Ultrasonography in surveillance for hepatocellular carcinoma in patients with non-alcoholic fatty liver disease
Abstract
International guidelines recommend six monthly ultrasounds as the primary surveillance tool for patients at risk of hepatocellular carcinoma (HCC). The dominant driver of liver disease in HCC surveillance populations is shifting, particularly in Europe and the United States, from chronic viral hepatitis (B or C), towards non-alcoholic fatty liver disease (NAFLD). Today, the population requiring HCC surveillance is also characterised by a high prevalence of overweight/obesity. These patient characteristics significantly impair ultrasound quality which can impede the detection of early HCC lesions. This diagnostic limitation has significant implications considering that eligibility for curative treatment depends upon the stage at which the cancer is detected. In this narrative review, we provide a comprehensive overview of the published evidence and national/international guidelines regarding ultrasound surveillance for HCC in people with NAFLD. We examine ultrasound sensitivity in this cohort for the detection of all stage and early HCC, the impact of steatosis and abdominal obesity on ultrasound performance, evidence for the addition of serum alpha-fetoprotein measurement, optimal timing of surveillance, emerging modalities for risk stratification and screening, and outline the challenges of case finding and surveillance eligibility criteria in this patient cohort. Finally, amalgamating all available evidence, we propose a pragmatic surveillance pathway for patients with NAFLD.
Keywords
INTRODUCTION
Primary liver cancer (PLC) is the 11th leading cause of cancer globally and 8th leading cause of cancer death, accounting for 12.5 million disability-adjusted life years[1]. Hepatocellular carcinoma (HCC) accounts for approximately 90% of PLC cases[2]. HCC is strongly associated with age, male gender, diabetes and carriage of the patatin-like phospholipase domain-containing protein 3 (PNPLA3) rs738409 C c.444C >G minor allele for patients with non-alcoholic fatty liver disease (NAFLD)[3,4]. HCC incidence is 89% and 78% higher in males and females, respectively, in the most deprived quintile compared with the least[5]. Incidence rates in Europe and America have increased since the 1990s, although in recent years, these have plateaued, most likely as a result of the success of direct-acting antiviral therapy for hepatitis C virus (HCV) infection[6]. Modelling from the UK suggests that HCC incidence is expected to increase 40% before 2035; however, mortality rates are predicted to rise further, in stark contrast to those of other cancers[7].
The estimated increase in HCC incidence reflects the emergence of NAFLD as a leading cause of PLC, driven by the obesity and type 2 diabetes (T2D) epidemics[8,9]. Estes et al. forecast that the incidence of NAFLD HCC will increase by 137% by 2030 in the United States[10]. When one reflects on the widespread use and effectiveness of direct-acting antiviral therapy for HCV and the rise in vaccination against hepatitis B virus (HBV) infection, it is clear that NAFLD will become the dominant aetiological driver of HCC.
Despite the potential for curative treatment, many patients with HCC are not eligible for this, either due to advanced tumour stage, poor liver function or low-performance status[11]. Early diagnosis of HCC is vital as the 1-yr survival is 78% (TNM stage 1) vs. 20% for those diagnosed at the latest stage (TNM stage 4)[12]. The importance of detection of small tumours translates into an increased likelihood of effective treatment and improvement in overall survival[13]. Better early detection of liver disease and HCC is a priority for health services[6,14]. Given that cirrhosis is the leading cause of HCC, international guidelines advise that all people with NAFLD-related cirrhosis are surveyed for HCC every 6 months via transabdominal ultrasonography (USS)[15-17]. While contrast-enhanced liver computed tomography (CT) and magnetic resonance imaging (MRI) have been shown to be more sensitive for HCC detection[18], the cost, availability, and impact on diagnostic services would prohibit their use as a surveillance tool using the current model of six monthly screening in all at-risk patients[19,20]. Their use also requires the need for contrast agents and radiation exposure in the case of CT. In this narrative review, we explore the challenges for HCC surveillance in patients with NAFLD, the performance of USS for people with NAFLD compared to other subpopulations, and the latest evidence regarding surveillance timing, the use of alpha-fetoprotein (AFP), contrast-enhanced USS and cost-effectiveness data. We discuss emerging surveillance tools and provide a summary of best clinical practices.
NON-ALCOHOLIC FATTY LIVER DISEASE
NAFLD is the leading cause of chronic liver disease in Europe, with an overall prevalence significantly higher in men than in women (39.7% vs. 25.6%, P < 0.0001)[21]. NAFLD encompasses a spectrum of clinical entities progressing from simple hepatic steatosis to non-alcoholic steatohepatitis (NASH), hepatic fibrosis and cirrhosis. Movement between these stages is dynamic prior to the development of cirrhosis. Prevalence rates of NAFLD are estimated to be 10%-30% in the general population, 50%-90% in people with obesity and 56% in people with T2D[22,23]. NAFLD is a metabolic disease with insulin resistance as a principal pathophysiological defect[24], and as such, a recent consensus group has proposed a change in nomenclature to metabolic-dysfunction associated fatty liver disease (MAFLD) characterised by liver steatosis (radiologically evident) and concomitant metabolic risk factors[25]. While excess fat accumulation in the liver itself, in many cases, may be of no long-term significance to liver health, hepatic steatosis can progress to liver fibrosis in up to 40%[26,27]. It is the presence of advancing liver fibrosis and cirrhosis that is associated with an increased risk of liver-related mortality including HCC[26,28,29], and incident cardiovascular disease[30]. While traditionally, liver fibrosis was staged at liver biopsy, there has been a huge expansion over the last two decades in the use of non-invasive fibrosis tests[31]. These include simple algorithms (e.g., fibrosis-4 (FIB-4) score, NAFLD fibrosis score), serum biomarkers [e.g., the enhanced liver fibrosis (ELF) test], and shear wave elastography [transient elastography, TE (Fibroscan®)[32], liver ultrasound elastography[33], and magnetic resonance elastography][34]. Sequential use of more than one test has been shown to reduce the need for liver biopsy[32]. In addition to the oncogenic risk posed by NAFLD, obesity and diabetes, observational studies suggest an elevated risk of HCC associated with the use of insulin and sulphonylureas[4,35]. There is also emerging evidence of an association between air pollution, NAFLD incidence[36] and HCC mortality[37]. Interestingly the molecular signature associated with NAFLD-HCC has recently been found to differ from that associated with non-NAFLD HCC, including higher rates of the ACVR2A mutations (a potential tumour suppressant)[38].
CHALLENGES PRESENTED BY THE EMERGENCE OF NON-ALCOHOLIC FATTY LIVER DISEASE AS A LEADING CAUSE OF HEPATOCELLULAR CARCINOMA
The emergence of NAFLD as a leading cause of HCC presents new and unique challenges for hepatologists, metabolic, obesity and diabetes physicians, oncologists and hepatobiliary surgeons.
Disease burden
Current and predicted prevalence levels of NAFLD means that the overall contribution of NAFLD to the global HCC burden is likely to surpass that of HCV[39]. Estimates of global trends in the burden of liver cancer using the methodology framework of the Global Burden of Disease study have identified that NASH has the fastest-growing age-standardised death rate for HCC[40]. A large cohort study from the United States Scientific Registry of Transplant Recipients has shown that 18% of individuals listed for a liver transplant, with an indication of HCC, have NASH (the 2nd most common cause after HCV), and that NAFLD is the fastest-growing cause of HCC in liver transplant candidates[41]. Case series from the UK (n = 632)[42] and US (n = 4,406)[43] have identified that 38% and 59% of cases of HCC may be attributable to NAFLD. While HCC incidence rates are similar between patients with NAFLD cirrhosis and HCV cirrhosis, NAFLD is associated with a comparatively low HCC risk compared to HCV overall [Table 1][44,45]. This has implications for the cost-effectiveness of USS-based HCC surveillance in this group.
