Artificial intelligence in coronary plaque characterization and risk assessment: from images to impact
Abstract
Accurate characterization of coronary atherosclerotic plaque and individualized cardiovascular risk assessment remain active challenges in clinical and interventional cardiology. In recent times, Artificial Intelligence (AI) has emerged as a powerful new tool able to support clinicians in determining diagnoses and prognoses from coronary imaging. This commentary focuses on the current applications of AI in coronary plaque imaging, particularly on coronary computed tomography angiography (CCTA), intravascular ultrasound (IVUS), and optical coherence tomography (OCT), evaluating its role in identifying high-risk plaque features and predicting future adverse cardiovascular events. We discuss limitations of conventional assessment methods, illustrating how AI algorithms can improve reproducibility, reduce operator dependence, and examine current evidence from registries and clinical studies. Furthermore, some key challenges remain to be addressed, including data quality, model generalizability, clinical integration, and regulatory concerns. We argue that AI’s promise lies not in replacing clinical expertise, but in empowering coronary risk stratification and characterization. Ongoing validation and clinician-AI collaboration will be essential to ensure meaningful patient outcomes.
Keywords
INTRODUCTION
The need for better risk stratification
Atherosclerotic cardiovascular disease remains the leading cause of death worldwide, accounting for an estimated 17.9 million deaths each year, representing approximately 32% of all global deaths, according to the World Health Organization[1]. The capability to detect and characterize coronary plaque morphology using non-invasive methods has recently improved with the advancement of imaging techniques[2]. However, current clinical practice still heavily relies on angiographic imaging evaluation to assess the severity of coronary stenoses, although it is now considered a marker with limited precision[2]. Indeed, angiographic stenosis provides only a luminal silhouette and correlates poorly with underlying plaque vulnerability, a key driver of acute coronary events[2]. Angiographic stenosis reflects only the degree of luminal narrowing and offers limited insight into the biological characteristics of the atherosclerotic plaque, which are more directly linked to the risk of rupture and acute coronary events[2,3]. With the increasing availability of large imaging datasets and computing power, artificial intelligence (AI) offers new opportunities to extract hidden information from images and provide more specific assessments of cardiovascular risk and prognosis[2-4].
IMAGING TECHNIQUES AND THEIR LIMITATIONS
Coronary computed tomography angiography (CCTA) is widely used for plaque detection, while intravascular ultrasound (IVUS) and optical coherence tomography (OCT) offer high-resolution views of the vessel wall[3]. Although evidence suggests that specific plaque characteristics, such as a thin fibrous cap, large lipid core, and signs of inflammation, effectively predict future cardiovascular events, intra-coronary imaging often performs suboptimally in cath labs, limited by interobserver variability, manual analysis, and suboptimal identification of vulnerable plaque features[4,5]. There is perhaps a critical need for methods able to integrate