Advances in minimally invasive esophagectomy (MIE): techniques, outcomes, and future directions
Abstract
Minimally invasive esophagectomy (MIE) has become a preferred surgical approach for treating esophageal cancer, offering significant advantages over traditional open surgery, such as reduced recovery times, fewer complications, and shorter hospital stays. This review highlights advancements in MIE techniques, with a focus on the growing role of robotic-assisted MIE (RAMIE) in enhancing surgical precision and patient outcomes. Recent trials, such as REVATE and ROBOT-2, highlight RAMIE’s advantages in lymph node resection and reduction in nerve injury rates, suggesting its potential to improve both short- and long-term outcomes. Further innovations, including artificial intelligence (AI) and telementoring, are enhancing surgical navigation and procedural safety, making MIE more accessible globally. Despite these advances, challenges remain, particularly regarding the steep learning curve. This review examines the evolution of MIE techniques, their clinical outcomes, and the future role of emerging technologies in optimizing esophageal cancer surgery.
Keywords
INTRODUCTION
Managing esophageal cancer presents significant challenges, not only due to the aggressive nature of the disease but also because of the technical complexity of its surgical treatment. Surgery remains the cornerstone of curative treatment for patients with localized disease, but traditional open esophagectomy has long been associated with significant morbidity, high complication rates, and prolonged recovery periods[1,2]. While these open techniques set an oncologic gold standard, their substantial morbidity led surgeons - particularly those in high-volume centers - to seek less invasive approaches that could preserve outcomes while improving recovery. Over the past few decades, advances in perioperative care and the development of minimally invasive esophagectomy (MIE) have offered a safer alternative with reduced surgical trauma and improved patient recovery.
Minimally invasive techniques, including thoracoscopic and laparoscopic approaches, have replaced many aspects of open surgery, reducing hospital stays, lowering complication rates, and allowing for a quicker return to normal function. More recently, robotic-assisted minimally invasive esophagectomy (RAMIE) has emerged as a promising advancement, providing enhanced dexterity, improved visualization, and greater control over complex anatomical dissections[3]. The growing use of robotic platforms has made it possible to achieve comparable, if not superior, oncologic outcomes compared to open and traditional MIE.
