Technical considerations for neovaginal canal creation during primary and revision gender-affirming vaginoplasty
Abstract
A critical component of gender-affirming vaginoplasty is the creation of a functional neovaginal canal capable of penetrative intercourse. Canal creation is inherently challenging due to the deep pelvic anatomy and risk of injury to the urethra, bladder, and rectum. These challenges are amplified in revision cases undertaken to correct neovaginal stenosis or convert minimal-depth to full-depth canals.
With increasing interest in and access to gender-affirming surgery, myriad techniques for canal creation have arisen. These utilize a variety of donor tissues to line the canal, including genital and extragenital skin grafts, xenografts, allografts, peritoneum, and intestine. Moreover, the rectoprostatic dissection necessary for canal creation can be performed via a perineal approach or with robotic assistance. However, few comparative studies exist to inform the choice of surgical approach or donor tissue.
In this article, we briefly review the donor tissue options available for neovaginal canal creation, discuss the associated advantages and disadvantages, and reference pertinent contemporary research. We additionally describe our institutional approach for canal creation in primary and revision vaginoplasties, highlighting technical nuances for success.
Keywords
INTRODUCTION
Gender-affirmation surgery comprises a host of procedures that reshape patients’ physical characteristics to reflect their gender identity. Feminizing gender-affirming procedures include breast augmentation, facial and vocal feminization, and genital reconstruction via vulvoplasty or vaginoplasty. While historically a minority of patients undergoing a transition proceeded to feminizing genital surgery, high satisfaction rates and the increasing availability and coverage of gender-affirming care have facilitated patients’ interest and access[1].
A critical aspect of gender-affirming vaginoplasty is the creation of a functional neovaginal canal suitable for penetrative intercourse. The ideal neovagina is elastic, moist, and hairless; prior studies have suggested a minimum depth of ≥ 10 cm and diameter of ≥ 3 cm[2,3]. Neovaginal canal dissection is challenging due to the deep perineal and pelvic anatomy, difficult exposure, and risk of injury to the adjacent urethra, bladder, and rectum. Gender-affirming surgeons employ various techniques to surmount these concerns[4].
In this article, we briefly review the tissue options available for creation of the neovaginal canal and describe our institutional practice for canal creation in primary and revision vaginoplasties. Other technical aspects of gender-affirming vaginoplasty include creation of a sensate clitoris, an aesthetic vulva, and an appropriately positioned feminine urethra with a downward-directed urinary stream. While equally important for a “successful” feminizing vaginoplasty, these fall outside the purview of this article, which will focus on neovaginal canal creation.
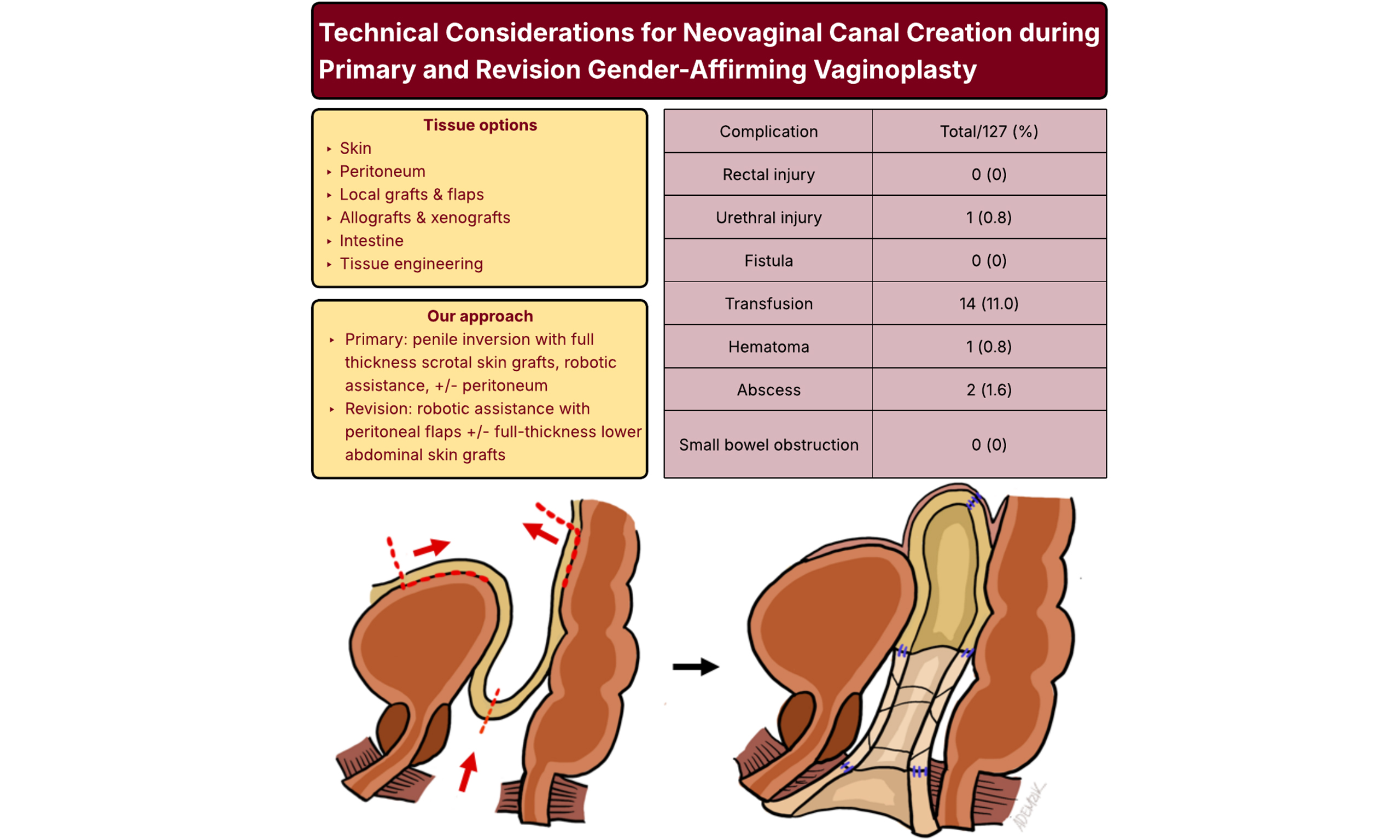
TISSUE OPTIONS FOR NEOVAGINAL CANAL CREATION
There are various donor tissues available to line the neovaginal canal during gender-affirming vaginoplasty. Choice of donor tissue must consider factors such as sensation, hair-bearing status, risk of secondary contracture, tissue availability, and donor site morbidity.
Skin
In 1957, Sir Harold Gillies pioneered early gender-affirming genital surgery via description of the penile inversion vaginoplasty (PIV), wherein the penis is deconstructed and a cylindrical inverted penile skin flap serves as the neovaginal canal[5]. The technique was later refined and popularized by French gynecologist Dr. Georges Burou[6]. Despite modifications over the subsequent decades, PIV and its variations represent a predominant technique for gender-affirming vaginoplasty.
