refer them to multidisciplinary treatment approaches. This decision-making process requires a comprehensive assessment of not only oncological factors but also the patient’s functional status, including liver function, performance status, and nutritional condition. Malnutrition has been well documented as a factor associated with poorer outcomes following liver resection[8,9]. Patients in this condition are more vulnerable to perioperative complications and often require prolonged hospitalization. Furthermore, their long-term survival may be unfavorably affected[10]. These effects are likely mediated by impaired immune function, delayed wound healing, and decreased ability to recover from surgical stress in malnourished individuals. The prognostic nutritional index (PNI), first proposed by Onodera et al., was originally developed to assess the surgical risk associated with gastrointestinal surgery, particularly to determine whether a patient could safely undergo intestinal anastomosis[11]. The PNI is easily calculated using serum albumin levels and lymphocyte counts, and it has since been applied across a wide range of malignancies as a simple yet effective nutritional and immunological marker[12,13]. Its ease of use, low cost, and strong correlation with postoperative outcomes have led to its widespread adoption in both clinical practice and research settings.
This study aimed to clarify the influence of malnutrition, defined according to the PNI, on surgical outcomes among patients undergoing LLR for HCC. Malnourished patients were identified using the PNI, and short-term outcomes were comprehensively evaluated using the textbook outcome (TO), while long-term outcomes were assessed based on overall survival (OS) and recurrence-free survival (RFS). By analyzing both short-term and long-term endpoints, our goal was to provide a more nuanced understanding of how preoperative nutritional status affects the overall clinical course following LLR for HCC.
In reporting this observational research, we adhered to the STROBE checklist to ensure transparency and completeness[14]. This adherence enhances transparency, reproducibility, and methodological rigor in reporting observational research.
METHODS
Participants
Between January 2011 and December 2019, 121 patients with HCC underwent primary LLR at our institution. We retrospectively reviewed their medical records to extract clinical variables, including demographic information (age, sex, and body mass index), recent weight loss, skeletal muscle index at the level of the third lumbar vertebra (L3-SMI), Eastern Cooperative Oncology Group performance status, American Society of Anesthesiologists physical status (ASA-PS), and indicators of liver function such as Child–Pugh score and indocyanine green retention at 15 min (ICGR15). Data extraction was performed independently by two trained researchers using a standardized data collection form. Any discrepancies in data interpretation were resolved by consensus or by consulting a senior investigator. This approach ensured the accuracy and consistency of the clinical data used in the analysis. Laboratory data included total lymphocyte count, serum albumin, aspartate aminotransferase, alanine aminotransferase, alpha-fetoprotein (AFP), and protein induced by vitamin K absence or antagonist-II (PIVKA-II). Tumor characteristics included size, number, stage, and presence of macroscopic vascular invasion. We also documented intraoperative blood loss, need for perioperative transfusion, occurrence of postoperative complications, and length of hospital stay. Skeletal muscle mass was quantified by calculating the L3-SMI, which was obtained from the cross-sectional muscle area (cm2) at the third lumbar vertebra on non-contrast CT images and adjusted for the square of patient height (m2). Liver function before surgery was evaluated using the Child–Pugh classification together with ICGR15. Tumor stage was assigned according to the General Rules for the Clinical and Pathological Study of Primary Liver Cancer[15]. Surgical procedures included laparoscopic partial resection, Couinaud segmentectomy, and sectionectomy. The severity of postoperative complications was graded using the Clavien–Dindo classification[16]. The nutritional status of patients was assessed using the PNI, which was calculated with the following formula: 10 × albumin (g/dL) + 0.005 × lymphocyte count (/μL), with a cutoff of < 40 indicating malnutrition[11]. All diagnoses and evaluations were made in accordance with the standard institutional protocols and current clinical guidelines for HCC management, ensuring uniformity across the study population.
Surgical procedure
Patients exhibiting evident ascites, clinical manifestations of portal hypertension, or distant metastases were deemed unsuitable for surgical resection and thus excluded. To evaluate tumor burden and exclude contraindications such as advanced disease or extra-hepatic metastasis, we used preoperative imaging modalities, namely contrast-enhanced computed tomography (CT) and magnetic resonance imaging (MRI). Surgical eligibility was determined by a multidisciplinary team consisting of surgeons, hepatologists, and radiologists. Since its introduction at our institution, LLR has been routinely performed using a five-port trocar technique. A flexible laparoscope was utilized to facilitate optimal visualization and maneuverability, particularly in posterior and superior liver segments. For parenchymal transection, we employed the clamp-crushing approach assisted by an ultrasonic vessel-sealing system (HARMONIC®; Ethicon Endo-Surgery Inc., Cincinnati, OH, USA). Bleeding control was achieved with a monopolar soft coagulation device (ERBE, Tübingen, Germany). During transection of the liver parenchyma, an intermittent Pringle maneuver was employed using a tourniquet to minimize intraoperative blood loss. The typical protocol involved 15 min of clamping followed by a 5-min release, repeated as needed. At the end of the procedure, a surgical drain was typically placed near the liver resection margin, and the abdominal wall was subsequently closed in layers. Drain output was assessed daily, and removal was based on the overall clinical course, taking into account the absence of bile leakage and a trend of decreasing output volume. Postoperative imaging and laboratory data were routinely monitored for early detection of complications.
TO
To comprehensively assess short-term outcomes, we used the concept of the TO, which has recently emerged as a valuable composite metric in surgical quality assessment. Unlike single outcome measures such as complication rate or length of stay, TO offers a multidimensional assessment that encompasses both surgical success and patient-centered recovery. This holistic approach provides a more robust reflection of perioperative quality, facilitating cross-study comparisons and benchmarking across institutions. Based on a review of prior literature on TO in liver surgery[10,17,18], we adopted five specific criteria to define TO in our study: absence of 30-day postoperative mortality, negative surgical margins, no unplanned readmission within 30 days, absence of major complications, and avoidance of prolonged postoperative hospitalization. These five indicators were selected due to their frequent adoption in previous hepatobiliary studies and their clinical relevance in the context of minimally invasive liver resection. Surgical margins were defined as negative when histopathology confirmed R0 resection. We defined major complications as adverse events classified as Grade III or higher according to the Clavien–Dindo system. Prolonged hospital stay was defined as a postoperative length of stay of 16 days or longer, corresponding to the 75th percentile of our overall patient cohort. This threshold is consistent with previous studies that employed TO as an outcome measure. TO was considered achieved only when all five criteria were simultaneously met. If even one of the conditions was not fulfilled, the case was classified as not achieving TO.
