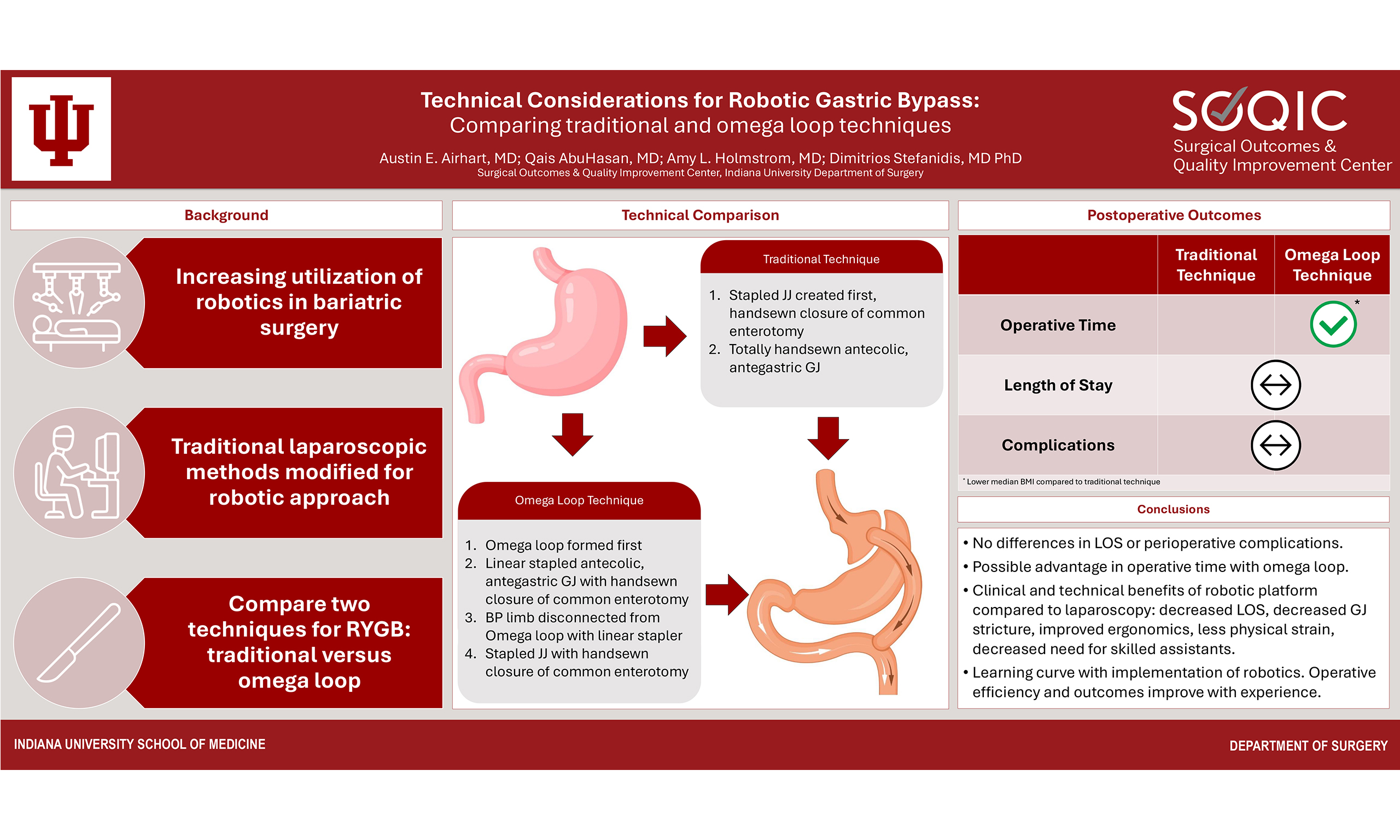
Technical considerations for robotic gastric bypass: comparing traditional and omega loop techniques
Abstract
Robotic surgery has become ubiquitous across a variety of surgical specialties including bariatric surgery. Application of this new technology to bariatric surgery requires some modifications to the traditional laparoscopic technique to accomplish the procedures effectively and efficiently. In this technical report, we describe our approach to robotic Roux-en-Y gastric bypass, outline potential benefits over laparoscopy, and highlight technical variations in the conduct of this operation that may be helpful to practicing bariatric surgeons, especially those who plan to adopt robotic surgery to their practice. We present handsewn vs. stapled gastrojejunostomy creation, omega loop technique vs. traditional construction of the jejunojejunostomy, and other technical variations. Each method has its own advantages and disadvantages which we have highlighted throughout this article. We also discuss pre- and postoperative management. When compared to traditional laparoscopic gastric bypass, robotic approaches remain less often used in current practice but are expected to surpass laparoscopy in just a few years. A robotic approach offers unique benefits for gastric bypass while still proving to be a safe and effective procedure for surgical weight loss.