This review aims to explore the evolution of MIE, detailing the various techniques currently in use, comparing outcomes with open surgery, and highlighting the latest advancements in robotic technology. Additionally, it examines the risk factors and patient selection criteria that determine the suitability of MIE and discusses the challenges that remain in optimizing surgical outcomes. As MIE continues to evolve, new innovations in artificial intelligence, and telementoring are expected to shape the future of esophageal surgery.
THORACOSCOPIC AND LAPAROSCOPIC ESOPHAGECTOMY
MIE techniques vary based on the combination of thoracic and abdominal approaches, each designed to optimize patient outcomes while maintaining the oncologic integrity of the procedure. The hybrid minimally invasive esophagectomy (HMIE) technique is a widely used approach that combines laparoscopic surgery for the abdominal phase with an open thoracotomy for the thoracic phase, reducing major intraoperative and postoperative complications (36% vs. 64%; RR 0.56, 95% CI 0.41-0.72; P < 0.001) compared to open esophagectomy in a multicenter RCT (n = 207, 13 centers). Notably, HMIE has been associated with a significantly lower incidence of pulmonary complications (18% vs. 30%) without compromising three-year overall survival[4]. In contrast, total laparoscopic esophagectomy (TLE), involving laparoscopic and video-assisted thoracoscopic surgery, utilizes laparoscopy for the abdominal component and thoracoscopy for the thoracic component. This approach, which avoids a thoracotomy altogether, has demonstrated reduced postoperative complications while maintaining oncologic efficacy, with recurrence-free survival rates of 71.6% (TLE) and 58.3% (open esophagectomy) in a single center cohort (n = 142); the adjusted hazard ratio for recurrence was 0.62, 95% CI 0.40-0.96[5,6].
According to the 2024 Society of Thoracic Surgeons (STS) General Thoracic Surgery Database, of the 6,854 esophagectomies from 210 U.S. hospitals between 2018 and 2022, 48.9% were performed minimally invasively. Among these, Ivor Lewis accounted for 75% and McKeown 16%[7]. Comparing the two techniques, minimally invasive McKeown esophagectomy (MIME) has been associated with higher perioperative morbidity, including greater blood loss, longer operating times, and an increased risk of pulmonary complications (OR 1.96), anastomotic leaks (OR 2.55), and vocal cord palsy (OR 5.62) compared to minimally invasive Ivor Lewis esophagectomy[8]. Because transhiatal esophagectomy is performed through traditional open abdominal and cervical incisions - and entirely avoids thoracic entry - it is not classified as a minimally invasive technique in this review. It represents only 8.7% of contemporary esophagectomy