Follow-up
Postoperative surveillance included routine blood testing every three months to assess tumor markers such as AFP and PIVKA-II. These intervals were chosen to facilitate early detection of recurrence and guide timely intervention, particularly in high-risk patients. All laboratory results were interpreted by hepatologists or attending surgeons, and any suspicious findings were discussed at multidisciplinary meetings. Imaging studies, including contrast-enhanced CT or MRI, were conducted at intervals of 3 to 6 months. Recurrence was diagnosed based on characteristic imaging features of HCC, such as arterial enhancement and washout in the venous phase, and was typically supported by rising tumor marker levels. When recurrence of HCC was detected, management followed the Clinical Practice Guidelines for the Management of Hepatocellular Carcinoma[19]. Treatment strategies, including repeat resection, transarterial chemoembolization, radiofrequency ablation (RFA), and systemic therapy, were determined according to tumor burden, liver function, and patient performance status, based on a multidisciplinary team approach. Patients were monitored until death, study censorship, or the end of the observation period in December 2023, whichever occurred first. Follow-up data were collected prospectively using a standardized template and reviewed by the research team at regular intervals to ensure data quality and completeness.
Statistical analysis
All analyses were conducted using JMP software (version 18.0.1; SAS Institute Inc., Cary, NC, USA). Continuous variables are expressed as medians, while categorical data are presented as frequencies and percentages. Comparisons of continuous variables were conducted using the the Mann-Whitney U test because the data were not normally distributed, whereas categorical variables were analyzed using Pearson’s chi-square or Fisher’s exact test, as appropriate. The definition of OS was the interval between surgery and either death from any cause or the date of last follow-up. RFS was defined as the interval between surgery and the first confirmed recurrence or death, whichever occurred first. OS and RFS were analyzed using the Kaplan–Meier method, and survival curves were compared using the log-rank test. To explore prognostic factors, both univariate and multivariate analyses were carried out with Cox proportional hazards models. For the multivariate models, variables were entered simultaneously using a forced-entry approach. Variables with a P value < 0.05 in the univariate analysis were included in the multivariate model. Statistical significance was defined as a two-sided P value lower than 0.05.
RESULTS
Patient characteristics
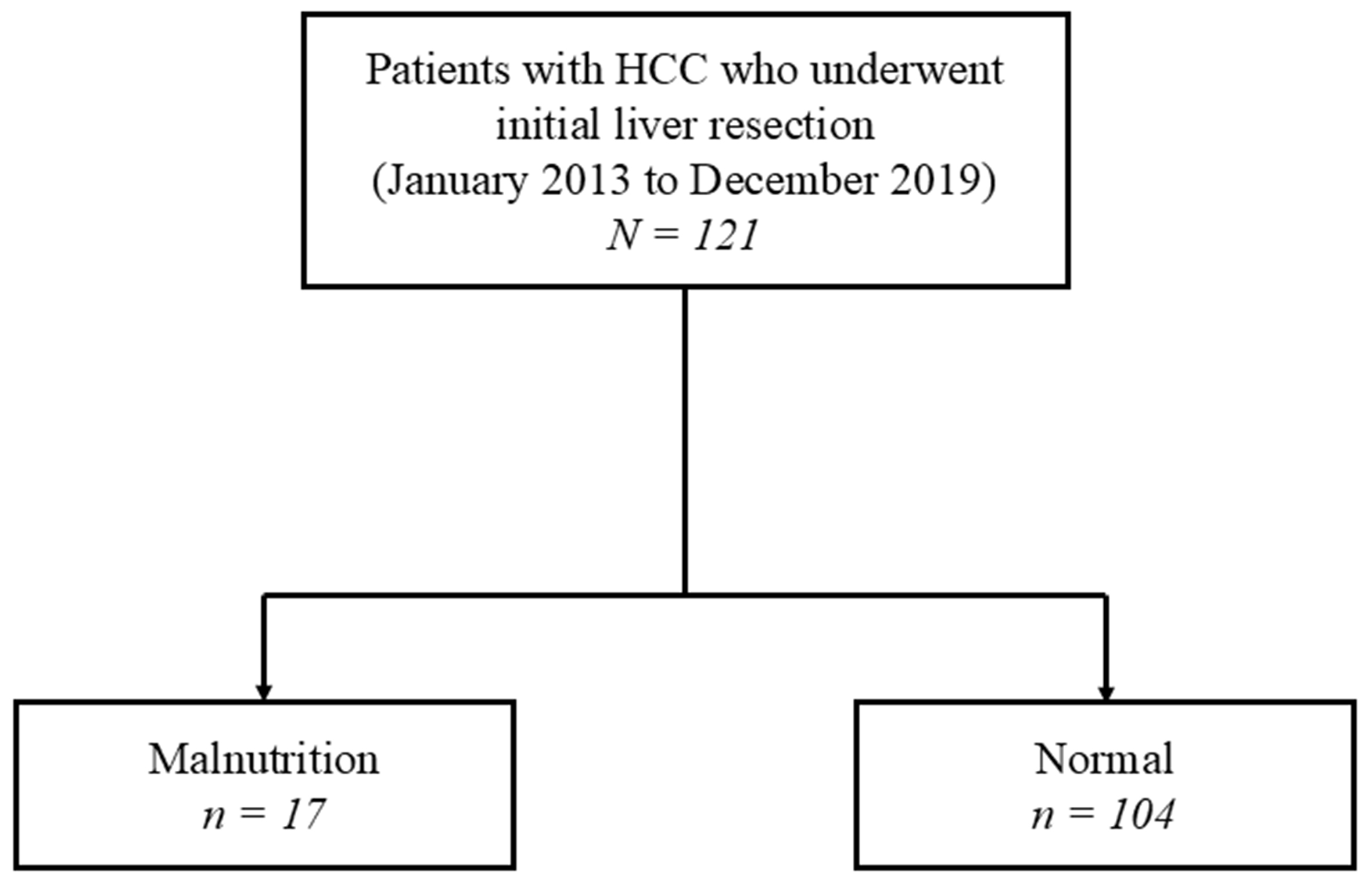
The PNI assessment identified 17 patients (14.0%) as having malnutrition (PNI < 40) and 104 patients (86.0%) as normal (PNI ≥ 40) [Figure 1]. Table 1 summarizes the comparison of clinical characteristics between the two groups. No significant differences were found in age, sex distribution, BMI, tumor size, tumor number, serum AFP, or PIVKA-II levels, suggesting well-balanced baseline oncologic characteristics. Likewise, the distribution of surgical procedures was similar between the malnutrition and normal groups (sectionectomy: 17.7% vs. 19.2%; segmentectomy: 11.8% vs. 8.7%; partial resection: 70.5% vs. 72.1%, respectively; P = 0.914). Therefore, surgical extent is unlikely to have influenced the observed differences in outcomes. However, several clinical parameters were significantly different between the groups. The malnutrition group demonstrated lower skeletal muscle mass with significantly reduced L3-SMI values (38.8 vs. 45.3 cm2/m2, P = 0.004), and poorer performance status (ASA-PS ≥ 3: 35.3% vs. 13.5%, P = 0.036). Both parameters of the PNI - serum albumin (3.4 vs. 4.1 g/dL, P < 0.001) and lymphocyte count (954 vs. 1,317/μL, P = 0.010) - were significantly lower in the malnutrition group, consistent with the nutritional classification. Additionally, the malnutrition group also exhibited significantly poorer liver function, including a greater proportion of Child–Pugh B cases (11.8% vs. 1.9%, P = 0.035) and elevated ICGR15 values (20.6% vs. 11.9%, P = 0.044). These findings suggest that the malnutrition group not only had biochemical markers of undernutrition but also impaired physical and liver functional reserves.
