Contemporary approaches to complex and contaminated hernias: innovations in mesh technology
Abstract
Ventral hernia repair in contaminated fields remains challenging, with evolving mesh technologies reshaping surgical approaches. While biologic mesh was traditionally preferred, recent evidence supports the use of permanent synthetic mesh in Centers for Disease Control (CDC) Class I-III wounds, showing lower recurrence rates and similar infection risks. Biosynthetic meshes, such as PhasixTM, provide reliable long-term durability and offer a cost-effective alternative to biologics, with promising infection resistance. A novel mesh suture (DurameshTM) improves fascial closure by distributing tension and integrating with tissue, showing early success in contaminated settings. This review outlines an approach to complex and contaminated hernias that incorporates these new technologies and discusses considerations for staged reconstruction. Permanent synthetic mesh remains the most reliable way to reduce hernia recurrence, while biosynthetic and mesh-suture technologies serve as valuable adjuncts for non-definitive repairs. Further research on the long-term safety and efficacy of biosynthetic meshes and mesh sutures is required to establish consensus in contaminated hernia repair.
Keywords
INTRODUCTION
Ventral hernia repair in contaminated fields remains a formidable challenge in abdominal wall reconstruction, with evolving considerations over time. Historically, wound contamination was considered an absolute contraindication to the placement of permanent synthetic mesh, and biologic mesh was favored because of its perceived lower risk of explantation[1]. However, several studies over the past decade have challenged this paradigm, highlighting concerns about the long-term performance of biologic mesh[2-4]. At the same time, growing evidence supports the selective use of permanent mesh in contaminated settings[5-7]. Against this backdrop, the medical device market has expanded rapidly, introducing new biologic, biosynthetic, and hybrid mesh designs, as well as novel suture materials. Consequently, in this paradox of expanding choices and evolving evidence, no consensus has yet been reached on the optimal approach to hernia repair in contaminated fields[8].
In this dynamic environment, a comprehensive understanding of the risks and benefits of different mesh types in contaminated settings is essential for tailoring surgical decision making. In addition, insights into the biomechanical properties of newer mesh innovations may help identify where they provide the greatest benefit for patients with complex, contaminated hernias. This review examines recent innovations in mesh technology and their role in such hernias. We summarize current evidence on permanent synthetic and biologic meshes in contaminated fields, highlight the emerging data supporting biosynthetic meshes, and introduce novel concepts such as mesh sutures. Finally, we outline our current approach to contaminated hernia repair, integrating these new technologies while considering patient, surgeon, and healthcare system factors.
AN UPDATE ON PERMANENT SYNTHETIC AND BIOLOGIC MESHES IN CONTAMINATED HERNIAS
Mesh infection has long been a dreaded complication of hernia repair due to its morbidity and the consequences of mesh explantation, enterocutaneous fistulas, and the risk of “burning a bridge” for future hernia repairs. For many years, wound contamination was considered a strict contraindication to the use of permanent synthetic mesh, and biologic mesh was regarded as the safer alternative[1]. Over the past decade, however, this dogma has been challenged, as the safe use of permanent synthetic mesh in Centers for Disease Control (CDC) class I-III cases has been repeatedly demonstrated[5-7]. Much of this progress can be attributed to shifts in mesh choice and placement: (1) monofilament instead of multifilament meshes; (2) macroporous over microporous meshes; (3) lightweight or medium-weight meshes rather than heavyweight meshes; and (4) retromuscular mesh placement - all factors shown to reduce the risk of mesh infection[9-11]. Preliminary data even suggest that heavyweight mesh, when placed in the retromuscular position, may be safe in contaminated hernia repair[12]. In head-to-head cohort studies and randomized control trials, permanent synthetic mesh has outperformed biologic mesh in CDC I-III wounds, showing lower rates of hernia recurrence and no increase - and possibly even lower rates - of surgical site occurrences (SSOs) or major complications[3,4,13,14]. Recently, two systematic reviews and meta-analyses comparing synthetic and biologic mesh in contaminated fields reported that synthetic mesh was associated with lower recurrence rates (10.3% vs. 24.5%, RR 0.44) and reduced rates of surgical site infection, complications, or reoperation[8,15]. A recurring caution in these studies is that complex hernia repairs were performed at high-volume centers by surgeons experienced in retromuscular/extraperitoneal repair in clean-contaminated and contaminated settings. In summary, synthetic mesh appears to be generally safe in CDC I-III repairs. Biologic mesh does not demonstrate significant advantages and is associated with substantially higher cost[14].
To address the shortcomings of biologic mesh, antibiotic-coated and hybrid meshes combining biologic components with permanent fibers have been developed. OviTex® Reinforced Tissue Matrix (TELA Bio, Malvern, PA), composed of sheep-derived extracellular matrix woven together with synthetic polypropylene, has shown safety in CDC I-III hernia repair with modest recurrence rates[16]. Zenapro® Hybrid Hernia Repair Device (Cook Biotech, West Lafayette, IN), which placed a lightweight macroporous polypropylene mesh between layers of porcine small intestinal submucosa, has since been withdrawn from the market. XenMatrixTM AB Surgical Graft (BD, Franklin Lakes, NJ), a non-crosslinked porcine dermal graft coated with rifampin and minocycline, has been used in CDC I-IV hernia repairs with a favorable safety profile and recurrence rate[17]. Further studies and clinical experience are needed before definitive conclusions can be drawn regarding the performance and indications of these newer materials.
INNOVATIONS IN BIOSYNTHETIC MESHES AND THEIR USE IN CONTAMINATED HERNIAS
With the limitations of biologic mesh and ongoing concerns about permanent mesh in contaminated fields, biosynthetic meshes were developed and introduced to the market. Also known as bioabsorbable meshes, these devices are composed of engineered polymers that provide a temporary scaffold for native cellular ingrowth after hernia repair, before gradually degrading. Compared with biologic meshes, biosynthetic meshes are more cost-effective[18] and generally degrade within a predictable timeframe through hydrolysis - a process unaffected by bacterial colonization, unlike the enzymatic degradation of most biologic meshes[19,20]. Currently available biosynthetic meshes for abdominal hernia repair [Table 1] include TIGR® Matrix (Novus Scientific, Uppsala, Sweden), GORE® BIO-A® (W.L. Gore & Associates, Inc., Newark, DE), PhasixTM (BD, Franklin Lakes, NJ), and TransorbTM (Medtronic, Minneapolis, MN).
