Sutureless clampless partial nephrectomy: where do we stand? A narrative review
Abstract
This review aims to evaluate the current literature on sutureless clampless partial nephrectomy (sl-oc PN) and assess its potential advantages in terms of renal function preservation, oncological safety, and perioperative outcomes. We conducted a literature search across multiple databases, including MEDLINE, PubMed, and Embase, selecting studies published in the last 10 years that reported on clampless and sutureless partial nephrectomy in cohorts of 10 or more patients. Ten studies from 2015 to 2025 were included, mostly involving tumors with low RENAL (Radius, Exophytic/endophytic properties, Nearness of tumor to collecting system or sinus, Anterior/posterior descriptor, and Location relative to polar lines) scores (< 5). Key outcomes analyzed were estimated blood loss, positive surgical margin rates, major complications (Clavien-Dindo grade ≥ 3), and postoperative renal function changes. Overall, the sutureless clampless technique demonstrated comparable oncological safety, low complication rates, and favorable preservation of renal function. These findings support the feasibility and safety of sl-oc PN, with careful case selection being essential.
Keywords
INTRODUCTION
The current gold standard for the management of localized renal cancer, particularly in tumors classified as T1 and, in selected instances, T2, is partial nephrectomy, as recommended by the most recent guidelines issued by the European Association of Urology (EAU)[1]. Since its initial implementation in clinical practice, the primary objective of partial nephrectomy has consistently been to maximize the preservation of healthy, functioning renal parenchyma, thereby maintaining optimal renal function in the postoperative setting. According to Mir et al.[2], the reduction in renal function following surgery is attributed to multiple contributing factors. These include the unavoidable loss of parenchymal tissue that is excised during the procedure, the dimensions and volume of the resected segment, the ischemic damage resulting from clamping of the renal artery, the oxidative stress and injury during the subsequent reperfusion phase, and finally, the trauma induced during renal reconstruction, commonly referred to as renorrhaphy. In addition to these procedural factors, other variables such as the anatomical location of the tumor within the kidney and the biological age of the patient have also been shown to significantly influence postoperative renal function[2].
Moreover, the ongoing debate regarding the optimal use of hilar clamping during partial nephrectomy remains unresolved and continues to generate discussion within the surgical and urological communities. Although a considerable volume of literature[3] has been published on the topic, conclusive evidence regarding its superiority or risks is still lacking. In the first randomized controlled trial (RCT) specifically designed to address this issue (the CLOCK trial; NCT02287987), Antonelli et al.[4] evaluated the safety and functional outcomes associated with various clamping strategies. Their findings revealed no statistically significant differences in postoperative renal function between patients who underwent off-clamp procedures and those who underwent traditional on-clamp techniques. Conversely, more recent studies[3] have highlighted that the act of suturing the renal parenchyma may inflict additional damage to the renal microvasculature, potentially leading to further parenchymal loss and a subsequent decline in global renal function.
In response to the recognized need to reduce ischemic injury, several authors[5,6] have advocated for innovative surgical approaches characterized by zero ischemia time. With the emergence and increasing adoption of robotic surgical platforms, some researchers have gone a step further by proposing a novel technique known as off-clamp and sutureless partial nephrectomy (sl-oc PN)[7-9]. This technique aims to completely avoid both clamping and parenchymal suturing, thereby minimizing intraoperative trauma and preserving renal function to a greater extent. Consequently, the present narrative review aims to comprehensively describe this emerging surgical technique and to critically evaluate its potential benefits in terms of both functional renal preservation and overall procedural safety.