cases. This technique is often favored for patients with significant pulmonary comorbidities, as it reduces the risk of thoracic complications but is associated with higher morbidity (43.4%) compared to minimally invasive Ivor Lewis (30.5%) and McKeown (40.3%) esophagectomy. Patients undergoing transhiatal esophagectomy also have increased infection rates and a lower lymph node harvest, which may affect oncologic outcomes[1]. While practice patterns continue to diverge, lower-volume centers still favor an open transhiatal approach for selected esophageal tumors, whereas most high-volume centers have transitioned almost entirely to thoracoscopic, laparoscopic, or robotic approaches.
ROBOTIC-ASSISTED MINIMALLY INVASIVE ESOPHAGECTOMY
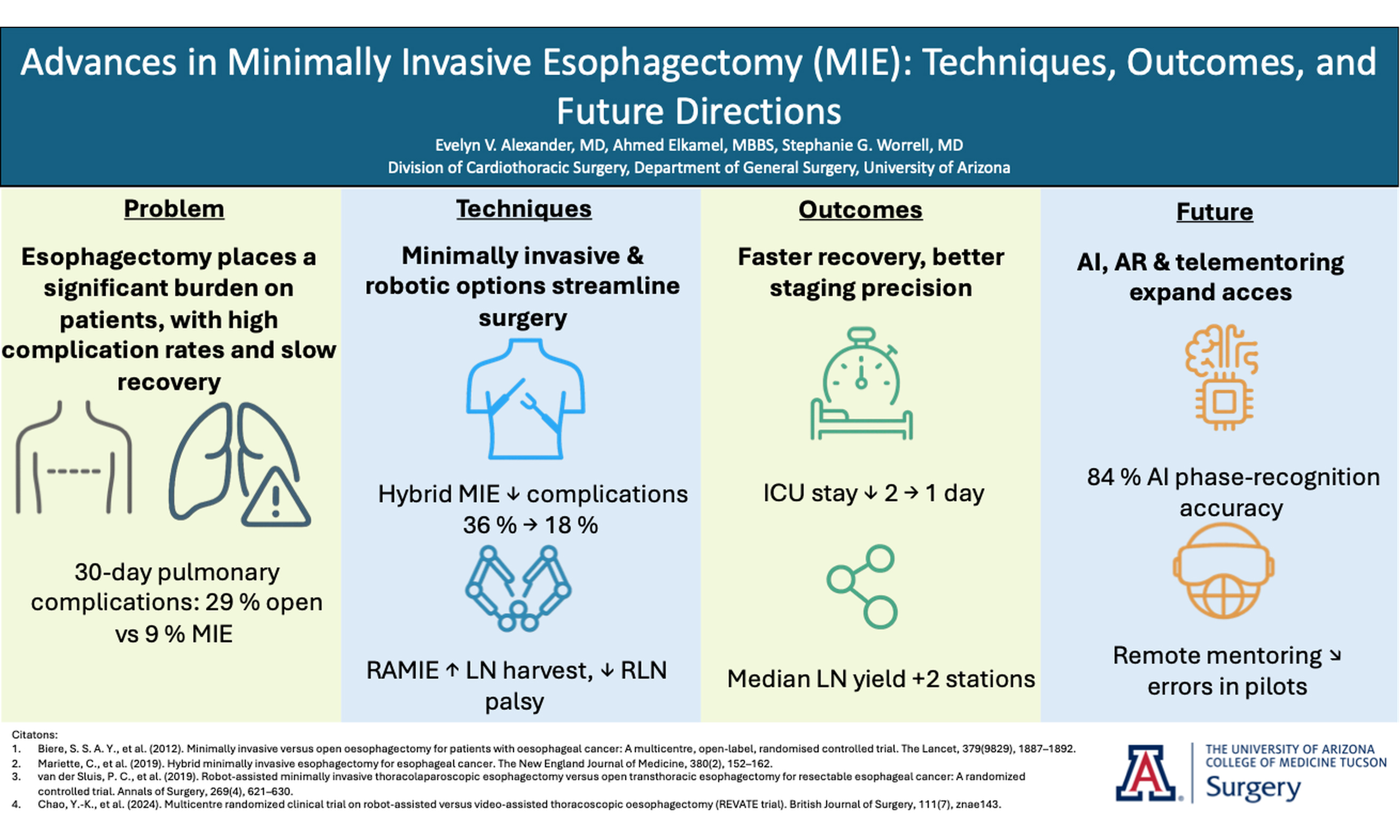
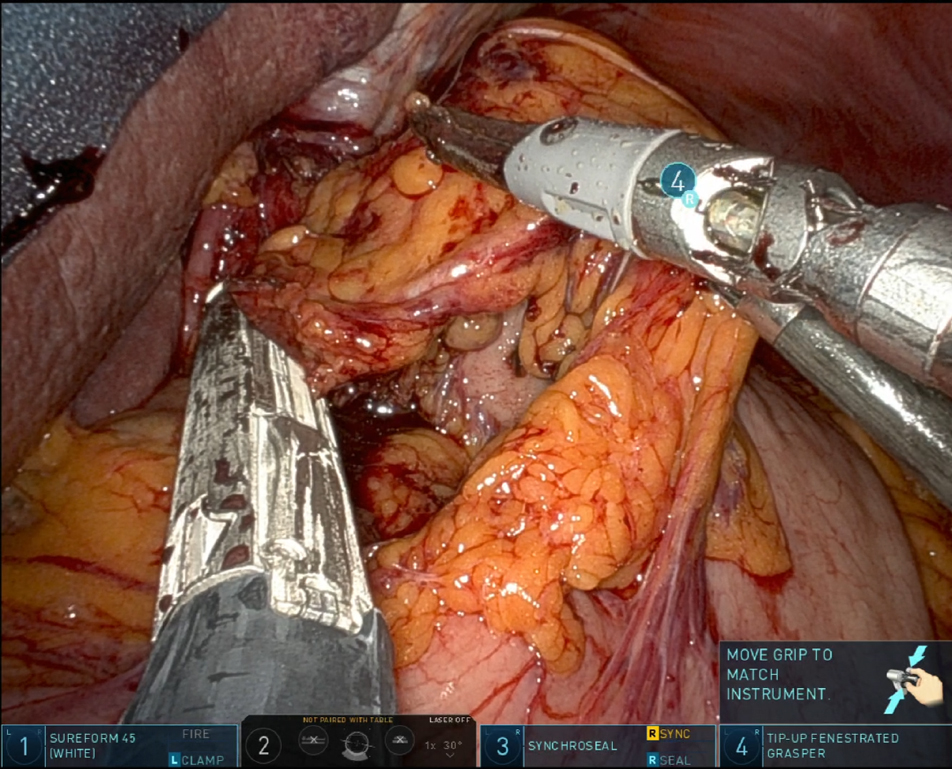
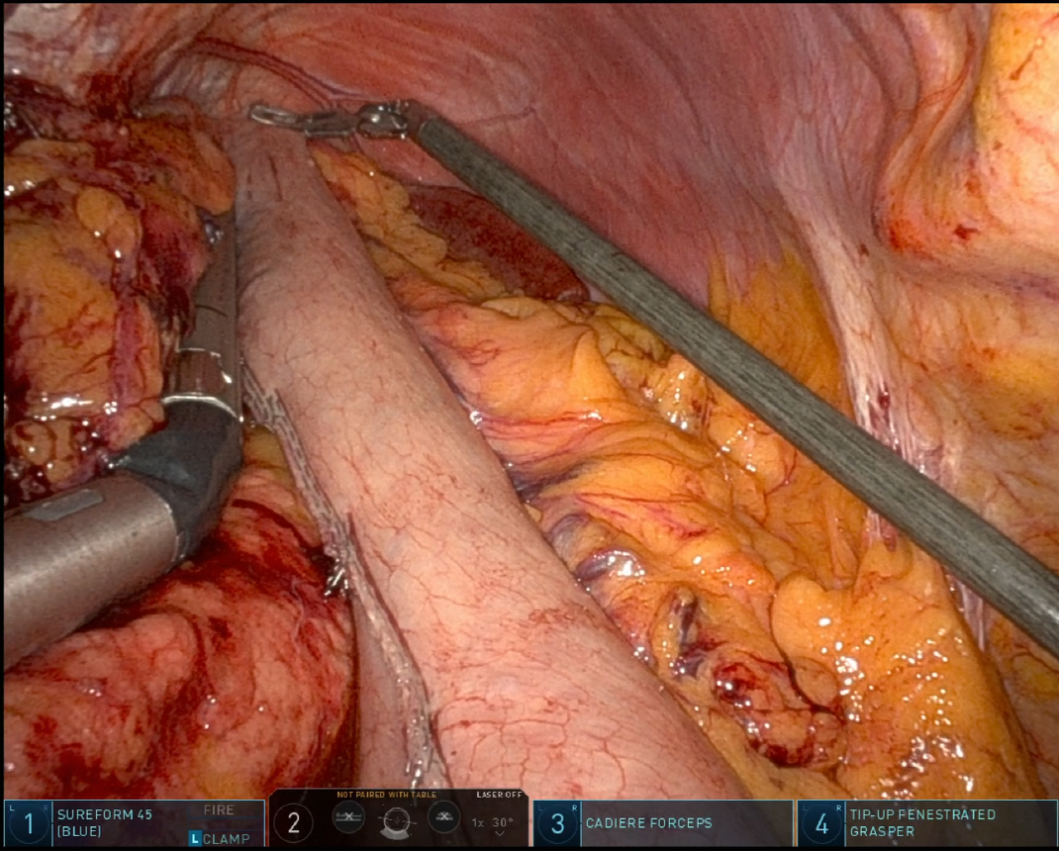
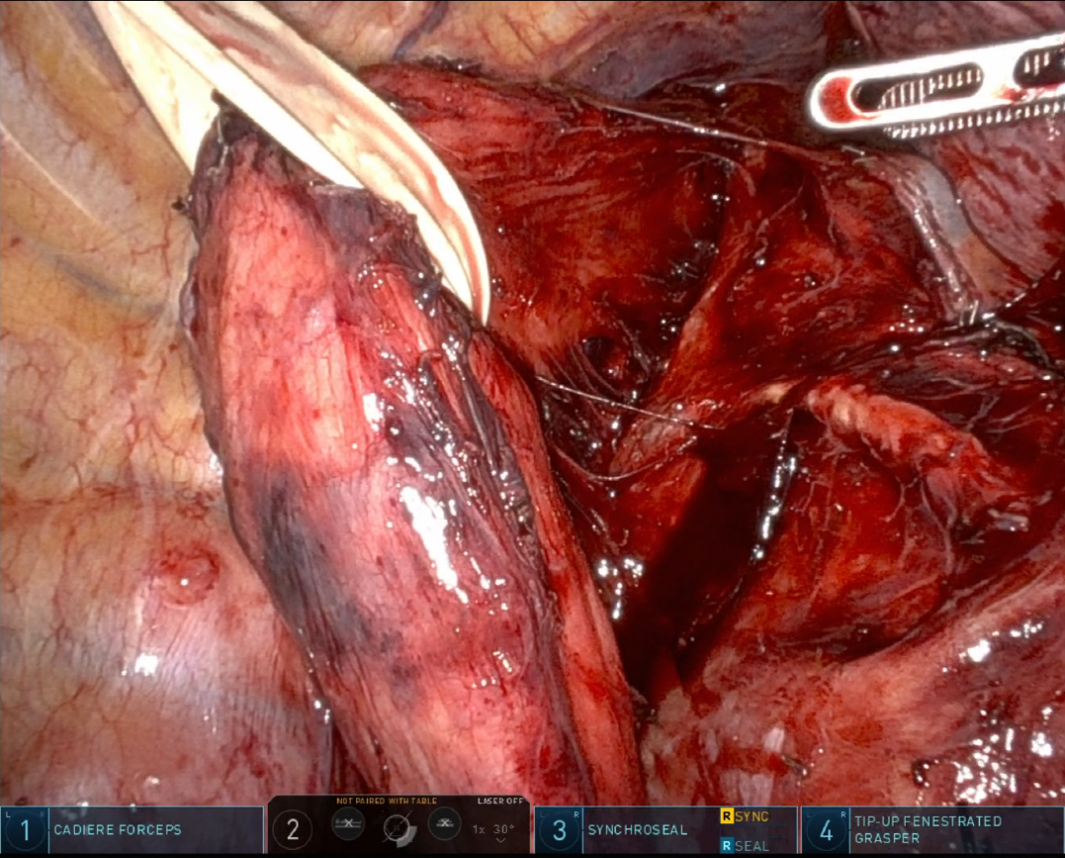
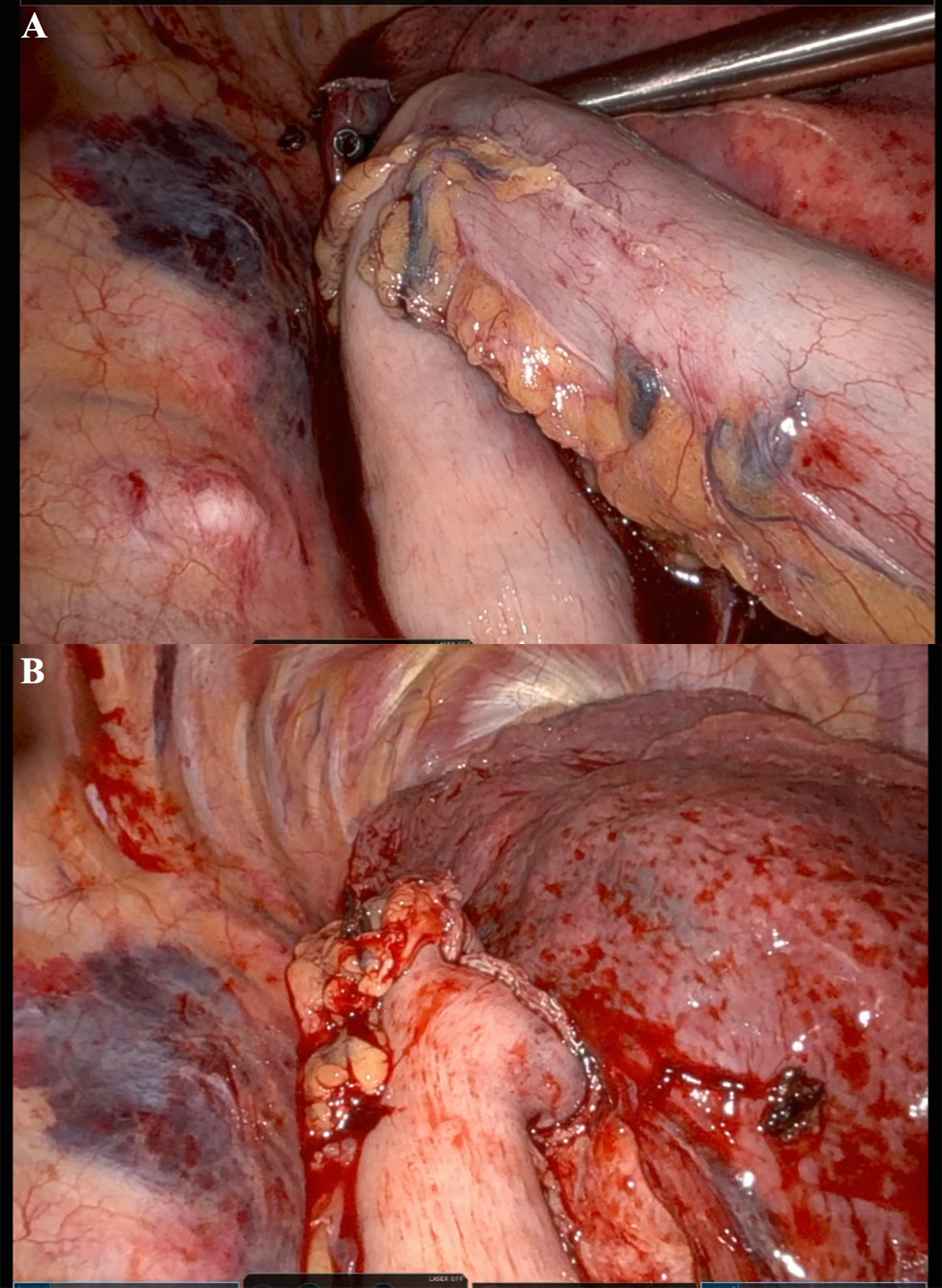
In recent years, RAMIE has gained traction as an alternative to traditional MIE. A 2012 series showed that robotic instruments could reproduce standard open dissection planes while keeping the pulmonary advantages of thoracoscopic surgery, supporting the adoption of RAMIE[9]. The use of robotic platforms, such as the Da Vinci surgical system, has been shown to enhance precision, ergonomics, and visualization. Figure 1 captures the abdominal phase, demonstrating the precision of RAMIE in performing extensive celiac lymphadenectomy and the meticulous stapling of the left gastric artery and vein. This step crucially minimizes blood loss and enhances the effectiveness of the lymphadenectomy, showcasing the advantages of RAMIE in managing complex surgical tasks. Figure 2 focuses on the creation of the gastric conduit using RAMIE, highlighting the system’s precision. This process not only allows for a more controlled conduit creation but also significantly reduces trauma, thereby speeding up patient recovery compared to more traditional methods. Figures 3, 4A and B further illustrate the mobilization of the esophagus and the strategic positioning of the gastric conduit within the thorax. These steps demonstrate RAMIE’s capability to ensure the structural integrity and functionality of the gastrointestinal tract, emphasizing its role in preserving critical anatomical relationships and enhancing patient outcomes. Together, these visualizations underscore the benefits of RAMIE, particularly its ability to improve surgical outcomes and reduce the risks associated with more invasive traditional approaches.
Figure 4. Thoracic phase of gastric conduit placement. (A) Gastric conduit being lifted into the thoracic cavity; (B) Gastric conduit positioning in the thoracic cavity.