anatomical, morphological, and potentially functional data in a consistent and scalable manner, increasing exam reproducibility and improving workflow in cath labs[6]. Coronary OCT resolves structures at roughly 10-20 µm axially and 20-40 µm laterally, with tissue penetration of about 1-2 mm, which helps distinguish thin fibrous caps and lipid pools with high contrast[7]. By comparison, conventional 40-MHz IVUS has lower spatial resolution (about 100-150 µm axially and 200-250 µm laterally) but penetrates deeper into the vessel wall. Higher-frequency (~60-MHz) IVUS improves axial resolution relative to
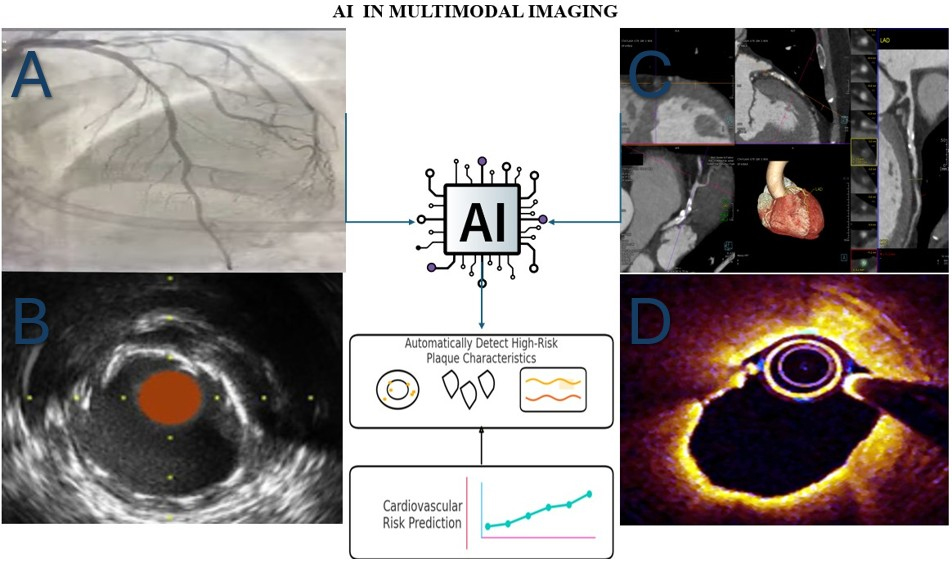
AI IN CORONARY PLAQUE CHARACTERIZATION
AI algorithms have demonstrated high accuracy in automating plaque detection in CCTA, quantifying plaque burden, and distinguishing high-risk morphologies, such as low-attenuation plaque, positive remodelling, or the napkin-ring sign[10,11]. Companies such as Cleerly, HeartFlow, and others have developed tools that provide rapid, reproducible, and quantitative assessments of plaque components on CCTA[12,13]. Similarly, deep learning models have shown reliability in analyzing OCT and IVUS images for core-lipid characterization and fibrous cap thickness[14,15]. The integration of AI into coronary imaging involves multiple stages, from image acquisition to automated plaque analysis and clinical decision support. Figure 1 illustrates a typical workflow for AI-assisted coronary plaque characterization across imaging modalities.
Figure 1. Schematic overview showing how AI integrates data from CCTA, OCT, and IVUS to identify high-risk plaque features, such as thin fibrous caps, lipid cores, and inflammation, for improved coronary risk assessment. (A) Image of coronary angiography from my clinical activity in Santa Maria Goretti Hospital, Latina; (B) Image of IVUS adapted from reference 26[26]. Licensed under CC BY 4.0
AI makes it possible to combine clinical information such as age, cholesterol levels, and symptoms with imaging findings, offering a more comprehensive assessment than traditional tools such as Coronary Artery Disease - Reporting and Data System (CAD-RADS) or Agatston scores[16]. Although the coronary artery calcium (CAC) score, most commonly calculated using the Agatston method, is a robust marker of total atherosclerotic burden and widely used for cardiovascular risk stratification, it has important limitations[17]. By design, the CAC score quantifies only macroscopic calcified plaque and fails to capture non-calcified components, which are often more vulnerable and prone to rupture[17]. Moreover, it does not differentiate between stable, densely calcified lesions and unstable plaques containing microcalcifications,
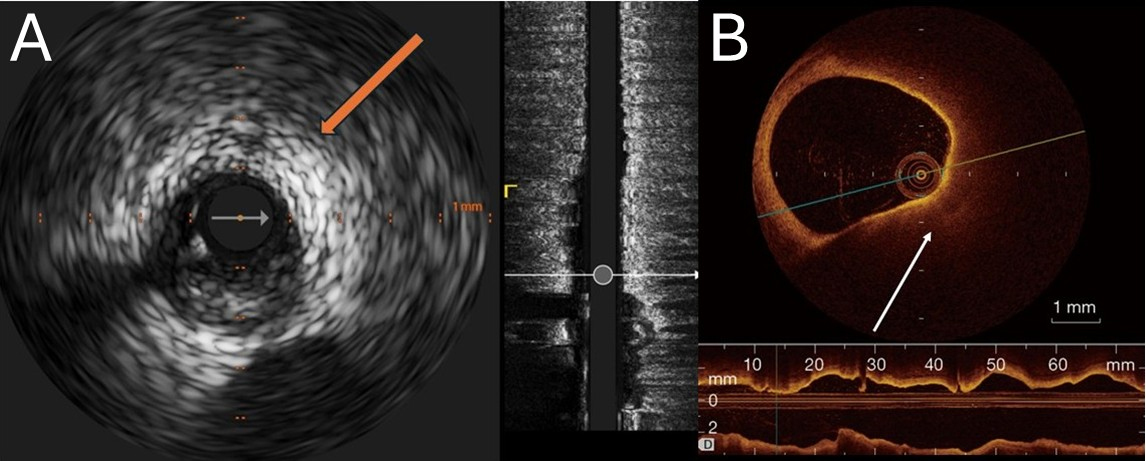
Figure 2. Representative intracoronary imaging of high-burden lipid-rich plaques. (A) Grayscale IVUS of the left circumflex artery showing an eccentric, predominantly hypoechoic plaque with marked deep ultrasound attenuation (arrow) and no bright calcific leading edge, consistent with attenuated plaque; (B) OCT from routine clinical imaging demonstrating a large, signal-poor, diffusely bordered lipid-rich plaque with long contiguous involvement on the longitudinal view. Scale bars =1 mm. IVUS: Intravascular ultrasound; OCT: optical coherence tomography. Images were acquired during routine care at our institution and are fully de-identified.
