The forehead flap in nasal reconstruction: overview and updates in evidence
Abstract
The paramedian forehead flap (PMFF) is an interpolated flap commonly used to reconstruct a variety of nasal defects. It is the preferred reconstructive method for moderate to large nasal defects, with advantages of robust blood supply, close color and texture matching with native nasal skin, relatively acceptable donor site morbidity, and ability to customize size and thickness. It may involve two or three stages to permit addition of cartilage grafts, debulking or other modifications, and pedicle division. Recent literature has supported numerous variations on the classically described forehead flap. These include shortened time to pedicle division, single-stage flaps, two-stage flaps for complex major reconstruction, pre-laminated flaps, and use of the forehead flap in total nasal reconstruction. Recent updates in the literature on PMFF in nasal reconstruction will be reviewed.
Keywords
INTRODUCTION
Reconstruction of nasal defects can be one of the most complex and challenging procedures within plastic surgery of the face. While some defects are amenable to secondary intent healing, primary closure or skin/composite grafting, this is often not the case. Careful consideration of both aesthetic and functional implications of the reconstructive method is crucial. Local flaps, including rotation and transposition flaps, are frequently employed for defects < 1-1.5 cm in favorable locations[1,2]. For larger defects, interpolated flaps such as the melolabial or paramedian forehead flap (PMFF) are commonly used - the latter a major workhorse in nasal reconstruction and the primary focus of this review.
The PMFF is an axial-pattern interpolated flap commonly used to reconstruct nasal defects larger than
OVERVIEW OF SURGICAL PRINCIPLES