With RAMIE, the patient can be positioned in the left lateral decubitus position, full lateral decubitus position, or prone position. Of those, the left lateral decubitus positioning, described by Lee et al., has further optimized operative exposure, improving procedural efficiency by reducing operative time (328 vs. 251 min) and blood loss (350 vs. 200 mL)[10]. Conversion to open thoracotomy was required in only 4.7% of cases, and these conversions were performed without altering patient positioning or causing major complications. Notably, there were no 30-day postoperative mortalities, underscoring the safety and feasibility of this technique[10]. Additionally, RAMIE has been associated with superior lymphadenectomy, reducing recurrent laryngeal nerve (RLN) injury rates and increasing mediastinal lymph node yield[11,12].
EMERGING TECHNIQUES
Beyond conventional techniques, emerging approaches such as Single-Incision Laparoscopic or Robotic Surgery and third-Space endoscopy are being explored to minimize scarring and accelerate recovery times. Single-port video-assisted thoracoscopic or robotic surgery has demonstrated several advantages over multi-port VATS, including significantly shorter drainage time, reduced blood loss, shorter operation time, and lower complication rates, all contributing to a reduced hospital stay[13]. Advancements in endoscopic technology have expanded the capabilities of minimally invasive surgery, enabling complex procedures such as endoscopic submucosal dissection (ESD) and endoscopic submucosal resection (EMR). New suturing devices, such as the Eagle Claw and OverstitchTM, have improved intestinal closure after ESD or EMR, while robotic platforms like the MASTER system have enhanced precision and accessibility. With growing evidence supporting the safety and feasibility of these techniques, endoscopic resections are increasingly used for early-stage tumors in the muscularis mucosa and some superficial submucosal tumors of the gastrointestinal tract, further advancing the field of minimally invasive surgery[14]. These innovations have the potential to significantly transform MIE and shift the focus toward preserving the esophagus.
CLINICAL OUTCOMES FROM KEY TRIALS
Minimally invasive esophagectomy has been widely studied in comparison to traditional open esophagectomy, with numerous clinical trials demonstrating clear benefits. Studies such as the TIME and MIRO trials have shown that MIE is associated with shorter intensive care unit stays, reduced overall hospitalizations, and fewer respiratory complications[4,15]. The TIME trial (n = 115, 5 centers) found that MIE led to fewer pulmonary complications (9% vs. 29%, P = 0.05), less blood loss (200 mL vs. 475 mL, P ≤ 0.001), and faster recovery compared to open esophagectomy[15]. Similarly, the MIRO trial (n = 207) highlighted the benefits of hybrid MIE, showing a significant reduction in intraoperative and postoperative complications [36% vs. 64%; relative risk (RR) 0.56, 95 % CI 0.41-0.72; P < 0.001], particularly pulmonary complications with in the first 30 days (18% vs. 30%; RR 0.60, 95% CI 0.38-0.95; P < 0.001), without compromising oncologic outcomes[16]. These advantages translate to faster recovery times and allow patients to begin adjuvant therapy sooner, which has been shown to significantly improve disease-free survival. These findings are consistent with other studies that have shown RAMIE to be associated with reduced perioperative complications[17,18]. Given these findings, MIE has become a compelling alternative to open surgery, particularly in centers where surgeons have expertise in minimally invasive techniques.
MIE, including RAMIE, has demonstrated favorable outcomes in multiple domains including lymph node yield, pulmonary morbidity, and intraoperative blood loss. Several high-quality randomized controlled trials (RCTs) have compared RAMIE or MIE to conventional approaches and provided critical insight into its efficacy and safety. A comparison of key outcomes across major RCTs is summarized in Table 1. These trials include the REVATE, TIME, and MIRO studies, which collectively represent the largest prospective data supporting the use of RAMIE in esophageal cancer surgery.
Comparative outcomes in key randomized control trials.
| Trial (Year) | Authors | Sample size | Comparison | Lymph node yield | Pulmonary complications | Blood loss (mL) | Anastomotic leak rate | Key findings |
| REVATE (2024) | Chao et al.[11] | 203 | RAMIE vs. VAMIE | 16 vs. 14 (P = 0.035) | 14% vs. 14% (P = 0.933) | 100 vs. 350 (P = 0.222) | 12% vs. 6% (P = 0.157) | RAMIE improved LN yield and reduced RLN palsy. There was no difference in pulmonary complication rates, blood loss or anastomotic rates |
| TIME Trial (2017) | Straatman et al.[15] | 115 | MIE vs. Open esophagectomy | 20 vs. 21 (P = 0.469) | 7% vs. 19% (p = 0.005) | 200 vs. 475 (P < 0.001) | 7% vs. 4% (P = 0.39) | MIE reduced pulmonary complications, blood loss and length of stay. There was no significant difference in lymph node yield or anastomotic leak rate |
| MIRO Trial (2019) | Nuytens et al.[16] | 207 | Hybrid MIE vs. Open esophagectomy | 21 vs. 22 (P = 0.10) | 18% vs. 30% (P < 0.001) | NR | 11% vs. 7% (P = 0.34) | Hybrid MIE lowered pulmonary complications with no significant difference in lymph node yield or anastomotic leak rate |
The REVATE trial (n = 203) compared RAMIE with video-assisted thoracoscopic esophagectomy (VAMIE) in patients with esophageal squamous cell carcinoma (SCC). They demonstrated that RAMIE led to a higher rate of successful left RLN lymph node dissection (88.3% vs. 69%, RR: 1.28, 95% CI 1.10-1.49, P < 0.001), along with lower short-term RLN palsy (20.4% vs. 34%, P = 0.029) and long-term RLN palsy (5.8% vs. 20%, P = 0.003). RAMIE also achieved a higher median mediastinal lymph node yield (16 vs. 14, P = 0.035)[11]. These findings support the growing use of RAMIE in experienced centers, where its precision and nerve-sparing advantages can be fully utilized. Additionally, supported by the REVATE trial, recent guidelines support the use of MIE for reducing perioperative pulmonary complications and improving quality of life. The American Society of Clinical Oncology and STS recommend MIE as it is associated with lower rates of pulmonary complications and improved postoperative recovery compared to open esophagectomy[19]. Additionally, the ongoing ROBOT-2 trial is expected to further clarify the potential benefits of RAMIE, specifically in patients with esophageal adenocarcinoma[12]. By directly comparing RAMIE to conventional MIE in a multicenter randomized setting, this study may help define the role of robotic-assisted techniques in modern esophageal cancer surgery and guide future recommendations for surgical approach.
Across studies, RAMIE consistently achieves comparable or improved lymph node harvest and significantly reduces pulmonary complications. However, anastomotic leak rates remain similar between RAMIE and conventional MIE, suggesting this complication is not yet overcome by technology alone. Thus, further innovations in perfusion assessment, surgical technique, and perioperative care remain essential.
ANASTOMOTIC LEAK: INCIDENCE AND MANAGEMENT STRATEGIES
Anastomotic leaks remain one of the most serious and challenging complications after esophagectomy, with significant implications for patient outcomes and recovery. In the International RAMIE Registry of 2,279 cases spanning 2018-2023, Kooij et al. reported a leak rate of 14.3% in the first study year, falling to 10% by year five (P < 0.01)[20]. Although single-institution series still report double-digit leaks, high-volume programs - such as the 380-case RAMIE cohort from Jehan et al. - now achieve “textbook outcomes” in 62% of patients, suggesting that structured training, ICG use, and protocolized postoperative care can meaningfully improve results[21].