Keywords
INTRODUCTION
Over the last decade, the utilization of robotic surgery has increased significantly, driven by advancements in technology, improved surgeon ergonomics, and enhanced dexterity compared to the laparoscopic approach[1,2]. In bariatric surgery, similar trends have emerged with robotic platforms being employed in one-third of cases as of 2022[3,4]. This shift toward robotic surgery in bariatrics can be particularly advantageous in complex procedures including Roux-en-Y gastric bypass (RYGB). The benefits can be even more pronounced in patients with challenging anatomy, such as those with a body mass index (BMI) exceeding 50 kg/m2 or those undergoing revisional surgery who are at increased risk of complications[5,6].
Studies have demonstrated that robotic revisional RYGB is associated with a lower rate of morbidity and a shorter hospital length of stay compared to its laparoscopic counterpart, possibly due to the increased precision and control provided by the robot[7]. Importantly, the increased utilization of robotic surgery and accumulated experience are associated with improved patient outcomes[4]. However, barriers to the adoption of robotic surgery remain, including cost and a perceived difficulty in transitioning from laparoscopy[8], despite studies demonstrating an easier learning curve for robotic surgery and transferable skills from laparoscopic surgery[9,10]. Additionally, structured curricula for surgeon training in robotic techniques for RYGB are limited.
In this technical “how I do it” styled paper, we describe two distinct techniques we use for robotic RYGB and the technical considerations of each. Prior to surgery, all patients undergo a comprehensive preoperative workup including endoscopy and other needed studies to assess eligibility for surgery. Patients routinely receive prophylactic intravenous antibiotics prior to incision and subcutaneous deep vein thrombosis chemoprophylaxis in preoperative holding. The patient is positioned supine with the arms