Overview of biosynthetic meshes
| Mesh name (Manufacturer) | Material | Design | Pore size | Mesh density | Degradation period |
| TIGR® matrix (Novus) | Fast-resorbing fibers (40% of weight): copolymer of glycolide, lactide, and TMC Slow-resorbing fibers (60% of weight): copolymer of lactide and TMC | Multifilament | 1-1.5 mm | 135 g/m2 | Partial - 4 mo Complete - 36 mo |
| GORE® BIO-A® (GORE®) | PGA: TMC | 3D scaffold of randomly oriented fibers | n/a | n/a | 6-7 mo |
| PhasixTM (BD) | P4HB | Monofilament | 0.26 mm | 182 g/m2 | 12-18 mo |
| TransorbTM (Medtronic) | PLLA/TMC | Monofilament | 1.4 mm | 170 g/m2 | 18-60 mo |
TIGR® Matrix is a multifilament macroporous mesh woven from two polymer fibers to enable a dual-stage degradation process via hydrolysis. The fast-resorbing fibers (40% of the mesh), a copolymer of glycolide, lactide, and trimethylene carbonate (TMC), maintain mechanical strength for about 2 weeks before being fully absorbed at 4 months. The slow-resorbing fibers (60% of the mesh), composed of lactide and TMC, retain strength for 6 months and are fully absorbed after approximately 36 months[20]. Use of TIGR® Matrix in contaminated hernia repair has been reported in a single study of 91 patients, which showed an SSO rate of 27% and a recurrence rate of 12%, decreased to 4.5% when a retromuscular approach was used. While its design is conceptually promising, further clinical experience is needed to confirm its effectiveness.
GORE® BIO-A® consists of a copolymer of polyglycolide (PGA) and TMC, spun into a 3-dimensional scaffold of randomly oriented fibers[21]. It undergoes degradation through a combination of hydrolytic and enzymatic pathways and is typically absorbed within 6-7 months. Because enzymatic degradation can be influenced by bacterial colonization, the rate of absorption may vary, although this has not been extensively studied[21]. Several investigations have assessed its use in contaminated settings[20,22]. The COBRA Study evaluated 104 patients undergoing contaminated hernia repair (CDC class II-III) with GORE® BIO-A®, placed retromuscularly in 90% of cases or intraperitoneally in 10%. Reported outcomes included a recurrence rate of 17% and an SSO rate of 28%[23]. Huang et al. examined 207 patients with hernia repairs across all CDC wound classes (I-IV) and reported a recurrence rate of 28.7% among those with contaminated wounds (CDC II-IV) after a mean follow-up of 55.4 months[24]. Some reports have also described complications requiring mesh excision. Overall, GORE BIO-A appears to be a safe option in contaminated hernia repair, with modest recurrence rates.
PhasixTM is a monofilament, macroporous mesh woven from poly-4-hydroxybutyrate (P4HB), a polymer produced by transgenic E. coli K12 bacteria. It undergoes degradation through hydrolysis and hydrolytic enzymatic digestion within 12-18 months. A variant, PhasixTM ST, incorporates additional polyglycolic acid fibers coated with sodium hyaluronate, carboxymethylcellulose, and a polyethylene-based hydrogel to create a “viscera-facing” surface that reduces adhesions when implanted intraperitoneally[25]. PhasixTM is the most widely studied biosynthetic mesh, particularly in contaminated hernia repairs[20]. Layer et al. (2023) conducted a retrospective review of 108 patients with VHWG grade 3/4 hernias who underwent repair with PhasixTM mesh. They reported an overall recurrence rate of 22.2% and an SSO rate of 36.1% after a median follow-up of 41 months[26]. Most meshes were placed in a retromuscular or intraperitoneal position. Similarly, Van den Dop et al. (2023) performed a subgroup analysis of 84 patients with VHWG grade 3 hernias who received retromuscular PhasixTM mesh repair as part of the PhasixTM Trial. They observed a recurrence rate of 15.9% at 5 years, with no mesh-related complications requiring explantation[27]. A systematic review and meta-analysis of PhasixTM use in ventral hernia repair reported a pooled recurrence rate of 4% (95%CI: 0%-12%) and a pooled complication rate of 50% (95%CI: 27%-72%). Several preclinical studies suggest additional advantages of PhasixTM in contaminated settings. Compared with other biosynthetic and hybrid biologic meshes, PhasixTM demonstrates greater resistance to abscess formation and microbial colonization, as well as a reduced inflammatory response[28,29]. These benefits are thought to derive from its macroporous, monofilament structure, which minimizes surface area while promoting angiogenesis and cellular ingrowth. Other animal studies suggest that P4HB may enhance the expression of antimicrobial peptides[30]. In contaminated environments, PhasixTM also maintains higher tensile strength than the native abdominal wall[29,31]. Taken together, PhasixTM and PhasixTM ST offer biomechanical properties that can be strategically applied by hernia surgeons operating in contaminated fields.
Newer biosynthetic meshes and hybrid constructs incorporating biosynthetic polymers are also in development, aiming to build on these favorable properties. One such example is TransorbTM, a macroporous, monofilament knitted mesh composed of poly-L-lactide and poly-TMC. It features proprietary self-fixating hooks and is fully resorbed within 24-36 months[32,33]. Early clinical trials in the United States are currently underway.
To date, no clinical trials have directly compared synthetic versus biosynthetic meshes, or different biosynthetic meshes against each other. A systematic review and meta-analysis found no significant differences in recurrence or SSO rates between PhasixTM and GORE® BIO-A®[20]. Two recent retrospective reviews from the Abdominal Core Health Quality Collaborative, including patients undergoing contaminated hernia repair (CDC II-III), found no differences in quality of life and pain scores, comparable 30-day outcomes, and lower 1-year recurrence rates with permanent synthetic mesh compared to biosynthetic mesh[34,35]. Similarly, a recent systematic review of ventral hernia repairs in contaminated fields (Ventral Hernia Working Group grade 2-3) reported no meaningful differences in recurrence or complication rates among synthetic, biosynthetic, and biologic meshes. However, the authors noted that the literature remains limited by study heterogeneity and the paucity of comparative trials[36]. Taken together, current evidence is insufficient to conclude that one biosynthetic mesh is superior to another, or that biosynthetic mesh should replace synthetic mesh as the primary option for contaminated hernia repair - except perhaps in CDC class IV wounds. We will describe how we have integrated these materials into our practice in subsequent sections.
MESH SUTURE - A NOVEL MESH DEVICE WITH PROMISING QUALITIES
Surgeons have long recognized the limitations of thin linear suture material for tissue reapproximation, particularly the issue of suture pull-through, where localized pressure at the suture-tissue interface cuts through tissue, leading to repair dehiscence[37]. To address this challenge, Souza et al. and Lanier et al. proposed the concept of “mesh suture” as a novel design to overcome the limitations of linear sutures[38,39]. The initial “hand-fashioned” mesh suture was created by cutting narrow strips of macroporous, uncoated polypropylene, attaching them to a suture needle, passing them through tissue, and tying them like conventional sutures. A proof-of-concept study in animal models demonstrated reduced suture pull-through and increased tensile strength[38]. Subsequently, hand-fashioned mesh suture was successfully used in contaminated abdominal wall closures and incisional hernia repairs (CDC II-IV), with minimal mesh-suture-related complications and hernia occurrence rates of 4% and 6% after mean follow-ups of 234 days and 11.8 months, respectively[39,40]. The se hernia repairs often involved anterior component separation to achieve midline closure.