LITERATURE RESEARCH
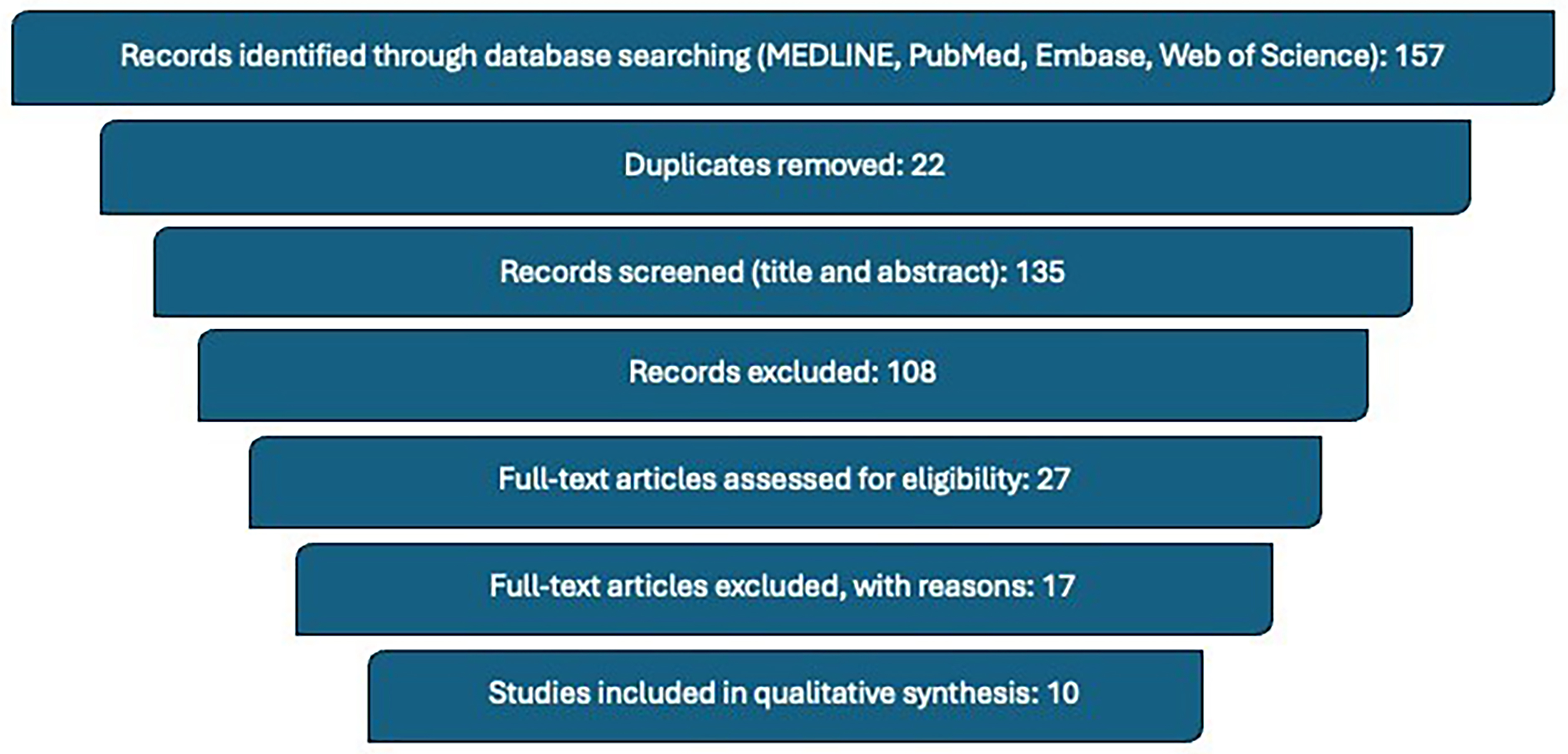
An electronic literature search was conducted using the MEDLINE database, PubMed, Embase and Web of Science databases, employing a combination of Medical Subject Headings (MeSH) terms and free-text keywords. The search was limited to studies published within the last ten years to ensure relevance to contemporary surgical practices. The specific search terms utilized for the research included the following combinations: “Robotic sutureless clampless partial nephrectomy”, “Laparoscopic sutureless clampless partial nephrectomy”, “off-clamp partial nephrectomy”, and “sutureless partial nephrectomy”. In addition to database searching, the reference lists of key review articles were thoroughly screened in order to identify any additional relevant studies that may not have been captured by the initial search strategy.
Only studies published in English were included in this review to ensure consistency and clarity in the interpretation of findings. Articles were deemed eligible if they reported clinical series involving more than ten patients and specifically described partial nephrectomy procedures incorporating both a clampless and sutureless surgical approach. The selection process began with an initial screening of titles and abstracts to identify potentially relevant articles. This was followed by a detailed full-text assessment of each candidate article to confirm that it met the predefined eligibility criteria.
Publications that did not present original data - such as editorials, expert commentaries, abstracts from conference proceedings, and book chapters - were systematically excluded from the analysis, as they were not considered to provide sufficient clinical detail or peer-reviewed evidence for the purposes of this review. The study selection process is summarized in Figure 1.
EVIDENCE SYNTHESIS
The surgical technique described in this manuscript specifically reflects the approach adopted at three institutions: Scientific Institute for Research, Hospitalization and Healthcare (IRCCS) Regina Elena of Rome (IFO), Sant’Andrea Hospital of Rome, and San Bassiano Hospital of Bassano del Grappa. While the review includes studies from multiple centers, the technique presented here corresponds to the standardized procedure employed at these sites. Nonetheless, some variability in operative steps and devices may exist across different centers, which is an important consideration when interpreting the collective evidence.