PROGNOSTIC APPLICATIONS AND RISK PREDICTION
AI models trained on large datasets have shown reliability in predicting major adverse cardiovascular events (MACEs), sometimes outperforming decisions made by clinicians[19]. Recent findings from the CONFIRM2 Registry highlight how advances in quantitative imaging, particularly AI-derived plaque characteristics, provide a detailed and powerful tool for predicting Major Adverse Cardiovascular Events[20]. These AI-based assessments offer a more comprehensive view of coronary artery disease burden than traditional human evaluations of stenosis severity alone[20]. The Scottish Computed Tomography of the Heart (SCOT-HEART) study’s semi-automatic-based sub-analyses demonstrated that plaque burden was more strongly associated with MACE than human-read stenosis assessments[21]. In a post hoc analysis involving 1,769 patients, those with low-attenuation plaque (LAP) burden exceeding 4% experienced a nearly fivefold increase in the risk of myocardial infarction (MI) compared to those with lower LAP burden {hazard ratio [HR] 4.65; 95%confidence interval [CI], 2.06-10.5; P < 0.001)[21]. Additionally, the presence of adverse plaque features, such as positive remodeling or
Comparison of conventional vs. AI-based coronary plaque assessment
| Dimension | Conventional imaging | AI-enhanced analysis |
| Median interpretation time | Manual quantitative plaque assessment by experts: ~25.7 ± 6.8 min per scan[29] | Automated plaque quantification ~5.7 ± 1.9 s (research setting); total report time remains site-dependent[29] |
| Interobserver variability | ICC 0.44-0.75 (CCTA stenosis)[30] | ICC > 0.90 (Quantitative Plaque Volume by advanced CT/AI)[29] |
| Diagnostic performance for ischemia vs. invasive FFR (per-patient) | CCTA: Sensitivity ~0.95, specificity ~0.35-0.45 (vs. FFR). CT-FFR: sensitivity ~0.85-0.90, specificity ~0.70-0.80)[31] | AI-QCT_ISCHEMIA AUC ~0.85 across CREDENCE/PACIFIC analyses; comparable to |
| Prognostic AUC for 5 years MACE | 0.73-0.75 (SCOT-HEART semi-quantitative scores)[34] | 0.85 (AI algorithm for CHD death)[35] |
| Change in management rate | 66% (DECODE). Management recommendations changed after adding AI-QCPA to the standard CCTA[32] | |
| Regulatory status | Multiple CE/FDA-cleared devices[31] | Several FDA-cleared AI tools[31] |
CHALLENGES IN IMPLEMENTATION
Data and model limitations
Despite recent advancements, several challenges continue to limit clinical translation[3,10,24]. Data heterogeneity across scanners, protocols, and populations constrains model robustness and large-scale applicability[3,10,24]. External validation remains limited, leaving generalizability uncertain[3,10,24]. Model opacity (“black-box” behavior) undermines clinician trust, alongside ethical concerns regarding bias, privacy, and accountability[3,10,24]. Plaque-specific issues include inconsistent feature definitions and reference standards across OCT, IVUS, and CCTA, and a paucity of prospective validation against hard MACE endpoints[3,10,24]. Addressing these gaps will require standardized multicenter datasets and outcome-anchored trials with harmonized endpoints[3,10,24].
Regulatory frameworks
Regulatory concerns exist regarding the appropriate clinical use, as every model must be validated and receive approval. Regulatory approval processes vary by country and the evolving nature of AI algorithms poses challenges related to recurrent regulatory revalidation.