Preventive strategies to reduce leaks continue to evolve. Early postoperative endoscopy in a high-volume Chinese center (n = 1,027) has been shown to lower leakage rates (9.8% vs. 22.7%; RR 0.43, 95% CI 0.30-0.61; P = 0.005) and shorten the median length of hospital stay by 3 days (17.6 vs. 20.9 days, P = 0.037) without increasing the risk of postoperative complications such as chyle leak, hypoproteinemia, pneumonia, or in-hospital mortality. These findings suggest that early endoscopy is a safe and effective tool for identifying ischemic complications that lead to anastomotic leaks and potentially improving postoperative outcomes[22,23]. Notably, techniques such as intraoperative fluorescence imaging have also been introduced to assess anastomotic perfusion in real time, reducing the risk of leaks. Indocyanine green fluorescence imaging (IGFI) has been shown to be a safe and effective tool for assessing anastomotic perfusion, enabling real-time visualization that aids intraoperative decision making. The technique can influence surgical plans by prompting adjustments to the resection margin when perfusion is inadequate. Notably, in Kooij et al.’s study, the Centers adopting routine perfusion assessment with indocyanine-green fluorescence saw the steepest decline in anastomosis leak rates (adjusted OR 0.72, 95 % CI 0.56-0.92)[20]. A systematic review and meta-analysis by Sozzi et al. (n = 3,291) found that ICG utilization during esophagectomy was associated with a reduced risk of postoperative anastomotic leaks, with a RR of 0.48 (95% CI 0.23-0.99; P = 0.05) compared to non-ICG-guided procedures[24]. These findings highlight IGFI’s role in improving the safety and outcomes of esophagojejunostomy and esophagogastrostomy by ensuring optimal blood supply to the anastomotic site, though its impact on reducing leaks remains variable[25].
Despite these promising results, the effectiveness of ICG remains somewhat controversial due to variability in study designs, definitions of anastomotic leaks, and heterogeneity in ICG use. For instance, the study by Banks et al. found higher rates of anastomotic leaks in the ICG group compared to the non-ICG group, suggesting potential issues with the technique or patient selection[23].
RISK FACTORS AND PATIENT SELECTION
Careful preoperative assessment is essential to optimize surgical outcomes. Several risk factors have been identified that can increase the likelihood of complications. Increasing age is a significant risk factor for postoperative complications and mortality following MIE. Patients over 75 years old, particularly those with lower skeletal muscle density, have higher postoperative mortality rates[26]. Similarly, higher BMI is associated with increased risk of postoperative complications, including anastomotic leak and prolonged surgery duration[27,28].
Diabetes mellitus is associated with a higher risk of postoperative complications, including anastomotic leak. A systematic review and meta-analysis demonstrated that diabetes mellitus significantly increases the risk of anastomotic leak after esophagectomy (OR = 1.63; 95% CI = 1.25-2.12, P < 0.001)[29]. Furthermore, congestive heart failure (CHF) is a significant predictor of perioperative morbidity and mortality. Patients with CHF undergoing noncardiac surgery, including MIE, have substantially higher risks of operative death and hospital readmission[30].
These findings highlight the critical role of preoperative risk assessment in identifying high-risk patients and optimizing their management to improve surgical outcomes.
Patients who have undergone complex gastrointestinal surgeries, such as Nissen fundoplication or gastric bypass, often have altered anatomies that complicate MIE. These technical challenges are associated with a higher incidence of postoperative complications, including anastomotic leaks, which occur in 21.5% of these cases, reoperations in 20% of patients, and an overall complication rate of 62.5%. Additionally, these patients tend to have greater intraoperative blood loss and may require alternative conduits if the stomach is unsuitable for esophageal replacement[31]. A summary of key patient-related risk factors and their associated impact on postoperative outcomes in MIE is presented in Table 2.
Patient risk factors and their impact on MIE outcomes
| Risk factor | Associated complication(s) | Effect on outcomes | Source |
| Age > 75 years | Higher mortality, pulmonary complications | ↑ Post-op mortality, longer recovery | Dezube et al.[32] |
| High BMI | Anastomotic leak, longer surgery duration | ↑ Leak risk, ↑ operative time | Conroy et al.[33] |
| Diabetes mellitus | Anastomotic leak | ↑ Leak risk | Li et al.[29] |
| CHF | Perioperative mortality | ↑ Risk of death and readmission | Turrentine et al.[34] |
| Prior gastrointestinal surgery | Technical difficulty, longer surgery duration | ↑ Operative time | Pontecorvo et al.[35] |
STS risk calculator is a tool for predicting outcomes in esophagectomy. The STS risk models, updated in 2024, provide robust preoperative risk estimation for operative mortality, major morbidity, and composite morbidity or mortality. The updated models include novel predictors such as body surface area and insurance payor type, and they demonstrate excellent calibration and discrimination, with a C statistic of 0.72 for operative mortality. These models are widely used for shared decision making, provider performance assessment, and quality improvement in thoracic surgery[7,36].
Preoperative predictive markers and imaging tools are increasingly being used to identify patients who may be at higher risk for complications. Advanced imaging modalities, including computed tomography (CT), positron emission tomography (PET), and endoscopic ultrasound (EUS), help to assess tumor characteristics and surgical feasibility. In addition to the well-established STS calculator, machine learning (ML) models have been developed with a focus on enhancing the predictions of outcomes in esophagectomy[37]. For instance, Winter et al. conducted a comparative retrospective case-control study to evaluate the performance of ML models versus the International Esodata Study Group (IESG) risk model for predicting 90-day mortality in oncologic esophagectomy[37]. The study included 552 patients and found that ML models outperformed the IESG model in terms of discrimination, with an area under the receiver operating characteristic curve (AUROC) of 0.64 compared to 0.44 for the IESG model. This suggests that ML models may provide more accurate preoperative risk stratification[38].
Furthermore, Klontzas et al. developed a multimodal ML model that integrates imaging and clinical data to predict anastomotic leakage (AL) following esophagectomy for esophageal cancer[39]. The study included 471 patients and utilized an XGBoost model with 10-fold cross-validation. The model achieved an AUROC of 79.2%, indicating a high level of accuracy in predicting AL. This approach highlights the potential of combining various data types to improve clinical outcome predictions[39]. These advances show the significant potential of artificial intelligence (AI) to enhance surgical planning and patient outcomes. However, further refinement and validation are essential for optimal integration into clinical practice.
ROLE OF AI AND IMAGING FOR CANCER MANAGEMENT
The integration of AI and advanced imaging techniques is enhancing the management of esophageal cancer across various stages of care. AI, particularly deep learning (DL) methods, are being increasingly utilized for image analysis in the diagnosis and management of esophageal cancer. DL algorithms have shown high accuracy in detecting dysplastic Barrett’s esophagus and esophageal cancer, often outperforming endoscopists[1]. These algorithms are also being applied to histologic diagnosis, segmentation of gross tumor volume, and prediction of treatment response[40].
In the context of MIE, AI has demonstrated utility in several areas. For instance, AI models have been developed to recognize recurrent laryngeal nerves during RAMIE, significantly improving the accuracy and speed of nerve identification, which is crucial for reducing the risk of nerve injury[41]. Additionally, AI-based systems for automated surgical-phase recognition during RAMIE have shown high accuracy, aiding in the standardization and efficiency of surgical procedures. The study by Takeuchi et al. aimed to develop an AI-based automated surgical-phase recognition system for RAMIE by analyzing robotic surgical videos[42]. The AI model was trained on videos annotated into nine surgical phases, including preparation, lower mediastinal dissection, upper mediastinal dissection, azygos vein division, subcarinal lymph node dissection (LND), right RLN LND, left RLN LND, esophageal transection, and post-dissection to completion of surgery. An additional phase, “no step”, was used to indicate sequences when the camera was removed from the thoracic cavity.
