Invasive assessment of culprit and non-culprit coronary lesions in patients with acute coronary syndromes
Abstract
For decades, coronary angiography has been the gold standard for identifying and evaluating culprit and non-culprit coronary lesions in acute coronary syndromes (ACS). However, angiography provides limited information on plaque composition and cannot assess the functional significance of lesions. To address these limitations, several invasive methods have been introduced and are now used in clinical practice, including intravascular imaging (IVI) and fractional flow reserve (FFR). Studies have compared FFR-guided complete revascularization with culprit-only revascularization, as well as FFR-guided versus angiography-guided revascularization in both ACS and stable angina. However, clinical trials in ACS have produced contradictory results: some studies indicate that FFR is feasible, safe, and associated with reduced major adverse cardiac events (MACE), while others are less conclusive. IVI has been evaluated in fewer ACS-specific studies, but initial findings are promising. Moreover, a novel strategy has emerged: preemptive stenting of high-risk, hemodynamically non-significant plaques identified by IVI to prevent future MACE. Nevertheless, the limited and sometimes contradictory data highlight the need for further research to determine the optimal diagnostic and treatment strategies for coronary lesions in patients with ACS.
Keywords
INTRODUCTION
Coronary artery disease (CAD) remains a major global health burden. Acute coronary syndrome (ACS), characterized by sudden onset, presents a considerable healthcare challenge and often results from the rapid progression of high-risk atherosclerotic plaques. ACS is responsible for approximately 2 million deaths worldwide annually[1]. Acute myocardial ischemia is typically associated with the abrupt destabilization of these high-risk plaques. In the context of ACS, it is critical not only to manage the culprit lesion but also to develop a strategy for evaluating and treating non-culprit lesions.
In patients with non-ST-segment elevation myocardial infarction (NSTEMI), multivessel CAD (MVD) is identified in approximately 40%-80% of cases, whereas roughly half of patients with ST-segment elevation myocardial infarction (STEMI) also exhibit MVD[2,3]. These patients generally experience worse clinical outcomes compared to those with single-vessel disease[4]. Notably, the Complete vs. Culprit-Only Revascularization Strategies to Treat Multivessel Disease After Early PCI for STEMI (COMPLETE) trial showed that complete revascularization significantly reduced major adverse cardiac events (MACE), compared to culprit-only percutaneous coronary intervention (PCI)[5].
Accurate identification of the culprit lesion in MVD is essential, as misidentification can lead to suboptimal revascularization strategies and worse outcomes. Although coronary angiography has long been considered the gold standard, it is limited by its inability to assess plaque composition and its reliance on two-dimensional imaging of contrast flow, which hinders accurate vessel sizing and functional evaluation[6-8]. To address these limitations, newer techniques have emerged. Intravascular imaging (IVI), particularly intravascular ultrasound (IVUS) and optical coherence tomography (OCT), along with physiological assessment using fractional flow reserve (FFR), now play key roles in clinical practice. This review explores the application of IVI and FFR in evaluating culprit and non-culprit lesions in ACS.
METHODOLOGY
For this narrative review, an exhaustive literature search of PubMed was performed in February 2025. The search terms were: “ST-elevation myocardial infarction”, “non ST-elevation myocardial infarction”, “acute coronary syndrome”, “coronary artery”, “coronary lesion”, “culprit-lesion”, “non-culprit lesion”, “fractional flow reserve”, “intravascular imaging”, “optical coherence tomography”, “intravascular imaging”, “multivessel disease”, “percutaneous coronary intervention”, “coronary revascularization”, “quantitative flow ratio”, and “instantaneous wave-free ratio”. More than 2000 studies were identified, and those published after 2005 were analyzed. For data presentation in tables, studies with a cohort size greater than 300 were selected for FFR, and greater than 200 for intravascular imaging. In addition to studies retrieved through this search, relevant trials cited in references, review articles, and previous meta-analyses were included when appropriate.
BASICS IVI AND FFR
Since its introduction in 1988, IVUS has become a cornerstone of interventional cardiology. It uses ultrasound via a mechanical or electronic catheter to image the vessel wall, with tissue penetration up to
Main differences between intravascular ultrasound and optical coherence tomography
| IVUS | OCT | |
| Energy source | Ultrasound | Near-infrared light |
| Resolution | 100-150 m | 10-15 m |
| Tissue penetration | 4-8 mm, can not penetrate calcium | 2-3 mm, can not penetrate lipid |
| Blood clearance | Not required | Required |
| Main features of high-risk plaque | Plaque burden | TCFA, lipid plaque |
| Use in specific clinical situations | IVUS vs. OCT | |
| ACS | OCT may be preferred as it may differentiate thrombus, allows for easier identification of culprit lesion and provide information regarding mechanism of ACS | |
| SCAD | IVUS may be better. OCT may provide easier interpretation of SCAD but contrast flush may propagate SCAD | |
| LMCA PCI | IVUS may be better as contrast flush in aorto-ostial lesions is difficult. There is more data for IVUS in LM disease | |
| Optimalization after stent implantation | OCT may be better as images obtained with higher resolution may be easier to interpret | |
| Stent failure | OCT may be better due to higher resolution and easier interpretation | |
Major differences and uses of Fractional Flow Reserve and Intravascular Imaging in daily clinical practice
| Fractional flow reserve | Intravascular imaging | |
| Assessment of significance of a coronary lesion | Yes - functional (physiological) significance of a coronary lesion | No - only exception is IVUS in LM |
| Assessment of plaque morphology | No | Yes (OCT provides more details due to higher resolution) |
| Role during PCI procedure | - Decide which lesions should be revascularized - After procedure may assess change in pressure gradient - evaluate efficacy of procedure | - Develop strategy for PCI and assess: • Plaque morphology • If any plaque modification techniques are needed • Landing zones, stent length and diameter - Evaluate PCI results • Stent expansion • Coverage of the lesion • Dissections |
| Endpoints as compared to angiography guided strategy in ACS patients (details in Table 3) | Unclear results, currently no major differences - new studies are needed. Fractional Flow Reserve guided complete revascularization is superior to culprit only revascularization | Reduction in major endpoints including target lesion and vessel failure, cardiac death. Better stent expansion and less procedural complications |
PHYSIOLOGY TESTING
Historically, lesion severity was assessed solely through angiography, using cutoff values of 50% for the left main (LM) artery and 70% for other coronary segments. Current guidelines for managing chronic coronary syndromes recommend FFR for intermediate lesions (40%-90% for non-LM and 40%-70% for LM) and in MVD to guide lesion selection[7]. In ACS, guidelines differentiate between STEMI and NSTEMI[8]. In STEMI, angiographic assessment is recommended for non-culprit lesions treated during the index procedure [Figure 1]. Evidence on the optimal timing of FFR for culprit and non-culprit lesions in STEMI patients remains limited[10]. In NSTEMI, FFR-guided assessment of non-culprit lesions in non-infarct-related arteries may be considered. Two key questions in ACS physiology testing are: (1) whether FFR-guided complete revascularization is superior to culprit-only revascularization; and (2) whether FFR-guided revascularization is superior to angiography-guided revascularization.
