Artificial intelligence for perioperative risk assessment in minimally invasive cardiac surgery: current applications and future perspectives
Abstract
Minimally invasive cardiac surgery (MICS) represents a significant advancement in cardiac surgical care, offering benefits such as reduced trauma, shorter hospital stays, and faster recovery. However, the complexity of perioperative management in MICS demands highly accurate risk stratification and decision-making. Artificial intelligence (AI) technologies are increasingly being integrated into perioperative workflows, providing clinicians with data-driven tools to enhance patient selection, predict complications, and optimize outcomes. This review explores current applications of AI in the perioperative assessment of patients undergoing minimally invasive cardiac procedures, with a focus on preoperative risk prediction, intraoperative monitoring, and postoperative management. It discusses the potential of AI to support precision medicine in MICS, highlights the technical and ethical challenges associated with its implementation, and outlines future directions for research and clinical integration. By bridging surgical innovation and computational intelligence, AI is poised to reshape the landscape of perioperative cardiac care.
Keywords
INTRODUCTION
Minimally invasive cardiac surgery (MICS) has gained increasing attention over the last three decades, offering reduced surgical trauma, shorter recovery time, and improved postoperative outcomes compared to conventional sternotomy approaches. Despite these advantages, MICS remains technically demanding and is still largely confined to high-volume centers with specialized expertise[1]. These procedures require precise patient selection and perioperative planning, as even small errors in surgical or anesthetic strategy can affect outcomes significantly[2].
To address these challenges, artificial intelligence (AI) is emerging as a powerful tool in cardiac surgery, with the potential to enhance perioperative care through data-driven decision-making, risk stratification, and real-time monitoring. Although AI has been widely explored in other medical domains, its integration into cardiac surgery - especially in minimally invasive contexts - is still in its early stages[2,3].
Early clinical experience has confirmed that minimally invasive techniques can offer long-term outcomes comparable to conventional surgery, provided that appropriate patient selection and intraoperative precision are ensured - two areas where AI may play a transformative role[4].
As surgical innovation advances, there is growing interest in leveraging machine learning (ML), deep learning (DL), and other AI-based methodologies to guide patient selection, predict complications, and optimize perioperative workflows in MICS[5].
Unlike broader reviews addressing AI in general surgical practice, the present work specifically focuses on MICS. This distinction is relevant because MICS entails unique challenges such as restricted access, the need for precise intraoperative navigation, and the progressive integration of robotic platforms - elements that are not fully captured in the wider surgical literature.
This review aims to summarize current applications of AI in perioperative risk assessment for MICS and to highlight its potential role in improving patient outcomes and supporting surgical decision-making in this evolving field.
CLASSICAL RISK STRATIFICATION IN CARDIAC SURGERY
Perioperative risk prediction is central to patient selection, shared decision-making, and surgical planning in cardiac surgery. Among the most widely adopted tools are the European System for Cardiac Operative Risk Evaluation (EuroSCORE) II and the Society of Thoracic Surgeons (STS) score, both of which offer structured, quantifiable estimations of surgical risk and are endorsed by major international guidelines. The 2021 European Society of Cardiology (ESC)/European Association for Cardio-Thoracic Surgery (EACTS) Guidelines on Valvular Heart Disease recommend their use within Heart Team discussions for patients undergoing valve surgery[6]. The 2022 ESC Guidelines on Myocardial Revascularization endorse their application in complex coronary disease management, while the 2023 American Heart Association (AHA) Guideline on Chronic Coronary Disease highlights their role in stratifying long-term risk in patients with stable ischemic syndromes[7,8].
These scores have become cornerstones in clinical practice due to their ease of use, broad accessibility, and validation across large datasets. EuroSCORE II, based on a European multicenter cohort, estimates in-hospital mortality using logistic regression and incorporates preoperative demographic and clinical variables. The STS score, derived from a large U.S. national database, is procedure-specific and includes additional endpoints such as stroke, renal failure, and prolonged ventilation. Their integration into surgical workflows has improved consistency in risk communication and facilitated evidence-based decisions, particularly in high-risk patients.
Multiple studies have confirmed their predictive performance across different settings. In a cohort of patients undergoing aortic or mitral valve surgery, both EuroSCORE II and STS demonstrated good discrimination for 30-day and 1-year mortality[9]. In a separate evaluation of off-pump coronary artery bypass grafting (CABG), EuroSCORE II maintained favorable accuracy among low-risk surgical candidates (Pathare). Similarly, its utility has been demonstrated in elderly populations, further supporting its generalizability across various cardiac surgical contexts[10].
