Artificial intelligence for enhancing decision-making in multidisciplinary tumor boards for HCC in China
Abstract
Hepatocellular carcinoma (HCC) exhibits high incidence and mortality rates in China, posing a significant public health burden. Established risk factors, including hepatitis B virus, hepatitis C virus, aflatoxin B1 exposure, alcohol consumption, and smoking, shape the unique epidemiological profile of HCC in China and exacerbate its marked tumor heterogeneity. This complexity leads to highly intricate prognostic assessment, management strategies, and predictive approaches across diverse patient populations. The updated “Guidelines for Diagnosis and Treatment of Primary Liver Cancer (2024 Edition)” reflect significant advancements in screening, diagnosis, staging, treatment, and follow-up, with particular emphasis on management strategies tailored to the Chinese context. The multidisciplinary tumor board (MDTB) serves as a cornerstone of modern oncology care. By integrating expertise from diverse medical specialties, the MDTB is crucial for developing individualized treatment plans for complex HCC cases. However, current MDTB practice faces significant challenges, primarily stemming from the rapid evolution of treatment options and the swift advancement of emerging technologies, particularly artificial intelligence (AI). This necessitates continuous learning among MDTB members to effectively integrate cutting-edge therapies and tools. This review focuses on the disease characteristics of HCC in China and the unmet needs within its clinical management. It delves into how AI technologies can enhance the capabilities of MDTBs, aiming to elucidate the transformative potential and persisting challenges of AI-driven multidisciplinary care models for HCC in China.
Keywords
INTRODUCTION
Liver cancer is the second most common cancer in China, accounting for approximately 51% of global annual deaths from the disease[1]. Major risk factors include chronic hepatitis B virus (HBV) and hepatitis C virus (HCV) infections. Although its incidence has declined since 1990, the burden of liver cancer remains heavy. In 2020, there were over 410,000 new cases and more than 390,000 deaths in China, highlighting the urgent need for improved management[2]. To enhance the quality of diagnosis and treatment and increase patient survival rates, the multidisciplinary team (MDT) model has been progressively promoted and implemented in hospitals across China[3]. This approach aims to optimize comprehensive hepatocellular carcinoma (HCC) care through interdisciplinary collaboration.
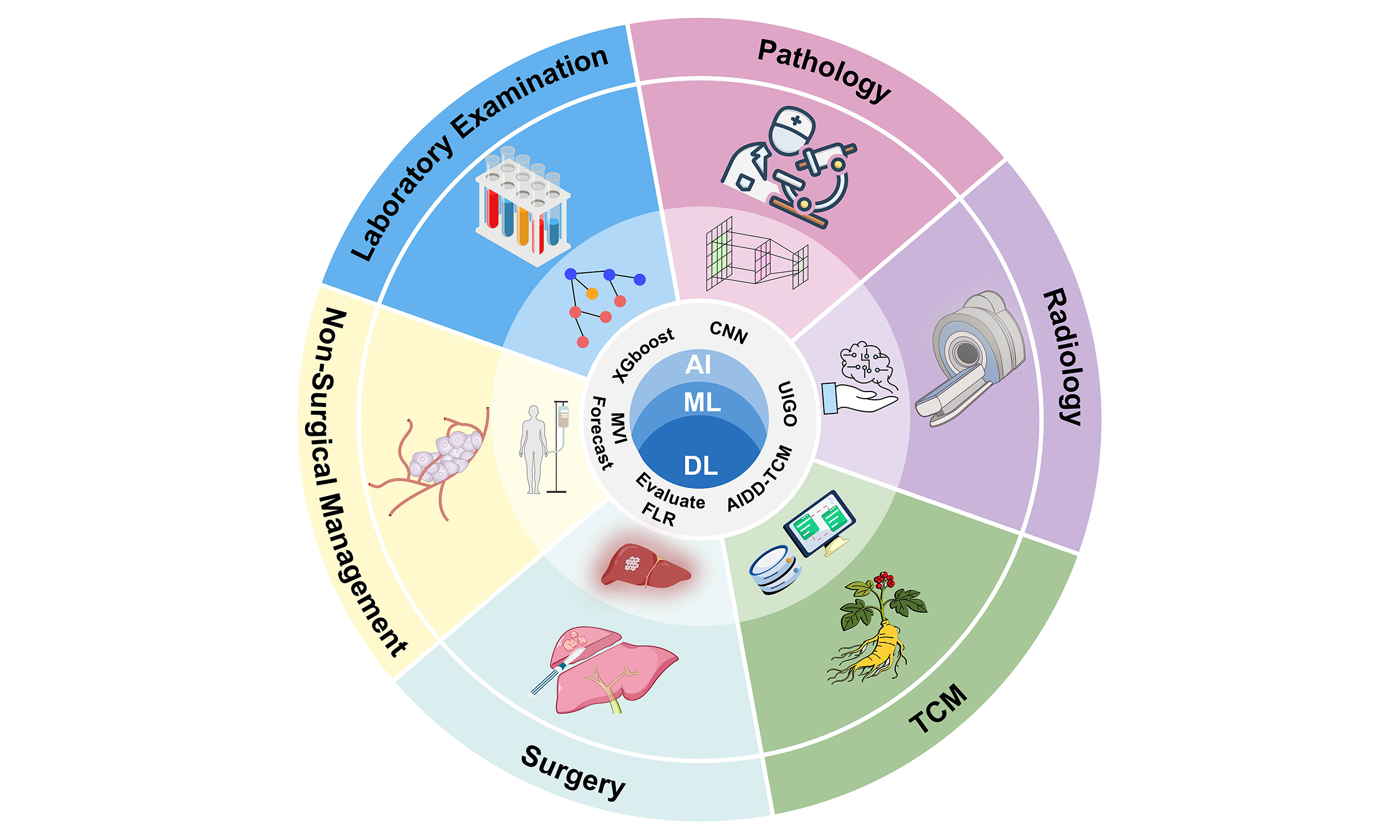
In this context, artificial intelligence (AI) is playing an increasingly vital role in multidisciplinary tumor boards (MDTBs). AI refers to computer systems capable of performing tasks that typically require human intelligence, such as perception, reasoning, and learning[4]. Machine learning (ML), a subset of AI, enables systems to learn autonomously from data and make predictions[5]. Deep learning (DL), a significant branch of ML, uses multi-layer artificial neural networks (ANNs) that simulate the connections of biological neurons to achieve automated feature extraction, making it particularly suited for handling complex tasks[6]. Among DL models, convolutional neural networks (CNNs) have shown remarkable advantages in medical image analysis. By applying convolutional filters to identify subtle patterns - such as edges and textures - in images, CNNs can assist in detecting imaging features that are challenging for the human eye to discern, such as irregular tumor margins or internal necrosis in HCC[7,8]. This significantly improves the prediction accuracy of prognostic factors including microvascular invasion (MVI)[9]. As shown in Table 1, these ML models are being applied across various domains of HCC management, from diagnosis and prognostic assessment to treatment planning, demonstrating their transformative potential in clinical practice.
Advances in the application of AI in HCC
| ML type | Application domain | Specific algorithm | Performance | Ref. |
| DL (CNN) | HCC prediction | Customized CNN for MRS data processing and classification | Early screening & risk prediction | Bae et al. (2025)[10] |
| Supervised learning (ensemble) | HCC prediction | M2P-HCC | Risk stratification & prognostic prediction | Hou et al. (2025)[11] |
| Supervised learning (survival analysis) | HCC prediction | aMAP-2Plus | Risk stratification & prognostic prediction | Potter et al. (2023)[12]; Hao et al. (2024)[13] |
| DL (transformer) | Pathology | Transformer-based model for feature extraction from H&E-stained WSIs | Differential diagnosis | Diao et al. (2022)[14] |
| Hybrid model (DL + optimization algorithm) | Radiomics | UIGO | Tumor detection and segmentation | Banerjee et al. (2025)[15] |
| Supervised learning (ensemble methods) | Multimodal diagnostic models | Ensemble models (XGBoost) based on serum peptidome profiles from MALDI-TOF MS | Early diagnosis & differential diagnosis | Prajumwongs et al. (2025)[16] |
| GNN | Multimodal diagnostic models | DenseGCN | Biomarker discovery | Zhang et al. (2022)[17] |
| Supervised learning (ensemble) | Multimodal diagnostic models | Pathway-based ML model | Biological mechanism exploration | Sucularli et al. (2025)[18] |
AI-DRIVEN PREDICTION OF HCC
In China, the primary high-risk groups for liver cancer include: individuals with HBV and/or HCV infection, excessive alcohol consumption, non-alcoholic fatty liver disease, history of aflatoxin exposure, cirrhosis of the liver due to various causes, and those with a family history of liver cancer[21-23]. At present, early screening for HCC primarily relies on imaging examinations, serum alpha-fetoprotein (AFP) testing, and detection of de-γ-carboxyprothrombin (PIVKA-II)[24]. However, the diagnostic accuracy of these methods has limitations; a meta-analysis revealed that the sensitivity of combined AFP and ultrasound (US) examination was only 63%[25]. To overcome the limitations of traditional screening methods, AI has become a crucial tool for analyzing vast amounts of complex data and constructing precise predictive models[26,27]. In the field of multi-omics liquid biopsy, AI models have demonstrated capabilities that surpass those of traditional biomarkers. For instance, the methylation, mutation, and protein (M2P)-HCC model integrates multi-dimensional HCC-related biomarkers - including gene methylation, somatic mutations, and proteins - through ML and has been incorporated into expert consensus guidelines[28]. Research indicates that M2P-HCC can detect early-stage HCC prior to the detection of hepatic lesions by abdominal US. However, despite the exceptional performance of such multi-omics models, they rely heavily on costly liquid biopsy and genetic sequencing technologies. For China’s vast population at high risk, the prohibitive expense and limited accessibility in primary healthcare settings represent core bottlenecks hindering their widespread adoption.