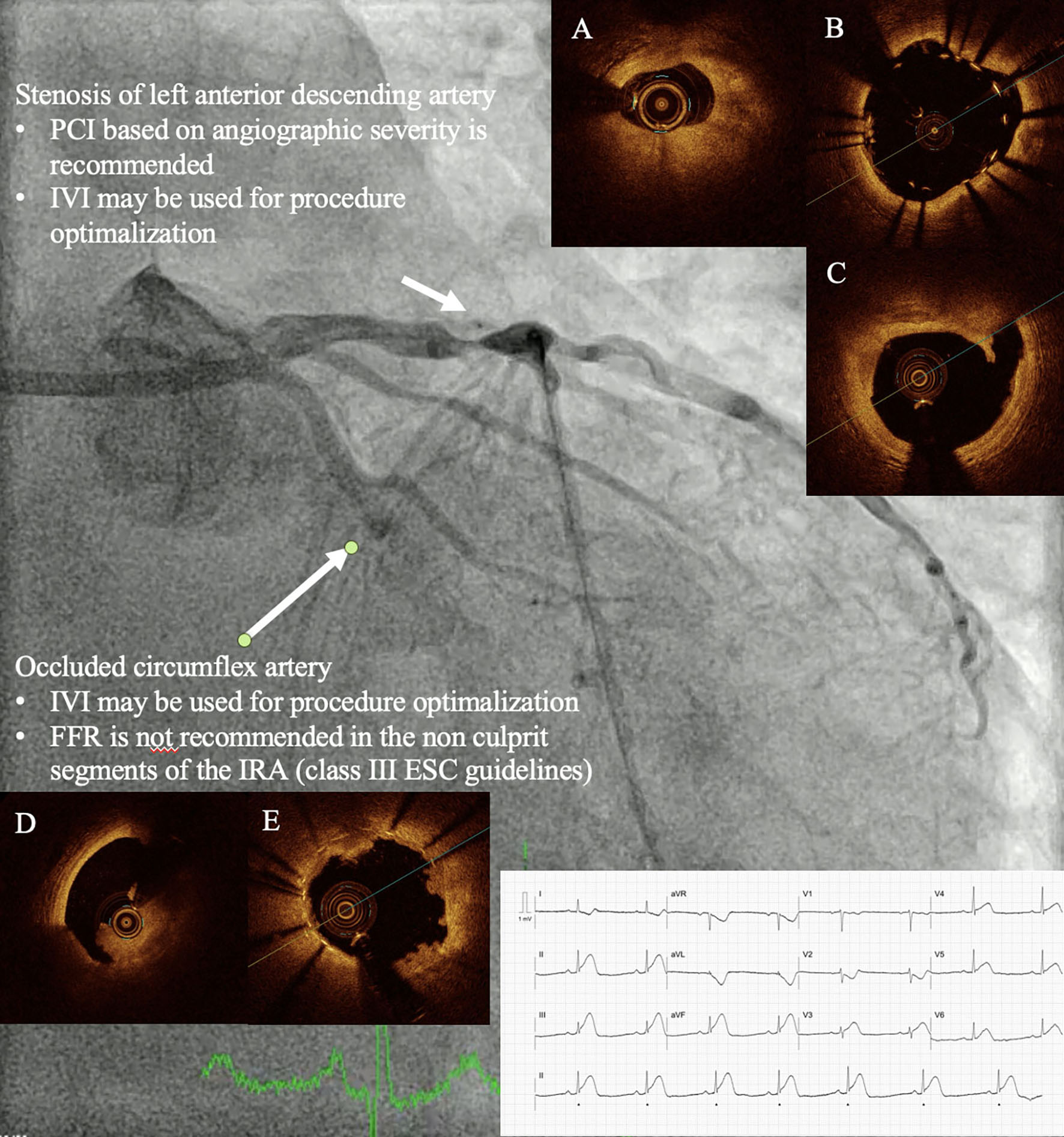
Figure 1. Patient with ST-elevation myocardial infarction and multivessel disease. Patient with STEMI (*) and an occluded circumflex artery who remained hemodynamically stable during PCI. Both PCI of the infarct-related artery and the non-culprit lesion were performed under OCT guidance. In the circumflex artery, OCT revealed thrombus (A), whereas in the left anterior descending artery, it showed a mixed fibrous and lipid plaque (B). Post-stenting OCT demonstrated stent malapposition (C), stent-edge dissection (D), and plaque protrusion after stent implantation (E). ESC: European Society of Cardiology; FFR: fractional flow reserve; IVI: intravascular imaging; OCT: optical coherence tomography; PCI: percutaneous coronary intervention; STEMI: ST-elevation myocardial infarction.
FFR-guided complete vs. culprit-only revascularization
FFR was initially validated in patients with stable angina (SA). Landmark trials have demonstrated that FFR-guided PCI significantly reduces MACE compared to angiography-guided strategies[11,12]. In a study of 2,400 patients with SA, Zimmermann et al. reported that FFR-guided PCI lowered the risk of myocardial infarction (MI) relative to medical therapy alone[13].
Several trials have demonstrated that FFR-guided complete revascularization, compared to culprit-only PCI, is safe and beneficial in ACS. Smits et al. showed that among 885 patients with STEMI, FFR-guided complete revascularization reduced the composite endpoint of MACE and cerebrovascular events compared to culprit-only PCI[5,14]. Patients in the culprit-only group required more repeat revascularizations during 1- and 3-year follow-ups, although no significant differences were observed in mortality or nonfatal MI. Cost analysis also indicated that FFR-guided complete revascularization decreased healthcare costs[14]. Comparable results were reported in the Danish Study of Optimal Acute Treatment of Patients with ST-Elevation Myocardial Infarction (DANAMI)-3 Primary PCI in Multivessel Disease (PRIMULTI) trial, which included 627 patients with STEMI[15]. Patients were randomized to either culprit-only PCI or FFR-guided full MVD revascularization. Patients in the full revascularization group had a significantly lower risk of a composite outcome comprising all-cause mortality, nonfatal reinfarction, and ischemia-driven revascularization than those in the culprit-only group. However, a more recent study reported contradictory findings[16]. In this trial of 1,542 patients with STEMI and high-risk NSTEMI, 764 were randomized to FFR-guided full revascularization and 778 to culprit-only PCI. Over a median follow-up of 4.8 years, no differences were observed in the primary outcome (a composite of all-cause death, MI, or unplanned revascularization) or in the broader composite of all-cause death, MI, or unplanned revascularization. Several factors may explain these unexpected findings. First, patients in this study were randomized within
FFR vs. angiography
Over the past decade, multiple trials have evaluated whether FFR-guided complete revascularization provides superior outcomes compared to angiography-guided approaches in ACS. The Fractional Flow Reserve in Unstable Angina and Non-ST-Segment Elevation Myocardial Infarction study demonstrated that FFR-guided PCI in MVD reduced MACE to a similar extent in patients with unstable angina or NSTEMI as in those with SA, with a 5.1% absolute risk reduction in MACE in the ACS subgroup at 2 years[17]. However, the subgroup analysis (n = 328) was underpowered to confirm superiority. In the study by Layland et al., 350 patients with NSTEMI were randomized to FFR-guided or angiography-guided revascularization
Major studies comparing Intravascular Imaging or Fractional Flow Reserve to angiography in patients with acute coronary syndrome and multivessel disease
| Study/Publication year | Study size | Groups | Time to PCI | Follow-up time | Events at follow-up |
| Layland et al.[18] FAMOUS-NSTEMI 2015 | 350 patients with NSTEMI* | Revascularization angiography guided (n = 174) vs. FFR guided (n = 176). FFR done in all lesions with 30% diameter stenosis and PCI/CABG was done if FFR was ≤ 0.80 | 3 days from myocardial ischemia | 1 year | - Patients who were free from coronary revascularization: 13.2% vs. 21.0% (P = 0.054) - Cardiac death, non-fatal myocardial infarction or unplanned hospitalization for heart failure: 8.6% vs. 8.0% (P = 0.89) - All cause death: 1.7% vs. 2.8% (P = 0.54) Index hospitalization: - Cost through hospitalization: 7,474 pounds vs.7,289 pounds (P = 0.61) - Number of patients treated with medical therapy: 13.2% vs. 22.7% (P = 0.022) |
| Puymirat et al.[19,20] FLOWER-MI 2021 and 2024 | 1,163 patients with STEMI* | Revascularization angiography guided (n = 577) vs. FFR guided (n = 586). FFR done in all lesions with > 50% diameter stenosis and PCI was done if FFR was ≤ 0.80 | From primary intervention: mean 2.7 days in angiography group and mean 2.6 days in FFR group. Staged intervention was done in 95% of patients | 1 and 3 years | 1 year: - Primary outcome (composite of death from any cause, nonfatal myocardial infarction, or unplanned hospitalization leading to urgent revascularization): 4.2% vs. 5.5% (P = ns) - Nonfatal MI: 1.7% vs. 3.1% (P = ns) - Any revascularization: 4.5% vs. 6.5% - Stent thrombosis: 1.0% vs. 0.7% (P = ns) - Hospitalization for recurrent ischemia: 3.3% vs. 5.5% (P = ns) 3 years: - Primary outcome: 8.2% vs. 9.4% (P = ns) - MI: 2.6% vs. 4.1% (P = ns) - Any revascularization: 7.6% vs. 9.6% - Stent thrombosis: 1.2% vs. 0.7% (P = ns) - Hospitalization for recurrent ischemia: 5.3% vs. 8.0% (P = ns) |
| Lee et al.[21] FRAME-AMI 2023 | 562 patients with STEMI (n = 265) and NSTEMI (n = 297)* | Revascularization angiography guided (n = 278) vs. FFR guided (n = 284). FFR done in all lesions with > 50% diameter stenosis and PCI was done if FFR was ≤ 0.80. In angiography group all lesions > 50% diameter stenosis had PCI | 60% patients during primary PCI, 40% of patients staged procedure during same hospitalization | 3.5 years (trial terminated earlier than planned) | - Death, myocardial infarction, and repeat revascularization: 19.7% vs. 7.4% (P = 0.003) - Procedure related MI: 4.0% vs. 1.1% - Spontaneous myocardial infarction: 5.0% vs. 1.4% (P = ns) - Repeat revascularization: 9.0% vs. 4.3% - Definite stent thrombosis: 0.4% vs. 0.0% |
| Meneveau et al.[46] DOCTORS study 2016 | 240 patients with NSTEMI | Revascularization angiography guided (n = 120) vs. OCT guided (n = 120) | - | 6 months | - FFR at the end of the procedure: - 2 TVR, 1 death and 1 MI in OCT group - 1 TVR and 1 MI in angiography group |