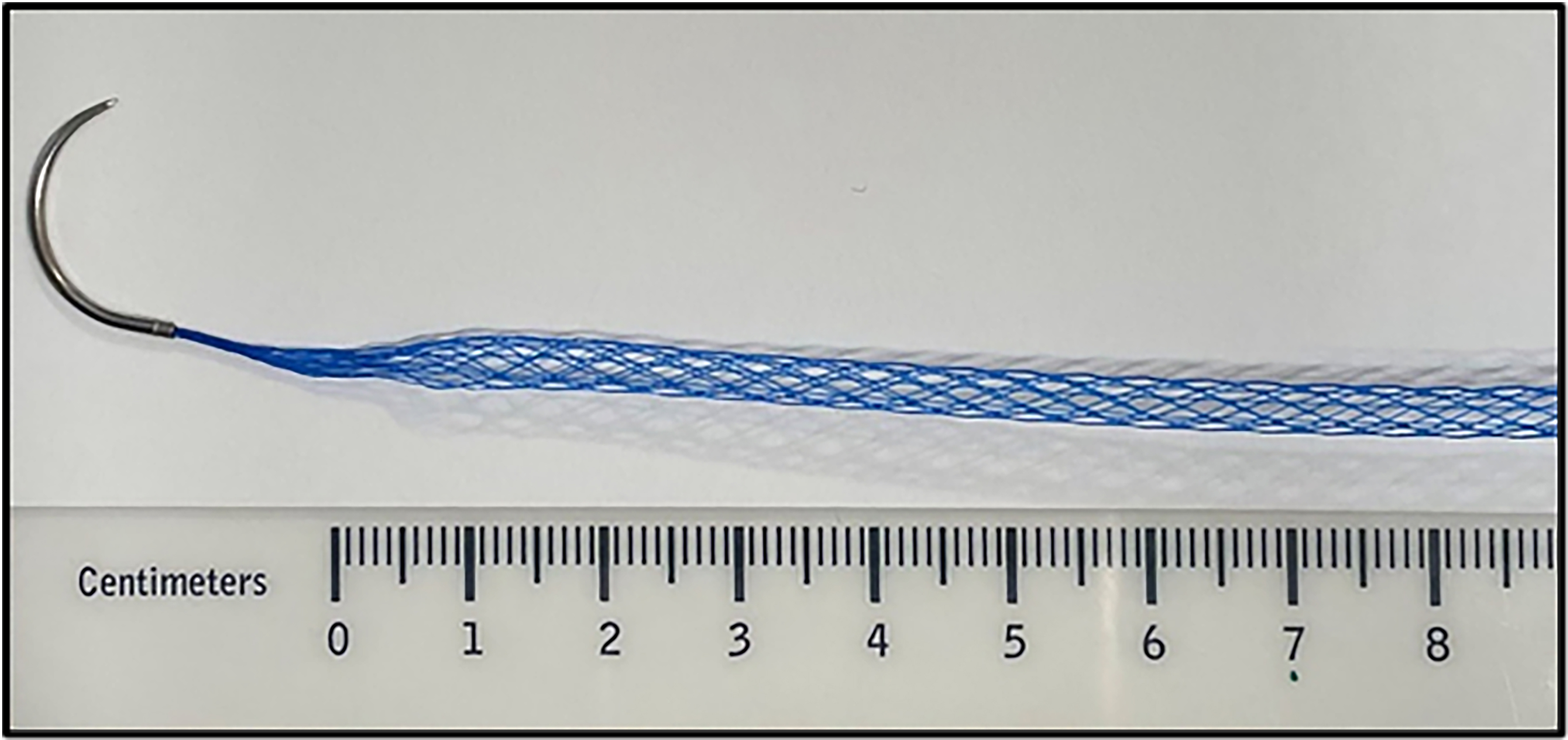
Building on these principles, a commercially manufactured mesh suture, DurameshTM (Mesh Suture Inc., Chicago, IL), was developed. DurameshTM consists of polypropylene filaments woven into a hollow cylindrical lattice [Figure 1] and swaged onto a needle[41,42]. Compared with 0-polypropylene suture, mesh suture demonstrates similar tensile strength, greater elasticity, improved resistance to suture pull-through, and enhanced tissue incorporation[42]. These benefits appear to result from its increased suture-tissue interface surface area, the distribution of tension across multiple filaments, and the allowance for tissue ingrowth and angiogenesis within its macroporous configuration[38,42,43]. Similar to macroporous mesh, this characteristic likely contributes to its safe use in contaminated fields. As DurameshTM was just approved by the US Food and Drug Administration (FDA) in 2022, long-term outcome data remain limited. In our initial experience with 63 patients in both clean and contaminated settings (CDC I-IV), we observed no mesh-suture-related complications or reoperations for mesh explanation after a mean follow-up of 45 days[44]. Hackenberger et al. (2024) reported outcomes for 379 patients, including 138 ventral/umbilical hernias (CDC I-III) and 93 abdominal incision closures (CDC I-IV). They found an overall surgical site infection rate of 6.1%, fascial dehiscence rate of 1.6%, and hernia development/recurrence rate of 0.6% after an average follow-up of 80 days, substantially better than outcomes reported for traditional suture alone or for biologic/biosynthetic mesh[45]. These early studies suggest that DurameshTM is safe for use in contaminated settings and has several advantages over traditional sutures for primary closure of abdominal wall defects. Future research should evaluate its long-term outcomes, compare its performance head-to-head with traditional suture in primary closure, and assess its efficacy in hernia repair relative to mesh.
APPROACH TO CONTAMINATED HERNIAS
The ongoing development of new mesh devices represents an important and exciting aspect of abdominal wall reconstruction; however, the adoption of these innovations should be cautious and deliberate. The mesh literature is limited by short follow-up durations, inconsistent definitions of recurrence and complications, and variability in surgical experience and technique, which may limit the generalizability of study findings to surgeons worldwide. It is also important to note that no mesh product is currently FDA-approved for CDC class II, III, or IV cases. With these factors in mind, we present our conservative approach to complex and contaminated hernias, emphasizing that it should be tailored to the surgeon’s experience, patient-specific factors, patient preferences and costs, and healthcare system considerations. We use generally accepted criteria to define complex hernias, including recurrence, contamination, large defects requiring component separation, presence of a fistula or stoma, infected prostheses, poor soft tissue quality, and emergency hernia repairs[46].
DECIDING BETWEEN DEFINITIVE AND PLANNED STAGED RECONSTRUCTION
Several key concepts emerge from the literature. First, the long-term durability of permanent synthetic mesh constructs is unparalleled[34,35]. In the absence of reliable predictors of recurrence with slowly absorbable mesh, the most effective way to minimize recurrence for most patients is the placement of a permanent mesh in an extraperitoneal/retromuscular position whenever feasible. Second, evidence continues to support the safe use of permanent synthetic mesh in CDC I-III hernia repairs. Finally, mesh infection remains a serious complication regardless of risk level, and mesh removal following a component separation can significantly limit future repair options. Therefore, efforts should be made to downstage hernia contamination whenever possible.
