Robot-assisted Partington-Rochelle procedure for chronic pancreatitis: a technical note with video presentation
Abstract
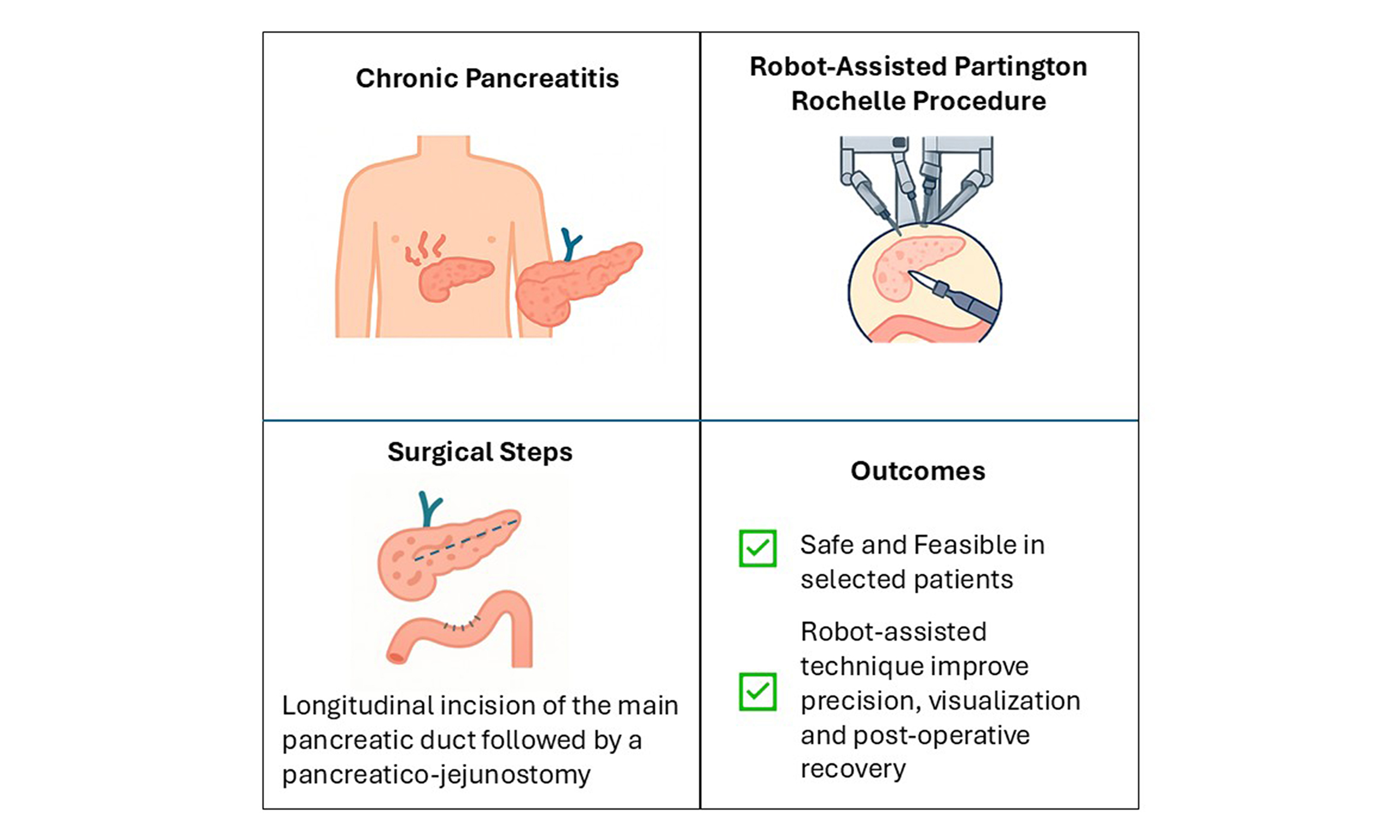
Chronic pancreatitis (CP) is a progressive inflammatory disease characterized by debilitating pain and exocrine insufficiency. When medical and endoscopic treatments fail, surgical decompression may be required. The Partington-Rochelle procedure, a well-established option for ductal decompression via pancreaticojejunostomy, is traditionally performed through open surgery. While the laparoscopic approach is technically challenging, robotic-assisted surgery offers enhanced precision, reduced trauma, and faster recovery. We present the case of a 45-year-old male with CP, recurrent pseudocyst formation, and persistent symptoms despite medical and endoscopic treatment. The patient underwent a robotic Partington-Rochelle procedure using the Da Vinci Xi system. The procedure was successfully completed in 240 min with and an estimated blood loss of 150 mL. Postoperative recovery was uneventful, and the patient was discharged on postoperative day 7 with no complications. At the 3-month follow-up, symptoms had completely resolved, and cyst size had decreased. At 30 months, the patient remained asymptomatic and no longer required analgesics. MRI showed stable pancreatic parenchyma with reduced cystic lesions, and fecal elastase levels indicated mild pancreatic insufficiency. This case highlights the feasibility and effectiveness of the robotic Partington-Rochelle procedure for CP management. The robotic approach provides superior visualization and precision, enabling optimal ductal decompression while overcoming the limitations of laparoscopic and open techniques. Compared to traditional open surgery, it offers faster recovery, reduced morbidity, and excellent long-term symptom control, making it a promising alternative for selected patients.