out. The patient is secured with a strap across the thighs, straps across both arms, and a footboard to avoid sliding during steep reverse Trendelenburg positioning. Appropriate padding of the arms, elbows, and other bony prominences is paramount especially in high BMI patients as positioning injuries are not rare.
TECHNICAL CONSIDERATIONS
Abdominal entry and port placement
Once the patient is adequately anesthetized, the abdomen is prepped and draped widely to allow exposure of the whole abdomen and flanks. It is important to position the top drape well above the xiphoid process, as otherwise, it may have to be repositioned for the placement of the liver retractor once insufflated. The bed is positioned in Trendelenburg position at 12 degrees.
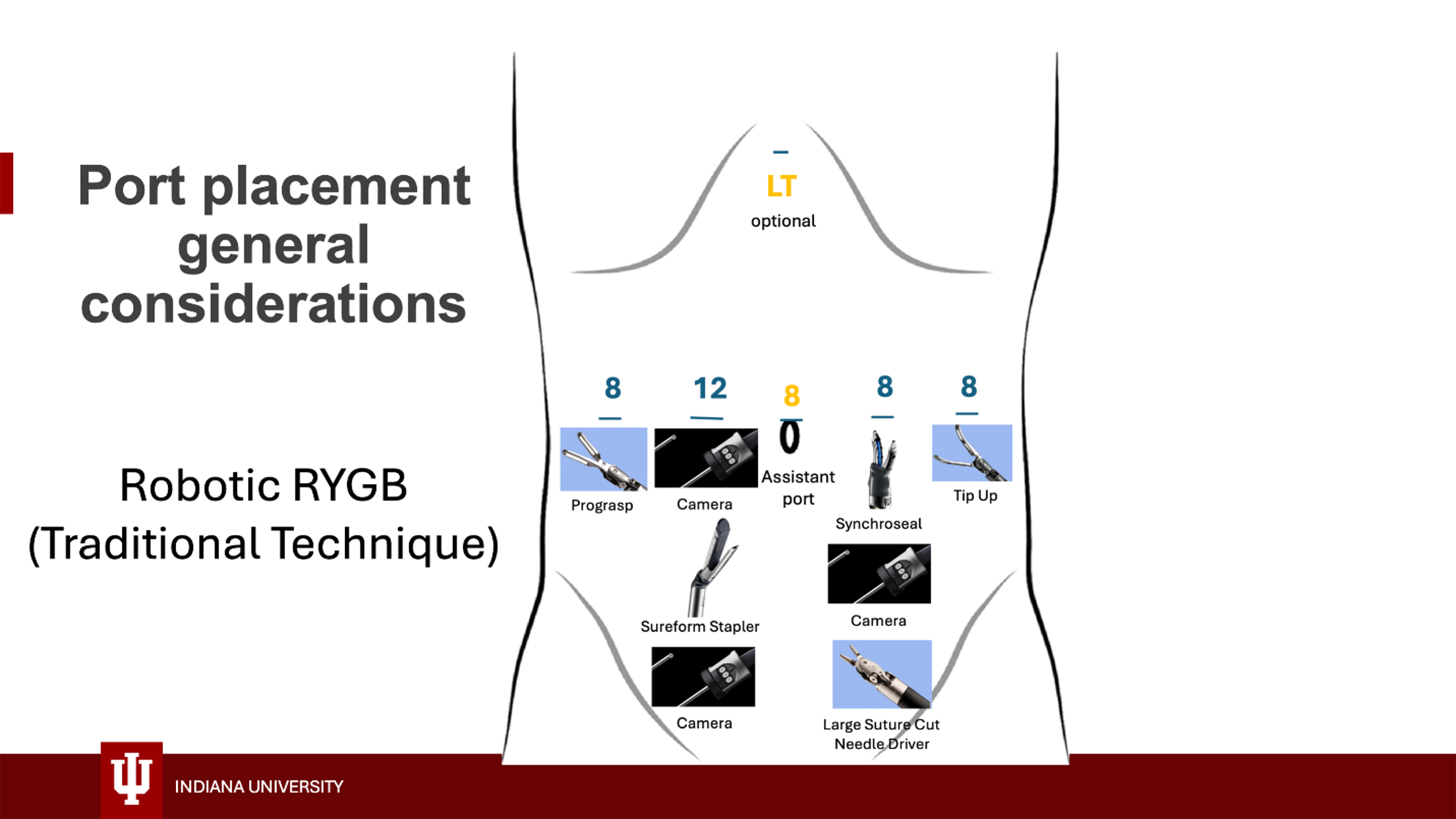
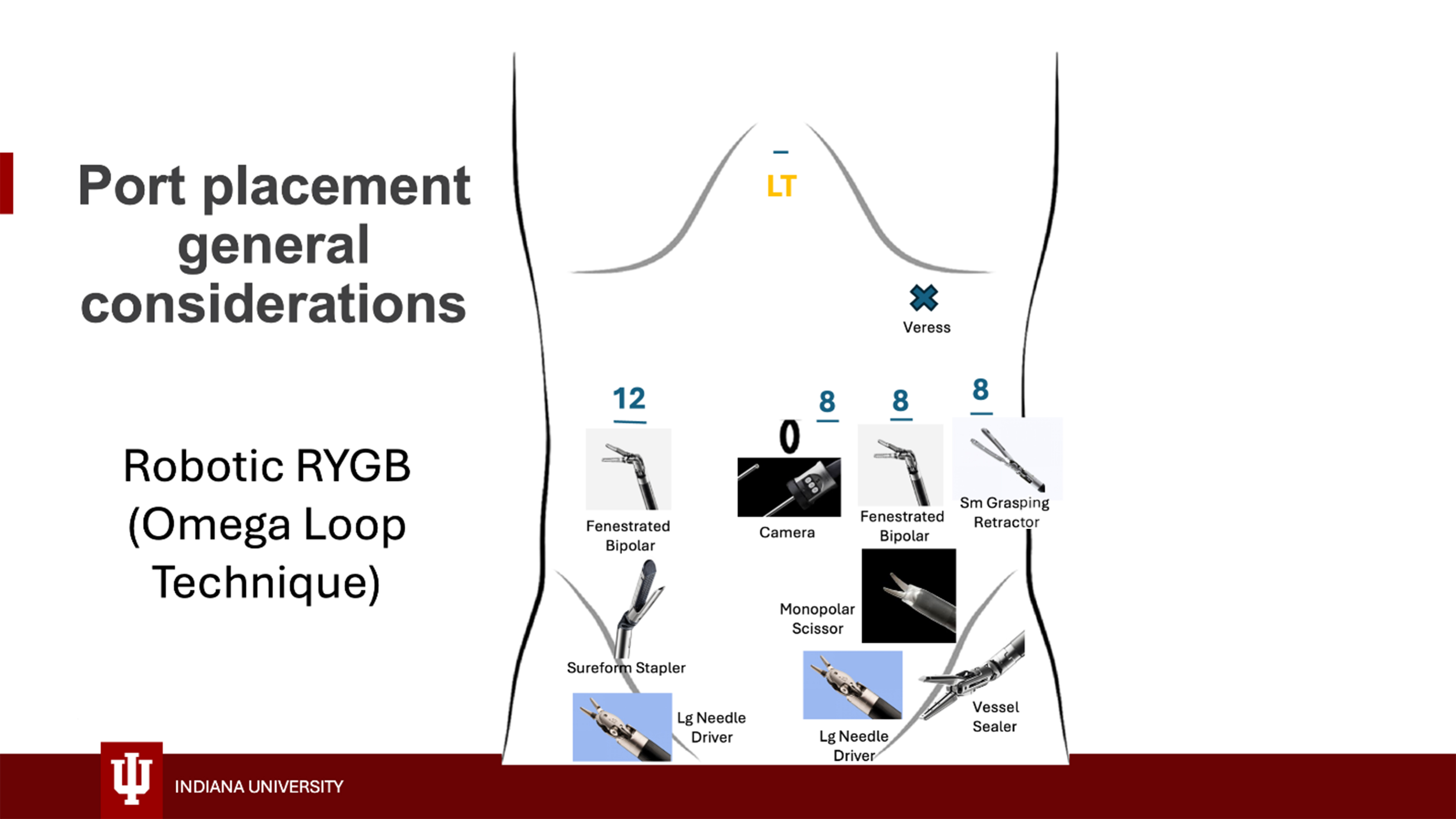
Different techniques can be used for initial entry, largely depending on the surgeon’s preference and experience, but closed techniques are preferred because the large subcutaneous tissue layer makes open techniques challenging. We use either direct entry with an 8 mm robotic trocar and a 5 mm 0-degree camera in the left upper quadrant using the Optiview technique or Veress needle entry at Palmer’s point. Pneumoperitoneum is established, and additional ports are placed under direct vision at the locations shown in Figures 1 and 2. Ports are generally placed with at least a palm’s-breadth distance between them, but specific measurements can also be made to determine their position: a port is placed approximately
Figure 1. Robotic port placement for traditional RYGB with handsewn GJ. RYGB: Roux-en-Y gastric bypass; GJ: gastrojejunostomy.
Surgical instruments
Instruments used for robotic RYGB and their location on the different arms are shown in Figures 1 and 2. Generally, the procedure can be safely accomplished using a Prograsp grasper, a tip-up grasper, a suture-cut needle driver, an energy device, and the 60 mm Sureform stapler. We use either the Synchroseal device or