Figure 1. Flowchart of patient selection and grouping according to nutritional status, as graded by the PNI. HCC: Hepatocellular carcinoma; PNI: prognostic nutritional index.
Comparison of clinical characteristics between nutritional status groups
| Variable | Malnutrition n = 17 | Normal n = 104 | P value | |
| Age (years)† | 73 (60-84) | 70 (45-93) | 0.290 | |
| Sex | Male | 11 (64.7) | 83 (79.8) | 0.207 |
| Female | 6 (35.3) | 21 (20.2) | ||
| BMI (kg/m2)† | 22.5 (17.0-34.1) | 24.1 (15.8-38.9) | 0.103 | |
| Weight loss (%)† | 2.30 (0-7.76) | 1.30 (0-10.42) | 0.435 | |
| L3-SMI (cm2/m2)† | 38.8 (25.3-53.9) | 45.3 (28.5-68.9) | 0.004 | |
| ECOG-PS (0/ ≥ 1) | 0 | 15 (88.2) | 96 (92.3) | 0.630 |
| ≥ 1 | 2 (11.8) | 8 (7.7) | ||
| ASA-PS | ≤ II | 11 (64.7) | 90 (86.5) | 0.036 |
| ≥ III | 6 (35.3) | 14 (13.5) | ||
| Child–Pugh class | A | 15 (88.2) | 102 (98.1) | 0.035 |
| B | 2 (11.8) | 2 (1.9) | ||
| ICGR15 (%)† | 20.6 (1.7-49.0) | 11.9 (3.8-47.3) | 0.044 | |
| Lymphocyte (/μL)† | 954 (240-1,754) | 1317 (407-2,910) | 0.010 | |
| Serum albumin (g/dL)† | 3.4 (2.7-3.7) | 4.1 (3.4-5.1) | < 0.001 | |
| Serum AST (IU/L)† | 27 (16-91) | 31 (13-86) | 0.568 | |
| Serum ALT (IU/L)† | 19 (7-91) | 30 (6-149) | 0.003 | |
| Serum AFP (ng/mL)† | 12 (2-8,229) | 7 (2-10,136) | 0.342 | |
| Serum PIVKA-II (mAU/mL)† | 37 (17-265,961) | 68 (12-29,944) | 0.244 | |
| Tumor size (mm)† | 30 (14-78) | 28 (10-75) | 0.417 | |
| Tumor number | Solitary | 14 (82.4) | 90 (86.5) | 0.706 |
| Multiple | 3 (17.7) | 14 (13.5) | ||
| Macroscopic vascular invasion | 0 (0) | 5 (4.8) | 1.000 | |
| Tumor stage | ≤ II | 15 (88.2) | 92 (88.5) | 1.000 |
| ≥ III | 2 (11.8) | 12 (11.5) | ||
| Operative blood loss (mL)† | 50 (10-900) | 50 (5-1,550) | 0.655 | |
| Perioperative blood transfusion | 2 (11.8) | 6 (5.8) | 0.312 | |
| Postoperative hospital stays (days)† | 10 (7-43) | 10 (5-130) | 0.854 | |
TO
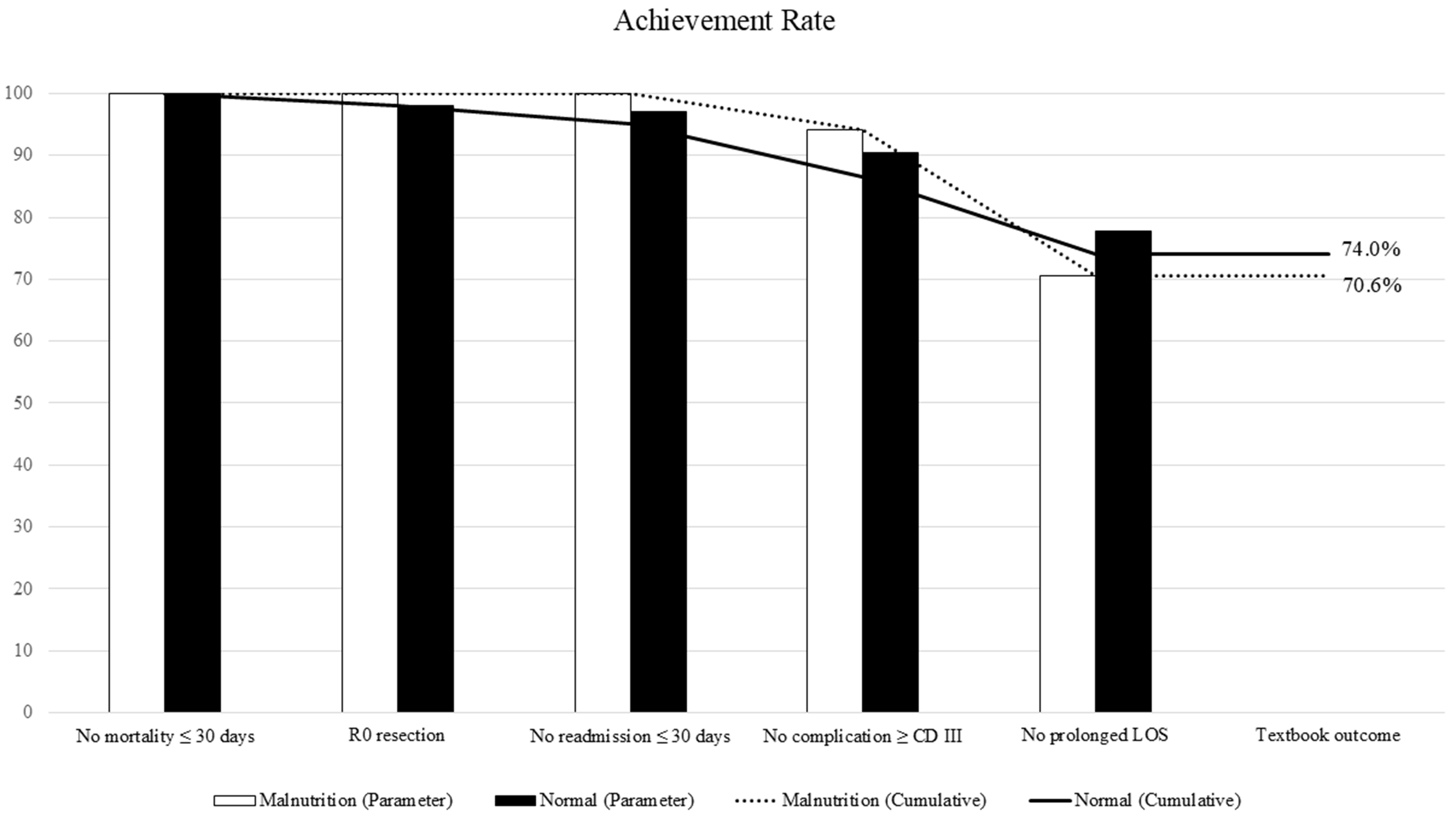
Table 2 and Figure 2 present the achievement rates of the five individual outcome components and composite TO. TO was achieved in 70.6% (12/17) of the malnutrition group and 74.0% (77/104) of the normal group (P = 0.771), with no significant difference between the groups. There were no 30-day postoperative deaths in either group. R0 resection was achieved in 100% of malnourished patients and 98.1% of normal patients. Major postoperative