Influence of the gut microbiota on the response to immunotherapy in hepatocellular carcinoma
Abstract
For hepatocellular carcinoma (HCC) patients, the clinical efficacy of immune checkpoint inhibitors (ICIs) remains limited by low response rates. The gut microbiome as a critical modulator of ICIs responsiveness in HCC. We systematically analyze the relevant gut microbial signatures distinguishing programmed death 1 therapy in responders and non-responders, with particular emphasis on prognostic taxa. Microbiome-targeted interventions, encompassing antibiotic modulation, probiotic supplementation, prebiotic administration, and fecal microbiota transplantation, may synergistically enhance the efficacy of ICIs by leveraging the immunomodulatory potential of gut-derived microbial metabolites. The mechanisms governing microbiome-mediated immunotherapeutic regulation involve multifaceted interactions, particularly through microbiota-driven immunomodulation within the tumor microenvironment. We identify key translational challenges of tumor heterogeneity in microbiomes. Future research directions emphasize the need for standardized protocols, longitudinal cohort studies, and innovative preclinical models to bridge existing knowledge gaps.
Keywords
INTRODUCTION
Hepatocellular carcinoma (HCC) represents a significant global health burden, ranking as the sixth most commonly diagnosed malignancy and the third leading contributor to cancer-associated mortality worldwide[1]. The pathogenesis of HCC is multifactorial, with established etiological factors encompassing chronic viral hepatitis like hepatitis B virus (HBV) and hepatitis C virus (HCV) infections, and chronic liver disease spectrum like alcoholic fatty liver disease (AFLD) and metabolic dysfunction-associated steatotic liver disease (MASLD)[2,3]. Therapeutic strategies for HCC are stratified according to disease stage and hepatic functional reserve. Early-stage HCC management necessitates comprehensive evaluation for potentially curative interventions, including percutaneous ablation techniques, anatomical hepatic resection, or orthotopic liver transplantation. The intermediate-stage disease typically warrants locoregional therapies, with transarterial chemoembolization (TACE), transcatheter arterial embolization (TAE), and transarterial radioembolization (TARE) constituting the primary therapeutic modalities[4].
Advanced or unresectable HCC that is not amenable to locoregional approaches requires systemic pharmacological intervention, predominantly comprising antiangiogenic agents and immune checkpoint inhibitors (ICIs)[5]. The antiangiogenic therapeutic armamentarium includes tyrosine kinase inhibitors (TKIs) such as sorafenib, lenvatinib, cabozantinib, and regorafenib, and monoclonal antibodies targeting vascular endothelial growth factor pathways like ramucirumab and bevacizumab. Current treatment paradigms designate FDA-approved agents sorafenib and lenvatinib as first-line therapies, with regorafenib, cabozantinib, and ramucirumab reserved for second-line treatment; however, these interventions demonstrate modest survival benefits[6,7]. The ICIs therapeutic class encompasses agents targeting the programmed death 1 (PD-1) axis (pembrolizumab and nivolumab), programmed death-ligand 1 (PD-L1) inhibitors (durvalumab and atezolizumab), and cytotoxic T lymphocyte-associated protein 4 (CTLA-4) antagonists (tremelimumab and ipilimumab)[8].
Prospective phase II and III clinical trials have demonstrated that approximately 15% of patients with advanced HCC achieve objective tumor responses following ICI therapy[9]. Nevertheless, a substantial proportion (> 30%) of advanced HCC patients exhibit intrinsic resistance to PD-1/PD-L1 blockade, with a subset manifesting paradoxical tumor hyperprogression following treatment initiation[10]. The clinical utility of ICIs in HCC is further constrained by two principal limitations: the potential for viral reactivation in hepatitis-associated cases and the current paucity of validated predictive biomarkers. These clinical challenges underscore the critical need for developing robust predictive biomarkers to optimize patient stratification and enhance therapeutic outcomes.
Currently, the FDA has recognized several molecular biomarkers for predicting ICI responsiveness, including tumor mutational burden (TMB) and genomic instability markers such as deficient mismatch repair (dMMR) and high microsatellite instability (MSI-H) status[11]. Recently, the gut microbiome has emerged as a promising predictive biomarker and therapeutic modulator in immuno-oncology. The intestinal microbiota plays a pivotal role in maintaining systemic immune homeostasis and has been implicated in the pathogenesis and progression of various malignancies, including HCC[12,13]. Gut microbial composition and functional capacity significantly influence host immune responses, particularly in modulating antitumor immunity during immunotherapy[14,15].
This review systematically examines the gut microbiota as both a predictive biomarker and therapeutic target in modulating ICI responses in HCC, integrating clinical observations with mechanistic insights to provide a comprehensive translational perspective. First, we characterize response-specific microbial signatures, identifying key taxa that correlate with improved clinical outcomes. Second, we evaluate microbiota-targeted interventions such as fecal microbiota transplantation and precision probiotics. Crucially, we elucidate the underlying biological mechanisms through which microbiota-derived metabolites and molecular patterns orchestrate antitumor immunity by reshaping the HCC tumor microenvironment. Furthermore, we highlight the bidirectional crosstalk between tumor heterogeneity and microbial composition. Despite these advances, critical knowledge gaps persist regarding patient-specific confounding factors, optimal intervention protocols, and the precise molecular pathways governing microbiota-immune-tumor interactions. By synthesizing these multifaceted aspects, this review not only establishes a mechanistic framework for understanding microbiota-mediated ICI responses in HCC but also identifies actionable research priorities to advance this promising therapeutic paradigm.
GUT MICROBIOME AS A PREDICTED MARKER OF THE RESPONSE TO IMMUNOTHERAPY
The intestinal microbiota comprises a complex ecosystem of approximately one trillion microorganisms, encompassing diverse bacterial taxa, archaea, viruses, fungi, and eukaryotic protists, forming a dynamic and metabolically active community that exhibits significant plasticity in response to environmental and host-derived factors[16]. This microbial consortium demonstrates substantial interindividual variability in both taxonomic composition and functional diversity, playing an indispensable role in maintaining systemic homeostasis and metabolic equilibrium[17]. Currently, lots of clinical evidence has established the gut microbiome as a critical determinant of therapeutic efficacy in immune checkpoint inhibition, with distinct microbial signatures correlating with significant differences in clinical outcomes, particularly progression-free survival (PFS) and overall survival (OS) endpoints[18].
