Novel penile prostheses for transmen: surgical outcomes of ZSI FTM penile implants after musculocutaneous latissimus dorsi neophalloplasty
Abstract
Aim: One of the main goals of neophalloplasty is to provide the possibility of penetrative sexual intercourse. For individuals undergoing neophalloplasty, the implantation of malleable or inflatable penile prostheses is currently the main option for achieving reliable erectile function. The aim of this study was to assess surgical outcomes after the implantation of specially designed malleable and inflatable penile implants for transgender men, following musculocutaneous latissimus dorsi free flap neophalloplasty.
Methods: Between January 2022 and January 2025, 20 transgender men underwent ZSI FTM 475 or ZSI FTM 100 penile prostheses insertion following musculocutaneous latissimus dorsi free flap neophalloplasty. The dorsal approach was used in all cases for prostheses insertion and plate fixation to the pubic periosteum. The space for the prosthesis was created in the neophallus, behind the muscle. For inflatable prostheses, the pump was placed into the left hemiscrotum and connected to the reservoir in the left inguinal region. Patient satisfaction was evaluated using the International Index of Erectile Function (IIEF).
Results: The mean follow-up period was 21 months (range 4-36 months), and the mean interval between neophalloplasty and prosthesis implantation was 44 months (range 6-120 months). No intraoperative complications occurred. The most significant complication observed was fracture of the penile implant (n = 2, 10%). All patients who engaged in penetrative sexual intercourse reported satisfactory penetration, with a mean IIEF score of 21.1.
Conclusion: These novel penile prostheses for transgender men demonstrate favorable surgical outcomes after latissimus dorsi neophalloplasty, with a high rate of patient satisfaction.
Keywords
INTRODUCTION
There are various surgical techniques for neophalloplasty in transgender men, each with its own advantages and disadvantages. A primary goal of neophalloplasty is to enable penetrative sexual intercourse. Since the neophallus lacks native erectile tissue, implantation of a penile prosthesis is necessary to obtain erectile rigidity. At our center, the musculocutaneous latissimus dorsi (MLD) free flap technique is the method of choice as it provides a neophallus with excellent volume and length, enabling safe penile prosthesis implantation. Its main drawback, however, is the lack of sensation in the neophallus[1-3].
Neophalloplasty is a multi-stage procedure; prosthesis placement is usually the last step in patients who require urethroplasty. Insertion of the penile implants is performed at least six months after the initial neophalloplasty to ensure adequate tissue healing and maturation before proceeding with further surgical intervention[2]. Although multiple techniques exist for prosthesis placement, current literature has not demonstrated the superiority of any one approach over others[4]. Device selection is often based on a combination of patient-specific factors, surgeon preference, and device availability. In neophalloplasty, erectile devices are associated with high complication rates that may directly compromise the neophallus[5]. Common complications include infection, migration, and protrusion, often resulting in prosthesis rejection and removal. One of the reasons for frequent complications is the lack of supportive tissue in the neophallus that would normally cover the implant, as is the case with cavernosal bodies in cismen. Until recently, only penile prostheses designed for cismen were available for transgender men, making high complication rates inevitable.
Despite numerous described techniques, no universal solution exists to achieve ideal penile rigidity in transgender men[2-5]. Even with significant surgical advances and improved knowledge, complications remain frequent. Therefore, the search for better solutions continues. Recently, penile implants specifically designed for transmen have been developed[6].
This study aimed to assess the surgical outcomes of novel malleable and inflatable penile prostheses specifically designed for transgender men, following MLD neophalloplasty.
METHODS
This retrospective, observational, descriptive study included all patients who underwent insertion of the specifically designed penile prostheses after primary MLD neophalloplasty at our center between January 2022 and January 2025, yielding a total of 20 patients. Patients who underwent other types of neophalloplasty or had a different type of prosthesis inserted were excluded. The mean age was 34.3 years (range 23-58). Data were collected from medical records and outpatient examinations, including patient demographics, age at surgery, type of surgical correction, complication rates, and sexual function. The dorsal neophallic approach was used in all cases.
The study was approved by the institutional review board, and informed consent was obtained from all patients.
Sexual function was assessed using the International Index of Erectile Function (IIEF) questionnaire, which evaluates five domains: erectile function, orgasmic function, sexual desire, intercourse satisfaction, and overall satisfaction[7].
Descriptive statistical analyses were performed using IBM® SPSS® Statistics 20.
Erectile devices
While a variety of penile implants are available, devices specifically designed for transgender men are limited. The differences between conventional penile implants and those tailored for transgender patients are summarized in Table 1[8-13].