While a comprehensive review of surgical technique is outside the scope of this writing, several key principles will be discussed. Conventional dogma dictates that the PMFF should be considered for nasal defects approximately 1.5 cm or larger. However, nasal size, patient aesthetic goals and preferences, and overall health must be considered when making a joint decision on reconstructive method. During surgical preparation, the nasal aesthetic subunits should be outlined to determine the extent of involvement of each subunit, as well as create a template from the contralateral side if uninvolved. It may be beneficial to excise additional native nasal tissue if the majority of a subunit has already been resected, so that the borders of the inset flap lie more inconspicuously along subunit boundaries[1]. Squaring of defect edges along subunit boundaries may also help prevent “pin cushioning” and better camouflage the reconstruction once healed. In cases of superficial defects, the wound bed is often thinned uniformly to the depth of underlying structural nasal framework. Cartilage grafts may be added for structural support and/or prevention of alar retraction. If the defect involves the adjacent medial cheek, this area is reconstructed first with cheek advancement, secured with deep sutures to the frontal process of the maxilla. For full-thickness defects reconstruction of the internal lining must be considered, which may involve septal or mucosal flaps, skin turn-in flaps, a folded or prelaminated forehead flap, or even free tissue transfer[5-7].
A template is then made of the final defect, and vertical position of the flap on the forehead is determined based on necessary pedicle length. In the senior author’s experience, the pedicle dissection may be carried out in the subperiosteal plane through the medial brow to maximize