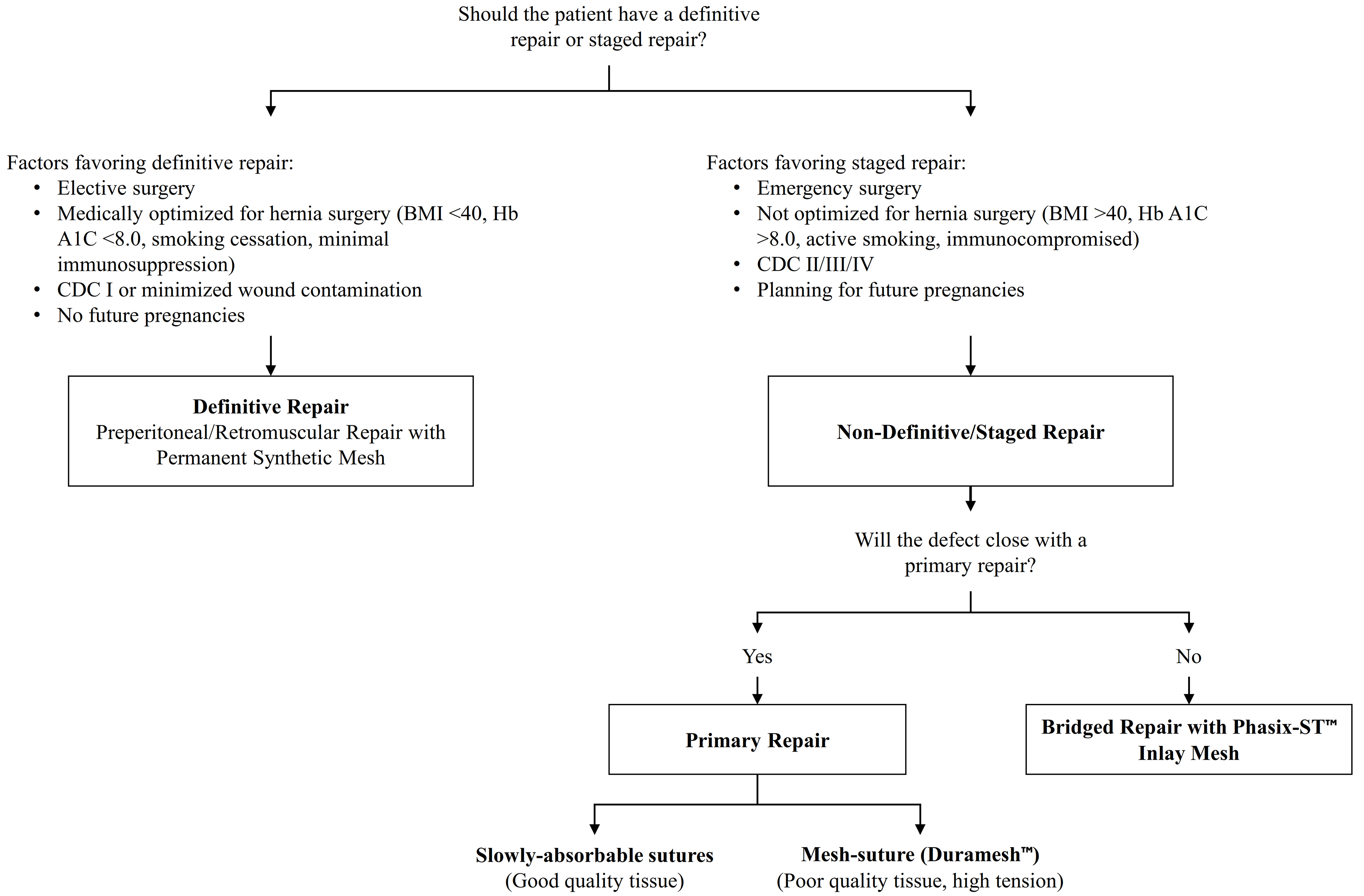
For these reasons, we often employ a staged approach for complex and contaminated hernias. Patients suitable for “definitive” repair undergo a single-stage approach with placement of a reduced-weight (light- or medium-weight) macroporous mesh in an extraperitoneal/retromuscular position. Patients unsuitable for a single-stage repair first undergo a “non-definitive” procedure with primary closure or bridging with slowly absorbable mesh, followed later by a second-stage definitive repair with permanent synthetic mesh. As described by us and others[47,48], the decision between definitive and staged reconstruction is guided by the following considerations:
(1) Is the procedure elective or emergent?
(2) What is the complexity of the planned definitive hernia repair?
(3) What is the skill set and experience of the operating team in abdominal wall reconstruction?
(4) Is the patient medically optimized for surgery (BMI < 40, HbA1C < 8.0, smoking cessation, minimal/no immunosuppression, and optimal cardiorespiratory function)? Does the patient have the capacity, motivation, and means to achieve optimization, and would optimization meaningfully improve postoperative outcomes? For example, a patient with a BMI of 41 and a small hernia requiring a minimally invasive repair would gain little from preoperative optimization, whereas a patient with a BMI of 50 and a loss-of-domain hernia requiring component separation would benefit substantially.
(5) What is the level of wound contamination, and is it within an acceptable range (CDC I-III)? Would staging reduce the risk of mesh infection? For example, a staged procedure can significantly lower contamination risk for a complex enteroprosthetic fistula, whereas staging offers little benefit for a patient with a permanent ostomy.
(6) If concurrent non-hernia surgery is planned, what is its complexity?
(7) What is the likelihood of future surgeries (e.g., for bleeding, anastomotic leaks, oncological recurrence, or future pregnancies)?
We generally do not use biologic or biosynthetic mesh as a substitute for permanent synthetic mesh in retromuscular positions with component separation, as it has not demonstrated superior or consistent outcomes in CDC class I-III wounds and incurs substantially higher costs. In CDC class IV wounds, we favor a staged approach over biosynthetic mesh to minimize infection risk and optimize long-term hernia durability.
There are situations where the decision between single-stage and staged reconstruction is not clear-cut. Staged reconstruction can involve higher costs for patients, hospitals, or healthcare systems than a one-stage approach, but these costs may be justified when considering the long-term expenses associated with hernia complications. In such cases, patient preferences, surgeon experience, and healthcare system factors should be carefully weighed.
An additional advantage of staged reconstruction is that some patients do not experience hernia recurrence - or symptomatic recurrence - after the initial, non-definitive repair with primary suture closure or bridging mesh. In our single-center retrospective review, 35.3% patients undergoing non-definitive repair (primary closure or bridging biologic/biosynthetic mesh) had no radiographic recurrence[47]. With this in mind, we discuss our approach to non-definitive hernia repair and how we have incorporated new mesh technologies to maximize the durability of these repairs.