complications (Clavien–Dindo Grade ≥ III) occurred in 1 patient (5.9%) in the malnutrition group and 10 patients (9.6%) in the normal group (P = 1.000). Unplanned readmission within 30 days occurred only in the normal group (6.7%); none were seen in the malnutrition group. Prolonged postoperative hospital stay (≥ 16 days) was noted in 5 patients (29.4%) in the malnutrition group and 21 patients (20.2%) in the normal group (P = 0.530). Among patients who did not achieve TO, the most common reason was prolonged hospitalization, followed by major complications. These results indicate that short-term surgical outcomes were comparable between the groups regardless of nutritional status.
Figure 2. Distribution of TO and its individual components. TO: Textbook outcome; LOS: length of hospital stay.
The comparison of achievement rate of TO between the nutritional status groups
| Outcome | Malnutrition n = 17 | Normal n = 104 | P value |
| TO | 12 (70.6) | 77 (74.0) | 0.771 |
| No mortality ≤ 30 days | 17 (100) | 104 (100) | 1.000 |
| R0 resection | 17 (100) | 102 (98.1) | 1.000 |
| No readmission ≤ 30 days | 17 (100) | 101 (97.1) | 1.000 |
| No complication ≥ Clavien–Dindo grade III | 16 (94.1) | 94 (90.4) | 1.000 |
| No prolonged length of hospital stays | 12 (70.6) | 82 (78.9) | 0.530 |
Survival analysis
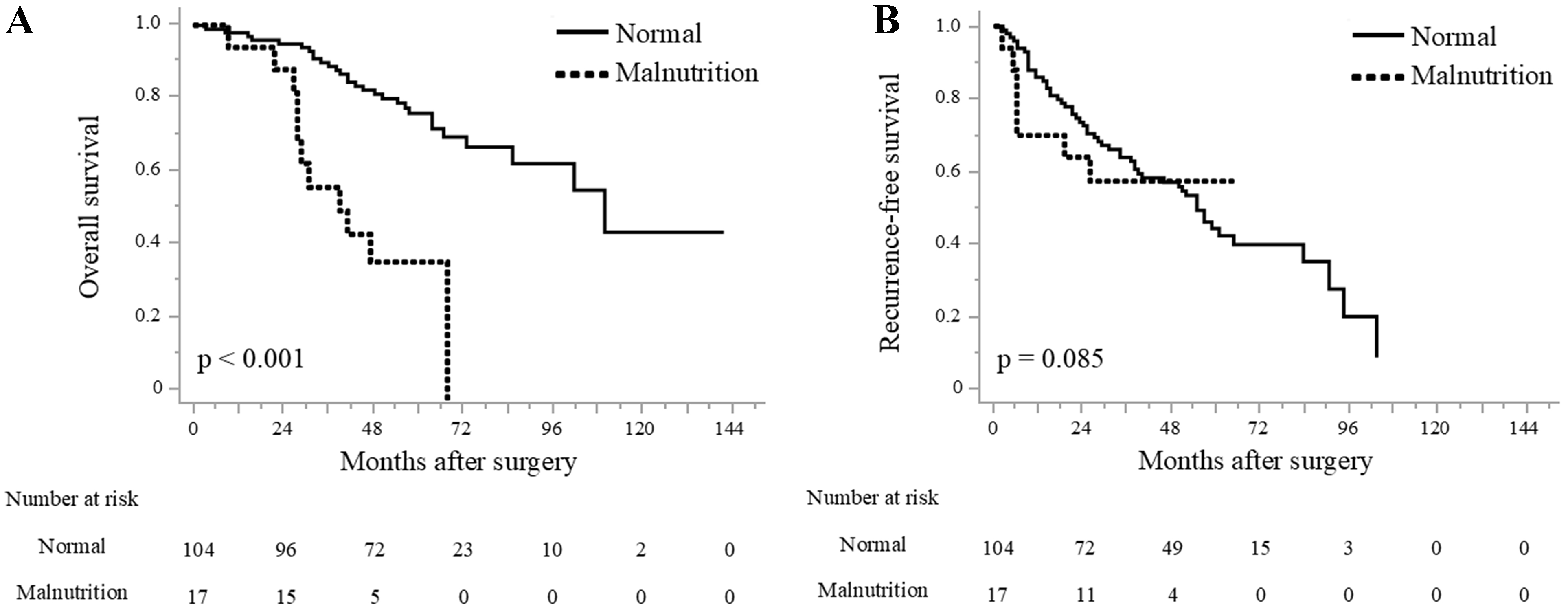
The Kaplan–Meier survival curves are shown in Figure 3. OS was significantly worse in the malnutrition group than in the normal group, with median survival times of 40 months vs. 107 months, respectively (P < 0.001; Figure 3A). RFS also showed a trend toward being worse in the malnutrition group, although this difference did not reach statistical significance (not reached vs. 57 months, P = 0.085; Figure 3B). The median RFS in the malnutrition group was not reached because of limited follow-up and the small number of recurrence events, but the trend indicates a potential association between poor nutritional status and early recurrence. The median follow-up duration for the overall cohort was 58 months. Among deceased patients, 5 of 11 in the malnutrition group died from HCC recurrence, while in the normal group, 12 of 30 deaths were attributed to tumor recurrence. This finding suggests that in the malnutrition group, both cancer-related and non-cancer-related factors contributed to the unfavorable OS.
