The new era of cholangiocarcinoma treatment: application of nano-based drug delivery systems
Abstract
Cholangiocarcinoma (CCA) is a rare primary cancer of the bile duct epithelium, accounting for about 3% of all gastrointestinal cancers worldwide. CCA incidence is notably higher in Southeast and East Asia, particularly in northeastern Thailand. The early diagnosis of CCA is limited, while the cancer tends to metastasize rapidly, contributing to high mortality rates. Current treatments for CCA, including conventional chemotherapies, often cause drug resistance and induce significant side effects due to the drug going off-target. This underlines the need for novel therapeutic strategies, including chemopreventive and adjuvant treatments. Targeted drug delivery systems using nano-based technologies offer a promising approach to enhance treatment specificity and effectiveness, thereby minimizing side effects. This review provides an overview of nanomedicine’s application in the treatment of CCA. Polymeric and lipid-based nanoparticles (NPs), as examples of passive targeting mechanisms such as the enhanced permeability and retention effect, are discussed. Additionally, functionalized NPs are described, focusing on their role in active targeting strategies in CCA therapy. This summary will support the development of more effective drugs for CCA.
Keywords
INTRODUCTION
Cholangiocarcinoma
Cholangiocarcinoma (CCA) is a primary cancer of the bile duct epithelium. CCA arises from the malignant transformation of cholangiocytes, the epithelial cells that line the biliary apparatus[1]. CCA is a rare cancer worldwide, representing approximately 3% of all gastrointestinal cancers[2,3]. However, the highest incidence of CCA was reported in Southeast and East Asia, especially in Northeastern Thailand[4]. The difficulty of early diagnosis and high metastasis are CCA’s main problems, leading to high mortality rates. Most patients are diagnosed in the advanced stage. Currently, complete tumor resection is considered the most effective treatment option for CCA[5]. However, since the patients are in an advanced stage with tumor metastasis, the operation has been limited. In the case of surgery, CCA patients still have poor outcomes, with 5-year survival rates ranging from 5% to 20%[6]. Chemotherapy (gemcitabine and cisplatin) and radiotherapy are other choices of CCA treatments, but their efficiency is still unsatisfactory due to cancer’s heterogeneity. The most effective treatment for CCA is surgery in the early stage, which can improve survival up to 4 years. Unfortunately, most patients are unresectable cases. The best choice for these patients is palliative care to improve quality of life[7,8].
The standard chemotherapeutic regimen for patients with advanced-stage CCA involves adding either durvalumab or pembrolizumab to the standard treatment of cisplatin and gemcitabine, offering a new first-line treatment option. Furthermore, advancements in comprehensive genomic profiling have enabled the identification of targetable genetic mutations, including isocitrate dehydrogenase 1, fibroblast growth factor receptor 2, human epidermal growth factor receptor 2 (HER2), B-Raf proto-oncogene, neurotrophic tropomyosin receptor kinase, rearranged during transfection, Kirsten rat sarcoma virus, and mouse double minute 2 homolog. This has paved the way for the development of precision medicine strategies for patients who have previously undergone treatment[9]. However, drug resistance in CCA patients leads to unsatisfactory outcomes and complications[8]. Therefore, for CCA treatment, novel drug development techniques are urgently required to improve the conventional efficiency of drugs. At present, nanotechnology, especially nano-based drug delivery systems, is considered a promising and cutting-edge technology for drug delivery that advances personalized medicine and improves patient outcomes.
CCA pathophysiology
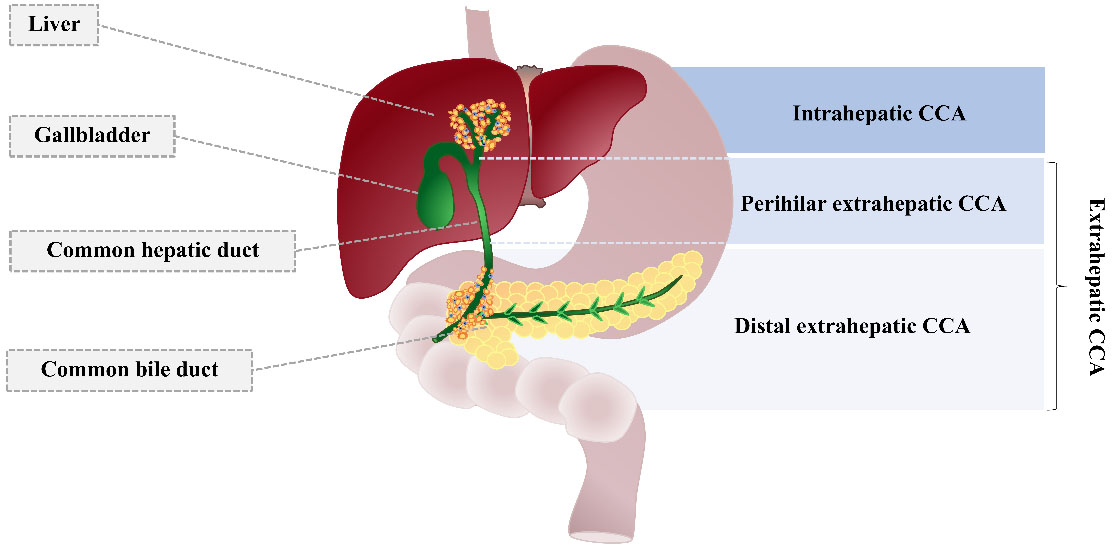
CCA is a malignancy of the bile ducts’ epithelium that arises from the malignant transformation of cholangiocytes. CCA is classified into two types according to the lesion’s location: intrahepatic and extrahepatic CCA [Figure 1]. An intrahepatic CCA tumor occurs within the liver, but extrahepatic CCA arises in the large bile duct[10]. Extrahepatic CCA is an adenocarcinoma with different histological variants, including papillary adenocarcinoma, signet-ring carcinoma, squamous cell or mucoepidermoid carcinoma, and a lymphoepithelioma-like form[11].
Figure 1. Cholangiocarcinoma classification. CCA is categorized into intrahepatic and extrahepatic types. Extrahepatic CCA is further divided into perihilar and distal types. Adapted from Blechacz et al.[1]. CCA: Cholangiocarcinoma.