Comparison of penile prostheses devices
| Feature | ZSI 100 FTM (malleable)[10] | ZSI 475 FTM (inflatable)[11,12] | Traditional malleable[13] | Traditional inflatable[8,9] |
| Base plate | Silicone-covered stainless steel | Silicone-covered stainless steel | N/A* | N/A* |
| Glans shape | Ogive-shaped removable glans stopper | Realistic glans shape | Cylindrical | Cylindrical |
| Size/Adjustability | Adjustable length (130-200 mm) | Multiple sizes available | Fixed length | Variable by cylinder size |
| Components | One-piece cylinder with fixation plate | 3-piece (cylinder, pump, reservoir) | Two separate cylinders | Cylinder, pump, reservoir |
| Primary application | Transgender men undergoing neophalloplasty | Transgender men undergoing neophalloplasty | Cisgender men with ED | Cisgender men with ED |
Malleable penile implant (ZSI 100 FTM)
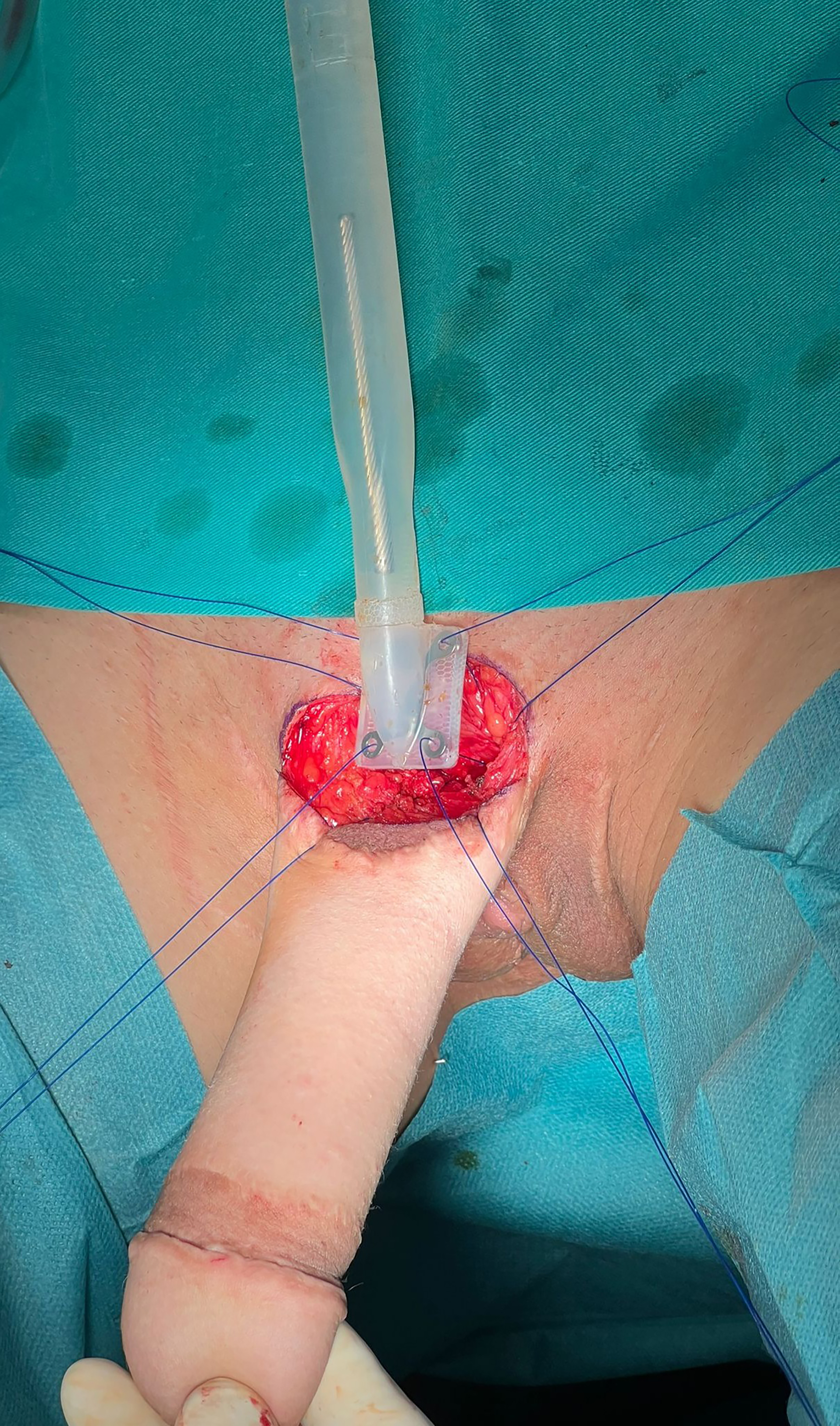
The ZSI 100 FTM is a one-piece malleable penile implant consisting of an adjustable cylinder, 22 mm in diameter, which can be shortened from 200 to 130 mm by sequentially trimming 5 mm from the distal end (ZSI 100 FTM). The distal tip features an ogive-shaped removable glans stopper. At the base of the cylinder, a silicone-coated, stainless-steel plate allows the prosthesis to be anchored to the pubic bone [Figure 1][10]. Another variant, the ZSI 100 D13 FTM, has a 13 mm diameter and can be adjusted up to 220 mm in length[10].
Inflatable penile implant (ZSI 475 FTM)
The ZSI 475 FTM is a 3-piece inflatable penile implant consisting of a cylinder with a realistic glans shape, a fixation plate at the base, a testicle-shaped pump, and a reservoir. In this study, the penile prosthesis comprises a single cylinder engineered to provide both stability and optimal cosmetic outcomes in both inflated and deflated states[11]. The device is available in four lengths - 120, 150, 180, and 210 mm - and has diameters ranging from 20 to 22 mm. Size selection was individualized based on patient anatomy and surgical planning to ensure proper fit and function. The fixation plate, made of silicone-coated stainless steel, allows the prosthesis to be anchored to the pubic bone[11,12].
Surgical candidacy
The implantation of a penile prosthesis is the final stage in neophallus reconstruction, providing neophallic rigidity. This procedure should be performed at least six months after the first stage to allow complete tissue healing.
A limitation of MLD free flap neophalloplasty is the limited tactile sensation of the neophallus, despite coaptation of the thoracodorsal nerve (primarily motor) to the ilioinguinal nerve (primarily sensory, with some motor fibers), which is a standard step in the procedure. Sensation is restricted to the proximal segment of the neophallus because the clitoris is incorporated at the base, and the urethra is formed via tubularization of the labia minora[1]. Hence, in this cohort, tactile sensation should not be used to guide the timing of surgery. The choice of penile prosthesis (ZSI vs. other devices) is influenced by several factors, including patient preference, surgeon experience, and anatomical considerations. Our center generally prefers ZSI devices, as they are specifically designed for transgender men and are relatively easier to implant. However, in cases of specific anatomical challenges or if a patient has a strong preference for a non-ZSI device, alternative options are considered.
Preoperative care
Patients are advised to stop smoking at least 1 month prior to surgery. A negative urine culture is required. All patients are instructed to shave the pubic and genital area and to use antimicrobial soap the day before surgery.
Vancomycin is administered intravenously 30 min prior to anesthesia induction.