Comparison of population prevalence and incidence of HCC for patients with NAFLD and hepatitis C virus infection
| NAFLD | Hepatitis C virus infection | |
| Population prevalence | 23%[45] | Pre DAA 2.0% (2015)[46] Post DAA 1.2% (2020)[47] |
| HCC incidence overall | 0.03 per 100 person-years[43] | Failed SVR (0.87 per 100 patient-years) SVR (0.24 per 100 patient-years)[44] |
| HCC incidence in cirrhotic population | 3.78 per 100 person-years[43] | Failed SVR (3.25 per 100 patient-years) SVR (1.97 per 100 patient-years)[44] |
Presentation of HCC outside of a surveillance programme
While overall survival appears comparable to other aetiologies of HCC, patients with NAFLD-HCC have been found to have a reduced disease-free survival than patients with non-NAFLD HCC[46]. This could be explained by the finding that patients with NAFLD-HCC are more likely to have presented with cancer detected outside of a surveillance programme (67.2% vs. 44.3% according to meta-analysis data), and with larger tumours (although overall Barcelona Clinic Liver Cancer (BCLC) stage is comparable)[46]. Several factors are likely to account for this. Two meta-analyses found that 38%-39% of patients with NAFLD-HCC were not cirrhotic at presentation vs. 14%-15% for other aetiologies of chronic liver disease[46,47]. This may occur as a result of genetic and oncogenic factors related to obesity, T2D, liver steatosis and hepatic oxidative stress[48]. Furthermore, a significant proportion of patients with NAFLD presenting with HCC, including those with cirrhosis, have undiagnosed pre-existing liver disease and thus are not enrolled in HCC surveillance[49].
Low sensitivity of USS for the detection of early HCC
As discussed below, ultrasound sensitivity for the detection of HCC in people with NAFLD is suboptimal, largely as a result of central obesity and the presence of hepatic steatosis. Reliance on this imaging modality for HCC surveillance, therefore, places patients with NAFLD at a disadvantage.
PERFORMANCE OF ULTRASOUND FOR DETECTION OF HEPATOCELLULAR CARCINOMA IN PATIENTS WITH CIRRHOSIS (ALL AETIOLOGIES) AND CHRONIC HEPATITIS B VIRUS INFECTION
USS has many favourable attributes as a surveillance tool: there are no associated risks, costs are moderate, and it has high levels of acceptability and achieves high sensitivities for the detection of HCC in certain patient groups (slim, non-cirrhotic). It is also possible to comment on features of cirrhosis and portal hypertension, and where Doppler is used, USS can detect portal vein thrombosis, all of which will guide the appropriate management of patients with HCC. Challenges include the detection of very early HCC, where there is a single cancerous nodule ≤ 2 cm (BCLC stage 0), and those that meet the Milan criteria (one nodule < 5 cm or three nodules each < 3 cm in diameter, without gross vascular invasion). The distinction between HCC and regenerating nodules, found in patients with cirrhosis, is also not possible on standard USS. Finally, the experience of the operator is another factor affecting the usefulness of ultrasound in this setting.
The use of USS for HCC surveillance was first recommended based on a landmark randomised trial in China of 18,816 patients with hepatitis B or chronic hepatitis[50]. The intervention group received six monthly USS plus AFP and these individuals experienced a 37% reduction in mortality. There have been no randomised trials in Western countries. A meta-analysis of prospective studies published up to 2007 confirmed that USS detected the majority of tumours before they presented clinically (94% sensitivity); however, it was less effective for the detection of early HCC (63% sensitivity)[51]. Biannual surveillance increased the sensitivity to 70% for detecting early-stage HCC[51]. In these studies, there was a mixed aetiology of liver disease, although the majority of patients had chronic HCV infection[51]. A systematic review of 14 cross-sectional studies reported that USS had a high level of specificity for HCC detection, but a sensitivity of only 60%[52]. Where studies used explanted liver as the gold standard, sensitivities for USS ranged from 58% to 89% in populations from the United States[53-55]. An analysis of 202 patients who received a liver transplant for HCC due to mixed aetiologies reported that USS had a 46% sensitivity for HCC detection compared to 65% for CT and MRI[18]. For lesions less than 2cm, sensitivity values were just 21% (USS), 40% (CT) and 47% (MRI)[18]. In 2018, Tzartzeva et al. published an updated meta-analysis of 32 studies (1990-2016; 13,367 patients) and identified that USS detected any stage of HCC with an 84% sensitivity (95%CI: 76-92) and early stage HCC with a sensitivity of just 47% (95%CI: 33%-61%)[56]. The aetiology of liver disease in this group was mixed, and it is unclear what proportion of people had NAFLD or were overweight.
In the absence of randomised control trials, a large number of observational studies have attempted to determine if biannual USS surveillance for HCC leads to a survival benefit. In 2022, Singal et al. performed a meta-analysis analysing the harms and benefits of ultrasound surveillance in people with cirrhosis (59 studies, 2014-2020; 145,396 participants)[57]. HCC surveillance was found to be associated with improved early-stage detection (OR 1.86, 95%CI: 1.73-1.98), receipt of curative treatment (OR 1.83, 95%CI: 1.69-1.97) and overall survival (HR 0.67, 95%CI: 0.61-0.72)[57]. However, only two studies examined whether HCC surveillance was associated with improved outcomes in people with NAFLD[57]. Four studies reported on harms associated with surveillance. These occurred in 8.8%-27.5% of patients and were mostly mild in severity[57].
PERFORMANCE OF ULTRASOUND FOR THE DETECTION OF HEPATOCELLULAR CARCINOMA IN THE CONTEXT OF NON-ALCOHOLIC FATTY LIVER DISEASE AND OBESITY
The study populations from the earliest trials looking at the effectiveness of USS surveillance for HCC[50,58] are not truly representative of today’s HCC surveillance population, particularly in the West. Specifically, they were based in Asian populations where chronic hepatitis B and C viruses were the predominant drivers of cancer. Patients in these studies had low rates of central obesity and liver steatosis, both of which are known to impair ultrasound image quality[59]. In addition, not all patients in these cohorts had cirrhosis[50], which can result in difficulties differentiating between an early HCC and regenerating nodules. In terms of cost-effectiveness, the annual incidence of HCC in populations with high rates of chronic viral hepatitis is over 5%; this is greater than that for NAFLD cirrhosis (approximately 0.7%-4.0%)[60-62]. As a result of the factors mentioned above, over 50% of tumours detected in these cohorts were small, leading to a greater than 50% five-year survival for patients who underwent surgery[63]. Survival rates may also have been positively influenced by the fact that patients with non-NAFLD causes of HCC are generally younger with fewer comorbidities than patients with NAFLD-HCC[46].