Regarding routine clinical data, the latest Guidelines for the Diagnosis and Treatment of Primary Liver Cancer recommend risk stratification using the age-Male-ALBI-Platelets (aMAP) score[29]. Building upon this, the aMAP-2 and aMAP-2Plus models - which further integrate multivariate longitudinal data with circulating free DNA (cfDNA) characteristics - can precisely identify ultra-high-risk populations with an annual HCC incidence risk as high as 12.5%[13]. Concurrently, classical ML algorithms such as extreme gradient boosting (XGBoost) have demonstrated outstanding performance in predicting HCC within specific studies, achieving accuracy rates as high as 87%[30]. However, such models face challenges in generalizability; a model validated within a specific population[31] must undergo rigorous external validation before application to China’s more diverse, HBV-predominant population.
Concurrently, DL has achieved breakthroughs in processing imaging and spectroscopic data. For instance, CNN-based models can analyze medical imaging data, identifying early-stage HCC with higher sensitivity and specificity than traditional methods[32-34]. However, the “black-box” nature of CNNs renders them insufficiently explainable, which remains a critical challenge in MDT decision-making requiring high levels of trust and accountability. Furthermore, the DL model developed by Bae et al. utilizes proton MR (1H-MR) spectroscopy to analyse the ratio of metabolites such as choline and lipids for HCC differentiation, achieving 94% sensitivity and 90.0% specificity[10]. However, the reliance on sophisticated spectroscopic instruments for model generation remains a significant barrier to widespread adoption in many developing countries and regions.
In summary, AI is propelling HCC early screening into an era of high precision by integrating multi-source heterogeneous data. Nevertheless, when adopting these tools, MDT practitioners must recognize that a model’s clinical value hinges not only on its reported accuracy but also on its cost-effectiveness, generalizability, and the interpretability of its decisions.
ROLE OF AI IN THE DIAGNOSIS OF HCC
Pathology
In MDTB, pathological diagnosis serves as the gold standard and cornerstone for all subsequent treatment decisions. However, traditional pathological diagnosis relies heavily on the individual experience of pathologists, proving not only time-consuming and labor-intensive but also prone to significant interobserver variability among physicians of differing experience levels, particularly in histological grading. This variability risks undermining the consistency of the standards upon which MDTs base their treatment planning.
AI technologies, particularly CNNs trained on whole-slide images (WSIs) of hematoxylin and eosin (HE) stained sections, are addressing these core challenges of efficiency and standardization in MDT[35-37]. Studies confirm that AI models can automatically identify and grade HCC regions with high accuracy[38-40]. For instance, models developed by Liu et al. and Diao et al.[14,40] have demonstrated performance surpassing that of junior and intermediate pathologists, achieving accuracy rates of 91%-97%[40], while significantly reducing diagnostic time. For MDT, this signifies that AI can provide faster, more consistent diagnostic evidence, mitigating uncertainties arising from individual experience variations.
However, this efficiency gain simultaneously exposes a critical bottleneck in interpretability. As studies indicate, while AI models are rapid, their diagnostic accuracy remains marginally below that of senior specialists, suggesting limitations in handling complex morphological nuances. For MDT, pathologists must not only assign grades but also explain their rationale. The “black-box” nature of AI models makes them difficult to fully trust, limiting their current potential as primary diagnostic tools and confining them more to an auxiliary screening role[41].
More significantly, AI’s contributions have extended beyond mere morphological recognition, venturing into the field of prognostic prediction - an area of greater concern for MDT[42,43]. AI is being employed to predict key prognostic factors such as MVI directly from HE-stained biopsy slides. This digital pathology biopsy capability offers MDT the potential for preoperative assessment of tumor aggressiveness. This holds significant value in guiding surgeons regarding surgical scope or oncologists in determining the necessity of neoadjuvant therapy.
However, the clinical translation of such advanced prognostic models immediately faces a formidable challenge: generalizability. As researchers have clearly demonstrated, model performance is constrained by the sample size and diversity of training data[44-46]. A high-performing model trained at Center A, with specific staining protocols and patient cohorts, will almost inevitably fail when deployed at Center B[47].
In summary, AI is propelling pathology from traditional morphological description toward the quantitative predictions required for MDT. However, for it to become a truly reliable decision-making partner within MDT, two core challenges must be addressed in the future: interpretability and multicenter external validation.
Radiomics
In MDT, imaging studies [computed tomography (CT)/magnetic resonance imaging (MRI)] serve as the core basis for defining tumor anatomical staging and guiding treatment decisions. However, traditional radiological diagnosis relies on radiologists’ visual assessment and qualitative descriptions (e.g., blurred margins, uneven enhancement). The advent of radiomics aims to advance imaging diagnosis from qualitative to quantitative by using AI to extract high-throughput quantitative features from imaging data that are imperceptible to the human eye[48-53].
The primary core value of AI in imaging diagnosis lies in classification and differential diagnosis, which is crucial for establishing the initial treatment pathway in MDT. Studies have demonstrated that CNN models based on radiomics excel in distinguishing HCC, intrahepatic cholangiocarcinoma (ICC), and benign lesions[54-56]. For instance, studies by Midya et al.[57] and Stollmayer et al.[58] both reported classification accuracies or specificities exceeding 90%. However, MDT requires not merely high accuracy but clinical robustness. These impressive figures are often achieved on rigorously curated, high-quality datasets. When confronted with the noisy, artefact-laden, and multi-vendor real-world images encountered in daily MDT practice, model performance almost inevitably suffers significant degradation.
The second key contribution of AI to MDT is Automatic Segmentation. Both surgeons and radiotherapists within MDT require precise tumor boundaries and volumes. AI models, such as the unified imaging omics optimization (UIGO) framework developed by Banerjee et al.[15], with a Dice coefficient exceeding 0.92, deliver rapid and reproducible segmentation results, liberating clinicians from time-consuming manual delineation. However, this efficiency gain introduces the challenge of human-machine conflict: AI segmentation logic differs from the anatomical reasoning of human radiologists. When AI-defined boundaries conflict with expert judgments, whom should the MDT trust? This “black-box” nature remains a primary implementation barrier[59,60].
The third and most forward-looking contribution of AI is non-invasive prognostic prediction. AI is being employed to analyze imaging features for predicting MVI, transarterial chemoembolization (TACE) treatment response, and patient overall survival (OS). This equips MDT with the ability to anticipate tumor biological behavior pre-treatment, enabling more precise selection between surgery, TACE, or stereotactic body radiotherapy (SBRT).
In summary, AI-driven radiomics is empowering MDT with enhanced quantitative analysis and predictive capabilities[61,62]. However, MDT must remain vigilant when adopting these tools: the high accuracy reported in current studies generally lacks multicenter, large-scale external validation[60], and the persistent lack of interpretability remains the core bottleneck preventing its adoption as a decision-making gold standard.
Multimodal diagnostic models
The era of precision medicine for HCC demands that MDT decision-making be grounded in a profound understanding of tumor molecular biology[63]. The true value of MDT lies in its interdisciplinary collaboration, yet its core challenge resides in systematically integrating vast amounts of heterogeneous data from diverse specialties (imaging, pathology, laboratory) to enable comprehensive preoperative assessment[64,65]. AI presents unprecedented opportunities for this purpose, with its core contribution being its role as a cross-disciplinary data fusion engine[66].