a vessel sealer for energy delivery; each has advantages and disadvantages, and their use largely depends on surgeon preference. Alternatively, a small grasping retractor, monopolar scissors, a fenestrated bipolar grasper, and two large needle drivers can be used [Figure 2]. The procedure can also be safely accomplished using a more cost-effective setup including a Prograsp, a tip-up gasper, a fenestrated bipolar, monopolar scissors, a suture cut needle driver, and the Sureform stapler (thus avoiding the cost of the energy device). We prefer the latter setup for revisional cases as the scissors work better for the dissection of scarred surgical planes. Please reference Supplementary Videos for visualization of surgical technique.
Operative approach
Pouch creation
Once the robot is docked, and an intragastric tube has been placed to decompress the stomach [a regular nasogastric tube or a 40 Fr ViSiGiTM suction calibration system (Visualize Sleeve Gastrectomy, Boehringer Laboratories, LLC, Phoenixville, PA, USA) can be used for this purpose], dissection begins at the angle of His to release the phrenoesophageal membrane. To accomplish this, the fundus of the stomach is retracted caudally and the epiphrenic fat pad is retracted to the patient’s right. The phrenoesophageal membrane is disrupted by blunt dissection using the remaining arm (usually the tip-up or small grasping retractor which are optimal for this task due to their length). Next, a perigastric dissection is performed to begin pouch formation. The location of perigastric window creation is identified by measuring about 5 cm from the gastroesophageal junction using the tip-up grasper which corresponds to the length of the metal part of this grasper. Alternatively, the small grasping retractor can be used for this measurement as the distance between its tips is 4.5 cm when the instrument is open. Generally, the location of the perigastric window is near the level of the second vascular bundle in the lesser curvature of the stomach.