The obesity epidemic began in the 1970s and has continued to rise exponentially; 42.4% of Americans currently have a body mass index (BMI) > 30 kg/m2[64]. The natural history of NAFLD, with potential progression towards cirrhosis and HCC[65], has meant that it is only in the last two decades that end-stage complications of chronic liver disease including HCC have been widely observed in this group. The rise of obesity and NAFLD also has significant implications for HCC surveillance. Reliance on USS for HCC surveillance presents challenges in the NAFLD population, 51% of whom are living with obesity[66]. The depth of the subcutaneous fat, with the inherent acoustic attenuation, leads to poor image definition, impairing earlier HCC detection. An example of how central obesity can obscure visualisation at ultrasound is shown in Figure 1 in comparison to images from a patient who is lean [Figure 2]. Recent cohort studies from Europe and America report that USS is inadequate (defined as detection of HCC outside of the Milan criteria) in 20.3%-32.2% of people[59,67]. In a retrospective cohort study of 941 patients, a higher BMI, NASH-related cirrhosis, Child-Pugh B or C cirrhosis and alcohol-related cirrhosis were all independently associated with inadequate USS quality in multivariable analysis[59]. Inadequate quality was observed in 9.3%, 18.0%, 22.8%, 35.5% and 39.3% of people of normal weight, overweight, obesity class I, obesity class II, and morbid obesity, respectively[59]. USS exams were inadequate in 34.6% of patients with NASH-related cirrhosis, in comparison to 15.0% of patients with other aetiologies of cirrhosis[59]. These factors lead to the under-recognition of small or early-stage HCC nodules. These findings were confirmed by Esfeh et al., who report that USS has a sensitivity for HCC detection of 59% in people without obesity and 19% for people with obesity in a population of 116 liver transplant recipients[68]. In a similar study of 352 consecutive patients undergoing liver transplant assessment for HCC, the univariate analysis identified obesity (sensitivity 76% for BMI ≥ 30 kg/m2vs. 87% for BMI < 30 kg/m2, P = 0.01), and an aetiology of NAFLD (sensitivity 59% vs. 84%; P = 0.02)[69]. In 2021, Kim et al. performed a meta-analysis to evaluate the incidence of USS surveillance failures in detection of early-stage HCC and to determine risk factors for this[70]. A total of 18 studies (21,467 individuals undergoing surveillance) were included. The pooled incidence of surveillance failure (defined as the number of surveillance failure patients/number of patients who underwent surveillance) was 2.2% (95%CI: 0.9%-5.4%) for USS alone and 2.6% (95%CI: 1.8%-3.9%) for USS plus AFP. A BMI of 30 or more was significantly associated with surveillance failure (OR 1.38,
Figure 1. Radiological images demonstrating poor ultrasound image quality due to central obesity, case 1. (A) Longitudinal section ultrasound image of the left lobe in a patient with large body habitus. Significant acoustic shadowing obscures the liver and a focal liver lesion cannot be excluded. The patient had a rise in AFP and so an MRI was arranged and a staging CT of the chest, abdomen and pelvis. (B) MRI demonstrating a 17mm focal lesion in the left lobe which on portal venous phase shows washout. (C) Arterial phase CT image shows a poorly defined low-density lesion with a subtle increased vascularity identified from the staging CT.
Figure 2. Radiological images demonstrating high ultrasound image quality in a lean patient, case 2. (A) Longitudinal section ultrasound image demonstrating a well defined 3cm hypoechoic liver lesion (yellow arrow) in the right lobe of liver close to the diaphragm (red arrow) visualised clearly in a patient with a thin body habitus. Usually this region can be difficult to assess on ultrasound. (B) MRI liver. The segment VIII liver lesion demonstrates rapid washout post contrast.
As part of their recent meta-analysis described above, Singal et al. performed a subgroup analysis of studies stratified according to the proportion of patients with NAFLD[57]. The researchers report similar point estimates for the association between surveillance and early detection of HCC (relative risk, RR 1.86, 2.23, and 2.04, respectively) and receipt of curative treatment (RR 1.79, 2.06, and 2.02, respectively) for studies with < 10%, 10%-20% and > 20% of patients with NAFLD[57]. Only two studies out of 59 specifically examined the benefits of HCC surveillance in the NAFLD cohort. Aby et al. (retrospective study, n = 99 NASH cirrhosis) reported no association with receipt of curative treatment (45.4% vs. 51.7%, P = 0.72)[71], whereas Lo et al. (retrospective study, n = 93 NAFLD cirrhosis) found a significant association with early-stage detection 68.2% vs. 29.7%, P = 0.001 with surveillance; however, this did not translate into improved survival[72].
SCREENING SCHEDULE FOR ULTRASOUND SURVEILLANCE
The literature suggests that it takes approximately 4-12 months for an undetectable lesion to reach 2 cm in size[73,74]. This has helped inform a six-monthly surveillance interval for people with cirrhosis with the aim of detecting tumours less than 3 cm in diameter. A 12-month interval has been shown to result in reduced detection rates for early cancer and reduced survival[75]. A multi-centre randomised trial reported no benefit for earlier detection of cancer with a three-monthly vs. six-monthly regime in people with cirrhosis, although this was found to be limited by the recall procedure[76]. Unfortunately, patients with NAFLD cirrhosis did not meet the inclusion criteria for this trial.
COST-EFFECTIVENESS OF ULTRASOUND SURVEILLANCE FOR HEPATOCELLULAR CARCINOMA DETECTION
Markov modelling suggests that 6-monthly USS surveillance for HCC in people with cirrhosis increases quality-adjusted life expectancy by 8.6 months overall and 3.5 years in patients with small treated tumours[20]. Biannual USS surveillance had an incremental cost-effectiveness ratio of $30,700 per quality-adjusted life year (QALY) gained and was more cost-effective than annual USS, biannual USS with AFP, annual/biannual CT, and annual MRI, using a threshold of $50,000 per QALY gained[20]. Where USS sensitivity dropped below 65%, or the specificity of AFP exceeded 95%, combined USS and AFP surveillance was preferred[20]. The incremental cost-effectiveness ratio of biannual CT and annual MRI consistently exceeds $100,000/QALY[20].
ADDITION OF ALPHA-FETOPROTEIN TO ULTRASOUND SURVEILLANCE
Elevated serum AFP concentration, particularly sustained high levels, can indicate a possible HCC; however, there is only weak evidence to support its use in HCC surveillance. In a study of 88 patients, the inclusion of AFP in the surveillance protocol led to the detection of an additional 6%-8% of HCC cases vs. those detected by USS alone[77]. Poor performance relates to the fact that up to 24% of early HCCs present with normal AFP levels (particularly patients without cirrhosis/normal transaminase levels, as frequently occurs with NAFLD-HCC)[78]. Indeed a greater proportion of people with NAFLD-related HCC are AFP non-secretors compared to people with other drivers of liver cancer[79,80]. Furthermore, AFP levels fluctuate with active liver inflammation leading to false positive results with viral and alcoholic hepatitis. In a meta-analysis by Singal et al. (2009, 1,116 patients), the pooled sensitivity of USS for the detection of early HCC increased from 63%-69% with the addition of AFP (although this did not reach statistical significance)[51]. A more recent meta-analysis (Tzartzeva et al., 2018, 13,367 patients) reported that AFP measurement improved sensitivity rates from 45% to 63% for early HCC; however, USS alone detected HCC with a higher specificity than USS plus AFP[56]. Few studies have looked at the use of AFP for surveillance, rather than as a diagnostic test. The only randomised trial of this type used AFP to see if this could lead to early cancer detection in a population of Chinese men who were hepatitis B surface antigen positive[81]. The study found that screening with AFP led to earlier diagnosis of HCC, but did not result in an overall reduction in mortality. There is an important evidence gap to examine the effectiveness and cost-effectiveness of AFP in addition to USS for surveillance in Western populations, including patients with cirrhosis and NAFLD.
The American Association for the Study of Liver Disease (AASLD) guidelines state that it is not possible to determine whether USS alone or the combination of USS plus AFP leads to a greater improvement in survival[16]. Similarly, the European Association for the Study of the Liver (EASL) concludes that “insufficient data are available regarding the diagnostic accuracy of AFP in patients with adequate treatment of the aetiological cause of liver disease making any calculation of the cost-effectiveness impossible to date”[15]. In contrast, the Asia-Pacific practice guidelines do recommend the use of AFP in addition to USS for surveillance, with the caveat that AFP is not recommended as a confirmatory test in small HCCs and the AFP cut-off value should be 200 ng/mL, although a lower value can be used in a population with hepatitis virus suppression or eradication[17]. While no specific recommendations have been made regarding people with NAFLD, the clinical utility of AFP in this setting is thought to be low[15,16]. AFP does, however, play an important prognostic role in patients with established HCC[82,83].