Etiology and carcinogenesis of CCA
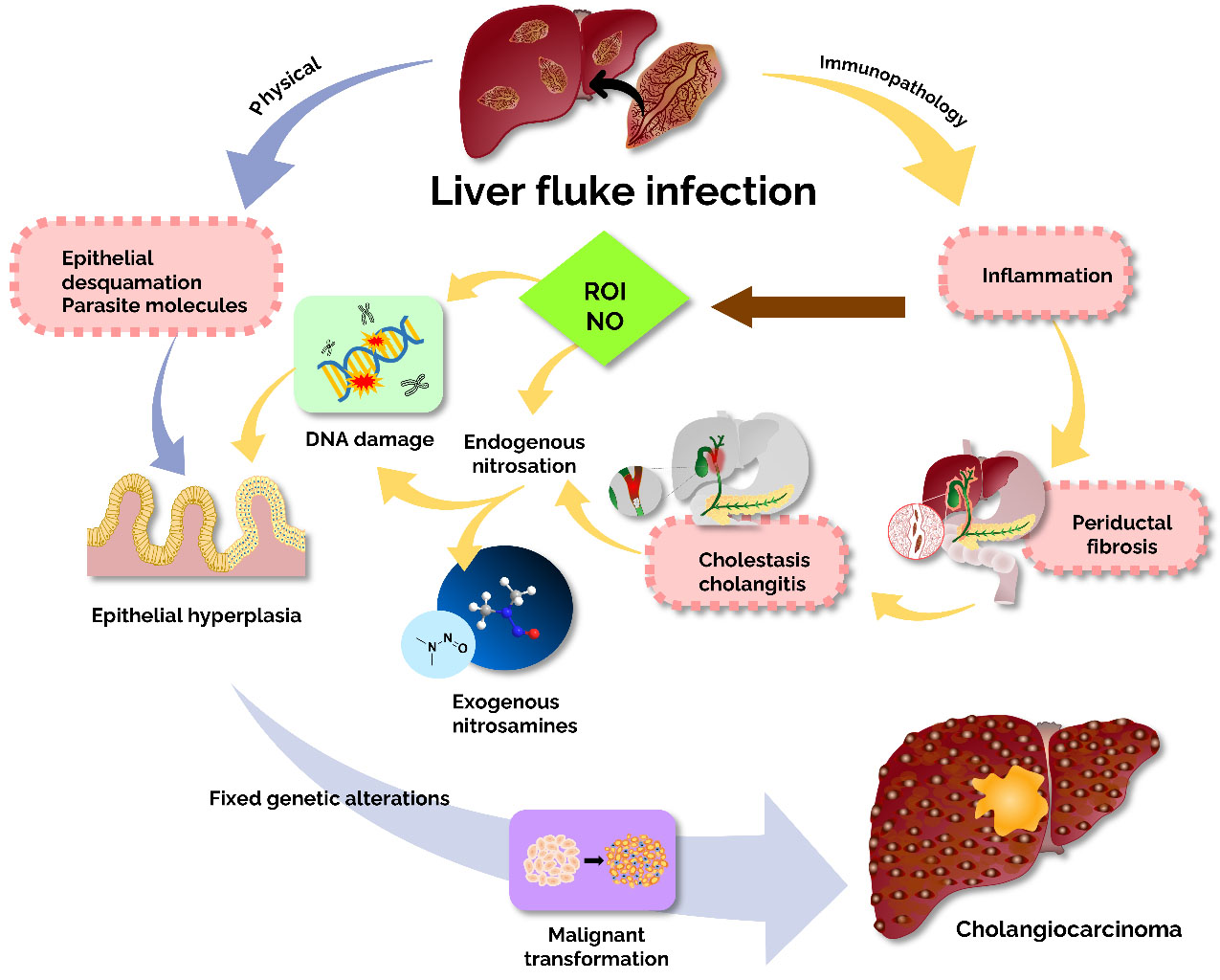
Most cases of CCA occur sporadically, and the exact etiology is unknown. However, the association of obstructed bile flow induces chronic inflammation, which is the main condition for CCA development[12]. The most common disease associated with CCA in Western countries is primary sclerosing cholangitis, while liver fluke [Opisthorchis viverrini (Ov), Clonorchis sinensis] infestation can cause CCA in Southeast Asia[13]. The endemic areas of these liver flukes are Japan and Southeast Asia, especially Thailand. Humans are infected by the consumption of undercooked fish containing adult worms. Worms lay their eggs in the host’s bile duct and persist there for several years, causing an inflammatory response that leads to CCA. The mechanisms by which Ov induces cholangiocarcinoma can be divided into two main categories: physical and immunopathological mechanisms [Figure 2]. Among the physical pathways, liver fluke infection leads to biliary damage. The flukes’ suckers attach to the biliary epithelium, causing damage to the bile ducts. When liver flukes mature, the lesions grow larger and become ulcerated, causing the formation of circumoval granulomas during chronic opisthorchiasis, which eventually leads to biliary periductal fibrosis. In addition to inflammation, the ulcers allow bile acids to enter, and prolonged exposure to these substances can increase the risk of malignant transformation, as these metabolites are known to be endogenous factors in the development of malignancy. In the immunopathology pathway, immune responses and immunopathological mechanisms play a key role in causing hepatobiliary damage in opisthorchiasis[14]. In hamsters with liver fluke infection in the bile duct, inflammation was observed around the infected bile ducts[15]. The immunopathology is caused by inflammation, particularly due to reactive oxygen intermediates and nitric oxide. These pathways intersect, causing genetic damage and uncontrolled cell proliferation. As DNA or genes are repeatedly replicated, the damage becomes stabilized, eventually leading to the malignant transformation of cholangiocytes, as shown in Figure 2[4,16].
Figure 2. The mechanistic insight of liver fluke-induced cholangiocarcinoma. Two main pathways are involved in liver fluke-induced bile duct epithelium damage: immunopathology to parasite antigens and mechanical damage. Adapted from Sripa et al.[4].
CCA pathogenesis
The exact molecular mechanisms underlying the development of CCA remain unclear. However, chronic inflammation is believed to promote cell proliferation and may increase the risk of DNA damage and somatic mutations[17,18]. Cholangiocytes secrete several proinflammatory cytokines, such as TNF-𝛼, IL-6, and nitric oxide. The reactive oxygen species interact with DNA together with nitric oxide and cause DNA damage, leading to mutation[19]. Moreover, IL-6 has also been found to be elevated in the serum of patients with CCA[20]. This cytokine is known to play a key role in cholangiocarcinogenesis and cell proliferation. A previous report showed that bile acids stimulate cell proliferation via the activation of growth factors, such as epithelial growth factors[21]. Non-alcoholic fatty liver disease is a potentially modifiable risk factor for cholangiocarcinoma and gallbladder cancer[22]. In addition, liver cirrhosis and viral hepatitis B and C are known risk factors for cholangiocarcinoma, particularly intrahepatic CCA[23,24]. Cholangiocarcinogenesis associated with viral hepatitis is likely connected to chronic inflammation and enhanced cell proliferation[25]. The impact of hepatitis infection on CCA also shows geographic differences, with variations between Western countries and Asia, where hepatitis viruses are endemic[26].
Challenges of CCA treatment
Currently, early diagnosis of CCA remains limited, and the disease is associated with a high likelihood of local metastases, including lymph node metastases. CCA is classified into five stages, from stage 0 (least advanced) to stage 4 (most advanced)[18]. The 5-year overall survival rates for stages 3 and 4 are less than 10% and 0%, respectively. Among patients eligible for curative surgery, the 5-year overall survival rate is between 15% and 40%[27]. However, most patients present to the hospital at an advanced, incurable stage. Although complete tumor resection is the best approach and remains the only potentially curative treatment for CCA, many patients are diagnosed too late to be eligible for surgery. Moreover, 10% to 35% of patients initially considered resectable are later found to be unresectable during exploratory laparotomy[28,29]. The median overall survival for patients with unresectable CCA is only 3-6 months[18]. The persistently low survival rates of CCA patients highlight the ongoing challenges in its treatment[5].
The incidence of both distant and locoregional recurrence remains high in patients with resected CCA, with around 60%-70% experiencing disease relapse. Until recently, adjuvant treatment was largely guided. A meta-analysis of adjuvant treatment demonstrated improved survival outcomes in patients who underwent R1 resection, positive for residual tumors. In recent years, the phase III Biliary Tract Cancer Adjuvant Capecitabine trial, which evaluated adjuvant capecitabine versus placebo, did not achieve its primary endpoint in the intention-to-treat analysis. Nevertheless, a preplanned sensitivity analysis revealed a survival advantage, which has led to the broad approval of capecitabine as the standard adjuvant treatment[30].
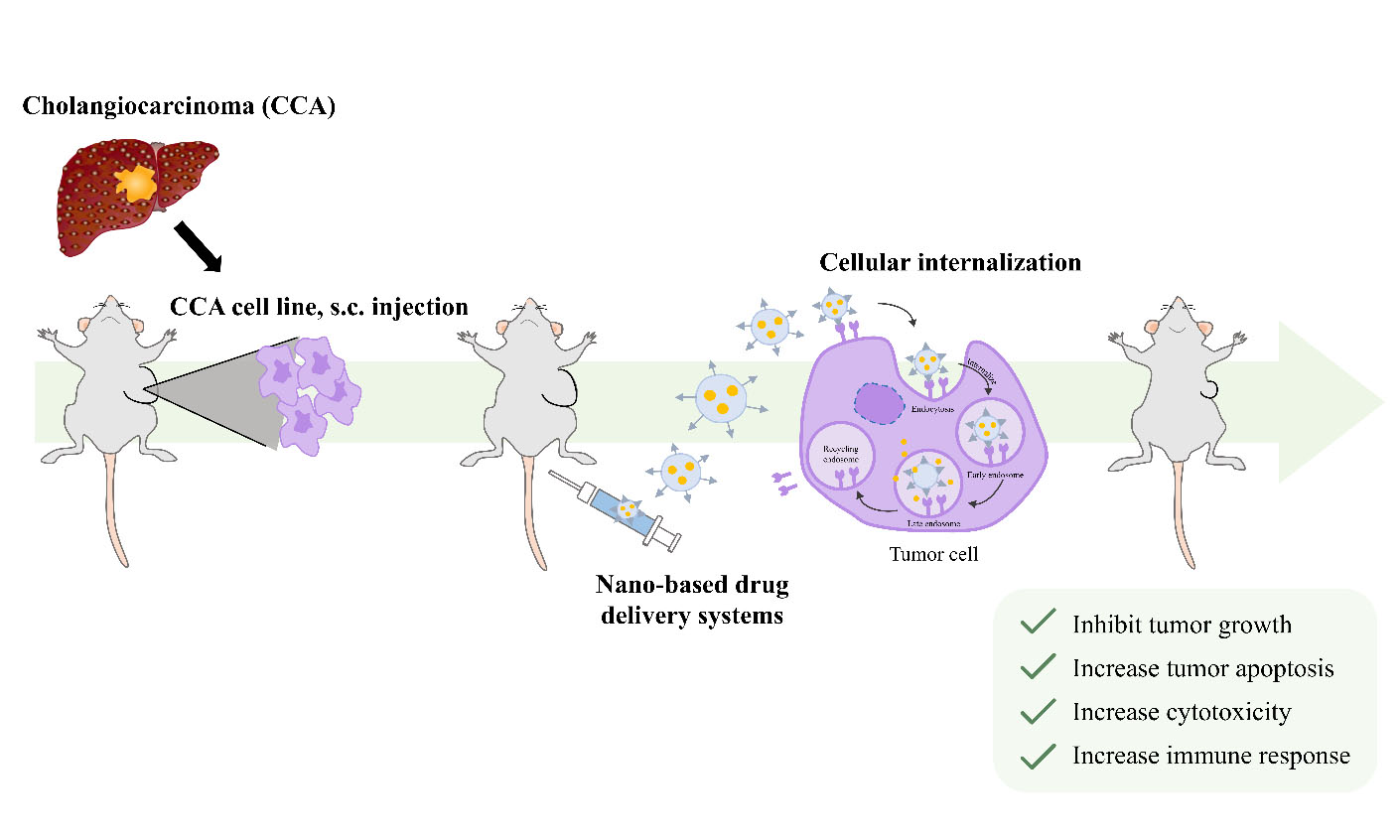
The treatment of CCA remains less effective and is associated with a high rate of complications. Conventional drug therapy via 5-FU, gemcitabine, doxorubicin, and cisplatin or their combination causes drug resistance in CCA patients. Therefore, novel treatment strategies for CCA, including chemopreventive and adjuvant therapeutics, must be developed. Conventional chemotherapeutic drugs are non-targeted cancer therapies that affect both tumor cells and numerous normal cells, leading to unfavorable side effects. Tumor-targeted drug delivery systems have been employed to increase the selectivity of drug accumulation and enhance internalization into tumor cells. The development of novel chemotherapeutic drugs via nano-based drug delivery systems has become an attractive approach to overcome the limitations of conventional drugs. Moreover, the exploitation of selective molecular targets that can inhibit tumor progression and improve clinical outcomes will be beneficial for drug development against CCA[31].