pedicle length while avoiding the hairline. The flap itself may be raised to a depth customized to the recipient site, in a subcutaneous plane and/or deeper subgaleal plane if greater thickness is desired. Classically, the recommended pedicle width is approximately 1.2 cm to ensure viability[8]. However, after identification of the supratrochlear artery with ultrasound, Doppler and/or indocyanine green (ICG) angiography, the senior author often designs a pedicle of 8-9 mm width. The flap is then raised and inset with minimal pedicle tension, and the forehead donor site is closed if able. If the donor site is too large for primary closure, it is preferred to allow healing by secondary intent, which yields superior aesthetic results compared to immediate skin grafting, and may be revised at a later time if needed[9].
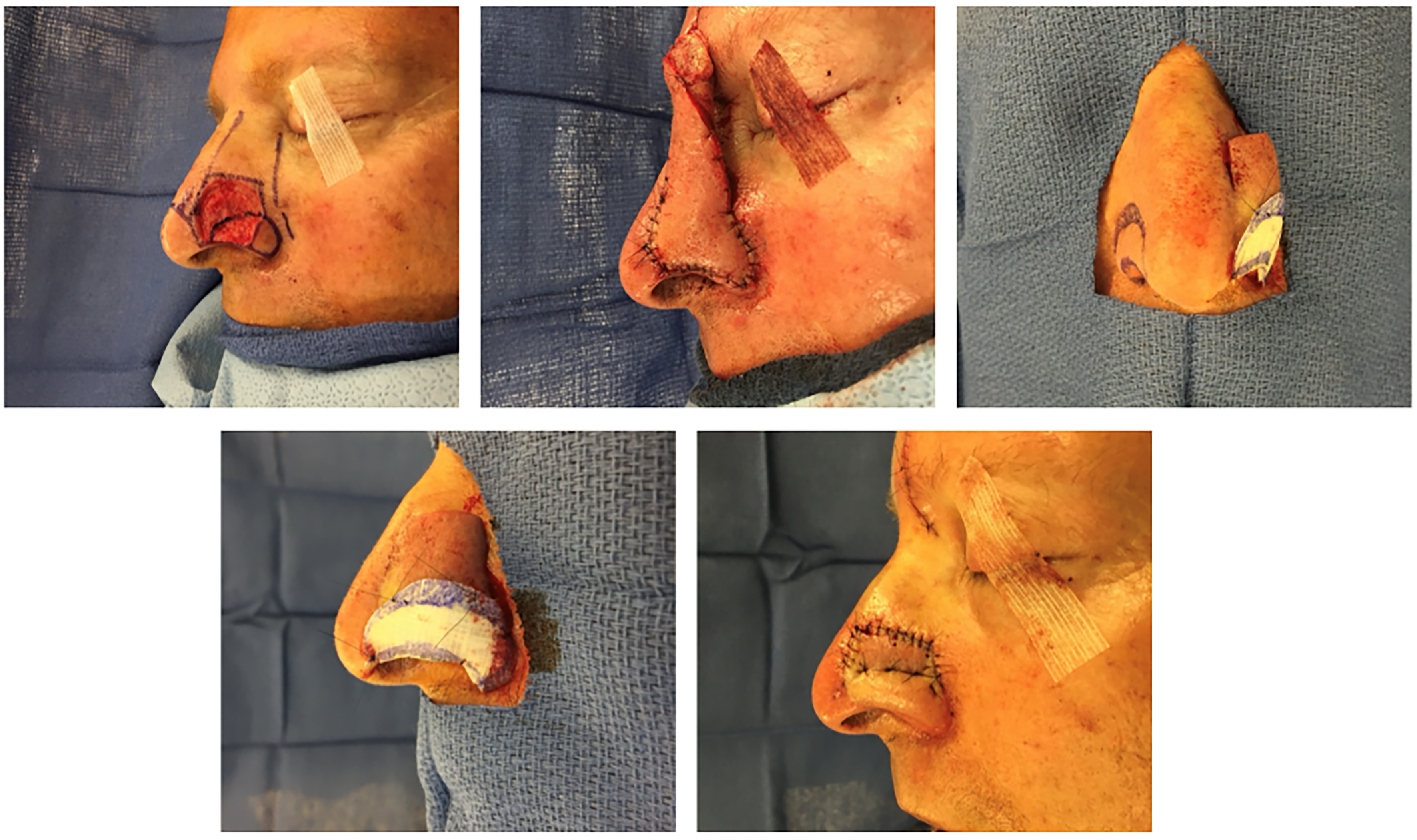
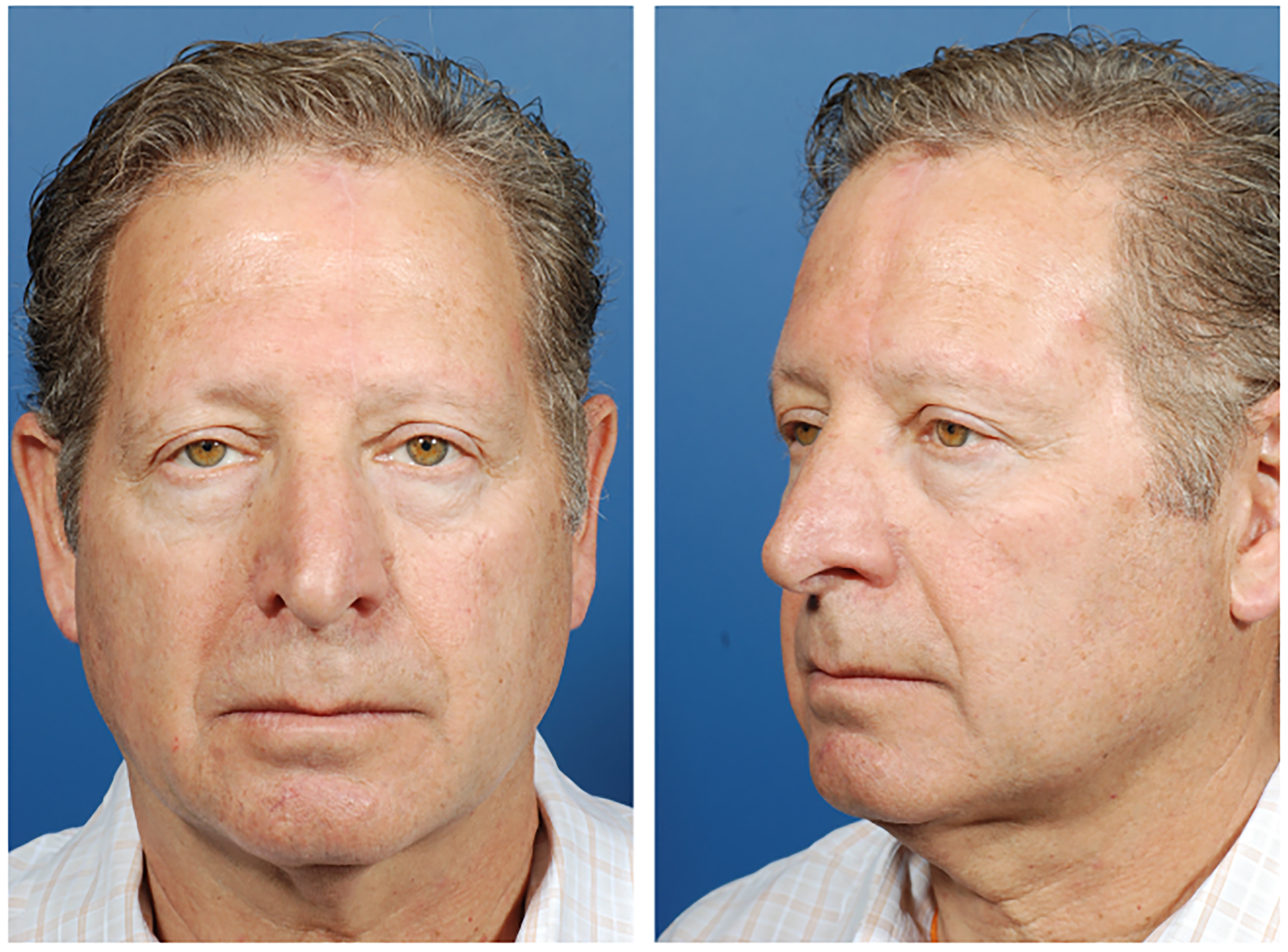
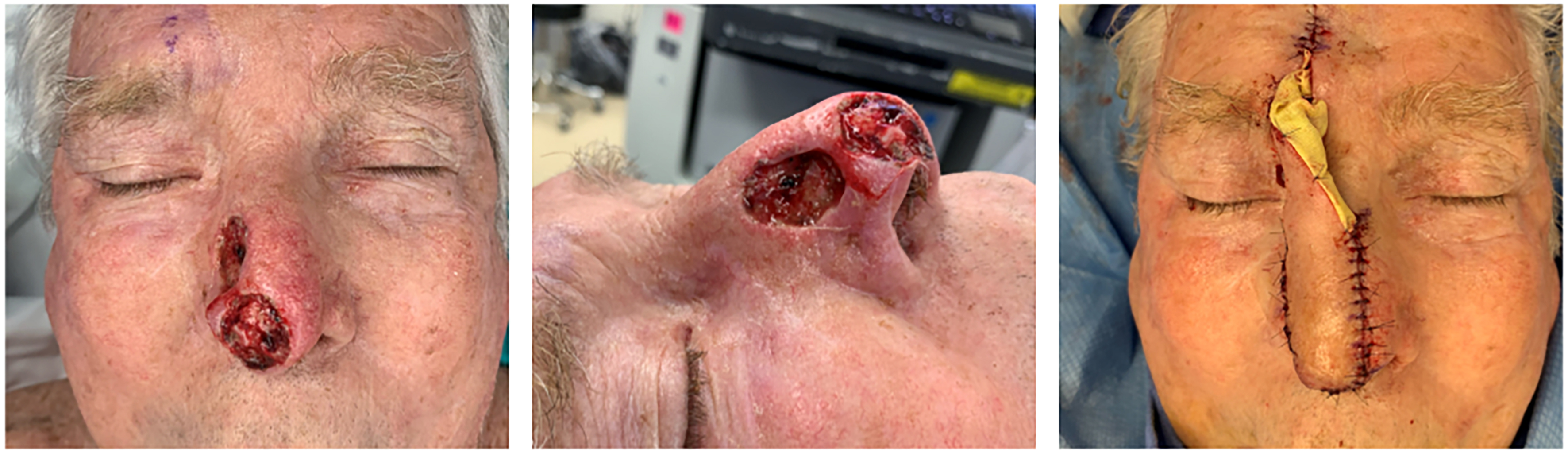
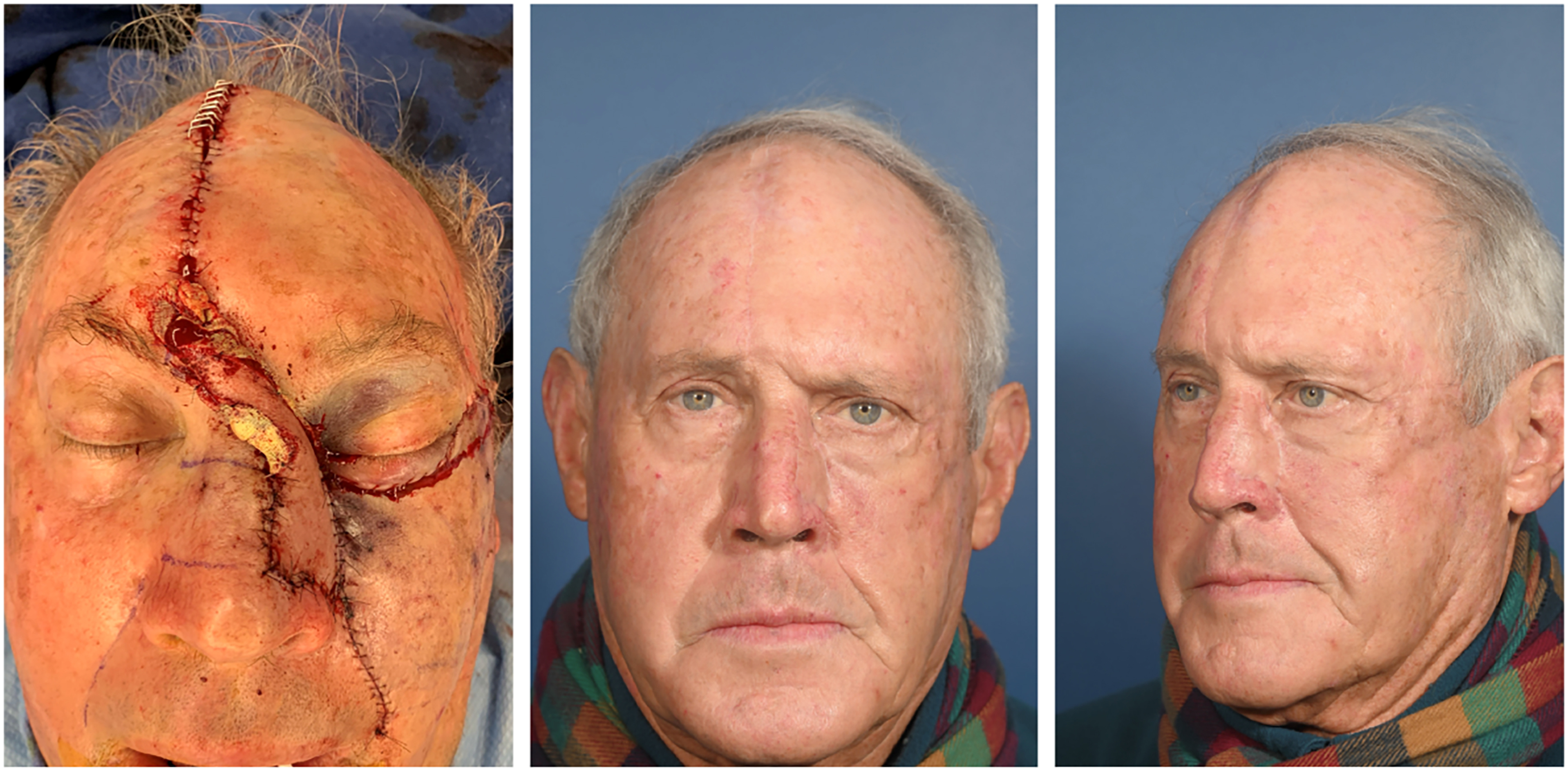
Figures 1 and 2 provide a patient example of two-stage PMFF reconstruction of a left alar defect using the above principles. Another case example in Figures 3 and 4 demonstrates unification of two large nasal defects via subunit excision and reconstruction with a single PMFF. The resultant defect, while larger, is better camouflaged with squared borders along subunit boundaries. Figure 5 provides an example of reconstruction of a nasal and medial cheek defect with cheek advancement and a PMFF. This case highlights the importance of first defining the nasal boundaries when the defect extends onto the medial cheek. In these circumstances, it is beneficial to first reconstruct the surrounding soft tissue defect prior to planning the nasal reconstruction, both to support the nasal reconstruction and create a natural-appearing nasofacial junction.
