Digital vessel for the diagnosis of cardiovascular diseases
Abstract
Over the past few decades, advances in physiological monitoring, imaging technologies, and artificial intelligence (AI) have greatly improved the diagnosis and treatment of cardiovascular diseases. However, traditional diagnostic methods have limitations, particularly in dynamic assessment and personalized care. Digital Twin technology offers a solution by creating virtual replicas of physical entities, enabling precise disease prediction and personalized interventions. This review introduces the concept of the digital vessel, a patient-specific cardiovascular digital twin that integrates imaging data, physiological signals, and AI-driven simulations. We define its core framework, summarize advances in image analysis, computational modeling, and predictive AI, and compare digital vessel models with conventional diagnostic tools in terms of data input, individualization, feedback, and scalability. Finally, we outline key challenges, including data integration, computational cost, clinical validation, and regulatory issues, and propose future research directions. By consolidating current knowledge, this review positions the digital vessel as a pathway toward precision diagnosis and personalized cardiovascular care.
Keywords
INTRODUCTION
The blood vessels, as a crucial component of the human circulatory system, play a key role in transporting blood and maintaining the normal physiological functions of tissues and organs. Cardiovascular diseases (CVDs), such as coronary artery disease, cerebrovascular disease, and peripheral vascular disease, are highly prevalent and pose significant threats to human health and life[1]. CVDs are the leading causes of morbidity and mortality worldwide[2], accounting for 17.9 million deaths per year, approximately one-third of all deaths globally. Moreover, the number of patients with CVD is on the rise due to population aging and unhealthy lifestyles.
For many chronic conditions, including CVD, significant deterioration is often attributed to delayed detection, misdiagnosis, or the failure to implement optimal treatment strategies. This can lead to significant health and economic losses for patients. Therefore, early disease prediction and precision medicine are of paramount importance. Currently, diagnostic and therapeutic approaches to CVD rely heavily on conventional population-level models and static clinical assessments[3]. Common diagnostic modalities include vascular imaging techniques, such as computed tomography angiography (CTA)[4] and magnetic resonance angiography (MRA)[5], which visualize vascular structures and help assess the extent of disease. Additionally, cardiovascular risk assessment often depends on standard biomarkers[6] such as blood pressure, blood glucose, cholesterol levels, and electrocardiography (ECG) data.
However, these methods come with inherent limitations. Imaging techniques provide only static assessments and often lack dynamic insights into disease progression or treatment feedback. Furthermore, generalized models used in risk prediction may overlook individual variability and fail to offer personalized care. Modern medicine is transitioning from a wait and react, curative discipline to a preventative, interdisciplinary science[7]. As a result, it is necessary to develop more efficient, precise, and individualized approaches for the prevention, early diagnosis, and treatment of CVD. Among the emerging technologies poised to meet these demands, digital twin (DT) technology stands out as a particularly promising frontier.
DT[8] technology creates a virtual representation of a real-world entity[9] that is continuously synchronized with its physical counterpart[10]. Originating in the 1970s when the National Aeronautics and Space Administration (NASA) used simulators as “twins” during the Apollo 13 program[11,12], DT has since evolved into a comprehensive framework that integrates multi-physics, multiscale, and probabilistic modeling with real-time sensor and historical data[13]. By reflecting the full lifecycle of its physical counterpart and updating dynamically[14], DT provides strong predictive capabilities that are increasingly applied in healthcare, including cardiology[15-17], oncology[18], and diabetes treatment[19].
The digital vessel is defined as a high-fidelity, patient-specific vascular replica that integrates imaging, physiological monitoring, and computational simulations in CVD research. As a specific application of DT, the digital vessel overcomes the limitations of static, generalized diagnostic tools by enabling continuous simulation, individualized risk prediction, and personalized therapeutic planning.
Given the significant potential of the digital vessel to advance cardiovascular medicine, a comprehensive understanding of its underlying technologies and recent progress is essential. Recent advancements in artificial intelligence (AI), machine learning, and computational modeling have greatly enhanced the capabilities of DT systems, enabling real-time updates based on continuous patient data. The integration of multimodal data, such as imaging, genetic information, and wearable sensor data, has allowed for more accurate and dynamic representations of the cardiovascular system. Moreover, the shift from traditional imaging techniques to more data-driven approaches has paved the way for the development of personalized disease models that can adapt to changes in the patient’s condition over time. This has significant implications for improving the early detection, monitoring, and treatment of CVDs.
This review aims to synthesize the current landscape of this emerging field. We will present the core technological approaches for implementing digital vessel, highlight recent groundbreaking applications in CVD research, and discuss the primary challenges and future directions. By providing this overview, our paper seeks to serve as a valuable resource for researchers and clinicians interested in leveraging DT technology for precision cardiovascular care.
EVOLUTION OF RESEARCH METHODOLOGIES IN CVD
The central challenge in CVD research has always revolved around how to accurately delineate, diagnose, and ultimately predict its development from complex physiological phenomena. The evolution of research methodologies, from classical observation to advanced simulation, directly responds to this enduring challenge. Traditional research methods have laid an important foundation for understanding and treating disease from multiple perspectives. Common research methods include anatomical and histological studies, physiological research, animal experimentation, and imaging studies.
Anatomical and histological studies, through direct observation or microscopic examination of vascular and myocardial structures, have established reference standards for normal cardiovascular morphology and provided intuitive insights into the pathological basis of CVDs. Physiological research examines regulatory mechanisms such as ECG, heart rate, and blood pressure to understand how the cardiovascular system adapts to different conditions. Additionally, animal models, such as mice and zebrafish, are widely used to explore CVD mechanisms, but they face challenges in translating findings directly to human diagnosis and therapy. Dynamic monitoring methods, including exercise stress tests, echocardiography under load, and 24 h ambulatory blood pressure monitoring, provide more detailed assessments by capturing cardiovascular function during stress or over time. These methods offer a more comprehensive view compared to static measurements, though they remain fragmented and non-continuous, limiting their integration into real-time clinical decision making.
Despite their contributions, traditional approaches in cardiovascular research face notable shortcomings. Anatomical and histological studies are static, offering only static insights from fixed specimens and often failing to capture dynamic processes such as vascular remodeling or plaque progression. Physiological studies, while informative for macroscopic parameters such as blood pressure and heart rate, cannot reproduce the complex, multifactorial scenarios observed in clinical practice. Animal models provide valuable mechanistic insights but suffer from interspecies differences, high costs, and ethical challenges, limiting their translational relevance. Collectively, these constraints underscore the need for dynamic, patient-specific methodologies capable of reflecting the temporal and multifactorial nature of CVD [Table 1].