Surgical technique
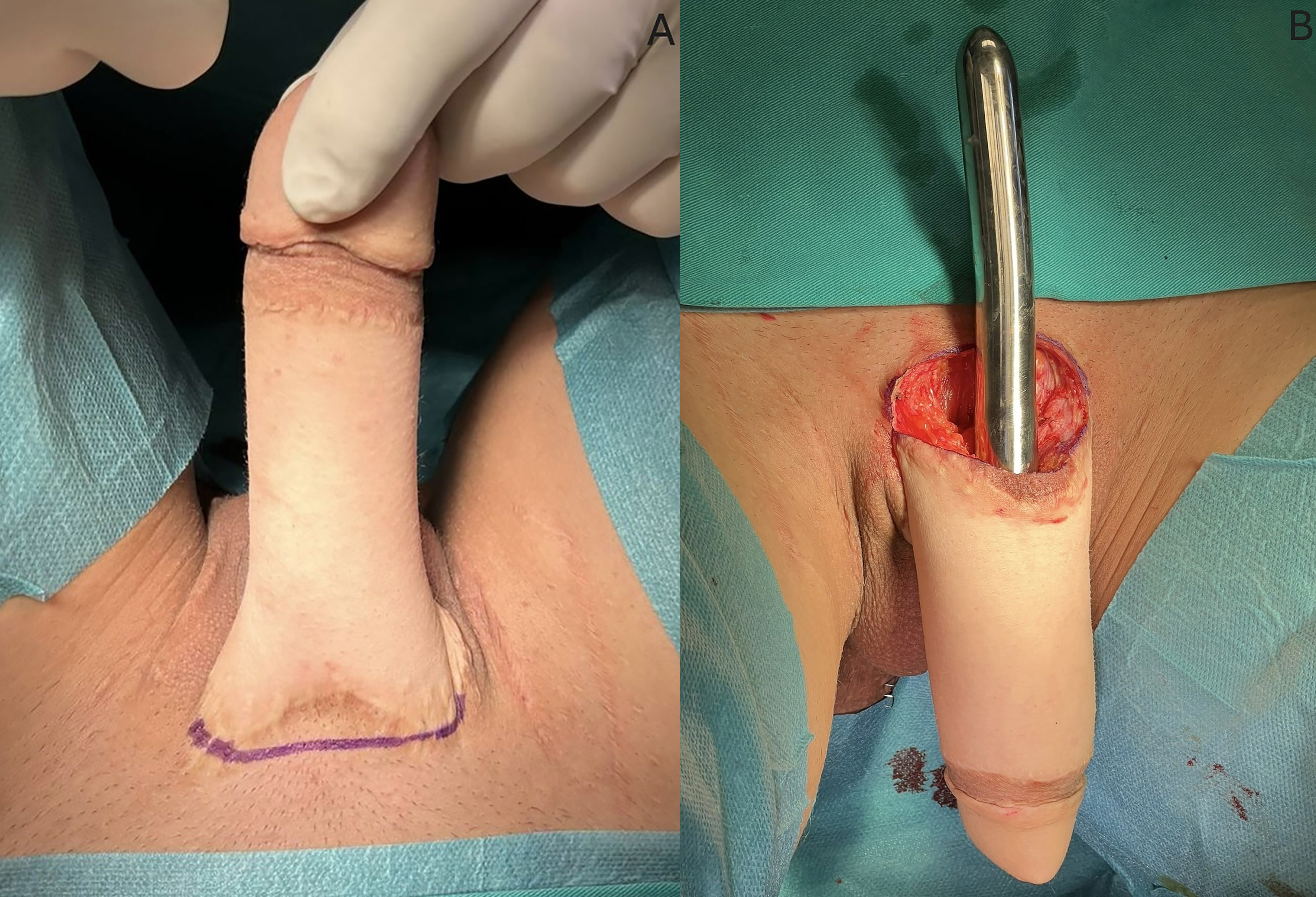
Patients are positioned supine. The genital region is scrubbed with chlorhexidine and povidone-iodine for
Figure 2. Approach for penile prostheses insertion in MLD neophalloplasty. (A) Semicircular incision marked on the dorsal aspect of the neophallus; (B) Adequate space for the penile implant created with proper dilation, reaching the tip of the neophallus. MLD: Musculocutaneous latissimus dorsi.
In cases with excessive girth or length of the neophallus, several strategies are considered, including shortening the neophallus, or reshaping the base of the neophallus simultaneously with its width. The specific approach is tailored to the individual patient’s anatomy and desired outcome.
Malleable prosthesis
The prosthesis base is anchored to the pubic symphysis with four interrupted non-resorbable monofilament sutures through the periosteum. The cylinder is then inserted into the neophallus. No drain is placed. The incision is closed in layers using absorbable sutures. A compressive dressing is applied, and the neophallus is fixed to the inferior abdominal wall.
Inflatable prosthesis
A hemiscrotal space is created through the same infrapubic incision on the contralateral side of the vascular pedicle using blunt dissection. In cases with a previously placed testicular implant, the implant is removed, and a new space is created next to the capsule.