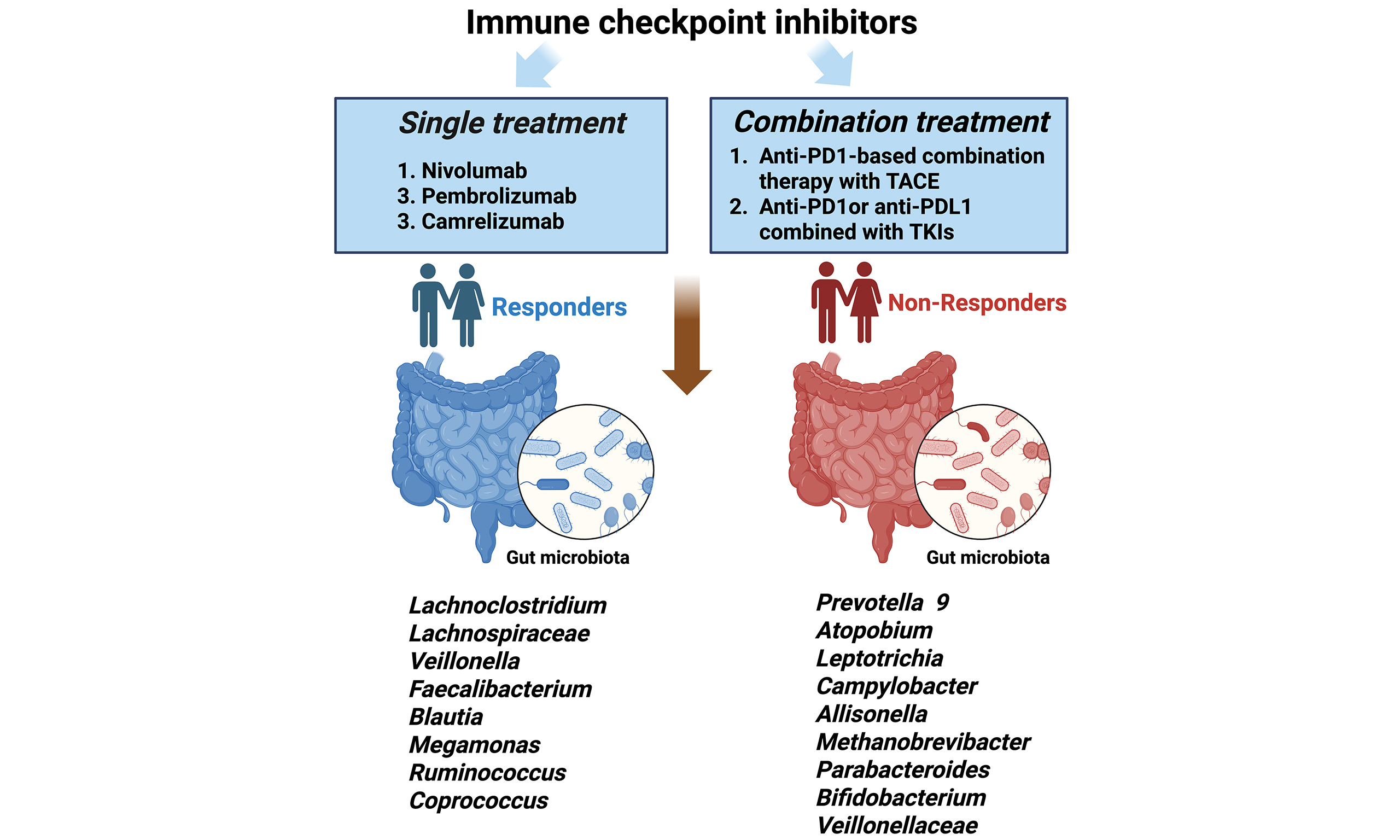
Single treatment
Accumulating studies from multiple clinical investigations have established a significant correlation between gut microbiota composition and therapeutic response to immunotherapy in HCC [Table 1]. Zheng et al. demonstrated that immunotherapy responders exhibited significantly enhanced microbial diversity, characterized by greater taxonomic richness and increased functional gene capacity compared to non-responders[19]. Longitudinal analysis revealed temporal microbial shifts, with Proteobacteria abundance progressively increasing in non-responders, becoming the dominant phylum by week 12 of treatment. Notably, responders showed specific enrichment of 20 microbial species, including Akkermansia muciniphila and various Ruminococcaceae species. Further supporting these findings, subsequent investigations have identified distinct α-diversity patterns, with responders demonstrating significantly higher Shannon diversity indices following nivolumab administration[20]. Taxonomic profiling revealed differential species abundance patterns, with non-responders showing relative predominance of Escherichia coli and Dialister pneumosintes, while responders were characterized by increased abundance of Citrobacter freundii, Azospirillum species, and Enterococcus durans. Importantly, specific microbial ratios have emerged as potential predictive biomarkers, with an elevated Firmicutes/Bacteroidetes ratio and reduced Prevotella/Bacteroides ratio associated with treatment resistance. Conversely, the presence of Akkermansia species has been consistently identified as a positive predictor of ICI response[20].
A summary of the gut microbiota associated with the efficacy of immunotherapy in HCC patients
| Study | Immunotherapy | No. of responders | No. of non-responders | Sequencing methods | Gut microbiota enriched in responders | Gut microbiota enriched in non-responders |
| Zheng et al.[19] | Camrelizumab (SHR-1210) | 3 | 5 | Metagenomic | Akkermansia muciniphila, Ruminococcaceae spp. | Proteobacteria |
| Chung et al.[20] | Nivolumab | 5 | 3 | 16S DNA | Citrobacter freundii, Azospirillum sp. and Enterococcus durans | Dialister pneumosintes, Escherichia coli, Lactobacillus reuteri, Streptococcus mutans, Enterococcus faecium, Streptococcus gordonii, Veillonella atypica, Granulicatella sp., and Trichuris trichiura |
Combination treatment
Despite the therapeutic promise of immune checkpoint inhibition, clinical trial data from phase III studies have demonstrated that monotherapy with either anti-PD-1 or anti-PD-L1 antibodies fails to confer significant OS benefits compared to sorafenib in advanced HCC populations[21,22]. Some studies highlight the critical role of gut microbiota composition in predicting therapeutic outcomes among advanced HCC patients undergoing single TKI treatment. For instance, sorafenib efficacy correlates with an increased abundance of probiotic Lactobacillus species (L. johnsonii, L. murinus, L. reuteri) and Bifidobacterium strains[23]. Similarly, lenvatinib treatment alters gut microbial profiles, suppressing pathogenic taxa (Bacteroides, Escherichia-Shigella, Prevotella, Eisenbergiella) while enriching beneficial genera like Faecalibacterium and Bifidobacterium[24]. Such microbiota-driven immunomodulation may underlie TKIs’ enhanced antitumor effects, providing a rationale for combining TKIs with ICIs to improve therapeutic outcomes.
Lots of studies mentioned that combinatorial regimens integrating ICIs with TKIs demonstrate superior clinical outcomes, establishing a novel therapeutic paradigm for advanced HCC management[25-29]. This therapeutic synergy has prompted investigations into the gut microbiome’s role in treatment response stratification, revealing significant microbial compositional differences between responders and non-responders to combination therapy. PD-1-based systemic therapies have identified distinct microbial signatures associated with treatment response. A summary of studies with anti-PD-1-based systemic therapy is provided in Table 2. Notably, Xin et al. demonstrated significant enrichment of Clostridiales taxa in responder populations, while non-responders exhibited predominant Bacteroidia colonization[28]. These findings are consistent with established patterns in immunotherapy-related microbiome research, which consistently identify Lachnospiraceae[25-27] and Ruminococcus[26,28] species as biomarkers of favorable treatment response. However, significant interstudy variability exists in the identification of response-associated microbial taxa, with notable discrepancies in specific bacterial associations. For instance, Shen et al.[29] reported increased Akkermansia abundance in non-responder populations, contrasting with Zheng et al.’s findings[19]. These inconsistencies likely stem from methodological variations across experimental protocols, encompassing factors ranging from fecal sample collection and preservation to bioinformatic processing and statistical analysis pipelines. Moreover, Cai et al.[30] systematically investigated the functional role of gut microbiota in regulating antitumor immunity through multi-dataset analysis. Their pathway enrichment analysis revealed four key microbial functional pathways that were significantly differentiated between responders (R) and non-responders (NR)[30]. These findings provide compelling evidence that specific microbial functional capacities, beyond mere taxonomic composition, play a crucial role in determining immunotherapy outcomes through multiple immunoregulatory mechanisms, offering new perspectives for microbiome-based therapeutic strategies in cancer treatment.
A summary of studies involving anti-PD-1-based systemic therapy in HCC patients
| StudyStudy | Immunotherapy | No. of responders | No. of non-responders | Sequencing methods | Gut microbiota enriched in responders | Gut microbiota enriched in non-responders |
| Lee et al.[23] | Nivolumab and Pembrolizumab combined with TKIs | 20 | 21 | 16S rRNA | Lachnoclostridium, Lachnospiraceae, and Veillonella | Prevotella 9 |
| Wu et al.[24] | Anti-PD-1-base systemic therapy | 16 | 19 | 16S rRNA | Faecalibacterium, Blautia, Lachnospiracea incertae Sedis, Megamonas, Ruminococcus, Coprococcus, Dorea and Haemophilus | Atopobium, Leptotrichia, Campylobacter, Allisonella, Methanobrevibacter, Parabacteroides, Bifidobacterium and Lactobacillus |
| Mao et al.[25] | Anti-PD-1-based systemic therapy | 17 | 13 | Metagenomic | Lachnospiraceae bacterium-GAM79, Alistipes sp., Marseille-P5997 | Veillonellaceae |
| Xin et al.[26] | Anti-PD-1-based combination therapy of TACE, Lenvatinib | 30 | 15 | Metagenomic | Collinsella genus, Ruminococcus-AM4211, and Ruminococcus-AF25_28AC | Bacteroides_AF20_13LB and Veillonella atypica |
| Shen et al.[27] | Anti-PD-1 or anti-PD-L1 combined with TKIs | 10 | 26 | 16S rRNA | Genera Succinivibrio and Tyzzerella | Genus Akkermansia |
THE GUT MICROBIOME COMPLEMENTS THE THERAPEUTIC EFFICACY OF IMMUNOTHERAPY
Comprehensive investigations into the gut microbiome’s role in HCC immunotherapy have established a compelling scientific rationale for microbiota-targeted therapeutic interventions. Encompassing antibiotic modulation, probiotic supplementation, prebiotic administration, and fecal microbiota transplantation (FMT) represent a novel therapeutic paradigm that potentially enhances immunotherapeutic efficacy through microbiome-mediated immunomodulation in HCC patients [Table 3].