One of AI’s most cutting-edge applications is radiogenomics - the use of AI to deeply analyze routine, cost-effective imaging data to predict complex, expensive molecular information. This holds significant implications for MDT’s preoperative assessment. For instance, studies by Gao et al.[67] and Cheng et al.[68] demonstrated that AI models can predict lysyl oxidases (LOX) molecular expression levels or epidermal growth factor receptor (EGFR) mutation status with moderate to high accuracy [area under the curve (AUC) 0.775-0.838] solely by analyzing CT radiomics features. This digital biopsy offers the first potential for MDT to preoperatively predict tumor molecular subtypes. However, the data completeness of such models presents a frequently overlooked major challenge: a model trained on integrated imaging, genomic, and pathological data may experience a precipitous decline in predictive performance when applied to patients from primary care hospitals with only CT imaging.
When multi-omics data become available, AI’s integrative capabilities are further enhanced. Algorithms such as least absolute shrinkage and selection operator (LASSO) regression, support vector machine (SVM), and graph neural networks (GNNs) can efficiently identify key biomarkers from vast datasets encompassing transcriptomics, DNA methylation, and proteomics[17,18]. For instance, Prajumwongs et al.[16] employed ML to analyze matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) spectral data, distinguishing HCC, ICC, and pancreatic cancer with over 85% accuracy. This capability enables MDT to extract actionable insights from the data deluge. However, MDT must adopt a critical stance when incorporating these findings, confronting the interrogation of biological plausibility: Is the correlation between AI-identified imaging features and molecular mutations a genuine biological link, or merely a spurious correlation specific to the dataset? Without biological interpretability, MDT struggles to rely on such predictions for high-stakes treatment decisions.
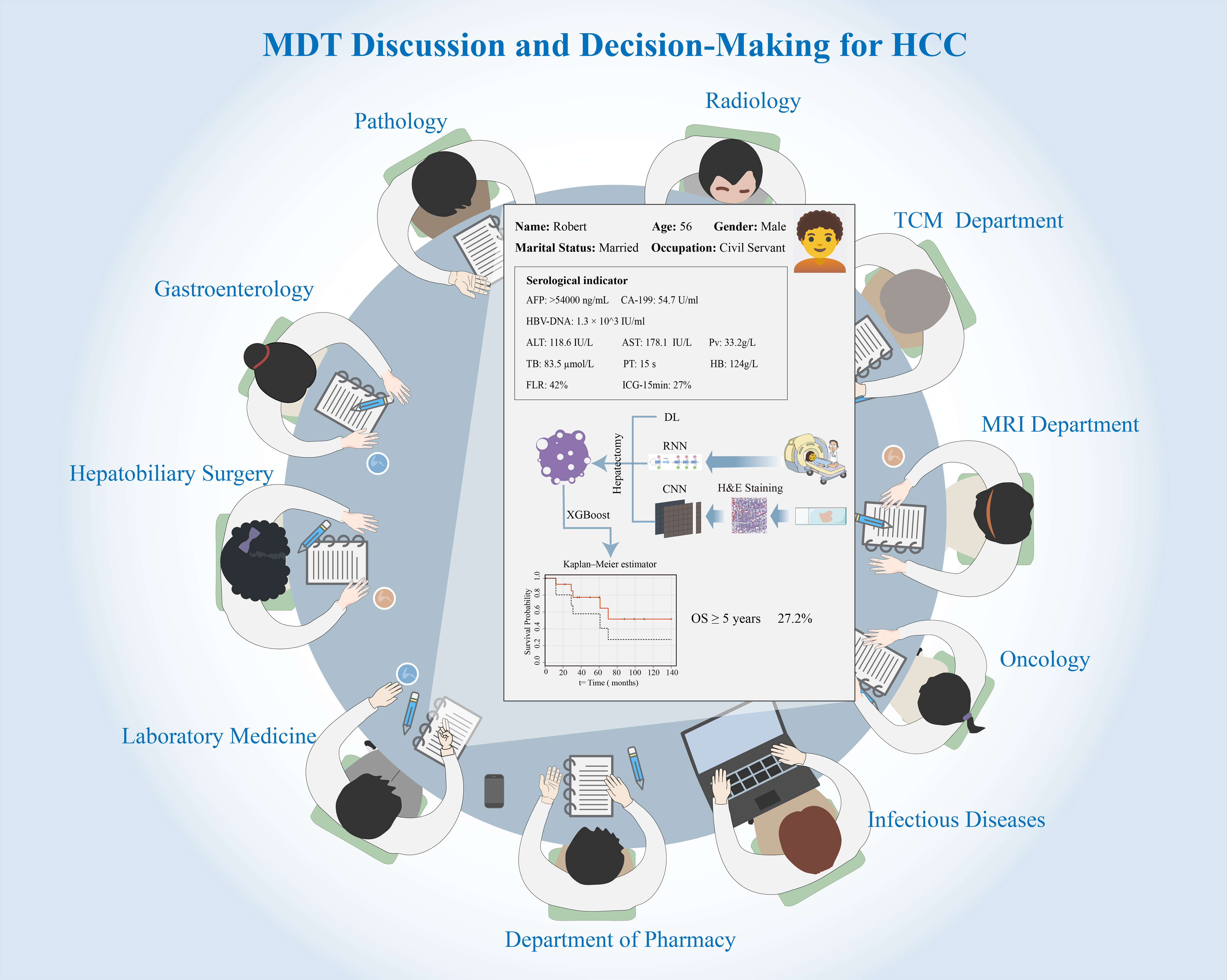
As illustrated in Figure 1, AI is evolving into the core engine driving MDT’s deep integration of multimodal data. By constructing comprehensive predictive models that transcend single-discipline knowledge, it provides MDT with more precise preoperative assessments. However, behind these high-accuracy reports lie deeper challenges MDT must overcome in clinical practice: data completeness dependency and biological plausibility validation.
Figure 1. MDT discussion and decision-making for HCC. Figure 1 illustrates how AI serves as the core engine for an integrated MDTB. The central panel represents an AI-driven decision support dashboard that integrates multimodal data (patient history, serological indicators, pathology, and radiology). AI models, such as CNNs for image analysis and RNNs for longitudinal data, process this information. The outputs, including quantitative predictions (e.g., survival probability calculated by XGBoost or Kaplan-Meier estimators) and treatment simulations, are presented to the MDT [including Hepatobiliary Surgery, Oncology, Radiology, TCM Department, etc.] to facilitate a unified, evidence-based, and personalized treatment plan. The illustration was created using Adobe Illustrator (version 2024). MDT: Multidisciplinary team; HCC: hepatocellular carcinoma; AI: artificial intelligence; MDTB: multidisciplinary tumor board; CNNs: convolutional neural networks; RNNs: recurrent neural networks; XGBoost: extreme gradient boosting; TCM: traditional Chinese medicine; MRI: magnetic resonance imaging.
AI IN WESTERN THERAPIES AND PROGNOSTIC ASSESSMENT
MDTB collaboration has become an indispensable component of treatment decision-making and prognostic management for HCC[69,70]. The role of AI is to enhance this process by providing quantitative, predictive, and personalized insights to the MDT, transcending static anatomical descriptions.
Application of AI in surgical management: integrating biology and anatomy
For surgical MDT, key decisions hinge on two factors: tumor biology and anatomical feasibility. AI provides critical decision support for both aspects.
Firstly, AI addresses tumor biology through preoperative prognostic prediction, a task previously reliant on postoperative pathology. Imaging informatics and DL models can now analyze preoperative CT/MRI scans to non-invasively estimate MVI probability, reporting AUC values as high as 0.77. This is pivotal for MDT, as high MVI risk prediction can alter the entire team’s strategy: it may prompt surgeons to favor more extensive anatomical resection or lead oncologists to recommend neoadjuvant therapy. However, the clinical translation of these MVI models faces a significant robustness challenge: while an AUC of 0.77[71] is promising, it may not be sufficiently robust for high-risk surgical decisions, and these models require large-scale, multicenter validation before they can be fully trusted by MDTs.