Despite their broad validation[9-11], both models face inherent limitations due to their static design, fixed variable sets, and inability to adjust to evolving intraoperative or postoperative conditions. Age and procedural complexity represent common sources of poor calibration. Kuplay et al. observed that in octogenarians undergoing cardiac surgery, EuroSCORE II tended to overpredict mortality in elective cases, while STS underestimated risk in urgent procedures and combined interventions[12]. These findings raise concerns about the generalizability of standard models to frail or borderline surgical candidates. Structural predictors, such as right ventricular dysfunction, pulmonary hypertension, and left atrial enlargement, are often overlooked in traditional risk scores. Espinoza Romero et al. proposed a staging model based on structural cardiac deterioration, demonstrating improved prognostic accuracy in patients undergoing surgical aortic valve replacement compared to both EuroSCORE II and STS[13]. Another limitation is the exclusion of intraoperative and early postoperative variables, which significantly impact clinical trajectories. Prins et al. emphasized that factors such as cardiopulmonary bypass duration, bleeding, and rhythm disturbances are unaccounted for in classical models, although they substantially influence short- and mid-term outcomes[14].
Moreover, existing models show reduced performance when applied to MICS. In these procedures, characterized by lower physiological impact but higher technical variability, classical scores may overestimate risk, as they were calibrated on populations undergoing conventional sternotomy[15].
Long-term predictive ability is also limited. Most traditional models are validated for in-hospital or 30-day mortality but do not reliably estimate outcomes such as late survival, reintervention, or functional recovery. These endpoints are increasingly relevant in contemporary practice, particularly for patients with stable chronic coronary syndromes. From a methodological standpoint, conventional scores rely on logistic regression, which limits their ability to capture non-linear associations or complex variable interactions. Argus et al. pointed out that these statistical frameworks, while interpretable and standardized, lack the flexibility needed to incorporate multimodal clinical data[16].
Finally, the rigidity of classical scores hinders personalization. As healthcare shifts toward precision medicine, there is an increasing need for dynamic models that adapt to patient variability, procedural innovation, and evolving intraoperative findings. ML algorithms, capable of integrating large-scale data and refining predictions over time, represent a promising alternative to traditional tools.
AI FOR PREOPERATIVE RISK PREDICTION
In recent years, AI has rapidly emerged as a transformative tool in cardiovascular medicine, offering promising alternatives to traditional risk scores through enhanced accuracy, adaptability, and personalization. In the context of cardiac surgery, AI-based models are being increasingly explored for preoperative risk prediction, aiming to overcome the limitations of conventional scoring systems such as EuroSCORE II and STS[17]. AI algorithms can dynamically incorporate large, heterogeneous datasets - including imaging, laboratory markers, and intraoperative data - and continuously improve through iterative learning[18].
One of the earliest and most impactful applications of AI in cardiac surgery has been the development of ML models for mortality prediction. These models use complex computational methods - such as random forests, gradient boosting, and deep neural networks - to identify non-linear relationships between variables, which are often missed by traditional logistic regression approaches. In a retrospective study including over 45,000 patients undergoing major cardiac surgery, a ML ensemble model significantly outperformed EuroSCORE II in predicting both in-hospital and 30-day mortality, with a higher area under the curve[19].
Similarly, another multicenter analysis employed supervised learning techniques, including random forest and support vector machines, to build predictive models for postoperative mortality and acute kidney injury. These models demonstrated strong discriminative power and calibration, particularly when incorporating novel variables such as preoperative hemoglobin, intraoperative hemodynamics, and intensive care unit (ICU) lab trends[20].
One of the major advantages of AI in this domain lies in its ability to personalize risk prediction at the patient level. Traditional scores assign risk based on broad population averages, often failing to capture subtle physiological patterns. In contrast, AI models can integrate granular variables - such as frailty indices, left atrial volume, systemic inflammation markers, or medication profiles - and generate predictions specific to the individual case. A study using DL to predict operative mortality in patients undergoing valvular surgery showed that even in moderate-risk populations, the AI model could detect critical interactions between comorbidities and procedural variables that were not weighted in standard scores[21]. Another significant application has been in the prediction of specific complications beyond mortality. For example, ML models have been used to forecast the risk of postoperative atrial fibrillation, prolonged ventilation, and stroke. In a cohort of 10,000 patients undergoing CABG or valve procedures, a gradient boosting model integrating over 100 preoperative features achieved higher accuracy in predicting new-onset atrial fibrillation compared to existing clinical models[22].
AI-based tools have also shown promise in enhancing risk stratification in high-risk or underrepresented populations, where traditional scores underperform. This includes elderly patients, those undergoing minimally invasive or redo surgery, and individuals with multiple comorbidities. In these settings, the flexibility of AI to reweight and reprioritize predictors based on the training dataset enables more nuanced decision-making. In one study focused on geriatric cardiac surgery patients, a neural network model demonstrated superior discrimination in predicting composite outcomes including mortality, stroke, and renal failure, compared to EuroSCORE II[23].