A Summary of studies examining the relationship between gut microbiota and clinical response to immunotherapy in HCC patients
| StudyStudyStud | Immunotherapy | Intervention | Enrollment | Phase | Outcome measures | Location | Status | |
| NCT05032014[36] | PD-1 inhibitors | Probio-M9 probiotic | 46 | NA | ORR, PFS and OS | China | Recruiting | |
| NCT04264975[41] | Anti-PD-L1 inhibitors | FMT | 60 | NA | ORR | Korea | Recruiting | |
| NCT05750030[42] | Atezolizumab plus bevacizumab | FMT | 12 | 2 | ORR, DCR, PFS and OS | Austria | Recruiting | |
| NCT05690048[43] | Atezolizumab/Bevacizumab | FMT/Vancomycin oral capsule | 48 | 2 | PFS and OS | Germany | Not recruiting | |
Antibiotics
Antibiotics (ATB) mediate profound immunomodulatory effects primarily through their capacity to induce gut microbiota dysbiosis. Clinical research consistently demonstrates that ATB exposure, particularly when administered either prior to or during the initial phase of ICI therapy, adversely affects clinical outcomes in HCC, as evidenced by reduced objective response rates, diminished PFS, and compromised OS[31-33]. Moreover, gut bacteria produce peptide antibiotics with significant therapeutic potential. For instance, researchers analyzed nearly 2,000 human gut microbiomes and used artificial intelligence to predict antimicrobial genetic sequences, synthesizing 78 candidate peptides. Over half of these peptides effectively inhibited bacterial growth in in vitro and in animal models[34]. One particularly promising peptide, prevotellin-2, demonstrated anti-infective capabilities comparable to the FDA-approved antibiotic polymyxin B[34].
The underlying mechanisms involve ATB-induced microbial community disruption, characterized by loss of taxonomic diversity and functional redundancy, which not only attenuates ICI efficacy but also potentially exacerbates immune-related adverse events[35]. These observations suggest that ATB administration may establish a preconditioned immunological microenvironment that impacts tumor-specific immune surveillance[36]. Contrasting these findings, a recent investigation reported enhanced immunotherapy efficacy associated with ATB administration within a 30-day window surrounding ICI initiation, independent of conventional disease- and treatment-related variables[37]. These divergent outcomes may be partially explained by methodological limitations, including the absence of comprehensive subgroup analyses accounting for critical variables such as antibiotic class, administration route, treatment duration, and pharmacokinetic parameters. The establishment of standardized, large-scale prospective studies incorporating detailed microbial and immunological profiling is essential to elucidate the complex interplay between antibiotic use and ICI outcomes in HCC patients[37].
Probiotics
Probiotics are live microorganisms or bioactive compounds that provide health benefits by modulating host physiology, particularly through interactions with the gut microbiota and the immune system. They serve as biochemical modulators, enhancing hepatic and intestinal function while strengthening immune responses. For instance, the Probio-M9 trial (NCT05032014) demonstrated that Lactobacillus rhamnosus supplementation can improve the efficacy of anti-PD-1 immunotherapy, with objective response rates (ORR) increasing from 21.7% in the control group to 39.1% in those receiving treatment[38,39]. Probiotics may also reduce aflatoxin B1-induced hepatocarcinogenesis by decreasing toxin absorption, correcting microbial imbalances, and lowering systemic LPS levels[40]. In murine studies, Lactobacillus plantarum C88 was shown to enhance fecal aflatoxin excretion and restore antioxidant defenses[40]. Similarly, probiotic yogurt containing L. rhamnosus and Streptococcus thermophilus reduced urinary aflatoxin metabolites in exposed children, likely through bacterial binding of toxins and decreased intestinal uptake[41]. Probiotics may play a role in suppressing HCC by downregulating oncogenic pathways and upregulating tumor suppressor genes, particularly through strains like Lactobacillus acidophilus and Bifidobacterium bifidum[42]. Additionally, probiotics may attenuate Toll-like receptor 4 (TLR4)-mediated inflammation, which is a key driver of HCC progression. Administration of Lactobacillus plantarum has been shown to reduce TLR4 expression and liver injury. Furthermore, both gut microbiota depletion and TLR4 inhibition have been found to suppress HCC development by 80%-90%[43].
While probiotics show promising therapeutic potential for liver diseases and HCC, their effects are strain- and context-dependent. Further large-scale clinical studies are necessary to optimize probiotic selection and validate their efficacy across diverse patient populations. Additionally, the mechanisms involved are complicated by significant functional overlap between administered strains and endogenous microbial taxa associated with the host response. This highlights the need for future investigations to adopt standardized methodologies that encompass next-generation microbiome sequencing platforms, comprehensive metabolomic profiling, and advanced bioinformatic analyses. Such approaches will be essential for optimizing the development and clinical application of next-generation probiotic formulations.
Prebiotics
Prebiotics represent a distinct category of non-digestible carbohydrates that serve as essential regulators of intestinal microbial balance[44]. These compounds, which include lactulose, inulin-type fructans (ITF), and galactooligosaccharides (GOS), possess unique structural characteristics that render them resistant to mammalian digestive enzymes while serving as preferential substrates for beneficial gut bacteria[45]. Through selective fermentation processes, prebiotics significantly alter gut microbial composition, particularly enhancing populations of Bifidobacterium and Lactobacillus species while suppressing potentially pathogenic microorganisms[46]. The metabolic byproducts of this fermentation, especially short-chain fatty acids (SCFAs) like propionate and butyrate, exhibit tissue-specific bioavailability and are essential for maintaining intestinal homeostasis[47]. Notably, preclinical studies have shown increased propionate levels in portal circulation following ITF administration, suggesting targeted hepatic delivery of this microbial metabolite with potential implications for liver pathophysiology[48,49].
The therapeutic potential of prebiotics primarily arises from the bioactive properties of SCFAs, which exert multifaceted effects on host physiology[50]. Propionate, in particular, has demonstrated significant antiproliferative activity against malignant cell lines, functioning through mechanisms that involve cell cycle arrest and apoptosis induction[51]. Moreover, the effects of prebiotics are mediated via specific G-protein-coupled receptors (FFA2/GPR43 and FFA3/GPR41), which are widely expressed in various tissues, including the intestinal epithelium and immune cells[52]. In hepatic applications, prebiotics such as lactulose have shown promise in accelerating post-surgical liver regeneration, potentially through the modulation of oxidative stress pathways and inflammatory responses[53].
Current research emphasizes the need for systematic investigations into strain-specific prebiotic effects and optimized dosing regimens to maximize therapeutic outcomes. Developing standardized protocols for prebiotic administration in clinical settings remains a critical challenge, necessitating rigorous evaluations of pharmacokinetic properties and potential drug interactions. Future studies should focus on elucidating the molecular mechanisms underlying prebiotic-mediated immunomodulation, particularly regarding their effects on tumor microenvironment remodeling and immune cell function.
Integrating prebiotic strategies with conventional anticancer therapies presents promising opportunities for synergistic treatment approaches; however, this integration requires careful validation through controlled clinical trials. As the field advances, a precision medicine approach that considers individual variations in gut microbiota composition and metabolic responses will be essential for realizing the full therapeutic potential of prebiotics in oncology and hepatology. Continued research efforts should prioritize both mechanistic studies and translational applications to bridge the gap between experimental findings and clinical implementation.