Surgical technique
The patient is placed in a lateral position, opposite to the side of the lesion. Transperitoneal access is established by inserting five ports for robotic and laparoscopic instruments. The camera is introduced at the umbilical level along the pararectal line. Two robotic ports are positioned along the midclavicular and anterior axillary lines, allowing for the use of Hot Shears monopolar curved scissors and ProGrasp forceps (Intuitive Surgical, Sunnyvale, CA, USA), respectively.
Additionally, two assistant ports (measuring 12 and 5 mm) are placed along the midline, situated between the camera port and the robotic arms, forming a “U”-shaped configuration around the tumor. These ports are used to insert one or two suction-irrigation tools, a Ligasure device, and a Weck clip applicator (Teleflex, Wayne, PA, USA), depending on surgical needs. Throughout the procedure, intra-abdominal pressure is maintained at 12 mmHg.
Regarding access during the laparoscopic technique, according to Introini et al.[10], the patient is placed in a flank position angled at the level of the twelfth rib. The first access is obtained using a free periumbilical trocar, followed by the placement, under direct vision, of one 10 mm and one 5 mm trocar in a triangular configuration, as well as an additional 5 mm subcostal trocar. Dell’Atti et al.[11] describe a similar trocar placement, also using a four-port transperitoneal configuration. A 12 mm trocar is inserted under direct vision in a supraumbilical position to establish the pneumoperitoneum, using carbon dioxide with an intra-abdominal pressure of 10 to 12 mmHg. A 45-degree endoscopic camera is then introduced, followed by the placement of two additional 10 mm trocars - one along the midclavicular line and the other midway between the iliac crest tubercle and the umbilicus. A fourth 5 mm trocar is placed laterally along the anterior axillary line to accommodate the laparoscopic aspiration instrument. As for the remaining stages of the surgical procedure, the laparoscopic technique resembles the robotic technique.
Toldt’s fascia is incised, allowing medial displacement of the colon. In most instances, the tumor can be directly accessed without the need for initial identification or dissection of the renal hilum. Alternatively, the renal artery may be isolated and encircled with a vessel loop, which is removed at the conclusion of the surgery. For tumors located at the kidney poles, the Gerota’s fascia is opened near the lesion without full mobilization of the kidney. A more extensive mobilization is reserved for posterior tumors, enabling improved access and direct visualization of the mass. When feasible, the fat overlying the tumor is left intact to support precise pathological staging. The tumor boundaries are carefully delineated and incised circumferentially using robotic scissors, gradually dissecting the lesion from surrounding healthy tissue along an avascular plane, with continuous monitoring for any bleeding. In more challenging or deeply located tumors, indocyanine green may be administered to evaluate vascular anatomy. If bleeding vessels are encountered, monopolar coagulation in forced mode is utilized for effective hemostasis. The tumor is dissected at both intermediate and base levels in accordance with the preplanned enucleation method. After complete excision, the tumor bed is further coagulated with monopolar energy until hemostasis is achieved. To avoid eschar sticking to the monopolar scissors, energy is applied with near-contact and concurrent gentle irrigation. Should there be an unintended breach of the calyceal system, it is repaired with a continuous suture using 4/0 absorbable monofilament. Once coagulation is complete, the surgical area is carefully inspected for two min to reassess hemostasis. A hemostatic agent (Floseal®) may be applied to the resection bed. Hemostasis is a crucial step to prevent secondary bleeding. Besides diathermy cauterization, commonly used devices include topical hemostatic agents and energy-based sealing tools. If a bleeding vessel branch is encountered, targeted cauterization or hemostatic agents are typically sufficient. In cases of significant bleeding, selective clamping or conversion to sutured renorrhaphy may be required to ensure safety. The excised tumor is placed in a 10-mm EndoCatch retrieval bag (Ethicon, Sommerville, NJ, USA) for removal. Closure of Gerota’s fascia and the peritoneum is achieved with a continuous barbed suture, and a drain is inserted into the renal fossa for a minimum of 24 h.
On the first postoperative day (POD 1), patients typically begin with liquids and gradually return to a regular diet. By POD 2, patients are mobilized, and both the drain and urinary catheter are usually removed[9].