The perigastric window is created using an energy device to divide the lesser curvature fat right along the gastric edge while the stomach is retracted upward toward the anterior abdominal wall and the lesser omentum is retracted toward the right hemiabdomen. The start of the dissection should stay close to the stomach to preserve the vagal nerve fibers. We do not use the pars flaccida technique that divides the vagal nerves. There are many small blood vessels in this area of dissection that require coagulation to prevent bleeding, but if bleeding is encountered, it generally will stop quickly with the application of pressure. The dissection is continued until the lesser sac is successfully entered and then proceeds perpendicularly to the long axis of the stomach. Creation of this tunnel in an upward oblique direction should be avoided as it leads to a longer path to the lesser sac.
Once the perigastric window is achieved and before the stomach is divided, it is important to remove the nasogastric tube or retract the ViSiGiTM toward the esophagus. Effective communication with the anesthesia team during this step is paramount to avoid stapling the gastric tube; a short time out to communicate with the anesthesia team is recommended. A 60 mm Sureform stapler with a blue load is then inserted into the perigastric window and fired perpendicularly to the long axis of the stomach to create the inferior border of the gastric pouch. The stapler can be inserted through arm 1 or arm 2 depending on the surgeon’s preference. A potential advantage of introducing the stapler through arm 2 is that it allows the surgeon to have a working hand on either side of the stapler which can optimize tissue manipulation and exposure during stapling. Advantages of using arm 1 for stapling include maximizing the distance from the target tissue which can make stapling easier and not having to change the camera location. If a ViSiGiTM is used to calibrate the pouch size, anesthesia is asked at this point to gently advance it to the horizontal staple line. The stomach is then divided vertically to create the lateral aspect of the pouch. Either white or blue loads can be used for this purpose (usually 2 or 3 fires) as the stomach is typically thin enough in this area to accommodate a white load, which allows superior hemostasis at the staple line. The direction of the vertical staple line targets the left crus of the diaphragm and previous angle of His dissection. Posterior attachments to the stomach must be taken down to allow stapler placement, especially for the second and third staple fires. Generally, the posterior dissection is more easily accomplished after the first vertical staple fire, which opens the space to allow more room for dissection. Using the tip-up or small grasping retractor in an inferior to superior sweeping motion helps detach the stomach from the left crus and creates the path required for stapler placement. After pouch creation, staple lines are inspected to ensure adequate formation and hemostasis; we oversew the staple line liberally if there are any concerns.
Stapled gastrojejunostomy and jejunojejunostomy creation (omega loop technique)
Omega loop creation
The greater omentum is split at the level of the distal transverse colon where the Roux limb is expected to cross it when antecolic; if the omentum is thin, it may not need to be divided [Supplementary Video 1]. The colon is then reflected cranially and the ligament of Treitz is identified. The small bowel is run for 50-
Disconnecting omega loop
Attention is then directed to disconnection of the BP limb from the Omega loop. The BP portion of the small bowel was previously positioned in the left lateral abdomen (screen right). Arm 4 uses a small grasping retractor to hold up on the BP side of the omega loop. The stapler is utilized in arm 1 to help retract and expose the Roux side of the omega loop, while the monopolar scissors dissect underneath the mesenteric side of the small bowel. Once a hole is created in the mesentery, the anvil side of the white load stapler is placed through and the stapler is fired, disconnecting the BP limb from the omega loop.
Jejunojejunostomy creation
Prior to formal creation of the Roux limb, an enterotomy is made in the newly disconnected BP limb. This enterotomy is enlarged to allow the cartridge side of the stapler to be inserted later. To create the Roux limb, the small bowel is run in a counterclockwise fashion 125 to 150 cm such that the Roux limb is positioned in the right lateral hemi abdomen (screen left). An enterotomy is performed, and the cartridge side of the stapler (using a white load) is placed in the BP limb with the anvil side in the Roux limb. A stapled jejunojejunostomy (JJ) is performed, and the common enterotomy is closed in the same fashion as the GJ.
Handsewn gastrojejunostomy with stapled jejunojejunostomy (traditional technique)
Jejunojejunostomy creation
As in the previously described technique, the transverse colon is reflected cranially to identify the ligament of Treitz [Supplementary Video 2]. The small bowel is run clockwise distally for 50 cm from the ligament of Treitz (measured with the tip-up grasper). A mesenteric window is created in this area and the small bowel is divided with a 60 mm white load of the Sureform stapler. The mesentery is then divided to ensure mobility. A Roux limb is measured to approximately 150 cm (again using the tip-up grapser) in a counterclockwise fashion and enterotomies are created on the antimesenteric border of the Roux limb and near the cut end of the BP limb. Enterotomies can be created either with monopolar scissors or the Synchroseal device. A 60 mm white load of the Sureform stapler is used to create a side-to-side jejunojejunal anastomosis. The enterotomies are then sutured closed with a running barbed absorbable suture taking care not to narrow the anastomosis.