Fecal microbiota transplantation
FMT represents a sophisticated therapeutic approach involving the transfer of processed donor stool material through either oral administration of lyophilized or cryopreserved capsules or direct endoscopic delivery via colonoscopy or gastroscopy[54]. Lots of clinical trials are evaluating the potential of FMT to overcome therapeutic resistance and prevent tumor recurrence by enhancing immunotherapy efficacy in refractory cases[55-57]. A phase I/II clinical trial (NCT04264975) identified a novel microbial species phylogenetically related to Prevotella sp. and Marseille-P4119, demonstrating significant immunostimulatory properties. This bacterial isolate exhibited potent activation of human CD4+ and CD8+ T lymphocytes, characterized by enhanced IFN-γ secretion and increased tumor-infiltrating lymphocyte populations in syngeneic murine models, resulting in significant tumor growth suppression. Furthermore, combination therapy with this microbial strain and anti-PD-1 blockade demonstrated synergistic antitumor effects, surpassing the efficacy of monotherapy[55].
Murine models have provided critical mechanistic insights into gut microbiomes in HCC interactions. However, divergences in gut-liver axis physiology, immune cell composition, and tumor biology, compounded by the artificial simplicity of laboratory mouse ecosystems, often obscure the relevance of preclinical findings to human HCC[58]. Although clinical observations partially validate murine data such as the conserved microbial signatures like Faecalibacterium depletion in HCC patients, discrepancies persist, particularly in microbiome-ICI response associations[59]. To bridge this gap, future research should prioritize multi-model validation, humanized platforms, and focus on conserved functional pathways rather than taxon-specific effects, while explicitly addressing murine study caveats, including lack of comorbidities and genetic diversity in translational interpretations. Moreover, while FMT has demonstrated promising clinical potential, safety concerns persist, with documented adverse events requiring more rigorous attribution analysis and standardized reporting protocols to establish causality. Future investigations should incorporate comprehensive safety assessments and long-term monitoring to better characterize the risk-benefit profile of FMT interventions.
MECHANISM BY WHICH THE GUT MICROBIOME INFLUENCES THE TREATMENT OF HCC WITH IMMUNOTHERAPY
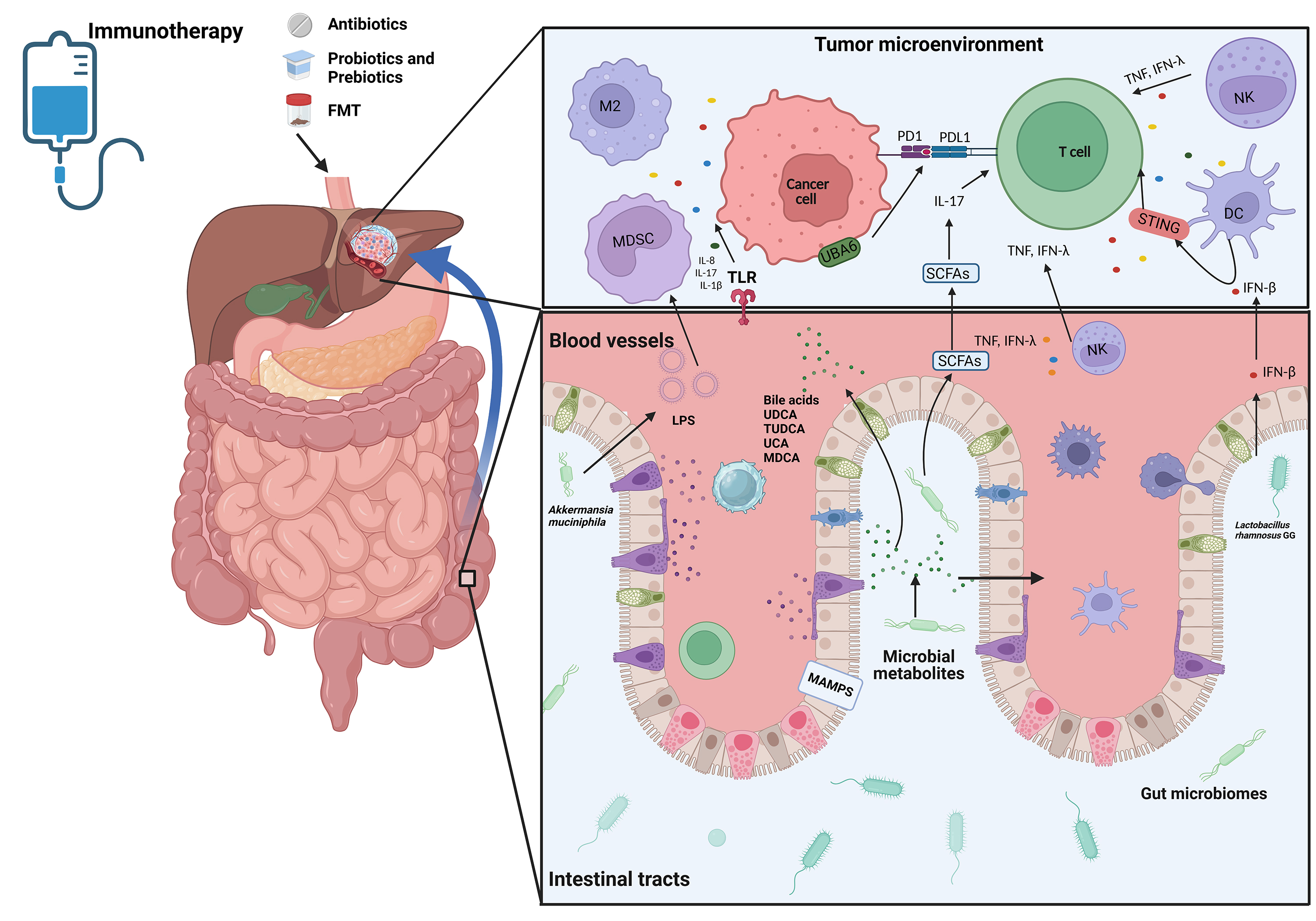
Gut microbiota-derived small molecules and metabolites reshape the tumor microenvironment (TME) with profound implications for immunotherapy responsiveness[59-61]. These microbial products critically regulate metabolic and inflammatory pathways, which interplay mechanistically with antitumor immunity, particularly in HCC[62,63]. Recent studies explored how microbiota-immune crosstalk modulates ICI outcomes, yet the precise molecular pathways governing this interaction in HCC demand systematic exploration[64]. This section delineates the immunomodulatory mechanisms through which gut microbiota influences ICI efficacy, focusing on two key aspects [Figure 1]: (1) Dynamic interactions between microbial signals and innate/adaptive immune cells that potentiate antitumor responses; (2) Metabolite-driven activation of immune effector pathways critical for ICI success.
Figure 1. A schematic diagram showing the mechanisms of microbiota in the regulation of ICI resistance in HCC patients. Created in BioRender.Lee, T. (2025) (https://BioRender.com/teyw5en). ICI: Immune checkpoint inhibitor; HCC: hepatocellular carcinoma; PD-L1: programmed death ligand 1; PD1: programmed death 1; FMT: fecal microbiota transplantation; MDSC: myeloid-derived suppressor cells; NK: natural killer; DC: dendritic cell; IFN-β: interferon-beta; IFN-γ: interferon-γ; TNF: tumor necrosis factor MAMPs: microbe-associated molecular patterns; SCFAs: short-chain fatty acids; IL-17: interleukin-17; IL-8: interleukin-8; IL-1β: interleukin-1 beta; UDCA: ursodeoxycholic acid; TUDCA: tauroursodeoxycholic acid; UCA: ursodeoxycholic acid; MDCA: murideoxycholic acid; TLR: toll-like receptor; LPS: Lipopolysaccharide; UBA6: ubiquitin-like modifier-activating enzyme 6; MDSC: myeloid-derived suppressor cells.
The gut microbiome modulates immunity, influencing immunotherapy
The gut microbiome orchestrates adaptive immune responses to enhance immunotherapy efficacy through multifaceted mechanisms, including TME remodeling via induction of CD8+ and CD4+ T cell populations and modulation of immunosuppressive cell subsets. Comprehensive immunophenotyping analyses utilizing flow cytometry and cytokine profiling have demonstrated that patients with elevated abundances of Clostridiales, Ruminococcaceae, and Faecalibacterium exhibit enhanced systemic immune activation, characterized by increased circulating effector CD4+ and CD8+ T cell frequencies and preserved cytokine responsiveness to anti-PD-1 therapy[65]. Conversely, Bacteroidales-dominant microbiota profiles are associated with immunosuppressive phenotypes, marked by expansion of regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs), correlating with diminished cytokine responses[66].