Summary of traditional cardiovascular research methods
| Method | Overview | Key contribution | Main limitations |
| Anatomy & Histology | Direct observation of organs and tissues, including gross anatomy and microscopic structure | Revealed macro- and micro-architecture; identified structural changes in disease[20,21] | Static, invasive[22,23] |
| Physiology (resting ECG, spot blood pressure, etc.) | Measurement of functional parameters such as heart rate, blood pressure, and cardiac output[24] | Simple, non-invasive assessment of cardiovascular function | Limited to discrete measurements; low resolution[25] |
| Animal models (mice[26], | Experimental systems mimicking human CVD mechanisms | Provided mechanistic insights; experimental testbeds | Interspecies differences, costly, ethical concerns |
| Dynamic assays (stress test, echo under load, 24 h blood pressure monitoring, etc.) | Functional tests performed under stress or over time | Evaluations performed at discrete visits or static imaging sessions | Still fragmented, not continuous |
| Medical imaging (CTA, MRA, DSA, etc.) | Visualization of vascular morphology and hemodynamics | High-resolution structural and functional information | Static only; heavy reliance on manual interpretation[28] |
Medical image processing plays a crucial role in cardiovascular research by providing high-resolution data on vascular morphology and structure. Techniques such as CTA, MRA, and digital subtraction angiography (DSA) each offer unique advantages. CTA uses X-rays to produce detailed cross-sectional images of the vascular system, helping to assess aneurysm size, location, and morphology, critical for surgical planning[29]. MRA, on the other hand, utilizes magnetic resonance principles to visualize vascular structures without the need for contrast agents, providing excellent soft-tissue contrast and aiding in the detection of early vascular pathologies such as atherosclerotic plaque[30]. DSA, which subtracts pre-contrast from post-contrast X-ray images, accentuates vascular morphology and blood flow dynamics, and is often used in real-time interventional procedures to guide operative maneuvers and ensure precision[31].
By virtue of their unique imaging principles and inherent advantages, these techniques play an indispensable role in the preliminary screening, precise diagnosis, treatment planning, and therapeutic efficacy evaluation of CVD. By complementing one another, these modalities significantly enhance the diagnostic and therapeutic capacity for CVD, thereby driving progress in the field of cardiovascular medicine. However, while these advanced imaging techniques provide unprecedented data, their sheer volume and complexity present a formidable challenge to manual interpretation, particularly in identifying subtle, early-stage pathologies. This context has set the stage for data-driven technologies to revolutionize medical data analysis.
To address the challenge of interpreting vast and complex imaging data, AI, particularly deep learning, has emerged as a transformative technology in cardiovascular medicine. Deep learning technology can autonomously learn discriminative feature representations from raw medical imaging and video data, thereby accurately identifying the dynamic patterns inherent within these datasets[32]. Compared with traditional methods, this technology does not rely on manually engineered features or cumbersome preprocessing pipelines. Its end-to-end learning capability confers significant advantages in medical image analysis, enabling efficient and accurate interpretation of complex imaging information and providing reliable support for clinical diagnosis. Cross-disciplinary integration of AI and biomedical sciences has significantly advanced CVD research, with an ever-growing number of AI models being developed to address diverse cardiovascular pathologies.
A prominent example involves the application of AI to cardiac magnetic resonance imaging (MRI) for screening and diagnosis of CVD, where researchers innovatively developed a two-stage AI model based on the Video Swin Transformer (VST)[33]. This model integrates cine MRI and late gadolinium enhancement (LGE)[34] datasets to achieve automated screening and high-precision diagnosis of CVD. By combining advanced deep learning architectures with multimodal imaging data, researchers have demonstrated that AI systems can not only match but even exceed the diagnostic performance of experienced clinicians. These approaches dramatically accelerate image interpretation, reduce manual workload, and enable the detection of subtle features such as early fibrosis or remodeling that are often missed by conventional analysis. Such results illustrate the broader potential of AI-augmented imaging to provide scalable, precise, and efficient tools for clinical cardiovascular care.
While AI excels at enhancing the analysis of existing data, its primary function is often reactive - identifying existing pathologies with remarkable accuracy. A more ambitious frontier seeks to move from this reactive analysis toward proactive, predictive simulation by creating holistic, dynamic models of an individual’s physiology. This has led to the emergence of the DT paradigm. Originating from industrial sectors where it is used to model and manage complex systems[35], the core idea of a DT is to create a
In healthcare, this paradigm has been adapted into the Medical Digital Twin (MDT), which is emerging as a critically important research frontier for enabling precision medicine. An MDT serves as a virtual representation of a patient, integrating multimodal data - from the genomic level to organ-wide dynamics - to simulate biological processes and forecast disease trajectories. Whereas standalone AI models are often trained for specific analytical tasks, an MDT aims to create a comprehensive, systems-level model that integrates these AI components into a broader, dynamic framework for personalized prediction and therapeutic planning[37-39].
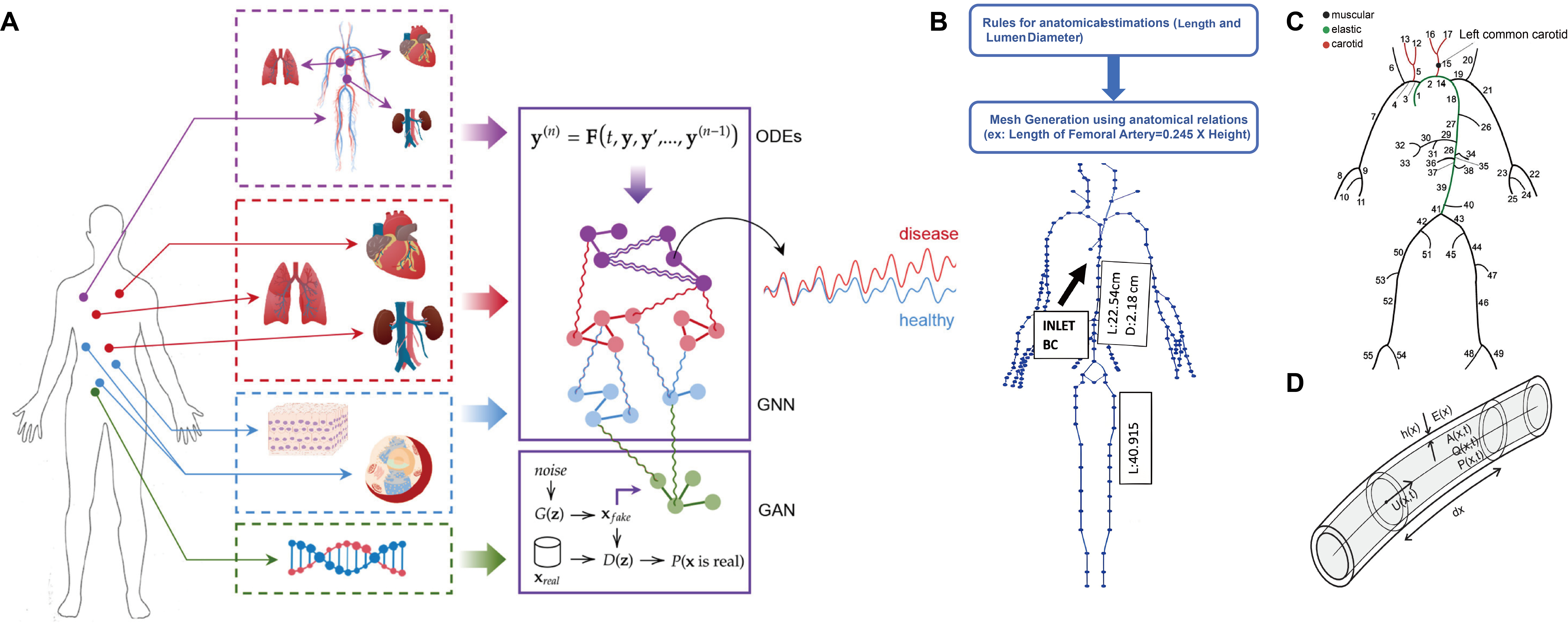
In the field of CVD research, DT has been realized in pioneering frameworks. For example, Barbiero et al.[7] introduced a pioneering DT framework. It utilizes graph representation learning and generative adversarial networks (GANs) to model multiscale physiological systems, with significant implications for CVD research. The study aims to address the challenge of integrating heterogeneous biological data from genomic to organ-level dynamics by constructing a DT for patients. The framework combines a Graph Neural Network (GNN) to forecast clinical endpoints (e.g., blood pressure) and a GAN to simulate transcriptomic crosstalk across tissues, enabling holistic modeling of CVD pathophysiology. The architecture of this framework, as depicted in Figure 1A, integrates a generative model with a graph-based network to achieve this holistic simulation.