An important methodological advance facilitated by AI is the integration of multimodal data sources into predictive models. In traditional scoring systems, most variables are manually selected and limited to clinical and demographic fields. In contrast, AI systems can ingest large volumes of imaging data [e.g., echocardiography, computed tomography (CT) angiography], laboratory values, electronic health record (EHR)-derived parameters, and even wearable device data. A recent pipeline incorporated left ventricular strain data and CT coronary calcium scoring into a model predicting early mortality and demonstrated significant improvement in calibration metrics[24].
Beyond predictive performance, AI systems are increasingly explored for their capacity to support dynamic risk reassessment throughout the perioperative course. In particular, integration with EHRs and real-time anesthetic monitoring allows continuous updating of clinical parameters, facilitating early detection of hemodynamic instability or organ dysfunction. Unlike traditional scores, which are fixed at baseline, AI-driven tools can adapt to evolving patient trajectories, adjusting risk profiles based on intraoperative events and postoperative trends. Such systems have already been implemented in anesthesia for real-time surveillance and physiological modeling, demonstrating improved accuracy in patient monitoring and decision-making[25].
AI IN MICS
MICS presents unique technical challenges due to limited visual and manual access, making it an ideal candidate for AI integration to enhance visualization, planning, navigation, and intraoperative decision-making.
In the preoperative phase, AI has enabled the development of three-dimensional anatomical reconstructions derived from high-resolution CT or magnetic resonance imaging datasets. These reconstructions, powered by ML algorithms, assist the surgical team in mapping individualized access routes and anticipating technical difficulties, thereby reducing operative time and enhancing control over procedural variables that impact risk[26].
Intraoperatively, computer vision (CV) and DL have been used to support surgeons by recognizing and segmenting anatomical landmarks in real time. This technology, initially applied in laparoscopic procedures, is now being extended to thoracoscopic and robotic-assisted cardiac surgeries. Algorithms trained on surgical video frames can assist in identifying key structures such as coronary arteries, valves, and conduction pathways. This has the potential to minimize errors caused by limited visual exposure or anatomical variation[27]. A major leap in this field is the development of AI-based surgical navigation systems. These systems integrate real-time video feeds with augmented reality (AR) overlays, highlighting anatomical structures or danger zones during dissection. In robotic MICS, where precision and spatial orientation are critical, such technology could augment surgeon performance and safety. While still under evaluation, early prototypes show promise in enhancing intraoperative awareness and reducing adverse events[28,29].
In addition to assisting during surgery, AI has demonstrated value in evaluating surgical skills. Video-based analysis combined with supervised learning can quantify surgical performance across domains such as tissue handling, dissection quality, and efficiency. These tools are being explored for training and certification purposes in endoscopic and robotic surgery. A standardized AI score, such as the “AI confidence score (AICS)”, has shown correlation with expert evaluations, opening the door to objective assessment in MICS[30].
Another important area of application is the prediction of intra- and postoperative complications. ML models trained on patient and procedural data have been used to forecast outcomes such as bleeding, arrhythmias, or prolonged ventilation following minimally invasive valve or bypass procedures. These models can assist clinicians in tailoring postoperative management and identifying high-risk patients who might benefit from more intensive monitoring[31]. Despite these advances, several technical and systemic barriers remain. Many AI models still face challenges in generalizability, especially when applied to varying camera types, surgical instruments, and patient populations. Moreover, intraoperative AI must contend with dynamic visual disturbances such as smoke, mist, or blood, which can obscure critical structures. This limits the accuracy of recognition models and highlights the need for robust datasets and adaptive algorithms[32].
The integration of robotic systems has further fueled interest in data-driven innovation. Unlike manual surgery, robotic platforms inherently collect kinematic and force data during procedures, creating a rich foundation for AI-driven analysis. These data streams can be used not only for skill evaluation but also for real-time error detection and workflow optimization. The feedback loop between robotic manipulation and AI interpretation opens possibilities for partially autonomous surgical steps in the future[33].
Commercial platforms are also beginning to enter this space. Tools such as Touch Surgery and Crowd-Sourced Assessment of Technical Skills (C-SATS) offer cloud-based video analysis, procedural mapping, and peer review, bringing AI-supported evaluation into clinical environments. While these platforms are currently more established in general or laparoscopic surgery, efforts are underway to tailor their functionality to the cardiac and thoracic domains[23]. Ethical and regulatory considerations are also crucial in the adoption of AI in MICS. Many models function as “black boxes”, limiting transparency in their decision-making processes. This lack of interpretability can hinder clinician trust and regulatory approval. Efforts are being made to improve model explainability and align AI outputs with clinically accepted rationale, especially in high-stakes settings such as cardiac surgery[22].