The inverted penile skin flap is well-vascularized and sensate. However, most patients will have insufficient neovaginal canal depth with penile skin alone. While the mean total penile length is 12.9 cm, penile skin alone often falls short of the desired neovaginal depth of 12-14 cm because some length is lost traversing the distance between the base of the penis and the future introitus; moreover, the proximal penile skin is repurposed for vulvar definition[7]. Many authors utilize full-thickness skin grafts (FTSGs) to line the remaining mid- and proximal neovaginal canal; scrotal skin is an apt donor, as it would otherwise be discarded. A notable disadvantage of its use is the need for preoperative laser or electrolysis hair removal, which imposes an additional cost and time burden for patients[8,9]. Hair removal can also be undertaken intraoperatively via mechanical, adhesive, or electrocautery depilation[10-12]. However, there are no comparative data to guide the choice of technique in the setting of gender-affirming vaginoplasty, and in our experience, intraoperative depilation is both tedious and unreliable.
Some patients have deficient genital skin due to hormone suppression, prior orchiectomy or other genital surgery, circumcision, or obesity[13]. In such circumstances, the combination of a penile inversion flap and scrotal skin graft still yields a neovagina of suboptimal depth. Additional donor tissues such as extragenital skin, biologic grafts, and peritoneum can further augment the canal.
Extragenital skin grafts can be harvested from multiple sites such as the lower abdomen, groin, buttocks, and thigh. Non-hair-bearing skin is preferred to avoid later neovaginal hair growth, which can be cumbersome to address. While both split-thickness and full-thickness grafts are feasible, the latter are generally favored due to the lower risk of secondary contracture and subsequent neovaginal stenosis. Despite their utility, extragenital skin grafts incur the morbidity of additional donor sites and may result in bothersome surgical scars.
Peritoneum
The use of peritoneum to augment the neovaginal canal was first described by Davydov in 1969 for the vaginal reconstruction of patients with Mayer-Rokitansky-Küster-Hauser syndrome[14]. The technique has since been adapted for laparoscopic and robotic approaches; the latter represents a major contemporary technique for both primary and revision gender-affirming vaginoplasty[15,16].
In the robotic Davydov peritoneal flap vaginoplasty, flaps of peritoneum are mobilized anteriorly from the posterior surface of the bladder and posteriorly from the anterolateral surface of the rectum. The inferior aspects of the flaps are sewn directly to the edge of the inverted penile tube, whereas the apices of the flaps are sewn together; the rectovesical pouch is thus incorporated as the neovaginal apex and excluded from the remaining peritoneal cavity. An alternative approach described in the context of revision vaginoplasty is the tubularized urachus-peritoneal hinge flap, which mobilizes a single inferiorly based flap of peritoneum spanning the bladder to the umbilicus[17]. This can be folded posteriorly to form a “cap” of canal apex or a nearly completely peritoneum-lined vaginal canal.
Utilizing peritoneal tissue yields a well-vascularized, hairless neovaginal apex, supplements canal depth, and obviates the need for extragenital skin grafting in most cases. Peritoneum is a semi-permeable, secretory membrane that may contribute to passive neovaginal canal lubrication. Despite this theoretical benefit, a recent systematic review suggested that neither skin, peritoneum, nor colon yields functional neovaginal lubrication and that lubricating potential should not factor into the choice of tissue for neovaginal lining[18]. Peritoneum produces a basal fluid rate unresponsive to sexual stimulation; patients may find such fluid more akin to near-constant discharge than to true erogenous lubrication.
The role of peritoneal flaps in primary and revision vaginoplasties is debated. A disadvantage of primary peritoneal flap vaginoplasty is the addition of an intra-abdominal component to an otherwise perineal dissection, thereby introducing the risks associated with abdominopelvic surgery. Moreover, primary peritoneal flap vaginoplasty may complicate the use of peritoneum during revision vaginoplasty, severely limiting donor tissue options and potentially necessitating intestinal vaginoplasty, which carries additional morbidity[19]. In a recent study of 17 high-volume surgeons who perform at least 40 gender-affirming vaginoplasties per year, five surgeons in the cohort reported using peritoneal flaps or grafts, while only one surgeon utilized peritoneal flaps in more than 50% of cases[7].
Local grafts and flaps
Additional tissue to augment the neovaginal canal can be gained from local tissue flaps and grafts. Several authors report using tunica vaginalis grafts as adjuncts to scrotal skin for lining the neovaginal canal, although supporting data are sparse[20,21]. The tunica vaginalis, embryologically derived from the abdominal peritoneum, envelops the testis as it descends through the inguinal canal. In patients without prior orchiectomy, it can be harvested and used as a supplemental graft to avoid the morbidity of an extragenital harvest site or peritoneal flaps. In the only published description of its routine use, the authors report incorporating tunica vaginalis grafts in 51.6% of patients undergoing primary gender-affirming vaginoplasty[22]. Another option is incorporating the spatulated urethra as a portion of the neovaginal canal, which can yield additional width, lubrication, and sensation[23,24]. Some authors have also posited repurposing the scrotal skin as a pedicled flap to form the posterior neovaginal canal and apex, rather than early harvest and thinning for use as a FTSG[25,26].
Allografts, xenografts, and tissue engineering
A promising option for neovaginal canal augmentation is the use of xenografts and allografts. These provide additional possibilities for canal coverage while eschewing the morbidity and requisite hair removal of a donor site, though the associated costs and dearth of long-term outcomes remain points of concern.
Rodriguez et al. (2020) first described the use of Nile tilapia fish skin in lieu of scrotal skin grafts during gender-affirming vaginoplasty. Nile tilapia skin is a viable option to scaffold epithelialization of the neovaginal canal due to its non-infectious microbiota, structural similarity to human skin, and good in vivo bio-resorption[27]. Similar to scrotal skin grafts, the biologic is tubularized and sutured to the inverted penile skin flap to form the neovaginal apex. Microscopic analysis of biopsied vaginal canal tissue at 180 days demonstrates hyperplastic stratified squamous epithelium with underlying fibrous connective tissue, much like a natal vaginal canal[27]. Other authors have described similar techniques for primary gender-affirming vaginoplasty utilizing decellularized ovine foregut extracellular matrix (Myriad; Aroa Biosurgery, San Diego, CA) in conjunction with peritoneum[28].
AlloDerm (LifeCell, Branchburg, NJ) is an acellular allograft consisting of the basement membrane and collagen scaffolding from human cadaveric skin. It has been described for use in cisgender vaginal reconstruction and, more recently, for revision vaginoplasty in combination with peritoneal flaps[29]. In such cases, Alloderm is sutured distally to the remnant neovaginal canal and proximally to newly mobilized peritoneal flaps, thereby “mending the gap” between the remnant canal and peritoneal cavity. After 3-6 weeks, the nonimmunogenic, acellular graft is epithelialized. One major drawback is the estimated cost of $30/cm2, which may restrict its use to complex revision cases.