Figure 1. From medical DTs to digital vessel. (A) Architecture of an integrated DT framework. This model combines data-driven and mechanistic approaches for multiscale physiological simulation. A Graph Neural Network (GNN) predicts organ-level hemodynamics, while a Generative Adversarial Network (GAN) simulates molecular-level transcriptomic crosstalk. These are integrated with Ordinary Differential Equations (ODEs) to capture system dynamics, enabling a holistic patient model[7]; (B) A conceptual workflow for generating a virtual patient database from clinical data using a one-dimensional hemodynamic model[42]; (C) A detailed 55-branch arterial network representing the geometric foundation of such a model[43]; (D) The core biophysical rule, which calculates vessel stiffness from diameter and age[43]. This figure is reproduced from Refs[7,42,43] under a Creative Commons Attribution 4.0 International (CC BY 4.0) license. DT: Digital twin.
For cardiovascular applications, DT frameworks specifically employ GNNs to represent the vascular system as a topological graph, where vascular segments and heart chambers are modeled as interconnected nodes. This approach enables dynamic prediction of blood pressure variability, achieving an accuracy of about 92% in simulated cases, thereby illustrating how conditions such as hypertension may propagate through the vascular network. In addition, GANs can address one of the key limitations in cardiovascular research-data scarcity-by generating synthetic datasets to support model training and improve robustness. Together, these methods highlight the potential of DTs to capture complex vascular dynamics and extend predictive modeling to scenarios where clinical data are limited.
In clinical validation, DT frameworks have been tested in patient-specific scenarios, such as hypertension and vascular complications. These models demonstrated the ability to simulate treatment responses and optimize therapeutic strategies, for example, by predicting how interventions could improve vascular compliance or mitigate risks of thrombosis. Such applications illustrate the potential of DTs to serve as virtual testbeds for personalized treatment planning, enabling therapies to be evaluated safely and efficiently before clinical implementation[40].
A representative clinical example of how digital vessel models can guide decision making is their application in coronary artery disease. Traditionally, when coronary stenosis is detected on CTA, clinicians must decide whether the narrowing is functionally significant enough to cause myocardial ischemia. This usually requires invasive catheter-based measurement of fractional flow reserve (FFR). By contrast, a digital vessel model can integrate patient-specific coronary CTA data with computational fluid dynamics (CFD) to create a virtual coronary circulation. Through this simulation, it is possible to calculate Computed Tomography (CT)-derived FFR
In practice, this diagnostic capability directly influences treatment decisions. If the simulated digital vessel shows that a stenosis does not reduce blood flow below the ischemic threshold, the patient can be managed conservatively with medication, avoiding unnecessary intervention. Conversely, if the model predicts impaired perfusion, clinicians can use the digital vessel to virtually plan a percutaneous coronary intervention - testing different stent positions and sizes in silico before performing the procedure. This not only reduces the risks of inappropriate stenting but also optimizes procedural strategy.
Such an approach illustrates how digital vessel models extend beyond static anatomical imaging: they provide functional, patient-specific insights that help clinicians decide whether to intervene, how to intervene, and what outcome to expect. This case exemplifies the potential of digital vessel to support precision cardiology by linking diagnostic imaging with personalized therapeutic planning.
Beyond integrating complex, multiscale data, another fundamental challenge in constructing physiological DTs is how to infer hard-to-measure internal states from easily accessible, non-invasive data. Addressing this, the work by Chakshu et al. (2021)[42] offers a pioneering solution for the cardiovascular system, providing a proof of concept for constructing active cardiovascular DTs through a deep learning-based inverse analysis framework. Their two-stage methodology first employs a Long Short-Term Memory (LSTM) network. This network is trained to accurately reverse engineer core vessel pressure waveforms (e.g., in the aorta) using only non-invasive blood pressure measurements from three peripheral sites: the carotid, brachial, and femoral arteries. A key innovation of this study is the creation of a large-scale virtual patient database to train the deep learning model. As shown in Figure 1B, the database uses a one-dimensional (1D) hemodynamic model to convert an individual’s basic information (such as age, height, and weight) and clinical indicators (such as mean arterial pressure and medical history) into personalized vascular geometry, boundary conditions, and material properties. This process generates thousands of highly realistic virtual cardiovascular system samples. A concrete illustration of the components within such a 1D hemodynamic model is provided by the work of Suriani et al. (2021)[43]. Their framework is built upon a detailed, 55-branch anatomical template of the arterial tree [Figure 1C], which is then parameterized with age-specific biophysical rules governing vessel stiffness and diameter [Figure 1D]. This approach enables the generation of a realistic virtual population ideal for studying age-related pathologies.
In the second stage, Suriani et al. (2021)[43] input the aortic pressure waveforms predicted through inverse analysis into a Convolutional Neural Network (CNN) and successfully achieved automatic detection and severity grading of abdominal aortic aneurysm (AAA). The accuracy for AAA detection reached 99.91%, while that for severity classification was 97.79%. The significance of this work lies in its clear demonstration of a technical pathway: how to reconstruct a high-fidelity, personalized internal physiological model from sparse,
Taken together, these two pioneering works highlight complementary pathways for building cardiovascular DTs. The framework from Barbiero et al.[7] addresses the “top-down” challenge of integrating heterogeneous, multiscale biological data, while the approach by Chakshu et al.[42] solves the critical “outside-in” problem of inferring internal physiological states from sparse, non-invasive measurements. The convergence of these approaches-integrating systemic biological models with real-time, non-invasive data streams-paves the way for a holistic DT capable of transforming personalized prediction and treatment in cardiovascular medicine.