Keywords
INTRODUCTION
Chronic pancreatitis (CP), a progressive inflammatory disease of the pancreas, typically manifests with debilitating abdominal pain and can lead to irreversible structural and functional damage. Management strategies range from conservative medical therapy to interventional procedures, depending on the severity and progression of the disease. Traditionally, a stepwise approach has been recommended, starting with endoscopic interventions and followed by surgery when medical treatment fails[1]. Endoscopic treatments, such as stenting and stone removal, are often considered the first-line interventions. They are less invasive and offer shorter recovery times, but may require repeated interventions and are not always successful in patients with complex pancreatic ductal anatomy or extensive calcifications[2].
Surgical approaches, in contrast, provide a more definitive solution by directly addressing the structural abnormalities within the pancreas that contribute to pain and dysfunction. Among these, the Partington-Rochelle procedure, a longitudinal pancreaticojejunostomy (LPJ), has traditionally been performed via open surgery, with favourable outcomes in terms of pain relief and preservation of pancreatic function[3].
Recent evidence suggests that earlier surgical intervention may yield better outcomes in pain control, functional preservation, and quality of life compared with delayed surgery following prolonged medical and endoscopic management[4].
A laparoscopic Partington-Rochelle procedure is feasible and offers advantages such as reduced postoperative pain, shorter hospital stay, and faster recovery. However, its technical demands are considerable due to the long pancreaticojejunal anastomosis, which can be particularly challenging in patients with multiple ductal strictures or limited pancreatic exposure[4].
With the advent of minimally invasive techniques, robot-assisted surgery has further advanced this approach, providing enhanced precision, reduced postoperative pain, and faster recovery.
Robot-assisted Partington-Rochelle procedures enable meticulous dissection and anastomosis with improved dexterity and visualization compared to conventional laparoscopy or open surgery[5].
This technical note aims to describe the surgical steps and discuss the feasibility of the robot-assisted Partington-Rochelle procedure in CP, focusing on efficacy, safety, and outcomes. The Supplementary Video presentation illustrates the technical details and postoperative results.
METHODS
Patient selection and preparation
A 45-year-old male had a history of alcohol and tobacco abuse, gallbladder microlithiasis, and hypercholesterolemia, all potential contributors to CP. Beginning in August 2020, he experienced recurrent emergency department admissions for acute exacerbations of CP, with imaging showing progressive pseudocyst formation and parenchymal changes. He was managed conservatively with analgesic therapy, including oral opioids at a daily morphine-equivalent dose of 40 mg.
In May 2022, due to persistent symptoms, the patient was referred to our unit. MRI revealed a 77 mm irregularly round mass in the pancreatic head with a heterogeneous fluid structure and internal solid septa, surrounded by a wall of irregular thickness. The main pancreatic duct (5 mm) and secondary ducts were dilated throughout the parenchyma, while the biliary tree was not significantly dilated and showed no obstructive endoluminal lesions. The gallbladder appeared dysmorphic with heterogeneous contents but without significant wall abnormalities. The case was reviewed at an internal multidisciplinary meeting, and early surgical intervention was recommended. In July 2022, the patient underwent a robotic Partington-Rochelle procedure.