Oncological and functional outcomes
The Medline database identified a total of 10 studies[5-14], from 2015 to 2025, that enrolled at least 10 patients. Most of them were retrospective, except for 1 prospective study[9]. The RENAL (Radius, Exophytic/endophytic properties, Nearness of tumor to collecting system or sinus, Anterior/posterior descriptor, and Location relative to polar lines) score was generally low and below a value of 5 with the exception of the study by Tanaka et al.[12] and Kihara et al.[14] which reported a mean RENAL/PADUA (Preoperative Aspects and Dimensions Used for an Anatomical) score of 8 and 7, respectively. The perioperative outcomes mostly assessed were blood loss [estimated blood loss (EBL)], surgical margin positivity (PSM), complications according to the Clavien-Dindo (CD) classification of grade 3 or higher, and finally, the loss of renal function. The latter was generally evaluated as the reduction in glomerular filtration rate three months after surgery, although two of the analyzed studies[6,11] considered the reduction in function six months post-surgery.
Table 1 presents the included studies and relevant results.
Analyzed studies and their main functional outcomes
| Author(s) | Sample | RENAL/PADUA | EBL (mL) | PSM | CD > 3 | Renal function |
| Franco et al. (2024)[9] | 21 | 4 (3.5-5) | 150 (50-300) | 2/21, 10% | 1/21, 5% | Decrease of 10 mL/min (3 months after surgery scintigraphy) |
| De Nunzio et al. (2024)[8] | 368 | - | - | 1/368, 0.27% | 7/368, 2% | Significant eGFR deterioration 20/368, 5% (postoperative GFR evaluation) |
| Brassetti et al. (2023)[7] | 175 | - | - | 3/175, 1.7% | 3/175, 2% | Significant reduction 10/175, 6% (Median follow-up time was 17 months) |
| Introini et al. (2020)[10] | 62 | 5 (4-6) | 165 (20-250) | 2/62, 3% | - | Decrease of 10 mL/min (Postoperative and at 3 months GFR evaluation) |
| Corongiu et al. (2019)[5] | 15 | 5 (5-7) | 310 (180-500) | 0/15 | 1/15, 6.7% | Decrease of 3.45 mL/min (postoperative GFR evaluation) |
| Zhang et al. (2019)[6] | 142 | 5 | 154 (10-800) | 2/142, 1.4% | - | Decrease of 2.3 mL/min (6 months follow-up with GFR evaluation) |
| Dell’Atti et al. (2018)[11] | 143 | 4.7 (4-6) | 110.2 (30-400) | 4/143, 2.7% | 1/143, 0.7% | Decrease of 0.8 mL/min (postoperative GFR and creatinine evaluation) |
| Tanaka et al. (2017)[12] | 74 | 8 (6-11) | 267 | - | 7/74, 9% | - |
| Li et al. (2016)[13] | 11 | - | - | - | - | - |
| Kihara et al. (2015)[14] | 103 | 7 (6-10) | 244 (110-530) | 4/103, 3.9% | 3/103, 3% | Decrease of 2.3 mL/min (3 months follow-up with GFR evaluation) |
DISCUSSION
Our study aimed to assess safety and functional outcomes of sl-oc PN by reviewing the existing data available in the literature.
A critical and highly debated step in partial nephrectomy is the clamping of the renal artery. The ischemia and subsequent reperfusion that result from this step could lead to a reduction in renal function[15]. The CLOCK trial[4], an important randomized trial published in 2019 by Antonelli et al., did not show any statistically significant differences in terms of safety of the two approaches, whether on-clamp or off-clamp. In particular, no differences were found regarding intraoperative EBL (off- vs. on-clamp, 100 vs. 100 mL, P = 0.7), postoperative complications rate (19% vs. 26%, P = 0.2), postoperative anemia [hemoglobin (Hb) decrease > 2.5 g/dl, 26% vs. 27%, P = 0.9; transfusion rate 3.4% vs. 6.3%, P = 0.5; re-intervention due to bleeding 1.1% vs. 4%, P = 0.4], acute kidney injury (AKI, 4% vs. 6%, P = 0.8), and PSM (3.5% vs. 8.2%, P = 0.1). Although randomized controlled data such as the CLOCK trial showed no significant functional benefit of off-clamp over on-clamp techniques, these findings do not specifically apply to sutureless approaches. The favorable renal function outcomes observed in the studies included in this review may reflect the additive benefits of avoiding both clamping and parenchymal suturing. Nevertheless, the limited availability of randomized data on sl-oc PN warrants cautious interpretation. Bertolo et al.[16] also showed no significant differences in perioperative outcomes between the two approaches (CLOCK II). Therefore, the off-clamp approach did not appear to increase the risk of bleeding or the rate of postoperative complications, contrary to findings from some other studies, which have suggested that off-clamp nephrectomy may be associated with higher rates of conversion to radical nephrectomy and increased need for blood transfusion, without providing a clear benefit in terms of renal function. A superselective clamping of the artery feeding the tumor has also been proposed over time, aiming to reduce hemorrhagic risks and maintain a zero-ischemia approach[15,17]. However, a real benefit for renal function was demonstrated by the results of Ayyad et al.[18], who found that off-clamp laparoscopic partial nephrectomy was safe in carefully selected patients, with a minimal increase in bleeding rate. According to their findings, except for the off-clamp group, the mean epidermal growth factor receptor (eGFR) changes were -1.40 (1.90) mL/min and -5.15 (2.50) in the on-clamp group, both statistically significant at the 0.05 level.