Gastrojejunostomy creation
Attention is then returned to the gastric pouch. The omentum is divided vertically using the vessel sealing device at a level where the Roux limb is expected to cross when brought up antecolic; if the omentum is thin, it may not need to be divided. It is also not divided if a retrocolic approach is used. The Roux limb is brought up to the gastric pouch in an antecolic, antegastric fashion and attached to the inferior staple line of the pouch with a running barbed absorbable suture. Enterotomies are then created in the gastric pouch and the adjacent Roux limb (either with scissors or the Synchroseal) that are approximately 2-2.5 cm in length. Two 6-inch, 3-0 Monocryl sutures tied together are then used to create the handsewn anastomosis. The suture line starts by connecting the middle of the enterotomies and is run to either side; once the corners of the anastomosis are reached, the anterior wall of the anastomosis is sewn together in a Connell fashion until the sutures meet and are tied together. A second layer is not typically used unless there is a concern related to the suture line.
Mesenteric defect closure
We routinely close all mesenteric defects created during the bypass to prevent future internal herniation. We use silk or barbed suture. Attention is paid to closing the defects completely in a running fashion. The closure of Peterson’s defect is typically continued to a level above the transverse colon.
Evaluation of gastrojejunal anastomosis
At this point, an endoscopic leak test is performed. A gastroscope is introduced and advanced under direct visualization into the gastric pouch. The gastrojejunal anastomosis and gastric staple lines are inspected to ensure hemostasis and anastomotic patency. The Roux limb is occluded using the tip-up grasper or the small grasping retractor and the anastomosis is submerged under a column of saline from the laparoscopic suction irrigator through an assistant port. The gastric pouch is gently insufflated with the endoscope and carefully inspected for leaks or bubbles from the anastomosis. We typically also affix the left upper quadrant omentum on top of the anastomosis. An alternative is to use indocyanine green (ICG) through the gastric tube to perform a leak test of the GJ using Firefly mode (The surgeon may need to adjust the sensitivities of the Firefly setting for this technique). Using ICG, the gastric pouch and proximal Roux limb appear distended and green in color; in the event of a leak, bright green ICG liquid will be visualized external to the anastomosis. This can then be confirmed with the placement of a sponge inside the abdomen to dry the area and visualize any further leakage of ICG. After the leak test is performed, the gastric tube may be removed. While the ICG technique is faster than endoscopic evaluation, it does not allow for intragastric assessment of hemostasis and anastomotic diameter.
Closure
We routinely close our 12 mm trocar site with a figure-of-eight 0-polydioxanone (PDS) suture using a Carter Thomason or Karl Storz suture passer. 8 mm ports are not closed at the fascial level.
A note regarding anastomotic technique
In this technical note, we have presented two differing techniques for GJ creation: totally handsewn (used in traditional technique) and linear stapled with handsewn closure of the common enterotomy (utilized in omega loop technique). While single-center studies have suggested conflicting advantages and disadvantages with each technique, multiple systematic reviews have failed to identify significant differences in clinical outcomes between these two methods[11,12]. Of note, these studies compared techniques during laparoscopic rather than robotic cases, and results may not be entirely applicable to the robotic platform, as robotic hand-sewing may be easier given additional range of motion compared to laparoscopy. Nonetheless, given that outcomes are largely comparable, technique selection may be based on surgeon preference and familiarity. A circular stapled technique is avoided due to association with higher rates of postoperative bleeding, marginal ulcer, and stricture[11,12]. Additionally, circular staplers are not currently available for the robotic platform used at our institution.
Postoperative course
On the first night after surgery, a clear liquid diet is allowed. The following day, the diet is advanced to bariatric full liquids with a goal of 4oz per hour. Once patients tolerate 4oz of oral intake per hour and meet other standard criteria for discharge, they are discharged home with clinic follow-up scheduled within two weeks and at one month.
POSTOPERATIVE OUTCOMES
In review of our own institutional data from 2024, primary RYGB procedures were identified and categorized according to operative technique (traditional vs. omega loop). Revisional cases were excluded from analysis along with cases in which a concomitant procedure was performed (e.g., hiatal hernia repair, ventral hernia repair, etc.). There were no significant differences in rate of postoperative complications between the two operative techniques. The majority of patients in both groups were discharged on postoperative day 1 or 2, and there were no differences in hospital length of stay between groups. Cases performed with the omega loop technique had a significantly shorter operative duration when compared to the traditional RYGB technique {Omega loop median = 129 m [interquartile range (IQR) = 100, 158] vs. traditional median = 161 m (IQR = 130, 192); P < 0.001}. However, the median BMI of patients who underwent omega loop technique was slightly lower [43 (IQR = 37, 49) vs. 50 (IQR = 37, 63); P = 0.006], which may account for the difference in operative duration. Differences in median BMI between patients undergoing each operative technique are attributed to differing referral patterns and number of years in practice between two surgeons. Additional research comparing postoperative outcomes between these two operative techniques is ongoing.