Prognostic factors
To identify variables influencing long-term prognosis, we applied both univariate and multivariate analyses using Cox proportional hazards models. Table 3 summarizes the results for OS. In univariate analysis, ASA-PS ≥ 3, multiple tumors, tumor size ≥ 50 mm, advanced tumor stage, and malnutrition (PNI < 40) were significantly associated with worse OS. In multivariate analysis, PNI-defined malnutrition emerged as the sole independent prognostic factor for OS [hazard ratio (HR) 3.88; 95% confidence interval (CI) 1.80-8.36; P < 0.001]. Tumor-related factors did not remain significant, possibly due to the limited sample size and strong correlation with nutritional status. For RFS [Table 4], only tumor size ≥ 50 mm was recognized as a significant prognostic factor in both univariate (HR 2.53; 95%CI 1.24-5.16; P = 0.010) and multivariate analyses (HR 2.86; 95%CI 1.38-5.90; P = 0.004). Nutritional status, TO achievement, and other baseline factors showed no significant association with RFS.
Univariate and Multivariate analysis for OS
| Variables | Univariate HR | 95%CI | P value | Multivariate HR | 95%CI | P value | |
| Age (years) | < 70 | Reference | |||||
| ≥ 70 | 1.81 | 0.95-3.44 | 0.068 | ||||
| Sex | Male | Reference | |||||
| Female | 2.08 | 0.81-5.33 | 0.124 | ||||
| ECOG-PS | 0 | Reference | |||||
| ≥ 1 | 2.23 | 0.86-5.75 | 0.095 | ||||
| ASA-PS | ≤ II | Reference | Reference | ||||
| ≥ III | 2.46 | 1.22-4.93 | 0.011 | 2.04 | 0.96-4.33 | 0.061 | |
| Child–Pugh class | A | Reference | |||||
| B | 1.80 | 0.43-7.51 | 0.418 | ||||
| ICGR15 (%) | < 10 | Reference | |||||
| ≥ 10 | 1.36 | 0.67-2.74 | 0.385 | ||||
| Serum AST (IU/L) | ≤ 50 | Reference | |||||
| > 50 | 1.18 | 0.52-2.70 | 0.684 | ||||
| Serum ALT (IU/L) | ≤ 50 | Reference | |||||
| > 50 | 1.26 | 0.55-2.89 | 0.573 | ||||
| Serum AFP (ng/mL) | < 20 | Reference | |||||
| ≥ 20 | 1.19 | 0.61-2.31 | 0.590 | ||||
| Serum PIVKA-II (mAU/mL) | < 40 | Reference | |||||
| ≥ 40 | 1.36 | 0.70-2.61 | 0.353 | ||||
| Tumor size (mm) | < 50 | Reference | Reference | ||||
| ≥ 50 | 2.40 | 1.05-5.49 | 0.037 | 2.24 | 0.95-5.28 | 0.064 | |
| Number of tumors | Solitary | Reference | Reference | ||||
| Multiple | 3.36 | 1.60-7.05 | 0.001 | 3.51 | 0.93-13.27 | 0.063 | |
| Macroscopic vascular invasion | Negative | Reference | |||||
| Positive | 1.82 | 0.43-7.60 | 0.411 | ||||
| Tumor stage | ≤ II | Reference | Reference | ||||
| ≥ III | 3.09 | 1.39-6.84 | 0.005 | 1.18 | 0.29-4.82 | 0.811 | |
| Operative blood loss (mL) | < 500 | Reference | |||||
| ≥ 500 | 1.23 | 0.43-3.49 | 0.691 | ||||
| Perioperative blood transfusion | No | Reference | |||||
| Yes | 1.83 | 0.64-5.18 | 0.252 | ||||
| TO | Yes | Reference | |||||
| No | 1.69 | 0.87-3.28 | 0.116 | ||||
| Nutritional status graded by the PNI | Normal | Reference | Reference | ||||
| Malnutrition | 4.35 | 2.12-8.92 | < 0.001 | 3.88 | 1.80-8.36 | < 0.001 | |
Univariate and Multivariate analysis for RFS
| Variables | Univariate HR | 95%CI | P value | Multivariate HR | 95%CI | P value | |
| Age (years) | < 70 | Reference | |||||
| ≥ 70 | 1.23 | 0.75-2.02 | 0.400 | ||||
| Sex | Male | Reference | |||||
| Female | 1.51 | 0.78-2.91 | 0.211 | ||||
| ECOG-PS | 0 | Reference | |||||
| ≥ 1 | 1.33 | 0.53-3.34 | 0.535 | ||||
| ASA-PS | ≤ II | Reference | |||||
| ≥ III | 0.88 | 0.43-1.79 | 0.727 | ||||
| Child–Pugh class | A | Reference | |||||
| B | 2.68 | 0.83-8.65 | 0.098 | ||||
| ICGR15 (%) | < 10 | Reference | |||||
| ≥ 10 | 1.58 | 0.89-2.80 | 0.114 | ||||
| Serum AST (IU/L) | ≤ 50 | Reference | |||||
| > 50 | 1.13 | 0.57-2.23 | 0.723 | ||||
| Serum ALT (IU/L) | ≤ 50 | Reference | |||||
| > 50 | 1.01 | 0.51-2.01 | 0.966 | ||||
| Serum AFP (ng/mL) | < 20 | Reference | |||||
| ≥ 20 | 1.17 | 0.68-1.99 | 0.556 | ||||
| Serum PIVKA-II (mAU/mL) | < 40 | Reference | |||||
| ≥ 40 | 1.35 | 0.80-2.26 | 0.250 | ||||
| Tumor size (mm) | < 50 | Reference | Reference | ||||
| ≥ 50 | 2.53 | 1.24-5.16 | 0.010 | 2.86 | 1.38-5.90 | 0.004 | |
| Number of tumors | Solitary | Reference | Reference | ||||
| Multiple | 2.95 | 1.56-5.57 | < 0.001 | 2.13 | 0.74-6.17 | 0.160 | |
| Macroscopic vascular invasion | Negative | Reference | |||||
| Positive | 2.06 | 0.64-6.67 | 0.224 | ||||
| Tumor stage | ≤ II | Reference | Reference | ||||
| ≥ III | 2.99 | 1.51-5.94 | 0.001 | 1.76 | 0.56-5.51 | 0.330 | |
| Operative blood loss (mL) | < 500 | Reference | |||||
| ≥ 500 | 1.63 | 0.74-3.60 | 0.222 | ||||
| Perioperative blood transfusion | No | Reference | |||||
| Yes | 2.06 | 0.88-4.81 | 0.092 | ||||
| TO | Yes | Reference | |||||
| No | 1.08 | 0.60-1.95 | 0.776 | ||||
| Nutritional status graded by the PNI | Normal | Reference | |||||
| Malnutrition | 1.12 | 0.50-2.48 | 0.772 | ||||
DISCUSSION
Minimally invasive liver resection, especially LLR, has been increasingly adopted worldwide because of its benefits, including reduced postoperative pain and morbidity, and shorter hospitalization duration compared with open liver resection[6,7]. These benefits are particularly relevant when considering surgery for frail populations, including older and malnourished patients. Recent studies have demonstrated that laparoscopic surgery is not only feasible but also safe for older patients and those with poor nutritional status, without compromising short-term outcomes. Nomi et al. reported that LLR was safely performed in older patients with HCC without increased morbidity[20]. Similarly, Chen et al. showed that laparoscopic gastrectomy in a malnourished cohort significantly improved both short- and long-term postoperative outcomes compared with open surgery in patients with gastric cancer[21]. Our present study confirms these findings by demonstrating that short-term surgical safety, as assessed by the TO, was maintained in