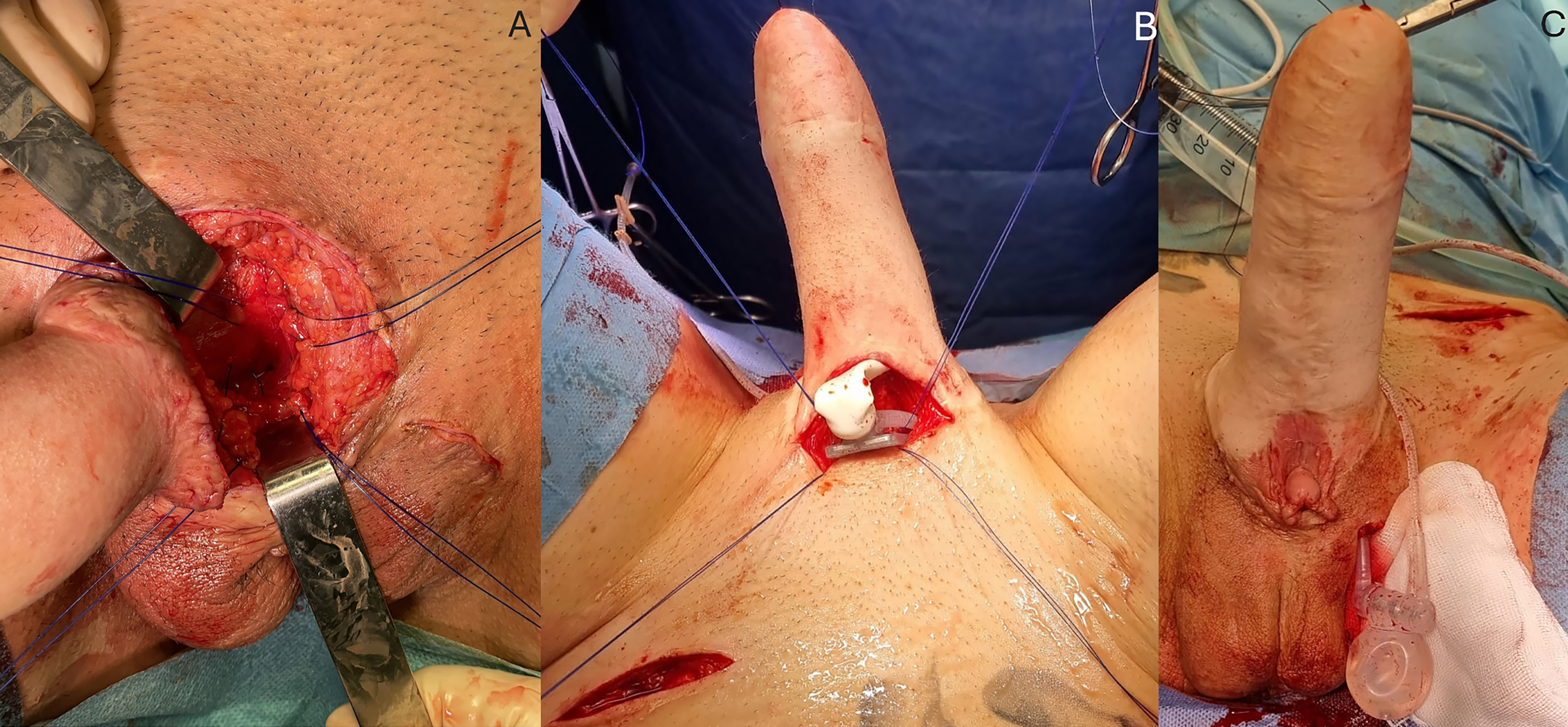
A second horizontal incision is made in the left iliac fossa, followed by blunt dissection of the Retzius space. The reservoir is then inserted and filled with saline. The prosthesis plate is anchored to the pubic symphysis with four interrupted sutures through the periosteum [Figure 3A and B]. The pump is positioned in the previously created hemiscrotal pocket [Figure 3C]. The cylinder is then inserted into the neophallus [Figure 3B]. The reservoir tubing is connected to the pump and cylinder tubing. Inflation is tested to confirm the proper functioning of the prosthesis. The prosthesis is inflated to 60% to maintain the dissected space. The incisions are closed, and a compressive dressing is applied.
Figure 3. Inflatable penile implant placement. (A) The periosteum of the pubic symphysis is identified, and four sutures are placed for subsequent fixation; (B) The cylinder is inserted into the neophallus, and sutures are prepared for implant fixation; (C) The testicular implant in the left hemiscrotum is removed, and a pump is placed. The cylinder is inflated, with the neophallus in an erect state.
Postoperative care
The urethral catheter is removed the day after surgery, and the patient is discharged. Oral antibiotics (amoxicillin and metronidazole) are prescribed for five days. The first postoperative visit is scheduled one week following the surgery. For patients with an inflatable ZSI 475 FTM, the cylinder is maintained at 60% inflation for four weeks to preserve the dissected space. Patients with a malleable penile implant are instructed to hold the neophallus upward to the abdominal wall for four weeks. During the first two months, patients should avoid strenuous activities, heavy lifting, and vigorous exercise to facilitate initial healing. Sexual activity is prohibited for two months.
Patient satisfaction
Currently, there is no validated, patient-reported outcome measure specifically for transmen, so surgical outcomes must be interpreted with caution. However, sexual function following prosthesis implantation was evaluated using the validated IIEF questionnaire[13].
RESULTS
The mean follow-up was 21 months (range 4-36) [Table 2]. Malleable ZSI 100 FTM and inflatable ZSI 475 FTM penile prostheses were implanted in 15 (75%) and 5 (25%) patients, respectively. In one case, a ZSI 475 FTM was implanted following the explanation of a prior inflatable prosthesis designed for cismen. The standard ZSI 100 FTM was placed in 12 patients, while the ZSI 100 D13 variant was used in 3 patients. Among the ZSI 475 FTM implants, 150 mm prostheses were used in 3 patients, and 180 mm prostheses in 2 patients. No modifications to the prosthesis glans cap were required for any of the patients.
Patient characteristics
| Patient characteristics and procedural variables | Values |
| Median (IQR) age, years | 34.3 (23-58) |
| Median (IQR) follow-up, months | 21 (4-36) |
| Comorbidities, n (%) - Smoking - Diabetes | 4 (20) 1 (5) |
| Type of neophalloplasty, n (%) - MLD free flap | 20 (100) |
| Prosthesis Implanted, n (%) - Malleable ZSI 100 FTM - Inflatable ZSI 475 FTM | 15 (75) 5 (25) |
| Prior erectile device, n (%) - AMS Ambicor | 1 (5) |
| Median (IQR) time between neophalloplasty and implantation, months | 44 (6-120) |
The median time from neophalloplasty to prosthesis implantation was 44 months (range 6-120 months). No intraoperative complications or issues with glans dilatation occurred. In this type of phalloplasty, the vascular bundle passes to the right of the pubic symphysis toward the groin; therefore, the pump is placed in the contralateral hemiscrotum to avoid potential vascular injury.