Following surgery, the patient experienced complete resolution of abdominal pain and discontinued analgesics. At the 30-month follow-up in January 2025, he remained asymptomatic, with imaging showing cyst size reduction and only mild pancreatic insufficiency (fecal elastase: 138 μg/g).
Surgical technique
The patient was placed supine with legs abducted and in the slight reverse Trendelenburg position. Pneumoperitoneum was established using the open technique, and a laparoscopic assistant port was inserted. A four-port robotic configuration was employed, including one optical port for the camera and three working ports for the robotic arms. The robotic ports were aligned along a supraumbilical longitudinal line, with the assistant port positioned at the umbilicus. The Da Vinci Xi robotic system was then docked.
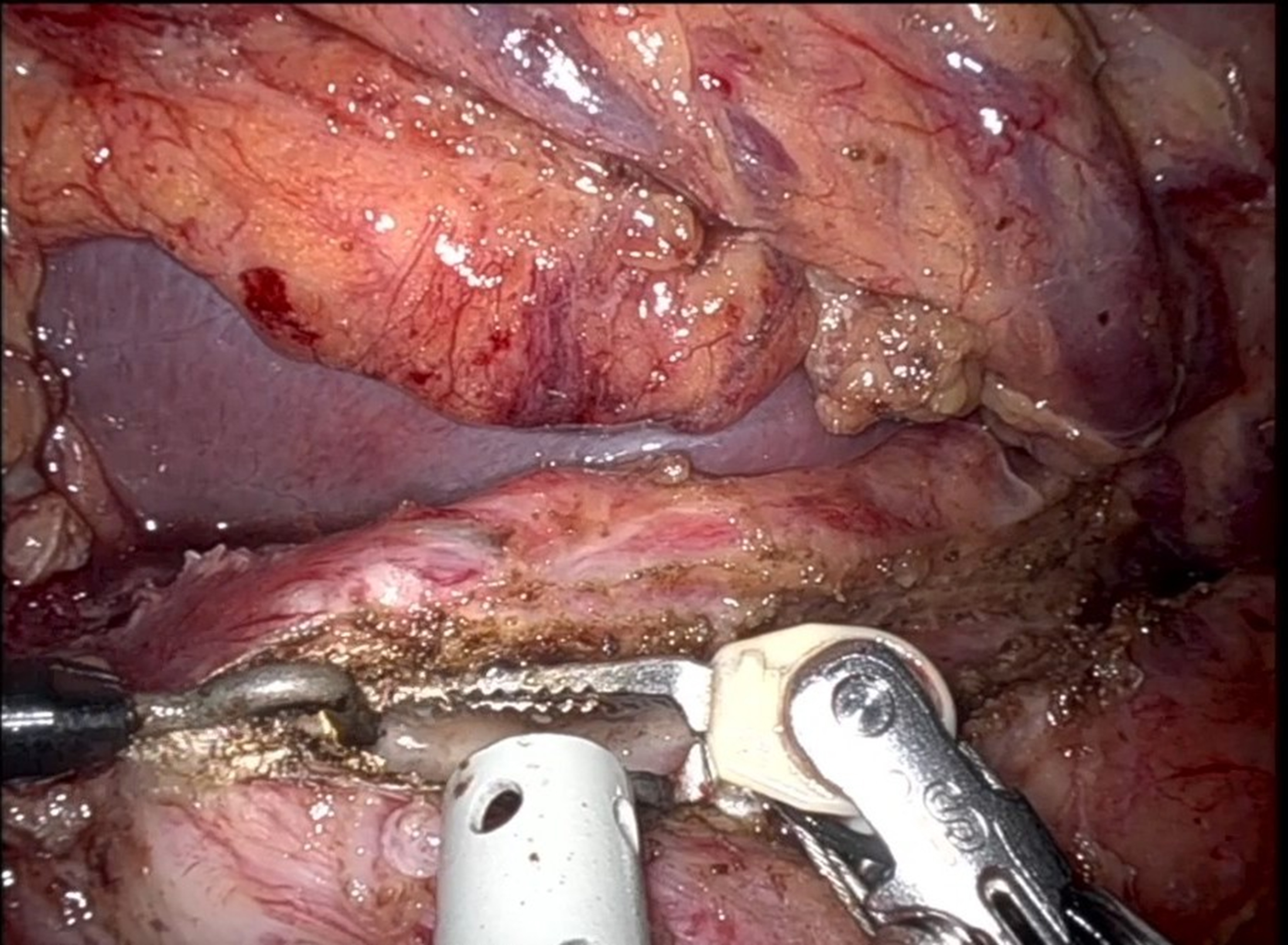
The procedure began with division of the gastrocolic ligament using the robotic Harmonic scalpel, providing access to the lesser sac and exposure of the anterior pancreatic surface. No acute inflammation was observed. The gastroepiploic artery was divided with a robotic stapler using a vascular load. Intraoperative ultrasound (IOUS) was conducted to evaluate the main pancreatic duct. Once the duct was identified, a 21-gauge needle was inserted, and leakage of pancreatic fluid confirmed the correct position. A longitudinal incision was then made along the duct from the tail to the head of the pancreas using a robotic monopolar hook [Figure 1]. After fully opening the duct, a Nelaton catheter was inserted into the duct to drain the pseudocyst in the pancreatic head. During this step, particular care was taken to avoid injury to the distal bile duct.