Secondly, AI enhances the assessment of anatomical feasibility. Surgeons’ decisions are constrained by the requirement to preserve sufficient future liver reserve (FLR)[72,73]. AI-driven automated segmentation of the liver, tumor, and vascular systems provides rapid, standardized volumetric measurements, optimizing FLR estimation[74,75]. This AI-driven 3D reconstruction (3D-RVT) is crucial for MDT planning of complex procedures such as associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) or portal vein embolization (PVE). Key barriers here are implementation and equity, particularly within the Chinese context. These advanced visualization systems require substantial computational power and specialized training, limiting their application to large urban centers and creating regional healthcare disparities.
Application of AI in non-surgical management: predicting response and standardizing planning
For the substantial cohort of patients unsuitable for resection, MDTBs must select from a complex array of non-surgical options [TACE, transarterial radioembolization (TARE), SBRT, and systemic therapies][16,76,77]. AI contributes through personalization and quantification.
AI’s most significant impact lies in predicting TACE response. MDTs have long strived to identify TACE-refractory patients early. CT/MRI-based radiomics and ML models can predict TACE response and survival prior to the initial procedure[78]. This enables MDTs to bypass ineffective TACE cycles, directing high-risk patients directly to more suitable therapies such as TARE, SBRT, or systemic treatment. A key limitation here is temporal validity, as many models were trained on data from the pre-immunotherapy era. Whether these same radiomics features can accurately predict TACE response in patients who have received or are about to receive immunotherapy remains a critical, unresolved question.
For ablation therapies, AI’s role is standardization. In radiotherapy, AI-driven automated segmentation accelerates SBRT planning, standardizing the delineation of the organ at risk. For TARE, voxel-based dosimetry supports personalized prescription[79]. The challenge here is clinical accountability; the “black-box” dilemma of AI has caused friction within MDT workflows, relegating it to a time-saving auxiliary tool rather than an independent decision-maker[71,80].
AI application in overall prognosis: a holistic perspective
Finally, after integrating all diagnostic and treatment-specific data, AI can provide MDT with a comprehensive prognostic assessment. Combined image-clinical ML models have demonstrated utility in predicting recurrence-free survival (RFS) and OS[81]. This is crucial for counselling patients and determining personalized postoperative monitoring intensity[82,83].
A significant challenge for these prognostic models, particularly in China, is the absence of traditional Chinese medicine (TCM) as a data variable. TCM represents a common adjunctive therapy in China. A prognostic model trained on western populations, which does not account for the immunomodulatory or symptom-relieving effects of concurrent TCM[84], will face questions regarding its generalizability when applied to MDT in China, owing to the omission of a key predictive feature. As AI approaches increasingly integrate imaging with genomics, they must also be adapted to incorporate regional therapeutic variables such as TCM to achieve true precision.
AI-driven integration of traditional Chinese and Western medicine for HCC
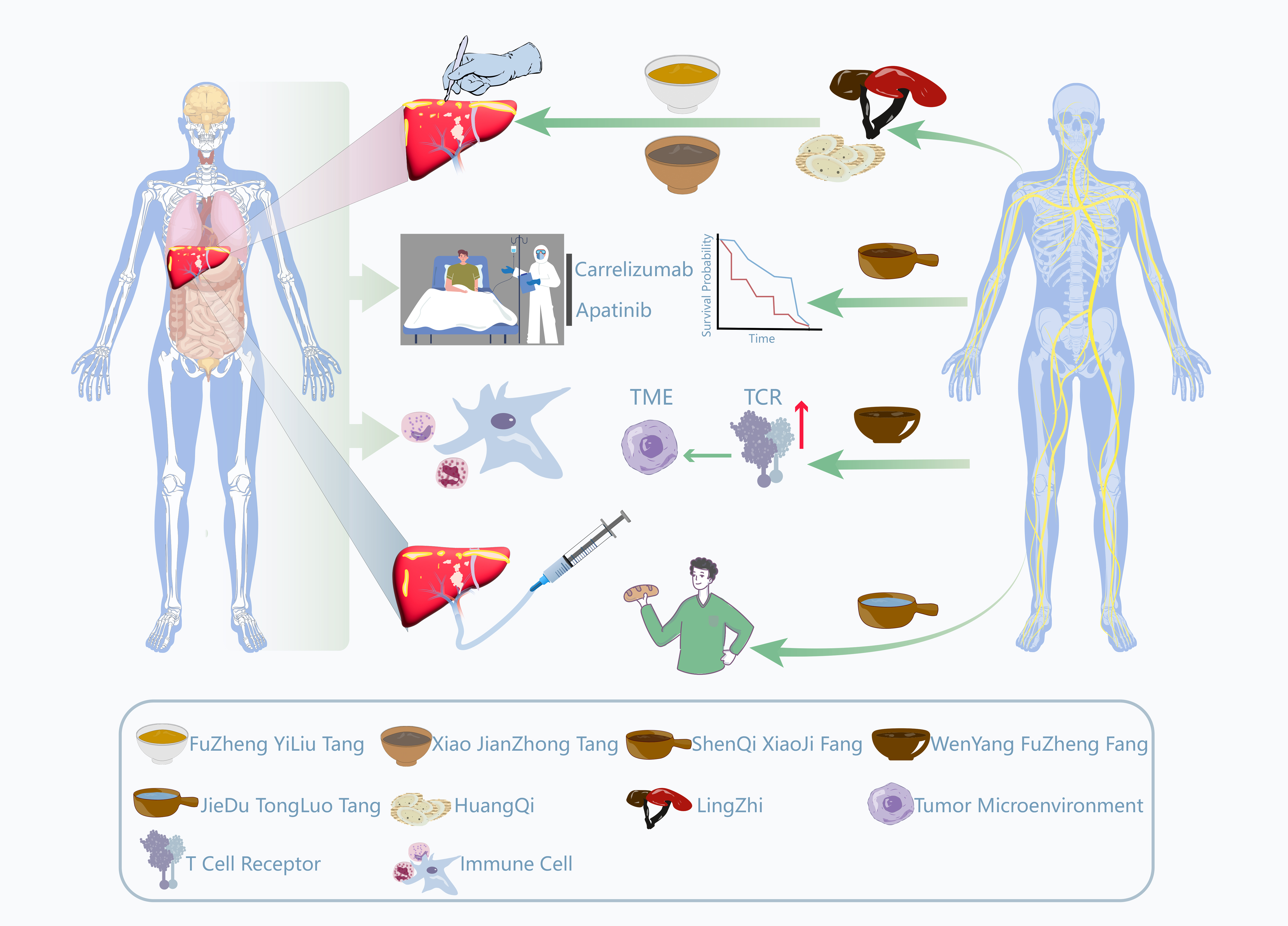
In China, integrated medicine (IM) constitutes a vital component of HCC clinical practice. TCM theory categorizes it under syndromes such as “mass accumulation” and “masses and masses”, emphasizing the pathological mechanisms of deficiency of vital energy and qi stagnation with blood stasis[85,86]. As a complementary therapy, TCM’s core principle lies in strengthening the healthy qi and expelling pathogenic factors. This involves enhancing host immunity and regulating bodily homeostasis to mitigate the toxic side effects of western medical treatments (such as TACE, chemotherapy, and immunotherapy) while improving patients’ quality of life[87-94]. As illustrated in Figure 2, TCM aims to modulate bodily homeostasis through multi-component, multi-targeted approaches. For instance, its active components, such as astragaloside IV and ginsenosides, have been demonstrated to exert synergistic antitumor effects with modern Western therapies through pathways including enhancement of the T-cell receptor (TCR) signaling pathway, optimization of cytokine networks, and remodeling of the tumor microenvironment (TME). Table 2 presents the specific constituents of various traditional Chinese medicinal formulas and their relevant applications in the field of HCC. However, despite TCM’s widespread application and growing clinical evidence, its integration into formal decision-making processes within MDT faces fundamental obstacles. The core issue lies in a translation barrier: TCM’s holistic, individualized diagnostic framework based on syndromes (Zheng, the TCM term for disease patterns) is incompatible with the data-driven, standardized, pathophysiology-based, evidence-based model relied upon by MDTs. This results in TCM’s clinical observations being difficult to objectively quantify and incorporate into unified decision models.