The complexity of a renal mass depends on factors such as its size, location, relationship to the renal pelvis and vessels, and nephrometric indices such as the RENAL and PADUA scores, which can assist the surgeon in selecting between on-clamp and off-clamp techniques. In a multicenter series involving patients with tumors characterized by high RENAL scores, outcomes of robot-assisted partial nephrectomy (RAPN) with (on-clamp) and without (off-clamp) renal vessel clamping were compared, demonstrating that the off-clamp approach may lead to improved postoperative renal function[19,20].
Suturing the renal parenchyma with single or double layers after the enucleation phase of the tumor mass is another debated issue. Renorrhaphy is indicated to close the renal breach for hemostatic purposes and to minimize the risk of urine extravasation and urinoma formation[21].
However, the potential harmful effects of renorrhaphy on renal function have recently been investigated, and alternative hemostatic techniques and renal parenchyma closure methods based on coagulation of the resection bed have been proposed[22]. As a matter of fact, Brassetti et al. recently showed promising results with the sutureless approach, demonstrating a good safety profile, low complication rates, and a reduced incidence of perioperative and short-term renal function decrease[7].
Several external studies have reported potential advantages of the sutureless technique over conventional sutured renorrhaphy, especially in the context of clampless partial nephrectomy for small renal masses. In a comparative study by Kilic et al.[23], patients undergoing clampless RAPN with the sutureless approach had a median EBL of 125 vs. 120 mL in the sutured group (P = 0.76). No statistically significant differences were observed in postoperative hemoglobin levels or drop in hemoglobin. At 3 months postoperatively, renal function remained stable in the sutureless group, whereas a mean eGFR decrease of 1.4 ± 9.8 mL/min was recorded in the sutured group, although this was not statistically significant (P > 0.05).
Similarly, Jin et al.[24] reported a lower incidence of AKI[25] in the sutureless laparoscopic partial nephrectomy group (3.1%) compared to the sutured group (14.1%), with comparable positive surgical margin rates (2.1% vs. 2.3%) and no significant difference in long-term renal function.
A narrative review by Cortés et al.[26] further confirmed the safety of the sutureless approach, highlighting that it does not increase the risk of perioperative complications, even when applied in selected challenging scenarios.
Finally, in a propensity-score matched analysis, Farinha et al.[27] compared sutureless or selective suturing techniques with standard sutured RAPN. They found that the sutureless/selective group had a shorter operative time (mean 118 vs. 130 min, P = 0.02) and a significantly lower rate of AKI (4.8% vs. 11.1%, P = 0.03), without compromising oncologic safety.
These findings, while not derived from the core studies included in our primary synthesis, provide supporting external evidence of the feasibility, safety, and potential functional benefits of sutureless approaches in carefully selected patients.
Studies on the safety and renal function of patients undergoing off-clamp sutureless robotic partial nephrectomy (RAPN) have also been conducted at our center[9]. Among the 21 patients evaluated, the reduction in renal function of the operated kidney ranged from 0 to 15 mL/s and from 0% to 40%, with median decreases of 4 mL/s and 12%, respectively. Notably, none of the patients experienced a reduction in eGFR of < 0.05. The learning curve of this surgical technique was also investigated through a multicenter study, the promising results reported by De Nunzio et al.[8] (2024) suggest that the sutureless clampless technique can be safely adopted even by surgeons with limited robotic experience. Their propensity-matched cohort study (23 patients per group) showed encouraging outcomes, supporting the feasibility of this approach beyond highly specialized centers. However, given the small sample size, further larger-scale studies are needed to confirm these findings and better define the learning curve associated with this technique.