THE ARCHITECTURE OF DIGITAL VESSEL
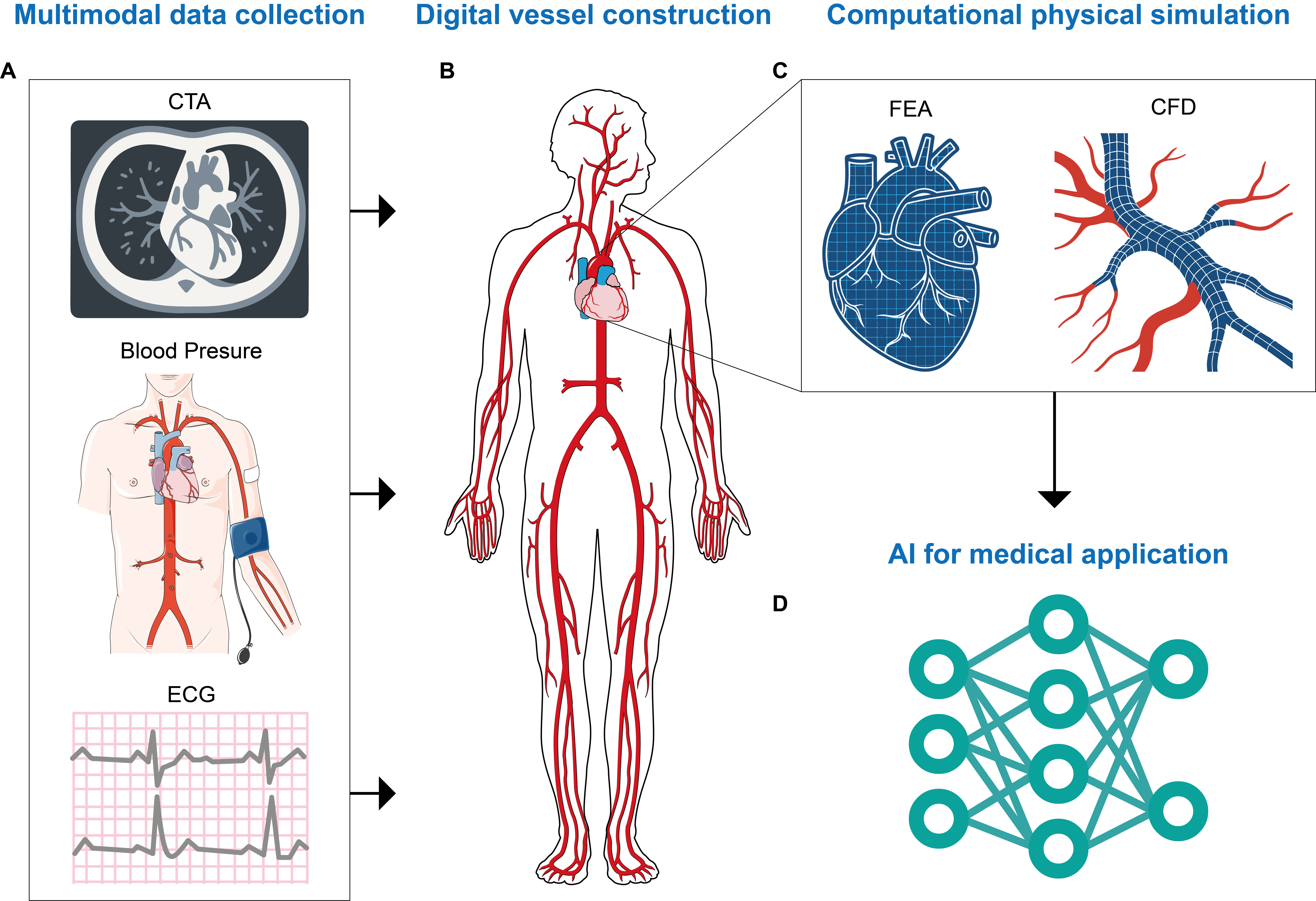
To provide readers with a comprehensive understanding of how DT technology is applied in CVD research, this review systematically presents the framework for constructing a digital vascular model. The entire process of the digital vessel framework is illustrated in Figure 2.
Figure 2. The framework of an AI-powered digital vessel. (A) The process begins with multimodal data collection, including medical imaging data (e.g., CTA) and physiological data (e.g., blood pressure, ECG); (B) Construct a digital vessel using the multimodal data; (C) Build up their computational physics static models for FEA and CFD simulations; (D) Finally, an AI Engine integrates these models for downstream applications. CTA: Computed tomography angiography; ECG: electrocardiography; FEA: finite element analysis; CFD: computational fluid dynamics; AI: artificial intelligence. Elements were manually prepared or modified from iconfont.cn and smart.servier.com.
Table 2 provides a structured comparison between conventional cardiovascular diagnostics (such as CTA, MRA, ECG, and population-based risk scores) and emerging digital vessel models. The comparison spans six dimensions, including data inputs, level of individualization, representation of temporal dynamics, predictive capability, feedback mechanisms, and clinical scalability. As shown, digital vessel models, grounded in AI-enabled DT methodologies, address key limitations of traditional approaches by enabling dynamic, patient-specific, and predictive simulations.
Comparison of traditional CVD diagnostic tools and digital vessel models
| Dimension | Traditional CVD diagnostic tools | Digital vessel models |
| Data input | Single-modality and static data sources such as ECG, spot blood pressure, lipid biomarkers, or static imaging sources[1] | Multimodal and longitudinal integration of imaging, physiological monitoring, biochemical markers, wearable sensor data, and electronic health records[17] |
| Individualization | Population-based reference ranges and risk scores; limited ability to reflect patient-specific anatomy or physiology[21] | Patient-specific virtual replicas that incorporate anatomy, hemodynamics, clinical history, and molecular data for tailored assessment[14] |
| Temporal dynamics | Static assessments[20] | Continuous updating with real-time or longitudinal data[35] |
| Predictive ability | Retrospective; describes current pathology[6] | Predictive; simulates disease progression and treatment response[37] |
| Real-time feedback | Limited; delayed updates after new tests[4] | Real-time monitoring and adaptive modeling[42] |
| Clinical scalability | Manual interpretation, time-consuming, and resource-intensive; scalability limited across large populations[5] | AI-driven automation, cloud-based simulations, and federated learning frameworks enable scalable applications across institutions and potential use in in silico clinical trials[44] |
Data collection
Constructing a vascular DT requires gathering multidimensional, multimodal data [Figure 2A]. These data are drawn from a broad array of medical devices and clinical records, providing a rich informational foundation for precise vascular modeling. Medical imaging data capture the geometric morphology and spatial orientation of blood vessels, and these datasets can later be used to construct the geometric model of the virtual digital vasculature. The advancement of wearable devices has opened new avenues for data acquisition in vascular DT applications. Wearable devices, such as smartbands and smartwatches, facilitate real-time monitoring of physiological parameters, including heart rate, blood pressure, and blood oxygen saturation.
Clinical records, containing detailed information on a patient’s medical history, symptomatology, diagnostic results, and treatment processes, are an essential data source for constructing vascular DT. A patient’s medical history, including chronic conditions such as hypertension, diabetes mellitus, and hyperlipidemia, is closely associated with the onset and progression of vascular diseases. Patients with chronic hypertension are exposed to prolonged elevated blood pressure, which can damage the vascular wall and significantly increase the risk of developing atherosclerosis[45,46]. Patients with diabetes are prone to vascular complications due to abnormal glucose metabolism[47,48]. By analyzing information from clinical records, it becomes possible to identify risk factors associated with vascular diseases. This comprehensive background knowledge supports the construction of accurate and personalized vascular DT models.