Figure 2. Schematic illustration of mechanisms and drug applications of combined Chinese and Western medicine in the treatment of HCC. Figure 2 illustrates the synergistic mechanisms between western medical treatments (e.g., surgery, immunotherapy, targeted therapy, and TACE) and TCM interventions. The role of AI is conceptual and computational: AI models analyze data derived from these pathways to quantify their interactions for the MDTB. AI can elucidate how TCM formulations (depicted as herbal bowls, such as FuZheng YiLiu Tang, Xiao JianZhong Tang, and ShenQi XiaoJi Fang) synergize with western therapies by modulating the TME or enhancing TCR signaling. This AI-driven integration enables the MDTB to objectively incorporate TCM into personalized treatment plans to enhance efficacy and reduce toxicity. The illustration was created using Adobe Illustrator (version 2024). HCC: Hepatocellular carcinoma; TACE: transarterial chemoembolization; TCM: traditional Chinese medicine; AI: artificial intelligence; MDTB: multidisciplinary tumor board; TME: tumor microenvironment; TCR: T-cell receptor; FuZheng YiLiu Tang: strengthening the body and anti-tumor decoction; Xiao JianZhong Tang: a formula designed to restore digestive balance by reducing inflammatory and stress responses; ShenQi XiaoJi Fang: Ginseng, Astragalus, and mass-eliminating formula; Wenyang Fuzheng Fang: a formula aimed at ameliorating the body’s deficient-cold state and remodeling the tumor microenvironment; Peiyuan Jiedu Tongluo Tang: a formula utilized to eliminate internal toxins and unblock microcirculation.
Details of TCM formulas
| Formula name | Key components | Main use | Ref. |
| Fuzheng Yiliu Tang (Strengthening the body and anti-tumor decoction) | Huangqi (Radix Astragali), Dangshen (Radix Codonopsis), Baizhu (Rhizoma Atractylodis Macrocephalae), Lingzhi (Ganoderma), etc. | Used post-radical resection for primary HCC to extend patient survival | Zhang et al.[89] |
| Xiao Jianzhong Tang (A formula designed to restore digestive balance by reducing inflammatory and stress responses) | Guizhi (Ramulus Cinnamomi), Shaoyao (Radix Paeoniae Alba), Shengjiang (Rhizoma Zingiberis Recens), Dazao (Fructus Jujubae), Zhigancao (Radix Glycyrrhizae Praeparata), Yitang (Maltosum) | Alleviates post-laparoscopic hepatectomy fatigue syndrome by reducing inflammation and stress responses | Zhang et al.[90] |
| Shenqi Xiaoji Fang (Ginseng, Astragalus, and mass-eliminating formula) | Dangshen (Radix Codonopsis), Huangqi (Radix Astragali), Ezhu (Rhizoma Curcumae), Sanleng (Rhizoma Sparganii) | Combined with Camrelizumab and Apatinib (immuno/targeted therapy) for advanced HCC with “Liver Qi Stagnation and Spleen Deficiency” syndrome to extend survival and improve quality of life | Zhang et al.[91] |
| Wenyang Fuzheng Fang (A formula aimed at ameliorating the body’s deficient-cold state and remodeling the tumor microenvironment) | Fuzi (Radix Aconiti Lateralis Praeparata), Rougui (Cortex Cinnamomi), Huangqi (Radix Astragali), Dangshen (Radix Codonopsis) | Combined with immunotherapy to improve symptoms, immune function, and tumor markers in HCC patients with “Yang Deficiency” syndrome | Zao et al.[93] |
| Peiyuan Jiedu Tongluo Tang (A formula utilized to eliminate internal toxins and unblock microcirculation) | Huangqi (Radix Astragali), Baihuasheshecao (Herba Hedyotidis Diffusae), Ezhu (Rhizoma Curcumae) | Combined with conventional western medicine to treat post-TACE syndrome, improving clinical efficacy, alleviating symptoms, and enhancing quality of life | Ge et al.[94] |
AI presents unprecedented opportunities to overcome this challenge by serving as both a computational engine and translator. The primary obstacle to TCM integration is not a lack of data, but the unstructured and qualitative nature of its data. AI is advancing TCM modernization by imparting the objectivity and standardization required for MDT decision-making. For instance, AI-driven network pharmacology and knowledge graphs (KG) are being employed to deconstruct the complexity of TCM formulas, elucidating their multi-component, multi-targeted anti-tumor pharmacological mechanisms in contemporary biomedical language[95]. Concurrently, DL models are establishing repeatable, objective quantitative standards for personalized TCM diagnosis by processing vast real-world data (RWD) - including clinical notes, tongue and pulse patterns, alongside radiomics and multi-omics data. The emergence of novel computational platforms such as AI-Driven TCM Drug Discovery Database (AIDD-TCM) provides the essential infrastructure for such large-scale analysis by integrating multi-omics, cheminformatics, and clinical data.
The ultimate role of AI within MDT is to act as an integrator. By merging TCM-derived variables - now quantified by AI, such as specific “syndrome” biomarkers or herb-target pathways - with standard clinical data (pathology, genetics, imaging), it constructs more complex, comprehensive multimodal predictive models. This will transform the decision of whether to integrate TCM from an empirical supplementary option into a computable, verifiable, evidence-based component of personalized precision treatment within MDT. Nevertheless, realizing this vision remains fraught with challenges: data heterogeneity and quality issues in real-world EMR data, the formidable task of standardizing TCM diagnostic terminology, the “black-box” nature of DL models hindering clinical trust, and quality control and batch consistency of TCM herbs themselves are all critical issues that must be resolved in the future.
AI-driven optimization of MDTB workflows and decision management
Beyond enhancing specific diagnostic or therapeutic tasks, AI holds significant potential in addressing systemic inefficiencies inherent in current MDTB operations. Traditional MDTBs frequently encounter challenges such as fragmented information, inconsistent guideline adherence, and resource constraints, which often impede timely and standardized decision-making. AI technologies offer targeted solutions to these management-level challenges. Firstly, a major bottleneck in MDTBs is the time-consuming manual aggregation of data. AI, particularly natural language processing (NLP)[96] technology, can automatically extract and structure key information (such as tumor burden, hepatic reserve capacity, and genetic profiles) from unstructured electronic medical records. By constructing a unified “MDTB dashboard” [Figure 1], AI ensures specialists gain instant access to comprehensive, visualized patient profiles, thereby reducing preparation time and minimizing information omissions. Secondly, human decision-making may occasionally deviate from standard protocols due to cognitive load or varying levels of experience. AI systems can function as real-time quality control tools during MDTB discussions. By instantly comparing proposed treatment plans against the latest relevant guidelines, algorithms flag potential discrepancies, prompting teams to verify decision rationale. This ensures MDTB decisions remain evidence-based and aligned with best practice. Finally, specialist resources within MDT meetings are finite. AI-driven triage models can pre-assess patient complexity, filtering out routine cases following standard pathways to prioritize complex, refractory cases genuinely requiring in-depth multidisciplinary deliberation. Furthermore, in the post-meeting phase, AI tools can assist in tracking patient adherence to treatment recommendations and monitoring follow-up outcomes, effectively closing the loop on multidisciplinary care management[97].
DISCUSSION
The integration of AI within MDTBs for HCC heralds a paradigm shift from experience-driven to data-driven decision-making. As outlined herein, AI has demonstrated potential to surpass human experts at multiple critical junctures of MDT decision-making, encompassing early prediction, pathological diagnosis, radiomics analysis, treatment planning, and even the modernization of integrated Chinese and Western medicine. AI models, particularly DL algorithms, can process vast multimodal datasets to uncover complex biological patterns elusive to human clinicians[27,39].
Despite impressive performance in trials, a significant implementation gap persists between AI model efficacy and actual clinical benefit[98,99]. This gap is multidimensional and constitutes the core obstacle to deploying AI-enabled MDT in real-world settings.
Firstly, the primary technical challenge lies in model robustness and generalization capability. The vast majority of current research remains confined to single-center, small-sample retrospective analyses, with highly homogeneous training data[100]. For instance, systematic reviews of published AI models for HCC diagnosis consistently indicate that the overwhelming majority - in some cases exceeding 80% - have never been tested using independent external validation sets[100]. This vulnerability leads to overfitting and spectral bias, severely compromising generalization capabilities. A high-accuracy model trained in one clinical setting will almost inevitably experience significant performance degradation when deployed in real-world environments featuring different equipment, protocols, and patient populations. Whilst this represents a global challenge, China’s vast population base, geographical disparities, and substantial equipment heterogeneity across hospital tiers impose even greater demands on model robustness.