Overall, the perioperative outcomes reported in the included studies were comparable to those documented in the existing literature on the standard RAPN technique. More specifically, the average EBL was 192 mL, a data comparable to the standard technique according to the study by Shvarts et al.[28]. The review by Minervini et al., in fact, shows an average of surgical margin positivity of 2.7% (0%-15%)[29]. The incidence of significant complications (CD grade ≥ 3) was low, occurring in an average of 4% of cases[29]. These findings are consistent with those reported for standard partial nephrectomy techniques. For example, Jiménez et al. documented a comparable rate, with an average of 22.2% of patients experiencing grade ≥ 3 complications[30]. According to the review by Mir et al.[2], compensatory hypertrophy in the contralateral kidney after partial nephrectomy in adults is marginal, and the decline in global renal function for patients with two kidneys averages around 10%, although this may vary depending on tumor size and location. Overall, the range of renal function preservation reported across different studies varies between 76% and 96%. Similarly, the studies analyzed in our review showed only a minimal reduction in renal function. Notably, the study by Franco et al.[9], which assessed renal filtration pre- and postoperatively using sequential renal scintigraphy, reported that the decrease in renal function in the operated kidney ranged from 0 to 15 mL/s and from 0% to 40%, with median values of 4 mL/s and 12%. In specific cases, such as patients with Von Hippel-Lindau (VHL) syndrome, the clampless and sutureless approach has been recommended to maximize renal function preservation, as VHL usually presents with multiple and bilateral lesions that may require multiple renal surgeries[31]. The safety and feasibility of the off-clamp sutureless technique have also been investigated using a laparoendoscopic single-port approach, with preliminary studies supporting the technique[32]. Certainly, further larger studies and RCTs are warranted to confirm the role of sl-oc PN, as artificial intelligence (AI) and three-dimensional (3D) models - augmented reality- may further enhance its role in the panorama of localized renal cancer management[33-35]. Recent developments in 3D imaging and reconstruction from computed tomography (CT) scans have significantly improved the ability to identify and selectively clamp tumor-feeding arterial branches during partial nephrectomy. This technique allows for precise ischemia of the tumor area while preserving maximal renal function by minimizing ischemic damage to healthy parenchyma. The choice between selective arterial clamping and a completely clampless approach depends on tumor complexity, vascular anatomy, and surgeon preference, with 3D imaging playing a crucial role in preoperative planning and intraoperative decision-making[36].
Table 2 shows the advantages and disadvantages related to the technique, as previously discussed.
Main advantages and disadvantages of the described surgical technique
| Advantages | Disadvantages |
| No manipulation of renal hilum | Increased risk of bleeding |
| Zero ischemia | Possible increased risk of PSM due to reduced visibility during the enucleation phase |
| Preservation of renal function | Limited to low Renal/Padua score masses? |
Our study is not devoid of limitations, and it is important to acknowledge these when considering the implications of our findings. Firstly, a significant proportion of the studies included in this review were retrospective in design, which inherently introduces certain methodological constraints. Retrospective studies are generally more prone to selection bias, incomplete data collection, and unmeasured confounding variables. Moreover, many of these studies involved a limited number of patients, often reflecting single-center experiences or small institutional series, which restricts the generalizability of their conclusions and diminishes the statistical power required to detect subtle differences in outcomes.
Secondly, the nature of our investigation as a narrative review is useful for providing an overview of current practices and highlighting emerging techniques; nevertheless, it lacks the methodological rigor and comprehensive data synthesis typically associated with more structured reviews. Our focus was specifically directed toward the sl-oc PN technique and its reported functional outcomes, with particular attention to postoperative renal function and perioperative safety. However, we recognize that the inclusion criteria, patient characteristics, surgical expertise, and operative approaches varied considerably among the selected studies. Such heterogeneity complicates cross-study comparisons and may influence the interpretation of the effectiveness and reproducibility of the technique across different clinical settings.
The adoption of the sutureless and clampless partial nephrectomy technique largely depends on the surgeon’s preference and experience. However, certain clinical and anatomical factors may limit its application. Cases involving large tumors, increased bleeding risk due to anticoagulant or antiplatelet therapy, coagulation disorders, or complex tumor locations are generally considered higher risk for significant hemorrhage. In such scenarios, conventional clamping of the renal artery and parenchymal suturing should be carefully considered to ensure optimal hemostasis and patient safety. Therefore, careful preoperative assessment and individualized surgical planning are crucial for appropriate case selection.
Future investigations with standardized methodologies, larger and more diverse patient cohorts, and prospective designs are necessary to validate the preliminary findings and better define the role of sl-oc PN within the broader context of nephron-sparing surgery. Moreover, studies with long-term follow-up (at least 5 years) are needed to clarify long-term oncologic and functional outcomes.