Data preprocessing
Raw data collected from various sources often contain noise, missing values, and outliers, and they vary in format and dimensionality. To ensure data quality and usability, a series of preprocessing steps is required. These operations lay a solid foundation for the subsequent modeling of vascular DT [Figure 2B].
Data cleaning effectively removes noise and erroneous information, while also correcting inconsistencies within the dataset. In medical imaging data, noise may arise due to limitations of imaging equipment, patient movement, and environmental interference. Common types of noise include speckle noise and Gaussian noise. Such noise can degrade image clarity and hinder the extraction of vascular features, making it necessary to apply filtering algorithms for effective denoising. Common filtering algorithms include mean filtering, median filtering, and Gaussian filtering. Feature extraction can extract key information representing vascular features from raw data. In medical imaging data, commonly used feature extraction methods include edge detection, region segmentation, and morphological analysis. Edge detection algorithms, such as the Canny and Sobel operators, can identify the boundaries of blood vessels, allowing for accurate delineation of vascular contours. Region segmentation algorithms-such as thresholding, Watershed Algorithm[49], and machine learning-based methods-are capable of isolating blood vessels from surrounding tissues.
To improve segmentation accuracy, many recent studies[50-54] have utilized deep learning methods such as U-Net (a U-shaped Convolutional Neural Network for biomedical image segmentation) and Mask region-based convolutional neural network (R-CNN). These models train neural networks to distinguish blood vessels from surrounding tissues, enabling the precise and efficient extraction of vascular geometric boundaries. Following image segmentation, the geometric information of the blood vessels is transformed into a digital dataset that represents their boundaries and morphology. Morphological analysis techniques-such as erosion, dilation, opening, and closing-can be applied to segmented vascular images for further refinement. These operations help remove residual noise, fill gaps, and smooth boundaries, which enhances the clarity and accuracy of vascular morphological features. In physiological parameter data, it is essential to extract features that reflect vascular function and hemodynamic changes. Key indicators include heart rate variability (HRV), blood pressure fluctuation amplitude, and trends in blood flow velocity. HRV refers to the subtle differences between successive cardiac cycles. Analyzing HRV allows for the assessment of autonomic regulation within the cardiovascular system[55]. Blood pressure fluctuation amplitude reflects the variation in blood pressure over a given period. Excessive fluctuations may be associated with an increased risk of developing CVD. Trends in blood flow velocity can indicate the patency of blood vessels and provide insights into the hemodynamic status of the circulatory system[56]. Dimensionality reduction involves compressing and transforming high-dimensional data while minimizing information loss. This process reduces data complexity and computational load, facilitating more efficient data processing.
Virtual geometric model of blood vessels
The virtual geometric model serves as the structural foundation of the digital vessel [Figure 2C]. The construction process begins with high-quality data derived from medical imaging [e.g., computed tomography (CT), MRI][57,58], which has undergone the preprocessing steps described previously (such as segmentation and feature extraction). This refined digital dataset, representing the vessel’s boundaries and morphology, is then used to generate a three-dimensional (3D) geometric representation. For instance, one study[59] has demonstrated the use of deep learning to reconstruct 3D coronary artery trees from two asynchronous X-ray projections. Further refinement can be achieved using 3D modeling software such as Blender[60], Autodesk Maya, and 3ds Max. These tools allow for manual or semi-automated adjustments to the vessel structure. Moreover, immersive technologies such as Virtual Reality (VR) and Augmented Reality (AR) are enhancing this process by transforming how these models are visualized and manipulated[61]. These platforms enable clinicians and engineers to interact with the geometric model in a true 3D space, moving beyond the limitations of a two-dimensional (2D) screen. This intuitive, hands-on interaction is not only crucial for refining the model’s anatomical accuracy but also serves as a powerful tool for pre-operative surgical planning and collaborative review, ensuring the final virtual vessel closely approximates the true form and function of its physical counterpart.
Blood flow simulation and fluid dynamics analysis
Blood flow simulation and fluid dynamics analysis are core modules within the digital vessel, enabling precise analysis of the blood flow conditions through numerical modeling techniques. This simulation system is used not only to study hemodynamic processes but also to predict the underlying mechanisms of vascular diseases such as arteriosclerosis and thrombosis. CFD, as a numerical simulation technique for fluid flow analysis, can quantify the movement patterns of blood within blood vessels in hemodynamic studies[62]. Finite Element Analysis (FEA) simulates the deformation and stress analysis of blood vessels, calculating their response under various blood flow conditions[63]. The collaborative, coupled modeling of CFD and FEA is particularly powerful, as it enables the simultaneous consideration of both hemodynamics and the mechanical response of the vessel wall[64], accurately characterizing the interaction between blood flow and the vessel wall, thereby providing support for vascular health assessment under various physiological and pathological conditions.
AI-driven predictive engine
Following the foundational construction of vascular geometric and computational models, the digital vessel framework culminates in its most dynamic and clinically significant component: an AI-driven predictive engine [Figure 2D]. This engine is designed to forecast vascular changes and disease progression by intelligently synthesizing a rich tapestry of multimodal patient data. The process begins with the sophisticated integration of heterogeneous data streams, which include static structural information from medical imaging such as CTA and MRA, dynamic physiological parameters such as real-time blood pressure and flow velocity, critical biochemical markers including lipid and glucose levels, and the patient’s longitudinal clinical history. To unlock their synergistic potential, advanced data fusion strategies are employed, ranging from early fusion, where raw features are concatenated, to late fusion, which combines the outputs of multiple models to form a holistic patient profile.
The architectural backbone of this predictive module is a multi-layered, hybrid model hierarchy tailored to the complexity of cardiovascular analysis. The 3D CNNs are leveraged for their power in volumetric image analysis, enabling the precise, automated quantification of structural abnormalities such as stenosis severity and plaque composition. Concurrently, LSTM networks process time-series data, capturing the temporal dependencies in physiological signals to model the dynamic behavior of the vascular system. Critically, Physics-Informed Neural Networks (PINNs) are integrated to bridge the gap between pure data-driven approaches and biophysical reality. By embedding the governing equations of fluid dynamics as soft constraints during training, PINNs ensure that the predicted hemodynamic parameters-such as Wall Shear Stress (WSS) and blood flow patterns-are not only accurate but also physically plausible.
Beyond primary predictions, the framework extends to advanced risk analysis using specialized models such as GNNs. These models treat the vascular network as an interconnected graph, making them exceptionally suited for analyzing systemic phenomena such as thrombosis risk and complex blood rheology[65], which depend on interactions across different vessel segments. To ensure these sophisticated models are robust, accurate, and generalizable for clinical application, rigorous training and validation protocols are essential. Methodologies include data augmentation to enrich sparse medical datasets, the implementation of
Ultimately, this AI-powered predictive module transforms the digital vessel from a descriptive replica into a proactive, intelligent system. It provides the data-driven foundation for enhancing early diagnosis, optimizing personalized treatment strategies, and delivering precise prognostic assessments, thereby fully realizing the potential of DT technology in cardiovascular medicine.
CHALLENGES, LIMITATIONS, AND FUTURE DIRECTIONS
While the concept of the digital vessel heralds a new era in cardiovascular medicine, its transition from a promising technological framework to a cornerstone of clinical practice hinges on overcoming several formidable challenges.