CONTRAST-ENHANCED ULTRASOUND FOR HEPATOCELLULAR CARCINOMA SURVEILLANCE
There has been an interest in using intravenous contrast agents (stabilised microbubbles containing air or other gases) to enhance the performance of USS for the detection of early HCC. These contrast agents are safe and are not really cleared (unlike iodinated agents used for CT or MRI). Contrast-enhanced USS (CEUS) allows real-time dynamic imaging (performed continuously for the first minute), permitting the detection of arterial neoangiogenesis. This is followed by intermittent scanning every 30-60 s for 5 min to examine washout. The degree and time of onset of the washout can help discriminate HCC (mild, late washout) from intrahepatic cholangiocarcinoma and other non-HCC tumours (marked, early washout)[84]. CEUS also benefits from the fact that arterioportal shunts seen at CT and MRI are not visible, and in the setting of cirrhosis, any lesion demonstrating arterial enhancement is likely to be malignant or pre-malignant[84]. CEUS does not enhance the ability of USS to detect small tumour foci, however[85]. This may be related to the fact that a comprehensive assessment of the whole liver cannot be performed during the short arterial phase. Also, not all well-differentiated HCCs show arterial enhancement[86]. CEUS is more expensive than non-contrast ultrasound, requiring expertise and specialised equipment. For the characterisation of known focal liver lesions, costs are comparable to CT, but CEUS is more cost-effective than MRI[87]. Therefore, CEUS is not recommended for surveillance but for diagnostic purposes in patients at high risk of HCC[15,16,84].
EMERGING TOOLS FOR HCC SURVEILLANCE
There has been some interest in adapting existing CT and MRI protocols to improve their acceptability as surveillance tools. In a prospective single-armed study, biannual two-phase low-dose CT has been trialled for HCC surveillance, which showed significantly higher levels of sensitivity (83.3% vs. 29.2%) than USS[88]. Abbreviated MRI examination involves using a shortened MRI protocol with fewer sequences, specifically designed to detect early-stage HCC[89]. Three strategies have been trialled: (i) non-contrast MRI;
Substantial investment is being directed towards exploring the use of biomarkers for detecting early disease. Published examples include the GALAD score (comprising age, gender, AFP, the lens culinaris agglutinin-reactive fraction of AFP, and Des-gamma-carboxy prothrombin (DCP) [also known as prothrombin induced by vitamin K absence II (PIVKA II)], which has been shown to detect any stage HCC in patients with NASH with an area under the curve (AUC) of 0.96, and HCC within the Milan criteria with an AUC of 0.91 (sensitivity of 68% and specificity of 95%)[91]. However, it requires specialist tests and has not yet been prospectively validated, although trials are underway (NCT05342350). DCP has been shown to have a sensitivity and specificity of 71% and 84%, respectively, for the detection of HCC[92]; however, DCP levels are associated with more advanced tumour stage and portal vein invasion, a limitation for early detection[93]. Recent observational studies from Japan have identified that IgM-free apoptosis inhibitor of macrophage (AIM) serum levels are a sensitive diagnostic marker for NASH-HCC, and that AIM activation appears before HCC is diagnostically detectable[94,95]. Larger validation studies are required, however. Another approach is a liquid biopsy [the analysis of tumour components, particularly circulating tumour DNA (ctDNA), circulating tumour cells or extracellular vesicles]. Oncoguard Liver (a composite of three ctDNA methylation genes, AFP and gender) has demonstrated high levels of sensitivity and specificity[96]. The HelioLiver Test (a multi‐analyte blood test combining ctDNA methylation panel, clinical variables, and protein tumour markers) has demonstrated an AUC of over 0.95 in a phase II study[97] and is currently undergoing prospective validation. Liquid biopsy is prone to detection errors, however, as ctDNA generally comprises < 2% of circulating DNA and less in early HCC.
In terms of risk stratification, the aMAP score was developed using data from prospective studies and randomized control trials and was found to be predictive of HCC at five years in patients with different aetiologies of liver disease; however, only 5% had NAFLD[98]. The “HCC risk score” developed using the Veterans Affairs healthcare dataset identifies patients with NAFLD cirrhosis at risk of HCC, but is not validated in people without cirrhosis[99]. Given the association noted between PNPLA3 and HCC[100], there has been an interest in whether this may serve as a useful tool for risk stratification. However, this association has been found to be less significant in patients with NAFLD[101], and a recent analysis of the performance of a polygenic risk score incorporating PNPLA3 reported only moderate accuracy in predicting which patients with NAFLD are at greatest risk of HCC[102]. The prognostic liver signature (PLS)-NAFLD, a 133 gene signature, has been shown to be predictive of HCC risk but is currently limited by availability and cost[103].
Ultimately better tools are needed to do risk stratification of individuals at risk of HCC and to tailor testing, and perhaps the time interval for surveillance accordingly to support a move towards precision screening for HCC[104]. Given the experience and time spent on HCC surveillance in many countries, improved patient selection and testing is urgently required. Consortia, including non-invasive biomarkers of metabolic liver disease (NIMBLE), Liver Investigation: Testing Marker Utility in Steatohepatitis (LITMUS) and early detection of hepatocellular liver cancer (DeLIVER), are pursuing the discovery of novel biomarkers for use in this clinical context, and may be used with USS, or when USS performance is suboptimal.
Shear wave elastography, a non-invasive marker for the prediction of liver fibrosis that uses a normal B-mode ultrasound probe, has been shown to be predictive of HCC risk in hepatitis B and C virus infection[105,106]. In addition, it may be clinically useful in distinguishing benign and malignant lesions[107]. Further validation is required, however.
Finally, while newer USS devices have the additional capacity to measure grades of liver steatosis[108], it is unclear how this would benefit the HCC surveillance population where the majority of patients will have cirrhosis.
BEST CLINICAL PRACTICE
International guidelines acknowledge the limited performance of USS in patients with central obesity and marked parenchymal heterogeneity. However, USS remains the primary recommended imaging technique for HCC surveillance [Table 2], considering its high sensitivity in the absence of these factors, safety and proven cost-effectiveness. The AASLD guidelines advise clinicians to utilise CT or MRI, with or without AFP, in patients every six months where USS is documented to be inadequate[16]. The latest American Gastroenterology Association (AGA) clinical practice update on screening and surveillance for HCC in patients with NAFLD supports this approach and emphasises that the adequacy of USS should be consistently reported, including descriptions of parenchyma heterogeneity, visualization of the entire liver, and beam attenuation, as these factors may be impaired in the presence of obesity[109]. These recommendations reflect the USS LI-RADS (Liver Reporting and Data System) visualisation scores (A - No or minimal limitation; B - Moderate limitation, the examination may obscure small masses; and C - Severe limitation, the examination may miss focal liver lesions)[110]. CT or MRI surveillance is advised where USS quality is graded as C, or in some cases B[109]. However, this grading system has not been validated and there is some uncertainty around the approach to patients in category B. The cost-effectiveness of this strategy has not been investigated, and this could have significant implications for health care providers and systems, as the number of patients qualifying for cross-sectional imaging is likely to continue to grow over the coming years[99].