INCORPORATING NEW MESH TECHNOLOGY FOR “NON-DEFINITIVE” STAGED RECONSTRUCTION
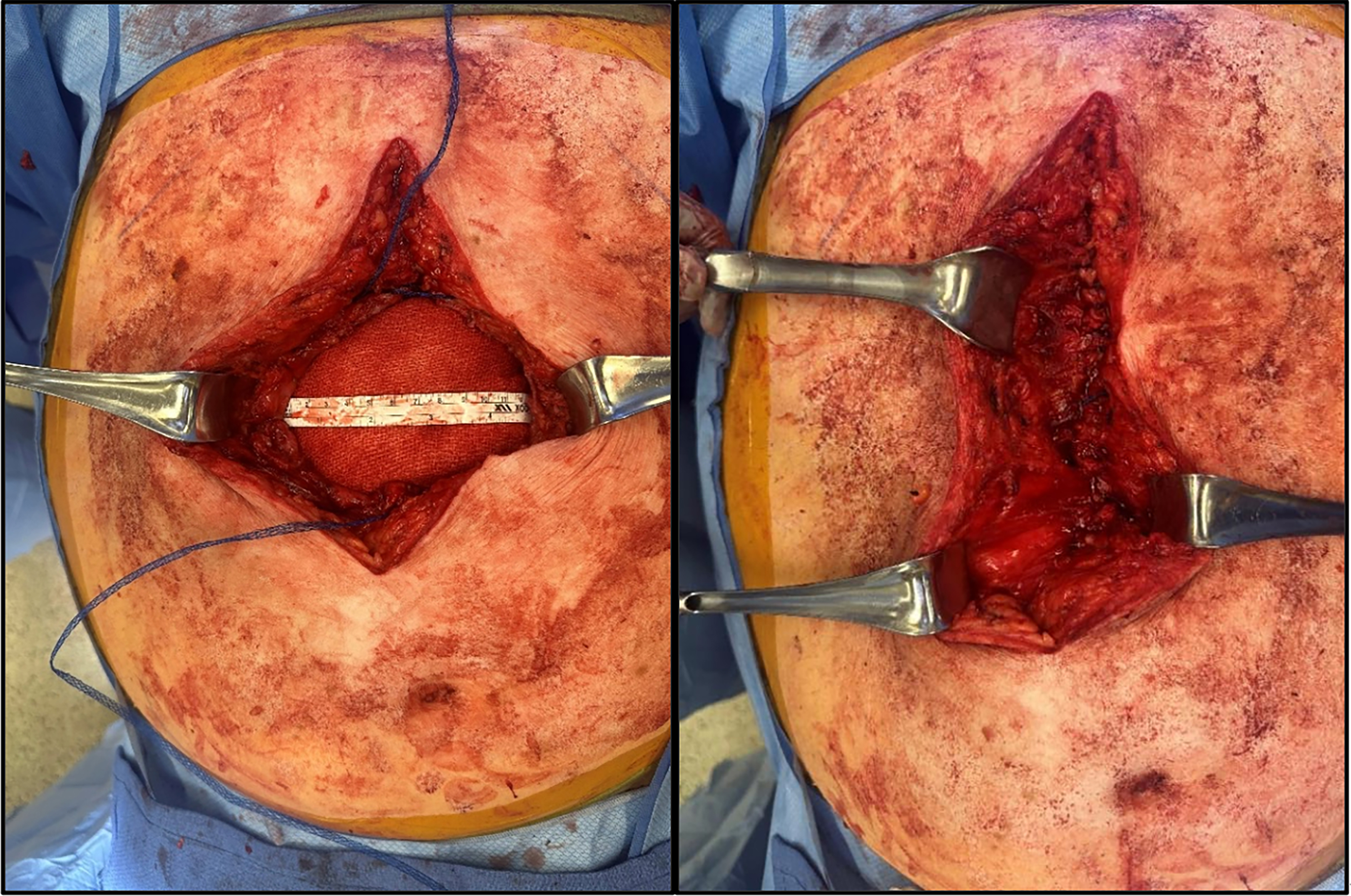
During the initial, “non-definitive” stage of a staged reconstruction, we prefer a “step-up” approach that balances simplicity, cost, and repair durability. Within this setting, newer innovations in hernia technology, particularly those suitable for contaminated environments, have proven useful. Most hernia defects can be closed primarily with interrupted figure-of-eight sutures using a slowly absorbable material. In areas with high tension, poor tissue quality, and/or contamination, we selectively reinforce the closure with mesh-suture to improve strength and reduce the risk of suture pull-through [Figure 2]. This technique has been particularly helpful in emergency primary parastomal hernia repairs, fascial closure following ostomy reversal, flank hernias, and incision closure after temporary abdominal closure in emergency general surgery.
Figure 2. Intraoperative use of Duramesh suture. Primary closure of a large incisional hernia in a contaminated case.
Defects that cannot be closed primarily with suture or mesh-suture are bridged with a slowly absorbable mesh. We prefer PhasixTM ST over rapidly absorbable meshes (e.g., VicrylTM, Ethicon, Somerville, NJ), biologics, or biosynthetics because:
(1) It is absorbed slowly over 12-18 months compared with 60-90 days for VicrylTM and 6 months for GORE® Bio-A®.
(2) Its degradation is unaffected by the acidic environment of contaminated fields, unlike VicrylTM, GORE® Bio-A®, and biologic meshes.
(3) It is less costly than biologic mesh.
Contrary to the commonly cited principle of “tension-free repair” in definitive hernia repair, we intentionally apply a moderate degree of tension to a bridged Phasix STTM repair and minimize the size of the bridged area. In our experience, this approach reduces hernia size at the second stage, thereby simplifying subsequent reconstruction and sometimes downstaging a patient from a transversus abdominus release (TAR) to a retrorectus repair alone. The PhasixTM ST mesh edges are sutured to the fascia with slowly absorbable suture, and the skin is closed over this repair whenever feasible [Figure 3]. Because PhasixTM is prone to seroma formation, we often leave a drain in place.