The journey toward creating these high-fidelity virtual counterparts begins with the very data that fuel them. A primary bottleneck lies in integrating the multidimensional, multimodal data previously described. The inherent heterogeneity of data formats, disparate temporal resolutions (e.g., a yearly CTA scan versus second-by-second wearable data), and inconsistent quality collectively present a significant data fusion challenge. Addressing this will require sophisticated AI-driven algorithms capable of harmonizing diverse data streams. Furthermore, as these DTs become increasingly personalized, ensuring robust data security and patient privacy is not merely a technical hurdle but an ethical imperative. To this end, advanced privacy-preserving techniques are emerging as critical solutions. Federated learning, for instance, allows models to be trained on decentralized data without compromising sensitive information, representing a promising path forward for digital health[44]. Ultimately, establishing robust data governance frameworks that ensure interoperability and ethical use remains a foundational prerequisite for progress.
Beyond the challenge of data acquisition lies the immense computational demand inherent in high-fidelity vascular modeling. The blood flow simulations and fluid dynamics analyses, powered by techniques such as CFD and FEA, are computationally intensive; a single detailed simulation can often take hours or even days. This latency currently precludes the real-time feedback required for urgent clinical decision making. Overcoming this computational bottleneck is a critical step, with future solutions pointing decisively towards a synergy between AI and high-performance computing. AI-driven surrogate models-data-driven emulators trained to approximate complex physical simulations-promise to replicate hemodynamic behaviors with high fidelity but at a fraction of the computational cost, making real-time analysis feasible. Innovations in this area, such as PINNs, are providing powerful deep learning frameworks for solving these complex problems[67]. The validation of these virtual models, however, remains the ultimate test of their utility. It is no longer sufficient to demonstrate mere correlation; the goal must be to establish causality. Future research must focus on rigorous validation against real-world patient outcomes through prospective in silico clinical trials, which are increasingly recognized as a credible pathway for evaluating new medical devices and therapies[68]. This process is essential to establish that a digital vessel’s predictions are causally linked to, and can improve upon, in vivo results.
Ultimately, the greatest hurdle may be the seamless translation of this technology into the clinical environment. For the digital vessel to be truly transformative, it must evolve from a complex research tool into an intuitive and indispensable asset for clinicians. A significant barrier to trust and adoption is the “black box” nature of many advanced AI models. Therefore, the development of explainable AI is paramount, enabling models to provide transparent, interpretable rationales for their predictions. This explainability is not only crucial for building clinical confidence but is also a key component in what has been termed “high-performance medicine”-the convergence of human and AI[69]. Furthermore, navigating the complex regulatory pathways of bodies such as the Food and Drug Administration (FDA) or the European Medicines Agency (EMA) presents a unique challenge. Traditional frameworks were designed for static medical devices, not for adaptive, learning AI systems. A new regulatory paradigm is required to address algorithms that continuously learn and evolve, ensuring their safety and efficacy throughout their entire lifecycle. This pressing need has already prompted new proposals from regulatory authorities.
Taken together, these issues highlight the current limitations of digital vessel. Key barriers include the difficulty of multimodal data integration, high computational costs that preclude real-time application, limited large-scale clinical validation, the opacity of many AI-driven models, privacy and ethical concerns associated with sensitive patient data, and the lack of standardized regulatory pathways. These factors collectively constrain the transition of digital vessel from research prototypes to routine clinical tools.
Addressing these limitations will be essential for the maturation of digital vessel technology. Priority areas include developing robust data fusion frameworks to integrate multimodal information, creating AI-driven surrogate models to reduce computational cost and enable real-time simulation, and conducting large-scale prospective validation studies to demonstrate clinical utility. Enhancing model interpretability through explainable AI will help build clinician trust, while implementing privacy-preserving strategies such as federated learning will safeguard patient data. Finally, the establishment of regulatory standards for adaptive, AI-enabled systems and the integration of environmental and behavioral data into digital vessel frameworks may further expand their utility in predictive and preventive cardiology.
While the path is complex, a convergence of advanced computing, privacy-preserving AI, and a deepening understanding of regulatory science is setting the stage for the digital vessel to fulfill its promise. By systematically addressing these challenges, the digital vessel will transition from a technological possibility to a clinical reality, fundamentally shifting cardiovascular care from a reactive discipline to a truly predictive, preventative, and personalized paradigm.
CONCLUSION
As scientific and technological paradigms evolve, so too do the research methodologies applied to CVD. DT technology, through its closed-loop framework of virtual-physical mapping, dynamic optimization, and intelligent decision making, is reshaping the diagnostic and therapeutic logic for vascular conditions. Its core advantages are not limited to the technical merits of non-invasiveness and precision; more profoundly, it propels a transformation of the medical model toward one that is predictive and personalized, offering an innovative and feasible pathway to address the global burden of CVD.
Although the development of digital vessel has shown significant promise, the path to widespread clinical adoption requires a concerted, multidisciplinary effort. Success does not hinge on solving any single challenge in isolation but on the synergistic integration of solutions. The immense computational demands, the need for standardized data fusion, and the persistent ethical and regulatory gaps are not merely obstacles but the very frontiers that define the field’s future direction. Looking ahead, the evolution of the digital vessel will be driven by targeted innovations. AI-driven surrogate models will be key to overcoming computational barriers, while the maturation of explainable AI and new regulatory frameworks will build the clinical and ethical trust necessary for translation.
Moreover, the interplay between vascular health and human movement represents a promising frontier for future research. Capturing whole-body biomechanics and physical activity patterns, from advanced animal models to high-resolution 3D human motion reconstruction[70,71], can complement digital vessel frameworks by providing critical insights into hemodynamic adaptation and vascular remodeling under different behavioral contexts[72]. By integrating digital behavior models with digital vascular models, it may become possible to non-invasively infer vascular health status through behavioral signatures alone, opening avenues for early detection and risk stratification without direct vascular imaging. This cross-domain linkage between digital behavior and digital vessel modeling stands to deepen our understanding of
Beyond these advancements, the digital vessel also provides a concrete implementation pathway for homeostasis medicine[73], a systems-oriented medical paradigm that targets the dynamic balance of internal physiological states as the primary goal of intervention. Rooted in the foundational biological principle of homeostasis, this approach shifts the clinical focus from episodic treatment of disease symptoms to the continuous preservation and restoration of system-level equilibrium[74,75]. By leveraging real-time digital models of vascular function, the digital vessel can detect subtle deviations from individual baselines, enabling early interventions that preserve physiological integrity. This paradigm aligns closely with the vision of predictive, preventive, and personalized medicine, and positions homeostasis not only as a biological concept but as a central computational and clinical objective. It is this convergence of technological power and homeostatic insight that will ultimately enable the digital vessel to move from a research concept to a clinical reality, making a tangible impact on human health.
DECLARATIONS
Acknowledgments
We acknowledge the resources from the iconfont.cn and smart.servier.com websites for the creation of the Figures in this paper.
Authors’ contributions
Conceptualization and Design: Han Y, Zhang W, Wei P
Writing-original draft preparation: Zhang W, Lin H, Han Y
Writing-review and editing: Zhang W, Lin H, Luan M, Han Y
All authors have read and agreed to the published version of the manuscript.