Another allograft option for neovaginal reconstruction is dehydrated human amnion/chorion membrane, commercially available as AmnioFix/EpiFix (MiMedx Group Inc., Marietta, GA). Amnion has long been utilized for complex burn and wound care due to its availability, cost-effectiveness, and low morbidity; in recent years, various authors have expanded its use for cisgender vaginoplasty in cases of Mayer-Rokitansky-Kuster-Hauser syndrome[30,31]. Amniotic membrane is favorable due to its immunologic properties, antifibroblastic activity, and propensity to epithelialize without hair growth or bothersome discharge[32]. However, additional study in the context of gender-affirming surgery is needed.
A nascent but encouraging realm is the use of tissue-engineered biomaterials in neovaginal reconstruction[33,34]. Tissue engineering carries the theoretical benefits of reproducibility, eschewing the need for a donor, and the ability to tailor specific growth factors to optimize the characteristics of the neovaginal mucosa.
Intestine
Intestinal vaginoplasty utilizes a pedicled flap of ileum, jejunum, or colon for the neovaginal canal and has historically served as the primary alternative to penile inversion vaginoplasty. Advantages include a robust, well-vascularized canal, natural lubrication from the intestinal mucosa, and plentiful donor tissue without the need for extragenital skin grafting[4]. The sigmoid colon is the most common intestinal segment utilized due to its large lumen, thick walls, and proximity to the pelvis[35]. However, with any type of intestinal vaginoplasty, there are unique risks related to bowel harvest and anastomosis, diversion colitis, inflammatory bowel disease, and the need for long-term surveillance of gastrointestinal malignancy within the neovaginal segment[2]. Additional disadvantages include the production of malodorous discharge and mucus. As such, intestinal flaps are less commonly offered for primary gender-affirming vaginoplasty; some authors consider it a favorable option for revision cases when facing a severe lack of suitable donor tissue[7].
OUR EXPERIENCE AND TECHNICAL CONSIDERATIONS FOR CANAL CREATION
Our institutional approach for primary and revision vaginoplasties reflects these considerations. We use the robotic platform to facilitate and standardize pelvic dissection and canal creation. For full-depth vaginoplasty, we primarily offer penile inversion vaginoplasty with scrotal skin grafts. For revision vaginoplasty, we utilize peritoneal flaps with or without extragenital FTSGs; the need for grafts depends on the depth of the remnant neovaginal canal.
Minimal-depth vaginoplasty
In some circumstances, patients pursue vaginoplasty without creation of a functional vaginal canal, termed minimal-depth vaginoplasty, zero-depth vaginoplasty, or vulvoplasty. This is a unique aspect of our institutional practice in that it comprises roughly 50% of our total vaginoplasty volume.
Patients seen in our clinic for gender-affirming genital feminization are counseled extensively regarding surgical options (minimal versus full-depth) and associated perioperative risks, long-term care, and implications for intimacy and intercourse. We elicit patients’ particular aesthetic and functional preferences. We also strive to entertain niche requests (e.g., penile-preserving vaginoplasty) so long as they are surgically feasible and in concordance with patients’ goals of care.
Patients in our practice who elect for minimal-depth vaginoplasty may prefer its relative “simplicity” over the demands of routine long-term dilation, which we stress as critical to the success of full-depth vaginoplasty. Some have cited the cost and time commitment of preoperative depilation as deterrents to electing full-depth vaginoplasty. Lastly, the high proportion of minimal-depth vaginoplasty in our practice may simply reflect regional variations in patient preference and sexual practices; put simply, such patients seek out the “low maintenance” option. We have similarly noted a high proportion of patients who seek gender-affirming orchiectomy only, with no plans for future vaginoplasty.
Minimal-depth vaginoplasty precludes penetrative vaginal intercourse, albeit with several advantages: (1) shortening operative times and minimizing perioperative morbidity, (2) avoiding the potentially hazardous rectoprostatic dissection, and (3) obviating the need for preoperative hair removal and long-term neovaginal canal douching and dilation[36]. Patients should consider this a relatively permanent decision, given that healthy scrotal skin is discarded. However, we and others have described techniques for the conversion of minimal-depth to full-depth neovaginas[37].
Though there is no true neovaginal canal created during minimal-depth vaginoplasty, we have noted several nuances critical for success. After taking down the perineal body, the neovagina is set at the level of the membranous urethra. After inverting the penile skin tube, the ventral skin (oriented posteriorly after inversion) is incised longitudinally to meet the perineal skin flap posteriorly; the perineal skin is similarly incised for tension-free apposition. These incisions determine the angle of the minimal-depth canal, which bears several consequences. Too steep an angle results in a pit-like canal, which can collect debris and urine despite its shallow depth. Insufficient angulation results in an unsightly straight-on appearance of the canal apex, which resembles the umbilicus.
Full-depth vaginoplasty
For primary full-depth vaginoplasty, we offer a modified penile inversion vaginoplasty utilizing robotic assistance for canal dissection. We prefer the combined perineal and robotic transperitoneal approach for several reasons. Foremost is that it minimizes the risks of rectal and urethral injury, which in large series are reported in 2.3%-2.6% and 1.1% of cases, respectively[38,39]. In our experience of 99 primary and 28 revision full-depth vaginoplasties, we have encountered zero rectal injuries or rectovaginal fistulae. One urethral injury (0.8%) occurred early in our learning curve and resolved with primary closure and prolonged Foley catheter drainage. These and other complications are summarized in Table 1.