both nutritional groups. The TO metrics - including lack of 30-day mortality, R0 resection, no Clavien–Dindo grade III or higher complications, no 30-day readmission, and no prolonged hospital stay - were comparable between groups stratified by nutritional status. This finding suggests that LLR provides a level of perioperative safety that is preserved even in patients with compromised nutritional reserves. Our results further suggest that the inherent advantages of laparoscopic surgery - such as reduced surgical stress and faster functional recovery - may play a particularly important role in protecting nutritionally vulnerable patients. By minimizing physiological burden and preserving postoperative immune competence, LLR may help maintain short-term outcomes in patients who would otherwise be considered at higher risk. The present study underscores the benefit of minimally invasive techniques in enabling curative surgery without significantly increasing perioperative risk, even in high-risk populations. Additionally, careful patient selection and meticulous perioperative management likely played essential roles in mitigating surgical risk.
Despite these encouraging short-term outcomes, long-term prognosis remains a concern for malnourished patients undergoing liver resection. While surgical resection remains the most definitive treatment for HCC, non-surgical treatments, such as RFA and radiation therapy (RT), are being increasingly recognized as viable alternatives, particularly for patients who are not candidates for surgery due to their nutritional status or overall condition. These treatments, which are less invasive than conventional surgery, have the advantage of good tolerability by patients with poor nutritional reserves. Recent studies have shown that RFA and RT can provide comparable survival outcomes to resection, particularly in patients with early-stage tumors or those with compromised liver function[22-24]. For malnourished patients, these techniques offer a means of tumor control while avoiding the additional stresses associated with major surgery. Given their reduced invasiveness, these treatments may be more appropriate for patients who would otherwise face significant risks during surgery. Incorporating RFA or RT into treatment plans for malnourished patients with HCC can provide a feasible, lower-risk option for managing disease progression and improving outcomes.
Malnutrition is known to compromise both innate and adaptive immunity, including decreases in natural killer cell activity, impaired T-cell responses, and altered cytokine production - all of which are critical for eliminating residual tumor cells postoperatively[25,26]. These deficits create a microenvironment favorable to tumor recurrence. For instance, a low PNI - a reflection of hypoalbuminemia and lymphopenia - has been associated with impaired antitumor immunity and early recurrence after cancer surgery[27,28]. Even in the context of minimally invasive surgery, this immunologic impact may be amplified in nutritionally compromised hosts. Our study observed a trend toward worse RFS in the malnutrition group, although statistical significance was not reached due to the limited sample size. Interestingly, when the OS was analyzed, the impact of malnutrition became more pronounced. The malnutrition group exhibited significantly worse OS despite not yet reaching median RFS. This discrepancy suggests that causes of death other than tumor recurrence may be disproportionately affecting this group. Among deceased patients, 5 of 11 in the malnutrition group died from HCC recurrence, compared with 12 of 30 in the normal group. This suggests that in malnourished patients, both cancer recurrence and non-cancer causes may contribute to inferior OS. Prior studies have reported that malnourished patients are more likely to die from non-cancer-related causes such as infections, cardiovascular events, and organ failure. Shoji et al. found that patients with malnutrition were at increased risk for non-cancer mortality following thoracic surgery, highlighting the systemic vulnerability of this population[29]. Although the proportion of cancer-related deaths was similar between nutritional groups, OS remained worse in the malnourished cohort. This suggests that the PNI may not solely reflect oncologic aggressiveness but instead a broader systemic vulnerability, encompassing both tolerance to disease progression and resilience to treatment-related stress. In addition to predicting perioperative risk, PNI may also serve as a surrogate marker of systemic frailty and immunological competence, offering a broader perspective on the patient’s physiological resilience. In contrast, patients with normal nutritional status may have greater physiologic reserve, enabling prolonged survival even in the context of cancer recurrence. To further minimize potential confusion due to differences in baseline liver function, we conducted an exploratory propensity score matching analysis that included Child–Pugh class, ICGR15, tumor stage, extent of resection, and age, using a caliper width of 0.2. This yielded 14 matched pairs. In the matched cohort, the achievement rate of TO was 64.3% in the malnutrition group and 85.7% in the normal group (P = 0.384), indicating no significant difference in short-term outcomes. However, median OS remained significantly worse in the malnutrition group (38 months vs. 99 months, P = 0.003), while RFS did not differ (not reached vs. 32.5 months, P = 0.655). Although limited by the small sample size, these results reinforce the robustness of our findings, suggesting that poor nutritional status independently contributes to inferior long-term survival even when baseline liver function is comparable.