COMMON PITFALLS
As with the acquisition of any new skill, adoption of the robotic platform follows a learning curve. Optimal intraoperative and clinical outcomes may only be achieved with time, experience, and skill refinement. Based on our own experience and those documented in the literature, operative time commonly increases early on as both surgeons and staff become acquainted with the robotic platform; however, as familiarity is gained, operative times quickly improve[13]. Likewise, perioperative morbidity follows a similar curve, decreasing as experience is gained over time[13]. With proper mentorship and clinical support, surgeons may further refine their operative skills, enhancing both efficiency and safety using the robotic platform.
CONCLUSION
This technical paper reviewed two detailed approaches for robotic gastric bypass surgery. While traditional laparoscopy remains the dominant approach nationally, the volume of robotic procedures has steadily increased in recent years[14]. Multiple systematic reviews with meta-analyses have suggested possible clinical benefits of the robotic platform for RYGB including decreased hospital length of stay and reduced rates of GJ stricture without increases in perioperative complications or readmissions[15,16]; however, data from individual centers often present contradictory results and remain highly variable and surgeon/center specific[17-20]. Anecdotally, we suggest several benefits of the robotic platform for the surgeon including decreased need for skilled assistants, improved ergonomics, and decreased physical strain compared to traditional laparoscopy. These benefits are most evident in select groups of patients, particularly those with BMI > 50 in which a thick abdominal wall may present significant difficulty with port placement and instrument manipulation laparoscopically[21]. In this population, large volumes of visceral fat also pose challenges with visualization and retraction. The additional mobility and degrees of freedom offered by the robotic platform can be of great assistance in these cases, allowing for more optimized dissection angles, improved retraction, better visualization, and finer dexterity. As a result, recent work has shown no increase in operative time for robotic approaches compared to traditional laparoscopy in this population[22].
Though longer operative times have traditionally been cited as a tradeoff of robotic approaches, when performed frequently, the robotic approach can be incredibly efficient and may reduce operative times and minimize morbidity in the hands of experienced surgeons at high-volume centers. As with all technology and tools, there is a learning curve when transitioning to the robotic platform[13]; both the operating surgeon and operating room (OR) staff must appropriately train on the robotic platform to become skilled and efficient. As the annual volume of robotic approaches for RYGB continues to rise and surgeons and OR staff gain more experience, traditional barriers to adopting robotic approaches will become less relevant as procedural time and operative costs decline. In summary, we have presented two techniques for robotic-assisted RYGB, each with its own distinct advantages and challenges. Both techniques employ a robotic approach, offering unique benefits compared to traditional laparoscopy, and have been proven to be safe and effective procedures for surgical weight loss.
DECLARATIONS
Author’s contributions
Study conception and design: Airhart AE, AbuHasan Q, Holmstrom AL, Stefanidis D
Data acquisition: Airhart AE, Holmstrom AL
Primary data analysis: Airhart AE
Video acquisition: Airhart AE, Holmstrom AL, Stefanidis D
Video editing and narration: Airhart AE
Manuscript writing: Airhart AE, AbuHasan Q
Manuscript editing: Holmstrom AL, Stefanidis D
Project supervision: Holmstrom AL, Stefanidis D
Availability of data and materials
All data and materials used in our study are available from the corresponding author upon reasonable request.
Financial support and sponsorship
None.
Conflicts of interest
Stefanidis D receives research support from Intuitive, Cook Medical, and Beckton Dickinson; Stefanidis D is a consultant for Johnson & Johnson and Applied Medical. The other authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
Supplementary Materials
REFERENCES
1. Sheetz KH, Claflin J, Dimick JB. Trends in the adoption of robotic surgery for common surgical procedures. JAMA Netw Open. 2020;3:e1918911.
2. Wee IJY, Kuo LJ, Ngu JC. A systematic review of the true benefit of robotic surgery: ergonomics. Int J Med Robot. 2020;16:e2113.
3. Bauerle WB, Mody P, Estep A, Stoltzfus J, El Chaar M. Current trends in the utilization of a robotic approach in the field of bariatric surgery. Obes Surg. 2023;33:482-91.