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Yeates K, Lohfeld L, Sleeth J, Morales F, Rajkotia Y, Ogedegbe O. A Global perspective on cardiovascular disease in vulnerable populations. Can J Cardiol. 2015;31:1081-93.
2. Spînu M, Onea LH, Homorodean C, Olinic M, Ober MC, Olinic DM. Optical coherence tomography-OCT for characterization of non-atherosclerotic coronary lesions in acute coronary syndromes. J Clin Med. 2022;11:265.
3. Sun X, Yin Y, Yang Q, Huo T. Artificial intelligence in cardiovascular diseases: diagnostic and therapeutic perspectives. Eur J Med Res. 2023;28:242.
4. Szczykutowicz TP. Computed tomography angiography: principles and advances. Radiol Clin North Am. 2024;62:371-83.
5. Kocaoglu M, Pednekar A, Fleck RJ, Dillman JR. Cardiothoracic magnetic resonance angiography. Curr Probl Diagn Radiol. 2024;53:154-65.
6. Upadhyay RK. Emerging risk biomarkers in cardiovascular diseases and disorders. J Lipids. 2015;2015:971453.
7. Barbiero P, Viñas Torné R, Lió P. Graph representation forecasting of patient’s medical conditions: toward a digital twin. Front Genet. 2021;12:652907.
8. Yao JF, Yang Y, Wang XC, Zhang XP. Systematic review of digital twin technology and applications. Vis Comput Ind Biomed Art. 2023;6:10.
9. Emmert-Streib F, Yli-Harja O. What is a digital twin? Experimental design for a data-centric machine learning perspective in health. Int J Mol Sci. 2022;23:13149.
10. Moztarzadeh O, Jamshidi MB, Sargolzaei S, et al. Metaverse and healthcare: machine learning-enabled digital twins of cancer. Bioengineering. 2023;10:455.
11. Barricelli BR, Casiraghi E, Fogli D. A survey on digital twin: definitions, characteristics, applications, and design implications. IEEE Access. 2019;7:167653-71.
12. Liu M, Fang S, Dong H, Xu C. Review of digital twin about concepts, technologies, and industrial applications. J Manuf Syst. 2021;58:346-61.
13. Emmert-streib F. Defining a digital twin: a data science-based unification. Mach Learn Knowl Extr. 2023;5:1036-54.
14. Sel K, Osman D, Zare F, et al. Building digital twins for cardiovascular health: from principles to clinical impact. J Am Heart Assoc. 2024;13:e031981.
15. Niederer SA, Sacks MS, Girolami M, Willcox K. Scaling digital twins from the artisanal to the industrial. Nat Comput Sci. 2021;1:313-20.
16. Bruynseels K, Santoni de Sio F, van den Hoven J. Digital twins in health care: ethical implications of an emerging engineering paradigm. Front Genet. 2018;9:31.
17. Corral-Acero J, Margara F, Marciniak M, et al. The ‘Digital Twin’ to enable the vision of precision cardiology. Eur Heart J. 2020;41:4556-64.
18. Hormuth DA 2nd, Jarrett AM, Lorenzo G, et al. Math, magnets, and medicine: enabling personalized oncology. Expert Rev Precis Med Drug Dev. 2021;6:79-81.
19. Shamanna P, Saboo B, Damodharan S, et al. Reducing HbA1c in Type 2 diabetes using digital twin technology-enabled precision nutrition: a retrospective analysis. Diabetes Ther. 2020;11:2703-14.
20. Oikonomou E, Theofilis P, Lampsas S, et al. Current concepts and future applications of non-invasive functional and anatomical evaluation of coronary artery disease. Life. 2022;12:1803.
21. Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317-25.
22. Dong Y, Ma G, Hou X, et al. Kindlin-2 controls angiogenesis through modulating Notch1 signaling. Cell Mol Life Sci. 2023;80:223.
23. Hou X, Ren C, Jin J, et al. Phosphoinositide signalling in cell motility and adhesion. Nat Cell Biol. 2025;27:736-48.
24. Benjamim CJR, Monteiro LRL, Pontes YMM, et al. Caffeine slows heart rate autonomic recovery following strength exercise in healthy subjects. Rev Port Cardiol. 2021;40:399-406.
25. Hunter PJ, Borg TK. Integration from proteins to organs: the Physiome Project. Nat Rev Mol Cell Biol. 2003;4:237-43.
26. Zadelaar S, Kleemann R, Verschuren L, et al. Mouse models for atherosclerosis and pharmaceutical modifiers. Arterioscler Thromb Vasc Biol. 2007;27:1706-21.
27. Bowley G, Kugler E, Wilkinson R, et al. Zebrafish as a tractable model of human cardiovascular disease. Br J Pharmacol. 2022;179:900-17.
28. Taylor CA, Figueroa CA. Patient-specific modeling of cardiovascular mechanics. Annu Rev Biomed Eng. 2009;11:109-34.
29. Members WG, Hiratzka LF, Bakris GL, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: a report of the American college of cardiology foundation/American heart association task force on practice guidelines, American association for thoracic surgery, American college of radiology, American stroke association, society of cardiovascular anesthesiologists, society for cardiovascular angiography and interventions, society of interventional radiology, society of thoracic surgeons, and society for vascular medicine. Circulation. 2010;121:e266-369.
30. Yuan C, Mitsumori LM, Ferguson MS, et al. In vivo accuracy of multispectral magnetic resonance imaging for identifying lipid-rich necrotic cores and intraplaque hemorrhage in advanced human carotid plaques. Circulation. 2001;104:2051-6.
31. Mosquera-Lopez C, Jacobs PG. Digital twins and artificial intelligence in metabolic disease research. Trends Endocrinol Metab. 2024;35:549-57.
33. Wang YJ, Yang K, Wen Y, et al. Screening and diagnosis of cardiovascular disease using artificial intelligence-enabled cardiac magnetic resonance imaging. Nat Med. 2024;30:1471-80.
34. Yu X, Chen J, Fang B, Wang W, Zhang LB, Lv Z. Cardiac LGE MRI segmentation with cross-modality image augmentation and improved U-Net. IEEE J Biomed Health Inform. 2023;27:588-97.
35. Thangaraj PM, Benson SH, Oikonomou EK, Asselbergs FW, Khera R. Cardiovascular care with digital twin technology in the era of generative artificial intelligence. Eur Heart J. 2024;45:4808-21.
36. Ali WA, Fanti MP, Roccotelli M, Ranieri L. A review of digital twin technology for electric and autonomous vehicles. Applied Sciences. 2023;13:5871.
37. Vallée A. Envisioning the future of personalized medicine: role and realities of digital twins. J Med Internet Res. 2024;26:e50204.
38. Laubenbacher R, Mehrad B, Shmulevich I, Trayanova N. Digital twins in medicine. Nat Comput Sci. 2024;4:184-91.
39. Kamel Boulos MN, Zhang P. Digital twins: from personalised medicine to precision public health. J Pers Med. 2021;11:745.
40. Vallée A. Digital twins for personalized medicine require epidemiological data and mathematical modeling: viewpoint. J Med Internet Res. 2025;27:e72411.