Figure 1. Partial thickness circular defect of the left ala and sidewall reconstructed with a paramedian forehead flap (top left). Note the planned subunit excision of the ala and squaring of defect edges along subunit boundaries. A template based on the non-operated side is used to facilitate symmetry. Tacking sutures are used to re-create the alar groove at the end of the second stage (pedicle division).
Figure 2. Postoperative photos after two-stage paramedian forehead flap reconstruction [Figure 1].
Figure 3. Reconstruction of two defects of the nasal tip, sidewall and dorsum. The defects are unified through subunit excision and reconstructed with a single paramedian forehead flap. Defects are shown prior to unification (left, center images) and following first stage reconstruction (right).
Figure 4. Postoperative photos after two-stage paramedian forehead flap reconstruction [Figure 3].
OUTCOMES AND RISK FACTORS IN FOREHEAD FLAP RECONSTRUCTION
A number of studies have published outcomes following PMFF nasal reconstruction. Little et al. analyzed 205 patients who underwent PMFF and found an overall major complication rate of 16.1% and minor complication rate of 31.2%. Only smoking and full-thickness defects were associated with postoperative complications[10]. Smoking was associated with flap necrosis, and full-thickness defects were associated with flap necrosis and alar notching. In this study, diabetes, vascular disease and age were not associated with major complications. Ji et al. analyzed outcomes from a variety of reconstructive methods following Mohs micrographic surgery and found that PMFFs (all two-stage) were more likely to have major revision surgery[11]. However, in this study PMFFs were compared to minor reconstructions such as skin grafts and primary closure. The largest study of PMFF complications to date was published by the senior author (SPM) in 2019. In this study of 2,175 patients, infection was the most common postoperative complication, and readmission risk factors included postoperative bleeding, alcohol use, and neurologic disorders[12].
A recent paper by Wu et al. evaluated complications within a cohort of 197 PMFFs to develop a risk prediction model. They found an overall complication rate of 25%, which included both impaired nasal function and more immediate postoperative flap complications. Factors associated with increased risk included hypoalbuminemia, female sex, prior excisions and rib cartilage grafting. Using the authors’ clinical risk calculator, intermediate- and high-risk categories increase the chance of any complication by 11- and 55-fold, respectively[13]. Interestingly, smoking was not associated with increased complications in this study, contrary to findings by Little et al.[10]. As sample size and classification of complications was similar in both studies, this could be due to different patient populations with various combinations of underlying comorbidities, though additional research would be necessary to make a definitive conclusion.
Interestingly, a recent survey of facial plastic and reconstructive surgeons performing PMFF reconstructions found a surgeon-reported complication rate of only 1%-5%, and an average of 1.1 total revision procedures following pedicle division[14]. It is possible that this reflects only more serious complications and not minor touch-up procedures to improve nasal cosmesis or function.
Number of stages
Historically, there has been conflicting evidence regarding indications for and differences between two- and three-stage PMFFs. Classically, three-stage reconstruction has been thought to be superior for complex reconstructions, and potentially offers improved cosmesis[15,16]. Menick argued for the addition of an intermediate stage in order to safely and aggressively thin the flap, re-contour, and add cartilage grafts if not placed during stage one[16]. Indeed, three-stage reconstructions are more commonly performed for such large, complex nasal reconstructions[17,18].
However, several recent studies have shown no increased risk of complications nor aesthetic concerns between two- and three-stage forehead flaps when comparing complex defects with similar size, depth and cartilage use, as well as similar medical comorbidities[17-19]. Some studies have even reported worse aesthetic outcomes in three-stage flaps. Stahl et al. (2015) found that three-stage procedures resulted in worse nasal ala bulk/thickening, even when controlling for defect size and use of cartilage[17]. Palmer et al. found that the only significant difference between similar two- and three-stage procedures is higher postoperative dyspigmentation in three-stage reconstructions[18].
These findings may challenge the conventional thought that three-stage reconstructions are necessary for complex defects to achieve the desired functional and cosmetic outcomes, as well as avoid postoperative flap complications. There may be some complex defects that are amenable to successful two-stage reconstruction. In addition, completing reconstruction in two stages reduces health care system costs and patient postoperative morbidity, allowing shorter duration of wound care and earlier return to work and other responsibilities.
TIMING OF PEDICLE DIVISION
ICG angiography
In addition to reducing number of reconstructive stages, decreasing time to pedicle division has also been heavily investigated in recent years. Many studies characterizing the vascular perfusion of PMFFs, as well as the safety and efficacy of early pedicle division, have relied on ICG angiography. Several methods have been described to assess intraoperative perfusion of cutaneous flaps, including handheld Doppler, duplex ultrasound, optical diffusion imaging spectroscopy, near-infrared spectroscopy, dynamic infrared thermography, fluorescein angiography, laser Doppler flowmetry, and laser-assisted ICG angiography[20-25]. An advantage of ICG angiography is the ability to pair this with SPY-Q software to extract quantitative perfusion metrics. Intravenous (IV) ICG binds to plasma proteins and has a 3-4 min half-life, and the SPY Elite Imaging System uses an 806 nm laser to illuminate ICG dye as it passes through the area of interest[20]. Relative perfusion of any point on the flap to a reference point elsewhere on the face may be calculated at any time during ICG passage, and the SPY-Q software also calculates time-independent relative flap perfusion.
This technology may be used in forehead flap planning to visualize the path of the supratrochlear arteries and compare perfusion intensity on the right and left sides. It may also be used to confirm distal flap perfusion after initial inset, prior to pedicle division, and immediately following pedicle division. Additionally, ICG angiography has been used to identify potential risk factors for decreased perfusion of PMFFs[26]. A 2019 study by the senior author retrospectively analyzed perfusion data from 71 forehead flap reconstructions and found that only cartilage graft use and time between stages significantly affected flap perfusion metrics[26]. Diabetes also appeared to negatively affect perfusion, though this did not reach statistical significance. Interestingly, tobacco use was not associated with decreased perfusion nor complications in this cohort.