CONCLUSION
Sutureless and clampless partial nephrectomy is increasingly recognized as a safe and effective technique for treating localized renal tumors. This approach avoids vascular clamping and parenchymal suturing, showing promising results in preserving renal function, minimizing complications, and achieving favorable oncological outcomes. When anatomical conditions are suitable and performed by experienced surgeons, this technique represents a viable alternative to conventional methods. However, careful patient selection is essential to optimize outcomes and reduce risks.
DECLARATIONS
Authors’ contributions
Conceptualization: Riolo S, Franco A
Methodology: Tema G, Nesi G, Silvestri T
Resources: De Nunzio C, Zeccolini G
Writing - original draft: Riolo S
Writing - review & editing: Franco A
Supervision: Celia A
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Ljungberg B, Bensalah K, Canfield S, et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol. 2015;67:913-24.
2. Mir MC, Ercole C, Takagi T, et al. Decline in renal function after partial nephrectomy: etiology and prevention. J Urol. 2015;193:1889-98.
3. Klatte T, Ficarra V, Gratzke C, et al. A Literature review of renal surgical anatomy and surgical strategies for partial nephrectomy. Eur Urol. 2015;68:980-92.
4. Antonelli A, Cindolo L, Sandri M, et al; AGILE Group (Italian Group for Advanced Laparo-Endoscopic Surgery). Safety of on- vs. off-clamp robotic partial nephrectomy: per-protocol analysis from the data of the CLOCK randomized trial. World J Urol. 2020;38:1101-8.
5. Corongiu E, Grande P, Di Santo A, et al. Safety and efficacy of retroperitoneal sutureless zero ischemia laparoscopic partial nephrectomy for low nephrometry score masses. Arch Ital Urol Androl. 2019:91.
6. Zhang F, Gao S, Chen XN, Wu B. Clampless and sutureless laparoscopic partial nephrectomy using monopolar coagulation with or without N-butyl-2-cyanoacrylate. World J Surg Oncol. 2019;17:72.
7. Brassetti A, Misuraca L, Anceschi U, et al. Sutureless purely off-clamp robot-assisted partial nephrectomy: avoiding renorrhaphy does not jeopardize surgical and functional outcomes. Cancers. 2023;15:698.
8. De Nunzio C, Tema G, Brassetti A, et al. Purely off-clamp sutureless robotic partial nephrectomy for novice robotic surgeons: a multi-institutional propensity score-matched analysis. J Clin Med. 2024;13:3553.
9. Franco A, Riolo S, Tema G, et al. Renal function preservation in purely off-clamp sutureless robotic partial nephrectomy: initial experience and technique. Diagnostics. 2024;14:1579.
10. Introini C, Di Domenico A, Ennas M, Campodonico F, Brusasco C, Benelli A. Functional and oncological outcomes of 3D clampless sutureless laparoscopic partial nephrectomy for renal tumors with low nephrometry score. Minerva Urol Nefrol. 2020;72:723-8.
11. Dell’Atti L, Scarcella S, Manno S, Polito M, Galosi AB. Approach for renal tumors with low nephrometry score through unclamped sutureless laparoscopic enucleation technique: functional and oncologic outcomes. Clin Genitourin Cancer. 2018;16:e1251-6.
12. Tanaka H, Fujii Y, Ishioka J, Matsuoka Y, Saito K, Kihara K. Absence of renal artery pseudoaneurysm on computed tomography after minimally-invasive partial nephrectomy using clampless and sutureless techniques. Int J Urol. 2017;24:472-3.
13. Li CC, Yeh HC, Lee HY, et al. Laparoscopic partial nephrectomy without intracorporeal suturing. Surg Endosc. 2016;30:1585-91.
14. Kihara K, Koga F, Fujii Y, et al. Gasless laparoendoscopic single-port clampless sutureless partial nephrectomy for peripheral renal tumors: perioperative outcomes. Int J Urol. 2015;22:349-55.
15. Simone G, Papalia R, Guaglianone S, Carpanese L, Gallucci M. Zero ischemia laparoscopic partial nephrectomy after superselective transarterial tumor embolization for tumors with moderate nephrometry score: long-term results of a single-center experience. J Endourol. 2011;25:1443-6.
16. Bertolo R, Bove P, Sandri M, et al; AGILE Group (Italian Group for Advanced Laparoendoscopic Surgery). Randomized clinical trial comparing on-clamp versus off-clamp laparoscopic partial nephrectomy for small renal masses (CLOCK II laparoscopic study): a intention-to-treat analysis of perioperative outcomes. Eur Urol Open Sci. 2022;46:75-81.