Summary of international guidelines pertaining to HCC surveillance in patients with NAFLD
| Guidelines | Surveillance criteria | Surveillance testing |
| American Gastroenterology Association Clinical Practice update on screening and surveillance for hepatocellular carcinoma in patients with non-alcoholic fatty liver disease: expert review (2020)[100] | ● Screening for HCC should be considered in all patients with cirrhosis due to NAFLD ● Patients with NAFLD with non- invasive markers showing evidence of advanced liver fibrosis or cirrhosis should be considered for HCC screening | ● Adequacy of USS in assessing the liver parenchyma for mass lesions should be documented when used for HCC screening in patients with cirrhosis due to NAFLD ● When the quality of USS is suboptimal for screening of HCC (e.g., due to obesity) future screening should be performed by either CT or MRI with or without AFP every 6 Months |
| Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases[16] | ● Cirrhotic patients, Child-Pugh stage A and B ● Cirrhotic patients, Child-Pugh stage C awaiting liver transplantation ● Surveillance of patients with HCV or NAFLD without cirrhosis is not recommended | ● Recommend surveillance using USS, with or without AFP every 6 months. Quality/Certainty of Evidence: low ● Novel biomarkers, outside of AFP, have shown promising results in case-control studies, but require further evaluation in phase III and IV biomarker studies before routine use ● CT and MRI are not recommended as the primary modality for the surveillance of HCC in patients with cirrhosis. However, in select patients with a high likelihood of having an inadequate USS, or if USS is attempted but inadequate, CT or MRI may be utilized |
| European Association for the Study of the Liver Clinical Practice Guidelines: management of hepatocellular carcinoma (2018)[15] | ● Cirrhotic patients, Child-Pugh stage A and B ● Cirrhotic patients, Child-Pugh stage C awaiting liver transplantation ● Non-cirrhotic fibrosis stage 3 patients, regardless of aetiology may be considered for surveillance based on an individual risk assessment ● The role of surveillance for patients with NAFLD without cirrhosis is unclear | ● Surveillance should be performed by experienced personnel in all high-risk populations using abdominal USS every six months ● Tumour biomarkers for accurate early detection are still lacking. The data available show that the biomarkers tested (i.e., AFP, AFP-L3 and DCP) are suboptimal in terms of cost-effectiveness for routine surveillance of early HCC |
| Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update[17] | ● Surveillance for HCC should be undertaken in high-risk groups of patients (cirrhotic hepatitis patients & chronic HBV carriers) | ● The combination of USS and serum AFP measurement ●performed biannually should be used as a surveillance ● strategy for HCC |
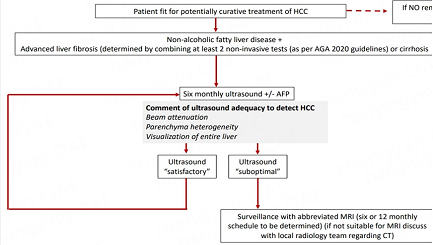
We propose a pragmatic approach based on practice in our centre, as described in Figure 3. We encourage documentation of the USS quality as either “satisfactory” or “suboptimal” for the detection of focal lesions [Box 1]. With suboptimal imaging, we suggest patients fit for curative treatment, instead receive an abbreviated MRI scan (we await the results of ongoing trials to determine if a 6- or 12-monthly surveillance schedule is preferable). Where MRI is contraindicated, we suggest physicians consult their radiology department regarding the use of CT and the optimal timing of this on a case-by-case basis. The effectiveness and cost-effectiveness of this approach have not been tested within a randomised control trial; the authors believe that this approach should be a priority for future research.
Figure 3. Suggested HCC surveillance pathway for patients with NAFLD. HCC: Hepatocellular carcinoma; AGA: American Gastroenterology Association; AFP alpha-fetoprotein; MRI: magnetic resonance imaging; CT: computerized tomography.
SHOULD NON-ALCOHOLIC FATTY LIVER DISEASE PATIENTS WITHOUT CIRRHOSIS UNDERGO HEPATOCELLULAR CARCINOMA SURVEILLANCE?
There is currently insufficient evidence to recommend HCC surveillance in patients with NAFLD without cirrhosis[111]. On the one hand, the overall risk of HCC is extremely low (15% HCC incidence at 10 years for people with NAFLD cirrhosis, vs. 2.7% for those without cirrhosis)[112], on the other hand, up to 40% of people with NAFLD develop HCC in the absence of cirrhosis[46]. People with NAFLD without cirrhosis are younger, have better liver function, and have fewer comorbidities and better performance status than those with NAFLD with cirrhosis, increasing their likelihood of receiving curative treatment if their tumour was diagnosed at an early stage. They would also have a lower probability of death from decompensated liver disease, extrahepatic cancer, and cardiovascular disease during surveillance. It is impractical to survey all patients with NAFLD for HCC; thus, more comprehensive risk stratification within this group would be beneficial. Liver fibrosis is a key risk factor for HCC in people with NAFLD. In a prospective study, the HCC incidence rate per 1,000 person-years was found to be 0.34 for advanced fibrosis vs. 0.04 for nil or minimally significant fibrosis defined histologically[113]. A similar association has been found where non-invasive serum fibrosis tests are used. Analysis of data from the Veterans Health Administration identified that a FIB-4 score > 2.67 is predictive of high incidence rates of HCC (0.39 per 1,000 person-years vs. 0.04 per 1,000 person-years in those with a persistently low FIB-4)[114]. There is also evidence of a positive relationship between liver stiffness measurement obtained at FibroScan and disease activity[115], including HCC incidence[116].
Guidelines disagree about the merits of HCC surveillance in patients with non-cirrhotic NAFLD [Table 2]. EASL, in contrast to the American (AASLD) and Asia-Pacific guidelines, recommends people with metabolic syndrome or NASH affected by severe fibrosis should undergo surveillance[15-17]. EASL state that all “non-cirrhotic fibrosis stage 3 patients, regardless of aetiology, may be considered for surveillance based on an individual risk assessment”[15]. The recent AGA update on HCC surveillance in NAFLD recommends HCC screening in those with evidence of “advanced liver fibrosis or cirrhosis” determined by combining at least two non-invasive tests: point-of-care tests (e.g., FIB-4 score), specialised blood tests (e.g., ELF test ), imaging-based tests (e.g., TE)[109]. The cut-offs selected (16.1 kPa for TE) are within the cirrhotic range, however[117]. This approach is largely driven by clinical consensus opinion as there is minimal evidence to support these cut-offs, or the use of non-invasive tests in this setting currently[104].
CONCLUSION
Mortality rates from HCC are increasing, driven largely by the continued rise in the prevalence of NAFLD in many countries. Outcomes for people with HCC are strongly associated with early detection; thus, optimisation of HCC surveillance techniques including USS is a major priority for research in this field. Patients with NAFLD are under-represented in HCC surveillance compared to other aetiologies of liver disease due to high rates of undetected disease in the community, and a higher prevalence of patients presenting with HCC in the absence of cirrhosis. Better tools are needed to help identify patients with NAFLD at risk of HCC. USS may be suboptimal for early disease detection for patients with obesity and NAFLD. Guidance from the AGA on recording the image quality of USS should be instituted, and additional imaging, with abbreviated MRI (or CT where MRI is contraindicated), should be decided on a case-by-case basis. Novel approaches, including the GALAD and AMAP score, in addition to other biomarkers, still require further evaluation prior to becoming part of routine surveillance.
DECLARATIONS
Authors’ contributionsWrote the manuscript draft: Hydes TJ
Contributed significantly to the reviewing and editing of the manuscript: Cuthbertson DJ, Palmer DH, Elshaarawy O, Johnson PJ, FernandoR, Cross TJ
Availability of data and materialsNot applicable.
Financial support and sponsorshipNone.
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2023.
REFERENCES
1. Kocarnik JM, Compton K, Dean FE, et al. Global Burden of Disease 2019 Cancer Collaboration. Cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life years for 29 cancer groups from 2010 to 2019: a systematic analysis for the global burden of disease study 2019. JAMA Oncol 2022;8:420-44.
2. Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers 2021;7:6.