Some may raise concerns regarding the cost and necessity of ICG angiography. Of note, a study by our group found an average cost savings of $177 per patient when ICG angiography was used to aid safe pedicle division at two weeks compared to patients divided at three weeks without angiography[27]. It may be an effective tool in reducing time to pedicle division, thus decreasing postoperative morbidity and allowing cost savings through patient productivity gains.
Pedicle division
A 2012 study by the senior author used ICG angiography to quantify forehead flap perfusion at weekly intervals following the initial reconstructive stage and found evidence of adequate vascular in-growth as early as one week postoperatively[20]. A subsequent 2015 study by the same group analyzed forehead flap perfusion 2 weeks after initial flap transfer in 10 patients with partial-thickness defects, vascularized tissue in more than 50% of the recipient bed, and no nicotine use. In this cohort, the flap was divided early at two weeks instead of the traditional three weeks, and there were no complications[28].
Most recently, our group prospectively assessed flap perfusion at one week post-flap transfer in 10 patients with the same inclusion criteria as the prior study, showing nearly the same perfusion at one week compared to two weeks. Pre-division perfusion was comparable to post-division perfusion, and all 10 patients had successful 7-day flap takedown with no flap-related complications[29]. This supports results from Somoano et al. on early takedown (average 7.2 days) in 26 PMFF patients, including those with tobacco use, diabetes and cartilage grafts. There was no increased risk of postoperative complications in the early division group, and none developed flap necrosis or major complications. A recent study also compared outcomes of “accelerated” (< 21-day) pedicle division without ICG angiography[30]. In this cohort of 39 two-stage PMFF patients, there were no major flap complications among the 7 who underwent accelerated takedown. There was no difference in revision rate or touch-up procedures between this cohort and those who underwent division at 3-4 weeks[18].