41. Guo B, Jiang M, Guo X, et al. Diagnostic and prognostic performance of artificial intelligence-based fully-automated on-site CT-FFR in patients with CAD. Sci Bull. 2024;69:1472-85.
42. Chakshu NK, Sazonov I, Nithiarasu P. Towards enabling a cardiovascular digital twin for human systemic circulation using inverse analysis. Biomech Model Mechanobiol. 2021;20:449-65.
43. Suriani I, Bouwman RA, Mischi M, Lau KD. An in silico study of the effects of cardiovascular aging on carotid flow waveforms and indexes in a virtual population. Am J Physiol Heart Circ Physiol. 2024;326:H877-99.
44. Rieke N, Hancox J, Li W, et al. The future of digital health with federated learning. NPJ Digit Med. 2020;3:119.
45. Boutouyrie P, Chowienczyk P, Humphrey JD, Mitchell GF. Arterial stiffness and cardiovascular risk in hypertension. Circ Res. 2021;128:864-86.
46. Wu S, Tian X, Chen S, et al. Arterial stiffness and blood pressure in treated hypertension: a longitudinal study. J Hypertens. 2023;41:768-74.
47. Islam K, Islam R, Nguyen I, et al. Diabetes mellitus and associated vascular disease: pathogenesis, complications, and evolving treatments. Adv Ther. 2025;42:2659-78.
48. Meir J, Huang L, Mahmood S, Whiteson H, Cohen S, Aronow WS. The vascular complications of diabetes: a review of their management, pathogenesis, and prevention. Expert Rev Endocrinol Metab. 2024;19:11-20.
49. Han Y, Chen K, Wang Y, et al. Multi-animal 3D social pose estimation, identification and behaviour embedding with a few-shot learning framework. Nat Mach Intell. 2024;6:48-61.
50. Fu Y, Guo B, Lei Y, et al. Mask R-CNN based coronary artery segmentation in coronary computed tomography angiography. In: Hahn HK, Mazurowski MA, Editors. Medical Imaging 2020: Computer-Aided Diagnosis; 2020 Feb 15-20; Houston, Texas, United States. SPIE, 2020;11314:1047-52.
51. Cui H, Wang Y, Li Y, et al. An improved combination of faster R-CNN and U-Net network for accurate multi-modality whole heart segmentation. IEEE J Biomed Health Inform. 2023;27:3408-19.
52. Ren K, Chang L, Wan M, Gu G, Chen Q. An improved U-net based retinal vessel image segmentation method. Heliyon. 2022;8:e11187.
53. Liu F, Zhu J, Lv B, et al. Auxiliary segmentation method of osteosarcoma MRI Image based on transformer and U-Net. Comput Intell Neurosci. 2022;2022:9990092.
54. Viedma IA, Alonso-Caneiro D, Read SA, Collins MJ. OCT retinal and choroidal layer instance segmentation using mask R-CNN. Sensors. 2022;22:2016.
55. Tiwari R, Kumar R, Malik S, Raj T, Kumar P. Analysis of heart rate variability and implication of different factors on heart rate variability. Curr Cardiol Rev. 2021;17:e160721189770.
56. Li J, Liu Z, Kong F, et al. An automatic Doppler angle analysis method for interventional blood flow velocity calibration. Ultrasonics. 2025;155:107706.
57. Abdelrahman KM, Chen MY, Dey AK, et al. Coronary computed tomography angiography from clinical uses to emerging technologies: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;76:1226-43.
58. Yin ZX, Xu HM. An unsupervised image segmentation algorithm for coronary angiography. BioData Min. 2022;15:27.
59. Wang Y, Banerjee A, Choudhury RP, et al. DeepCA: deep learning-based 3D coronary artery tree reconstruction from two 2D non-simultaneous X-ray angiography projections. In: 2025 IEEE/CVF Winter Conference on Applications of Computer Vision (WACV); 2025 Feb 26 - 2025 Mar 6; Tucson, AZ, USA. IEEE; 2025. pp. 337-46,.
60. Bruns N. Blender: universal 3D processing and animation software. Unfallchirurg. 2020;123:747-50.
61. Kim B, Loke YH, Mass P, et al. A novel virtual reality medical image display system for group discussions of congenital heart disease: development and usability testing. JMIR Cardio. 2020;4:e20633.
62. Siddqi ZF. Computational fluid dynamics: modeling and analysis of blood flow in arteries. In: Singh RE, editor. Motion Analysis of Biological Systems. Cham: Springer International Publishing; 2024. pp. 89-121.
63. Kurniatie MD, Noviyadi NR, Mayasari DA, et al. Finite element analysis on blood vessels: understanding clipping phenomenon. In: 2024 International Seminar on Application for Technology of Information and Communication (iSemantic); 2024 Sep 21-22; Semarang, Indonesia. IEEE; 2024. pp. 107-11.
64. Syed F, Khan S, Toma M. Modeling dynamics of the cardiovascular system using fluid-structure interaction methods. Biology. 2023;12:1026.
65. Meystre S, van Stiphout R, Goris A, Gaitan S. AI-based gut-brain axis digital twins. In: Hägglund M, Blusi M, Bonacina S, Nilsson L, Cort Madsen I, Pelayo S, Moen A, Benis A, Lindsköld L, Gallos P, editors. Caring is Sharing - Exploiting the Value in Data for Health and Innovation. IOS Press; 2023. pp. 1007-8.
66. Ran X, Shi J, Chen Y, Jiang K. Multimodal neuroimage data fusion based on multikernel learning in personalized medicine. Front Pharmacol. 2022;13:947657.
67. Raissi M, Perdikaris P, Karniadakis G. Physics-informed neural networks: a deep learning framework for solving forward and inverse problems involving nonlinear partial differential equations. J Comput Phys. 2019;378:686-707.
68. Viceconti M, Henney A, Morley-fletcher E. In silico clinical trials: how computer simulation will transform the biomedical industry. Int J Clin Trials. 2016;3:37.
69. Topol EJ. High-performance medicine: the convergence of human and artificial intelligence. Nat Med. 2019;25:44-56.
70. Huang K, Han Y, Chen K, et al. A hierarchical 3D-motion learning framework for animal spontaneous behavior mapping. Nat Commun. 2021;12:2784.
71. Lyu K, Chen H, Liu Z, Zhang B, Wang R. 3D human motion prediction: a survey. Neurocomputing. 2022;489:345-65.
72. Han Y, Jiang Z, Ju F, Wang L, Liu Q, Wei P. BL-BERT: Extracting Body Language From Behavior Sequences In Freely Moving Mice. In: Liu Q, Qu Y, Wu H, Qi Y, Zeng A, Pan D, editors. Human Brain and Artificial Intelligence. Singapore: Springer Nature; 2025. pp. 181-91.
73. Wang S, Qin L. Homeostatic medicine: a strategy for exploring health and disease. Curr Med. 2022;1:16.
74. Li X, Jiang O, Chen M, Wang S. Mitochondrial homeostasis: shaping health and disease. Curr Med. 2024;3:32.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Copyright
Data & Comments
Data
















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].