Complications of full-depth and revision vaginoplasty with robotic assistance (n = 127)
| Complication | Full-depth vaginoplasty (%) | Revision vaginoplasty (%) | Total (%) |
| No. | 99 | 28 | 127 |
| Rectal injury | 0 (0) | 0 (0) | 0 (0) |
| Urethral injury | 1 (1.0) | 0 (0) | 1 (0.8) |
| Fistula | 0 (0) | 0 (0) | 0 (0) |
| Transfusion | 14 (14.0) | 0 (0) | 14 (11.0) |
| Hematoma | 1 (1.0) | 0 (0) | 1 (0.8) |
| Abscess | 2 (2.0) | 0 (0) | 2 (1.6) |
| Small bowel obstruction | 0 (0) | 0 (0) | 0 (0) |
| Neovaginal stenosis or loss of depth, | 7 (7.1) | 7 (25.0) | 14 (11.0) |
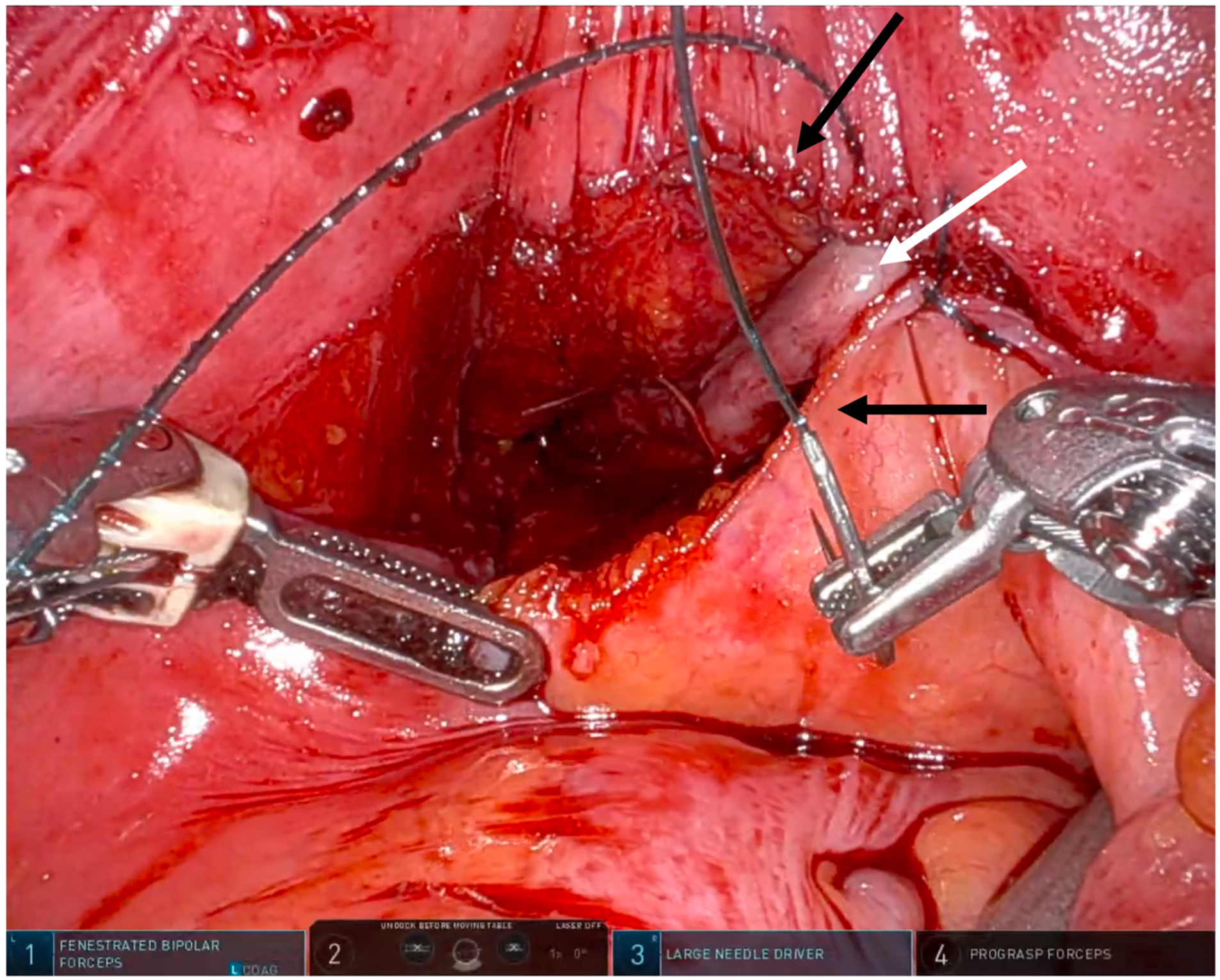
Second, the robotic platform standardizes neovaginal canal creation. The neovagina is pexied to the peritoneal reflection, and in most cases, this yields a canal of sufficient depth without concern for later prolapse. We do not routinely employ peritoneal flaps in primary full-depth cases, as we reliably gain sufficient canal depth in this manner. This eschews the relatively rare but notable risks of small bowel obstruction, internal herniation, and neurogenic lower urinary tract dysfunction associated with peritoneal flap harvest[19,40]. In our technique, the peritonotomy is closed as the neovaginal apex is pexied, thereby sequestering the canal from the intra-abdominal contents [Figure 1]. It is critical to note that each case is individualized: for patients with penoscrotal hypoplasia or refractory genital hair growth, we do not hesitate to proceed with peritoneal flap vaginoplasty to preserve sufficient skin for satisfactory vulvar definition.
Figure 1. Robotic view of robotic primary full depth vaginoplasty closure. The robotic surgeon simultaneously closes the peritonotomy (black arrows) while suspending the apex of the neovaginal canal (white arrow), which is composed of inverted penile skin and scrotal FTSG. FTSG: Full-thickness skin graft.
After emptying the bladder, canal dissection begins via a perineal approach. We leave the bulbospongiosus muscle attached posteriorly until canal dissection is complete, which maintains the correct plane of dissection and, in the event of a rectal or urethral injury, serves as a well-vascularized flap for interposition. The perineal body is taken down transversely and the dissection is continued along Denonvillier’s fascia to develop the potential space between the rectum and prostatic apex. Laterally, dissection continues in the fatty tissue just posterior to the inferior pubic ramus.
We then continue canal dissection via a robotic transperitoneal approach. In the steep Trendelenberg position, pneumoperitoneum is established and robotic ports are placed; for a multi-port system, these are configured in a manner similar to that for a robotic prostatectomy. It is prudent to minimize the distance between ports (no greater than 8-9 mm), as the lateral-most ports will be restricted by the narrow inlet of the “male” pelvis.
A peritonotomy is made in the rectovesical pouch and dissection continues caudad, posterior to the vas deferens. This is akin to the dissection plane during a Retzius-sparing radical prostatectomy. Dissection can be either in the intrafascial (between the leaflets of Denonvillier’s fascia) or infrafascial (posterior to both leaflets) plane. We prefer the latter, as it leads rapidly to the pelvic floor musculature laterally and allows us to “break through” (i.e., connect the perineal and abdominal surgical fields) off midline, minimizing the risk of rectal injury. This space is easily found through blunt dissection by the perineal surgeon. The levator musculature is incised over an assistant’s gloved finger or instrument just posterior to the inferior pubic ramus. Once the bilateral spaces are widened sufficiently, a finger can be hooked around the remaining rectourethralis midline attachments. These are taken down perineally over a finger, which allows for tactile feedback to avoid urethral or rectal injury. Finally, the lateral pelvic floor musculature is further incised to maximize vaginal width.
One drawback of infrafascial dissection is proximity to the rectum and theoretical risk of injury, particularly in younger, thinner patients with minimal peri-rectal fat. Additionally, the smaller, softer prostate in these patients requires careful anterior dissection to avoid violating the prostatic capsule. Early recognition of missteps allows for straightforward correction. Intrafascial dissection is also a valid alternative, connecting perineal and robotic planes at the midline[16,41]. In our hands, however, infrafascial dissection enables rapid, safe, and reproducible connection of the perineal and robotic planes, allowing blunt access to the lateral urogenital triangle and tactile guidance for midline attachments, reducing the need for routine digital rectal examination or specialized retractors and maintaining a low intraoperative injury rate [Table 1].