While the PNI has proven useful for evaluating nutritional and immunological status in surgical patients, it is not the only available indicator. Other indices, such as the controlling nutritional status score, geriatric nutritional risk index, and albumin-bilirubin grade, have also been validated in hepatobiliary surgery and oncology settings[30-32]. Each marker reflects a slightly different aspect of patient physiology - such as lipid metabolism, renal function, or hepatic reserve - and may be more suitable depending on the clinical context. Although the PNI offers the advantage of simplicity and widespread familiarity, it does not incorporate liver-specific parameters or markers of systemic inflammation. In this regard, using multiple nutritional indices in parallel or developing composite scoring systems could offer a more holistic assessment of patient frailty. Future studies might consider comparing these indices directly to determine the most reliable predictors for postoperative outcomes in patients with HCC undergoing liver resection. In addition to biochemical markers, body composition metrics such as the L3-SMI have emerged as important predictors of outcomes in hepatobiliary surgery. Sarcopenia, or the progressive loss of skeletal muscle mass and function, has been associated with increased postoperative morbidity, longer hospital stays, and diminished long-term survival[33]. In the present study, L3-SMI values were significantly lower in the malnutrition group, suggesting a concordance between sarcopenia and poor nutritional status as assessed by PNI. However, discordance between these two parameters can also occur; for example, a patient may present with low muscle mass but retain adequate serum albumin and lymphocyte counts. Therefore, a combined assessment using both PNI and L3-SMI - or potentially incorporating emerging criteria such as those proposed by the Global Leadership Initiative on Malnutrition (GLIM)[34] - may provide a more robust framework for risk stratification and clinical decision making. Prospective validation of such combined models would be a valuable direction for future research. Preoperative nutritional intervention may offer a promising approach to improve postoperative outcomes in malnourished patients. Several studies have suggested that optimizing nutritional status through targeted supplementation or immunonutrition prior to surgery can enhance immune responses, reduce complications, and potentially improve long-term survival[35,36]. However, evidence in the context of HCC and liver resection remains limited and warrants further investigation.
As this study was retrospective and restricted to a single institution, confirmation through prospective multicenter investigations is recommended to strengthen the robustness and generalizability of the findings. A larger sample size drawn from diverse institutions would provide greater statistical power to detect subgroup differences and refine the predictive value of nutritional indices such as the PNI. Moreover, incorporating a standardized protocol for perioperative nutritional intervention and functional assessment - such as muscle strength or physical performance - would allow for a more nuanced evaluation of frailty. In the era of personalized medicine, developing a risk stratification model that integrates nutritional, oncologic, and surgical factors could guide decision making and optimize patient selection for liver resection. Such models could also inform prehabilitation strategies and help identify patients who may benefit from nutritional optimization before undergoing curative treatment. Collaborative research efforts across institutions and disciplines will be essential to establish evidence-based guidelines that improve outcomes in malnourished patients with HCC.
This study includes several limitations. First, the sample size was limited, reducing the statistical power to detect subtle differences in outcomes such as RFS or in subgroup analyses. Second, due to the retrospective design, selection bias is inherently present. Notably, even among patients classified as having poor nutritional status, only those deemed fit for surgery were included in this study, potentially underestimating the true impact of malnutrition. Third, the TO criteria used - while based on previous literature - do not reflect a universally accepted standard. Different studies may define TO differently, limiting the generalizability of our findings. Moreover, nutritional status was assessed solely using the PNI, which, although validated, may not fully capture the multidimensional aspects of malnutrition, such as sarcopenia or micronutrient deficiencies. Combining the PNI with body composition analysis may provide a more comprehensive understanding of patient frailty. Finally, the cause-of-death analysis in our study was limited by its retrospective nature and reliance on available medical records. Some deaths may have been multifactorial or incorrectly classified, particularly in malnourished patients with multiple comorbidities. A more detailed prospective follow-up could help clarify the mechanisms underlying the observed association between low PNI and worse OS.
In conclusion, LLR may be a safe and feasible surgical option for HCC, even in patients with compromised nutritional status. However, poor preoperative nutritional status - as assessed by the PNI - remains a significant predictor of inferior long-term outcomes, particularly OS. These findings underscore the importance of comprehensive preoperative assessment and the potential value of nutritional intervention strategies in improving oncologic outcomes. A multidisciplinary approach that integrates nutritional evaluation, perioperative optimization, and individualized treatment planning may contribute to improved survival and quality of care in this high-risk population.
DECLARATIONS
Authors’ contributions
Conceptualization and design: Oji K, Urade T
Administrative support: Oji K, Omiya S, Urade T
Provision of study materials or patients: Oji K, Urade T, Omiya S, Kido M, Komatsu S, Fukushima K, Yoshida T, Tai K, Arai K, Iguchi K, Yanagimoto H, Fukumoto T
Collection and assembly of data: Oji K, Omiya S, Urade T
Data analysis and interpretation: Oji K, Urade T
Manuscript writing: Oji K, Urade T
Critical revision: Omiya S, Kido M, Komatsu S, Fukushima K, Yoshida T, Tai K, Arai K, Iguchi K, Lee D, Akita M, Mizumoto T, Ishida J, Nanno Y, Asari S, Yanagimoto H, Fukumoto T
Final approval of manuscript: Oji K, Urade T, Omiya S, Kido M, Komatsu S, Fukushima K, So S, Yoshida T, Tai K, Arai K, Iguchi K, Lee D, Akita M, Mizumoto T, Ishida J, Nanno Y, Asari S, Yanagimoto H, Fukumoto T
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Availability of data and materials
The data are not publicly available due to the inclusion of information that could compromise the privacy of research participants.
Financial support and sponsorship
None.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
This investigation received approval from the Institutional Ethics Committee at Kobe University Hospital (Approval No. B220120) and was performed in accordance with the Declaration of Helsinki. Owing to the retrospective design, the board waived the requirement for written informed consent. Instead, information regarding the study and an opt-out option was made publicly available on the hospital’s official website. All patient data were anonymized prior to analysis to protect personal information, and access to the dataset was restricted to the study investigators.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6.
2. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-49.
3. Tsilimigras DI, Bagante F, Sahara K, et al. Prognosis After Resection of Barcelona Clinic Liver Cancer (BCLC) Stage 0, A, and B hepatocellular carcinoma: a comprehensive assessment of the current BCLC classification. Ann Surg Oncol. 2019;26:3693-700.
4. Sorin Y, Ikeda K, Kawamura Y, et al. Effectiveness of particle radiotherapy in various stages of hepatocellular carcinoma: a pilot study. Liver Cancer. 2018;7:323-34.
5. Qin S, Chen M, Cheng AL, et al.; IMbrave050 investigators. Atezolizumab plus bevacizumab versus active surveillance in patients with resected or ablated high-risk hepatocellular carcinoma (IMbrave050): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2023;402:1835-47.
6. Tsai KY, Chen HA, Wang WY, Huang MT. Long-term and short-term surgical outcomes of laparoscopic versus open liver resection for hepatocellular carcinoma: might laparoscopic approach be better in early HCC? Surg Endosc. 2019;33:1131-9.