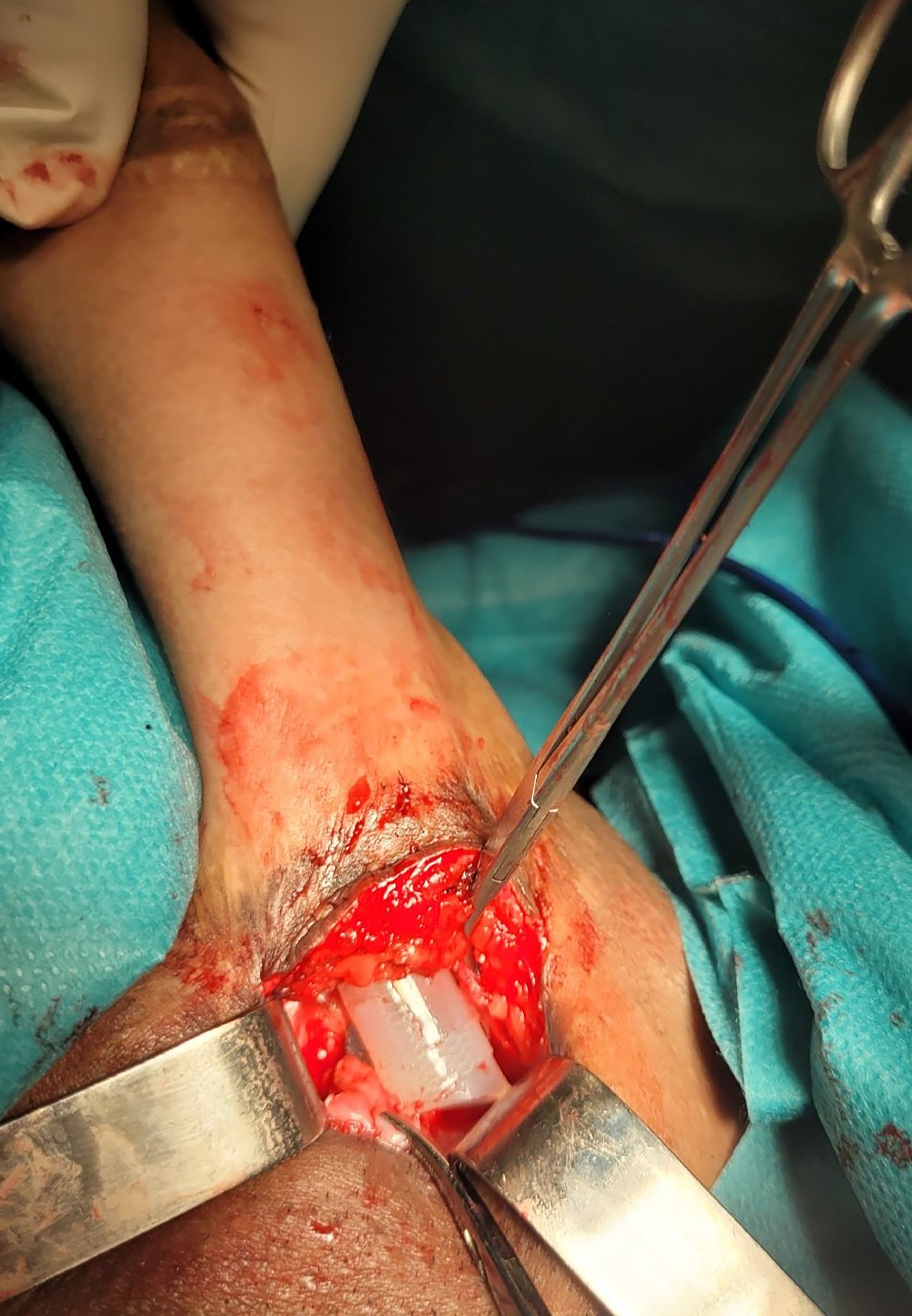
There were 6 cases (30%) with postoperative complications in total [Table 3]. Three cases (15%) of wound dehiscence were successfully treated conservatively. Three additional cases (15%) required revision surgery: two due to implant fracture and one due to implant detachment. Implant fractures occurred 6 and 9 months after implantation [Figure 4]. In this follow-up period, no complications such as rejection, protrusion, erosion, malfunction, or infection were encountered.
Figure 4. Revision surgery via the same dorsal approach revealed a fracture of the malleable penile implant.
Implant characteristics and outcomes
| Outcome variables | Values | Time to revision surgery post-prostheses insertion, months |
| Size of implanted prosthesis, n (%) Malleable - ZSI 100 FTM - ZSI 100 FTM D13 Inflatable - ZSI 475 FTM 150 mm - ZSI 475 FTM 180 mm | 12 (80) 3 (20) 3 (60) 2 (40) | |
| Median (IQR) duration of surgery, min | 65 (45-75) | |
| Urethral catheter used during surgery, n (%) | 20 (100) | |
| Postoperative complications conservatively treated, n (%) - Wound dehiscence | 3 (15) | |
| Postoperative complications requiring revision surgery, n (%) - Penile implant fracture - Detachment of the penile implant | 2 (10) 1(5) | - 6 and 9 - 16 |
In those two cases with penile implant fracture, we had initially inserted ZSI 100 D13. Patients requiring revision received new penile implants, all of which were replaced with the ZSI 100 FTM device. One patient in this group requested removal of the penile implant, expressing a preference to avoid additional surgery in case of future complications. In one patient (5%), prosthesis detachment occurred 16 months after the primary implantation, requiring an additional surgical procedure for refixation. In this study group, three cases (15%) experienced mild complications (Grade I according to the Clavien-Dindo classification), including partial wound dehiscence, which was managed conservatively. In one case, there were some difficulties with pump manipulation, which were resolved through reinforced patient training after confirming that there was no mechanical failure of the device. No complications related to the MLD flap or any other further complications developed.