3. Burton A, Balachandrakumar VK, Driver RJ, et al. HCC-UK/BASL/NCRAS Partnership. Regional variations in hepatocellular carcinoma incidence, routes to diagnosis, treatment and survival in England. Br J Cancer 2022;126:804-14.
4. Hydes TJ, Cuthbertson DJ, Graef S, et al. The impact of diabetes and glucose-lowering therapies on hepatocellular carcinoma incidence and overall survival. Clin Ther 2022;44:257-68.
5. UK CR. Cancer Research UK. Liver cancer incidence statistics. Available from: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/liver-cancer/incidence#ref-2 [Last accessed on 30 Mar 2023].
6. National Cancer Institute. Cancer stat facts: liver and intrahepatic bile duct cancer, 2022. Available from: https://seer.cancer.gov/statfacts/html/livibd.html [Last accessed on 30 Mar 2023].
7. Smittenaar CR, Petersen KA, Stewart K, Moitt N. Cancer incidence and mortality projections in the UK until 2035. Br J Cancer 2016;115:1147-55.
8. Burton A, Tataru D, Driver RJ, et al. HCC-UK/BASL/NCRAS Partnership Steering Group. Primary liver cancer in the UK: Incidence, incidence-based mortality, and survival by subtype, sex, and nation. JHEP Rep 2021;3:100232.
9. Younossi ZM, Otgonsuren M, Henry L, et al. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology 2015;62:1723-30.
10. Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018;67:123-33.
11. Association for the Study of the Liver. Electronic address: [email protected], European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of alcohol-related liver disease. J Hepatol 2018;69:154-81.
12. Office for National Statistics. cancer survival by stage at diagnosis for England.; 2019. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/datasets/cancersurvivalratescancersurvivalinenglandadultsdiagnosed [Last accessed on 30 Mar 2023].
13. De Toni EN, Schlesinger-Raab A, Fuchs M, et al. Age independent survival benefit for patients with hepatocellular carcinoma (HCC) without metastases at diagnosis: a population-based study. Gut 2020;69:168-76.
14. Williams R, Aspinall R, Bellis M, et al. Addressing liver disease in the UK: a blueprint for attaining excellence in health care and reducing premature mortality from lifestyle issues of excess consumption of alcohol, obesity, and viral hepatitis. Lancet 2014;384:1953-97.
15. Association for the Study of the Liver. Electronic address: [email protected], European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2018;69:182-236.
16. Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the american association for the study of liver diseases. Hepatology 2018;68:723-50.
17. Omata M, Cheng AL, Kokudo N, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int 2017;11:317-70.
18. Yu NC, Chaudhari V, Raman SS, et al. CT and MRI improve detection of hepatocellular carcinoma, compared with ultrasound alone, in patients with cirrhosis. Clin Gastroenterol Hepatol 2011;9:161-7.
19. Sangiovanni A, Manini MA, Iavarone M, et al. The diagnostic and economic impact of contrast imaging techniques in the diagnosis of small hepatocellular carcinoma in cirrhosis. Gut 2010;59:638-44.
20. Andersson KL, Salomon JA, Goldie SJ, Chung RT. Cost effectiveness of alternative surveillance strategies for hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol 2008;6:1418-24.
21. Riazi K, Azhari H, Charette JH, et al. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2022;7:851-61.
22. Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther 2011;34:274-85.
23. Stål P. Liver fibrosis in non-alcoholic fatty liver disease - diagnostic challenge with prognostic significance. World J Gastroenterol 2015;21:11077-87.
24. Targher G, Tilg H, Byrne CD. Non-alcoholic fatty liver disease: a multisystem disease requiring a multidisciplinary and holistic approach. Lancet Gastroenterol Hepatol 2021;6:578-88.
25. Eslam M, Newsome PN, Sarin SK, et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol 2020;73:202-9.
26. De A, Duseja A. Natural history of simple steatosis or nonalcoholic fatty liver. J Clin Exp Hepatol 2020;10:255-62.
27. Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R. Fibrosis progression in nonalcoholic fatty liver
28. Dulai PS, Singh S, Patel J, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology 2017;65:1557-65.
29. Ekstedt M, Hagström H, Nasr P, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology 2015;61:1547-54.
30. Baratta F, Pastori D, Angelico F, et al. Nonalcoholic fatty liver disease and fibrosis associated with increased risk of cardiovascular events in a prospective study. Clin Gastroenterol Hepatol 2020;18:2324-2331.e4.
31. Association for the Study of the Liver. Electronic address: [email protected], Clinical Practice Guideline Panel, Chair:, EASL Governing Board representative:, Panel members:. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis - 2021 update. J Hepatol 2021;75:659-89.
32. Mózes FE, Lee JA, Selvaraj EA, et al. LITMUS Investigators. Diagnostic accuracy of non-invasive tests for advanced fibrosis in patients with NAFLD: an individual patient data meta-analysis. Gut 2022;71:1006-19.
33. Dietrich CF, Bamber J, Berzigotti A, et al. EFSUMB guidelines and recommendations on the clinical use of liver ultrasound elastography, update 2017 (long version). Ultraschall Med 2017;38:e16-47.
34. Singh S, Venkatesh SK, Loomba R, et al. Magnetic resonance elastography for staging liver fibrosis in non-alcoholic fatty liver disease: a diagnostic accuracy systematic review and individual participant data pooled analysis. Eur Radiol 2016;26:1431-40.
35. Lee JY, Jang SY, Nam CM, Kang ES. Incident hepatocellular carcinoma risk in patients treated with a sulfonylurea: a nationwide, nested, case-control study. Sci Rep 2019;9:8532.
36. Guo B, Guo Y, Nima Q, et al. China Multi-Ethnic Cohort (CMEC) collaborative group. Exposure to air pollution is associated with an increased risk of metabolic dysfunction-associated fatty liver disease. J Hepatol 2022;76:518-25.
37. Gan T, Bambrick H, Tong S, Hu W. Air pollution and liver cancer: a systematic review. J Environ Sci (China) 2023;126:817-26.
38. Pinyol R, Torrecilla S, Wang H, et al. Molecular characterisation of hepatocellular carcinoma in patients with non-alcoholic steatohepatitis. J Hepatol 2021;75:865-78.
39. Baffy G. Hepatocellular carcinoma in non-alcoholic fatty liver disease: epidemiology, pathogenesis, and prevention. J Clin Transl Hepatol 2013;1:131-7.
40. Huang DQ, Singal AG, Kono Y, Tan DJH, El-Serag HB, Loomba R. Changing global epidemiology of liver cancer from 2010 to 2019: NASH is the fastest growing cause of liver cancer. Cell Metab 2022;34:969-977.e2.
41. Younossi Z, Stepanova M, Ong JP, et al. Global nonalcoholic steatohepatitis council. nonalcoholic steatohepatitis is the fastest growing cause of hepatocellular carcinoma in liver transplant candidates. Clin Gastroenterol Hepatol 2019;17:748-755.e3.
42. Dyson J, Jaques B, Chattopadyhay D, et al. Hepatocellular cancer: the impact of obesity, type 2 diabetes and a multidisciplinary team. J Hepatol 2014;60:110-7.
43. Sanyal A, Poklepovic A, Moyneur E, Barghout V. Population-based risk factors and resource utilization for HCC: US perspective. Curr Med Res Opin 2010;26:2183-91.
44. Orci LA, Sanduzzi-Zamparelli M, Caballol B, et al. Incidence of hepatocellular carcinoma in patients with nonalcoholic fatty liver disease: a systematic review, meta-analysis, and meta-regression. Clin Gastroenterol Hepatol 2022;20:283-292.e10.
45. Ioannou GN, Green PK, Berry K. HCV eradication induced by direct-acting antiviral agents reduces the risk of hepatocellular carcinoma. J Hepatol ;2017:25-32.