| Yamashita et al.[50] Kumamoto Intervention Conference Study Registry 2021 | 6,025 patients with STEMI (n = 3,096) and NSTEMI/UA (n = 2,929) | Revascularization angiography guided (n = 2,412) vs. IVI guided(n = 3,613). IVI group always had IVUS, could have additionally OCT but there is no data on how many OCTs were done | - | 1 year | - Total cardiac events: 4.1% vs. 1.9% - Non-fatal MI: 1.5% vs. 0.6% (P < 0.001) - Stent thrombosis: 0.6% vs. 0.8% (P = 0.017) - Cardiac death: 2.7% vs. 1.3% (P < 0.001) |
| Kim et al.[49] From KAMIR-NIH Registry 2022 | 13,104 patients with STEMI (n = 4,541) and NSTEMI (n = 4,466) | Revascularization angiography guided (n = 7,120) vs. IVUS guided (n = 1,887) | - | 3 years | - Target lesion failure: 8.0% vs. 4.8% - Target vessel MI: 1.2% vs. 0.6% (P = 0.019) - Any MI: 2.9% vs. 2.4% (P = 0.136) - Cardiac death: 5.5% vs. 3.1% (P < 0.001) |
| Li et al.[51] IVUS-ACS 2024 | 3,505 patients with STEMI (n = 973) and NSTEMI/UA (n = 2,532) | Revascularization angiography guided (n = 1,752) vs. IVUS guided (n = 1,753) | Patients could have procedure up to 30 days after ACS | 1 year | - Target vessel failure: 7.3% vs. 4.0% - Target vessel myocardial infarction: 3.8% vs. 2.5% (P = 0.018) - Clinically driven target vessel revascularization: 3.2% vs. 1.4% (P = 0.001) - Clinically driven target lesion revascularization: 2.5% vs. 1.3% (P = 0.014) - Definite or probable stent thrombosis: 0.9% vs. 0.6% (P = ns) |
The Multivessel PCI Guided by FFR or Angiography for Myocardial Infarction (FLOWER-MI) trial enrolled 1,171 patients with STEMI and MVD and found no significant differences between FFR-guided and angiography-guided complete revascularization at 1- and 3-year follow-ups[19]. As expected, patients in the FFR arm had fewer treated lesions (55.7% vs. 90.5%). Surprisingly, the angiography group had three times fewer periprocedural MIs (as discussed later). No differences in the primary outcomes were observed during the 1-year follow-up. At the 3-year follow-up, results remained consistent, with no significant differences in the primary outcomes between the two arms[20]. Secondary outcomes, including stent thrombosis or revascularization, also did not differ significantly.
By contrast, the more recent FRAME-AMI trial (2023) demonstrated that FFR-guided revascularization of non-culprit lesions in patients with ACS (both NSTEMI and STEMI) was superior to routine PCI based on angiographic diameter stenosis for non-infarct-related arteries[21]. The analysis included 562 patients, and during a median follow-up of 3.5 years, the FFR group showed lower rates of all-cause death (2.1% vs. 8.5%), cardiac death (1.4% vs. 8.2%), and procedure-related MI (1.1% vs. 4.0%). Notably, no significant differences were observed in repeat revascularization, definite stent thrombosis, or contrast-induced nephropathy. These results were primarily driven by the NSTEMI subgroup (27 primary outcome events vs. 9) compared with the STEMI subgroup (13 vs. 9). Similar to FLOWER-MI, patients in the angiography-guided arm underwent treatment of more lesions, with a higher mean number of stents (1.3 vs. 0.9 in the FFR-guided group). Importantly, in FLOWER-MI, 7 of 9 deaths in the FFR arm were non-cardiac, whereas 7 of 10 deaths in the angiography-guided arm were cardiac, which may partially explain the differing results between the two studies. In FLOWER-MI, more patients in the FFR group experienced procedure-related MI, mainly due to lower TIMI flow at the end of the procedure. Direct comparison of these two trials is not feasible, as they included different patient subsets (STEMI vs. NSTEMI + STEMI); in addition, the follow-up duration and timing of PCI for non-infarct-related arteries varied. In FLOWER-MI, 95% of patients underwent staged intervention, whereas in FRAME-AMI, 60% of patients received intervention during primary PCI. Finally, FRAME-AMI was terminated prematurely.
In a network meta-analysis, Elbadawi et al. included 8,195 patients with STEMI from 11 trials[22]. Although the authors confirmed that complete revascularization was safer than culprit-only revascularization, they found no evidence that FFR-guided revascularization was superior to angiography-guided approaches. Similar findings were reported in another meta-analysis published in the same year[23].
In addition to the limitations noted above, the inconclusive results of these studies may also be attributed to microvascular dysfunction, diffuse vasoconstriction, and altered hyperemic response during the acute phase[10,24,25]. Catecholamine-driven metabolic changes can further compromise FFR reliability by impairing myocardial oxygen utilization[22,26]. These changes increase oxygen demand, reduce glycogen stores, decrease myocardial oxidation-reduction enzyme activity, and disrupt mitochondrial function. Animal studies have also demonstrated functional abnormalities in nonischemic regions during acute ischemia[22]. Nonetheless, several clinical studies involving patients with STEMI and NSTEMI have found no significant differences in index FFR values between the acute and subacute phases[10,24,27].
Finally, growing evidence suggests that high-risk plaques that are not angiographically or hemodynamically significant may be more likely to cause MACE than severe lesions without high-risk features[28]. Although such high-risk plaque features cannot be detected by FFR, plaque composition and stenting outcomes can be assessed using IVI.
ANATOMY GUIDANCE
Guidelines for chronic coronary syndrome recommend IVI (IVUS or OCT) during PCI for complex anatomical lesions, including those involving the left main stem, bifurcations, and long lesions[7]. IVUS is also advised for evaluating intermediate stenosis in the left main stem before revascularization. Earlier ACS guidelines recommend considering IVI to guide PCI, particularly OCT in cases with ambiguous culprit lesions[8].
Imaging to determine lesion significance
A key limitation of IVI is its inability to directly assess the functional significance of a lesion, unlike FFR. IVUS, but not OCT, can be used to evaluate the left main stem. In Western populations, a minimum lumen area (MLA) of 6 mm2 by IVUS correlates with FFR < 0.75[29], while in Asian populations, where vessels are typically smaller, an MLA of 4.5-4.8 mm2 is considered sufficient[30,31]. For accurate assessment of the left main artery, IVUS pullback should be performed from both the left anterior descending and circumflex arteries.
For non-left main lesions, attempts to define an anatomic threshold for clinically significant stenosis using IVUS have yielded inconsistent results, partly because IVUS and OCT cannot assess the amount of viable myocardium at risk[29]. Reported IVUS MLA cutoffs range from 2.1 to 4.4 mm2, and OCT cutoffs range from 1.6 to 2.4 mm2[29]. These thresholds provide relatively high negative predictive value but low positive predictive value, making them insufficiently reliable for daily practice.