The neovaginal skin tube, composed of penile shaft skin and scrotal skin grafts, is tubularized and inverted over a #7 purple, 3.7 cm-diameter Soul Source silicone pelvic trainer (Soul Source, North Hollywood, CA). In cases with adequate penoscrotal skin, the robotic surgeon closes the initial peritoneotomy while simultaneously securing the apex of the skin tube using running 3-0 V-loc sutures (Medtronic, Minneapolis, MN) [Figure 1]. At the end of the procedure, an ALLURA silicone shell vaginal stent (PMT Corporation, Chanhassen, MN) is placed and filled with saline or air under laparoscopic guidance. The stent, generally 12-15 cm in length, remains in place until postoperative day 5.
Revision vaginoplasty, including conversion from minimal to full-depth vaginoplasty
An obstinate complication after vaginoplasty is neovaginal canal stenosis, reported in 4.5%-12% of patients[42,43]. Loss of canal depth and width can result from poor graft take, contracture, suboptimal initial canal creation, insufficient dissection of the levator musculature, and/or nonadherence to postoperative dilation regimens. Many patients suffer from pre-existing pelvic floor dysfunction or sexual trauma, which further hinders adherence. Thus, a reliable and reproducible technique for revision vaginoplasty is essential.
Revision vaginoplasty is beset by several challenges: (1) re-operating in the deep pelvis, (2) risk of injury to adjacent structures such as the bladder, urethra, and rectum, and (3) limited residual donor tissue for canal lining. Similar to our approach for primary full-depth vaginoplasty, we prefer a combined perineal and robotic transperitoneal approach, which facilitates dissection and suturing within the previously explored pelvis. For donor tissue, we utilize a combination of robotically harvested peritoneal flaps and