CANCER NANOMEDICINE
Cancer remains one of the leading causes of mortality worldwide. Although modern treatments such as immunotherapy and conventional medicines are widely used, a major challenge in drug delivery is off-target effects, which can lead to undesirable side effects. To address this, nanotechnology has been applied in the form of nanocarriers to enhance therapeutic efficacy by specifically delivering drug payloads to targeted organs or cells.
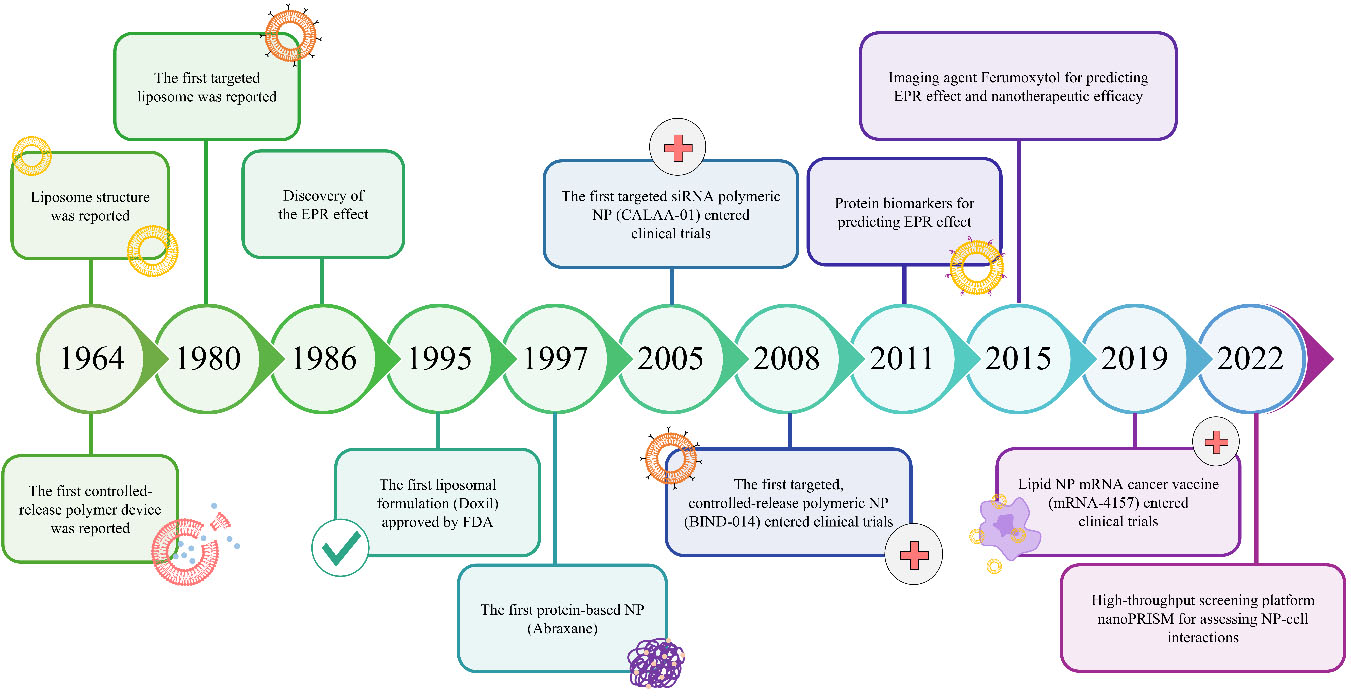
Colloidal gold particles were used for medicinal purposes in the early history of nanomedicine[32]. The term “nanomedicine” was first introduced by Robert A. Freitas in 1999[33]. Nanomedicine has been sought due to conventional cancer drugs’ deficiencies, such as low specificity, rapid drug clearance, biodegradation, and limited targeting. Nanoparticles (NPs) as drug carriers have been proposed as an alternative strategy for cancer treatment. Furthermore, the surface modification of drug-carried NPs yields a multifunctional drug delivery system. Such a multifunctional drug delivery system can be tailored via surface modification of drug-loaded NPs, resulting in a specific controlled release[34]. In comparison with conventional anti-cancer drugs, the targeted nano-therapeutic approach has exhibited higher efficacy with reduced toxicity, enhanced permeability, sustained controlled release, and increased plasma half-life of drugs[35]. Key milestones in the development of nano-based drug delivery systems are illustrated in the timeline shown in Figure 3.
Figure 3. Timeline of nanomedicine developments. Adapted from Fan et al.[36]. EPR: Enhanced permeability and retention; NP: nanoparticle.
For almost three decades, research focused on cancer nanomedicine has expanded[36]. Various types of NPs, including inorganic, polymeric, and lipid-based NPs, have been employed to deliver chemotherapeutic agents, immunotherapeutic drugs, and nucleic acids to tumor sites. At present, 15 anti-cancer-based nanomedicines are approved globally, whereas up to 200 novel nanomedicines are under investigation in clinical trials[36]. Upon advances in nanotechnology, the series of nanomedicines has been continuously developed, focusing on improving therapeutic efficiency and minimizing off-target effects[37].
