Antithrombotic therapy in patients undergoing transcatheter aortic valve replacement (TAVR): from current evidence to perspective
Abstract
The use of transcatheter aortic valve replacement (TAVR) for care of symptomatic severe aortic stenosis has increased over the last years; after initially treating patients at prohibitive or high surgical risk, nowadays the procedure can be considered for intermediate or low surgical risk. Although thrombotic events (ischemic stroke, myocardial infarction, and leaflet thrombosis) decreased in patients at lower risk, antithrombotic therapy after TAVR is still recommended. However, the optimal antithrombotic regimen is a still matter of debate due to the lack of randomized data and the concomitant increased risk of bleeding events. In the present review, we analyze current data, recommendations of international guidelines and consensus documents, and potential future scenarios with a rational approach of separation of patients with or without a pre-procedural indication for long-term oral anticoagulant therapy.
Keywords
INTRODUCTION
Transcatheter aortic valve replacement (TAVR) can be considered as the treatment of choice in patients with severe symptomatic aortic stenosis (AS) at prohibitive or high surgical risk and as an alternative to surgical valve replacement (SAVR) in patients at lower risk[1].
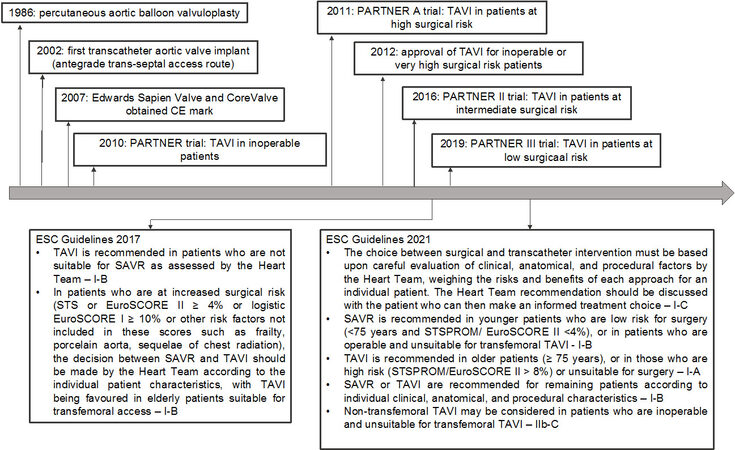
The growing of elderly population in Europe and US has increased the prevalence of AS over the last decades[2]; furthermore, positive results of clinical trials in patients at intermediate/low risk[1] and the evolution of device technologies are expected to lead to treating a larger number of patients both young and elderly with more comorbidities [Figure 1].
Patients undergoing TAVR can be considered by themselves at higher risk of thrombotic events for advanced age and comorbidities.
The currently available transcatheter aortic bioprostheses include different types of stents that work as a support for a xenograft tissue with three leaflets of porcine or bovine pericardium.
As for coronary stents, an endothelization of the struts usually occurs within one month; however, this process is not present at the level of struts far from the aortic vessel wall, with a potential increased risk of embolic events related to this “incomplete endothelization”. Furthermore, thrombosis can occur at the level of the leaflets[3].
Therefore, peri- and post-procedural antithrombotic therapy is mandatory to prevent ischemic events, but its optimal regimen is still a matter of debate.
Table 1 reports the current recommendation of European and American guidelines for antithrombotic therapy after TAVR.
Current recommendations of European and American guidelines for antithrombotic therapy after TAVR[1,5]
| COR | LOE | |
| 2021 ESC/EACTS Guidelines for the management of valvular heart disease | ||
| Oral anticoagulation is recommended lifelong for TAVR patients who have other indications for anticoagulation | I | B |
| Lifelong SAPT is recommended after TAVR in patients with no baseline indication for OAC | I | A |
| Routine use of OAC is not recommended after TAVR in patients with no baseline indication for OAC | III | B |
| 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease | ||
| For patients with a bioprosthetic TAVR, aspirin 75 to 100 mg daily is reasonable in the absence of other indications for oral anticoagulants | 2a | B-R |
| For patients with a bioprosthetic TAVR who are at low risk of bleeding, dual antiplatelet therapy with aspirin 75 to 100 mg and clopidogrel 75 mg may be reasonable for 3 to 6 months after valve implantation | 2b | B-NR |
| For patients with a bioprosthetic TAVR who are at low risk of bleeding, anticoagulation with a VKA to achieve an INR of 2.5 may be reasonable for at least 3 months after valve implantation | 2b | B-NR |
| For patients with bioprosthetic TAVR, treatment with low-dose rivaroxaban (10 mg daily) plus aspirin (75-100 mg) is contraindicated in the absence of other indications for oral anticoagulants | 3 | B-R |
Briefly, based on a recent European position paper and the 2021 European guidelines, patients not requiring long-term oral anticoagulant therapy (OAC) and without recent coronary stent implantation (< 3 months) should be treated with single antiplatelet therapy (SAPT), aspirin (ASA), or clopidogrel; if there is an indication for OAC, a vitamin K antagonist (VKA) or a direct oral anticoagulant (DOAC) should be continued with no addition of single antiplatelet therapy (SAPT)[1-4].