A summary of selected AI applications in MICS is provided in Table 1.
Selected applications of AI in MICS
| Application area | Clinical relevance | Key references |
| Preoperative evaluation | Enhances risk prediction beyond EuroSCORE/STS, with personalized stratification | [19,20] |
| Surgical planning | Supports access route definition via imaging-based 3D reconstructions | [21,26] |
| Intraoperative guidance | Enables anatomical recognition and augmented navigation in thoracoscopic/robotic MICS | [27,31] |
| Skill assessment and feedback | Provides objective evaluation from surgical video and robotic kinematics | [30,32] |
| Implementation challenges | Data heterogeneity, workflow disruption, lack of prospective validation | [33-35] |
CHALLENGES AND FUTURE DIRECTIONS
Despite the substantial promise of AI enhancing perioperative decision-making and technical precision in MICS, numerous challenges must be addressed before these tools can achieve widespread clinical integration. One of the foremost limitations is the quality, completeness, and representativeness of the data used to develop predictive models. Many current AI applications are trained on retrospective datasets from single centers, often reflecting specific surgical populations, protocols, and technologies. This restricts the external validity of models when applied in real-world contexts that differ from their development environment, particularly in the heterogeneous field of MICS, where procedural variability is high[33]. In addition to data issues, interpretability remains a critical obstacle. A large proportion of high-performing models rely on DL architectures that operate as black boxes, generating accurate but opaque outputs. In a clinical environment that demands accountability, traceability, and understanding, the absence of transparent decision pathways undermines clinician confidence and limits practical utility[34]. This has fueled interest in the development of explainable AI (XAI), which seeks to make complex predictions intelligible to human users. However, current XAI methods remain largely experimental and have yet to be robustly validated in cardiac surgical applications. Another significant challenge is the integration of AI into existing clinical workflows. Implementation often necessitates not only software infrastructure, but also hardware upgrades, user training, and reconfiguration of perioperative routines. These requirements can be resource-intensive and logistically complex, especially in low-volume centers or health systems with limited digital integration. Furthermore, surgical AI systems must interface with imaging, monitoring, and robotic platforms in real time - an operational feat that is rarely trivial[35].
Beyond the technical dimension, the intraoperative environment poses unique stressors to AI reliability: dynamic fields, occlusion by instruments, motion artifacts, and variable lighting conditions can reduce the accuracy of real-time recognition systems. In MICS, where visual access is already limited, such degradation in performance may compromise safety rather than enhance it[36]. Equally important are the regulatory and clinical validation gaps. While retrospective studies frequently show superior discrimination of AI models over traditional risk scores, very few tools have undergone prospective testing in large multicenter trials. Without such validation, these models cannot be credibly endorsed in guidelines or approved for use as decision-support systems in high-stakes environments such as cardiac surgery[37]. The ethical and medico-legal implications of AI use also remain unresolved. There is currently no clear consensus on accountability in the event of adverse outcomes linked to AI-guided recommendations, nor are patients routinely informed that their care may be partially influenced by algorithmic systems. This lack of transparency raises concerns about informed consent and medical liability. In parallel, data governance and privacy are major concerns, especially when large datasets are used to train centralized or commercial models. The risk of patient re-identification, misuse of sensitive data, or algorithmic bias based on sociodemographic variables is non-negligible[38]. Lastly, cultural resistance within the surgical community may act as a barrier in itself. Surgeons, anesthesiologists, and clinical teams have deeply rooted practices and rely heavily on clinical intuition and experiential judgment. The introduction of algorithmic tools - particularly those perceived as intrusive, opaque, or prescriptive - may be met with skepticism or even rejection unless accompanied by rigorous education and evidence of benefit. To overcome these challenges, the next phase of development should prioritize interdisciplinary collaboration, ensuring that AI systems are designed with clinicians, validated in diverse environments, explained in accessible terms, and embedded within the operative culture without disrupting the essential human dimension of surgical care[39,40].
IMPROVING VIDEO-BASED AI SKILL ASSESSMENT TOOLS
To enhance the robustness and clinical relevance of video-based AI models such as AICS, several strategies should be prioritized. First, the creation of large, annotated, and multicenter surgical video datasets is essential. These should ideally include contributions from surgeons with diverse experience levels and procedural styles, in order to capture real-world variability and minimize bias. The current literature shows that most existing studies rely on small, single-center datasets, often lacking detailed annotation protocols or inter-rater validation, which limits generalizability[41]. Second, expert-derived consensus metrics - such as precision in tissue handling, clarity of anatomical dissection, and procedural fluidity - should be used to guide model training and evaluation, ensuring that AI systems align with clinically meaningful standards[24]. Lastly, AI models must be trained with technical heterogeneity in mind: incorporating variations in surgical techniques, instruments, and institutional workflows can increase model adaptability and fairness across different MICS settings. This approach would not only improve reproducibility but also promote equity in access to advanced AI tools, regardless of a center’s volume or technological infrastructure[42,43].