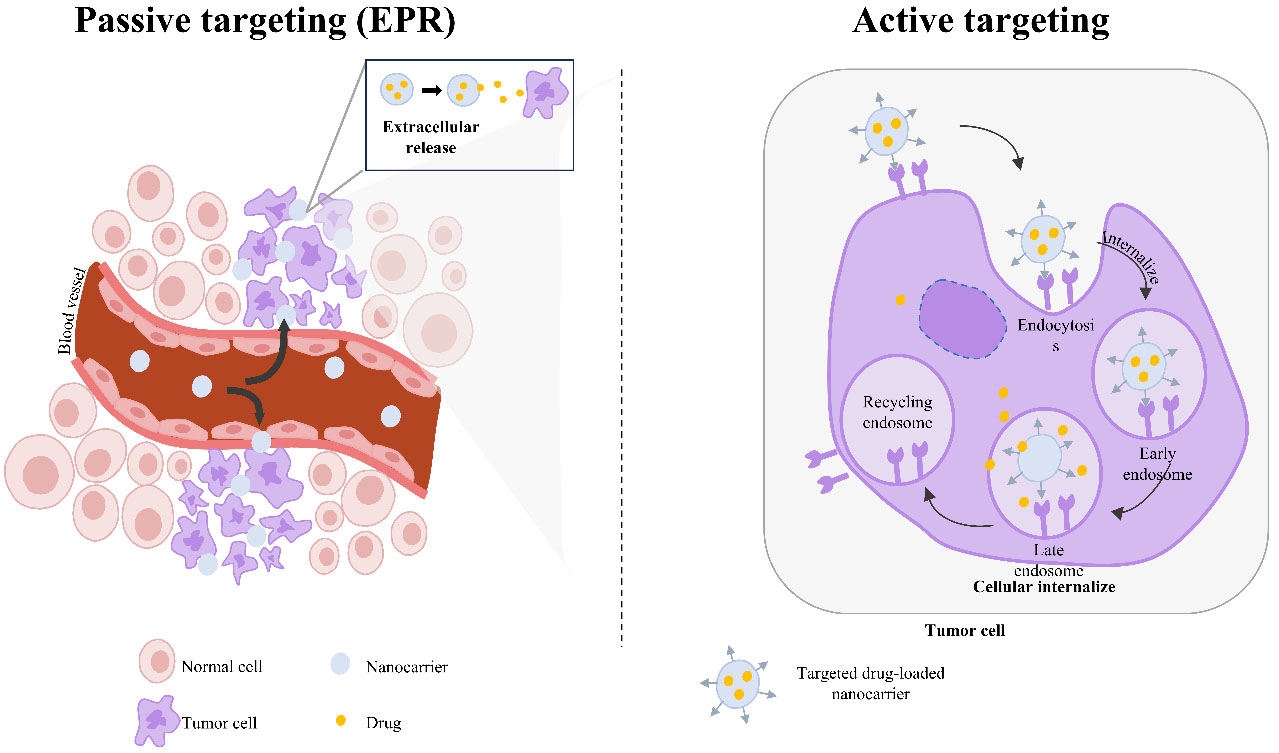
In tumor tissues, the therapeutic efficacy of nanomedicine is primarily achieved through passive accumulation in solid tumors via the enhanced permeability and retention (EPR) effect. The EPR effect arises from the leaky vasculature and deficient lymphatic drainage characteristic of tumor tissues, allowing NPs to penetrate and accumulate within the tumor microenvironment[38] [Figure 4]. The first nanomedicine approved by the FDA was pegylated liposomal doxorubicin (Doxil®), indicated for the treatment of breast cancer. The latest FDA-approved nanomedicine is paclitaxel micellar (Apealea®). Both Doxil® and Apealea® rely on EPR-mediated passive targeting to accumulate in tumor tissues[36].
Figure 4. Schematic representation of the mechanism whereby nanomedicines achieve cancer therapeutic efficiency via passive targeting (EPR effect) and active targeting (ligand binding). Adapted from R. Masoudifar et al.[41]. EPR: Enhanced permeability and retention.
To improve specificity and enhance nanotherapeutics’ cellular internalization into tumor cells through cell-specific targeting, second-generation cancer nanomedicines have been developed[39]. Nanocarriers can be functionalized using targeting moieties that selectively bind to antigens or receptors expressed on the tumor cell membrane. The targeting moieties can be carbohydrates, small molecules, peptides, antibodies, or nucleic acids. Conjugated nanocarriers can increase cellular uptake and increase therapeutic efficiency
As an emerging class of cancer treatments, nanomedicines have drawn increasing attention from both the scientific community and the wider public. Nanomedicine-based therapy has emerged as a promising approach for targeting cancer cells while not affecting normal healthy cells[42,43]. The distinct advantages of nanomedicines have been well-documented, including tumor accumulation via the enhanced permeability and retention effect, protection of drugs from enzymatic and mechanical degradation, prolonged drug release, triggered or stimulus-responsive activation, specific targeting at the tumor site, and the ability to overcome biological barriers[44]. For instance, stromal-rich cancers such as pancreatic ductal adenocarcinoma and cholangiocarcinoma are among the most challenging to treat due to their distinct pathobiological characteristics. These cancers feature a dense desmoplastic stroma and poorly vascularized, hypoperfused tumor vessels, which greatly limit the effectiveness of conventional small-molecule drugs. However, nanomedicines offer promising potential to overcome these barriers[45]. Furthermore, the ability to combine multiple therapeutic strategies within a single nanoplatform is one of the advantages of nanomedicine[42]. Certain biological vesicles, such as exosomes, have also been explored as nanomedicine carriers[46]. Due to their inherent tumor-homing capabilities, MSCs and MSC-derived exosomes show potential as targeted delivery vehicles for cancer treatment. They can precisely deliver antitumor molecules, RNA, or chemotherapeutic drugs, effectively reducing the viability and invasiveness of tumor cells[46].
In this review, we summarize the current progress in nanomedicine, with a particular focus on its application to CCA treatment. Polymeric and lipid-based NPs are described as representatives of passive targeting via the EPR effect. Additionally, NPs with specific functionalization or surface modifications are discussed as examples of active targeting nanomedicine.
NANOMEDICINES FOR CCA TREATMENT
Nanomedicine for CCA treatment via passive targeting
Polymeric NPs
Polymeric NPs (PNPs) are nanoscale solid particles composed of biocompatible polymers. An active pharmaceutical ingredient (API), such as an anti-cancer drug, can be either encapsulated within the PNP or conjugated to its surface. Based on the source of the polymer, PNPs are generally categorized into two types: natural (or naturally derived) polymers and synthetic polymers[47].
Natural polymeric NPs
Chitosan is deacetylated chitin, which can be obtained from a variety of natural sources, including marine organisms, insects, fungi, and animals. The physicochemical characteristics of chitosan largely depend on its deacetylation degree and molecular weight[48]. Due to its excellent biocompatibility and biodegradability, chitosan-based nanosystems have been widely applied in cancer diagnosis and treatment[49]. In CCA treatment, chitosan-based NPs have been developed to deliver chemotherapeutic[50] and photodynamic therapeutic drugs[51], demonstrating superior efficacy over free APIs.
Moreover, a chitosan-based delivery system can be designed to promote the delivery to cancer cells, particularly those with multidrug resistance, which exhibit a unique acidic pH and elevated glutathione concentration[52]. In 2013, doxorubicin (DOX) was incorporated into histidine (His)-modified chitosan-based NPs, and its therapeutic effectiveness on DOX-resistant CCA was investigated for the first time. The DOX-loaded His-modified chitosan-based NPs (DOX-His-CNPs) demonstrated significantly higher cytotoxicity to DOX-resistant CCA compared to free DOX in both in vitro and in vivo studies[50].
Unlike chemotherapy, each API used in photodynamic therapy (PDT) is non-toxic to both cancerous and noncancerous cells in the absence of light activation. The cytotoxic effects of PDT primarily depend on the generation of reactive oxygen species (ROS), which are produced through biochemical reactions involving light and photosensitizing agents. These ROS play a crucial role in inducing apoptosis and necrosis in cells. Therefore, the selectivity of PDT for cancer cells is a key factor determining its therapeutic efficacy[53]. Chlorin E6 (C6)-loaded ursodeoxycholic acid-modified chitosan has been shown to enhance C6 accumulation in CCA cells, increase ROS production, and improve phototoxicity compared to free C6[51].
Synthetic polymeric NPs
Poly (lactic-co-glycolic acid) (PLGA) is a synthetic, biocompatible, and biodegradable polymer approved by the US FDA for clinical use[54]. PLGA-based NPs are well known for their ability to sustain and prolong the release of encapsulated APIs[55]. Currently, PLGA-based NPs have been applied to both chemotherapeutic drugs, such as doxorubicin[56], and phytochemical agents for CCA treatment, including ursolic acid[39] and atractylodin (ATD)[57]. Incorporating these APIs into PLGA NPs effectively overcomes their solubility limitations, resulting in enhanced cytotoxicity against CCA cells and reduced toxicity to noncancerous cells compared to the free APIs. Notably, ATD-loaded PLGA NPs have demonstrated higher oral bioavailability and improved biodistribution relative to free ATD[57].
Lipid-based NPs
Lipid-based NPs (LNPs) mainly consist of lipid components such as phospholipids, cholesterol, and triglycerides. Due to their structural similarity to biological membranes, LNPs exhibit excellent biocompatibility[58]. Currently, various types of LNPs have been utilized in cancer therapy, with liposomes being the most notable LNPs investigated for CCA treatment. A recent retrospective study reported that CCA patients receiving a combination of irinotecan-loaded liposomes and chemotherapeutic drugs, including leucovorin and 5-fluorouracil as a second‑line treatment, achieved an overall survival of 12.4 months after initiation[59]. Despite these promising results, the clinical application of liposomes remains limited. However, several studies have been conducted in vitro or in animal models, as summarized below.
Gemcitabine (GEM), a chemotherapeutic agent commonly used to treat CCA, exerts its cytotoxic effects by inhibiting DNA synthesis[60]. However, GEM is rapidly inactivated intracellularly, leading to a short half-life and reduced chemosensitivity[61]. By incorporating GEM into photosensitizer-modified liposomes, targeted delivery to CCA tissues can be achieved, and local laser irradiation can further promote GEM release from the carrier. This strategy has shown enhanced anti-cancer activity and reduced side effects[62]. In addition to chemotherapeutic drugs, photosensitizers can also be encapsulated within liposomes. For example, 5-aminolevulinic acid (ALA), a precursor to protoporphyrin IX, generates cytotoxic ROS upon light exposure. ALA-loaded liposomes demonstrated superior cellular internalization and higher photocytotoxicity compared to free ALA[63]. An overview of nanomedicines for CCA treatment via passive targeting is provided in Table 1.