The study enrolled 31 patients and divided them into early and late periods to assess the relationship between surgical-phase duration and surgical periods. The AI model achieved an accuracy of 84% in phase recognition. Significant reductions in the duration of the preparation, post-dissection to completion of surgery, and “no step” phases were observed in the late period compared to the early period, indicating improved efficiency over time.
Moreover, AI is being used to predict postoperative complications such as anastomotic leakage. A multimodal machine learning model integrating imaging and clinical data has shown promise in predicting anastomotic leakage with a high degree of accuracy, which can be particularly valuable in preoperative planning and risk stratification[39]. For instance, the Winter et al. model included demographic, surgical and clinical data[43], while Klontzas et al. included preoperative CT imaging and clinical variables[39]. The models provided probabilities for each patient, indicating the likelihood of 90-day mortality or anastomotic leaks. Overall, the integration of AI and advanced imaging techniques in the management of esophageal cancer, particularly in the context of MIE, holds significant potential for improving diagnostic accuracy, surgical outcomes, and personalized treatment strategies.
CURRENT CHALLENGES IN MIE AND RAMIE
Surgeon experience and case volume play a critical role in the success of MIE, particularly RAMIE. The learning curve for RAMIE is steep, with estimates suggesting proficiency requires as few as 20 cases and up to 175 cases[20]. Kooij et al. identified that significant improvements in operative time, blood loss, and conversion rates were observed after 40 cases, with further improvements in anastomotic leakage and vocal cord palsy rates after 80 cases. Additional studies have shown that anastomotic leakage rates decreased from 22.5% to 8.1% and pneumonia rates from 25% to 4% after achieving proficiency[44]. Similarly, Pickering et al. noted that learning curves for RAMIE typically range from 18 to 73 cases for lymph node yields and operative times, with formal training programs significantly reducing the learning curve length[45]. This has led to a push for centralizing MIE procedures in high-volume centers where expertise is more readily available.
Prior experience in laparoscopic or robotic surgery can also accelerate the learning process. For instance, Zhang et al. found that surgeons with prior experience in open and thoraco-laparoscopic esophagectomy required only cases to gain early proficiency in RAMIE[46]. Additionally, van der Sluis et al. demonstrated that a structured proctoring program could reduce the learning phase to 24 cases for a newly introduced surgeon, compared to 70 cases for the proctor[47].
GLOBAL ACCESSIBILITY AND TRAINING
The development of telementoring, where experienced surgeons provide real-time remote guidance to less experienced colleagues. This approach leverages advanced technologies to bridge the gap between expert and novice surgeons, enhancing surgical training and patient outcomes.
In the context of laparoscopic sleeve gastrectomy, the Society of American Gastrointestinal and Endoscopic Surgeons conducted a multi-institutional quality improvement initiative. This study found that real-time telementoring using the Visitor1 remote presence system was highly effective, with both mentors and mentees rating the experience positively. The mentees reported learning new techniques and expressed high satisfaction with the educational value of the sessions[48]. Another innovative approach is the mobile internet-based mixed-reality interactive telecollaboration system used in neurosurgical procedures. This system enables complex visual and verbal communication between remote and local surgeons, allowing for precise intraoperative guidance. The system has been successfully implemented in neuroendoscopic surgeries, demonstrating excellent image resolution and minimal latency, which are critical for effective telecollaboration[49]. Additionally, the System for Telementoring with Augmented Reality (STAR) has been shown to improve surgical performance and confidence among less experienced surgeons. Participants using STAR performed procedures with fewer errors and reported higher confidence levels compared to those who did not use the system[50]. Meanwhile, Tsai et al. highlighted the success of telementoring in rural Uganda, where it facilitated the establishment of a minimally invasive surgical center, where they telemonitored 37 appendectomies and 24 cholecystectomies. They reported no intraoperative complications and a low postoperative infection rate of 3.3%, suggesting the safety and feasibility of telementoring in resource-limited settings[51].
In the context of esophagectomies, studies such as those by Tsai et al. and Den Boer et al. have showcased its effectiveness and feasibility[51,52]. Den Boer et al. demonstrated that telementoring is a viable method for training surgeons in RAMIE within high-volume centers, especially after initial onsite training[52]. Another study by Ebihara et al. explored the use of a double-surgeon cockpit for tele-robot-assisted minimally invasive esophagectomy on cadavers, showing stable communication and effective telesurgical support[53]. This suggests its potential for wider adoption in surgical education. Together, these findings underline the value of telementoring in enhancing surgical education, improving outcomes, and expanding access to high-quality minimally invasive surgery across diverse environments. Remote proctoring and tele-mentoring can provide real-time guidance and support to surgeons performing MIE and RAMIE, thereby improving surgical outcomes and expanding the reach of these advanced techniques. This approach can also facilitate the dissemination of best practices and the standardization of surgical techniques globally[54,55].
FUTURE DIRECTIONS
Emerging technologies such as augmented reality (AR) and advanced robotics hold promise for refining MIE approaches and outcomes. AR can enhance visualization and precision during surgery, potentially reducing complications and improving surgical accuracy. According to a systematic review by Arjomandi Rad et al., AR has been utilized for preoperative planning, intraoperative guidance, and training in thoracic surgery[56]. This includes the generation of 3D renderings of the thoracic cavity and esophageal anatomy, which can enhance procedural accuracy and surgical confidence. Additionally, Sadeghi et al. discuss the application of AR in cardiothoracic surgery, highlighting its potential for intraoperative guidance and navigation, which can be extrapolated to esophagectomies[57]. These AR systems provide real-time visualization of anatomical structures, aiding in the precise identification and dissection of the esophagus and surrounding tissues.
Advanced robotic systems, with improved dexterity and control, can further enhance the surgeon's ability to perform complex dissections and anastomoses with greater precision. The integration of these technologies into surgical practice is expected to improve patient outcomes and reduce the learning curve for new surgeons[58,59].
There are several gaps in current knowledge that need to be addressed through large-scale trials. These include the long-term survival and quality of life outcomes post-MIE and RAMIE. While early results from trials such as the RAMIE trial have demonstrated the safety and feasibility of RAMIE, long-term data are still pending. Future research should focus on comparing the oncologic outcomes, recurrence rates, and patient-reported quality of life between RAMIE and conventional MIE. Additionally, studies should investigate the cost-effectiveness of these advanced surgical techniques to justify their widespread adoption[60,61].
CONCLUSION
MIE has revolutionized the surgical management of esophageal cancer, offering substantial advantages over traditional open techniques, including reduced morbidity, shorter recovery times, and comparable or superior oncologic outcomes. The evolution of RAMIE, supported by trials such as REVATE and ROBOT-2, underscores its potential to enhance precision, lymph node yield, and nerve preservation, thereby improving both short- and long-term patient outcomes. Innovations in AI, fluorescence imaging, and telementoring further augment procedural safety and accessibility, bridging gaps in surgical training and resource-limited settings.
Despite these advancements, challenges persist, particularly the steep learning curve associated with RAMIE, which necessitates centralized expertise in high-volume centers to optimize outcomes. Patient selection remains critical, with preoperative risk stratification and advanced imaging playing pivotal roles in mitigating complications. Emerging technologies, such as augmented reality and next-generation robotics, promise to refine surgical precision and democratize access to minimally invasive techniques globally.
Future directions must prioritize large-scale trials to validate long-term survival benefits, cost-effectiveness, and quality-of-life metrics. Collaborative efforts to standardize training through telementoring and structured proctoring programs will be essential to broaden adoption. As MIE continues to evolve, the integration of technological innovation, evidence-based practice, and global surgical education will solidify its role as the cornerstone of curative esophageal cancer treatment, improving outcomes for patients worldwide.