Further mechanistic studies have revealed that Faecalibacterium enrichment promotes Th1 polarization while suppressing Treg populations in peripheral circulation, potentially contributing to the durable clinical responses observed with ipilimumab treatment[67]. Enhanced microbial diversity has been positively correlated with the expansion of memory CD8+ T cell and natural killer (NK) cell subsets during PD-1 blockade, suggesting microbiome-mediated modulation of immune memory formation[68]. The spatial distribution of specific microbial taxa, particularly Bifidobacterium, within the tumor microenvironment has been shown to enhance NK cell activation through mechanisms involving increased intestinal permeability, thereby potentiating antitumor immunity[69].
At the molecular level, Lactobacillus rhamnosus GG has been demonstrated to activate the cGAS/STING/TBK1/IRF7 signaling axis in dendritic cells, stimulating IFN-β production and enhancing cross-priming of tumor-specific CD8+ T cells, which synergizes with anti-PD-1 therapy[70]. Similarly, Akkermansia muciniphila exerts immunostimulatory effects through activation of the intratumoral IFN-I-NK-DC axis via STING pathway signaling, while simultaneously promoting intestinal barrier integrity through reduction of serum lipopolysaccharide (LPS) and bile acid metabolites and suppressing immunosuppressive m-MDSC and M2 macrophage populations[71,72].
The gut microbiota also mediates direct antitumor effects through novel mechanisms. The ubiquitin-like modifier activating enzyme 6 (UBA6) expressed on tumor cells interacts with microbial components to enhance tumor cell immunogenicity, thereby sensitizing tumors to ICI therapy[73]. Additionally, Lactobacillus reuteri produces the antimicrobial compound reuterin, which exerts selective antitumor activity through the induction of protein oxidation and inhibition of ribosomal biogenesis and protein translation in malignant cells[74].
Gut microbiome-related metabolites modulate immunotherapy
The gut microbiome-derived metabolome, comprising microbe-associated molecular patterns (MAMPs), SCFAs, and bile acid metabolites, plays a pivotal role in modulating therapeutic responses to immunotherapy in HCC [Table 4]. MAMPs exhibit trans-epithelial translocation capacity, potentially exacerbating microbial dysbiosis and contributing to hepatocarcinogenesis through multiple mechanisms[75,76]. This dysbiotic state facilitates the production of carcinogenic metabolites while promoting hepatic fibrogenesis and cirrhosis progression, mediated through immune dysregulation, altered microbial metabolic networks, and compromised intestinal barrier function[77,78]. SCFAs serve as crucial mediators of intestinal homeostasis, maintaining epithelial barrier integrity while exerting direct antitumor effects through inhibition of tumor cell proliferation and induction of apoptotic pathways[79]. Furthermore, SCFAs demonstrate immunomodulatory properties by regulating the differentiation and functional polarization of both immunosuppressive Tregs and proinflammatory IL-17+ γδ T cell populations[80].
A summary of studies comprehensively links microbial metabolites to their target immune pathways and functional impacts on HCC progression
| Microbial metabolite | Involved immune pathway | Functional impact on HCC | References |
| Short-chain fatty acids (SCFAs) | Treg differentiation; IL-17 + γδ T cell polarization | Maintains intestinal barrier integrity; Inhibits tumor cell proliferation; Induces apoptosis | [65,66] |
| Secondary bile acids (UDCA, TUDCA, MDCA) | NKT cell activation (IFN-γ, TNF); TLR signaling | Enhances antitumor immunity; Predictive biomarker for PD-1 response; Modulates hepatic metabolic reprogramming | [67-71] |
| Microbe-associated molecular patterns (MAMPs) | TLR activation (IL-8, IL-17, IL-1β); Inflammasome signaling | Promotes immune cell recruitment; Induces oxidative stress and genomic instability; Exacerbates dysbiosis-linked carcinogenesis | [61-64,72-74] |
| Reuterin (L. reuteri-derived) | Ribosomal biogenesis inhibition; Protein oxidation | Direct antitumor activity via metabolic disruption in cancer cells | [71,72] |
| LPS (Lipopolysaccharide) | TLR4/NF-κB pathway; M2 macrophage polarization | Promotes fibrogenesis and cirrhosis; Disrupts intestinal barrier function | [71,72] |
Bile acids, synthesized through hepatic-cholangiocyte crosstalk, play essential roles in lipid metabolism and energy homeostasis. The bidirectional interaction between host cells and gut microbiota regulates bile acid metabolism, influencing hepatic metabolic programming and contributing to liver cancer pathogenesis[81]. Microbial biotransformation of primary to secondary bile acids modulates natural killer T (NKT) cell dynamics, with activated NKT cells secreting key antitumor cytokines including IFN-γ and TNF, thereby orchestrating tumor-specific immune responses[82,83]. Clinical investigations have identified distinct bile acid signatures associated with PD-1 therapy response, characterized by elevated concentrations of ursodeoxycholic acid (UDCA), tauroursodeoxycholic acid (TUDCA), ursodeoxycholic acid (UCA), and murideoxycholic acid (MDCA) in responder populations. These metabolic profiles correlate with increased abundances of Lachnoclostridium, Lachnospiraceae, and Veillonella, alongside decreased Prevotella 9 representation[84]. Integrative analyses of clinical datasets suggest that serum bile acids may function as molecular mediators of gut microbiome-host transcriptome crosstalk. Furthermore, microbial markers associated with tumor immune microenvironment modulation and bile acid metabolism demonstrate significant predictive value for clinical outcomes, achieving an area under the curve (AUC) of 81% in prognostic models[85].
At the molecular interface of microbial-host interactions, pathogen-associated molecular patterns (PAMPs) engage TLRs to initiate cytokine and chemokine cascades, [84] including IL-8, IL-17, and IL-1β, which promote immune cell recruitment to hepatic tissues. These inflammatory mediators concurrently induce oxidative stress and genomic instability, potentially initiating hepatocarcinogenic processes[86-88].
TUMOR HETEROGENEITY AND GUT MICROBIOME IN HCC IMMUNOTHERAPY
The reciprocal relationship between tumor heterogeneity and gut microbial composition establishes a dynamic biological network that significantly influences HCC immunotherapy outcomes. This complex interplay necessitates a multidimensional analytical framework to develop optimized therapeutic strategies. Below, we systematically examine how distinct layers of tumor heterogeneity interact with the gut microbiome to modulate ICI efficacy.
Etiology heterogeneity
The etiology of HCC critically shapes both tumor biology and associated gut microbiome profiles, leading to divergent responses to immunotherapy. HBV/HCV-induced HCC exhibits microbiome signatures characterized by elevated levels of Enterobacteriaceae and reduced microbial diversity, correlating with enhanced inflammatory responses[89]. Moreover, the gut microbiota modulates viral hepatitis progression through TLR activation and interferon signaling, potentially priming the immune microenvironment for improved ICI sensitivity[90]. In contrast, NASH-related HCC demonstrates a distinct microbial profile enriched in Bacteroides and Ruminococcus, accompanied by altered bile acid metabolism. These microbial-derived secondary bile acids, particularly deoxycholic acid, promote immunosuppression via farnesoid X receptor (FXR) signaling and Tregs expansion, contributing to the observed reduction in ICI efficacy in this subgroup[90]. This etiology-specific microbial imprinting suggests that therapeutic microbiome modulation should be tailored according to HCC pathogenesis.
Tumor genetic heterogeneity
Tumor genetic alterations exert profound effects on both local immunity and systemic microbial ecology. CTNNB1-mutant HCCs frequently exhibit immune-excluded phenotypes, characterized by markedly reduced CD8+ T cell infiltration due to the activation of the Wnt/β-catenin pathway[91]. This immunosuppressive milieu may selectively enrich gut microbial taxa such as Fusobacterium nucleatum, which further exacerbates immune evasion through MDSC recruitment[91]. In contrast, TP53 loss-of-function correlates with enhanced proinflammatory cytokine production and gut barrier dysfunction. The resulting microbial translocation of LPS perpetuates hepatic inflammation via TLR4 signaling, creating a paradoxical environment where chronic inflammation coexists with impaired anti-improve immunity[92]. These findings underscore how driver mutations influence microbial ecosystems while simultaneously shaping the tumor immune microenvironment.