Examples of polymeric and lipid-based nanomedicines for CCA treatment via passive targeting
| Nanocarrier | Payload | Characterization | In Vitro/In Vivo Model | Application | Ref. |
| DOX-His-CNPs | DOX | < 200 nm | Exhibited higher cytotoxicity against DOX-resistant HuCCT-1 cholangiocarcinoma cells compared to free DOX in both in vitro and in vivo studies | Promoted DOX release in the tumor microenvironment (low pH and high glutathione) | [50] |
| C6-UA-CNPs | C6 | < 400 nm | Showed higher cytotoxicity toward HuCCT-1 cholangiocarcinoma cells and lower toxicity to noncancerous cells compared to free C6 | Enhanced cellular uptake of C6 and increased phototoxicity in cancer cells | [51] |
| DOX-DexbLG | DOX | 100-200 nm | Demonstrated greater penetration and cytotoxicity against DOX-resistant HuCCT-1 cells compared to free DOX | Improved DOX penetration into cancer cells | [56] |
| ATD-PLGA NPs | ATD | 214 nm, -30 mV, 68% EE | Showed higher cytotoxicity to HuCCT-1 and CL-6 cholangiocarcinoma cell lines compared to free ATD | Exhibited higher oral bioavailability and improved biodistribution compared to free ATD | [57] |
| IRI-Lip | IRI | N/A | The combination with leucovorin and 5-fluorouracil as second-line therapy demonstrated promising overall survival one year after initiation | Used as second-line treatment for CCA | [59] |
| GEM-PheoA-Lip | GEM | 100 nm, +0.04 mV, 38% EE | Showed higher anti-cancer activity than GEM alone in both in vitro (HuCCT-1 cells) and in vivo studies | Enabled specific delivery of GEM to HuCCT-1 cells, with local irradiation enhancing cytotoxicity | [62] |
| ALA-Lip | ALA | 87-510 nm, 4.4-5.1% EE | Demonstrated superior cellular internalization and higher photocytotoxicity compared to free ALA | Effective candidate for ALA delivery in cholangiocarcinoma cells | [63] |
| UA-PLGA NPs | UA | 240 nm, -16 mV, 98% EE | Exhibited superior cytotoxicity toward KKU-213A (JCRB1557) and KKU-055 (JCRB1551) cholangiocarcinoma cell lines compared to free UA | Showed anti-cancer activity with excellent hemocompatibility | [64] |
Inorganic nanoparticles
Inorganic nanoparticles are nanoparticles that do not contain carbon[65]. Compared with organic nanoparticles, they exhibit biocompatibility, non-toxicity, hydrophilicity, and high stability[66]. Common types of inorganic nanoparticles include magnetic nanoparticles, quantum dots, gold nanoparticles, and metal oxide nanoparticles. The delivery of 5-fluorouracil using magnetic Fe3O4 nanoparticles[67] and modified gold nanoparticles[68] has demonstrated higher efficiency than the free drug in treating CCA in vitro and in vivo, respectively. Inorganic nanoparticles can also be modified for targeted drug delivery. For example, gemcitabine-loaded zeolitic imidazolate framework-67 (GEM-ZIF97) significantly reduced CCA cell viability, maintained higher viability of normal cells, and decreased tumor size in in vivo studies[69], demonstrating the potential of inorganic nanoparticles in cancer therapy. Additionally, carbon dots have been reported to inhibit the growth of CCA cells[70]. However, inorganic nanoparticles are more often efficient when used in combination with other therapies.
Biomimetic nanoparticles
Exosomes
Exosomes are extracellular structures enclosed by a lipid bilayer, which safeguards their cargo from degradation. Notably, exosomes secreted by various cell types can partially reflect the heterogeneity of their cells of origin, providing them with superior biocompatibility compared to other delivery systems such as liposomes and lipid-based nanoparticles. Recent studies have demonstrated that exosomes are effective carriers for delivering chemotherapeutic drugs, successfully inhibiting tumor cell proliferation and growth both in vitro and in vivo. For example, exosomes derived from human bone marrow mesenchymal stem cells have been investigated as carriers for 5-fluorouracil (5-FU). The effects of these 5-FU-loaded exosomes on the growth of CCA cells were evaluated in vitro. Compared to free 5-FU, exosome-loaded 5-FU resulted in a significantly greater reduction in CCA cell viability. These findings suggest that 5-FU-loaded exosomes could represent an effective chemotherapeutic strategy for CCA treatment[71].
Nanomedicine for CCA treatment via targeted NPs
Nanotechnology has transformed cancer treatment by enabling precise drug delivery systems. Passive targeting via the EPR effect was one of the earliest strategies, taking advantage of the leaky vasculature in tumors to facilitate NP accumulation in cancerous tissues. However, variability in tumor vascularization and the limited specificity of passive targeting often result in suboptimal treatment outcomes[72].
Active targeting enhances selectivity by functionalizing NPs with ligands, such as antibodies, peptides, or small molecules, that specifically recognize overexpressed markers or proteins on CCA cells. By utilizing these molecular markers, targeted NPs significantly improve cellular uptake and therapeutic efficacy while minimizing off-target effects, offering promising avenues for the precision treatment of this aggressive malignancy[72]. The importance of these molecular targets and their application in NP functionalization for CCA treatment are discussed below.
Takeda G protein-coupled receptor 5
One promising strategy involves using liposomes to deliver curcumin, a compound known for its anti-cancer properties but limited by poor solubility and stability. Deoxycholic acid (DCA), a secondary bile acid, has been utilized to formulate DCA-based curcumin-containing liposomes (dLip/Cur) based on the hypothesis that DCA can bind to the Takeda G protein-coupled receptor 5 (TGR5), which is overexpressed in CCA cells. Studies have shown that these DCA-formulated liposomes significantly improve cytotoxicity against CCA cell lines. They promote caspase-dependent apoptosis more effectively than curcumin, suggesting that this novel delivery system could enhance curcumin’s therapeutic potential against cholangiocarcinoma[3].
Folic Acid Receptor
The overexpression of folic acid receptors in cancer cells relative to normal tissues offers a unique opportunity for targeted therapy. Researchers have developed gold NPs (AuNPs) conjugated with 5FU and folic acid (FA), further functionalized with polyethylene glycol (PEG) as a linker (AuNPs-PEG-5FU-FA). This targeted delivery system has demonstrated increased cytotoxicity in CCA cell lines expressing FA receptors, specifically M139 and M213 cells, compared to free 5FU. Moreover, the cytotoxicity is closely correlated with FA receptor expression, and its mechanism of action is linked to the activation of the mitochondrial apoptotic pathway[68].
Human epidermal growth factor receptor 2
HER2 is a member of the tyrosine kinase family that is crucial for cellular proliferation and apoptosis. HER2 overexpression has been observed in various cancer cells, including cholangiocarcinoma. The upregulation of HER2 can promote tumor growth and aggressiveness and is often linked to poor prognosis in patients with CCA[73].
Cell membrane-derived nanovesicles have been developed as alternative nanotechnological drug delivery systems due to their superior biocompatibility and reduced clearance by the reticuloendothelial system. Moreover, the surface of these nanovesicles can be effectively modified with a wide range of ligands. In one study, indocyanine green (ICG), a medical dye used for diagnostics and photothermal therapy, was incorporated into nanovesicles and functionalized with an HER2-specific affibody (A-NVs@ICG) to target HER2-overexpressing perihilar cholangiocarcinoma for precision theranostics. This functionalization markedly enhanced the specificity toward HER2-expressing cells and increased cellular uptake of ICG in SK-Cha-1 cells (a human intrahepatic cholangiocarcinoma cell line). The improved cellular uptake led to significantly higher in vitro photothermal cytotoxicity and greater tumor volume reduction in vivo[74].
Epithelial cellular adhesion molecule
Epithelial cellular adhesion molecule (EpCAM) is a transmembrane glycoprotein commonly expressed in CCA cells, making it a promising target for drug delivery[75]. A study investigated the use of milk-derived nanovesicles (MNVs) decorated with EpCAM-specific aptamers to deliver RNA therapeutics targeting programmed death-ligand 1 (PD-L1) in CCA. PD-L1 is a key immune checkpoint that enables cancer cells to evade immune detection and is highly expressed in CCA[76]. The researchers assessed the effects of these EpCAM-targeted nanovesicles on PD-L1 expression in human CCA cell lines HuCCT-1 and SNU1079. The MNVs were loaded with either small interfering RNA (siRNA-tMNVs) or ribonucleoproteins (RNP-tMNVs) specific for PD-L1, resulting in an approximately 50% reduction in PD-L1 expression. Additionally, co-culture experiments with T cells and natural killer cells showed increased immune activity, including enhanced degranulation and cytokine release, indicating that targeted delivery effectively modulated the immune response against tumor cells. The study also utilized multicellular spheroids to better mimic the tumor microenvironment, further validating the immunomodulatory effects of the engineered nanovesicles.