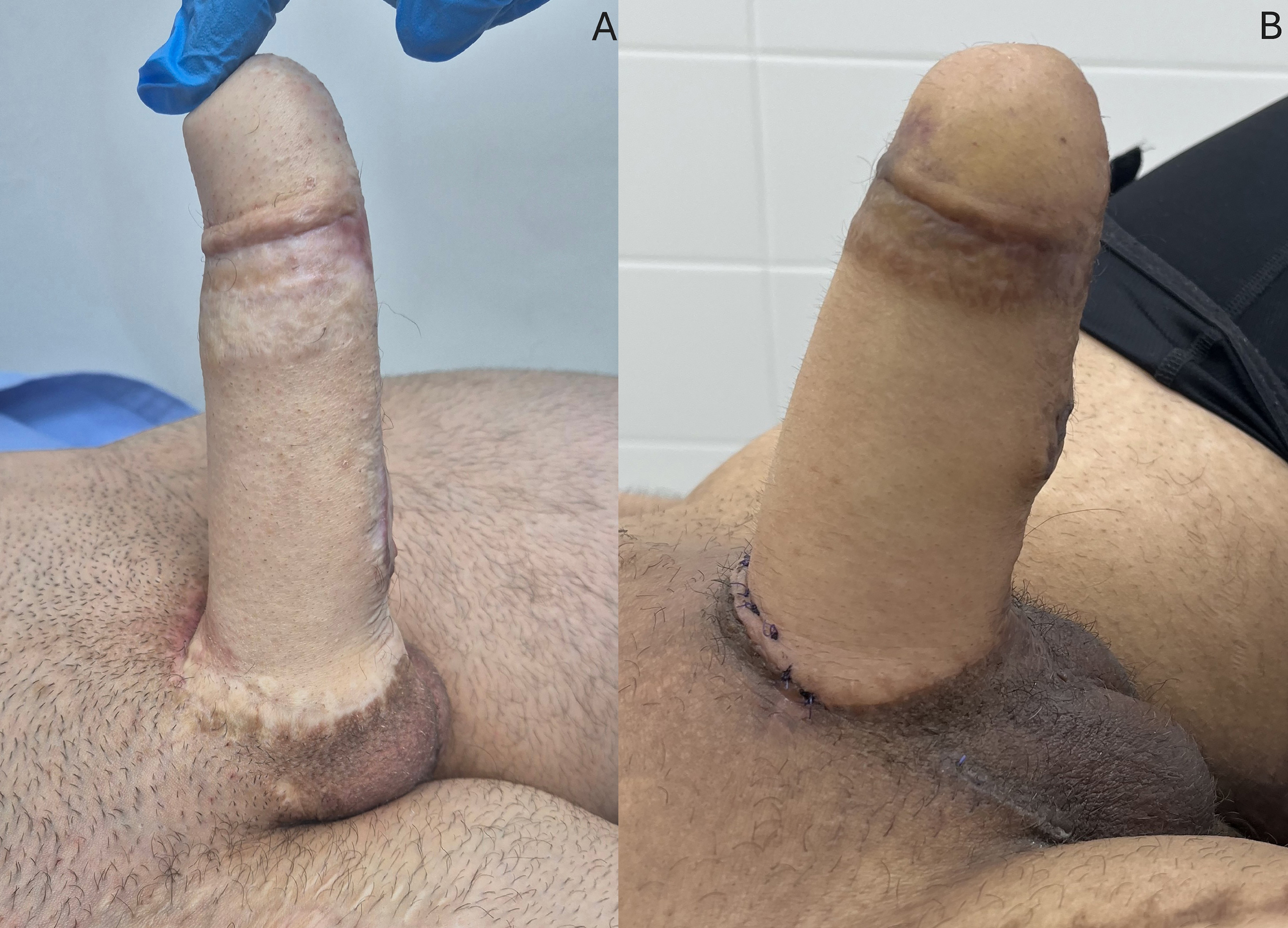
Intercourse with complete penetration was feasible in all cases. All patients completed the IIEF questionnaires (100%) and reported satisfactory penetration, with a mean IIEF of 21.1. Average scores were 21.3 (range 19-26) in the erectile function domain and 8.7 (range 7-10) in the overall satisfaction domain. Three patients (15%) scored below 21 in the erectile function domain, indicating erectile dysfunction. At the end of the follow-up, 19 patients (95%) still had prostheses, with an overall revision rate of 15% [Figure 5A and B].
DISCUSSION
Adequate erectile function is an important aspect of many men´s quality of life[14-16]. Consequently, achieving penetrative sexual function is a key surgical goal for transmen undergoing neophalloplasty[16]. An erectile device is usually necessary to attain this goal; however, it is associated with a high complication rate[16,17]. Transgender men and surgeons dealing with these shared goals have to grapple with complications that jeopardize the patient’s quality of life.
Recognizing the specific needs of transmen, specific erectile devices were developed in the form of a single cylinder device with a fixation plate, which better conforms to the anatomy of a neophallus[6].
This study assessed the initial surgical outcomes after implantation of the malleable ZSI 100 FTM and the inflatable ZSI 475 FTM prostheses in transgender men after MLD neophalloplasty. Several previous studies have reported outcomes for prostheses specifically designed for transgender men following radial free forearm flap and anterolateral thigh flap neophalloplasty[14,18-20]. However, no information is available on the implantation of these innovative erectile devices after MLD free flap neophalloplasty.
In a native penis, overall complication rates (infection, erosion or device dysfunction) for penile prosthesis implantation are < 2%, significantly lower than the rates observed in neophallus implants[21]. In transgender men, studies of penile prostheses designed for cismen following neophalloplasty report overall complication rates of 23.9%-47.6%, while the rates for transmen-specific prostheses were 28.4%-44%[15,22,23].
