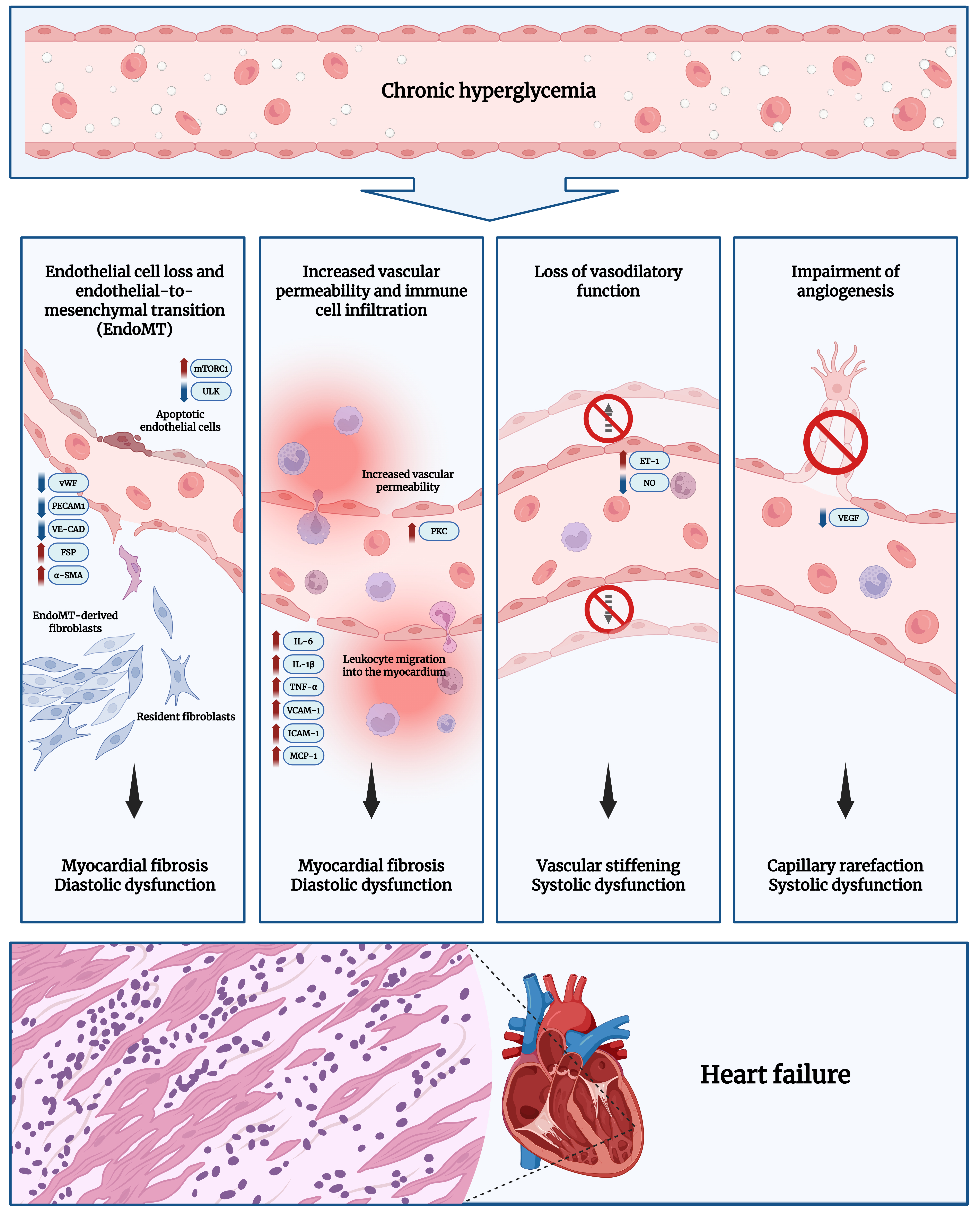
fig1
Figure 1. Pathogenesis of DCM. Prolonged hyperglycemia leads to metabolic changes in the myocardium, which converge on ED. This impairs normal endothelial function, manifesting as increased EC loss, increased transformation of ECs into fibroblasts (through EndoMT), elevated vascular permeability with immune cell infiltration into the myocardium, impaired vasodilation, and defective angiogenesis[3,4,7,10]. Excessive fibroblast transition and heightened inflammatory signaling drive progressive myocardial fibrosis, reducing ventricular relaxation and leading to diastolic dysfunction. Meanwhile, impaired endothelial vasodilation and angiogenesis causes vascular stiffening and rarefaction, reducing myocardial perfusion. The resulting nutrient and oxygen deficiency in