46. Tan DJH, Ng CH, Lin SY, et al. Clinical characteristics, surveillance, treatment allocation, and outcomes of non-alcoholic fatty liver disease-related hepatocellular carcinoma: a systematic review and meta-analysis. Lancet Oncol 2022;23:521-30.
47. Stine JG, Wentworth BJ, Zimmet A, et al. Systematic review with meta-analysis: risk of hepatocellular carcinoma in non-alcoholic steatohepatitis without cirrhosis compared to other liver diseases. Aliment Pharmacol Ther 2018;48:696-703.
48. Liu YL, Patman GL, Leathart JB, et al. Carriage of the PNPLA3 rs738409 C >G polymorphism confers an increased risk of non-alcoholic fatty liver disease associated hepatocellular carcinoma. J Hepatol 2014;61:75-81.
49. Hydes TJ, Matthews C, Kumar V, et al. P21 Lack of NAFLD-related HCC surveillance may explain more advanced stage at diagnosis and worse median survival. BASL annual meeting. Gut 2022;71:A46-A47.
50. Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol 2004;130:417-22.
51. Singal A, Volk ML, Waljee A, et al. Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther 2009;30:37-47.
52. Colli A, Fraquelli M, Casazza G, et al. Accuracy of ultrasonography, spiral CT, magnetic resonance, and alpha-fetoprotein in diagnosing hepatocellular carcinoma: a systematic review. Am J Gastroenterol 2006;101:513-23.
53. Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology 2001;33:1394-403.
54. Teefey SA, Hildeboldt CC, Dehdashti F, et al. Detection of primary hepatic malignancy in liver transplant candidates: prospective comparison of CT, MR imaging, US, and PET. Radiology 2003;226:533-42.
55. Gambarin-Gelwan M, Wolf DC, Shapiro R, Schwartz ME, Min AD. Sensitivity of commonly available screening tests in detecting hepatocellular carcinoma in cirrhotic patients undergoing liver transplantation. Am J Gastroenterol 2000;95:1535-8.
56. Tzartzeva K, Obi J, Rich NE, et al. Surveillance imaging and alpha fetoprotein for early detection of hepatocellular carcinoma in patients with cirrhosis: a meta-analysis. Gastroenterology 2018;154:1706-1718.e1.
57. Singal AG, Zhang E, Narasimman M, et al. HCC surveillance improves early detection, curative treatment receipt, and survival in patients with cirrhosis: A meta-analysis. J Hepatol 2022;77:128-39.
58. Chen TH, Chen CJ, Yen MF, et al. Ultrasound screening and risk factors for death from hepatocellular carcinoma in a high risk group in Taiwan. Int J Cancer 2002;98:257-61.
59. Simmons O, Fetzer DT, Yokoo T, et al. Predictors of adequate ultrasound quality for hepatocellular carcinoma surveillance in patients with cirrhosis. Aliment Pharmacol Ther 2017;45:169-77.
60. Sanyal AJ, Banas C, Sargeant C, et al. Similarities and differences in outcomes of cirrhosis due to nonalcoholic steatohepatitis and hepatitis C. Hepatology 2006;43:682-9.
61. Ascha MS, Hanouneh IA, Lopez R, Tamimi TA, Feldstein AF, Zein NN. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology 2010;51:1972-8.
62. Yatsuji S, Hashimoto E, Tobari M, Taniai M, Tokushige K, Shiratori K. Clinical features and outcomes of cirrhosis due to non-alcoholic steatohepatitis compared with cirrhosis caused by chronic hepatitis C. J Gastroenterol Hepatol 2009;24:248-54.
63. Tang Z, Yu Y, Zhou X. An important approach to prolonging survival further after radical resection of AFP positive hepatocellular carcinoma. Journal of Experimental Clinical Cancer Research. J Exp Clin Cancer Res 1984;3:359-66.
64. National Centre for Health Statistics. National health and nutrition examination survey, 1999-2018. Available from: https://www.cdc.gov/nchs/nhanes/index.htm [Last accessed on 30 Mar 2023].
65. Adams LA, Lymp JF, St Sauver J, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology 2005;129:113-21.
66. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73-84.
67. Poggio P, Olmi S, Ciccarese F, et al. Italian Liver Cancer (ITA.LI.CA) Group.. Factors that affect efficacy of ultrasound surveillance for early stage hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol 2014;12:1927-33.e2.
68. Esfeh JM, Hajifathalian K, Ansari-Gilani K. Sensitivity of ultrasound in detecting hepatocellular carcinoma in obese patients compared to explant pathology as the gold standard. Clin Mol Hepatol 2020;26:54-9.
69. Samoylova ML, Mehta N, Roberts JP, Yao FY. Predictors of ultrasound failure to detect hepatocellular carcinoma. Liver Transpl 2018;24:1171-7.
70. Kim DH, Hong SB, Choi SH, et al. Surveillance failure in ultrasound for hepatocellular carcinoma: a systematic review and meta-analysis. Gut 2022;71:212-3.
71. Aby E, Phan J, Truong E, Grotts J, Saab S. Inadequate hepatocellular carcinoma screening in patients with nonalcoholic steatohepatitis cirrhosis. J Clin Gastroenterol 2019;53:142-6.
72. Lo S, Gane E, Bartlett A, Or D. Clinical features and survival of nonalcoholic fatty liver disease-related hepatocellular carcinoma. Hepatology International 2016;10:S437.
73. Barbara L, Benzi G, Gaiani S, et al. Natural history of small untreated hepatocellular carcinoma in cirrhosis: a multivariate analysis of prognostic factors of tumor growth rate and patient survival. Hepatology 1992;16:132-7.
74. Ebara M, Ohto M, Shinagawa T, et al. Natural history of minute hepatocellular carcinoma smaller than three centimeters complicating cirrhosis. A study in 22 patients. Gastroenterology 1986;90:289-98.
75. Santi V, Trevisani F, Gramenzi A, et al. Italian Liver Cancer (ITALI.CA) Group. Semiannual surveillance is superior to annual surveillance for the detection of early hepatocellular carcinoma and patient survival. J Hepatol 2010;53:291-7.
76. Trinchet JC, Chaffaut C, Bourcier V, et al. Groupe d’Etude et de Traitement du Carcinome Hépatocellulaire (GRETCH). Ultrasonographic surveillance of hepatocellular carcinoma in cirrhosis: a randomized trial comparing 3- and 6-month periodicities. Hepatology 2011;54:1987-97.
77. Biselli M, Conti F, Gramenzi A, et al. A new approach to the use of α-fetoprotein as surveillance test for hepatocellular carcinoma in patients with cirrhosis. Br J Cancer 2015;112:69-76.
78. Giannini EG, Sammito G, Farinati F, et al. Italian Liver Cancer (ITALI.CA) Group. Determinants of alpha-fetoprotein levels in patients with hepatocellular carcinoma: implications for its clinical use. Cancer 2014;120:2150-7.
79. Piscaglia F, Svegliati-Baroni G, Barchetti A, et al. HCC-NAFLD Italian Study Group. Clinical patterns of hepatocellular carcinoma in nonalcoholic fatty liver disease: a multicenter prospective study. Hepatology 2016;63:827-38.
80. Paradis V, Zalinski S, Chelbi E, et al. Hepatocellular carcinomas in patients with metabolic syndrome often develop without significant liver fibrosis: a pathological analysis. Hepatology 2009;49:851-9.
81. Chen JG, Parkin DM, Chen QG, et al. Screening for liver cancer: results of a randomised controlled trial in Qidong, China. J Med Screen 2003;10:204-9.