These studies suggest that, in appropriately selected patients with amenable defects and relatively minor comorbidities, forehead flap division as early as seven days after initial flap transfer is safe and effective. Early division has several advantages, including decreased time with a noticeable reconstructive deformity, less time away from work and other obligations, shorter duration of wound care, and limiting bothersome sequelae of an intact pedicle including visual field obstruction and difficulty wearing glasses. An analysis of the psychological effects of PMFF nasal reconstruction found that psychological distress is greatest prior to reconstruction and improves with each subsequent stage[31]. Thus, safe methods for shortening the reconstructive time window may also be beneficial for mental health and disease experience. However, a 2024 survey of facial plastic and reconstructive surgeons found that > 80% perform pedicle division at least 3 weeks after flap transfer[14]. Given the data outlined here, early division should be considered in select patients as a safe alternative to division at 3 weeks.
SINGLE STAGE FOREHEAD FLAPS
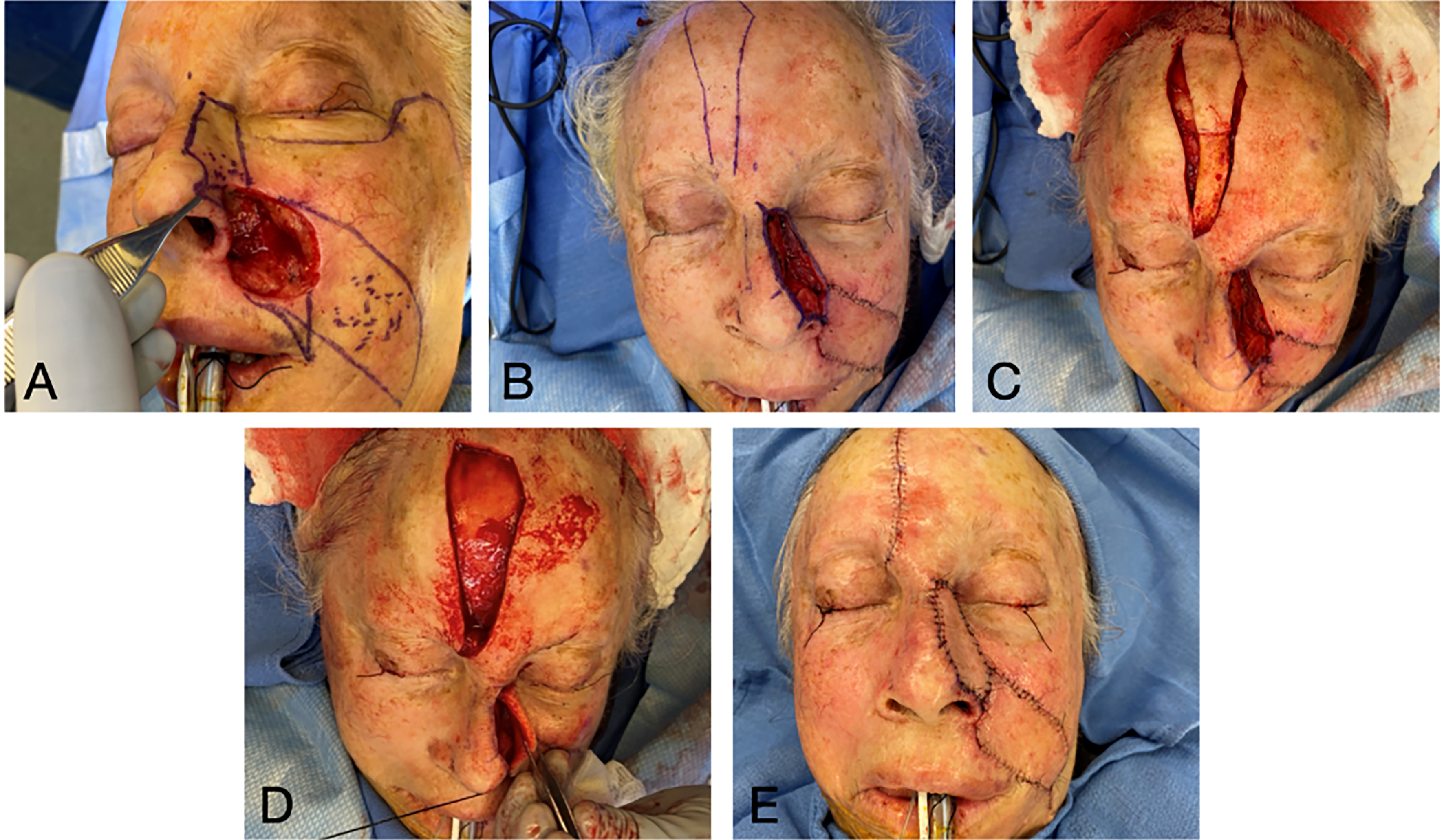
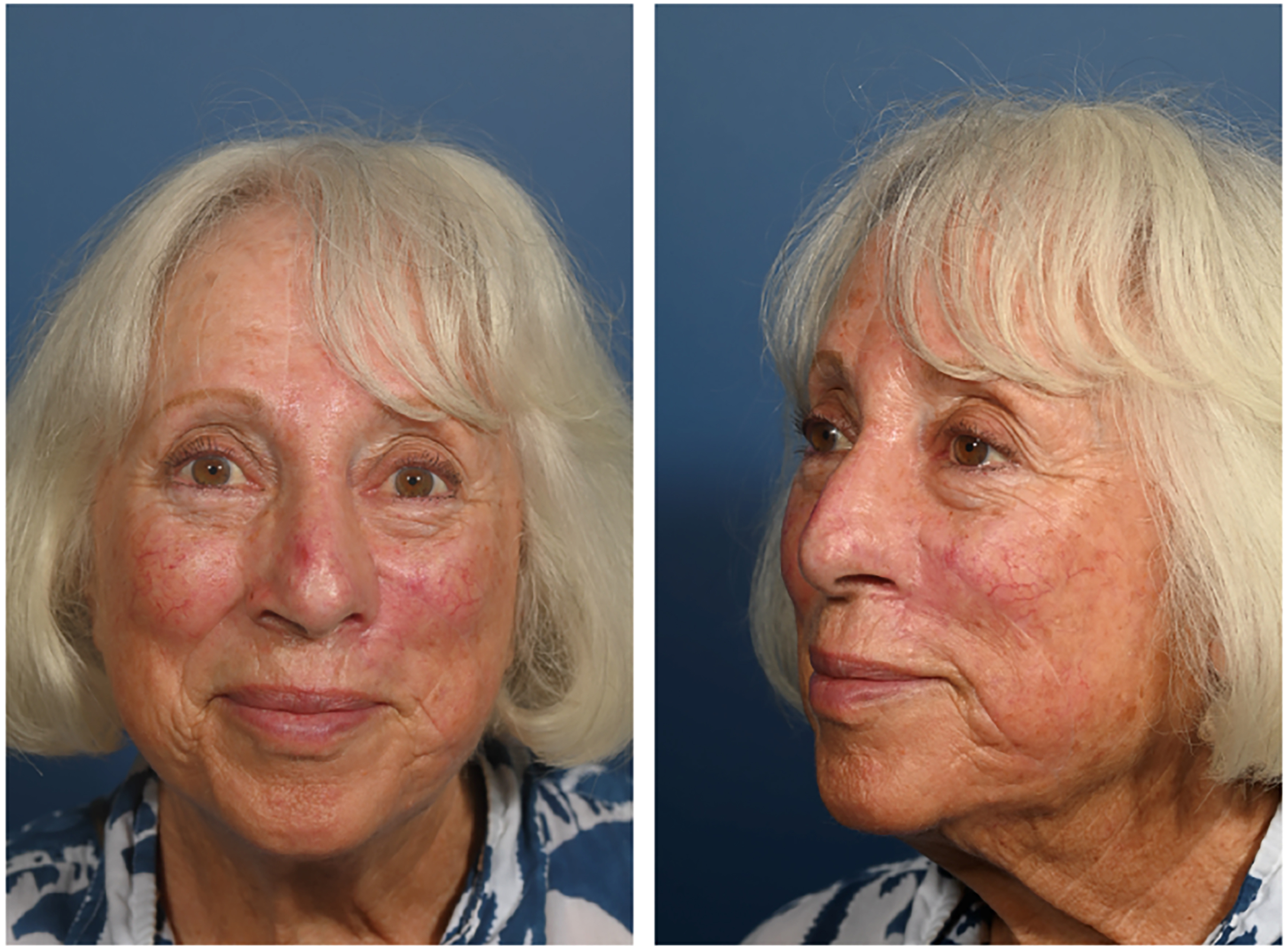
In contrast to the traditional multi-stage flap, the single-stage PMFF has been described as a viable reconstructive option in some patients. Park et al. described the single-stage forehead flap technique in which the soft tissue surrounding the vascular pedicle is de-epithelialized and thinned, and the forehead island flap is tunneled within the soft tissue of the glabella to the recipient nasal defect[32,33]. It is most suitable for defects that extend to the upper third of the nasal dorsum or sidewall. This limits the distance over which the pedicle is tunneled, theoretically decreasing risk of pedicle compression. A shorter tunneled distance also permits any pedicle bulk to be concealed within the thicker soft tissue of the glabella[33]. A patient example of a single-stage PMFF is provided in Figures 6 and 7.
Figure 6. Planning and execution of single-stage forehead flap. (A) Original defect after debridement, and planning for V-Y advancement closure of cheek and internal lining flap; (B) Internal lining and V-Y flaps completed, with ear cartilage graft in place; (C) Forehead flap raised. Note de-epithelialized area; (D) Forehead flap tunneled within the glabella; (E) Forehead flap in place. V-Y: V-Y advancement flap, a local tissue flap design where a V-shaped incision is advanced and closed in a Y-shaped configuration for defect reconstruction.