The therapeutic potential of EpCAM-targeted MNVs was further evaluated in an immunocompetent mouse model of CCA. Mice were treated with siRNA-loaded MNVs in combination with gemcitabine to assess tumor burden reduction compared to control groups. The results showed that this combination therapy significantly reduced tumor size and improved overall survival rates. The study highlights the promising application of EpCAM-targeted nanovesicles for the delivery of RNA therapeutics[77].
Hyaluronic acid receptor
GEM is an anti-cancer drug commonly prescribed for the treatment of CCA[78]. To enhance its efficacy against drug-resistant CCA and reduce adverse effects, researchers have encapsulated GEM within zeolitic imidazolate frameworks-67 (GEM@ZIF-67-HA), a type of metal-organic framework (MOF) characterized by its crystalline porous nanostructure. This nanoparticle system offers tunable properties and versatile functionalization options. Furthermore, hyaluronic acid was conjugated onto these NPs to target hyaluronic Acid (HA) receptors, such as CD44, which are frequently overexpressed in various cancer cells, including CCA. The ZIF-67 system enables passive targeting through pH-responsive drug release, facilitating drug release specifically under acidic conditions. HA conjugation enhances the cellular uptake of ZIF-67 in QBC939 cells (CD44-positive cholangiocarcinoma cells), while showing minimal uptake in HIBEC cells (normal biliary epithelial cells). In vitro cytotoxicity assays demonstrated increased toxicity in QBC939 cells without significant effects on HIBEC cells. Additionally, in vivo studies revealed that the GEM@ZIF-67-HA-targeted group exhibited lower tumor growth rates and reduced tumor weight compared to the group treated with free gemcitabine[69].
Another promising approach used HA to target CCA cells with a dual-targeting nanoplatform. In this design, micelles formed by octadecylamine (ODA) encapsulate the Polo-like kinase 1 (PLK1) inhibitor Ro3280 (Ro). These micelles are functionalized with HA to target HA receptors on CCA cells and with an anisamide-derived ligand (AA) to target sigma receptors on cancer-associated fibroblasts (CAFs). The resulting AA-HA-ODA/Ro micelles exhibited significantly higher affinity toward HuCCT-1 cells and activated NIH/3T3 cells compared to normal hepatocytes (LO2 cells). Moreover, AA-HA-ODA/Ro demonstrated stronger antitumor effects in organoids and multicellular spheroids. In vivo studies further confirmed uniform tumor penetration, sequential release, and reductions in liver pathological lesions, liver weight, and tumor-positive area percentage[79]. The nanomedicines designed for CCA treatment via actively targeted NPs are summarized in Table 2.
Examples of actively targeted nanomedicines for CCA treatment
| Nanocarrier | Payload | Characterization | Targeting Moiety | Targeted CElls/ Tissue | In vitro/In vivo effects | Application | Ref. |
| AuNPs-PEG-5FU-FA | 5FU | 15.17 ± 1.69 nm, 90% EE | Folic acid | M139, M213 | Increased cellular uptake, enhanced cytotoxicity, activated mitochondrial apoptotic pathways | Platform to improve specificity to CCA cells | [68] |
| GEM@ZIF-67-HA MOFs | Gemcitabine | 103.6 nm, PDI 0.145 | HA | ZIF-67, QBC939 | Improved cell internalization, increased in vitro cytotoxicity, enhanced in vivo antitumor effects | pH-responsive platform enabling passive and active targeting | [69] |
| A-NVs@ ICG | ICG | ~ 200 nm, 72.7% EE,-14.9 mV | HER2 | SK-Cha-1 | Increased specificity to HER2-positive CCA cells, enhanced in vitro photothermal cytotoxicity, improved in vivo antitumor effects | Theranostics | [75] |
| siRNA-tMNVs and RNP-tMNVs | siRNA, RNP | N/A | EpCAM | HuCCT-1, SNU-1079 | Reduced PD-L1 expression, enhanced immune response against cancer cells, reduced tumor size, improved survival in animal models | Immunotherapy | [77] |
| AA-HA-ODA/Ro micelles | PLK1 inhibitor (Ro3280) | ~ 40 nm | HA, AA | HuCCT-1, CAFs | Improved selectivity for CCA cells and CAFs, enhanced organoid and 3D spheroid cytotoxicity, improved in vivo antitumor effects | Dual-targeting nanoplatform | [79] |
| dLip/Cur | Curcumin | N/A | TGR5 | KKU-213A, KKU-213B | Enhanced in vitro cytotoxicity, promoted caspase-3/7-dependent apoptosis pathways | Platform for improved drug solubility and targeted delivery to CCA cells | [80] |
CONCLUSION AND PERSPECTIVE
Although the significant benefits of nanotechnology are widely recognized, concerns about its potential risks to human health and the environment are only starting to surface. Due to the limited availability of supporting data, critics have raised several warnings about the potentially harmful effects of nanotoxicity on both human health and ecological systems. As with most new technologies, the emphasis on benefits has been tempered by considerable debate about the safety and appropriate use of nanotechnology[81,82]. The exact mechanisms underlying nanotoxicity remain a subject of debate. However, the physicochemical characteristics of NPs are the major factors that determine their toxicity pathways. The concept of nanotoxicology is grounded in several key parameters, including particle size, surface area, shape (morphology), chemical composition, surface properties, and the tendency of nanoparticles to agglomerate or aggregate. Each of these factors plays a crucial role in determining the appropriate nanoparticle dosage and is essential for accurately evaluating their toxicity[83]. In multicellular organisms, nanotoxicity primarily results from cell injury, which may be reversible due to cellular repair mechanisms or, if more severe and irreversible, can cause long-term cellular damage or even cell death[84]. The literature suggests that various mechanisms of toxicity have been reported depending on the type of NPs. These mechanisms include the generation of ROS, disruption of the cell membrane, damage to the cytoskeleton and cellular organelles, DNA damage, and the triggering of inflammatory responses[85,86]. NPs also have the potential to cause kidney damage, leading to conditions such as inflammation, oxidative stress, fibrosis, and apoptosis, which can progress to acute or chronic renal failure. Several types of NPs, including silver nanoparticles, titanium dioxide nanoparticles, carbon nanotubes, and quantum dots, have been associated with nephrotoxicity[87]. To mitigate nanotoxicity during the development of nanoparticle-based systems, various scientific approaches and strategies have been explored. These include techniques such as coating, doping, grafting, ligation, and the incorporation of antioxidants - all aimed at enhancing the biocompatibility and safety of nanoparticles while preserving their functionality.
Although nanomedicine is widely accepted and has been implemented as a novel approach to revolutionize cancer treatment, its translation from basic research to clinical application has encountered significant challenges. In studies of other cancers, nanomedicines that showed promising therapeutic results in preclinical studies often failed in clinical trials. The main obstacle to nano-based drug delivery is the inability to overcome the complex physiological barriers present in the human body. To reduce the high attrition rates in clinical development, more advanced approaches are required. Specifically for CCA, clinical trials are urgently needed to evaluate the efficacy of NPs. Consequently, the study of targeted NPs has garnered significant interest, as they offer the potential to improve CCA treatment by enhancing tumor accumulation, facilitating efficient cellular internalization, and ensuring precise subcellular localization.
DECLARATIONS
Acknowledgments
This work was supported by the National Research Council of Thailand (NRCT) (Grant no. N41A640102) to Saengkrit N.
Authors’ contributions
Conceived and designed the review: Saengkrit N, Dana P
Searched the literature: Dana P, Tanyapanyachon P, Klibaim S, Rattanatayarom M, Chonniyom W
Drafted the manuscript text: Dana P, Tanyapanyachon P, Klibaim S, Rattanatayarom M, Chonniyom W
prepared the tables and figures: Dana P, Tanyapanyachon P, Klibaim S, Rattanatayarom M, Chonniyom W
All authors have read and approved the final manuscript.
Availability of data and materials
Not applicable.
Financial support and sponsorship
This work was financially supported by the National Research Council of Thailand (NRCT) (Grant no. N41A640102).
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Blechacz B, Komuta M, Roskams T, Gores GJ. Clinical diagnosis and staging of cholangiocarcinoma. Nat Rev Gastroenterol Hepatol. 2011;8:512-22.