Identifying the culprit lesion
Although IVI cannot reliably assess the functional significance of coronary lesions in most cases, it can help identify culprit lesions when angiography alone is inconclusive. For example, in non-Q-wave MI, more than one-third of patients have either no identifiable culprit lesion or multiple potential culprits[32]. In a study of 1,107 patients with NSTEMI, angiography alone failed to identify the culprit lesion in 14% of cases[3], whereas IVI detected the culprit in 96.4%. Both IVUS and OCT can identify thrombi, but the higher resolution of OCT enables more reliable thrombus detection[28].
Likewise, OCT is superior to IVUS for detecting plaque rupture due to its higher resolution. IVI is also useful in patients with MI with nonobstructive coronary arteries (MINOCA; Figure 2). In one study of 116 patients with MINOCA, OCT identified possible culprit lesions in 46.2% of cases[33]. In a more recent study of 190 MINOCA patients undergoing OCT, the underlying cause was identified in 61.1% of cases[34]. The etiologies included plaque erosion (33.7%), plaque rupture (17.4%), calcified nodule (1.1%), spontaneous coronary artery dissection (4.2%), and coronary spasm (4.7%). Importantly, in spontaneous coronary artery dissection, IVUS may be preferred over OCT for safety reasons, as OCT requires contrast injection.
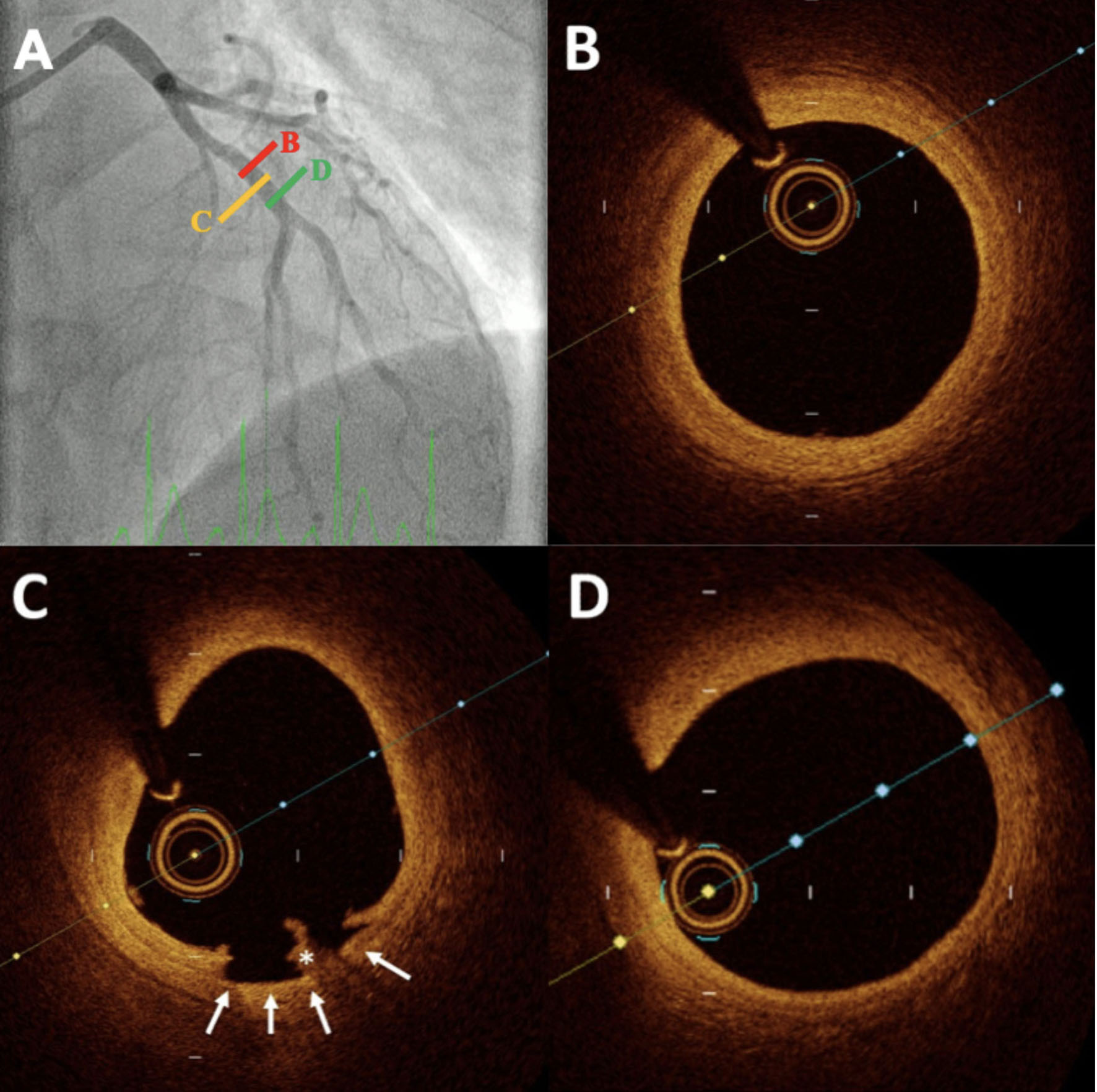
Figure 2. Example of a patient with myocardial infarction and nonobstructive coronary artery disease. Angiography revealed no significant abnormalities in the main coronary arteries (A). OCT of the LAD revealed a normal coronary artery with a well-defined three-layer structure (B), followed by a thrombus (*) associated with a small dissection (C), and then a return to a normal vessel (D). LAD: Left anterior descending artery; OCT: optical coherence tomography
Identification of high-risk plaques with IVI
One reason FFR studies may yield inconclusive results is that FFR cannot characterize lesion morphology. Lesions considered insignificant by angiography or FFR may still harbor high-risk plaques capable of precipitating future cardiovascular events [Figure 3]. A high-risk plaque is defined as a thrombosis-prone lesion or a plaque likely to progress rapidly into a culprit lesion and trigger ACS[28]. High-risk plaques represent the precursors of plaque rupture, plaque erosion, or eruptive calcified nodules - three substrates known to cause luminal thrombosis. Pathological research further suggests that lesion behavior may vary within the same patient, with plaques transitioning from phenotypes associated with instability [e.g., thin-cap fibroatheroma (TCFA)] to those linked with stability (e.g., fibrocalcific plaques without rapid progression or flow-limiting stenosis), and vice versa[28]. Moreover, plaque progression is frequently silent, as it may occur through the formation of nonocclusive thrombi. Depending on the study, features of high-risk plaques include TCFA, small MLA, macrophage infiltration (inflammation), lipid-rich composition, and high plaque burden[28].