82. Duvoux C, Roudot-Thoraval F, Decaens T, et al. Liver Transplantation French Study Group. Liver transplantation for hepatocellular carcinoma: a model including α-fetoprotein improves the performance of Milan criteria. Gastroenterology 2012;143:986-94.e3; quiz e14.
83. Kadalayil L, Benini R, Pallan L, et al. A simple prognostic scoring system for patients receiving transarterial embolisation for hepatocellular cancer. Ann Oncol 2013;24:2565-70.
84. Chernyak V, Fowler KJ, Kamaya A, et al. Liver imaging reporting and data system (LI-RADS) version 2018: imaging of hepatocellular carcinoma in at-risk patients. Radiology 2018;289:816-30.
85. Lencioni R, Piscaglia F, Bolondi L. Contrast-enhanced ultrasound in the diagnosis of hepatocellular carcinoma. J Hepatol 2008;48:848-57.
86. Nicolau C, Catalá V, Vilana R, et al. Evaluation of hepatocellular carcinoma using SonoVue, a second generation ultrasound contrast agent: correlation with cellular differentiation. Eur Radiol 2004;14:1092-9.
87. Smajerova M, Petrasova H, Little J, et al. Contrast-enhanced ultrasonography in the evaluation of incidental focal liver lesions: a cost-effectiveness analysis. World J Gastroenterol 2016;22:8605-14.
88. Yoon JH, Lee JM, Lee DH, et al. A comparison of biannual two-phase low-dose liver CT and US for HCC surveillance in a group at high risk of HCC development. Liver Cancer 2020;9:503-17.
89. An JY, Peña MA, Cunha GM, et al. Abbreviated MRI for hepatocellular carcinoma screening and surveillance. Radiographics 2020;40:1916-31.
90. Gupta P, Soundararajan R, Patel A, Kumar-M P, Sharma V, Kalra N. Abbreviated MRI for hepatocellular carcinoma screening: a systematic review and meta-analysis. J Hepatol 2021;75:108-19.
91. Best J, Bechmann LP, Sowa JP, et al. GALAD score detects early hepatocellular carcinoma in an international cohort of patients with nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol 2020;18:728-735.e4.
92. Zhu R, Yang J, Xu L, et al. Diagnostic performance of Des-γ-carboxy prothrombin for hepatocellular carcinoma: a meta-analysis. Gastroenterol Res Pract 2014;2014:529314.
93. Hagiwara S, Kudo M, Kawasaki T, et al. Prognostic factors for portal venous invasion in patients with hepatocellular carcinoma. J Gastroenterol 2006;41:1214-9.
94. Koyama N, Yamazaki T, Kanetsuki Y, et al. Activation of apoptosis inhibitor of macrophage is a sensitive diagnostic marker for NASH-associated hepatocellular carcinoma. J Gastroenterol 2018;53:770-9.
95. Okanoue T, Yamaguchi K, Shima T, et al. Serum levels of immunoglobulin M-free inhibitors of macrophage/CD5L as a predictive and early diagnostic marker for nonalcoholic steatohepatitis-associated hepatocellular carcinoma. Hepatol Res 2022;52:998-1008.
96. Chalasani NP, Porter K, Bhattacharya A, et al. Validation of a novel multitarget blood test shows high sensitivity to detect early stage hepatocellular carcinoma. Clin Gastroenterol Hepatol 2022;20:173-182.e7.
97. Lin N, Lin Y, Xu J, et al. A multi-analyte cell-free DNA-based blood test for early detection of hepatocellular carcinoma. Hepatol Commun 2022;6:1753-63.
98. Fan R, Papatheodoridis G, Sun J, et al. aMAP risk score predicts hepatocellular carcinoma development in patients with chronic hepatitis. J Hepatol 2020;73:1368-78.
99. Ioannou GN, Green P, Kerr KF, Berry K. Models estimating risk of hepatocellular carcinoma in patients with alcohol or NAFLD-related cirrhosis for risk stratification. J Hepatol 2019;71:523-33.
100. Singal AG, Manjunath H, Yopp AC, et al. The effect of PNPLA3 on fibrosis progression and development of hepatocellular carcinoma: a meta-analysis. Am J Gastroenterol 2014;109:325-34.
101. Yang J, Trépo E, Nahon P, et al. PNPLA3 and TM6SF2 variants as risk factors of hepatocellular carcinoma across various etiologies and severity of underlying liver diseases. Int J Cancer 2019;144:533-44.
102. Bianco C, Jamialahmadi O, Pelusi S, et al. Non-invasive stratification of hepatocellular carcinoma risk in non-alcoholic fatty liver using polygenic risk scores. J Hepatol 2021;74:775-82.
103. Fujiwara N, Kubota N, Crouchet E, et al. Molecular signatures of long-term hepatocellular carcinoma risk in nonalcoholic fatty liver disease. Sci Transl Med :14.
104. Singal AG, Sanduzzi-Zamparelli M, Nahon P, et al. International Liver cancer Association (ILCA) white paper on hepatocellular carcinoma risk stratification and surveillance. J Hepatol ;2023:S0168-8278(23)00111.
105. Hamada K, Saitoh S, Nishino N, et al. Shear wave elastography predicts hepatocellular carcinoma risk in hepatitis C patients after sustained virological response. PLoS One 2018;13:e0195173.
106. Zhang T, Zhang G, Deng X, et al. APS (age, platelets, 2d shear-wave elastography) score predicts hepatocellular carcinoma in chronic hepatitis B. Radiology 2021;301:350-9.
107. Nacheva-georgieva EL, Doykov DI, Andonov VN, Doykova KA, Tsvetkova SB. Point shear wave elastography and 2-dimensional shear wave elastography as a non-invasive method in differentiating benign from malignant liver lesions. Gastroenterology Insights 2022;13:296-304.
108. Cassinotto C, Jacq T, Anselme S, et al. Diagnostic performance of attenuation to stage liver steatosis with mri proton density fat fraction as reference: a prospective comparison of three US machines. Radiology 2022;305:353-61.
109. Loomba R, Lim JK, Patton H, El-Serag HB. AGA clinical practice update on screening and surveillance for hepatocellular carcinoma in patients with nonalcoholic fatty liver disease: expert review. Gastroenterology 2020;158:1822-30.
110. LI-RADS® v2017 US Core. Available from: https://www.acr.org/-/media/ACR/Files/RADS/LI-RADS/LI-RADS-US-Algorithm-Portrait-2017.pdf [Last accessed on 30 Mar 2023].
111. Plaz Torres MC, Bodini G, Furnari M, et al. Surveillance for hepatocellular carcinoma in patients with non-alcoholic fatty liver disease: universal or selective? Cancers (Basel) 2020;12:1422.
112. Reig M, Gambato M, Man NK, et al. Should patients with NAFLD/NASH Be Surveyed for HCC? Transplantation 2019;103:39-44.
113. Sanyal AJ, Van Natta ML, Clark J, et al. NASH Clinical Research Network (CRN). prospective study of outcomes in adults with nonalcoholic fatty liver disease. N Engl J Med 2021;385:1559-69.
114. Kanwal F, Kramer JR, Mapakshi S, et al. Risk of hepatocellular cancer in patients with non-alcoholic fatty liver disease. Gastroenterology 2018;155:1828-1837.e2.
115. Newsome PN, Sasso M, Deeks JJ, et al. FibroScan-AST (FAST) score for the non-invasive identification of patients with non-alcoholic steatohepatitis with significant activity and fibrosis: a prospective derivation and global validation study. Lancet Gastroenterol Hepatol 2020;5:362-73.
116. Shearer JE, Jones R, Parker R, Ferguson J, Rowe IA. The natural history of advanced chronic liver disease defined by transient elastography. Clin Gastroenterol Hepatol 2023;21:694-703.e8.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.



















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].