ETHICAL AND EQUITY CONSIDERATIONS IN SURGICAL AI
The integration of AI into MICS raises concrete and well-documented ethical and equity challenges. First, algorithmic opacity remains a critical barrier. As many high-performing AI models rely on DL architectures, their decision processes are often unintelligible to clinicians and patients alike. This lack of interpretability may erode trust, limit shared decision-making, and complicate liability attribution in case of adverse events[44].
Bias in training data is equally concerning. Even minor imbalances in demographic or procedural representation can lead to skewed outputs that disadvantage specific subgroups. In MICS, where anatomical access, surgical technique, and perioperative protocols vary substantially across centers and patient populations, such biases are not only likely but also clinically relevant[45,46]. Without systematic efforts to ensure dataset diversity, AI tools risk reinforcing structural disparities in surgical outcomes.
Financial and infrastructural limitations further complicate equitable deployment. Most AI platforms require advanced imaging, cloud-based data integration, and dedicated personnel - resources not universally available. As a result, the implementation of AI-enhanced MICS workflows may be feasible only in well-funded, high-volume centers, creating an uneven landscape of access and benefit[47].
Moreover, recent literature warns that AI may shift the ethical focus of care. By embedding assumptions about ideal surgical behavior or outcomes, AI systems risk reconfiguring the role of the surgeon into that of a passive executor, potentially displacing clinical judgment with prescriptive algorithmic norms[48]. This technological framing can introduce subtle pressures toward conformity, rather than personalization of care.
To mitigate these risks, future development must prioritize interpretability, dataset equity, and implementation models that are both cost-sensitive and adaptable across diverse clinical environments[49].
CONCLUSION
The convergence of MICS and AI represents a pivotal frontier in the evolution of cardiac care. Traditional risk prediction models such as EuroSCORE II and the STS score have played a foundational role in guiding surgical decision-making for decades. However, as cardiac surgery becomes increasingly nuanced, especially in the context of minimally invasive approaches, the limitations of these classical tools - rigid structures, static inputs, and poor adaptability to real-time change - have become more apparent. AI offers a paradigm shift: by leveraging ML, DL, and advanced data integration, it enables dynamic, personalized, and continuously learning systems that can enhance preoperative assessment, intraoperative precision, and postoperative management.
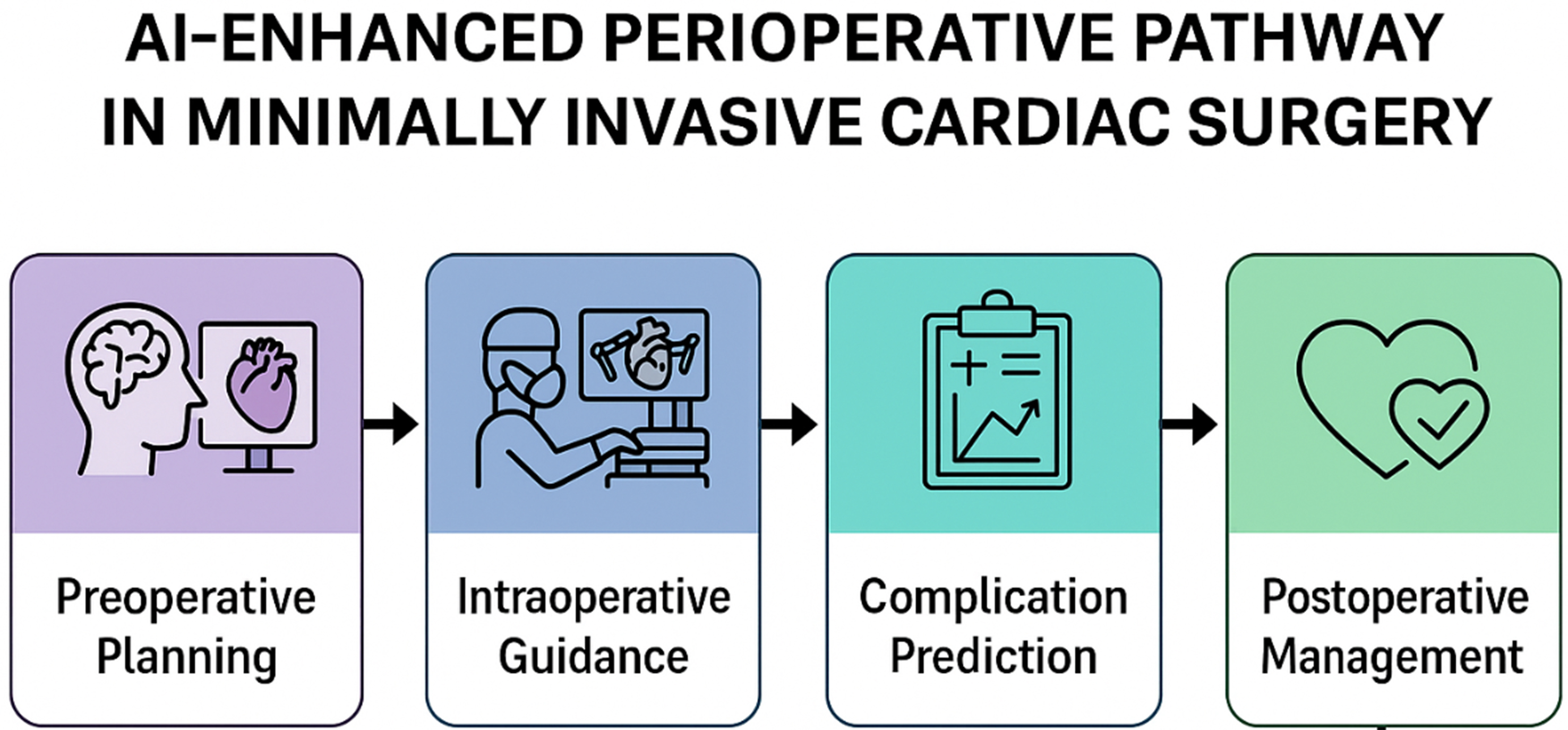