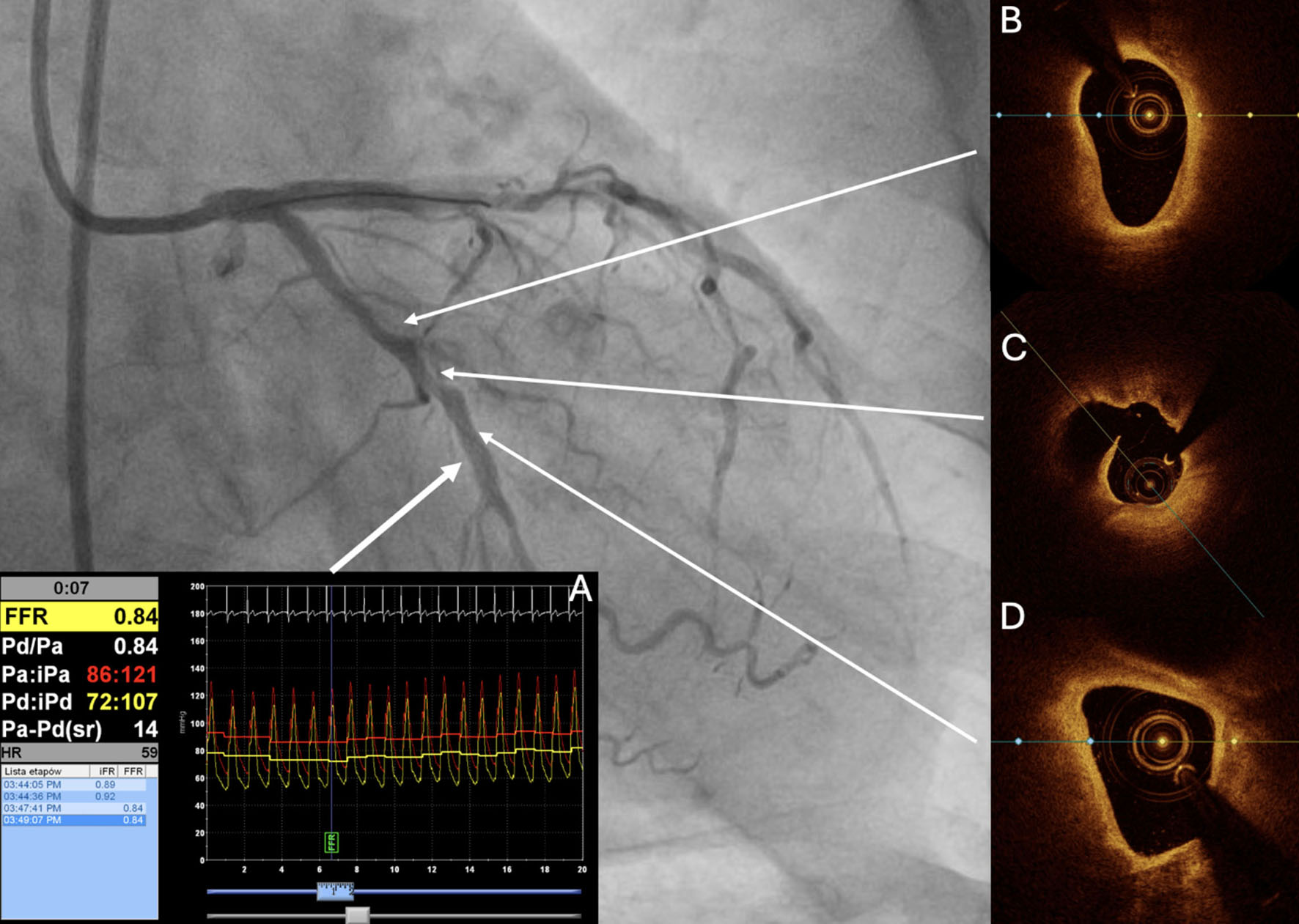
Figure 3. Evaluation of a non-culprit lesion in non-ST-segment elevation myocardial infarction. Evaluation of a non-culprit lesion in non-ST-segment elevation myocardial infarction. FFR (A) was performed in the non-culprit circumflex artery, yielding a value of 0.84 (not hemodynamically significant). However, OCT revealed a long lipid plaque with TCFA (B, D) and a probable silent plaque rupture site (C). Ongoing studies aim to determine whether such hemodynamically non-significant but high-risk lesions should be stented. FFR: Fractional flow reserve; OCT: optical coherence tomography; TCFA: thin-cap fibroatheroma.
One of the first direct in vivo links between TCFA and subsequent adverse events was presented in the Providing Regional Observations to Study Predictors of Events in the Coronary Tree (PROSPECT) study[35]. Among 697 patients, 313 had TCFA in 596 angiographically non-culprit lesions. In lesion-level analysis, plaque burden ≥ 70%, TCFA, and MLA ≤ 4.0 mm2 were independent predictors of non-culprit lesion-related MACE. The more recent PROSPECT II study enrolled 898 patients with 3,629 non-culprit lesions[36]. Only patients within 4 weeks of ACS were included, and non-culprit, non-flow-limiting lesions were assessed using IVI (IVUS + near-infrared spectroscopy). Over a median follow-up of 3.7 years, high lipid content and large plaque burden were independent predictors of lesion-related MACE. In a PROSPECT II substudy, the investigators reported that lipid content at the culprit site was not associated with in-stent MACE; however, residual lipid and uncovered plaque burden at the stent edge were linked to a higher risk of stent edge-related culprit lesion MACE[37].
An OCT study involving patients with SA and ACS showed that, over 6 years of lesion-level follow-up, only non-culprit lesions identified as lipid-rich at baseline progressed to ACS[38]. Notably, 33% of baseline lipid-rich plaques with TCFA caused ACS during follow-up.
Significant findings were also reported by Kedhi et al. in the COMBINE OCT-FFR trial[39]. This study included patients with diabetes mellitus and either SA or ACS (approximately one-quarter of the total). Non-culprit plaques were first assessed with FFR and then with OCT. Patients with FFR-negative lesions and TCFA had a significantly higher risk of the primary endpoint (cardiac death, target vessel MI, clinically driven target lesion revascularization, or hospitalization due to unstable or progressive angina) than those without TCFA (13.3% vs. 3.1%, respectively). Clinically driven target lesion revascularization occurred in 11.2% of TCFA-positive patients compared to 1.4% of TCFA-negative patients. The results remained consistent at the 5-year follow-up[40]. The PECTUS-obs study, which included only post-ACS patients, yielded similar findings[41]. OCT was performed for FFR-negative non-culprit lesions, as in the COMBINE OCT-FFR trial. During 2 years of follow-up, unplanned revascularization occurred in 9.8% of patients with OCT-defined high-risk lesions, compared to 4.3% of those with non-high-risk lesions (P < 0.05). Target vessel revascularization was also significantly more frequent among patients with high-risk lesions.
Procedure optimization with IVI
In daily clinical practice, IVI is increasingly used for procedural planning and optimization. With IVI, operators can determine the need for lesion preparation, select the proper stent size and length, and optimize outcomes after stent implantation. To date, both randomized trials and meta-analyses have demonstrated that IVI use is associated with a reduction in MACE compared to angiography-guided interventions[42]. These studies also reported significant reductions in all-cause and cardiovascular mortality, MI, and stent thrombosis. Compared with angiography guidance, IVI-guided PCI optimization has been associated with more frequent post-dilatation, larger minimal stent areas, and greater use of stents to minimize geographic miss and treat edge dissections[31]. Furthermore, in patients with NSTEMI versus SA, a strategy of FFR-directed, IVUS-guided optimization achieved comparable improvements in FFR, MLA, and minimal stent area[43].
Most IVI studies have been conducted in patients with SA, with relatively few in patients with ACS. Antonsen et al. studied 100 patients with NSTEMI and showed that OCT guidance improved Nobori stent strut coverage at 6 months compared with angiography[44]. Kala et al. investigated 201 patients with STEMI and found that those who underwent OCT-guided PCI had a smaller in-segment area of stenosis at 9 months compared with those in the angiography-guided arm[45]. Moreover, OCT guidance led to post-PCI optimization in 29% of cases. In the Does Optical Coherence Tomography Optimize Results of Stenting study, OCT-guided PCI was associated with higher post-procedural FFR compared with angiography-guided PCI in patients with NSTEMI[46].
For IVUS use in ACS, registry studies have suggested that IVUS guidance during long-term follow-up reduces the incidence of MACE compared to angiography guidance[47,48]. In the Korean registry of 13,104 ACS patients, 1,887 underwent IVUS-guided PCI and 7,120 underwent angiography-guided PCI[49]. After 3 years, in patients who underwent PCI under IVUS guidance, a significant reduction in target lesion failure was observed, mainly driven by cardiac death and target vessel MI. This analysis focused on patients treated with second-generation drug-eluting stents. Similarly, a Japanese registry including 6,025 ACS patients showed that IVI use reduced adverse events compared to angiography-guided PCI[50]. In a recent multicenter trial conducted in China, Pakistan, and the UK, 3,505 ACS patients were randomized to IVUS- or angiography-guided PCI[51]. After 1 year, the IVUS group had a significantly lower rate of target vessel failure (4.0% vs. 7.3%), although no differences were observed in stent thrombosis or all-cause mortality.
To the best of our knowledge, only one study has directly compared IVUS with OCT in ACS patients[52]. The Optical Frequency Domain Imaging vs. Intravascular Ultrasound in Percutaneous Coronary Intervention ACS randomized trial enrolled 158 patients (19 of whom were excluded). Clinical follow-up was scheduled at 8 and 12 months post-PCI. At 8 months, the mean MLA was 4.91 mm2 in the OCT group and 4.76 mm2 in the IVUS group. The incidence of MACE was similar between groups. The authors concluded that both OCT- and IVUS-guided PCI are safe and feasible in ACS patients. However, the study was not sufficiently powered to evaluate the clinical impact of IVI in this setting.