A cholecystectomy was subsequently performed. Calot’s triangle was carefully dissected, and the critical view of safety was obtained. The cystic artery was divided between laparoscopic metal clips, and the cystic duct was opened for biliary exploration. A white dye test was performed by injecting diluted Lipidol into the cystic duct to verify the absence of communication between the biliary system and the pseudocyst. After exploration, the cystic duct was closed with Hem-o-lok clips. A transmesocolic Roux-en-Y limb was then prepared. The jejunum was divided approximately 40-50 cm distal to the ligament of Treitz, and an enteroenterostomy was performed using a robotic stapler. The enterotomy created for the stapler was closed with a running 4-0 barbed suture to ensure a watertight closure and prevent leakage.
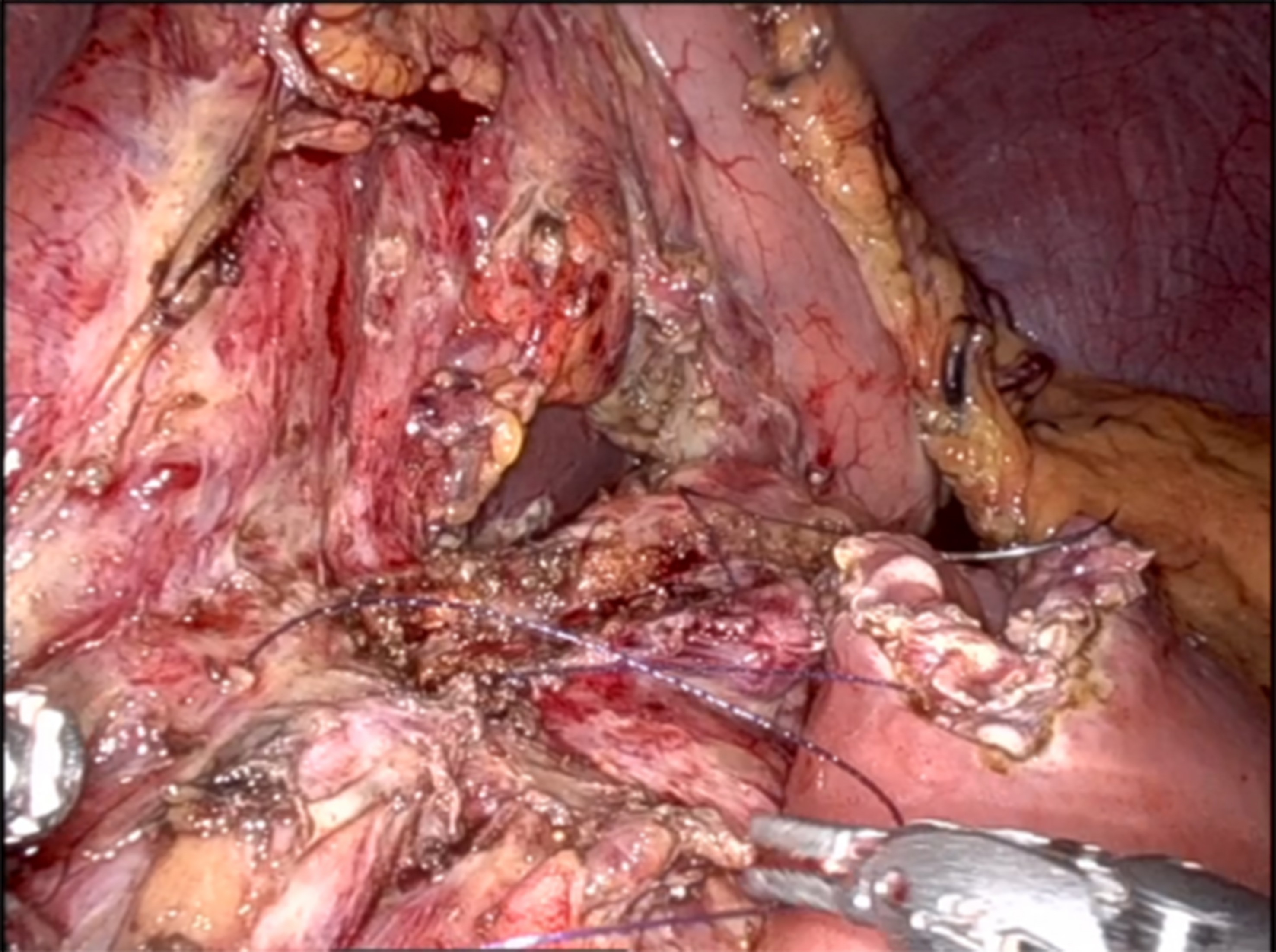
The pancreatico-jejunal anastomosis [Figure 2] was fashioned in two layers: the fourth robotic arm elevated the stomach to expose the anterior surface of the pancreas, while the second and third arms created the anastomosis.
• Posterior layer: A 4-0 barbed suture was placed in a semicontinuous fashion, securing the posterior wall of the pancreatic duct to the posterior wall of the jejunal loop.
• Anterior layer: The same technique was applied to the anterior wall, ensuring accurate, tension-free approximation.
A 24 Fr drain was placed near the pancreatico-jejunal anastomosis to monitor for postoperative pancreatic leaks or fistulas. After confirming hemostasis, the robotic arms were disengaged, and the procedure was completed. Total operative time was 240 min, with an estimated blood loss of 150 mL.