Secondly, barriers exist in data, trust, and regulation. Data privacy, security, and institutional data silos constrain the development and sharing of high-quality models. The sensitive multimodal data required for MDT (imaging, pathology, genomics, follow-up) is fragmented across institutions, constituting a major bottleneck in AI research and development. Furthermore, algorithmic opacity undermines clinical trust. The “black-box” nature of DL runs counter to the accountable consensus pursued by MDT[101]. Unless an AI model can provide clear, credible, and pathophysiologically grounded explanations for its treatment recommendations, it will remain an isolated predictive tool rather than a partner deeply integrated into clinical decision-making workflows. Regulatory lag is a key issue: AI tools for diagnosis and treatment are categorized as medical devices and are therefore subject to stringent approval processes. In China, while the National Medical Products Administration (NMPA) has expedited approvals for AI imaging software, significant challenges persist in establishing clear guidelines for more complex, multimodal, or DL-based decision support systems. This objectively delays their clinical translation.
Finally, substantial organizational and human barriers exist. AI implementation is not merely a technical issue but a complex change management challenge. MDT operates within highly mature, high-stakes, established workflows. To ensure adoption, staff acceptance must become a core consideration, extending beyond mere usability. This necessitates adherence to human-centered design principles, involving clinicians early and iteratively in the development process to ensure tools adapt to their workflow rather than disrupt it. Furthermore, successful integration relies on robust training programs and sustained institutional support to overcome natural resistance to altering established diagnostic and decision-making heuristics.
Challenges and insights in the context of China’s national circumstances
The unique contribution of this review lies in its examination of AI within the specific context of HCC management in China. In recent years, China has persistently promoted the deep integration of AI with healthcare at the national level. The corresponding top-level design provides policy support for AI applications in scenarios such as auxiliary diagnosis and treatment, as well as health management. However, China’s unique national circumstances also present distinct challenges and opportunities.
Firstly, AI assumes a dual role within TCM integration. Within China’s MDT environment, the most complex non-imaging data requiring AI analysis is often TCM’s syndrome data. As previously noted, TCM’s individualized, qualitative framework historically excluded it from formal MDT decision-making due to incompatibility with modern medicine’s standardized evidence-based paradigm. This presents a unique entry point for AI. In China, AI must not only process imaging and genomic data but also function as a quantification engine, analyzing vast RWD to elucidate correlations between syndrome patterns and objective biomarkers[95]. Consequently, the future of AI within China’s MDT landscape is not merely a technical issue but fundamentally a methodological challenge: how to construct integrated combined Chinese and Western medicine data models.
Secondly, AI’s potential impact on health equity warrants attention. The high cost and accessibility challenges of advanced AI models[102,103] are particularly acute in China, potentially constituting major implementation barriers. China’s HCC management faces significant urban-rural and regional disparities in healthcare resources. Should AI development continue to rely on high-end infrastructure and substantial expenditure, it risks exacerbating rather than alleviating health inequalities. Thus, we propose a novel perspective: future AI research in China must prioritize cost-effectiveness and accessibility alongside the pursuit of higher accuracy. For instance, exploring models for initial risk stratification based solely on routine US imaging and serum AFP levels - data readily available in primary care settings - could yield greater public health significance than models reliant on costly omics data.
Future directions
Looking ahead, the successful implementation of AI-enabled MDT requires a shift from model development to clinical implementation science, focusing on addressing China’s specific challenges. Firstly, model robustness must be addressed through mandatory multicenter, prospective external validation and the establishment of a national, diverse HCC database. Secondly, to tackle data fragmentation and security concerns, privacy-preserving computing techniques such as federated learning represent a key technological trend for enabling cross-institutional collaborative modelling.
More critically, future development must confront two core challenges in China. (1) Regarding TCM integration, sustained research investment is needed in AI-driven TCM translation and quantification. This encompasses developing explainable AI to enhance algorithm transparency and integrating evaluations of AI-TCM interventions into international clinical trial standards such as SPIRIT and CONSORT, thereby enabling genuine TCM integration into MDT; (2) Regarding health inequalities, priority should be given to developing low-cost, high-efficiency AI tools to ensure technological advances benefit the broadest patient cohorts. Furthermore, AI’s value should be expanded throughout the entire MDT workflow. For instance, generative AI could simulate virtual cases for MDT training, or large language models could automatically generate patient-friendly explanations of complex treatment plans.
In summary, China is advancing the deep integration of AI with healthcare through policy guidance and practical exploration. By addressing data, trust, and regulatory challenges through technological innovation, and deepening human-machine collaboration through expanded applications, AI is poised to transcend its tool-based attributes. It will become a core driver propelling China’s HCC management towards precision, standardization, and equity[84,104].
DECLARATIONS
Authors’ contributions
Conceptualization: Li D, Wang H
Writing - original draft, visualization: Li D, Gao F
Supervision, conceptualization, writing - review and editing: Fu X, Han J
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Qin Y, Tang C, Li J, Gong J. Liver cancer in China: the analysis of mortality and burden of disease trends from 2008 to 2021. BMC Cancer. 2024;24:594.
2. Shen C, Jiang X, Li M, Luo Y. Hepatitis virus and hepatocellular carcinoma: recent advances. Cancers. 2023;15:533.
3. Oncology Society of Chinese Medical Association. [Chinese Medical Association guideline for clinical diagnosis and treatment of lung cancer (2024 edition)]. Zhonghua Zhong Liu Za Zhi. 2024;46:805-43.
4. Sharma S. Benefits or concerns of AI: a multistakeholder responsibility. Futures. 2024;157:103328.
5. Yüksel N, Börklü HR, Sezer HK, Canyurt OE. Review of artificial intelligence applications in engineering design perspective. Eng Appl Artif Intell. 2023;118:105697.
6. Sarker IH. Deep learning: a comprehensive overview on techniques, taxonomy, applications and research directions. SN Comput Sci. 2021;2:420.
7. Mienye ID, Swart TG, Obaido G, Jordan M, Ilono P. Deep convolutional neural networks in medical image analysis: a review. Information. 2025;16:195.
8. Nguyen-Tat TB, Hung TQ, Nam PT, Ngo VM. Evaluating pre-processing and deep learning methods in medical imaging: combined effectiveness across multiple modalities. Alex Eng J. 2025;119:558-86.
9. Xia TY, Zhou ZH, Meng XP, et al. Predicting microvascular invasion in hepatocellular carcinoma using CT-based radiomics model. Radiology. 2023;307:e222729.
10. Bae JS, Lee HH, Kim H, Song IC, Lee JY, Han JK. Deep learning-aided 1H-MR spectroscopy for differentiating between patients with and without hepatocellular carcinoma. Magn Reson Med Sci. 2025:mp.2025-0064.
11. Hou J, Berg T, Vogel A, et al. Comparative evaluation of multimarker algorithms for early-stage HCC detection in multicenter prospective studies. JHEP Rep. 2025;7:101263.
12. Potter LN, Yap J, Dempsey W, Wetter DW, Nahum-Shani I. Integrating intensive longitudinal data (ILD) to inform the development of dynamic theories of behavior change and intervention design: a case study of scientific and practical considerations. Prev Sci. 2023;24:1659-71.
13. Hao S, Lin S, Wang J, Zhong Q. Dynamic modeling for multivariate functional and longitudinal data. J Econometrics. 2024;239:105573.
14. Diao S, Tian Y, Hu W, et al. Weakly supervised framework for cancer region detection of hepatocellular carcinoma in whole-slide pathologic images based on multiscale attention convolutional neural network. Am J Pathol. 2022;192:553-63.
15. Banerjee T, Singh DP, Kour P, et al. A novel unified Inception-U-Net hybrid gravitational optimization model (UIGO) incorporating automated medical image segmentation and feature selection for liver tumor detection. Sci Rep. 2025;15:29908.
16. Prajumwongs P, Titapun A, Thanasukarn V, et al. Serum peptide biomarkers by MALDI-TOF MS coupled with machine learning for diagnosis and classification of hepato-pancreato-biliary cancers. Sci Rep. 2025;15:29169.
17. Zhang G, Peng Z, Yan C, Wang J, Luo J, Luo H. A novel liver cancer diagnosis method based on patient similarity network and DenseGCN. Sci Rep. 2022;12:6797.
18. Sucularli C. Machine learning-based identification of diagnostic and prognostic mitotic cell cycle genes in hepatocellular carcinoma. PLoS One. 2025;20:e0331118.
19. Xie DY, Zhu K, Ren ZG, Zhou J, Fan J, Gao Q. A review of 2022 Chinese clinical guidelines on the management of hepatocellular carcinoma: updates and insights. Hepatobiliary Surg Nutr. 2023;12:216-28.
20. Xu X, Li J, Zhu Z, et al. A comprehensive review on synergy of multi-modal data and AI technologies in medical diagnosis. Bioengineering. 2024;11:219.