2. Khan SA, Tavolari S, Brandi G. Cholangiocarcinoma: epidemiology and risk factors. Liver Int. 2019;39 Suppl 1:19-31.
3. Sarantis P, Tzanetatou ED, Ioakeimidou E, et al. Cholangiocarcinoma: the role of genetic and epigenetic factors; current and prospective treatment with checkpoint inhibitors and immunotherapy. Am J Transl Res. 2021;13:13246-60.
4. Sripa B, Pairojkul C. Cholangiocarcinoma: lessons from Thailand. Curr Opin Gastroenterol. 2008;24:349-56.
5. Gibson RN, Yeung E, Hadjis N, et al. Percutaneous transhepatic endoprostheses for hilar cholangiocarcinoma. Am J Surg. 1988;156:363-7.
6. Wu Q, Fan H, Lang R, et al. Overexpression of 14-3-3σ modulates cholangiocarcinoma cell survival by PI3K/Akt signaling. Biomed Res Int. 2020;2020:3740418.
7. Qi S, Yan H. Effect of percutaneous transhepatic cholangial drainag + radiofrequency ablation combined with biliary stent implantation on the liver function of patients with cholangiocarcinoma complicated with malignant obstructive jaundice. J Transl Res. 2021;13:1817-24.
8. Li S, Qian H, Peng Y, Jia H, Lin G. Differentiating peripheral cholangiocarcinoma in stages T1N0M0 and T2N0M0 from hepatic hypovascular nodules using dynamic contrast-enhanced MRI. Sci Rep. 2017;7:8084.
9. Zanuso V, Tesini G, Valenzi E, Rimassa L. New systemic treatment options for advanced cholangiocarcinoma. J Liver Cancer. 2024;24:155-70.
10. Hirohashi K, Uenishi T, Kubo S, et al. Macroscopic types of intrahepatic cholangiocarcinoma: clinicopathologic features and surgical outcomes. Hepatogastroenterology. 2002;49:326-9.
12. Mosconi S, Beretta GD, Labianca R, Zampino MG, Gatta G, Heinemann V. Cholangiocarcinoma. Crit Rev Oncol Hematol. 2009;69:259-70.
13. Su CH, Shyr YM, Lui WY, P'Eng FK. Hepatolithiasis associated with cholangiocarcinoma. Br J Surg. 1997;84:969-73.
14. Flavell DJ, Flavell SU. Opisthorchis viverrini: pathogenesis of infection in immunodeprived hamsters. Parasite Immunol. 1986;8:455-66.
15. Sripa B, Kaewkes S. Localisation of parasite antigens and inflammatory responses in experimental opisthorchiasis. Int J Parasitol. 2000;30:735-40.
16. Sripa B, Deenonpoe R, Brindley PJ. Co-infections with liver fluke and Helicobacter species: a paradigm change in pathogenesis of opisthorchiasis and cholangiocarcinoma? Parasitol Int. 2017;66:383-9.
18. Doherty B, Nambudiri VE, Palmer WC. Update on the diagnosis and treatment of cholangiocarcinoma. Curr Gastroenterol Rep. 2017;19:2.
19. Pinlaor S, Sripa B, Ma N, et al. Nitrative and oxidative DNA damage in intrahepatic cholangiocarcinoma patients in relation to tumor invasion. World J Gastroenterol. 2005;11:4644-9.
20. Sripa B, Brindley PJ, Mulvenna J, et al. The tumorigenic liver fluke Opisthorchis viverrini--multiple pathways to cancer. Trends Parasitol. 2012;28:395-407.
21. Lozano E, Sanchez-Vicente L, Monte MJ, et al. Cocarcinogenic effects of intrahepatic bile acid accumulation in cholangiocarcinoma development. Mol Cancer Res. 2014;12:91-100.
22. Park JH, Hong JY, Kwon M, et al. Association between non-alcoholic fatty liver disease and the risk of biliary tract cancers: a South Korean nationwide cohort study. Eur J Cancer. 2021;150:73-82.
23. Gatselis NK, Tepetes K, Loukopoulos A, et al. Hepatitis B virus and intrahepatic cholangiocarcinoma. Cancer Invest. 2007;25:55-8.
24. Matsumoto K, Onoyama T, Kawata S, et al. Hepatitis B and C virus infection is a risk factor for the development of cholangiocarcinoma. Intern Med. 2014;53:651-4.
25. Ralphs S, Khan SA. The role of the hepatitis viruses in cholangiocarcinoma. J Viral Hepat. 2013;20:297-305.
26. Kirstein MM, Vogel A. Epidemiology and risk factors of cholangiocarcinoma. Visc Med. 2016;32:395-400.
27. Ramírez-Merino N, Aix SP, Cortés-Funes H. Chemotherapy for cholangiocarcinoma: an update. World J Gastrointest Oncol. 2013;5:171-6.
28. Forner A, Vidili G, Rengo M, Bujanda L, Ponz-Sarvisé M, Lamarca A. Clinical presentation, diagnosis and staging of cholangiocarcinoma. Liver Int. 2019;39 Suppl 1:98-107.
29. Saha SK, Zhu AX, Fuchs CS, Brooks GA. Forty-year trends in cholangiocarcinoma incidence in the U.S.: intrahepatic disease on the rise. Oncologist. 2016;21:594-9.
30. Rizzo A, Brandi G. BILCAP trial and adjuvant capecitabine in resectable biliary tract cancer: reflections on a standard of care. Expert Rev Gastroenterol Hepatol. 2021;15:483-5.
31. Sirica AE. Cholangiocarcinoma: molecular targeting strategies for chemoprevention and therapy. Hepatology. 2005;41:5-15.
33. R. A. J. Freitas, Nanomedicine, Volume I: Basic Capabilities. 1st ed. Boca Raton: CRC Press; 1999.
34. Chamundeeswari M, Jeslin J, Verma ML. Nanocarriers for drug delivery applications. Environ Chem Lett. 2019;17:849-65.
35. Priem B, van Leent MMT, Teunissen AJP, et al. Trained immunity-promoting nanobiologic therapy suppresses tumor growth and potentiates checkpoint inhibition. Cell. 2020;183:786-801.e19.
36. Fan D, Cao Y, Cao M, Wang Y, Cao Y, Gong T. Nanomedicine in cancer therapy. Signal Transduct Target Ther. 2023;8:293.
37. Manzari MT, Shamay Y, Kiguchi H, Rosen N, Scaltriti M, Heller DA. Targeted drug delivery strategies for precision medicines. Nat Rev Mater. 2021;6:351-70.
38. Rosenblum D, Joshi N, Tao W, Karp JM, Peer D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat Commun. 2018;9:1410.
39. Youn YS, Bae YH. Perspectives on the past, present, and future of cancer nanomedicine. Adv Drug Deliv Rev. 2018;130:3-11.
40. Bertrand N, Wu J, Xu X, Kamaly N, Farokhzad OC. Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Adv Drug Deliv Rev. 2014;66:2-25.
41. Masoudifar R, Pouyanfar N, Liu D, et al. Surface engineered metal-organic frameworks as active targeting nanomedicines for mono- and multi-therapy. Applied Materials Today. 2022;29:101646.
42. Doroudian M, Azhdari MH, Goodarzi N, O’Sullivan D, Donnelly SC. Smart nanotherapeutics and lung cancer. Pharmaceutics. 2021;13:1972.
43. Farjadian F, Faghih Z, Fakhimi M, Iranpour P, Mohammadi-Samani S, Doroudian M. Glucosamine-modified mesoporous silica-coated magnetic nanoparticles: A “Raisin-Cake”-like structure as an efficient theranostic platform for targeted methotrexate delivery. Pharmaceutics. 2023;15:2491.
44. Wang C, Zhang S. Advantages of nanomedicine in cancer therapy: a review. ACS Appl Nano Mater. 2023;6:22594-610.
45. Adiseshaiah PP, Crist RM, Hook SS, McNeil SE. Nanomedicine strategies to overcome the pathophysiological barriers of pancreatic cancer. Nat Rev Clin Oncol. 2016;13:750-65.
46. Rahimian S, Mirkazemi K, Kamalinejad A, Doroudian M. Exosome-based advances in pancreatic cancer: the potential of mesenchymal stem cells. Crit Rev Oncol Hematol. 2025;207:104594.
47. Yadav HK, Almokdad AA, shaluf SI, Debe MS. Polymer-based nanomaterials for drug-delivery carriers. nanocarriers for drug delivery. In: Mohapatra SS, Ranjan S, Dasgupta N, Mishra KR, Thomas S, editors. Nanocarriers for Drug Delivery: Nanoscience and Nanotechnology in Drug Delivery. Amsterdam: Elsevier; 2019. p. 531-56.
48. Wang JJ, Zeng ZW, Xiao RZ, et al. Recent advances of chitosan nanoparticles as drug carriers. Int J Nanomedicine. 2011;6:765-74.