Challenges in penile prosthesis implantation in the neophallus begin with understanding the unique anatomy, which guides the selection of the appropriate erectile device and the safest approach to minimize potential intraoperative complications. Inadequate supportive tissue compared to the native penile anatomy is a major contributor to severe and devastating complications after erectile device implantation in transgender men.
Unlike a native penis, the neophallus lacks corpora cavernosa and tunica albuginea (native erectile tissue), structures generally used for prosthesis fixation and coverage. The ZSI 100 and ZSI 475 FTM devices address this anatomical difference with a fixation plate at the base, allowing anchoring to the pubic bone.
Malpositioning with transmen-specific erectile devices has been reported in 4.7%-7% of cases, compared to 12.6%-14.6% for prostheses developed for cismen[17,19,22]. In this series, there was one case of prosthesis detachment with malpositioning and two cases of implant fracture.
Reported revision rates range from 19%-44% for transmen-specific devices and 19%-43.4% for cismen devices[17-27]. However, follow-up periods were considerably shorter in studies of transmen-specific prostheses (6.3-16 months) than in studies of devices designed for cismen (20-48 months)[17-27].
In contrast to an encouragingly low number of complications related to malpositioning and cylinder protrusions, infection rates after prosthesis implantation in a neophallus, either with standard (4.2%-11.9%) or transmen-specific (12%-14.2%) devices, are higher compared with the 1.1% infection rate observed after prosthesis implantation in a native penis[25-28]. The restricted vascular supply and significant scarring associated with neophallus free flaps hinder the healing process and increase infection risk[6]. No infections were observed during the follow-up period in our study.
In a recent study by Marchand et al., a high complication rate of 53.1% was reported following ZSI475 FTM implantation[14]. However, their study primarily evaluated outcomes after inflatable penile implant insertion, whereas in our cohort, most of the patients underwent implantation of malleable penile prostheses. Consequently, we observed a lower overall complication rate. Marchand et al. also reported a high rate of urethral complications (71%) and noted a correlation between urethral and mechanical complications. The discrepancy in complication rates between the studies may be attributable to differences in surgical technique and study populations: Marchand et al.’s cohort primarily underwent radial forearm free flap neophalloplasty (56.2%), whereas all patients in our study underwent MLD free flap neophalloplasty, with other types of neophalloplasty excluded. Marchand et al. (2025) additionally described improvements in implant design, including tubing reinforcement and adjustments to the anchoring plate, which may contribute to reduced mechanical dysfunction. The lower complication rates observed in our study may also reflect the inherent advantages of the MLD flap, which provides substantial neophallus volume and muscular support for the implanted prosthesis, potentially reducing stress on the device and surrounding tissues. Implant placement behind the muscle further minimizes the risk of urethral injury. Nonetheless, detachment of the penile cylinder remains a significant concern. It is important to acknowledge that our study’s smaller sample size (n = 20) and shorter follow-up period, compared to Marchand et al.’s larger cohort (n = 89), may limit the detection of less frequent or delayed complications. Despite differences in complication rates, both studies highlight the role of penile prosthesis implantation in achieving satisfactory sexual function for transmen following neophalloplasty. Longer-term studies with larger cohorts are needed to fully elucidate the long-term outcomes of ZSI 475 FtM implantation.
Evaluation of patient satisfaction is limited by the lack of validated questionnaires designed specifically for this population. In this study, we used the IIEF, a commonly used questionnaire for evaluating sexual satisfaction. While informative, the IIEF may not fully capture the nuances of the transgender experience. Since this study was initiated, the GENDER-Q - a trans-specific series of patient-reported outcome measures (PROMs) has been developed and could provide a more comprehensive assessment in future studies[29]. Most previous studies (66%) have reported results based on non-validated questionnaires[16,17,30]. In our cohort, a median IIEF score of 21.1 was recorded, which is consistent with normal erectile function. Additional analyses and longer-term follow-up are needed to obtain more information about satisfaction and long-term complications following this complex reconstructive procedure.
Limitations of this study include its retrospective design, small sample size, absence of a standardized, validated questionnaire, and lack of a control group, which restricts comparative analyses. Long-term outcomes remain to be determined.
Overall, penile prostheses specifically designed for transmen in our cohort appeared to have enabled favorable surgical outcomes and high patient satisfaction. MLD free flap neophalloplasty provided adequate neophallus volume and sufficient blood supply for safe penile prosthesis implantation. The most significant complication observed was penile implant fracture. Long-term studies are needed for further analysis.
DECLARATIONS
Acknowledgements
All figures were created by Professor Miroslav Djordjevic and his team at the Belgrade Center for Urogenital Reconstructive Surgery.
Authors’ contributions
Contributed substantially to the conception and design of the study and performed data analysis and interpretation: Djordjevic ML, Bencic M, Yepes C
Provided administrative, technical, and data acquisition support: Bizic M, Pusica S, Stojanovic B
Drafted the work and substantively revised it, contributed substantially to the conception and design of the study: Stojanovic B, Bizic M, Djordjevic ML
Availability of data and materials
All the data and materials used in our study are available from the corresponding author upon reasonable request.
Financial support and sponsorship
None.
Conflicts of interest
Djordjevic ML is an Editorial Board member of Plastic and Aesthetic Research and a Guest Editor of the Special Issue “The Clinical Complexities and Ingenious Solutions within Gender-Affirming Surgery.” Bencic M and Stojanovic B are Guest Editors of the same Special Issue. Djordjevic ML, Bencic M and Stojanovic B were not involved in any aspect of the editorial process for this manuscript, including reviewer selection, manuscript handling, or decision making. The other authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Belgrade Center for Urogenital Reconstructive Surgery (No. 2233/XI - 16, 2024). Written informed consent was obtained from all participants to ensure adherence to the ethical standards for research involving human subjects.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Djordjevic ML, Bencic M, Kojovic V, et al. Musculocutaneous latissimus dorsi flap for phalloplasty in female to male gender affirmation surgery. World J Urol. 2019;37:631-7.