21. Yang T, Wang MD, Xu XF, Li C, Wu H, Shen F. Management of hepatocellular carcinoma in China: seeking common grounds while reserving differences. Clin Mol Hepatol. 2023;29:342-4.
22. Xu Y, Xia C, Li H, et al. Survey of hepatitis B virus infection for liver cancer screening in China: a population-based, cross-sectional study. Chin Med J. 2024;137:1414-20.
23. Zhang CH, Cheng Y, Zhang S, Fan J, Gao Q. Changing epidemiology of hepatocellular carcinoma in Asia. Liver Int. 2022;42:2029-41.
25. Wang X, Zhang Y, Yang N, et al. Evaluation of the combined application of AFP, AFP-L3%, and DCP for hepatocellular carcinoma diagnosis: a meta-analysis. Biomed Res Int. 2020;2020:5087643.
26. Tu X, He Z, Huang Y, Zhang Z, Yang M, Zhao J. An overview of large AI models and their applications. Vis Intell. 2024;2:65.
27. Alqahtani T, Badreldin HA, Alrashed M, et al. The emergent role of artificial intelligence, natural learning processing, and large language models in higher education and research. Res Social Adm Pharm. 2023;19:1236-42.
28. Yu X, Lei X. Application of the multi-omics liquid biopsy method M2P-HCC in early liver cancer screening for high-risk individuals with hepatitis B-related liver cancer. Diagnostics. 2023;13:2484.
29. Zhou J, Sun H, Wang Z, et al. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (2019 Edition). Liver Cancer. 2020;9:682-720.
30. El Atifi W, El Rhazouani O, Khan FM, Sekkat H. Optimizing ensemble machine learning models for accurate liver disease prediction in healthcare. PLoS One. 2025;20:e0330899.
31. Sang H, Lee H, Lee M, et al. Prediction model for cardiovascular disease in patients with diabetes using machine learning derived and validated in two independent Korean cohorts. Sci Rep. 2024;14:14966.
32. Abdelhamed W, El-Kassas M. Integrating artificial intelligence into multidisciplinary evaluations of HCC: opportunities and challenges. Hepatoma Res. 2025;11:8.
33. Seven İ, Bayram D, Arslan H, et al. Predicting hepatocellular carcinoma survival with artificial intelligence. Sci Rep. 2025;15:6226.
34. Zhang ZM, Huang Y, Liu G, et al. Development of machine learning-based predictors for early diagnosis of hepatocellular carcinoma. Sci Rep. 2024;14:5274.
35. Chartampilas E, Rafailidis V, Georgopoulou V, Kalarakis G, Hatzidakis A, Prassopoulos P. Current imaging diagnosis of hepatocellular carcinoma. Cancers. 2022;14:3997.
36. Vengateswaran HT, Habeeb M, You HW, Aher KB, Bhavar GB, Asane GS. Hepatocellular carcinoma imaging: exploring traditional techniques and emerging innovations for early intervention. Med Nov Technol Devices. 2024;24:100327.
37. Tu J, Wang B, Wang X, et al. Current status and new directions for hepatocellular carcinoma diagnosis. Liver Res. 2024;8:218-36.
38. Romeo M, Dallio M, Napolitano C, et al. Clinical applications of artificial intelligence (AI) in human cancer: is it time to update the diagnostic and predictive models in managing hepatocellular carcinoma (HCC)? Diagnostics. 2025;15:252.
39. Chatzipanagiotou OP, Loukas C, Vailas M, et al. Artificial intelligence in hepatocellular carcinoma diagnosis: a comprehensive review of current literature. J Gastroenterol Hepatol. 2024;39:1994-2005.
40. Liu Z, Liu Y, Hong Y, et al. Deep learning for prediction of hepatocellular carcinoma recurrence after resection or liver transplantation: a discovery and validation study. arXiv 2021;arXiv:2106.00090. Available from: https://doi.org/10.48550/arXiv.2106.00090 [accessed 18 Dec 2025].
41. Ennab M, Mcheick H. Enhancing interpretability and accuracy of AI models in healthcare: a comprehensive review on challenges and future directions. Front Robot AI. 2024;11:1444763.
42. Tiwari A, Mishra S, Kuo TR. Current AI technologies in cancer diagnostics and treatment. Mol Cancer. 2025;24:159.
43. Yates J, Van Allen EM. New horizons at the interface of artificial intelligence and translational cancer research. Cancer Cell. 2025;43:708-27.
44. Richter M, Emden D, Leenings R, et al.; MBB consortium, FOR2107 consortium, PRONIA consortium. Generalizability of clinical prediction models in mental health. Mol Psychiatry. 2025;30:3632-9.
45. Futoma J, Simons M, Panch T, Doshi-Velez F, Celi LA. The myth of generalisability in clinical research and machine learning in health care. Lancet Digit Health. 2020;2:e489-92.
46. Yang J, Soltan AAS, Clifton DA. Machine learning generalizability across healthcare settings: insights from multi-site COVID-19 screening. NPJ Digit Med. 2022;5:69.
47. Chang Q, Yan Z, Zhou M, et al. Mining multi-center heterogeneous medical data with distributed synthetic learning. Nat Commun. 2023;14:5510.
48. Harding-Theobald E, Louissaint J, Maraj B, et al. Systematic review: radiomics for the diagnosis and prognosis of hepatocellular carcinoma. Aliment Pharmacol Ther. 2021;54:890-901.
49. Yao S, Ye Z, Wei Y, Jiang HY, Song B. Radiomics in hepatocellular carcinoma: a state-of-the-art review. World J Gastrointest Oncol. 2021;13:1599-615.
50. Jiang C, Cai YQ, Yang JJ, et al. Radiomics in the diagnosis and treatment of hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2023;22:346-51.
51. Avery E, Sanelli PC, Aboian M, Payabvash S. Radiomics: a primer on processing workflow and analysis. Semin Ultrasound CT MR. 2022;43:142-6.
52. Dong D, Liu S, Liu Z, et al. Radiomics and multiomics research. In: Liu S, editor. Artificial intelligence in medical imaging in China. Singapore: Springer Nature; 2024. pp. 63-81.
53. Lambin P, Leijenaar RTH, Deist TM, et al. Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol. 2017;14:749-62.
54. Gurzu S, Szodorai R, Jung I, Banias L. Combined hepatocellular-cholangiocarcinoma: from genesis to molecular pathways and therapeutic strategies. J Cancer Res Clin Oncol. 2024;150:270.
55. An L, Zheng R, Zhang S, et al. Hepatocellular carcinoma and intrahepatic cholangiocarcinoma incidence between 2006 and 2015 in China: estimates based on data from 188 population-based cancer registries. Hepatobiliary Surg Nutr. 2023;12:45-55.
56. Mirbabaie M, Stieglitz S, Frick NRJ. Artificial intelligence in disease diagnostics: a critical review and classification on the current state of research guiding future direction. Health Technol. 2021;11:693-731.
57. Midya A, Chakraborty J, Srouji R, et al. Computerized diagnosis of liver tumors from CT scans using a deep neural network approach. IEEE J Biomed Health Inform. 2023;27:2456-64.
58. Stollmayer R, Budai BK, Tóth A, et al. Diagnosis of focal liver lesions with deep learning-based multi-channel analysis of hepatocyte-specific contrast-enhanced magnetic resonance imaging. World J Gastroenterol. 2021;27:5978-88.
59. Maniaci A, Lavalle S, Gagliano C, et al. The integration of radiomics and artificial intelligence in modern medicine. Life 2. 24;14:1248.
60. Youssef A, Pencina M, Thakur A, Zhu T, Clifton D, Shah NH. External validation of AI models in health should be replaced with recurring local validation. Nat Med. 2023;29:2686-7.
61. Khalifa M, Albadawy M. AI in diagnostic imaging: revolutionising accuracy and efficiency. Comput Methods Programs Biomed Update. 2024;5:100146.
62. Vrettos K, Triantafyllou M, Marias K, Karantanas AH, Klontzas ME. Artificial intelligence-driven radiomics: developing valuable radiomics signatures with the use of artificial intelligence. BJR AI. 2024;1:ubae011.
63. Yan T, Yu L, Zhang N, et al. The advanced development of molecular targeted therapy for hepatocellular carcinoma. Cancer Biol Med. 2022;19:802-17.
64. Chan YT, Zhang C, Wu J, et al. Biomarkers for diagnosis and therapeutic options in hepatocellular carcinoma. Mol Cancer. 2024;23:189.