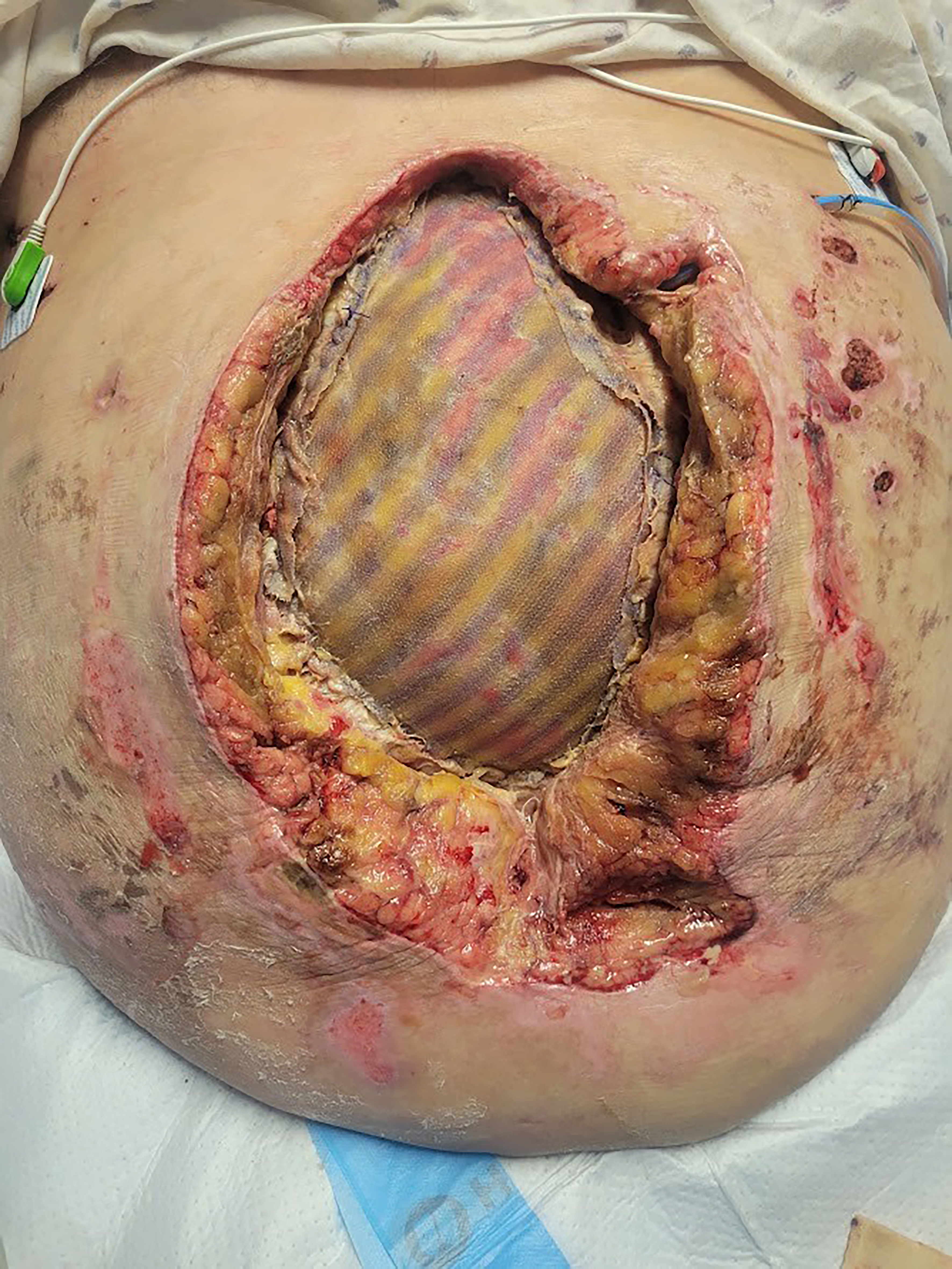
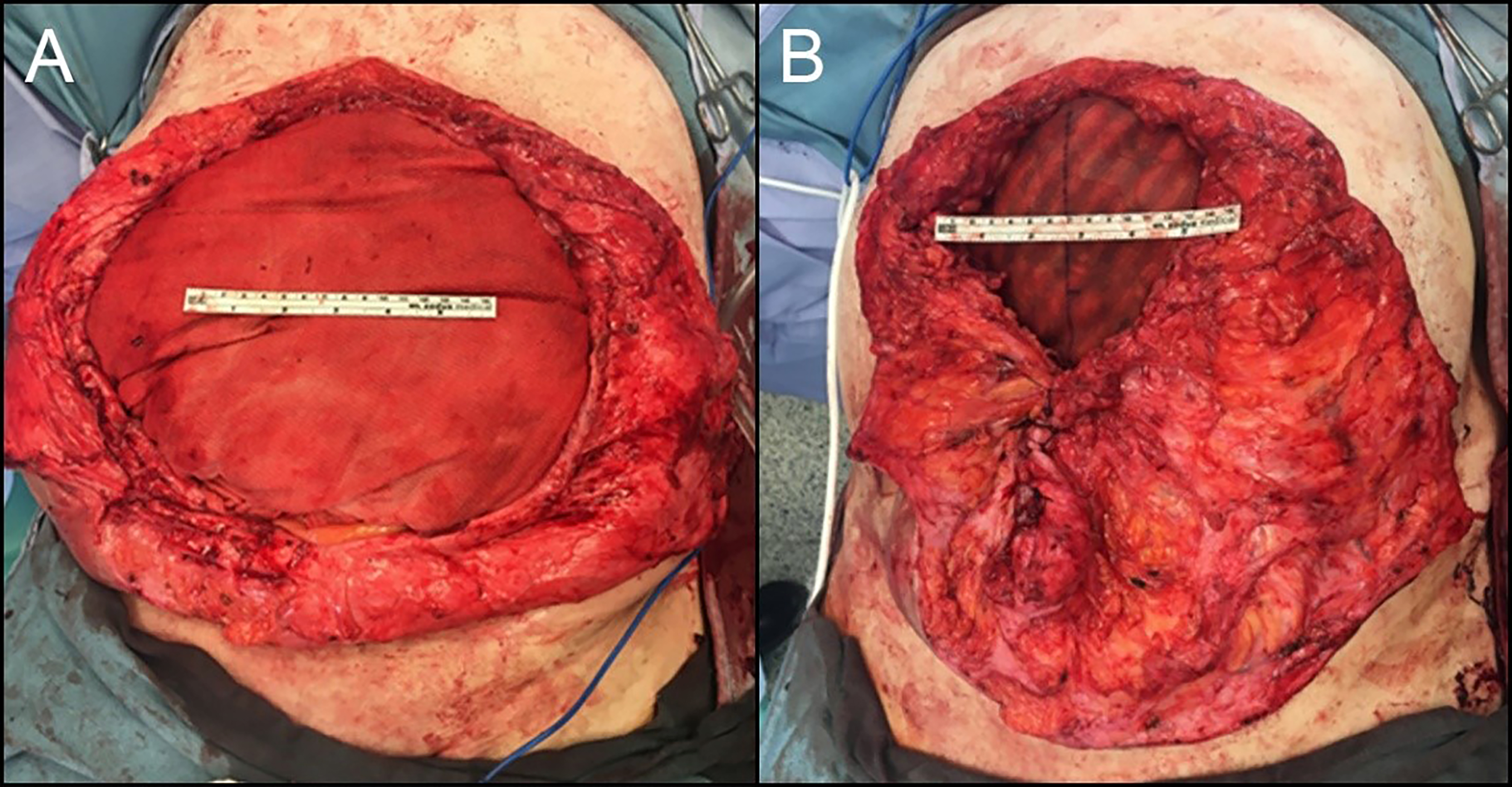
It must be emphasized that, according to the manufacturer’s Instructions for Use, PhasixTM ST mesh is not intended for bridged repairs, as this theoretically results in eventual hernia formation. Despite this warning, we are comfortable using it in this setting because we recognize that complete mesh reabsorption will lead to an abdominal wall defect and potentially a symptomatic hernia requiring definitive repair. Since the purpose of the first stage is not definitive repair, all patients are counseled accordingly. In some cases, a combination of approaches is required. For example, certain portions of the fascial defect may be closed primarily, while others require bridging with absorbable mesh. In such scenarios, we close as much of the defect as possible and then place PhasixTM ST as a pure inlay bridge under physiologic tension [Figure 4].