In this review, we have highlighted how AI-based models outperform conventional scores in predictive accuracy, particularly in complex and high-risk populations, and how their applications extend beyond mortality to include complications, functional outcomes, and even skill evaluation. Within the MICS landscape, AI is already demonstrating value in imaging reconstruction, surgical planning, video annotation, and real-time navigation. These tools are not merely augmenting human capacity - they are redefining what is technically feasible and operationally safe in modern cardiac surgery. Yet the road ahead is not without obstacles. Data heterogeneity, model opacity, integration complexity, ethical uncertainty, and regulatory ambiguity all present formidable challenges that must be addressed through rigorous research, transparent methodologies, and interdisciplinary collaboration. If AI is to truly become an ally in the surgical suite, it must be explainable, validated, interoperable, and trusted - not just by developers and institutions, but by the clinicians who will ultimately bear responsibility for its application. The integration of AI spans the entire perioperative continuum in MICS, enhancing risk prediction and clinical decision-making at each stage [Figure 1].
Figure 1. AI-enhanced perioperative pathway in MICS. AI contributes across the surgical timeline. AI contributes at multiple stages: during preoperative planning, AI supports patient selection and imaging-based risk stratification; in intraoperative guidance, CV and AR assist anatomical recognition and robotic navigation; in complication prediction, ML models integrate patient and procedural data to forecast adverse events; and in postoperative management, AI-driven monitoring tools help optimize recovery, personalize follow-up, and improve long-term outcomes. Together, these components illustrate how AI can provide continuous, data-driven support across the surgical timeline in MICS. AI: Artificial intelligence; MICS: minimally invasive cardiac surgery; CV: computer vision; AR: augmented reality; ML: machine learning.
The future of cardiac surgery will not be shaped by technology alone, but by the ability to integrate that technology into workflows that are humane, ethical, and clinically sound. As we stand at the intersection of surgical innovation and computational intelligence, the goal is not to replace the surgeon’s judgment, but to refine it - to provide decision-making tools that are not only faster or more precise, but wiser. With continued progress and a commitment to clinical relevance, AI may not only support MICS - it may help define its next era.
DECLARATIONS
Authors’ contributions
Conceptualization and writing - original draft: Veneziano FA
Writing - review and editing: Mistrulli R, Gioia FA
Supervision and critical revision: De Luca L
All authors have read and approved the final version of the manuscript.
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Holubec T, Dahle G, Bonaros N. Editorial: Minimally invasive cardiac surgery: state of the art and current challenges. Front Cardiovasc Med. 2023;10:1286868.
2. Ilcheva L, Risteski P, Tudorache I, et al. Beyond conventional operations: embracing the era of contemporary minimally invasive cardiac surgery. J Clin Med. 2023;12:7210.