65. Nair M, Sandhu SS, Sharma AK. Cancer molecular markers: a guide to cancer detection and management. Semin Cancer Biol. 2018;52:39-55.
66. Dwivedi YK, Hughes L, Ismagilova E, et al. Artificial intelligence (AI): multidisciplinary perspectives on emerging challenges, opportunities, and agenda for research, practice and policy. Int J Inf Manag. 2021;57:101994.
67. Gao K, Yaermaimaiti M, Wang Y, Xia G, Xu T, Wang H. Bi-regional machine learning radiomics based on CT noninvasively predicts LOX expression level and overall survival in hepatocellular carcinoma. Cancer Med. 2025;14:e71154.
68. Cheng B, Deng H, Zhao Y, et al. Predicting EGFR mutation status in lung adenocarcinoma presenting as ground-glass opacity: utilizing radiomics model in clinical translation. Eur Radiol. 2022;32:5869-79.
69. European Association for the Study of the Liver. EASL Clinical Practice Guidelines on the management of hepatocellular carcinoma. J Hepatol. 2025;82:315-74.
70. Singal AG, Llovet JM, Yarchoan M, et al. AASLD Practice Guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology. 2023;78:1922-65.
71. Li S, Li XG, Zhou F, et al. Automated segmentation of liver and hepatic vessels on portal venous phase computed tomography images using a deep learning algorithm. J Appl Clin Med Phys. 2024;25:e14397.
72. Memeo R, Conticchio M, Deshayes E, et al. Optimization of the future remnant liver: review of the current strategies in Europe. Hepatobiliary Surg Nutr. 2021;10:350-63.
73. Kaplan DE, Ripoll C, Thiele M, et al. AASLD Practice Guidance on risk stratification and management of portal hypertension and varices in cirrhosis. Hepatology. 2024;79:1180-211.
74. Zeng X, Tao H, Dong Y, et al. Impact of three-dimensional reconstruction visualization technology on short-term and long-term outcomes after hepatectomy in patients with hepatocellular carcinoma: a propensity-score-matched and inverse probability of treatment-weighted multicenter study. Int J Surg. 2024;110:1663-76.
75. Zeng L, Zhu Y, Guo P. Meta-analysis of the effects of three-dimensional visualized medical techniques hepatectomy for liver cancer with and without the treatment of sorafenib. Evid Based Complement Alternat Med. 2022;2022:4507673.
76. Chansangrat J, Gadani S. Radioembolization for hepatocellular carcinoma: updated strategies and evolving clinical applications. Hepatoma Res. 2024;10:49.
77. Yang Y, Yu H, Qi L, et al. Combined radiofrequency ablation or microwave ablation with transarterial chemoembolization can increase efficiency in intermediate-stage hepatocellular carcinoma without more complication: a systematic review and meta-analysis. Int J Hyperthermia. 2022;39:455-65.
78. Bartnik K, Krzyziński M, Bartczak T, et al. A novel radiomics approach for predicting TACE outcomes in hepatocellular carcinoma patients using deep learning for multi-organ segmentation. Sci Rep. 2024;14:14779.
79. Kokabi N, Arndt-Webster L, Chen B, et al. Voxel-based dosimetry predicting treatment response and related toxicity in HCC patients treated with resin-based Y90 radioembolization: a prospective, single-arm study. Eur J Nucl Med Mol Imaging. 2023;50:1743-52.
80. Bibault JE, Giraud P. Deep learning for automated segmentation in radiotherapy: a narrative review. Br J Radiol. 2024;97:13-20.
81. Lee CL, Freeman M, Burak KW, et al. Real-world outcomes of atezolizumab with bevacizumab treatment in hepatocellular carcinoma patients: effectiveness, esophagogastroduodenoscopy utilization and bleeding complications. Cancers. 2024;16:2878.
82. European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236.
83. Kinsey E, Lee HM. Management of hepatocellular carcinoma in 2024: the multidisciplinary paradigm in an evolving treatment landscape. Cancers. 2024;16:666.
84. Xu F, Zhang H, Chen J, et al. Recent progress on the application of compound formulas of traditional Chinese medicine in clinical trials and basic research in vivo for chronic liver disease. J Ethnopharmacol. 2024;321:117514.
85. Guan Y, He Q. Liver cancer: Zheng classification of Qi stagnation and blood stasis. Pharmacol Pharm. 2014;5:75-82.
86. Gu Z, Qi X, Zhai X, et al. Study on TCM syndrome differentiation of primary liver cancer based on the analysis of latent structural model. Evid Based Complement Alternat Med. 2015;2015:761565.
87. Yang SR, Chen L, Luo D, Wang YY, Liang FX. Unlocking the potential: how acupuncture reshapes the liver-centered lipid metabolism pattern to fight obesity. J Integr Med. 2024;22:523-32.
88. Lei Y, Chen C. Bibliometric analysis of traditional Chinese medicine in cancer treatment via immune system modulation (2015-2025). Front Immunol. 2025;16:1581885.
89. Zhang H, Dai Q, Zeng M, et al. Investigating the metabolic level of endogenous and Exogenous Substances On The Intervention Of Traditional Chinese medicine Fuzheng Yiliu Decoction in a rat orthotopic liver cancer model. Cancer Manag Res. 2022;14:2785-801.
90. Zhang JX, Bao SC, Chen J, et al. Xiaojianzhong decoction prevents gastric precancerous lesions in rats by inhibiting autophagy and glycolysis in gastric mucosal cells. World J Gastrointest Oncol. 2023;15:464-89.
91. Zhang L, Yang JX, Li XH, Zhang XY, Wang HJ. Effect of modified Huqi prescription on quality of life in patients with primary liver cancer of Zhengqi deficiency and toxin-stasis binding syndrome after transcatheter arterial chemoembolization: a retrospective cohort study. J Tradit Chinese Med. 2019;60:306-10.
92. Yu YX, Wang S, Liu ZN, et al. Traditional Chinese medicine in the era of immune checkpoint inhibitor: theory, development, and future directions. Chin Med. 2023;18:59.
93. Zao X, Cao X, Liang Y, et al. The Chinese herbal KangXianYiAi formula inhibits hepatocellular carcinoma by reducing glutathione and inducing ferroptosis. Pharmacol Res Mod Chin Med. 2023;8:100276.
94. Ge Y, Shi X, Zhang W, et al. Effect of the self-designed peiyuan jiedu tongluo decoction combined with conventional western medicine on postoperative syndrome of liver cancer patients treated with transcatheter arterial chemoembolization. Hebei Med J. 2023;45:3581-3.
95. Han L, Ma Y, Wu W, et al. Research progress on the therapeutic effects of effective components of traditional Chinese medicine in the treatment of gastric cancer precursors through modulation of multiple signaling pathways. Front Oncol. 2025;15:1555274.
96. Bilal M, Hamza A, Malik N. NLP for analyzing electronic health records and clinical notes in cancer research: a review. J Pain Symptom Manage. 2025;69:e374-94.
97. Shi Z, Wu B, Hu B, et al. A large language model for clinical outcome adjudication from telephone follow-up interviews: a secondary analysis of a multicenter randomized clinical trial. Nat Commun. 2025.
98. Zhang H, Zhang Z, Zhang K, Gao Z, Shen Z, Shen W. CT-based deep learning radiomics model for predicting proliferative hepatocellular carcinoma: application in transarterial chemoembolization and radiofrequency ablation. BMC Med Imaging. 2025;25:363.
99. Liang J, Weng S, Zhang J, et al. Diagnostic performance of [18F]FAPI-04 PET/CT in suspected recurrent hepatocellular carcinoma: prospective comparison with contrast-enhanced CT/MRI. Eur J Nucl Med Mol Imaging. 2025;52:3951-62.
100. Rompianesi G, Pegoraro F, Ceresa CD, Montalti R, Troisi RI. Artificial intelligence in the diagnosis and management of colorectal cancer liver metastases. World J Gastroenterol. 2022;28:108-22.
101. Magrabi F, Ammenwerth E, McNair JB, et al. Artificial intelligence in clinical decision support: challenges for evaluating AI and practical implications. Yearb Med Inform. 2019;28:128-34.
102. Tan S, Xin X, Wu D. ChatGPT in medicine: prospects and challenges: a review article. Int J Surg. 2024;110:3701-6.
103. Hou H, Zhang R, Li J. Artificial intelligence in the clinical laboratory. Clin Chim Acta. 2024;559:119724.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Special Topic
Copyright
Data & Comments
Data















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].