System integration
Furthermore, AI models, to be effective, need to be incorporated into picture archiving and communication systems (PACS) and electronic health records. Current limitations in interoperability represent barriers to the adoption of these systems[25]. To highlight the technological evolution, Table 2 lists key patents underpinning AI applications in coronary imaging modalities, spanning angiography, IVUS, and OCT.
Patents on artificial intelligence in coronary imaging modalities
| Modality | Patent number | Title | Key features | Year |
| Coronary angiography | US9962124B2 | Automated analysis of vasculature in coronary angiograms | Machine learning for segmentation and stenosis detection in angiograms | 2018 |
| Coronary angiography | US20250114150A1 | Artificial intelligence for coronary angiography | AI to analyze coronary angiography images for enhanced diagnostic accuracy | 2025 |
| Coronary IVUS | US7397935B2((based on WO2005107366A2) | Method for segmentation of IVUS image sequences | Statistical methods for detecting vessel boundaries in IVUS | 2008 |
| Coronary OCT | CN107993221A | Automatic identification of vulnerable plaques in cardiovascular OCT images | Machine learning to identify vulnerable plaque types | 2018 |
| Coronary OCT | WO2017214421A1 | Automated coronary plaque characterization and risk assessment using OCT | AI for plaque characterization and risk assessment in OCT | 2017 |
| CCTA | WO2015058044A1 | ML-based assessment of fractional flow reserve | Predicts FFR using machine learning on coronary CT angiograms | 2015 |
| CCTA | US11263188B2 | Generation and management of an artificial intelligence (AI) model | AI model management applicable to CCTA data analysis | 2022 |
| QCA | US20240273723A1 | Automated analysis of coronary angiograms | ML-based classification and estimation of stenosis severity | 2024 |
FUTURE DIRECTIONS
The future of artificial intelligence in plaque characterization will depend on several factors: the launch of large, multicenter prospective studies that assess real clinical outcomes; the use of federated learning to train AI models across institutions without sharing raw patient data, improving both privacy and generalizability. Current literature points toward the integration of AI models in daily practice to support clinicians, rather than replace human expertise. Progress will also be linked to the development of hybrid platforms that bring together imaging data, such as perfusion and plaque morphology, with molecular, genetic, or biomarker information to provide a more comprehensive view of disease. Another promising direction lies in the integration of imaging-derived data with clinical, laboratory, and non-invasive instrumental parameters[13]. Combining high-sensitivity biomarkers [e.g., troponin, C-Reactive Protein (CRP)], clinical features (e.g., symptom presentation, risk factor burden), and echocardiographic findings (e.g., regional wall motion abnormalities) with imaging data could enable the development of complex and multimodal AI systems[3,13]. To illustrate how hybrid platforms integrating imaging and clinical data may enhance coronary risk stratification in practice, we propose the following hypothetical clinical scenario: A 63-year-old man with hypertension and a history of elevated low-density lipoprotein (LDL) cholesterol presents with atypical chest pain. He undergoes coronary Computed Tomography (CT) angiography, which is analyzed using an advanced platform that integrates imaging findings with clinical and laboratory data. The automated report identifies high-risk plaque features, such as low-attenuation plaque and positive remodeling, and combines these with his risk factors, including blood pressure and lipid profile, to estimate his individualized cardiovascular risk. Although no significant stenosis is present, the overall assessment supports intensification of medical therapy with high-dose statins and lifestyle changes. The platform allows for follow-up imaging and biomarker tracking, enabling more precise monitoring over time and personalized adjustments to therapy.
These platforms may substantially enhance risk stratification and support personalized decision making in coronary artery disease. An overview of commercially available AI-based systems, including their imaging modality, availability, and core features, is provided in Table 3.