7. Takahara T, Wakabayashi G, Beppu T, et al. Long-term and perioperative outcomes of laparoscopic versus open liver resection for hepatocellular carcinoma with propensity score matching: a multi-institutional Japanese study. J Hepatobiliary Pancreat Sci. 2015;22:721-7.
8. Famularo S, Di Sandro S, Giani A, et al. The impact of age and ageing on hepatocarcinoma surgery: short- and long-term outcomes in a multicentre propensity-matched cohort. Liver Int. 2019;39:894-904.
9. Omiya S, Urade T, Komatsu S, et al. Impact of GLIM criteria-based malnutrition diagnosis on outcomes following liver resection for hepatocellular carcinoma. HPB. 2023;25:1555-65.
10. Oji K, Urade T, Omiya S, et al. Achieving textbook outcome in liver resection for hepatocellular carcinoma: malnutrition’s pivotal role. Langenbecks Arch Surg. 2025;410:139.
11. Onodera T, Goseki N, Kosaki G. [Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients]. Nihon Geka Gakkai Zasshi. 1984;85:1001-5.
12. Tanemura A, Mizuno S, Hayasaki A, et al. Onodera’s prognostic nutritional index is a strong prognostic indicator for patients with hepatocellular carcinoma after initial hepatectomy, especially patients with preserved liver function. BMC Surg. 2020;20:261.
13. Xishan Z, Ye Z, Feiyan M, Liang X, Shikai W. The role of prognostic nutritional index for clinical outcomes of gastric cancer after total gastrectomy. Sci Rep. 2020;10:17373.
14. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335:806-8.
15. Kudo M, Kitano M, Sakurai T, Nishida N. General Rules for the Clinical and Pathological Study of Primary Liver Cancer, Nationwide Follow-Up Survey and Clinical Practice Guidelines: the outstanding achievements of the Liver Cancer Study Group of Japan. Dig Dis. 2015;33:765-70.
16. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-13.
17. Tsilimigras DI, Sahara K, Moris D, et al. Assessing textbook outcomes following liver surgery for primary liver cancer over a 12-year time period at major hepatobiliary centers. Ann Surg Oncol. 2020;27:3318-27.
18. Ruzzenente A, Poletto E, Conci S, et al. Factors related to textbook outcome in laparoscopic liver resections: a single western centre analysis. J Gastrointest Surg. 2022;26:2301-10.
19. Kokudo N, Makuuchi M. Evidence-based clinical practice guidelines for hepatocellular carcinoma in Japan: the J-HCC guidelines. J Gastroenterol. 2009;44 Suppl 19:119-21.
20. Nomi T, Hirokawa F, Kaibori M, et al. Laparoscopic versus open liver resection for hepatocellular carcinoma in elderly patients: a multi-centre propensity score-based analysis. Surg Endosc. 2020;34:658-66.
21. Chen WZ, Yu DY, Zhang XZ, et al. Comparison of laparoscopic and open radical gastrectomy for gastric cancer patients with GLIM-defined malnutrition. Eur J Surg Oncol. 2023;49:376-83.
22. Wang JH, Wang CC, Hung CH, Chen CL, Lu SN. Survival comparison between surgical resection and radiofrequency ablation for patients in BCLC very early/early stage hepatocellular carcinoma. J Hepatol. 2012;56:412-8.
23. Hatanaka T, Kakizaki S, Hiraoka A, et al.; Real-life Practice Experts for HCC (RELPEC) Study Group. Comparison of surgical resection and radiofrequency ablation for early-stage HCC patients with Child-Pugh Class B. J Gastroenterol Hepatol. 2025;40:2068-77.
24. Fu Y, Yang Z, Liu S, et al. Comparison of resection, ablation, and stereotactic body radiation therapy in treating solitary hepatocellular carcinoma ≤ 5 cm: a retrospective, multicenter, cohort study. Int J Surg. 2025;111:1535-40.
25. Neeman E, Ben-Eliyahu S. Surgery and stress promote cancer metastasis: new outlooks on perioperative mediating mechanisms and immune involvement. Brain Behav Immun. 2013;30 Suppl:S32-40.
26. Donlon NE, Davern M, Hayes C, et al. The immune response to major gastrointestinal cancer surgery and potential implications for adjuvant immunotherapy. Crit Rev Oncol Hematol. 2022;175:103729.
27. Kazi M, Gori J, Sasi S, et al. Prognostic nutritional index prior to rectal cancer resection predicts overall survival. Nutr Cancer. 2022;74:3228-35.
28. Uri I, Horváth A, Tamás L, Polony G, Dános K. Prognostic nutritional index (PNI) correlates with survival in head and neck cancer patients more precisely than other nutritional markers - real world data. Eur Arch Otorhinolaryngol. 2024;281:6599-611.
29. Shoji F, Haratake N, Akamine T, et al. The preoperative controlling nutritional status score predicts survival after curative surgery in patients with pathological stage I non-small cell lung cancer. Anticancer Res. 2017;37:741-7.
30. Tsunematsu M, Haruki K, Fujiwara Y, et al. Preoperative controlling nutritional status (CONUT) score predicts long-term outcomes in patients with non-B non-C hepatocellular carcinoma after curative hepatic resection. Langenbecks Arch Surg. 2021;406:99-107.
31. Tsukagoshi M, Araki K, Igarashi T, et al. Lower geriatric nutritional risk index and prognostic nutritional index predict postoperative prognosis in patients with hepatocellular carcinoma. Nutrients. 2024;16:940.
32. Zhao S, Wang M, Yang Z, et al. Comparison between Child-Pugh score and Albumin-Bilirubin grade in the prognosis of patients with HCC after liver resection using time-dependent ROC. Ann Transl Med. 2020;8:539.
33. Giakoustidis A, Papakonstantinou M, Chatzikomnitsa P, et al. The effects of sarcopenia on overall survival and postoperative complications of patients undergoing hepatic resection for primary or metastatic liver cancer: a systematic review and meta-analysis. J Clin Med. 2024;13:3869.
34. Cederholm T, Jensen GL, Correia MITD, et al.; GLIM Core Leadership Committee, GLIM Working Group. GLIM criteria for the diagnosis of malnutrition - a consensus report from the global clinical nutrition community. Clin Nutr. 2019;38:1-9.
35. Ren L, Fu Y, Wang Z, Ma C, Ahn S, Meng Q. Effectiveness of the CANCER-AIMS intervention on nutritional status and symptom management in patients with gastric cancer following gastrectomy: a randomized controlled trial. Int J Nurs Stud. 2024;159:104873.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Copyright
Data & Comments
Data

















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].