This study’s authors recently characterized perfusion of single-stage PMFF reconstructions using ICG angiography[34]. This is the first study providing such quantitative measurements of single-stage forehead flap perfusion in nasal reconstruction. This cohort of eight patients demonstrated excellent perfusion of the distal flap relative to a cheek reference point, and perfusion data were comparable to multi-stage, non-tunneled forehead flaps. Such data offers reassurance regarding concerns for degree of pedicle rotation, venous congestion and pedicle compression in the tunneled flap. In addition, there were no incidents of flap necrosis or other complications related to vascular compromise such as dehiscence, infection or epidermolysis. Only one patient underwent a revision procedure for glabellar debulking, suggesting that glabellar fullness is an uncommon concern postoperatively.
A single-stage surgery in which the pedicle remains intact may be ideal for certain patients with vascular disease, risk factors such as smoking or diabetes, need for timely initiation of adjuvant radiation therapy, or other medical comorbidities that increase general anesthesia-associated risks. Other advantages include optimizing utilization of health resources, patient convenience, and easier postoperative wound care[27]. As previously mentioned, a single stage flap is not an ideal option for defects that do not extend to the upper third of the nose. However, for large, full-thickness defects of the lower third, a single-stage flap may still be considered if the remaining sidewall subunit skin is converted to a turn-down flap for internal nasal lining, with a resultant skin defect that extends to the upper third of the nose.
OTHER TECHNICAL ADVANCES
Internal lining reconstruction
For large full-thickness or subtotal nasal defects, both reconstruction of the internal lining and adequate, appropriately sized soft tissue for external reconstruction must be considered. Reconstruction of the internal lining is crucial to prevent wound contracture and nasal collapse and provide coverage for structural grafts[35,36]. This may be challenging for larger defects, and local solutions such as mucosal/septal flaps or epithelial turn-in flaps are often inadequate or unavailable.
For small to moderate nasal ala lining defects or partial internal lining reconstruction of the ala, the melolabial flap may be an excellent option. Moyer described a two-stage interpolated melolabial flap, which may be covered with a skin graft at the initial stage to prevent wound contracture. Alternatively, structural grafting and formal external reconstruction may be performed simultaneously, and the melolabial flap pedicle divided at a subsequent stage[37]. Subsequently, the senior author described the novel single-stage melolabial flap for ala internal lining reconstruction. The flap is tunneled through an intact skin bridge at the junction of the alar base and nasolabial fold following de-epithelialization of the tunneled segment, preserving the alar-facial junction. In cases in which it is not possible to preserve the skin isthmus, a non-tunneled flap may be used and the isthmus incorporated into the flap for internal lining[38].
Pre-laminated flaps have also been described as a solution for internal lining reconstruction[36]. With this technique, the first stage involves designing and raising the forehead flap, harvesting a split- or full-thickness skin graft from another site and contouring and suturing this to the undersurface of the forehead flap, and laying the composite flap back down in the donor site. While both have been described, it is thought that full thickness grafts demonstrate less contracture than split thickness grafts[5,39]. The composite flap is then raised approximately 4 weeks later, the vascularized skin graft is trimmed and customized to the lining defect, and the flap is inset. No structural support is placed at this stage, though nasal packing or other alternative support is necessary to prevent significant soft tissue contracture. During the third stage strategic incisions are made to elevate the skin and subcutaneous tissue off of the frontalis muscle, which remains adherent to and nourishes the skin graft. Cartilage grafts are added, the soft tissue is thinned, and the flap is draped down over the new framework. The pedicle may be divided in 3-4 weeks during a fourth and final stage[5,39]. This technique provides an ample source of customizable internal lining and pre-contracture prior to flap transfer, though it does necessitate an additional surgical stage. Pre-lamination may provide superior aesthetic results, less bulk and improved nasal function compared to free tissue transfer for large internal lining defects[39].
Subtotal nasal reconstruction
The PMFF has also been used for successful subtotal and total nasal reconstruction. Free tissue transfer, pre-lamination or a folded forehead flap may be used for multi-layer reconstruction[5,6,39,40]. Ziegler et al. describe using a 3D printed model created from the pre-resection CT to guide flap design in subtotal nasal reconstruction[39]. In this case, a pre-laminated forehead flap without tissue expansion was used.
CONCLUSION
The PMFF remains a robust workhorse for nasal reconstruction. It provides functionally and aesthetically favorable outcomes for moderate to large nasal defects with acceptable donor site morbidity. Recent modifications in forehead flap technique include single-stage flaps, two-stage flaps for complex major defects, ICG angiography-supported pedicle division at 1-2 weeks, pre-lamination for internal lining, and subtotal nasal reconstruction. These modifications expand the utility of the forehead flap in nasal reconstruction and may improve patient outcomes and reduce postoperative morbidity.
DECLARATIONS
Authors’ contributions
Conceptualization, data curation, data review, data analysis, content, writing-draft, writing-editing and review, writing-final, supervision: Longino ES, Most SP
Figure creation: Most SP
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
Both authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Consent has been gained for all images and no Institutional Review Board (IRB) approval is required. Informed consent has been obtained from patients.
Consent for publication
Written informed consent for publication has been obtained for all patient images.
Copyright
© The Author(s) 2025.
REFERENCES
2. Park SS. Reconstruction of nasal defects larger than 1.5 centimeters in diameter. Laryngoscope. 2000;110:1241-50.
3. Correa BJ, Weathers WM, Wolfswinkel EM, Thornton JF. The forehead flap: the gold standard of nasal soft tissue reconstruction. Semin Plast Surg. 2013;27:96-103.
5. Arianpour K, Byrne PJ. Nasal lining reconstruction with prelaminated forehead flap. Facial Plast Surg Clin North Am. 2024;32:239-46.
6. Aliotta RE, Meleca J, Vidimos A, Fritz MA. Free vascularized fascia lata flap for total columella reconstruction. Am J Otolaryngol. 2022;43:103226.
7. Howard BE, Patel S, Shockley WW, Clark JM. Total nasal reconstruction: advances in free tissue transfer for internal lining and structural support. Facial Plast Surg Clin North Am. 2024;32:247-59.
8. Menick FJ. Aesthetic refinements in use of forehead for nasal reconstruction: the paramedian forehead flap. Clin Plast Surg. 1990;17:607-22.