AI systems in coronary imaging modalities - summary table
| System (Company) | Modality | First release | Core DL architecture | Performance metrics | Validation dataset |
| Ultreon 1.0 (Abbott)[36] | OCT | 2021 | Not publicly disclosed | No peer-reviewed, product-specific accuracy/AUC for the AI features | Pan-London PCI cohort study |
| AVVIGO+ (Boston Sci)[37] | IVUS | 2023 | Automated lesion assessment (ALA), architecture not disclosed | No peer-reviewed, product-specific accuracy/AUC for the AI features | Multiple studies, including a 48-patient study on its machine learning[38] |
| AI-QCA platform (Various)[39] | QCA | 2024 | Ensemble of DeepLabV3+, U-Net++, and U2-Net | Randomized FLASH trial: post-PCI MSA 6.3 ± 2.2 vs. 6.2 ± 2.2 mm2 (AI-QCA vs. OCT), met noninferiority; OCT-defined endpoints comparable | FLASH RCT, 400 patients, 13 centers |
| HeartFlow plaque[40] | CCTA | 2023 | Proprietary algorithms Proprietary 3-D CNN | No peer-reviewed, product-specific accuracy/AUC for the AI features | REVEALPLAQUE study, 258 patients, prospective, multicenter |
| Cleerly coronary[41] | CCTA | 2021 | Proprietary machine learning algorithms; generates 3D model, 3-D CNN | AI-QCT AUC vs. MPI for ≥ 50% stenosis 0.88 vs. 0.66; ≥ 70% 0.92 vs. 0.81 | CREDENCE and PACIFIC-1 studies |
| Siemens AIrad companion[42] | CCTA | 2019 | Deep learning algorithms; specific architecture not explicitly confirmed for cardiac features in provided materials | AUC 0.90, NPV 98% (CAD-RADS ≥ 4A), and plaque-burden agreement (κ_w 0.97 for CACS; 0.79 for SIS)[43] | Multiple studies and extensive datasets, with patient counts ranging from dozens to hundreds, for each module[43] |
| Nanox HealthCCSng 2.0[44] | Non-contrast CT | 2024 | Not explicitly stated in provided materials | No peer-reviewed, product-specific accuracy/AUC for the AI features | Multiple real-world studies, with patient cohorts from hundreds to thousands[45] |
CONCLUSION
AI-based analysis of coronary plaque constitutes a great technological advancement with an increasing role in personalized cardiovascular care. Beyond its technical capabilities, the true value of AI in coronary imaging lies in its progressive integration into daily cardiology practice. AI tools can significantly reduce interpretation time by automating plaque quantification and risk assessment, freeing clinicians to focus on decision making and patient care. Embedding AI outputs directly into PACS or electronic health records can substantially improve communication and collaboration across the care team, supporting a shared understanding of risk profiles. Future improvements in accuracy, reproducibility, and scalability of
DECLARATIONS
Authors’ contributions
conceived the idea for the commentary, performed the literature review, and wrote the first draft: Lauretti A
contributed to the critical revision of the manuscript and provided additional references and insights on clinical applications: Borgi M
provided supervision, critically reviewed the manuscript for intellectual content, and approved the final version: Versaci F
All authors read and approved the final manuscript.
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Nedkoff L, Briffa T, Zemedikun D, Herrington S, Wright FL. Global trends in atherosclerotic cardiovascular disease. Clin Ther. 2023;45:1087-91.
2. Labrecque Langlais É, Corbin D, Tastet O, et al. Evaluation of stenoses using AI video models applied to coronary angiography. NPJ Digit Med. 2024;7:138.
3. Abdelrahman KM, Chen MY, Dey AK, et al. Coronary computed tomography angiography from clinical uses to emerging technologies: JACC state-of-the-art review. J Am Coll Cardiol. 2020;76:1226-43.
4. Dzaye O, Razavi AC, Blaha MJ, Mortensen MB. Evaluation of coronary stenosis versus plaque burden for atherosclerotic cardiovascular disease risk assessment and management. Curr Opin Cardiol. 2021;36:769-75.
5. Veelen A, van der Sangen NMR, Henriques JPS, Claessen BEPM. Identification and treatment of the vulnerable coronary plaque. Rev Cardiovasc Med. 2022;23:39.
6. Chen Q, Pan T, Wang YN, et al. A coronary CT angiography radiomics model to identify vulnerable plaque and predict cardiovascular events. Radiology. 2023;307:e221693.
7. Tearney GJ, Regar E, Akasaka T, et al. Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: a report from the international working group for intravascular optical coherence tomography standardization and validation. J Am Coll Cardiol. 2012;59:1058-72.
8. Radu MD, Yamaji K, García-García HM, et al. Variability in the measurement of minimum fibrous cap thickness and reproducibility of fibroatheroma classification by optical coherence tomography using manual versus semi-automatic assessment. EuroIntervention. 2016;12:e987-97.