Figure 4. Combining primary closure and inlay biosynthetic mesh reconstruction for massive loss-of-domain hernias. (A) Loss-of-domain defect prior to closure; (B) Abdominal closure with mesh suture and inlay Phasix-ST Mesh.
We have accumulated over 8 years of unpublished experience using Phasix-ST in contaminated/dirty cases (CDC III: 12.2%, CDC IV: 40.8%) across more than 40 patients at multiple institutions. Our preliminary data show acceptable rates of SSOs requiring procedural intervention (SSOPI, 35.4%) and hernia recurrence (34.7%) after a mean follow-up of 495 days (IQR 157-699). Recurrence was determined either by the operating surgeon or by cross-sectional imaging when available. There was only one case of mesh infection requiring excision. Importantly, despite full absorption of the Phasix-ST mesh, not all patients developed a symptomatic bulge, and the majority (> 85%) did not request or require additional hernia repair at follow-up. While more rigorous comparative trials are necessary, these findings suggest that our approach is both rational and safe. In summary, we have not yet adopted PhasixTM for definitive hernia repair in the retromuscular position. At present, its most valuable role is in restoring abdominal continuity for non-definitive repairs in contaminated fields.
We have summarized our overall strategy in a decision-making algorithm [Figure 5]. This approach allows us to achieve abdominal closure with materials that are safe, predictable across all wound classes, provide prolonged tensile strength, and preserve options for future abdominal wall reconstruction. We acknowledge that this is a conservative strategy and expect it to evolve as: (1) more evidence emerges regarding the long-term comparative performance of different mesh constructs; and (2) we develop more reliable ways to identify which patients can safely achieve a durable, single-stage repair without permanent synthetic mesh.
CONCLUSION
Advances in mesh technology have expanded the options available for abdominal wall reconstruction in contaminated settings. Biosynthetic meshes show promise, offering advantages such as cost-effectiveness and predictable absorption; however, their long-term outcomes and direct comparisons with synthetic meshes remain under investigation. The development of novel materials such as mesh suture also holds great potential. Despite these innovations, a cautious, individualized approach remains essential. Ultimately, integrating new technologies with thoughtful surgical strategies and a growing understanding of mesh behavior in contaminated environments may pave the way for safer and more effective hernia repairs in the future.
DECLARATIONS
Acknowledgments
The authors would like to thank Dr. McKell Quattrone and Dr. Eric Moyer for their contributions to the collection of photographs in this article.
Authors’ contributions
Made substantial contributions to the conception and design of the study and performed data analysis and interpretation: Fung BSC, Pauli EM
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
Pauli EM is a speaker for Becton-Dickinson and Medtronic and is a consultant for Boston Scientific Corp., Actuated Biomedical, Inc., Cook Biotech, Neptune Medical, Surgimatix, Noah Medical, Allergan, Intuitive Surgical, ERBE, Integra, STERIS, Vicarious Surgical, TELA Bio, and Mesh Suture, Inc. He has royalties in UpToDate, Inc. and Springer and financial interests in IHC, Inc., Cranial Devices Inc., and Actuated Medical. Fung BSC declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Breuing K, Butler CE, Ferzoco S, et al; Ventral Hernia Working Group. Incisional ventral hernias: review of the literature and recommendations regarding the grading and technique of repair. Surgery. 2010;148:544-58.
2. Rosen MJ, Krpata DM, Ermlich B, Blatnik JA. A 5-year clinical experience with single-staged repairs of infected and contaminated abdominal wall defects utilizing biologic mesh. Ann Surg. 2013;257:991-6.
3. Harris HW, Primus F, Young C, et al. Preventing recurrence in clean and contaminated hernias using biologic versus synthetic mesh in ventral hernia repair: the PRICE randomized clinical trial. Ann Surg. 2021;273:648-55.
4. Olavarria OA, Bernardi K, Dhanani NH, et al. Synthetic versus biologic mesh for complex open ventral hernia repair: a pilot randomized controlled trial. Surg Infect. 2021;22:496-503.
5. Carbonell AM, Criss CN, Cobb WS, Novitsky YW, Rosen MJ. Outcomes of synthetic mesh in contaminated ventral hernia repairs. J Am Coll Surg. 2013;217:991-8.
6. Slater NJ, Knaapen L, Bökkerink WJV, et al. Large contaminated ventral hernia repair using component separation technique with synthetic mesh. Plast Reconstr Surg. 2015;136:796e-805.
7. Warren J, Desai SS, Boswell ND, et al. Safety and efficacy of synthetic mesh for ventral hernia repair in a contaminated field. J Am Coll Surg. 2020;230:405-13.
8. Morris MP, Mellia JA, Christopher AN, et al. Ventral hernia repair with synthetic mesh in a contaminated field: a systematic review and meta-analysis. Hernia. 2021;25:1035-50.
9. Ellis R, Miller BT. Mesh selection in abdominal wall reconstruction: an update on biomaterials. Surg Clin North Am. 2023;103:1019-28.
10. Engelsman AF, van der Mei HC, Busscher HJ, Ploeg RJ. Morphological aspects of surgical meshes as a risk factor for bacterial colonization. Br J Surg. 2008;95:1051-9.
11. Engelsman AF, van der Mei HC, Ploeg RJ, Busscher HJ. The phenomenon of infection with abdominal wall reconstruction. Biomaterials. 2007;28:2314-27.
12. Ellis RC, Maskal SM, Messer N, et al. Short-term outcomes of heavyweight versus mediumweight synthetic mesh in a retrospective cohort of clean-contaminated and contaminated retromuscular ventral hernia repairs. Surg Endosc. 2024;38:4006-13.
13. Majumder A, Winder JS, Wen Y, Pauli EM, Belyansky I, Novitsky YW. Comparative analysis of biologic versus synthetic mesh outcomes in contaminated hernia repairs. Surgery. 2016;160:828-38.
14. Rosen MJ, Krpata DM, Petro CC, et al. Biologic vs synthetic mesh for single-stage repair of contaminated ventral hernias: a randomized clinical trial. JAMA Surg. 2022;157:293-301.
15. Siddiqui A, Lyons NB, Anwoju O, et al. Mesh type with ventral hernia repair: a systematic review and meta-analysis of randomized trials. J Surg Res. 2023;291:603-10.
16. DeNoto G 3rd, Ceppa EP, Pacella SJ, et al. 24-month results of the BRAVO study: a prospective, multi-center study evaluating the clinical outcomes of a ventral hernia cohort treated with OviTex® 1S permanent reinforced tissue matrix. Ann Med Surg. 2022;83:104745.