3. Dieberg G, Smart NA, King N. Minimally invasive cardiac surgery: a systematic review and meta-analysis. Int J Cardiol. 2016;223:554-60.
4. Reuthebuch O, Stein A, Koechlin L, et al. Five-year survival of patients treated with minimally invasive direct coronary artery bypass (MIDCAB) compared with the general swiss population. Thorac Cardiovasc Surg. 2024;72:404-12.
5. Claessens J, Rottiers R, Vandenbrande J, et al. Quality of life in patients undergoing minimally invasive cardiac surgery: a systematic review. Indian J Thorac Cardiovasc Surg. 2023;39:367-80.
6. Vahanian A, Beyersdorf F, Praz F, et al; ESC/EACTS Scientific Document Group. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2022;43:561-632.
7. Neumann FJ, Sousa-Uva M, Ahlsson A, et al; ESC Scientific Document Group. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40:87-165.
8. Virani SS, Newby LK, Arnold SV, et al; Peer Review Committee Members. 2023 AHA/ACC/ACCP/ASPC/NLA/PCNA Guideline for the Management Of Patients With Chronic Coronary Disease: a report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation. 2023;148:e9-119.
9. Zhuo DX, Bilchick KC, Shah KP, et al. MAGGIC, STS, and EuroSCORE II risk score comparison after aortic and mitral valve surgery. J Cardiothorac Vasc Anesth. 2021;35:1806-12.
10. Bradbury JL, Rodrigues TS, Kumarasiri M, et al. Utility of the Euroscore II in predicting long-term outcomes in a contemporary Australian cohort with complex coronary artery disease. Am J Cardiol. 2025;256:18-22.
11. Shahian DM, Blackstone EH, Edwards FH, et al; STS workforce on evidence-based surgery. Cardiac surgery risk models: a position article. Ann Thorac Surg. 2004;78:1868-77.
12. Kuplay H, Bayer Erdoğan S, Baştopçu M, Karpuzoğlu E, Er H. Performance of the EuroSCORE II and the STS score for cardiac surgery in octogenarians. Turk Gogus Kalp Damar Cerrahisi Derg. 2021;29:174-82.
13. Espinoza Romero C, Rosa VEE, Octavio Kormann S, et al. Impact of a new preoperative stratification based on cardiac structural compromise in patients with severe aortic stenosis undergoing valve replacement surgery. Diagnostics. 2024;14:2250.
14. Prins C, de Villiers Jonker I, Botes L, Smit FE. Cardiac surgery risk-stratification models. Cardiovasc J Afr. 2012;23:160-4.
15. Gao F, Shan L, Wang C, et al. Predictive ability of European Heart Surgery Risk Assessment System II (EuroSCORE II) and the Society of Thoracic Surgeons (STS) score for in-hospital and medium-term mortality of patients undergoing coronary artery bypass grafting. Int J Gen Med. 2021;14:8509-19.
16. Argus L, Taylor M, Ouzounian M, Venkateswaran R, Grant SW. Risk prediction models for long-term survival after cardiac surgery: a systematic review. Thorac Cardiovasc Surg. 2024;72:29-39.
17. Khera R, Oikonomou EK, Nadkarni GN, et al. Transforming cardiovascular care with artificial intelligence: from discovery to practice: JACC state-of-the-art review. J Am Coll Cardiol. 2024;84:97-114.
18. Sulague RM, Beloy FJ, Medina JR, et al. Artificial intelligence in cardiac surgery: a systematic review. World J Surg. 2024;48:2073-89.
19. Amin A, Cardoso SA, Suyambu J, et al. Future of artificial intelligence in surgery: a narrative review. Cureus. 2024;16:e51631.
20. Hassan AM, Rajesh A, Asaad M, et al. Artificial intelligence and machine learning in prediction of surgical complications: current state, applications, and implications. Am Surg. 2023;89:25-30.
21. Kokkinakis S, Kritsotakis EI, Lasithiotakis K. Artificial intelligence in surgical risk prediction. J Clin Med. 2023;12:4016.
22. Bignami EG, Cozzani F, Del Rio P, Bellini V. The role of artificial intelligence in surgical patient perioperative management. Minerva Anestesiol. 2021;87:817-22.
23. Yoon HK, Yang HL, Jung CW, Lee HC. Artificial intelligence in perioperative medicine: a narrative review. Korean J Anesthesiol. 2022;75:202-15.
24. Kenig N, Monton Echeverria J, Muntaner Vives A. Artificial intelligence in surgery: a systematic review of use and validation. J Clin Med. 2024;13:7108.
25. Oei SP, Bakkes THGF, Mischi M, Bouwman RA, van Sloun RJG, Turco S. Artificial intelligence in clinical decision support and the prediction of adverse events. Front Digit Health. 2025;7:1403047.