FTSGs from non-hair-bearing lower abdomen or groin skin when residual canal tissue is minimal. Patients undergoing conversion from minimal-depth to full-depth vaginoplasty are managed similarly to revision cases. This technique has been previously presented in video format[37].
Revision begins with assessment of the remnant neovaginal canal depth, usually achievable through an in-office pelvic exam. Severe introital stenosis may preclude accurate assessment; in such cases, cross-sectional imaging is obtained to identify a dilated or sequestered canal. These patients may benefit from introital revision alone.
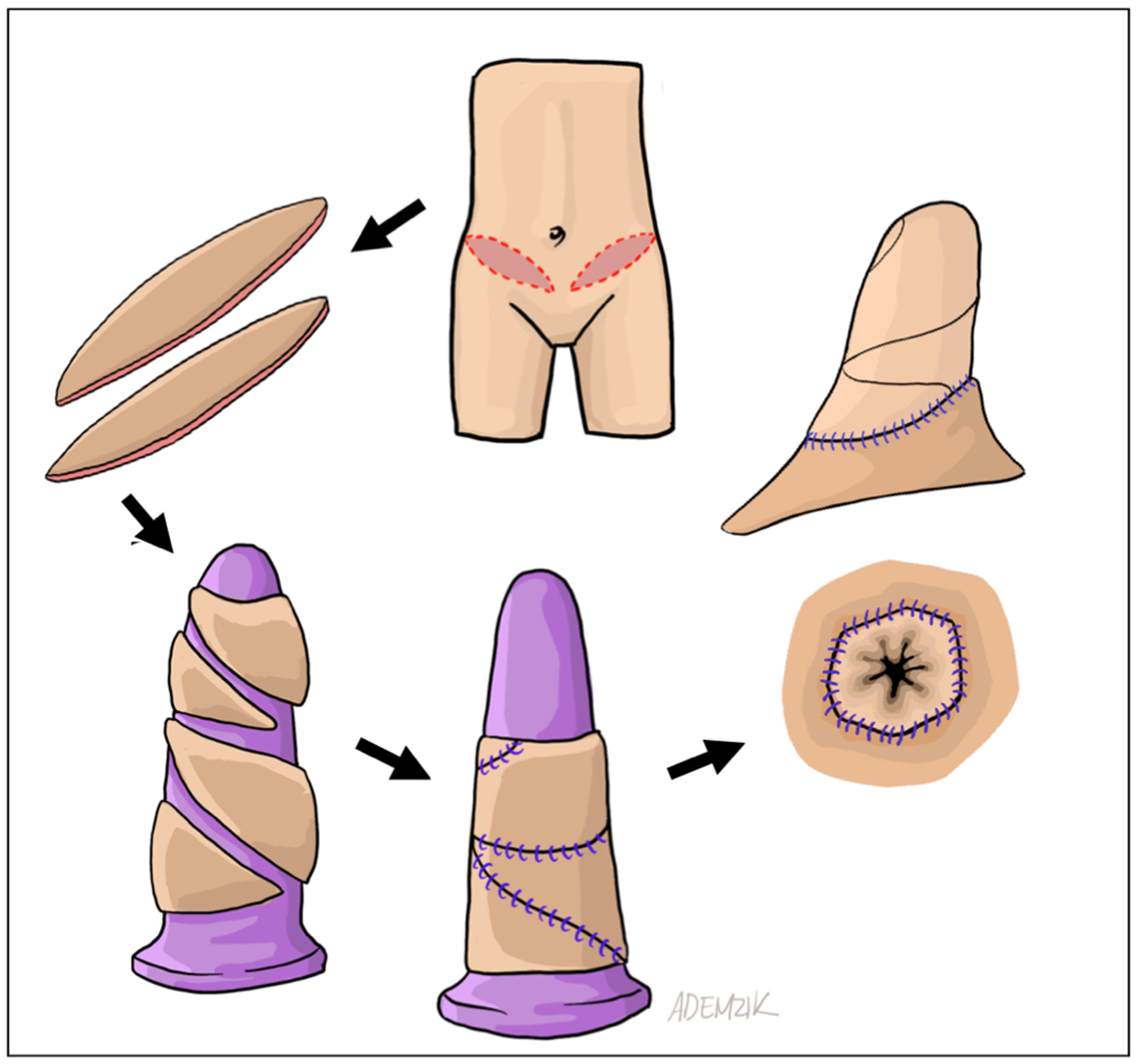
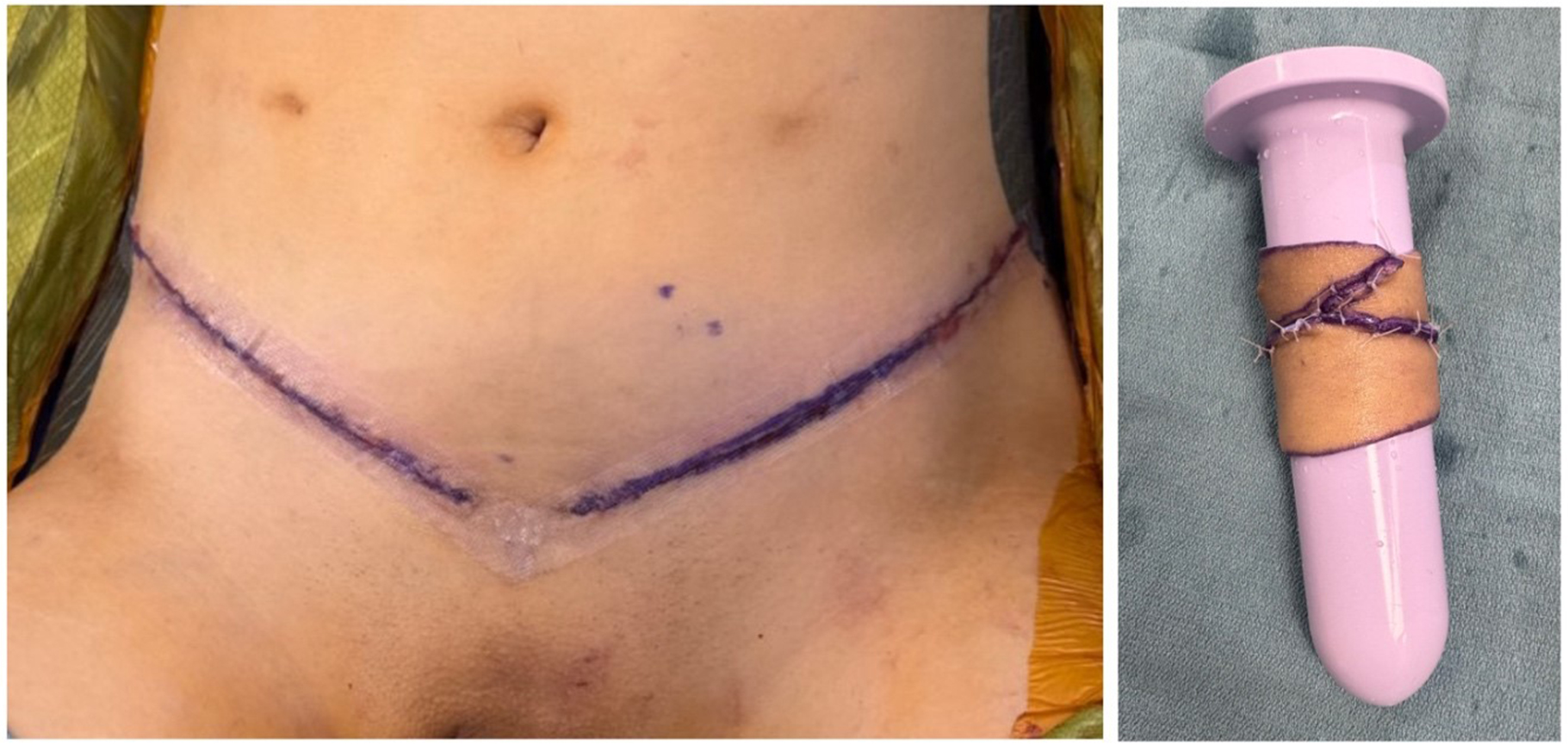
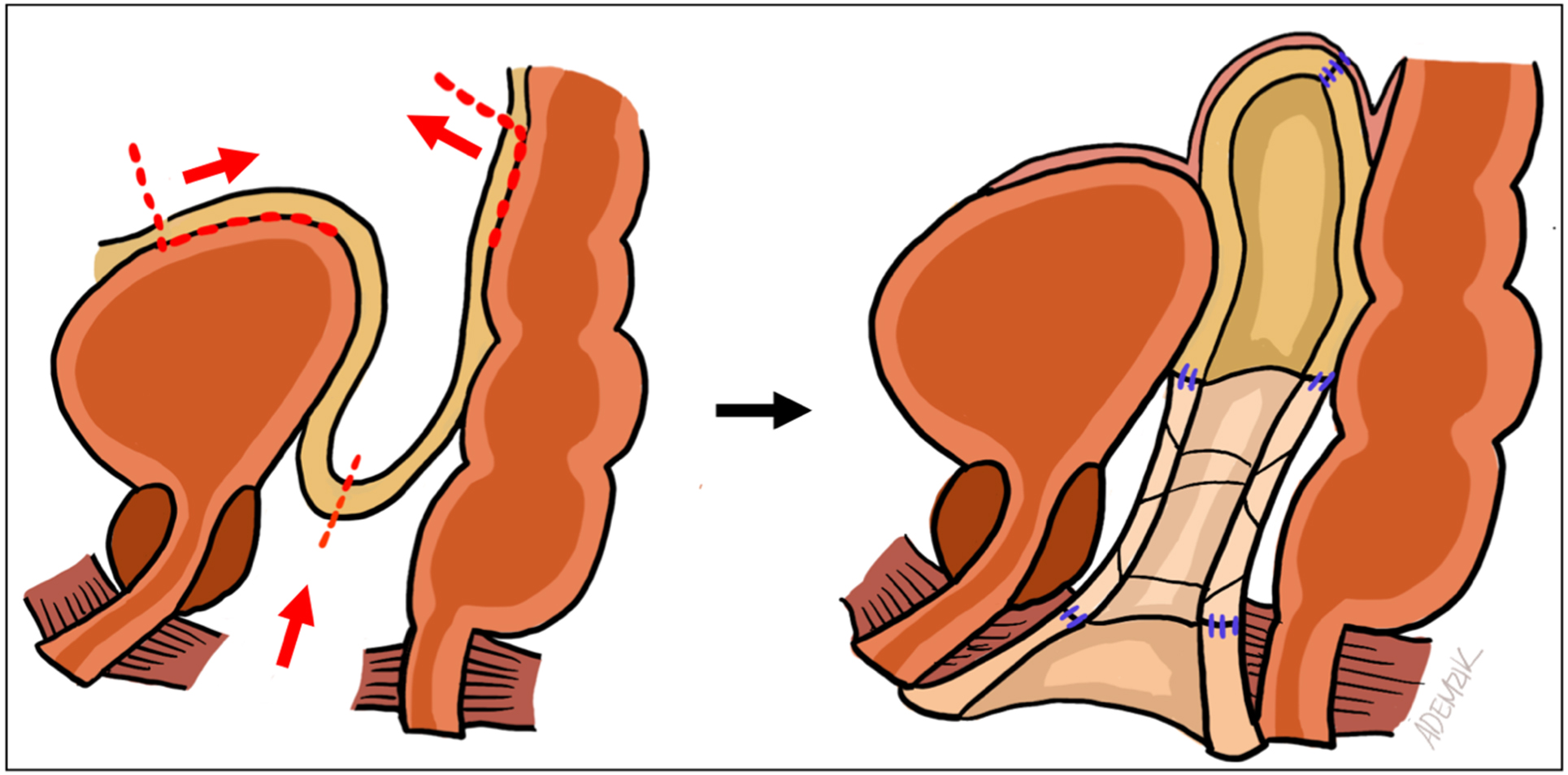
When the canal apex is easily visible perineally, severe stenosis is present. In such cases, peritoneal flaps alone cannot reach the remnant apex, leaving a gap within the rectoprostatic space. This is universally true for conversion from minimal- to full-depth vaginoplasty. We proceed with bilateral ellipsoid FTSG harvest from non-hair-bearing lower abdomen or inguinal skin. The grafts are thinned, tubularized over a vaginal dilator, and sutured distally to the remnant canal apex and proximally to the peritoneal edge [Figure 2]. Donor sites are closed primarily in layers [Figure 3]. Although some authors have proposed the use of AlloDerm (LifeCell Corp, Branchburg, NJ) or other biologics to avoid the morbidity and operative time associated with FTSG harvest[29], we still prefer autologous grafts due to their reliability. In our experience, patients generally tolerate the additional donor sites well. Nevertheless, prospective comparative studies between autografts (e.g., FTSG) and biologic allografts or xenografts in primary and revision gender-affirming vaginoplasty remain necessary.