Postoperative management
The patient was initially monitored in the Post-Anesthesia Care Unit (PACU) and transferred to the inpatient ward 24 h postoperatively. Postoperative care included early mobilization, a gradual transition to a full oral diet beginning on postoperative day (POD) 3, and prophylactic anticoagulation with low-molecular-weight heparin (LMWH) 4000 UI, adjusted for body weight.
Amylase levels in the drainage fluid were measured on POD 1 and POD 3, both of which were within normal limits. The abdominal drain was removed on POD 4, and the patient was discharged on POD 7 without complications.
At the two-week outpatient follow-up, blood tests and physical examination showed no abnormalities. MRI and MRCP performed at three months revealed a normal-sized pancreas with multiple subcentimeter cysts, as shown in Supplementary Video.
At the 30-month follow-up in January 2025, the patient remained in good general condition, reporting no abdominal pain and no need for analgesics. MRI showed a reduction in cyst size. Fecal elastase was mildly reduced at 138 μg/g (normal > 200 μg/g), indicating minimal pancreatic insufficiency. No signs of diabetes were present, and all other laboratory results were within normal limits.
DISCUSSION
This case underscores the feasibility and safety of the robot-assisted Partington-Rochelle procedure in the surgical management of CP outside of acute exacerbations, even when complicated by recurrent pseudocyst formation.