DECLARATIONS
Acknowledgments
The authors would like to acknowledge Margaret Schrieber, for her support of this project, specifically her work to obtain IRB approval from the University of Arizona..
Authors’ contributions
Conception, design of the study, data analysis and interpretation: Alexander E, Elkamel A, Worrell SG
Data analysis and interpretation: Alexander E, Elkamel A
Conceptual design of study and review of data and manuscript: Alexander E, Elkamel A, Worrell SG
Data acquisition, analysis, and interpretation: Alexander E, Elkamel A
Availability of data and materials
Not Applicable.
Financial support and sponsorship
None.
Conflicts of interest
Worrell SG. is a speaker for Intuitive. The other authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Dyas AR, Stuart CM, Bronsert MR, Schulick RD, McCarter MD, Meguid RA. Minimally invasive surgery is associated with decreased postoperative complications after esophagectomy. J Thorac Cardiovasc Surg. 2023;166:268-78.
2. Sakamoto T, Fujiogi M, Matsui H, Fushimi K, Yasunaga H. Comparing perioperative mortality and morbidity of minimally invasive esophagectomy versus open esophagectomy for esophageal cancer: a nationwide retrospective analysis. Ann Surg. 2021;274:324-30.
3. Pointer DT Jr, Saeed S, Naffouje SA, et al. Outcomes of 350 robotic-assisted esophagectomies at a high-volume cancer center: a contemporary propensity-score matched analysis. Ann Surg. 2022;276:111-8.
4. Mariette C, Markar SR, Dabakuyo-Yonli TS, et al; Fédération de Recherche en Chirurgie (FRENCH) and French Eso-Gastric Tumors (FREGAT) Working Group. Hybrid minimally invasive esophagectomy for esophageal cancer. N Engl J Med. 2019;380:152-62.
5. Kinjo Y, Kurita N, Nakamura F, et al. Effectiveness of combined thoracoscopic-laparoscopic esophagectomy: comparison of postoperative complications and midterm oncological outcomes in patients with esophageal cancer. Surg Endosc. 2012;26:381-90.
6. Yao F, Wang J, Yao J, Xu L, Qian J, Cao Y. Is thoracoscopic-laparoscopic esophagectomy a better alternative to thoracoscopic esophagectomy? Int J Surg. 2017;48:105-9.
7. Velotta JB, Seder CW, Bonnell LN, et al; Society of Thoracic Surgeons General Thoracic Surgery Database Task Force. 2024 update of the society of thoracic surgeons short-term esophagectomy risk model: more inclusive and improved calibration. Ann Thorac Surg. 2024;118:834-42.
8. Deng J, Su Q, Ren Z, et al. Comparison of short-term outcomes between minimally invasive McKeown and Ivor Lewis esophagectomy for esophageal or junctional cancer: a systematic review and meta-analysis. Onco Targets Ther. 2018;11:6057-69.
9. Weksler B, Sharma P, Moudgill N, Chojnacki KA, Rosato EL. Robot-assisted minimally invasive esophagectomy is equivalent to thoracoscopic minimally invasive esophagectomy. Dis Esophagus. 2012;25:403-9.
10. Lee S, Tamura T, Miki Y, et al. Robot-assisted minimally invasive esophagectomy for esophageal cancer in the left lateral decubitus position. Surg Endosc. 2024;38:7208-16.
11. Chao YK, Li Z, Jiang H, et al. Multicentre randomized clinical trial on robot-assisted versus video-assisted thoracoscopic oesophagectomy (REVATE trial). Br J Surg. 2024:111.
12. Tagkalos E, van der Sluis PC, Berlth F, et al. Robot-assisted minimally invasive thoraco-laparoscopic esophagectomy versus minimally invasive esophagectomy for resectable esophageal adenocarcinoma, a randomized controlled trial (ROBOT-2 trial). BMC Cancer. 2021;21:1060.
13. Li Y, Dai T. Meta-analysis comparing the perioperative efficacy of single-port versus two and multi-port video-assisted thoracoscopic surgical anatomical lung resection for lung cancer. Medicine (Baltimore). 2023;102:e32636.
14. Yip HC, Chiu PW. Recent advances in natural orifice transluminal endoscopic surgery†. Eur J Cardiothorac Surg. 2016;49 Suppl 1:i25-30.
15. Straatman J, van der Wielen N, Cuesta MA, et al. Minimally invasive versus open esophageal resection: three-year follow-up of the previously reported randomized controlled trial: the TIME trial. Ann Surg. 2017;266:232-6.
16. Nuytens F, Dabakuyo-Yonli TS, Meunier B, et al; Fédération de Recherche en Chirurgie (FRENCH) and French Eso-Gastric Tumors (FREGAT) Working Groups. Five-year survival outcomes of hybrid minimally invasive esophagectomy in esophageal cancer: results of the MIRO randomized clinical trial. JAMA Surg. 2021;156:323-32.
17. Groot EM, Goense L, Kingma BF, van den Berg JW, Ruurda JP, van Hillegersberg R. Implementation of the robotic abdominal phase during robot-assisted minimally invasive esophagectomy (RAMIE): results from a high-volume center. Surg Endosc. 2023;37:1357-65.
18. Li XK, Xu Y, Zhou H, et al. Does robot-assisted minimally invasive oesophagectomy have superiority over thoraco-laparoscopic minimally invasive oesophagectomy in lymph node dissection? Dis Esophagus. 2021;34:doaa050.
19. Worrell SG, Goodman KA, Altorki NK, et al. The society of thoracic surgeons/American society for radiation oncology updated clinical practice guidelines on multimodality therapy for locally advanced cancer of the esophagus or gastroesophageal junction. Ann Thorac Surg. 2024;117:15-32.
20. Kooij CD, de Jongh C, de Jongh BF, et al. The current state of robot-assisted minimally invasive esophagectomy (RAMIE): outcomes from the upper GI international robotic association (UGIRA) esophageal registry. Ann Surg Oncol. 2025;32:823-33.
21. Jehan F, Brady M, Attwood K, Hochwald SN, Kukar M. Achieving textbook outcomes with robotic-assisted Ivor Lewis esophagectomy: a single-center experience with 150 consecutive patients. Surgery. 2025;29:101979.
22. Ma s, Brady M, Attwood K, Hochwald SN, Kukar M. Early postoperative endoscopy for predicting anastomotic leakage after minimally invasive esophagectomy: a large-volume retrospective study. Surgery. 2024;175:1305-11.
23. Banks KC, Barnes KE, Wile RK, et al. Outcomes of anastomotic evaluation using indocyanine green fluorescence during minimally invasive esophagectomy. Am Surg. 2023;89:5124-30.
24. Sozzi A, Bona D, Yeow M, et al. Does indocyanine green utilization during esophagectomy prevent anastomotic leaks? J Clin Med. 2024;13:4899.
25. Wang P, Tian Y, Du Y, Zhong Y. Intraoperative assessment of anastomotic blood supply using indocyanine green fluorescence imaging following esophagojejunostomy or esophagogastrostomy for gastric cancer. Front Oncol. 2024;14:1341900.
26. Cossu A, Palumbo D, Battaglia S, et al; SISME - Società Italiana per lo Studio delle Malattie dell’Esofago - Italian society of disease of the esophagus. sarcopenia and patient’s body composition: new morphometric tools to predict clinical outcome after Ivor Lewis esophagectomy: a multicenter study. J Gastrointest Surg. 2023;27:1047-54.