4. AbuHasan Q, Miller PM, Li WS, Burney CP, Yuce TK, Stefanidis D. Racial disparities in the utilization and outcomes of robotic bariatric surgery: an 8-year analysis of Metabolic and Bariatric Surgery Accreditation Quality Improvement Program data. Surg Obes Relat Dis. 2025;21:158-65.
5. Zhang L, Tan WH, Chang R, Eagon JC. Perioperative risk and complications of revisional bariatric surgery compared to primary Roux-en-Y gastric bypass. Surg Endosc. 2015;29:1316-20.
6. Verhoeff K, Mocanu V, Dang J, et al. Five years of MBSAQIP Data: characteristics, outcomes, and trends for patients with super-obesity. Obes Surg. 2022;32:406-15.
7. Spurzem GJ, Broderick RC, Kunkel EK, et al. Robotic bariatric surgery reduces morbidity for revisional gastric bypass when compared to laparoscopic: outcome of 8-year MBSAQIP analysis of over 40,000 cases. Surg Endosc. 2024;38:6294-304.
8. Benmessaoud C, Kharrazi H, MacDorman KF. Facilitators and barriers to adopting robotic-assisted surgery: contextualizing the unified theory of acceptance and use of technology. PLoS One. 2011;6:e16395.
9. Starnes CC, Gochnour DC, Hall B, Wilson EB, Snyder BE. The economy of motion of the totally robotic gastric bypass: technique, learning curve, and outcomes of a fellowship-trained, robotic bariatric surgeon. J Laparoendosc Adv Surg Tech A. 2015;25:411-8.
10. Behera K, McKenna M, Smith L, et al. Transferring laparoscopic skills to robotic-assisted surgery: a systematic review. J Robot Surg. 2024;18:11.
11. Fakas S, Elias M, Lim D, Meytes V. Comparison of gastrojejunostomy techniques and anastomotic complications: a systematic literature review. Surg Endosc. 2021;35:6489-96.
12. Jiang HP, Lin LL, Jiang X, Qiao HQ. Meta-analysis of hand-sewn versus mechanical gastrojejunal anastomosis during laparoscopic Roux-en-Y gastric bypass for morbid obesity. Int J Surg. 2016;32:150-7.
13. Hirri F, Pickering OJ, Carter NC, van Boxel GI, Pucher PH. Learning curves for adoption of robotic bariatric surgery: a systematic review of safety, efficiency and clinical outcomes. J Robot Surg. 2024;18:349.
14. Scarritt T, Hsu CH, Maegawa FB, Ayala AE, Mobily M, Ghaderi I. Trends in utilization and perioperative outcomes in robotic-assisted bariatric surgery using the MBSAQIP database: a 4-year analysis. Obes Surg. 2021;31:854-61.
15. Economopoulos KP, Theocharidis V, McKenzie TJ, Sergentanis TN, Psaltopoulou T. Robotic vs. laparoscopic Roux-en-Y gastric bypass: a systematic review and meta-analysis. Obes Surg. 2015;25:2180-9.
16. Markar SR, Karthikesalingam AP, Venkat-Ramen V, Kinross J, Ziprin P. Robotic vs. laparoscopic Roux-en-Y gastric bypass in morbidly obese patients: systematic review and pooled analysis. Int J Med Robot. 2011;7:393-400.
17. Koeller E, Luhrs AR, Giorgi M. Changes in utilization of robotic bariatric surgery and effect on patient outcomes from 2015-2020. J Robot Surg. 2023;17:2041-5.
18. Caiazzo R, Bauvin P, Marciniak C, et al; SOFFCO-mm Study Group. Impact of robotic assistance on complications in bariatric surgery at Expert Laparoscopic Surgery Centers: a retrospective comparative study with propensity score. Ann Surg. 2023;278:489-96.
19. Rogula T, Koprivanac M, Janik MR, et al. Does robotic Roux-en-Y gastric bypass provide outcome advantages over standard laparoscopic approaches? Obes Surg. 2018;28:2589-96.
20. Lainas P, Kassir R, Benois M, et al. Comparative analysis of robotic versus laparoscopic Roux-en-Y gastric bypass in severely obese patients. J Robot Surg. 2021;15:891-8.
21. Marincola G, Procopio PF, Pennestrì F, et al. Robot-assisted vs laparoscopic bariatric procedures in super-obese patients: clinical and economic outcomes. J Robot Surg. 2024;18:34.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Copyright
Data & Comments
Data

















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].