Figure 2. Schematic representation pf FTSG harvest, tubularization, and intra-abdominal passage. The distal anastomosis is visualized perineally, while the proximal anastomosis is seen robotically. FTSG: Full-thickness skin graft.
Figure 3. Appearance of FTSG harvest sites after layered closure. Harvest from the non-hair bearing lower abdomen and groin yields plentiful, robust donor tissue. FTSG: Full-thickness skin graft.
For less severe canal stenosis, peritoneal flaps alone may suffice to augment depth. A helpful preoperative indicator is that a neovagina accommodating a finger to the proximal interphalangeal joint (second knuckle) likely has sufficient length for revision using only peritoneal flaps. This can guide preoperative counseling and set realistic expectations regarding the need for multiple donor sites.
Intraoperatively, robotic dissection begins in the rectovesical pouch. Placement of a vaginal dilator or end-to-end anastomosis (EEA) sizer in the remnant canal is helpful. The neovaginal cuff is opened, and surrounding scar tissue and levator musculature are incised to accommodate the 3.7 cm dilator. In cases of severe stenosis, the tubularized skin graft is sutured to the introitus using absorbable sutures in a clock-face pattern and inserted into the vaginal space, easily visualized robotically.
Peritoneal flaps are mobilized robotically as described by Jacoby et al. (2019), bounded anteriorly by the vas deferens and medial umbilical ligaments and posteriorly by the ureters and sacral promontory[16]. The inferior edges of the anterior and posterior peritoneal flaps (i.e., cut edges of the initial peritonotomy) are sutured with 3-0 V-loc to the remnant canal or tubularized FTSG inverted over a vaginal dilator. Lateral flap edges are then approximated to form a new neovaginal apex within the rectovesical pouch [Figure 4]. Gradual reduction of insufflation during apical closure can minimize suture-line tension; mobilization of the bladder may also reduce tension.
Figure 4. Sagittal diagram of neovaginal canal components during revision vaginoplasty. The canal is formed distally from remnant canal tissue, in its mid-section from FTSGs, and proximally from peritoneal flaps. FTSGs: Full-thickness skin grafts.
Our approach offers several advantages. The robotic transperitoneal technique simultaneously enables peritoneal flap harvest, intra-abdominal suturing, and safe dissection within the re-operative pelvis. The peritoneum provides well-vascularized, readily accessible donor tissue for reconstructing the neovaginal apex. Additionally, FTSGs can bridge the gap between a stenotic canal and the peritoneum with minimal additional cost, operative time, and morbidity. This hybrid anastomosis is considerably simpler and faster than trying to “stretch” the peritoneum to the introitus, which often leads to a technically challenging deep pelvic anastomosis, tearing of donor tissues, and an increased risk of subsequent re-stenosis. We encountered such challenges early in our experience and now prefer to “mend the gap” using additional donor grafts[29].
In an unpublished review of our institutional revision vaginoplasty series, patients achieved a median neovaginal depth increase of 13.7 cm. While we have applied this technique for several patients undergoing revision after primary peritoneal flap vaginoplasty, these cases remain technically demanding, often requiring extensive peritoneal flap harvest and/or broad bladder mobilization. Whether this approach is widely reproducible, or whether patients presenting for revision after prior peritoneal flap vaginoplasty are better suited to alternatives (e.g., intestinal vaginoplasty) remains to be determined.
CONCLUSION
The challenges of gender-affirming vaginoplasty have prompted a wave of innovations and outcomes research aimed at optimizing surgical approach, donor tissue selection, and functional results. Despite substantial progress over the past few decades, some patients continue to require improved solutions. Through careful application of technical innovations, adherence to principles of tissue transfer, and targeted research, we can continue to expand and refine the surgical options available for these patients.
DECLARATIONS
Authors’ contributions
Conception and design: Chao BW, Pariser JJ
Data collection and analysis: Chao BW, Keiner C
Manuscript drafting: Chao BW, Keiner C
Manuscript editing: Chao BW, Pariser JJ
Oversight and supervision: Pariser JJ
Original drawings and artwork: Demzik A
Availability of data and materials
The dataset generated and analyzed during this study is available from the corresponding author upon reasonable request.
Financial support and sponsorship
None.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Chart review necessary for this manuscript was deemed exempt from individual informed consent as part of a retrospective study regarding outcomes after gender-affirming surgery (IRB protocol #000025711).
Consent for publication
All figures and images featured in the manuscript are original. Operative images were taken intraoperatively with patient permission.
Copyright
© The Author(s) 2025.
REFERENCES
1. Canner JK, Harfouch O, Kodadek LM, et al. Temporal trends in gender-affirming surgery among transgender patients in the United States. JAMA Surg. 2018;153:609-16.
2. Morrison SD, Claes K, Morris MP, Monstrey S, Hoebeke P, Buncamper M. Principles and outcomes of gender-affirming vaginoplasty. Nat Rev Urol. 2023;20:308-22.
3. Karim RB, Hage JJ, Mulder JW. Neovaginoplasty in male transsexuals: review of surgical techniques and recommendations regarding eligibility. Ann Plast Surg. 1996;37:669-75.
4. Coon D, Morrison SD, Morris MP, et al. Gender-affirming vaginoplasty: a comparison of algorithms, surgical techniques and management practices across 17 high-volume centers in North America and Europe. Plast Reconstr Surg Glob Open. 2023;11:e5033.
5. Gillies HD, Millard DR. The principles and art of plastic surgery. London: Butterworth; 1957.
6. Hage JJ, Karim RB, Laub DR Sr. On the origin of pedicled skin inversion vaginoplasty: life and work of Dr Georges Burou of Casablanca. Ann Plast Surg. 2007;59:723-9.
7. Bene NC, Ferrin PC, Xu J, Dy GW, Dugi D 3rd, Peters BR. Tissue options for construction of the neovaginal canal in gender-affirming vaginoplasty. J Clin Med. 2024;13:2760.
8. Yuan N, Feldman AT, Chin P, Zaliznyak M, Rabizadeh S, Garcia MM. Comparison of permanent hair removal procedures before gender-affirming vaginoplasty: why we should consider laser hair removal as a first-line treatment for patients who meet criteria. Sex Med. 2022;10:100545.
9. Mankowski P, Mukherjee S, Kumar S, et al. Barriers to completing preoperative hair removal for penile inversion vaginoplasty. Arch Sex Behav. 2024;53:2003-10.
10. Dorafshar AH, Gitman M, Roughton M, Gottlieb LJ. Adhesive depilation for scalp graft hair removal. Plast Reconstr Surg. 2010;125:152e-4e.