Current evidence highlights the superiority of surgical drainage over endoscopic treatment in achieving sustained pain relief and preserving pancreatic function in CP[4]. Surgical options for CP are generally categorized into drainage, resection, and hybrid procedures, with the choice guided by pancreatic duct morphology and the presence of an inflammatory head mass. The Partington-Rochelle procedure, a pure drainage technique, is indicated for patients with a significantly dilated main pancreatic duct in the absence of a dominant head mass. By contrast, the Frey and Beger procedures combine drainage with pancreatic head resection, making them preferable in cases where an inflammatory mass contributes to symptoms. The Frey procedure involves limited resection of the pancreatic head while preserving duodenal continuity, whereas the Beger procedure requires transection of the pancreas above the portal vein. The Izbicki procedure extends the drainage concept by incorporating a longitudinal V-shaped excision of the pancreatic parenchyma, offering an alternative for patients with small duct disease. Selecting the appropriate technique is crucial for balancing pain relief, preserving pancreatic function, and minimizing surgical morbidity[6]. Among these approaches, the Partington-Rochelle procedure, a modification of the Puestow procedure, has long been considered the gold standard for longitudinal pancreatic duct decompression in patients with main pancreatic duct dilation and refractory pain[3]. However, in its open form, the procedure is associated with significant morbidity, prolonged hospitalization, and delayed recovery[4].
Over recent decades, minimally invasive techniques, particularly laparoscopy, have been developed to reduce these drawbacks. Laparoscopic surgery offers advantages such as shorter hospital stays, reduced postoperative pain, and faster recovery[7]. Nonetheless, it presents notable technical challenges, particularly in pancreatic procedures requiring precise ductal incision and meticulous anastomosis. Limitations of laparoscopy include two-dimensional visualization, restricted instrument mobility, longer operative times, and a steep learning curve. A systematic review by Ramia et al. analyzed 82 cases of laparoscopic longitudinal pancreaticojejunostomy (LLPJ), including 72 laparoscopic and 10 robotic-assisted procedures. The laparoscopic approach was deemed safe and feasible, with a low conversion rate (5%), minimal intraoperative bleeding (mean 57 mL), and an 11.4% morbidity rate, primarily due to minor complications. No mortality was reported. The mean operative time was 218 minutes (range 103-377), and the hospital stay averaged 5.6 days. Pain relief was achieved in 90% of patients, underscoring its effectiveness. However, technical limitations were noted: (1) difficult visualization and exposure of the pancreas, requiring stomach suspension or retraction; (2) difficulty identifying the main pancreatic duct, leading to conversions to open surgery, especially when IOUS was unavailable; and (3) challenges with suturing, as constructing a precise pancreaticojejunostomy requires advanced laparoscopic skills. In contrast, robotic assistance, though reported in only 10 cases, facilitated suturing and anastomosis by providing enhanced visualization, tremor elimination, and improved ergonomics. Nevertheless, robotic procedures were associated with longer operative times (mean 268 min) and higher costs, limiting widespread adoption. The review concluded that LLPJ is a promising alternative to open surgery but emphasized the need for high-quality comparative studies between laparoscopic, robotic, and open approaches[8].
Robotic-assisted surgery offers several advantages that directly address the limitations of both open and laparoscopic approaches. The robotic system provides three-dimensional high-definition imaging, enhanced dexterity, and tremor elimination, enabling precise dissection and suturing. These features are particularly beneficial for pancreatico-jejunal anastomoses, where achieving a tension-free, watertight anastomosis is critical to reducing the risk of pancreatic fistula[9]. Kirks et al. reported one of the largest case series comparing robotic-assisted and open LPJ for CP. Operative times were comparable (268 vs. 236 min), but the robotic approach was associated with significantly lower intraoperative blood loss, shorter hospital stays, and reduced postoperative medication costs. Although robotic procedures incurred higher operative supply costs, these were offset by faster recovery and decreased morbidity, supporting their feasibility and potential advantages in selected patients[10].
In our case, robotic assistance facilitated precise pancreatic duct incision, safe cholecystectomy with intraoperative biliary exploration, optimal pancreatico-jejunal anastomosis, and improved retroperitoneal exposure. The patient’s postoperative course was uneventful, with complete resolution of abdominal pain, cessation of analgesic use, and a marked reduction in pseudocyst size. At 30 months postoperatively, the patient remained asymptomatic, demonstrating the long-term clinical effectiveness of this approach. Recovery was smooth, characterized by early mobilization, drain removal on POD 4, and discharge on POD 7 without complications.