Tumor metabolic heterogeneity
Tumoral metabolic diversity creates spatially distinct microniches that exert selective pressures on microbial communities. Stabilization of hypoxia-inducible factors (HIF-1α/2α) drives anaerobic glycolysis, resulting in lactate accumulation. This microenvironment favors the expansion of lactate-utilizing bacteria, which in turn generate immunosuppressive metabolites like succinate[93,94]. Additionally, impaired tumor vasculature leads to irregular oxygen and nutrient gradients, establishing intratumoral zonation patterns through hypoxia-mediated selection pressures[95]. This spatial metabolic compartmentalization, characterized by region-specific metabolic reprogramming, may ultimately enhance the tumor’s adaptive capacity and proliferative potential[95].
FURTHER DIRECTIONS
The gut microbiota exerts a profound influence on therapeutic responses to ICIs, positioning it as a potential predictive biomarker for stratifying responder and non-responder patient populations[96]. Despite significant advancements in understanding microbiota-mediated modulation of cancer therapy, several critical knowledge gaps warrant further investigation.
Multi-omics approaches
Methodological heterogeneity in fecal sample analysis presents a substantial challenge. While 16S rRNA sequencing and whole-genome sequencing represent the predominant techniques for microbiome characterization, variability in analytical pipelines and reference databases has contributed to inconsistent findings across studies. This underscores the urgent need for standardization of microbiome profiling methodologies across all analytical stages, from sample processing to bioinformatic interpretation. Moreover, the predominant reliance on single-omics approaches in existing research has limited our understanding of the complex, multifactorial interactions within the tumor-microbiome-immune axis, consequently hindering therapeutic optimization. The inherent biological diversity among patients, encompassing variations in immune status and comorbidities, further complicates the identification of universal ICI response biomarkers. Therefore, the development of integrated multi-omics frameworks incorporating gut transcriptomic, proteomic, and metabolomic profiling is essential for the comprehensive characterization of microbiota-mediated therapeutic responses. The mechanistic underpinnings of microbiome-mediated ICI response modulation remain incompletely characterized. Significant research opportunities exist in elucidating the complex microbial-host interactions governing antitumor immunity and identifying specific microbial taxa with pivotal immunomodulatory functions. To address these knowledge gaps, a multidimensional research strategy integrating computational modeling, multi-omics approaches, and functional validation is imperative.
Large-scale clinical trials
The implementation of large-scale, multiregional clinical trials encompassing diverse patient populations is crucial for establishing the clinical relevance of microbial signatures and interventions. Such studies should be complemented by the development of microbiome-targeted therapeutic strategies, potentially enabling personalized immunotherapy approaches. To ensure data comparability and reproducibility, international collaborative efforts must establish standardized protocols for sample collection, storage, processing, and analysis. Furthermore, longitudinal monitoring of microbial dynamics throughout the therapeutic course is essential, given the temporal plasticity of the gut microbiome and its dynamic interplay with immunotherapeutic interventions.
Ethical considerations and logistical challenges
The major ethical considerations and logistical challenges that must be resolved to responsibly integrate microbiome-based biomarkers and therapies into clinical practice. The selection and screening of donors for FMT represents one of the most pressing ethical challenges in microbiome medicine. Unlike conventional drug manufacturing, where raw materials are chemically defined and quality-controlled, FMT relies on human-derived biological material with inherent variability that directly impacts therapeutic efficacy and safety. This donor-dependent efficacy raises fundamental questions about equitable access to effective treatments and the ethical obligations surrounding donor recruitment and compensation. Key considerations include establishing consensus on "healthy" donor microbiomes, preventing commercialization that limits access, overcoming geographic and socioeconomic barriers to treatment availability, resolving intellectual property issues, and creating standardized manufacturing processes for both FMT and defined microbial consortia, all while ensuring robust safety monitoring and adapting regulatory approaches to balance innovation with patient protection across diverse populations and healthcare settings.
CONCLUSION
In conclusion, accumulating preclinical and clinical evidence substantiates the pivotal role of the gut microbiome in modulating therapeutic responses to immunotherapy in HCC. Microbiota-targeted interventions, encompassing antibiotic modulation, probiotic supplementation, prebiotic administration, and FMT, represent promising therapeutic avenues that may potentiate the efficacy of ICIs through synergistic microbial-metabolite-immune interactions. The mechanisms underlying microbiome-mediated antitumor responses are multifaceted, involving a complex interplay between microbial communities, host immunity, and metabolic networks. However, clinical translation faces significant hurdles, including methodological variability in microbiome profiling, patient-specific confounders, and unresolved questions regarding optimal microbial consortia for therapeutic manipulation. This synthesis advances the field by proposing a unified framework that bridges microbial ecology with immuno-oncology, while outlining three priority directions for future research: the development of microbiome-based predictive biomarkers through multi-omics integration, standardization of microbiota-modulating protocols for clinical use, and mechanistic dissection of microbe-immune-metabolic networks using gnotobiotic models. Addressing these challenges will be paramount for harnessing the full potential of microbiome-ICI synergy to overcome current limitations in HCC treatment.
DECLARATIONS
Authors’ contributions
Conceptualized, designed, drafted, and revised the manuscript: Wu XQ, Lee TKW
Availability of data and materials
Not applicable.
Financial support and sponsorship
This study was supported by the RGC Theme-based Research Scheme (RGC TRS T12-705-24-R)
Conflicts of interest
Terence K.W. Lee is an Editorial Board member of the journal Hepatoma Research. Terence K.W. Lee is also a Guest Editor for the Special Issue “The Gut Microbiome and HCC: Implications for Early Diagnostic Biomarkers and Novel Therapies.” Terence K.W. Lee was not involved in any steps of editorial processing, notably including reviewer selection, manuscript handling, or decision making. The other authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-49.
2. Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16:589-604.
3. Rinella ME, Lazarus JV, Ratziu V, et al; NAFLD Nomenclature consensus group. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol. 2023;79:1542-56.
4. Zhou J, Sun H, Wang Z, et al. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (2019 edition). Liver Cancer. 2023;12:405-44.
5. Singal AG, Llovet JM, Yarchoan M, et al. AASLD practice guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology. 2023;78:1922-65.
6. Llovet JM, Pinyol R, Kelley RK, et al. Molecular pathogenesis and systemic therapies for hepatocellular carcinoma. Nat Cancer. 2022;3:386-401.
7. Lazzaro A, Hartshorn KL. A comprehensive narrative review on the history, current landscape, and future directions of hepatocellular carcinoma (HCC) systemic therapy. Cancers. 2023;15:2506.
8. Martins F, Sofiya L, Sykiotis GP, et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. 2019;16:563-80.
9. Sangro B, Sarobe P, Hervás-Stubbs S, Melero I. Advances in immunotherapy for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2021;18:525-43.
10. Kim CG, Kim C, Yoon SE, et al. Hyperprogressive disease during PD-1 blockade in patients with advanced hepatocellular carcinoma. J Hepatol. 2021;74:350-9.
11. Yamaguchi H, Hsu JM, Sun L, Wang SC, Hung MC. Advances and prospects of biomarkers for immune checkpoint inhibitors. Cell Rep Med. 2024;5:101621.
12. Ha S, Wong VW, Zhang X, Yu J. Interplay between gut microbiome, host genetic and epigenetic modifications in MASLD and MASLD-related hepatocellular carcinoma. Gut. 2024;74:141-52.
13. Schwabe RF, Greten TF. Gut microbiome in HCC - mechanisms, diagnosis and therapy. J Hepatol. 2020;72:230-8.
14. Mao J, Wang D, Long J, et al. Gut microbiome is associated with the clinical response to anti-PD-1 based immunotherapy in hepatobiliary cancers. J Immunother Cancer. 2021;9:e003334.
15. Lee PC, Wu CJ, Hung YW, et al. Gut microbiota and metabolites associate with outcomes of immune checkpoint inhibitor-treated unresectable hepatocellular carcinoma. J Immunother Cancer. 2022;10:e004779.