9. Conduto D, Antunes de Almeida A, Pinto A, Nixon Martins A, Pinheiro C. Healing by secondary intention or skin grafting for the paramedian forehead flap donor area. Cureus. 2025;17:e77076.
10. Little SC, Hughley BB, Park SS. Complications with forehead flaps in nasal reconstruction. Laryngoscope. 2009;119:1093-9.
11. Ji J, Alexander N, Enin K, Spataro E. Factors associated with outcomes of facial reconstruction after mohs micrographic surgery. Craniomaxillofac Trauma Reconstr. 2024;17:NP131-7.
12. Chen CL, Most SP, Branham GH, Spataro EA. Postoperative complications of paramedian forehead flap reconstruction. JAMA Facial Plast Surg. 2019;21:298-304.
13. Wu SS, Patel V, Oladeji T, Knackstedt R, Gastman B. Development of a risk prediction model for complications following forehead flaps for nasal and periorbital reconstruction. J Craniofac Surg. 2023;34:362-7.
14. DeSisto NG, Arnaud EH, Chowdhury N, et al. Forehead flap practices: a cross-sectional survey of facial plastic and reconstructive surgeons. Facial Plast Surg Aesthet Med. 2024;26:256-61.
15. Menick FJ. A 10-year experience in nasal reconstruction with the three-stage forehead flap. Plast Reconstr Surg. 2002:109;1856-61.
17. Stahl AS, Gubisch W, Haack S, Meisner C, Stahl S. Aesthetic and functional outcomes of 2-stage versus 3-stage paramedian forehead flap techniques: a 9-year comparative study with prospectively collected data. Dermatol Surg. 2015;41:1137-48.
18. Palmer WJ, Amin D, Kaki P, et al. Outcomes and complications of 2-stage versus 3-stage paramedian forehead flaps. Otolaryngol Head Neck Surg. 2025;172:1888-96.
19. Santos Stahl A, Gubisch W, Fischer H, Haack S, Meisner C, Stahl S. A cohort study of paramedian forehead flap in 2 stages (87 flaps) and 3 stages (100 flaps). Ann Plast Surg. 2015;75:615-9.
20. Woodard CR, Most SP. Intraoperative angiography using laser-assisted indocyanine green imaging to map perfusion of forehead flaps. Arch Facial Plast Surg. 2012;14:263-9.
21. Eskiizmir G, Tanyeri Toker G, Ozgur E, Tarhan S, Cengiz Ozyurt B. Hemodynamic changes in paramedian forehead flap. J Craniofac Surg. 2018;29:159-62.
22. Colwell AS, Craft RO. Near-infrared spectroscopy in autologous breast reconstruction. Clin Plast Surg. 2011;38:301-7.
23. de Weerd L, Mercer JB, Setså LB. Intraoperative dynamic infrared thermography and free-flap surgery. Ann Plast Surg. 2006;57:279-84.
24. Singer R, Lewis CM, Franklin JD, Lynch JB. Fluorescein test for prediction of flap viability during breast reconstructions. Plast Reconstr Surg. 1978;61:371-5.
25. Hölzle F, Loeffelbein DJ, Nolte D, Wolff KD. Free flap monitoring using simultaneous non-invasive laser Doppler flowmetry and tissue spectrophotometry. J Craniomaxillofac Surg. 2006;34:25-33.
26. Abdelwahab M, Kandathil CK, Most SP, Spataro EA. Utility of indocyanine green angiography to identify clinical factors associated with perfusion of paramedian forehead flaps during nasal reconstruction surgery. JAMA Facial Plast Surg. 2019;21:206-12.
27. Calloway HE, Moubayed SP, Most SP. Cost-effectiveness of early division of the forehead flap pedicle. JAMA Facial Plast Surg. 2017;19:418-20.
28. Surowitz JB, Most SP. Use of laser-assisted indocyanine green angiography for early division of the forehead flap pedicle. JAMA Facial Plast Surg. 2015;17:209-14.
29. Rudy SF, Abdelwahab M, Kandathil CK, Most SP. Paramedian forehead flap pedicle division after 7 days using laser-assisted indocyanine green angiography. J Plast Reconstr Aesthet Surg. 2021;74:116-22.
30. Somoano B, Kampp J, Gladstone HB. Accelerated takedown of the paramedian forehead flap at 1 week: indications, technique, and improving patient quality of life. J Am Acad Dermatol. 2011;65:97-105.
31. Hsu FY, Hsiao YC, Su YJ, Chang CS, Yen CI. A prospective study of psychological adjustment during and after forehead flap nasal reconstruction. J Craniomaxillofac Surg. 2024;52:692-6.
32. Park SS. The single-stage forehead flap in nasal reconstruction: an alternative with advantages. Arch Facial Plast Surg. 2002;4:32-6.
33. Park SS. Revisiting the single-stage forehead flap in nasal reconstruction. JAMA Facial Plast Surg. 2013;15:383-4.
34. Longino ES, Kandathil CK, Most SP. Comparing perfusion of single-stage and multi-staged paramedian forehead flaps using indocyanine green angiography. Facial Plast Surg Aesthet Med. 2025;27:357-60.
35. Burget GC, Menick FJ. The subunit principle in nasal reconstruction. Plast Reconstr Surg. 1985;76:239-47.
36. Gassner H, Sadick H, Haubner F, Artinger V, Kuehnel T. Prelamination to reconstruct internal nasal lining. Facial Plast Surg. 2013;29:411-6.
37. Griffin GR, Chepeha DB, Moyer JS. Interpolated subcutaneous fat pedicle melolabial flap for large nasal lining defects. Laryngoscope. 2013;123:356-9.
38. Okland TS, Akkina SR, Perrault D, Most SP. The single-stage melolabial flap for internal lining of full thickness defects of the nasal ala. Am J Otolaryngol. 2023;44:103804.
39. Ziegler JP, Oyer SL. Prelaminated paramedian forehead flap for subtotal nasal reconstruction using three-dimensional printing. BMJ Case Rep. 2021;14:e238146.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Special Topic
Copyright
Data & Comments
Data

















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].