Despite these promising outcomes, several limitations must be acknowledged. As this is a single case, the findings cannot be generalized. Larger prospective studies are necessary to validate the superiority of the robotic approach over conventional methods. Comparative analyses should assess not only clinical outcomes but also cost-effectiveness, patient quality of life, and surgeon learning curves[11].
CONCLUSION
The robotic Partington-Rochelle procedure represents a safe, effective, and technically feasible alternative for the management of CP in carefully selected patients. Its advantages over both open and laparoscopic techniques, particularly in terms of precision, visualization, and postoperative recovery, support its value in the evolving landscape of pancreatic surgery. Ongoing technological advancements and growing clinical experience are likely to broaden its application and further define its role in pancreatic surgery.
DECLARATIONS
Authors’ contributions
Concept and study design: Nasto RA, Montalti R
Data acquisition and technical support: Loiaco G, Rompianesi G, Giglio MC, Campanile S, Caggiano M, Zinno G, Benassai G
Data analysis and interpretation: Nasto RA, Roberto M, Troisi RI
Manuscript drafting: Nasto RA, Montalti R, Troisi RI
Critical revision of the manuscript for important intellectual content: Nasto RA, Montalti R, Loiaco G, Rompianesi G, Giglio MC, Campanile S, Caggiano M, Zinno G, Benassai G, Troisi RI
All authors have read and approved the final version of the manuscript.
Availability of data and materials
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Financial support and sponsorship
None.
Conflicts of interest
Montalti R is an Editorial Board member of the journal Mini-invasive Surgery. Montalti R was not involved in any steps of editorial processing, notably including reviewer selection, manuscript handling, or decision making. The other authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
This study is a case report presented as a Technical Note. Therefore, approval from an ethics committee was not required according to the policies of the Federico II University Hospital, Naples, Italy. Informed consent was obtained from the patient.
Consent for publication
Written informed consent for publication of this case report, including medical details, images, and videos, was obtained from the patient.
Copyright
© The Author(s) 2025.
Supplementary Materials
REFERENCES
1. Issa Y, Kempeneers MA, Bruno MJ, et al; Dutch Pancreatitis Study Group. Effect of early surgery vs endoscopy-first approach on pain in patients with chronic pancreatitis: the ESCAPE randomized clinical trial. JAMA. 2020;323:237-47.
2. Fuchs M, Reimann FM, Gaebel C, Ludwig D, Stange EF. Treatment of infected pancreatic pseudocysts by endoscopic ultrasonography-guided cystogastrostomy. Endoscopy. 2000;32:654-7.
3. Partington PF, Rochelle RE. Modified Puestow procedure for retrograde drainage of the pancreatic duct. Ann Surg. 1960;152:1037-43.
4. Kempeneers MA, Issa Y, Ali UA, et al; Working group for the International (IAP - APA - JPS - EPC) Consensus Guidelines for Chronic Pancreatitis. International consensus guidelines for surgery and the timing of intervention in chronic pancreatitis. Pancreatology. 2020;20:149-57.
5. Gagner M, Pomp A. Laparoscopic pancreatic resection: is it worthwhile? J Gastrointest Surg. 1997;1:20-5; discussion 25.
7. Kalayarasan R, Shukla A. Changing trends in the minimally invasive surgery for chronic pancreatitis. World J Gastroenterol. 2023;29:2101-13.
8. Ramia JM, Azagra JS, De la Plaza R, Manuel A, Latorre R, Lopez-Marcano A. Laparoscopic longitudinal pancreaticojejunostomy for chronic pancreatitis: systematic review of the literature. Surgeon. 2020;18:137-41.
9. Asbun HJ, Moekotte AL, Vissers FL, et al; International Study Group on Minimally Invasive Pancreas Surgery (I-MIPS). The Miami International Evidence-based Guidelines on Minimally Invasive Pancreas Resection. Ann Surg. 2020;271:1-14.
10. Kirks RC, Lorimer PD, Fruscione M, et al. Robotic longitudinal pancreaticojejunostomy for chronic pancreatitis: comparison of clinical outcomes and cost to the open approach. Int J Med Robot. 2017;13:e1832.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Special Issue
Copyright
Data & Comments
Data

















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].