Deferred stenting with IVI
Patients presenting with ACS are typically treated with PCI and stenting, regardless of the underlying pathology. However, in certain cases, treatment can be personalized [Figure 4]. A prime example is plaque erosion detected with OCT. It is well established that patients with plaque rupture have a worse prognosis than those with plaque erosion[53]. In the EROSION study, 55 patients with OCT-confirmed plaque erosion, residual vessel stenosis < 70%, and thrombolysis in myocardial infarction (TIMI) grade III flow on angiography received conservative treatment with antithrombotic therapy rather than stent implantation[54]. At the 1-year follow-up, 49 of 53 evaluable patients remained free from MACE[55]. At 4 years, 11 patients required target lesion revascularization; however, no hard endpoints (death, MI, stroke, bypass surgery, or heart failure) were reported[56].
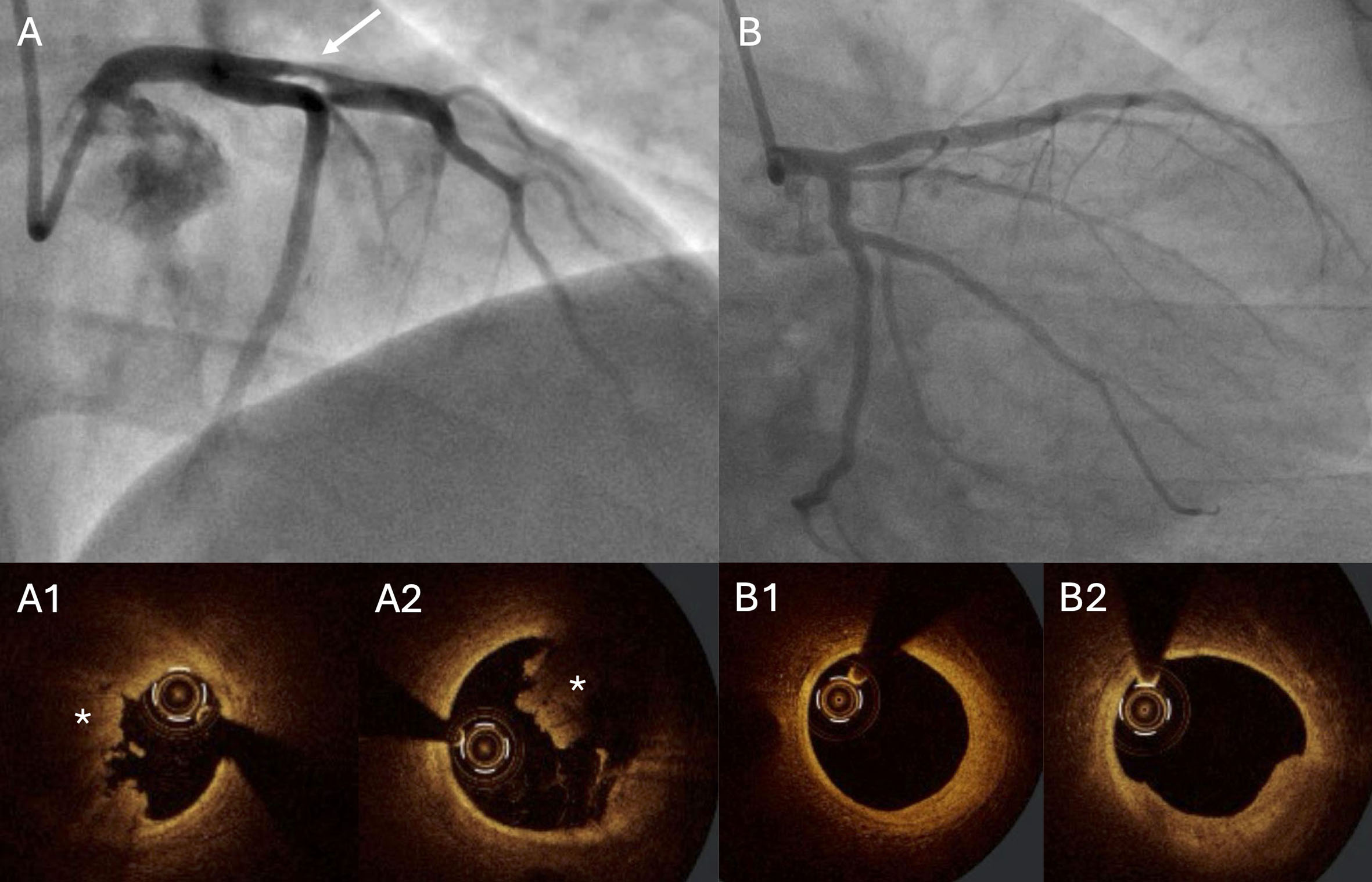
Figure 4. Patient admitted with ST-elevation myocardial infarction. A 34-year-old patient was admitted with anterior wall STEMI. Angiography revealed significant stenosis in the proximal LAD (A; arrow). Thrombectomy was performed, followed by OCT. The OCT image showed a high thrombus burden (A1 and A2; asterisk). PCI was deferred, and the patient received DAPT and anticoagulation (enoxaparin). Angiography with OCT was repeated one week later (B), showing only a small thrombus, fibrous plaque without significant stenosis, and no signs of plaque rupture (B1 and B2). The operators decided not to stent the proximal LAD and recommended OMT. DAPT: Dual antiplatelet therapy; LAD: left anterior descending artery; OCT: optical coherence tomography; OMT: optimal medical therapy; PCI: percutaneous coronary intervention; STEMI: ST-elevation myocardial infarction
NOVEL CONCEPTS AND MODALITIES
Results from studies such as PROSPECT, COMBINE OCT-FFR, and PECTUS-obs have prompted evaluation of mechanical stabilization of non-culprit, hemodynamically non-significant plaques. In the Prospective Randomized Evaluation of the Vascular Effects of Norvasc Trial (PREVENT), 1,606 patients with FFR-negative lesions (≥ 0.8) were included[57]. Lesions were assessed using IVUS, virtual histology intravascular ultrasound (VH-IVUS), OCT, or NIRS. Those with ≥ 2 high-risk plaque features were randomized to PCI or optimal medical therapy (OMT). High-risk plaque features were defined as plaque burden > 70%, MLA ≤ 4.0 mm2, TCFA on OCT or VH-IVUS, and lipid-rich plaque on NIRS. At 2 years, the primary outcome occurred in 3patients (0.4%) in the PCI group compared with 27 patients (3.4%) in the OMT group. However, the study had some limitations. Fewer than 4% of patients presented with either STEMI or NSTEMI. Moreover, plaque vulnerability was mainly determined by MLA or plaque burden; fewer than 7% of high-risk lesions had TCFA, and only approximately 27% were lipid-rich. Currently, the COMBINE-INTERVENE trial is underway. Like the PREVENT study, it focuses on FFR-negative lesions, but high-risk features will be defined exclusively using OCT. If these trials yield positive results, they may lead to a shift in clinical practice toward stenting FFR-negative, high-risk lesions.
Novel physiology-based assessment modalities
To reduce costs, shorten procedure times, and minimize risks associated with pharmacological agents or invasive measurements, new physiology-based modalities have been introduced. Among these, the instantaneous wave-free ratio (iFR) is most widely used. However, in patients with ACS, iFR may overestimate lesion severity[25]. Another approach, the quantitative flow ratio (QFR), is an angiography-based, non-invasive technique for evaluating lesion severity. Musto et al. studied 209 patients with STEMI and MVD who underwent iFR-guided revascularization[58]. At 1-year follow-up, deferring PCI in non-culprit lesions based on iFR was shown to be safe, with low adverse event rates. Bär et al. showed that
Despite these promising findings, evidence supporting iFR and QFR in ACS remains limited due to small sample sizes. Larger randomized trials are therefore needed to clarify their clinical utility, especially in light of the results of the Functional Assessment versus Angiography for Multivessel Evaluation III trial[65].