16. Zhang C, Liu H, Sun L, et al. An overview of host-derived molecules that interact with gut microbiota. Imeta. 2023;2:e88.
17. Martinez-Guryn K, Leone V, Chang EB. Regional diversity of the gastrointestinal microbiome. Cell Host Microbe. 2019;26:314-24.
18. Shui L, Yang X, Li J, Yi C, Sun Q, Zhu H. Gut microbiome as a potential factor for modulating resistance to cancer immunotherapy. Front Immunol. 2019;10:2989.
19. Zheng Y, Wang T, Tu X, et al. Gut microbiome affects the response to anti-PD-1 immunotherapy in patients with hepatocellular carcinoma. J Immunother Cancer. 2019;7:193.
20. Chung MW, Kim MJ, Won EJ, et al. Gut microbiome composition can predict the response to nivolumab in advanced hepatocellular carcinoma patients. World J Gastroenterol. 2021;27:7340-9.
21. Yau T, Park JW, Finn RS, et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022;23:77-90.
22. Abou-Alfa GK, Lau G, Kudo M, et al. Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evid. 2022;1:EVIDoa2100070.
23. Yu H, Lin G, Jiang J, et al. Synergistic activity of Enterococcus Faecium-induced ferroptosis via expansion of IFN-γ+CD8+ T cell population in advanced hepatocellular carcinoma treated with sorafenib. Gut Microbes. 2024;16:2410474.
24. Chai X, Tang Y, Li X, et al. Lenvatinib improves the relative abundance of probiotics in intestinal flora of patients with primary liver cancer. Research Square. 2024;Research Square:rs-4024621. Available from https://doi.org/10.21203/rs.3.rs-4024621/v1 [accessed 28 May 2025].
25. Simpson RC, Shanahan ER, Scolyer RA, Long GV. Towards modulating the gut microbiota to enhance the efficacy of immune-checkpoint inhibitors. Nat Rev Clin Oncol. 2023;20:697-715.
26. Wu H, Zheng X, Pan T, et al. Dynamic microbiome and metabolome analyses reveal the interaction between gut microbiota and anti-PD-1 based immunotherapy in hepatocellular carcinoma. Int J Cancer. 2022;151:1321-34.
27. Lu Y, Yuan X, Wang M, et al. Gut microbiota influence immunotherapy responses: mechanisms and therapeutic strategies. J Hematol Oncol. 2022;15:47.
28. Xin Y, Peng G, Song W, Zhou X, Huang X, Cao X. Gut microbiota as a prognostic biomarker for unresectable hepatocellular carcinoma treated with anti-PD-1 therapy. Front Genet. 2024;15:1366131.
29. Shen YC, Lee PC, Kuo YL, et al. An exploratory study for the association of gut microbiome with efficacy of immune checkpoint inhibitor in patients with hepatocellular carcinoma. J Hepatocell Carcinoma. 2021;8:809-22.
30. Cai X, Cho JY, Chen L, et al. Enriched pathways in gut microbiome predict response to immune checkpoint inhibitor treatment across demographic regions and various cancer types. iScience. 2025;28:112162.
31. Cheung KS, Lam LK, Seto WK, Leung WK. Use of antibiotics during immune checkpoint inhibitor treatment is associated with lower survival in hepatocellular carcinoma. Liver Cancer. 2021;10:606-14.
32. Alshammari K, Alsugheir F, Aldawoud M, et al. Association between antibiotic exposure and survival in patients with hepatocellular carcinoma treated with nivolumab. J Clin Oncol. 2021;39:e16186.
33. Spahn S, Roessler D, Pompilia R, et al. Clinical and genetic tumor characteristics of responding and non-responding patients to PD-1 inhibition in hepatocellular carcinoma. Cancers. 2020;12:3830.
34. Torres MDT, Brooks EF, Cesaro A, et al. Mining human microbiomes reveals an untapped source of peptide antibiotics. Cell. 2024;187:5453-67.e15.
35. Pierrard J, Seront E. Impact of the gut microbiome on immune checkpoint inhibitor efficacy - a systematic review. Curr Oncol. 2019;26:395-403.
36. Pinato DJ, Howlett S, Ottaviani D, et al. Association of prior antibiotic treatment with survival and response to immune checkpoint inhibitor therapy in patients with cancer. JAMA Oncol. 2019;5:1774-8.
37. Ramirez J, Guarner F, Bustos Fernandez L, Maruy A, Sdepanian VL, Cohen H. Antibiotics as major disruptors of gut microbiota. Front Cell Infect Microbiol. 2020;10:572912.
38. Zhang W, Zhang Y, Li Y, Ma D, Zhang H, Kwok LY.
39. ClinicalTrials.gov. Qiang Xu, Jiangxi Provincial Cancer Hospital. Available from https://classic.clinicaltrials.gov/ct2/show/NCT05032014 [accessed 28 May 2025].
40. Huang L, Duan C, Zhao Y, et al. Reduction of aflatoxin B1 toxicity by lactobacillus plantarum C88: a potential probiotic strain isolated from Chinese traditional fermented food “tofu”. PLoS One. 2017;12:e0170109.
41. Russo E, Fiorindi C, Giudici F, Amedei A. Immunomodulation by probiotics and prebiotics in hepatocellular carcinoma. World J Hepatol. 2022;14:372-85.
42. Heydari Z, Rahaie M, Alizadeh AM, Agah S, Khalighfard S, Bahmani S. Effects of Lactobacillus acidophilus and Bifidobacterium bifidum probiotics on the expression of MicroRNAs 135b, 26b, 18a and 155, and their involving genes in mice colon cancer. Probiotics Antimicrob Proteins. 2019;11:1155-62.
43. Elshaer AM, El-Kharashi OA, Hamam GG, Nabih ES, Magdy YM, Abd El Samad AA. Involvement of TLR4/CXCL9/PREX-2 pathway in the development of hepatocellular carcinoma (HCC) and the promising role of early administration of lactobacillus plantarum in Wistar rats. Tissue Cell. 2019;60:38-47.
44. Wilson B, Whelan K. Prebiotic inulin-type fructans and galacto-oligosaccharides: definition, specificity, function, and application in gastrointestinal disorders. J Gastroenterol Hepatol. 2017;32 Suppl 1:64-8.
45. Hutkins R, Walter J, Gibson GR, et al. Classifying compounds as prebiotics - scientific perspectives and recommendations. Nat Rev Gastroenterol Hepatol. 2025;22:54-70.
46. Liu Y, Wang J, Wu C. Modulation of gut microbiota and immune system by probiotics, pre-biotics, and post-biotics. Front Nutr. 2022;8:634897.
47. Meyers G, Samouda H, Bohn T. Short chain fatty acid metabolism in relation to gut microbiota and genetic variability. Nutrients. 2022;14:5361.
48. Hao Y, Hao Z, Zeng X, Lin Y. Gut microbiota and metabolites of cirrhotic portal hypertension: a novel target on the therapeutic regulation. J Gastroenterol. 2024;59:788-97.
49. Mager LF, Burkhard R, Pett N, et al. Microbiome-derived inosine modulates response to checkpoint inhibitor immunotherapy. Science. 2020;369:1481-9.
50. Ashaolu TJ, Ashaolu JO, Adeyeye SAO. Fermentation of prebiotics by human colonic microbiota in vitro and short-chain fatty acids production: a critical review. J Appl Microbiol. 2021;130:677-87.
51. Kim K, Kwon O, Ryu TY, et al. Propionate of a microbiota metabolite induces cell apoptosis and cell cycle arrest in lung cancer. Mol Med Rep. 2019;20:1569-74.
52. Teyani R, Moniri NH. Gut feelings in the islets: the role of the gut microbiome and the FFA2 and FFA3 receptors for short chain fatty acids on β-cell function and metabolic regulation. Br J Pharmacol. 2023;180:3113-29.
53. Cornide-Petronio ME, Álvarez-Mercado AI, Jiménez-Castro MB, Peralta C. Current knowledge about the effect of nutritional status, supplemented nutrition diet, and gut microbiota on hepatic ischemia-reperfusion and regeneration in liver surgery. Nutrients. 2020;12:284.