2. Rezaei SJ, Miller AS, Miranda N, Ganor O. Gender-affirming surgeries for transgender and gender diverse individuals and associated health outcomes. Behav Sci Law. 2024;42:588-606.
3. Dennis M, Granger A, Ortiz A, Terrell M, Loukos M, Schober J. The anatomy of the musculocutaneous latissimus dorsi flap for neophalloplasty. Clin Anat. 2018;31:152-9.
4. Lee WG, Christopher N, Ralph D. Phalloplasty: which technique should I choose? J Sex Med. 2025;22:554-5.
5. Alba B, Nolan IT, Weinstein B, et al. Gender-affirming phalloplasty: a comprehensive review. J Clin Med. 2024;13:5972.
6. ZSI. Gender affirmation surgery: options and medical innovations for transgender individuals. Available from: https://zephyr-surgical-implants.webflow.io/pathologies-trans-solutions/transgender-solutions. [Last accessed on 28 Sep 2025].
7. Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49:822-30.
8. Boston Scientific. AMS 700 inflatable penile prosthesis. Available from: https://www.ncbi.nlm.nih.gov/books/NBK547852/. [Last accessed on 28 Sep 2025].
9. Coloplast. Titan® penile implant. Available from: https://iu.coloplast.us/products/titan-penile-implants/. [Last accessed on 28 Sep 2025].
10. ZSI-100-FTM surgeon documents. Available from: https://web.zsimplants.ch/products/zsi-100-ftm/zsi-100-ftm/zsi-100-ftm. [Last accessed on 30 Sep 2025].
11. Verla W, Goedertier W, Lumen N, et al. Implantation of the ZSI 475 FTM erectile device after phalloplasty: a prospective analysis of surgical outcomes. J Sex Med. 2021;18:615-22.
12. ZSI 475 FTM surgeons documents. Available from: https://web.zsimplants.ch/products/zsi-475-ftm/zsi-475-ftm/documents/surgeon. [Last accessed on 30 Sep 2025].
13. Coloplast. Genesis® malleable penile prosthesis. Available from: https://iu.coloplast.us/products/malleable-penile-prosthesis/. [Last accessed on 28 Sep 2025].
14. Marchand S, Morel-Journel N, Carnicelli D, Boucher F, Ruffion A, Neuville P. Surgical outcomes of the ZSI475 FtM inflatable erectile prosthesis implantation after phalloplasty. Urology. 2025;198:217-21.
15. Levine LA, Becher EF, Bella AJ, et al. Penile prosthesis surgery: current recommendations from the international consultation on sexual medicine. J Sex Med. 2016;13:489-518.
16. Zhang X, Neuville P, Skokan AJ. Sexual health in transgender and gender diverse people. Curr Opin Urol. 2024;34:330-5.
17. Pigot GLS, Sigurjónsson H, Ronkes B, Al-Tamimi M, van der Sluis WB. Surgical experience and outcomes of implantation of the ZSI 100 FtM malleable penile implant in transgender men after phalloplasty. J Sex Med. 2020;17:152-8.
18. Falcone M, Peretti F, Preto M, et al. The outcomes of inflatable penile prosthesis implantation in the context of genital gender affirming surgery in assigned female at birth patients: a comparative study between cis-male Dacron modified and Zephyr Surgical Implants 475 female to male penile prosthesis. Int J Impot Res. 2025;Epub ahead of print.
19. Neuville P, Morel-Journel N, Cabelguenne D, Ruffion A, Paparel P, Terrier JE. First outcomes of the ZSI 475 FtM, a specific prosthesis designed for phalloplasty. J Sex Med. 2019;16:316-22.
20. Fascelli M, Hennig F, Dy GW. Penile and testicular prosthesis following gender-affirming phalloplasty and scrotoplasty: a narrative review and technical insights. Transl Androl Urol. 2023;12:1568-80.
21. Carson CC 3rd, Mulcahy JJ, Harsch MR. Long-term infection outcomes after original antibiotic impregnated inflatable penile prosthesis implants: up to 7.7 years of followup. J Urol. 2011;185:614-8.
22. Falcone M, Garaffa G, Gillo A, Dente D, Christopher AN, Ralph DJ. Outcomes of inflatable penile prosthesis insertion in 247 patients completing female to male gender reassignment surgery. BJU Int. 2018;121:139-44.
23. Zuckerman JM, Smentkowski K, Gilbert D, et al. Penile prosthesis implantation in patients with a history of total phallic construction. J Sex Med. 2015;12:2485-91.
24. Gottlieb L, Cripps C. An update on gender-affirming phallus construction using the radial forearm free-flap. Neurourol Urodyn. 2023;42:963-72.
25. Pang KH, Christopher N, Ralph DJ, Lee WG. Insertion of inflatable penile prosthesis in the neophallus of assigned female at birth individuals: a systematic review of surgical techniques, complications and outcomes. Ther Adv Urol. 2023;15:17562872231199584.
26. Neuville P, Morel-Journel N, Maucourt-Boulch D, Ruffion A, Paparel P, Terrier JE. Surgical outcomes of erectile implants after phalloplasty: retrospective analysis of 95 procedures. J Sex Med. 2016;13:1758-64.
27. Sun HH, Isali I, Mishra K, et al. Surgical outcomes at a single institution of infrapubic insertion of malleable penile prosthesis in transmen. Urology. 2023;173:209-14.
28. Zhang TR, Harel D, Rivera A, et al. Incidence, complications, and long-term outcomes of gender-affirming phalloplasty: analysis of a large statewide population-based dataset. Urology. 2024;185:27-33.
29. Kaur MN, Morrison SD, Kennedy SL, et al. International study to develop a patient-reported outcome measure to evaluate outcomes of gender-affirming care - the GENDER-Q. J Patient Rep Outcomes. 2024;8:134.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Special Topic
Copyright
Data & Comments
Data


















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].