27. Peters AK, Juratli MA, Roy D, et al. Factors influencing postoperative complications following minimally invasive Ivor Lewis esophagectomy: a retrospective cohort study. J Clin Med. 2023;12:5688.
28. Yu B, Liu Z, Zhang L, et al. Pre- and intra-operative risk factors predict postoperative respiratory failure after minimally invasive oesophagectomy. Eur J Cardiothorac Surg. 2024;65:ezae107.
29. Li SJ, Wang ZQ, Li YJ, et al. Diabetes mellitus and risk of anastomotic leakage after esophagectomy: a systematic review and meta-analysis. Dis Esophagus. 2017;30:1-12.
30. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American college of cardiology/American heart association task force on practice guidelines. Circulation. 2014;130:e278-333.
31. Shen KR, Harrison-Phipps KM, Cassivi SD, et al. Esophagectomy after anti-reflux surgery. J Thorac Cardiovasc Surg. 2010;139:969-75.
32. Dezube AR, Cooper L, Mazzola E, et al. Long-term outcomes following esophagectomy in older and younger adults with esophageal cancer. J Gastrointest Surg. 2022;26:1119-31.
33. Conroy MA, O'Connor AL, Qureshi AP, Wood SG. Impact of morbid obesity on post-esophagectomy leak rate: a NSQIP analysis. J Gastrointest Surg. 2023;27:1539-44.
34. Turrentine FE, Sohn MW, Jones RS. Congestive Heart failure and noncardiac operations: risk of serious morbidity, readmission, reoperation, and mortality. J Am Coll Surg. 2016;222:1220-9.
35. Pontecorvo AA, Cornejo J, Alomari M, et al. Outcomes of esophagectomy in patients with previous foregut surgery: a matched retrospective cohort study. Surg Endosc. 2025;39:3970-8.
36. Raymond DP, Seder CW, Wright CD, et al. Predictors of major morbidity or mortality after resection for esophageal cancer: a society of thoracic surgeons general thoracic surgery database risk adjustment model. Ann Thorac Surg. 2016;102:207-14.
37. Winter A, van de Water RP, Pfitzner B, et al. Enhancing preoperative outcome prediction: a comparative retrospective case-control study on machine learning versus the international esodata study group risk model for predicting 90-day mortality in oncologic esophagectomy. Cancers (Basel). 2024;16:3000.
38. Jung JO, Pisula JI, Bozek K, et al. Prediction of postoperative complications after oesophagectomy using machine-learning methods. Br J Surg. 2023;110:1361-6.
39. Klontzas ME, Ri M, Koltsakis E, et al. Prediction of anastomotic leakage in esophageal cancer surgery: a multimodal machine learning model integrating imaging and clinical data. Acad Radiol. 2024;31:4878-85.
40. Low SW, Lentz RJ, Chen H, et al. Shape-sensing robotic-assisted bronchoscopy vs. digital tomosynthesis-corrected electromagnetic navigation bronchoscopy: a comparative cohort study of diagnostic performance. Chest. 2023;163:977-84.
41. Furube T, Takeuchi M, Kawakubo H, et al. Usefulness of an artificial intelligence model in recognizing recurrent laryngeal nerves during robot-assisted minimally invasive esophagectomy. Ann Surg Oncol. 2024;31:9344-51.
42. Takeuchi M, Kawakubo H, Saito K, et al. Automated surgical-phase recognition for robot-assisted minimally invasive esophagectomy using artificial intelligence. Ann Surg Oncol. 2022;29:6847-55.
43. Winter A, van de Water RP, Pfitzner B, et al. Enhancing preoperative outcome prediction: a comparative retrospective case-control study on machine learning versus the international esodata study group risk model for predicting 90-day mortality in oncologic esophagectomy. Cancers (Basel). 2024;16:3000.
44. Yang Y, Li B, Hua R, et al; Written on behalf of the AME Thoracic surgery collaborative group. Assessment of quality outcomes and learning curve for robot-assisted minimally invasive McKeown esophagectomy. Ann Surg Oncol. 2021;28:676-84.
45. Pickering OJ, van Boxel GI, Carter NC, Mercer SJ, Knight BC, Pucher PH. Learning curve for adoption of robot-assisted minimally invasive esophagectomy: a systematic review of oncological, clinical, and efficiency outcomes. Dis Esophagus. 2023;36:doac089.
46. Zhang H, Chen L, Wang Z, et al. The learning curve for robotic McKeown esophagectomy in patients with esophageal cancer. Ann Thorac Surg. 2018;105:1024-30.
47. der Sluis PC, Ruurda JP, van der Horst S, Goense L, van Hillegersberg R. Learning curve for robot-assisted minimally invasive thoracoscopic esophagectomy: results from 312 cases. Ann Thorac Surg. 2018;106:264-71.
48. Nguyen NT, Okrainec A, Anvari M, et al. Sleeve gastrectomy telementoring: a SAGES multi-institutional quality improvement initiative. Surg Endosc. 2018;32:682-7.
49. Zhang S, Li F, Zhao Y, et al. Mobile internet-based mixed-reality interactive telecollaboration system for neurosurgical procedures: technical feasibility and clinical implementation. Neurosurg Focus. 2022;52:E3.
50. Rojas-Muñoz E, Cabrera ME, Lin C, et al. The system for telementoring with augmented reality (STAR): a head-mounted display to improve surgical coaching and confidence in remote areas. Surgery. 2020;167:724-31.
51. Tsai C, Damoi JO, Zhang LP, et al. Collaborative international partnership: enabling the development of a state-of-the-art minimally invasive surgical center in rural Uganda through telementoring. World J Surg. 2024;48:1602-8.
52. den Boer RB, de Jongh C, van Boxel GI, et al. Feasibility of telementoring during robot-assisted minimally invasive esophagectomy. Dig Surg. 2025;42:1-8.
53. Ebihara Y, Hirano S, Shichinohe T, et al. Tele-robot-assisted minimally invasive esophagectomy using a double-surgeon cockpit on a cadaver. Surg Today. 2025.
54. Grimminger PP, van der Horst S, Ruurda JP, van Det M, Morel P, van Hillegersberg R. Surgical robotics for esophageal cancer. Ann N Y Acad Sci. 2018;1434:21-6.
55. Yang Y, Li B, Yi J, et al. Robot-assisted Versus Conventional minimally invasive esophagectomy for resectable esophageal squamous cell carcinoma: early results of a multicenter randomized controlled trial: the RAMIE trial. Ann Surg. 2022;275:646-53.
56. Arjomandi Rad A, Vardanyan R, Thavarajasingam SG, et al. Extended, virtual and augmented reality in thoracic surgery: a systematic review. Interact Cardiovasc Thorac Surg. 2022;34:201-11.
57. Sadeghi AH, Mathari SE, Abjigitova D, et al. Current and future applications of virtual, augmented, and mixed reality in cardiothoracic surgery. Ann Thorac Surg. 2022;113:681-91.
58. Yip HC, Shirakawa Y, Cheng CY, Huang CL, Chiu PWY. Recent advances in minimally invasive esophagectomy for squamous esophageal cancer. Ann N Y Acad Sci. 2020;1482:113-20.
59. Boxel GI, Kingma BF, Voskens FJ, Ruurda JP, van Hillegersberg R. Robotic-assisted minimally invasive esophagectomy: past, present and future. J Thorac Dis. 2020;12:54-62.
60. der Sluis PC, van Hillegersberg R. Robot assisted minimally invasive esophagectomy (RAMIE) for esophageal cancer. Best Pract Res Clin Gastroenterol. 2018;36-37:81-3.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Special Issue
Copyright
Data & Comments
Data
















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].