49. Moghaddam FD, Zare EN, Hassanpour M, et al. Chitosan-based nanosystems for cancer diagnosis and therapy: stimuli-responsive, immune response, and clinical studies. Carbohydr Polym. 2024;330:121839.
50. Yang JI, Lee HL, Yun JJ, et al. pH and redox-dual sensitive chitosan nanoparticles having methyl ester and disulfide linkages for drug targeting against cholangiocarcinoma Cells. Materials (Basel). 2022;15:3795.
51. Lee HM, Jeong YI, Kim DH, et al. Ursodeoxycholic acid-conjugated chitosan for photodynamic treatment of HuCC-T1 human cholangiocarcinoma cells. Int J Pharm. 2013;454:74-81.
52. Kennedy L, Sandhu JK, Harper ME, Cuperlovic-Culf M. Role of glutathione in cancer: from mechanisms to therapies. Biomolecules. 2020;10:1429.
53. Correia JH, Rodrigues JA, Pimenta S, Dong T, Yang Z. Photodynamic therapy review: principles, photosensitizers, applications, and future directions. Pharmaceutics. 2021;13:1332.
54. Sadat Tabatabaei Mirakabad F, Nejati-Koshki K, Akbarzadeh A, et al. PLGA-based nanoparticles as cancer drug delivery systems. Asian Pac J Cancer Prev. 2014;15:517-35.
55. Hines DJ, Kaplan DL. Poly(lactic-co-glycolic) acid-controlled-release systems: experimental and modeling insights. Crit Rev Ther Drug Carrier Syst. 2013;30:257-76.
56. Jeong YI, Kim DH, Chung CW, et al. Doxorubicin-incorporated polymeric micelles composed of dextran-b-poly(DL-lactide-co-glycolide) copolymer. Int J Nanomedicine. 2011;6:1415-27.
57. Omar AI, Plengsuriyakarn T, Chittasupho C, Na-Bangchang K. Enhanced oral bioavailability and biodistribution of atractylodin encapsulated in PLGA nanoparticle in cholangiocarcinoma. Clin Exp Pharmacol Physiol. 2021;48:318-28.
58. Sheoran S, Arora S, Samsonraj R, Govindaiah P, Vuree S. Lipid-based nanoparticles for treatment of cancer. Heliyon. 2022;8:e09403.
59. Allo G, Can AD, Wahba R, et al. Nanoliposomal irinotecan in combination with leucovorin and 5-fluorouracil in advanced biliary tract cancers. Mol Clin Oncol. 2022;16:52.
60. Plunkett W, Huang P, Xu YZ, Heinemann V, Grunewald R, Gandhi V. Gemcitabine: metabolism, mechanisms of action, and self-potentiation. Semin Oncol. 1995;22(4 Suppl 11):3-10.
61. Gilbert JA, Salavaggione OE, Ji Y, et al. Gemcitabine pharmacogenomics: cytidine deaminase and deoxycytidylate deaminase gene resequencing and functional genomics. Clin Cancer Res. 2006;12:1794-803.
62. Kim DH, Im BN, Hwang HS, Na K. Gemcitabine-loaded DSPE-PEG-PheoA liposome as a photomediated immune modulator for cholangiocarcinoma treatment. Biomaterials. 2018;183:139-50.
63. Choi KH, Chung CW, Kim CH, Kim DH, Jeong YI, Kang DH. Effect of 5-aminolevulinic acid-encapsulate liposomes on photodynamic therapy in human cholangiocarcinoma cells. J Nanosci Nanotechnol. 2014;14:5628-32.
64. Maphanao P, Phothikul Y, Choodet C, et al. Development and in vitro evaluation of ursolic acid-loaded poly(lactic-co-glycolic acid) nanoparticles in cholangiocarcinoma. RSC Adv. 2024;14:24828-37.
65. Alshammari BH, Lashin MMA, Mahmood MA, et al. Organic and inorganic nanomaterials: fabrication, properties and applications. RSC Adv. 2023;13:13735-85.
66. Paul W, Sharma C. Inorganic nanoparticles for targeted drug delivery. In: Sharma CP, editor. Biointegration of Medical Implant Materials (2nd ed). Cambridge: Woodhead Publishing; 2010. p. 204-35.
67. Tang T, Zheng JW, Chen B, et al. Effects of targeting magnetic drug nanoparticles on human cholangiocarcinoma xenografts in nude mice. Hepatobiliary Pancreat Dis Int. 2007;6:303-7.
68. Ngernyuang N, Seubwai W, Daduang S, Boonsiri P, Limpaiboon T, Daduang J. Targeted delivery of 5-fluorouracil to cholangiocarcinoma cells using folic acid as a targeting agent. Mater Sci Eng C Mater Biol Appl. 2016;60:411-5.
69. Long C, Peng H, Yang W, et al. Targeted delivery of gemcitabine for precision therapy of cholangiocarcinoma using hyaluronic acid-modified metal-organic framework nanoparticles. ACS Omega. 2024;9:11998-2005.
70. Xia J, Kawamura Y, Suehiro T, Chen Y, Sato K. Carbon dots have antitumor action as monotherapy or combination therapy. Drug Discov Ther. 2019;13:114-7.
71. Chen M, Li Y, Ma N, Zang J. Mesenchymal stem cell-derived exosomes loaded with 5-Fu against cholangiocarcinoma in vitro. Mol Med Rep. 2022;25:213.
72. Chen Z, Kankala RK, Long L, Xie S, Chen A, Zou L. Current understanding of passive and active targeting nanomedicines to enhance tumor accumulation. Coord Chem Rev. 2023;481:215051.
73. Haaft BH, Pedregal M, Prato J, Klümpen HJ, Moreno V, Lamarca A. Revolutionizing anti-HER2 therapies for extrahepatic cholangiocarcinoma and gallbladder cancer: Current advancements and future perspectives. Eur J Cancer. 2024;199:113564.
74. Zhang X, Zhang Y, Zhang Y, et al. Bio-engineered cell membrane nanovesicles as precision theranostics for perihilar cholangiocarcinoma. Biomater Sci. 2020;8:1575-9.
75. Liu Y, Wang Y, Sun S, et al. Understanding the versatile roles and applications of EpCAM in cancers: from bench to bedside. Exp Hematol Oncol. 2022;11:97.
76. Ahn S, Lee JC, Shin DW, Kim J, Hwang JH. High PD-L1 expression is associated with therapeutic response to pembrolizumab in patients with advanced biliary tract cancer. Sci Rep. 2020;10:12348.
77. Gondaliya P, Sayyed AA, Yan IK, Driscoll J, Ziemer A, Patel T. Targeting PD-L1 in cholangiocarcinoma using nanovesicle-based immunotherapy. Mol Ther. 2024;32:2762-77.
78. Gabriel E, Gandhi S, Attwood K, Kuvshinoff B, Hochwald S, Iyer R. Gemcitabine and capecitabine for advanced biliary cancer. J Gastrointest Oncol. 2017;8:728-36.
79. Zhou Y, Xu L, Wang Z, et al. Sequentially targeting and intervening mutual polo-like kinase 1 on CAFs and tumor cells by dual targeting nano-platform for cholangiocarcinoma treatment. Theranostics. 2022;12:3911-27.
80. P. K. W. C. S. P. and K. V. P. Srikoon, “Deoxycholic acid-formulated curcumin enhances caspase 3/7-dependent apoptotic induction in cholangiocarcinoma cell lines,” Journal of Bacic and Applied Pharmacology, 2021;1. Available from: https://www.researchgate.net/publication/361332214_Deoxycholic_acid-formulated_curcumin_enhances_caspase_37-dependent_apoptotic_induction_in_cholangiocarcinoma_cell_lines (accessed on 2025-7-4).
82. Dreher KL. Health and environmental impact of nanotechnology: toxicological assessment of manufactured nanoparticles. Toxicol Sci. 2004;77:3-5.
83. Zielińska A, Costa B, Ferreira MV, et al. Nanotoxicology and nanosafety: safety-by-design and testing at a glance. Int J Environ Res Public Health. 2020;17:4657.
84. Donahue ND, Acar H, Wilhelm S. Concepts of nanoparticle cellular uptake, intracellular trafficking, and kinetics in nanomedicine. Adv Drug Deliv Rev. 2019;143:68-96.
85. Knaapen AM, Borm PJ, Albrecht C, Schins RP. Inhaled particles and lung cancer. Part A: mechanisms. Int J Cancer. 2004;109:799-809.
86. Sharma N, Kurmi BD, Singh D, et al. Nanoparticles toxicity: an overview of its mechanism and plausible mitigation strategies. J Drug Target. 2024;32:457-69.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Special Issue
Copyright
Data & Comments
Data

















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].