17. Ng CK, Gill IS, Patil MB, et al. Anatomic renal artery branch microdissection to facilitate zero-ischemia partial nephrectomy. Eur Urol. 2012;61:67-74.
18. Ayyad M, Ayaad O, Alkhatatbeh H, Sawaqed F, Al-Rawashdeh S. Laparoscopic partial nephrectomy: off-clamp versus on clamp. Asian Pac J Cancer Prev. 2022;23:1719-23.
19. Ferriero M, Brassetti A, Mastroianni R, et al. Off-clamp robot-assisted partial nephrectomy for purely hilar tumors: technique, perioperative, oncologic and functional outcomes from a single center series. Eur J Surg Oncol. 2022;48:1848-53.
20. Tuderti G, Mastroianni R, Anceschi U, et al. Assessing the trade-off between the safety and effectiveness of off-clamp robotic partial nephrectomy for renal masses with a high RENAL score: a propensity score-matched comparison of perioperative and functional outcomes in a multicenter analysis. Eur Urol Focus. 2023;9:1037-43.
21. Gill IS, Aron M, Gervais DA, Jewett MAS. Clinical practice. Small renal mass. N Engl J Med. 2010;362:624-34.
22. Bahler CD, Sundaram CP. Effect of renal reconstruction on renal function after partial nephrectomy. J Endourol. 2016;30 Suppl 1:S37-41.
23. Kilic S, Ates M. Sutureless versus conventional suture renorrhaphy in clampless robotic partial nephrectomy: a single center propensity score matching analysis. Actas Urol Esp. 2025;49:501704.
24. Jin D, Ren D, Zhang J, et al. A propensity score-matched comparison between sutureless and suture techniques in laparoscopic nephron-sparing surgery: a retrospective non-randomized observational study. J Laparoendosc Adv Surg Tech A. 2020;30:1314-9.
25. Sherer MV, Deka R, Salans MA, Nelson TJ, Sheridan P, Rose BS. Androgen deprivation therapy and acute kidney injury in patients with prostate cancer undergoing definitive radiotherapy. Prostate Cancer Prostatic Dis. 2023;26:276-81.
26. Cortés JC, González García J, Caño Velasco J, Aragón Chamizo J, Subirá Rios D. Reconstruction techniques after partial nephrectomy: classic vs. sutureless approach - a narrative review. Curr Urol Rep. 2024;25:49-54.
27. Farinha R, Rosiello G, Paludo AO, et al. Selective suturing or sutureless technique in robot-assisted partial nephrectomy: results from a propensity-score matched analysis. Eur Urol Focus. 2022;8:506-13.
28. Shvarts O, Tsui KH, Smith RB, KERNION JB, Belldegrun A. Blood loss and the need for transfusion in patients who undergo partial or radical nephrectomy for renal cell carcinoma. J Urol. 2000;164:1160-3.
29. Minervini A, Campi R, Sessa F, et al. Positive surgical margins and local recurrence after simple enucleation and standard partial nephrectomy for malignant renal tumors: systematic review of the literature and meta-analysis of prevalence. Minerva Urol Nefrol. 2017;69:523-38.
30. Jiménez MT, Calvo DC, Moscatiello P, et al. Robotic partial nephrectomy for treating renal masses: outcomes and complications. Arch Esp Urol. 2024;77:858-64.
31. Peraire Lores M, Domínguez J, Bravi CA, et al. Robot-assisted sutureless partial nephrectomy for the treatment of fifteen bilateral renal masses in a patient with Von Hippel-Lindau syndrome: a case report from a high-volume robotic center. CEN Case Rep. 2023;12:335-40.
32. Yasuda Y, Saito K, Tanaka H, et al. Outcomes of gasless laparoendoscopic single-port partial nephrectomy in 356 consecutive patients: feasibility of a clampless and sutureless technique. Int J Urol. 2021;28:302-7.
33. Porpiglia F, Fiori C, Checcucci E, Amparore D, Bertolo R. Hyperaccuracy three-dimensional reconstruction is able to maximize the efficacy of selective clamping during robot-assisted partial nephrectomy for complex renal masses. Eur Urol. 2018;74:651-60.
34. Lombardo R, Gallo G, Stira J, et al. Quality of information and appropriateness of Open AI outputs for prostate cancer. Prostate Cancer Prostatic Dis. 2025;28:229-31.
35. Michiels C, Khene ZE, Prudhomme T, et al. 3D-image guided robotic-assisted partial nephrectomy: a multi-institutional propensity score-matched analysis (UroCCR study 51). World J Urol. 2023;41:303-13.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Copyright
Data & Comments
Data
















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].