26. Caballero D, Sánchez-Margallo JA, Pérez-Salazar MJ, Sánchez-Margallo FM. Applications of artificial intelligence in minimally invasive surgery training: a scoping review. Surgeries. 2025;6:7.
27. Arakaki S, Takenaka S, Sasaki K, et al. Artificial intelligence in minimally invasive surgery: current state and future challenges. JMA J. 2025;8:86-90.
28. Venkatesan M, Mohan H, Ryan JR, et al. Virtual and augmented reality for biomedical applications. Cell Rep Med. 2021;2:100348.
29. Riddle EW, Kewalramani D, Narayan M, Jones DB. Surgical simulation: virtual reality to artificial intelligence. Curr Probl Surg. 2024;61:101625.
30. Miles TJ, Ghanta RK. Machine learning in cardiac surgery: a narrative review. J Thorac Dis. 2024;16:2644-53.
31. Gadhachanda KR, Marsool Marsool MD, Bozorgi A, et al. Artificial intelligence in cardiovascular procedures: a bibliometric and visual analysis study. Ann Med Surg. 2025;87:2187-203.
32. Kankanamge D, Wijeweera C, Ong Z, et al. Artificial intelligence based assessment of minimally invasive surgical skills using standardised objective metrics - a narrative review. Am J Surg. 2025;241:116074.
33. Bellini V, Valente M, Bertorelli G, et al. Machine learning in perioperative medicine: a systematic review. J Anesth Analg Crit Care. 2022;2:2.
34. Leivaditis V, Beltsios E, Papatriantafyllou A, et al. Artificial intelligence in cardiac surgery: transforming outcomes and shaping the future. Clin Pract. 2025;15:17.
35. Theriault-Lauzier P, Suc G, Sengupta PP, et al. Artificial intelligence in valvular heart disease: current evidence and future perspectives. Eur Heart J Valvular Cardiol. 2025;1:xwaf002.
36. Graeßner M, Jungwirth B, Frank E, et al. Enabling personalized perioperative risk prediction by using a machine-learning model based on preoperative data. Sci Rep. 2023;13:7128.
37. Nashef SAM, Ali J. Artificial intelligence and cardiac surgery risk assessment. Eur J Cardiothorac Surg. 2023;63:ezad226.
38. Abdel Malek M, van Velzen M, Dahan A, et al. Generation of preoperative anaesthetic plans by ChatGPT-4.0: a mixed-method study. Br J Anaesth. 2025;134:1333-40.
39. Henckert D, Malorgio A, Schweiger G, et al. Attitudes of anesthesiologists toward artificial intelligence in anesthesia: a multicenter, mixed qualitative-quantitative study. J Clin Med. 2023;12:2096.
40. Brandenburg JM, Müller-Stich BP, Wagner M, van der Schaar M. Can surgeons trust AI? Perspectives on machine learning in surgery and the importance of eXplainable artificial intelligence (XAI). Langenbecks Arch Surg. 2025;410:53.
41. King A, Fowler GE, Macefield RC, et al. Use of artificial intelligence in the analysis of digital videos of invasive surgical procedures: scoping review. BJS Open. 2025;9:zraf073.
42. Pedrett R, Mascagni P, Beldi G, Padoy N, Lavanchy JL. Technical skill assessment in minimally invasive surgery using artificial intelligence: a systematic review. Surg Endosc. 2023;37:7412-24.
43. Guo C, He Y, Shi Z, Wang L. Artificial intelligence in surgical medicine: a brief review. Ann Med Surg. 2025;87:2180-6.
44. Arjomandi Rad A, Vardanyan R, Athanasiou T, Maessen J, Sardari Nia P. The ethical considerations of integrating artificial intelligence into surgery: a review. Interdiscip Cardiovasc Thorac Surg. 2025;40:ivae192.
45. Cross JL, Choma MA, Onofrey JA. Bias in medical AI: implications for clinical decision-making. PLOS Digit Health. 2024;3:e0000651.
46. Khanna NN, Maindarkar MA, Viswanathan V, et al. Economics of artificial intelligence in healthcare: diagnosis vs. treatment. Healthcare. 2022;10:2493.
47. De Simone B, Deeken G, Catena F. Balancing ethics and innovation: can artificial intelligence safely transform emergency surgery? A narrative perspective. J Clin Med. 2025;14:3111.
48. Fangerau H. Artifical intelligence in surgery: ethical considerations in the light of social trends in the perception of health and medicine. EFORT Open Rev. 2024;9:323-8.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Special Topic
Copyright
Data & Comments
Data
















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].