11. Opoku-Agyeman JL, Humenansky K, Burkey B. A simple technique for intraoperative scalp skin graft depilation using Dermabond®. Cureus. 2020;12:e9786.
12. Dubin DP, Routt ET, Lin MJ, Torbeck RL, Khorasani H. Intraoperative electrosurgical depilation of a skin graft. J Cutan Aesthet Surg. 2020;13:257-8.
13. Sineath RC, Butler C, Dy GW, Dugi D 3rd. Genital hypoplasia in gender-affirming vaginoplasty: prior orchiectomy, penile length, and other factors to guide surgical planning. J Urol. 2022;208:1276-87.
14. Davydov SN. Colpopoeisis from the peritoneum of the uterorectal space. Akush Ginekol. 1969;45:55-7.
15. Bianchi S, Berlanda N, Brunetti F, Bulfoni A, Ferrero Caroggio C, Fedele L. Creation of a neovagina by laparoscopic modified Davydov vaginoplasty in patients with partial androgen insensitivity syndrome. J Minim Invasive Gynecol. 2017;24:1211-7.
16. Jacoby A, Maliha S, Granieri MA, et al. Robotic Davydov peritoneal flap vaginoplasty for augmentation of vaginal depth in feminizing vaginoplasty. J Urol. 2019;201:1171-6.
17. Smith SM, Yuan N, Stelmar J, et al. An alternative option for gender-affirming revision vaginoplasty: the tubularized urachus-peritoneal hinge flap. Sex Med. 2022;10:100572.
18. O’Dwyer C, Kumar S, Wassersug R, et al. Vaginal self-lubrication following peritoneal, penile inversion, and colonic gender-affirming vaginoplasty: a physiologic, anatomic, and histologic review. Sex Med Rev. 2023;11:212-23.
19. Dy GW, Blasdel G, Shakir NA, Bluebond-Langner R, Zhao LC. Robotic peritoneal flap revision of gender affirming vaginoplasty: a novel technique for treating neovaginal stenosis. Urology. 2021;154:308-14.
20. Bowers ML, Gynecology
21. Suporn Clinic. The Suporn Clinic. Available from: http://supornclinic.com/. [Last accessed on 18 Aug 2025].
22. Bustos SS, Rios-Sanchez M, Fahradyan V. Penile inversion vaginoplasty: an evolving technique. Cureus. 2024;16:e68949.
23. Perovic SV, Stanojevic DS, Djordjevic ML. Vaginoplasty in male transsexuals using penile skin and a urethral flap. BJU Int. 2000;86:843-50.
24. Amend B, Seibold J, Toomey P, Stenzl A, Sievert KD. Surgical reconstruction for male-to-female sex reassignment. Eur Urol. 2013;64:141-9.
25. Gentile G, Martino A, Nadalin D, et al. Penile-scrotal flap vaginoplasty versus inverted penile skin flap expanded with spatulated urethra: a multidisciplinary single-centre analysis. Arch Ital Urol Androl. 2020:92.
26. Nijhuis THJ, Özer M, van der Sluis WB, et al. The bilateral pedicled epilated scrotal flap: a powerful adjunctive for creation of more neovaginal depth in penile inversion vaginoplasty. J Sex Med. 2020;17:1033-40.
27. Dias MTPM, Bilhar APM, Rios LC, et al. Neovaginoplasty using Nile Tilapia Fish Skin as a new biologic graft in patients with Mayer-Rokitansky-Küster-Hauser syndrome. J Minim Invasive Gynecol. 2020;27:966-72.
28. Mishra K, Sellke N, Gupta S. (179) Tubularized Augmented Peritoneal Cap (TAPCAP) vaginoplasty. J Sex Med. 2024;21:qdae167.175.
29. Parker A, Brydges H, Blasdel G, Bluebond-Langner R, Zhao LC. Mending the gap: AlloDerm as a safe and effective option for vaginal canal lining in revision robotic assisted gender affirming peritoneal flap vaginoplasty. Urology. 2023;173:204-8.
30. Sarwar I, Sultana R, Nisa RU, Qayyum I. Vaginoplasty by using amnion graft in patients of vaginal agenesis associated with Mayor-Rokitansky-Kuster-Hauser syndrome. J Ayub Med Coll Abbottabad. 2010;22:7-10.
31. Bhandari S, Dangal G, Karki A, et al. Vaginoplasty with amnion graft: management of Mayer-Rokitansky-Kuster-Hauser syndrome. J Nepal Health Res Counc. 2024;21:530-3.
32. Vatsa R, Bharti J, Roy KK, et al. Evaluation of amnion in creation of neovagina in women with Mayer-Rokitansky-Kuster-Hauser syndrome. Fertil Steril. 2017;108:341-5.
33. Zhu L, Zhou H, Sun Z, Lou W, Lang J. Anatomic and sexual outcomes after vaginoplasty using tissue-engineered biomaterial graft in patients with Mayer-Rokitansky-Küster-Hauser syndrome: a new minimally invasive and effective surgery. J Sex Med. 2013;10:1652-8.
34. Sueters J, Groenman FA, Bouman MB, et al. Tissue engineering neovagina for vaginoplasty in Mayer-Rokitansky-Küster-Hauser syndrome and gender dysphoria patients: a systematic review. Tissue Eng Part B Rev. 2023;29:28-46.
35. Bouman MB, van der Sluis WB, Buncamper ME, Özer M, Mullender MG, Meijerink WJHJ. Primary total laparoscopic sigmoid vaginoplasty in transgender women with penoscrotal hypoplasia: a prospective cohort study of surgical outcomes and follow-up of 42 patients. Plast Reconstr Surg. 2016;138:614e-23e.
37. Loftus CJ, Ratanawong JP, Pariser JJ. V09-01 An operative technique for conversion of minimal to full depth gender affirming peritoneal flap vaginoplasty. J Urol. 2023:209.
38. Buncamper ME, van der Sluis WB, van der Pas RSD, et al. Surgical outcome after penile inversion vaginoplasty: a retrospective study of 475 transgender women. Plast Reconstr Surg. 2016;138:999-1007.
39. Cristofari S, Bertrand B, Leuzzi S, et al. Postoperative complications of male to female sex reassignment surgery: a 10-year French retrospective study. Ann Chir Plast Esthet. 2019;64:24-32.
40. Robinson IS, Blasdel G, Bluebond-Langner R, Zhao LC. The management of intra-abdominal complications following peritoneal flap vaginoplasty. Urology. 2022;164:278-85.
41. Peters BR, Martin LH, Butler C, Dugi D, Dy GW. Robotic peritoneal flap vs. perineal penile inversion techniques for gender-affirming vaginoplasty. Curr Urol Rep. 2022;23:211-8.
42. Horbach SER, Bouman MB, Smit JM, Özer M, Buncamper ME, Mullender MG. Outcome of vaginoplasty in male-to-female transgenders: a systematic review of surgical techniques. J Sex Med. 2015;12:1499-512.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Special Issue
Copyright
Data & Comments
Data

















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].