54. Li X, Zhang S, Guo G, Han J, Yu J. Gut microbiome in modulating immune checkpoint inhibitors. EBioMedicine. 2022;82:104163.
55. Kim Y, Kim G, Kim S, et al. Fecal microbiota transplantation improves anti-PD-1 inhibitor efficacy in unresectable or metastatic solid cancers refractory to anti-PD-1 inhibitor. Cell Host Microbe. 2024;32:1380-93.e9.
56. Pomej K, Frick A, Scheiner B, et al. Study protocol: fecal microbiota transplant combined with atezolizumab/bevacizumab in patients with hepatocellular carcinoma who failed to achieve or maintain objective response to atezolizumab/bevacizumab - the FAB-HCC pilot study. PLoS One. 2025;20:e0321189.
57. Xiao K, Li K, Xiao K, Yang J, Zhou L. Gut microbiota and hepatocellular carcinoma: metabolic products and immunotherapy modulation. Cancer Med. 2025;14:e70914.
58. Cigliano A, Liao W, Deiana GA, Rizzo D, Chen X, Calvisi DF. Preclinical models of hepatocellular carcinoma: current utility, limitations, and challenges. Biomedicines. 2024;12:1624.
59. Jugder BE, Kamareddine L, Watnick PI. Microbiota-derived acetate activates intestinal innate immunity via the Tip60 histone acetyltransferase complex. Immunity. 2021;54:1683-97.e3.
60. Zheng D, Liwinski T, Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020;30:492-506.
61. He Y, Fu L, Li Y, et al. Gut microbial metabolites facilitate anticancer therapy efficacy by modulating cytotoxic CD8+ T cell immunity. Cell Metab. 2021;33:988-1000.e7.
62. Microbiota-induced IFN-I signaling promotes an antitumor microenvironment. Cancer Discov. 2021;11:2955.
64. Liu X, Li S, Wang L, Ma K. Microecological regulation in HCC therapy: gut microbiome enhances ICI treatment. Biochim Biophys Acta Mol Basis Dis. 2024;1870:167230.
65. Araji G, Maamari J, Ahmad FA, Zareef R, Chaftari P, Yeung SJ. The emerging role of the gut microbiome in the cancer response to immune checkpoint inhibitors: a narrative review. J Immunother Precis Oncol. 2022;5:13-25.
66. Peng Z, Cheng S, Kou Y, et al. The gut microbiome is associated with clinical response to anti-PD-1/PD-L1 immunotherapy in gastrointestinal cancer. Cancer Immunol Res. 2020;8:1251-61.
67. Davar D, Dzutsev AK, McCulloch JA, et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science. 2021;371:595-602.
68. Jin Y, Dong H, Xia L, et al. The diversity of gut microbiome is associated with favorable responses to anti-programmed death 1 immunotherapy in Chinese patients with NSCLC. J Thorac Oncol. 2019;14:1378-89.
69. Rizvi ZA, Dalal R, Sadhu S, et al. High-salt diet mediates interplay between NK cells and gut microbiota to induce potent tumor immunity. Sci Adv. 2021;7:eabg5016.
70. Si W, Liang H, Bugno J, et al.
71. Lam KC, Araya RE, Huang A, et al. Microbiota triggers STING-type I IFN-dependent monocyte reprogramming of the tumor microenvironment. Cell. 2021;184:5338-56.e21.
72. Wu XQ, Ying F, Chung KPS, et al. Intestinal Akkermansia muciniphila complements the efficacy of PD1 therapy in MAFLD-related hepatocellular carcinoma. Cell Rep Med. 2025;6:101900.
73. Zhang L, Jiang L, Yu L, et al. Inhibition of UBA6 by inosine augments tumour immunogenicity and responses. Nat Commun. 2022;13:5413.
74. Bell HN, Rebernick RJ, Goyert J, et al. Reuterin in the healthy gut microbiome suppresses colorectal cancer growth through altering redox balance. Cancer Cell. 2022;40:185-200.e6.
75. Ma J, Li J, Jin C, et al. Association of gut microbiome and primary liver cancer: a two-sample Mendelian randomization and case-control study. Liver Int. 2023;43:221-33.
76. Liu X, Li S, Wang L, Ma K. Microecological regulation in HCC therapy: gut microbiome enhances ICI treatment. Biochim Biophys Acta Mol Basis Dis. ;1870:167230.
77. Hsu CL, Schnabl B. The gut-liver axis and gut microbiota in health and liver disease. Nat Rev Microbiol. 2023;21:719-33.
78. Tilg H, Adolph TE, Trauner M. Gut-liver axis: pathophysiological concepts and clinical implications. Cell Metab. 2022;34:1700-18.
79. Zheng Z, Wang B. The gut-liver axis in health and disease: the role of gut microbiota-derived signals in liver injury and regeneration. Front Immunol. 2021;12:775526.
80. Lee J, d’Aigle J, Atadja L, et al. Gut microbiota-derived short-chain fatty acids promote poststroke recovery in aged mice. Circ Res. 2020;127:453-65.
81. Song Y, Lau HC, Zhang X, Yu J. Bile acids, gut microbiota, and therapeutic insights in hepatocellular carcinoma. Cancer Biol Med. 2023;21:144-62.
82. Zhang X, Coker OO, Chu ES, et al. Dietary cholesterol drives fatty liver-associated liver cancer by modulating gut microbiota and metabolites. Gut. 2021;70:761-74.
83. Lynch L, Nowak M, Varghese B, et al. Adipose tissue invariant NKT cells protect against diet-induced obesity and metabolic disorder through regulatory cytokine production. Immunity. 2012;37:574-87.
84. Behary J, Amorim N, Jiang XT, et al. Gut microbiota impact on the peripheral immune response in non-alcoholic fatty liver disease related hepatocellular carcinoma. Nat Commun. 2021;12:187.
85. Huang H, Ren Z, Gao X, et al. Integrated analysis of microbiome and host transcriptome reveals correlations between gut microbiota and clinical outcomes in HBV-related hepatocellular carcinoma. Genome Med. 2020;12:102.
86. Zhou J, Tripathi M, Sinha RA, Singh BK, Yen PM. Gut microbiota and their metabolites in the progression of non-alcoholic fatty liver disease. Hepatoma Res. 2021;7:11.
87. Silveira MAD, Bilodeau S, Greten TF, Wang XW, Trinchieri G. The gut-liver axis: host microbiota interactions shape hepatocarcinogenesis. Trends Cancer. 2022;8:583-97.
88. Zhou A, Tang L, Zeng S, Lei Y, Yang S, Tang B. Gut microbiota: a new piece in understanding hepatocarcinogenesis. Cancer Lett. 2020;474:15-22.
89. Laface C, Lauricella E, Ranieri G, et al. HCC and immunotherapy: the potential predictive role of gut microbiota and future therapeutic strategies. Onco. 2025;5:9.
90. Monti E, Vianello C, Leoni I, et al. Gut microbiome modulation in hepatocellular carcinoma: preventive role in NAFLD/NASH progression and potential applications in immunotherapy-based strategies. Cells. 2025;14:84.
91. Murai H, Kodama T, Maesaka K, et al. Multiomics identifies the link between intratumor steatosis and the exhausted tumor immune microenvironment in hepatocellular carcinoma. Hepatology. 2023;77:77-91.
92. Chen K, Shuen TWH, Chow PKH. The association between tumour heterogeneity and immune evasion mechanisms in hepatocellular carcinoma and its clinical implications. Br J Cancer. 2024;131:420-9.
93. Suthen S, Lim CJ, Nguyen PHD, et al. Hypoxia-driven immunosuppression by Treg and type-2 conventional dendritic cells in HCC. Hepatology. 2022;76:1329-44.
94. Pral LP, Fachi JL, Corrêa RO, Colonna M, Vinolo MAR. Hypoxia and HIF-1 as key regulators of gut microbiota and host interactions. Trends Immunol. 2021;42:604-21.
95. Berndt N, Eckstein J, Heucke N, et al. Metabolic heterogeneity of human hepatocellular carcinoma: implications for personalized pharmacological treatment. FEBS J. 2021;288:2332-46.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Special Issue
Copyright
Data & Comments
Data















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].