CONCLUSIONS AND FUTURE PERSPECTIVES
Most studies on IVI and FFR have been conducted in patients with SA. Findings regarding FFR in ACS, particularly in STEMI, remain inconsistent, which is reflected in current ESC guideline recommendations. By contrast, IVI studies focused exclusively on ACS are limited but encouraging. In the near future, advancements in equipment and technology (including artificial intelligence) may shift clinical practice from FFR toward novel physiology-based modalities such as QFR or iFR. However, additional studies are required, as current evidence remains insufficient and inconsistent. Given the conflicting results of FFR studies, there is increasing emphasis on identifying high-risk plaque characteristics rather than relying solely on hemodynamic significance. In many countries, financial constraints limit the combined use of FFR and IVI within the same procedure. This will hopefully change as growing evidence supports the complementary use of both modalities to improve patient outcomes. Nevertheless, further research is necessary to establish optimal diagnostic and therapeutic strategies for managing non-culprit, high-risk plaques in patients with acute coronary syndromes.
DECLARATIONS
Authors’ contributions
Conceptualization: Bryniarski K, Jang IK
Methodology: Bryniarski K, Gasior P, Bulnes J, Kleczynski P
Writing - original draft preparation: Bryniarski K, Niewiara L, Szolc P
Writing - review and editing: Bryniarski K, Gasior P, Camilleri W, Tomaniak M, Ntantou E, Bulnes J, Niewiara L, Szolc P, Kleczynski P, Legutko J, Jang IK
Table preparation: Bryniarski K
Figures preparation: Bryniarski K, Gasior P, Szolc P, Niewiara L, Legutko J
Supervision: Jang IK, Legutko J
Availability of data and materials
The images used were obtained from the Authors’ database. The datasets that support the findings of this study are available from the corresponding author upon reasonable request.
Financial support and sponsorship
None.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Bhatt DL, Lopes RD, Harrington RA. Diagnosis and treatment of acute coronary syndromes: a review. JAMA. 2022;327:662-75.
2. Park DW, Clare RM, Schulte PJ, et al. Extent, location, and clinical significance of non-infarct-related coronary artery disease among patients with ST-elevation myocardial infarction. JAMA. 2014;312:2019-27.
3. Balbi MM, Scarparo P, Tovar MN, et al. Culprit lesion detection in patients presenting with non-ST elevation acute coronary syndrome and multivessel disease. Cardiovasc Revasc Med. 2022;35:110-8.
4. Sorajja P, Gersh BJ, Cox DA, et al. Impact of multivessel disease on reperfusion success and clinical outcomes in patients undergoing primary percutaneous coronary intervention for acute myocardial infarction. Eur Heart J. 2007;28:1709-16.
5. Mehta SR, Wood DA, Storey RF, et al. Complete revascularization with multivessel PCI for myocardial infarction. N Engl J Med. 2019;381:1411-21.
6. Tonino PA, Fearon WF, De Bruyne B, et al. Angiographic versus functional severity of coronary artery stenoses in the FAME study fractional flow reserve versus angiography in multivessel evaluation. J Am Coll Cardiol. 2010;55:2816-21.
7. Vrints C, Andreotti F, Koskinas KC, et al. 2024 ESC guidelines for the management of chronic coronary syndromes: developed by the task force for the management of chronic coronary syndromes of the European Society of Cardiology (ESC) Endorsed by the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2024;45:3415-537.
8. Byrne RA, Rossello X, Coughlan JJ, et al. 2023 ESC guidelines for the management of acute coronary syndromes: developed by the task force on the management of acute coronary syndromes of the European Society of Cardiology (ESC). Eur Heart J. 2023;44:3720-826.
9. Fabris E, Kedhi E, Verdoia M, et al. Current role of intracoronary imaging for implementing risk stratification and tailoring culprit lesion treatment: a narrative review. J Clin Med. 2023;12:3393.
10. Ganzorig N, Pompei G, Jenkins K, et al. Role of physiology in the management of multivessel disease among patients with acute coronary syndrome. AsiaIntervention. 2024;10:157-68.
11. Tonino PA, De Bruyne B, Pijls NH, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360:213-24.
12. De Bruyne B, Pijls NH, Kalesan B, et al. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med. 2012;367:991-1001.
13. Zimmermann FM, Omerovic E, Fournier S, et al. Fractional flow reserve-guided percutaneous coronary intervention vs. medical therapy for patients with stable coronary lesions: meta-analysis of individual patient data. Eur Heart J. 2019;40:180-6.
14. Smits PC, Laforgia PL, Abdel-Wahab M, et al. Fractional flow reserve-guided multivessel angioplasty in myocardial infarction: three-year follow-up with cost benefit analysis of the Compare-Acute trial. EuroIntervention. 2020;16:225-32.
15. Engstrøm T, Kelbæk H, Helqvist S, et al. Complete revascularisation versus treatment of the culprit lesion only in patients with ST-segment elevation myocardial infarction and multivessel disease (DANAMI-3 - PRIMULTI): an open-label, randomised controlled trial. Lancet. 2015;386:665-71.
16. Böhm F, Mogensen B, Engstrøm T, et al. FFR-Guided Complete or Culprit-Only PCI in patients with myocardial infarction. N Engl J Med. 2024;390:1481-92.
17. Sels JW, Tonino PA, Siebert U, et al. Fractional flow reserve in unstable angina and non-ST-segment elevation myocardial infarction experience from the FAME (Fractional flow reserve versus Angiography for Multivessel Evaluation) study. JACC Cardiovasc Interv. 2011;4:1183-9.
18. Layland J, Oldroyd KG, Curzen N, et al. Fractional flow reserve vs. angiography in guiding management to optimize outcomes in non-ST-segment elevation myocardial infarction: the British Heart Foundation FAMOUS-NSTEMI randomized trial. Eur Heart J. 2015;36:100-11.
19. Puymirat E, Cayla G, Simon T, et al. Multivessel PCI guided by FFR or angiography for myocardial infarction. N Engl J Med. 2021;385:297-308.
20. Puymirat E, Cayla G, Simon T, et al. Three-year outcomes with fractional flow reserve-guided or angiography-guided multivessel percutaneous coronary intervention for myocardial infarction. Circ Cardiovasc Interv. 2024;17:e013913.
21. Lee JM, Kim HK, Park KH, et al. Fractional flow reserve versus angiography-guided strategy in acute myocardial infarction with multivessel disease: a randomized trial. Eur Heart J. 2023;44:473-84.
22. Elbadawi A, Dang AT, Hamed M, et al. FFR- versus angiography-guided revascularization for nonculprit stenosis in STEMI and multivessel disease: a network meta-analysis. JACC Cardiovasc Interv. 2022;15:656-66.
23. Omar A, Senguttuvan NB, Ueyama H, et al. Meta-analysis comparing fractional flow reserve and angiography-guided complete revascularization of nonculprit artery for ST-elevation myocardial infarction. Am J Cardiol. 2022;183:8-15.
24. Musto C, De Felice F, Rigattieri S, et al. Instantaneous wave-free ratio and fractional flow reserve for the assessment of nonculprit lesions during the index procedure in patients with ST-segment elevation myocardial infarction: the WAVE study. Am Heart J. 2017;193:63-9.
25. van der Hoeven NW, Janssens GN, de Waard GA, et al. Temporal changes in coronary hyperemic and resting hemodynamic indices in nonculprit vessels of patients with ST-segment elevation myocardial infarction. JAMA Cardiol. 2019;4:736-44.
26. Vikhert AM, Cherpachenko NM. Changes in metabolism of undamaged sections of myocardium following infarction. Circ Res. 1974;35:182-91.
27. Ntalianis A, Sels JW, Davidavicius G, et al. Fractional flow reserve for the assessment of nonculprit coronary artery stenoses in patients with acute myocardial infarction. JACC Cardiovasc Interv. 2010;3:1274-81.
28. Legutko J, Bryniarski KL, Kaluza GL, et al. Intracoronary imaging of vulnerable plaque-from clinical research to everyday practice. J Clin Med. 2022;11:6639.
29. Mintz GS, Guagliumi G. Intravascular imaging in coronary artery disease. Lancet. 2017;390:793-809.
30. Jang JS, Shin HC, Bae JS, et al. Diagnostic performance of intravascular ultrasound-derived minimal lumen area to predict functionally significant non-left main coronary artery disease: a meta-analysis. Korean Circ J. 2016;46:622-31.
31. Mintz GS, Matsumura M, Ali Z, Maehara A. Clinical utility of intravascular imaging: past, present, and future. JACC Cardiovasc Imaging. 2022;15:1799-820.