9. Huisman J, Egede R, Rdzanek A, et al. Multicenter assessment of the reproducibility of volumetric radiofrequency-based intravascular ultrasound measurements in coronary lesions that were consecutively stented. Int J Cardiovasc Imaging. 2012;28:1867-78.
10. Klüner LV, Chan K, Antoniades C. Using artificial intelligence to study atherosclerosis from computed tomography imaging: a state-of-the-art review of the current literature. Atherosclerosis. 2024;398:117580.
11. Xu P, Liu T, Zhou F, et al. Artificial intelligence in coronary computed tomography angiography. Medicine Plus. 2024;1:100001.
12. Rinehart S, Raible SJ, Ng N, et al. Utility of artificial intelligence plaque quantification: results of the DECODE study. J Soc Cardiovasc Angiogr Interv. 2024;3:101296.
13. Cho GW, Anderson L, Quesada CG, et al. Serial analysis of coronary artery disease progression by artificial intelligence assisted coronary computed tomography angiography: early clinical experience. BMC Cardiovasc Disord. 2022;22:506.
14. Chandramohan N, Hinton J, O’Kane P, Johnson TW. Artificial intelligence for the interventional cardiologist: powering and enabling OCT image interpretation. Interv Cardiol. 2024;19:e03.
15. Zhang C, Guo X, Guo X, et al. Machine learning model comparison for automatic segmentation of intracoronary optical coherence tomography and plaque cap thickness quantification. Comput Model Eng Sci. 2020;123:631-46.
16. Nurmohamed NS, Bom MJ, Jukema RA, et al. AI-guided quantitative plaque staging predicts long-term cardiovascular outcomes in patients at risk for atherosclerotic CVD. JACC Cardiovasc Imaging. 2024;17:269-80.
17. Greenland P, Blaha MJ, Budoff MJ, Erbel R, Watson KE. Coronary calcium score and cardiovascular risk. J Am Coll Cardiol. 2018;72:434-47.
18. Criqui MH, Denenberg JO, Ix JH, et al. Calcium density of coronary artery plaque and risk of incident cardiovascular events. JAMA. 2014;311:271-8.
19. Muzammil MA, Javid S, Afridi AK, et al. Artificial intelligence-enhanced electrocardiography for accurate diagnosis and management of cardiovascular diseases. J Electrocardiol. 2024;83:30-40.
20. Feuchtner GM, Lacaita PG, Bax JJ, et al. AI-Quantitative CT coronary plaque features associate with a higher relative risk in women: CONFIRM2 registry. Circ Cardiovasc Imaging. 2025;18:e018235.
21. Williams MC, Kwiecinski J, Doris M, et al. Low-attenuation noncalcified plaque on CCTA predicts myocardial infarction: results from the multicenter SCOT-HEART trial (Scottish computed tomography of the HEART). Circulation. 2020;141:1452-62.
22. Kim Y, Yoon HJ, Suh J, et al; FLASH Trial Investigators. Artificial intelligence-based fully automated quantitative coronary angiography vs optical coherence tomography-guided PCI: The FLASH Trial. JACC Cardiovasc Interv. 2025;18:187-97.
23. Rosenbacke R, Melhus Å, McKee M, Stuckler D. How Explainable artificial intelligence can increase or decrease clinicians’ trust in AI applications in health care: systematic review. JMIR AI. 2024;3:e53207.
24. Pinna A, Boi A, Mannelli L, et al. Machine learning for coronary plaque characterization: a multimodal review of OCT, IVUS, and CCTA. Diagnostics. 2025;15:1822.
25. Tejani AS, Cook TS, Hussain M, Sippel Schmidt T, O’Donnell KP. Integrating and adopting AI in the radiology workflow: a primer for standards and integrating the healthcare enterprise (IHE) profiles. Radiology. 2024;311:e232653.
26. Dobrolińska MM, Gąsior PM, Pociask E, et al. Performance of integrated near-infrared spectroscopy and intravascular Ultrasound (NIRS-IVUS) system against quantitative flow ratio (QFR). Diagnostics. 2021;11:1148.
27. Wikimedia Commons. CCTA CAD-RADS 4a.png. Available from: https://commons.wikimedia.org/wiki/File:CCTA_CAD-RADS_4a.png [Last accessed on 15 Sep 2025].