17. Ilahi ON, Velmahos G, Janis JE, et al. Prospective, multicenter study of antimicrobial-coated, noncrosslinked, acellular porcine dermal matrix (XenMatrixTM AB Surgical Graft) for hernia repair in all centers for disease control and prevention wound classes: 24-month follow-up cohort. Ann Med Surg. 2023;85:1571-7.
18. Charleux-Muller D, Romain B, Boisson C, Velten M, Brigand C, Lejeune C. Cost-effectiveness analysis of resorbable biosynthetic mesh in contaminated ventral hernia repair. J Visc Surg. 2022;159:279-85.
19. Jacobsen G, DuCoin C. Biodegradable meshes in abdominal wall surgery. In: Novitsky Y, Editors. Hernia surgery. Springer, Cham; 2016. pp. 71-8.
20. Perrone G, Giuffrida M, Bonati E, Petracca GL, Catena F. Biosynthetic meshes in contaminated fields: where are we now? Hernia. 2023;27:765-80.
21. GORE® BIO-A® Tissue reinforcement - instructions for use. Available from: https://www.goremedical.com/products/bio-a-tissue. [Last accessed on 22 Sep 2025].
22. Claessen JJM, Timmer AS, Atema JJ, Boermeester MA. Outcomes of mid-term and long-term degradable biosynthetic meshes in single-stage open complex abdominal wall reconstruction. Hernia. 2021;25:1647-57.
23. Rosen MJ, Bauer JJ, Harmaty M, et al. Multicenter, prospective, longitudinal study of the recurrence, surgical site infection, and quality of life after contaminated ventral hernia repair using biosynthetic absorbable mesh: the COBRA study. Ann Surg. 2017;265:205-11.
24. Huang EY, Broderick RC, Spurzem GJ, et al. Long-term outcomes of PGA-TMC absorbable synthetic scaffold in both clean and contaminated ventral hernia repairs. Surg Endosc. 2024;38:2231-9.
25. PhasixTM mesh - a resorbable mesh with resorbable hydrogel coating for soft tissue reconstruction - instructions for open and laparoscopic use. Available from: https://www.bd.com/content/dam/bd-assets/na/surgery/documents/instructions-for-use/Phasix-ST-E-IFU-with-hiatal-final.pdf. [Last accessed on 28 Sep 2025].
26. Layer T, Benammi S, Dubuisson V, et al. Incisional hernia repair with a slowly absorbable P4HB mesh: what happens after the mesh disappears? Hernia. 2023;27:387-94.
27. Van den Dop LM, Van Rooijen MMJ, Tollens T, et al. Five-year follow-up of a slowly resorbable biosynthetic P4HB Mesh (Phasix) in VHWG grade 3 incisional hernia repair. Ann Surg Open. 2023;4:e366.
28. Lake SP, Stoikes NFN, Badhwar A, Deeken CR. Contamination of hybrid hernia meshes compared to bioresorbable PhasixTM Mesh in a rabbit subcutaneous implant inoculation model. Ann Med Surg. 2019;46:12-6.
29. Stoikes NFN, Scott JR, Badhwar A, Deeken CR, Voeller GR. Characterization of host response, resorption, and strength properties, and performance in the presence of bacteria for fully absorbable biomaterials for soft tissue repair. Hernia. 2017;21:771-82.
30. Molina C, Hussey GS, Liu A, Eriksson J, D’Angelo WA, Badylak SF. Role of 4-hydroxybutyrate in increased resistance to surgical site infections associated with surgical meshes. Biomaterials. 2021;267:120493.
31. Martin DP, Badhwar A, Shah DV, et al. Characterization of poly-4-hydroxybutyrate mesh for hernia repair applications. J Surg Res. 2013;184:766-73.
32. TransorbTM self-gripping resorbable mesh - technique guide. Available from: https://www.medtronic.com/en-us/healthcare-professionals/products/hernia-repair/synthetic-mesh/transorb-self-gripping-resorbable-mesh.html. [Last accessed on 22 Sep 2025].
33. Vestberg R, Lecuivre J, Radlovic A, Payet E, Bayon Y, Bouré L. A novel self-gripping long-term resorbable mesh providing temporary support for open primary ventral and incisional hernia. J Mater Sci Mater Med. 2023;34:59.
34. Rodriguez-Quintero JH, Estrada A, Arias-Espinosa L, et al. Elective complex ventral hernia repair in contaminated fields: a propensity score-matched analysis of long-term quality of life and outcomes between different prostheses. Surgery. 2024;176:1668-75.
35. Rodriguez-Quintero JH, Romero-Velez G, Lima DL, Huang LC, Sreeramoju P, Malcher F. Permanent vs absorbable mesh for ventral hernia repair in contaminated fields: multicenter propensity-matched analysis of 1-year outcomes using the abdominal core health quality collaborative database. J Am Coll Surg. 2023;236:374-86.
36. Morales-Conde S, Hernández-Granados P, Tallón-Aguilar L, Verdaguer-Tremolosa M, López-Cano M. Ventral hernia repair in high-risk patients and contaminated fields using a single mesh: proportional meta-analysis. Hernia. 2022;26:1459-71.
38. Souza JM, Dumanian ZP, Gurjala AN, Dumanian GA. In vivo evaluation of a novel mesh suture design for abdominal wall closure. Plast Reconstr Surg. 2015;135:322e-30.
39. Lanier ST, Dumanian GA, Jordan SW, Miller KR, Ali NA, Stock SR. Mesh sutured repairs of abdominal wall defects. Plast Reconstr Surg Glob Open. 2016;4:e1060.
40. Dumanian GA, Lanier ST, Souza JM, et al. Mesh sutured repairs of contaminated incisional hernias. Am J Surg. 2018;216:267-73.
41. Dumanian GA. Suturable mesh demonstrates improved outcomes over standard suture in a porcine laparotomy closure model. Plast Reconstr Surg Glob Open. 2021;9:e3879.
42. Dumanian GA, Tulaimat A, Dumanian ZP. Experimental study of the characteristics of a novel mesh suture. Br J Surg. 2015;102:1285-92.
43. Scheiber CJ, Kurapaty SS, Goldman SM, Dearth CL, Liacouras PC, Souza JM. Suturable mesh better resists early laparotomy failure in a cyclic ball-burst model. Hernia. 2020;24:559-65.
44. Quattrone M, Moyer ED, Zolin SJ, et al. Short-term outcomes of mesh-suture repair in the treatment of ventral hernias: a single-center study. Surg Endosc. 2025;39:2129-35.
45. Hackenberger PN, Mittal M, Fronza J, Shapiro M. Duramesh registry study: short-term outcomes using mesh suture for abdominal wall closure. Front Surg. 2023;10:1321146.
46. Slater NJ, Montgomery A, Berrevoet F, et al. Criteria for definition of a complex abdominal wall hernia. Hernia. 2014;18:7-17.
47. DeLong CG, Crowell KT, Liu AT, et al. Staged abdominal wall reconstruction in the setting of complex gastrointestinal reconstruction. Hernia. 2024;28:97-107.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Special Topic
Copyright
Data & Comments
Data
















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].