32. Kerensky RA, Wade M, Deedwania P, Boden WE, Pepine CJ; Veterans Affairs Non-Q-Wave Infarction Stategies in-Hospital (VANQWISH) Trial Investigators. Revisiting the culprit lesion in non-Q-wave myocardial infarction. Results from the VANQWISH trial angiographic core laboratory. J Am Coll Cardiol. 2002;39:1456-63.
33. Reynolds HR, Maehara A, Kwong RY, et al. Coronary optical coherence tomography and cardiac magnetic resonance imaging to determine underlying causes of myocardial infarction with nonobstructive coronary arteries in women. Circulation. 2021;143:624-40.
34. Zeng M, Zhao C, Bao X, et al. Clinical characteristics and prognosis of MINOCA caused by atherosclerotic and nonatherosclerotic mechanisms assessed by OCT. JACC Cardiovasc Imaging. 2023;16:521-32.
35. Stone GW, Maehara A, Lansky AJ, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364:226-35.
36. Erlinge D, Maehara A, Ben-Yehuda O, et al. Identification of vulnerable plaques and patients by intracoronary near-infrared spectroscopy and ultrasound (PROSPECT II): a prospective natural history study. Lancet. 2021;397:985-95.
37. Kjøller-Hansen L, Maehara A, Kelbæk H, et al. Impact of lipidic plaque on in-stent and stent edge-related events after PCI in myocardial infarction: a PROSPECT II substudy. Circ Cardiovasc Interv. 2024;17:e014215.
38. Kubo T, Ino Y, Mintz GS, et al. Optical coherence tomography detection of vulnerable plaques at high risk of developing acute coronary syndrome. Eur Heart J Cardiovasc Imaging. 2021;22:1376-84.
39. Kedhi E, Berta B, Roleder T, et al. Thin-cap fibroatheroma predicts clinical events in diabetic patients with normal fractional flow reserve: the COMBINE OCT-FFR trial. Eur Heart J. 2021;42:4671-9.
40. Fabris E, Berta B, Hommels T, et al. Long-term outcomes of patients with normal fractional flow reserve and thin-cap fibroatheroma. EuroIntervention. 2023;18:e1099-107.
41. Mol JQ, Volleberg RHJA, Belkacemi A, et al. Fractional flow reserve-negative high-risk plaques and clinical outcomes after myocardial infarction. JAMA Cardiol. 2023;8:1013-21.
42. Stone GW, Christiansen EH, Ali ZA, et al. Intravascular imaging-guided coronary drug-eluting stent implantation: an updated network meta-analysis. Lancet. 2024;403:824-37.
43. Groenland FTW, Neleman T, Ziedses des Plantes AC, et al. Fractional flow reserve directed percutaneous coronary intervention optimization using high-definition intravascular ultrasound in non-ST-segment elevation acute coronary syndrome versus chronic coronary syndrome. Catheter Cardiovasc Interv. 2025;106:12-22.
44. Antonsen L, Thayssen P, Maehara A, et al. Optical coherence tomography guided percutaneous coronary intervention with nobori stent implantation in patients with non-ST-segment-elevation myocardial infarction (OCTACS) trial: difference in strut coverage and dynamic malapposition patterns at 6 months. Circ Cardiovasc Interv. 2015;8:e002446.
45. Kala P, Cervinka P, Jakl M, et al. OCT guidance during stent implantation in primary PCI: A randomized multicenter study with nine months of optical coherence tomography follow-up. Int J Cardiol. 2018;250:98-103.
46. Meneveau N, Souteyrand G, Motreff P, et al. Optical coherence tomography to optimize results of percutaneous coronary intervention in patients with non-ST-elevation acute coronary syndrome: results of the multicenter, randomized DOCTORS study (does optical coherence tomography optimize results of stenting). Circulation. 2016;134:906-17.
47. Maluenda G, Lemesle G, Ben-Dor I, et al. Impact of intravascular ultrasound guidance in patients with acute myocardial infarction undergoing percutaneous coronary intervention. Catheter Cardiovasc Interv. 2010;75:86-92.
48. Okura H, Saito Y, Soeda T, et al. Frequency and prognostic impact of intravascular imaging-guided urgent percutaneous coronary intervention in patients with acute myocardial infarction: results from J-MINUET. Heart Vessels. 2019;34:564-71.
49. Kim Y, Bae S, Johnson TW, et al. Role of intravascular ultrasound-guided percutaneous coronary intervention in optimizing outcomes in acute myocardial infarction. J Am Heart Assoc. 2022;11:e023481.
50. Yamashita T, Sakamoto K, Tabata N, et al. Imaging-guided PCI for event suppression in Japanese acute coronary syndrome patients: community-based observational cohort registry. Cardiovasc Interv Ther. 2021;36:81-90.
51. Li X, Ge Z, Kan J, et al. Intravascular ultrasound-guided versus angiography-guided percutaneous coronary intervention in acute coronary syndromes (IVUS-ACS): a two-stage, multicentre, randomised trial. Lancet. 2024;403:1855-65.
52. Otake H, Kubo T, Hibi K, et al. Optical frequency domain imaging-guided versus intravascular ultrasound-guided percutaneous coronary intervention for acute coronary syndromes: the OPINION ACS randomised trial. EuroIntervention. 2024;20:e1086-97.
53. Nakajima A, Sugiyama T, Araki M, et al. Plaque rupture, compared with plaque erosion, is associated with a higher level of pancoronary inflammation. JACC Cardiovasc Imaging. 2022;15:828-39.
54. Jia H, Dai J, Hou J, et al. Effective anti-thrombotic therapy without stenting: intravascular optical coherence tomography-based management in plaque erosion (the EROSION study). Eur Heart J. 2017;38:792-800.
55. Xing L, Yamamoto E, Sugiyama T, et al. EROSION study (effective anti-thrombotic therapy without stenting: intravascular optical coherence tomography-based management in plaque erosion): a 1-year follow-up report. Circ Cardiovasc Interv. 2017;10:e005860.
56. He L, Qin Y, Xu Y, et al. Predictors of non-stenting strategy for acute coronary syndrome caused by plaque erosion: four-year outcomes of the EROSION study. EuroIntervention. 2021;17:497-505.
57. Park SJ, Ahn JM, Kang DY, et al. Preventive percutaneous coronary intervention versus optimal medical therapy alone for the treatment of vulnerable atherosclerotic coronary plaques (PREVENT): a multicentre, open-label, randomised controlled trial. Lancet. 2024;403:1753-65.
58. Musto C, Scappaticci M, Biondi-Zoccai G, et al. Instantaneous wave-free ratio-guided revascularization of nonculprit lesions in STEMI patients with multivessel coronary disease: the WAVE registry. Catheter Cardiovasc Interv. 2022;100:351-9.
59. Bär S, Kavaliauskaite R, Ueki Y, et al. Quantitative flow ratio to predict nontarget vessel-related events at 5 years in patients with ST-segment-elevation myocardial infarction undergoing angiography-guided revascularization. J Am Heart Assoc. 2021;10:e019052.
60. Lauri FM, Macaya F, Mejía-Rentería H, et al. Angiography-derived functional assessment of non-culprit coronary stenoses in primary percutaneous coronary intervention. EuroIntervention. 2020;15:e1594-601.
61. Erriquez A, Campo G, Guiducci V, et al. QFR for the revascularization of nonculprit vessels in MI patients: insights from the FIRE trial. JACC Cardiovasc Interv. 2024;17:1425-36.
62. Erbay A, Penzel L, Abdelwahed YS, et al. Prognostic impact of quantitative flow ratio (QFR)-consistent complete revascularization in patients with myocardial infarction and multivessel coronary artery disease. Am Heart J. 2024;276:22-30.
63. Barauskas M, Žiubrytė G, Jodka N, Unikas R. Quantitative flow ratio vs. angiography-only guided PCI in STEMI patients: one-year cardiovascular outcomes. BMC Cardiovasc Disord. 2023;23:136.
64. Xu X, Fang C, Jiang S, et al. Functional or anatomical assessment of non-culprit lesions in acute myocardial infarction. EuroIntervention. 2025;21:e217-28.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Special Issue
Copyright
Data & Comments
Data
















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].