28. Viscusi MM, La Porta Y, Migliaro G, et al. Current applications and new perspectives in optical coherence tomography (OCT) coronary atherosclerotic plaque assessment: from PCI optimization to pharmacological treatment guidance. Photonics. 2023;10:158.
29. Lin A, Manral N, McElhinney P, et al. Deep learning-enabled coronary CT angiography for plaque and stenosis quantification and cardiac risk prediction: an international multicentre study. Lancet Digit Health. 2022;4:e256-65.
30. Choudhary G, Atalay MK, Ritter N, et al. Interobserver reliability in the assessment of coronary stenoses by multidetector computed tomography. J Comput Assist Tomogr. 2011;35:126-34.
31. Zhuang B, Wang S, Zhao S, Lu M. Computed tomography angiography-derived fractional flow reserve (CT-FFR) for the detection of myocardial ischemia with invasive fractional flow reserve as reference: systematic review and meta-analysis. Eur Radiol. 2020;30:712-25.
32. Lipkin I, Telluri A, Kim Y, et al. Coronary CTA With AI-QCT interpretation: comparison with myocardial perfusion imaging for detection of obstructive stenosis using invasive angiography as reference standard. AJR Am J Roentgenol. 2022;219:407-19.
33. Nurmohamed NS, Danad I, Jukema RA, et al; CREDENCE and PACIFIC-1 Investigators. Development and validation of a quantitative coronary CT angiography model for diagnosis of vessel-specific coronary ischemia. JACC Cardiovasc Imaging. 2024;17:894-906.
34. Maclean E, Cronshaw R, Newby DE, Nicol E, Williams MC. Prognostic utility of semi-quantitative coronary computed tomography angiography scores in the SCOT-HEART trial. J Cardiovasc Comput Tomogr. 2023;17:393-400.
35. Johnson KM, Johnson HE, Zhao Y, Dowe DA, Staib LH. Scoring of coronary artery disease characteristics on coronary CT angiograms by using machine learning. Radiology. 2019;292:354-62.
36. Abbott. UltreonTM 1.0 software: experience the power of automation. Available from: https://www.cardiovascular.abbott/int/en/hcp/products/percutaneous-coronary-intervention/intravascular-imaging/ultreon-software/ultreon-1-0.html [Last accessed on 15 Sep 2025].
37. Boston Scientific. AVVIGOTM+ Multi-Modality Guidance System. Available from: https://www.bostonscientific.com/en-US/products/ffr-ivus-systems/avvigo-guidance-system.html [Last accessed on 15 Sep 2025].
38. Galo J, Chaturvedi A, Al-Qaraghuli A, et al. Machine learning in intravascular ultrasound: validating automated lesion assessment for complex coronary interventions. Catheter Cardiovasc Interv. 2025;105:1320-8.
39. In Kim Y, Roh JH, Kweon J, et al. Artificial intelligence-based quantitative coronary angiography of major vessels using deep-learning. Int J Cardiol. 2024;405:131945.
40. HeartFlow. Heartflow introduces next generation interactive plaque analysis platform to assess patient risk in suspected coronary artery disease. Available from: https://www.heartflow.com/press-release/Heartflow-plaque-interactive/ [Last accessed on 15 Sep 2025].
41. Cleerly. Not all plaque analysis software are the same: roviding multi-modality imaging decision support. Available from: https://cleerlyhealth.com/plaque-analysis [Last accessed on 15 Sep 2025].
42. Siemens Healthineers. AI-Rad Companion: providing multi-modality imaging decision support. Available from: https://www.siemens-healthineers.com/en-us/digital-health-solutions/ai-rad-companion [Last accessed on 15 Sep 2025].
43. Kay FU, Canan A, Kukkar V, et al. Diagnostic accuracy of on-premise automated coronary CT Angiography analysis based on coronary artery disease reporting and data system 2.0. Radiology. 2025;315:e242087.
44. NANOX. Nanox receives FDA clearance for HealthCCSng V2.0, upgraded version of advanced AI cardiac solution empowering physicians in assessment of coronary artery calcium. Available from: https://investors.nanox.vision/news-releases/news-release-details/nanox-receives-fda-clearance-healthccsng-v20-upgraded-version [Last accessed on 15 Sep 2025].
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Special Issue
Copyright
Data & Comments
Data
















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].