The American guidelines consider ASA 75-100 mg/daily as a reasonable treatment after TAVR without an indication for OAC (Class IIa); however, in patients at low risk of bleeding, dual antiplatelet therapy (DAPT) (ASA 75-100 mg/daily plus clopidogrel 75 mg/daily for 3-6 months) or a VKA with a target international normalized ratio (INR) of 2.5 for at least three months may both be considered after the procedure (Class IIb)[5].
THROMBOTIC AND BLEEDING RISK AFTER TAVR
The main adverse thrombotic events occurring in patients undergoing TAVR are stroke, myocardial infarction (MI), and bioprosthesis valve thrombosis.
The incidence of cerebrovascular accidents ranges in different studies from 0% to 5%[6], with a higher rate reported within the first months after the procedure[7]. Early events (< 1 month after procedure) are usually related to the embolization of debris from the aortic valve or the aortic wall[8]. Later events are more likely related to patient risk factors such as age and comorbidities, mainly atrial fibrillation (AF)[7].
About half of patients undergoing TAVR present a concomitant coronary artery disease (CAD); however, the rate of subsequent myocardial infarctions is low[9].
Data on thrombosis of the bioprosthesis are currently the most debated: thrombus can present as hypo-attenuated thickening (HALT), as HALT with reduced leaflet motion (RLM), or as clinical thrombosis with increased transvalvular gradients. In the Evolut Low Risk trial analysis, including patients treated with surgical or transcatheter procedures, at one-year follow-up, HALT and RLM were detected with CT scan in 30.9% and 31% of cases treated with self-expanding transcatheter bioprosthesis, respectively[10]. The clinical effects of these findings are uncertain and controversial; in some studies, it was associated with an increased rate of cerebrovascular events[11].
Conversely, patients undergoing TAVR are at a concomitant increased risk of bleeding[12]. However, the rate of major bleeding in clinical trials has decreased over time with an incidence of about 20% in inoperable patients[13] and 7.7% in patients at low surgical risk [14]. The reduction of sheath size, the improved experience of operators, and the treatment of younger patients have led to a decrease in procedural bleeding events. Female sex, Society of Thoracic Surgeons (STS) score, chronic kidney disease, low hemoglobin at baseline, atrial fibrillation or flutter at baseline or 30 days, post-procedural moderate/severe paravalvular leak at 30 days, and a greater left ventricular mass have been reported as independent predictors of bleeding complications[12,15].
Later major bleeding complications (> 30 days after procedure) occurred in about 6% of patients undergoing TAVR, with a gastrointestinal event in more than half of the cases[12].
In real world, the incidence of bleeding was not different between access-site and non-access-site events, but the latter occurred later (> 30 days after procedure) in more cases. Even though both were associated with adverse outcomes, mortality was higher in patients who experienced a non-access-site event[15].
Table 2 summarizes rates of death, myocardial infarction, stroke, and bleeding events from the main randomized clinical trials according to surgical risk and type of bioprosthesis (i.e., self-expandable vs. balloon-expandable valves).
Rates of death from any cause, death from cardiovascular causes, myocardial infarction, major stroke, and major bleeding events divided by surgical risk and type of prosthetic valve
| Incidence (%) | ||
| High-risk patients | PARTNER trial | CoreValve US pivotal trial |
| Death from any cause at 30 days | 5% | 3.3% |
| Death from any cause at 1 year | 30.7% | 14.2% |
| Death from cardiovascular causes at 30 days | 4.5% | 3.1% |
| Death from cardiovascular causes at 1 year | 20.5% | 10.4% |
| Myocardial infarction at 30 days | 0% | 0.8% |
| Myocardial infarction at 1 year | 0.6% | 1.9% |
| Major stroke at 30 days | 5% | 3.9% |
| Major stroke at 1 year | 7.8% | 5.8% |
| Major bleeding events at 30 days | 16.8% | 28.1% |
| Major bleeding events at 1 year | 22.3% | 29.5% |
| Intermediate-risk patients | PARTNER 2 trial | SURTAVI trial |
| Death from any cause at 30 days | 3.9% | 2.2% |
| Death from any cause at 2 years | 16.7% | 11.4% |
| Death from cardiovascular causes at 30 days | 3.3% | 2% |
| Death from cardiovascular causes at 2 years | 10.1% | 7.7% |
| Myocardial infarction at 30 days | 1.2% | 0.9% |
| Myocardial infarction at 2 years | 3.6% | 2.8% |
| Major stroke at 30 days | 5.5% | 3.4% |
| Major stroke at 2 years | 9.5% | 6.2% |
| Major bleeding events at 30 days | 10.4% | 12.1% |
| Major bleeding events at 2 years | 17.3% | - |
| Low-risk patients | PARTNER 3 trial | Evolut low risk trial |
| Death from any cause at 30 days | 0.4% | 0.5% |
| Death from any cause at 1 year | 1% | 2.4% |
| Death from cardiovascular causes at 30 days | 0.4% | 0.5% |
| Death from cardiovascular causes at 1 year | 0.8% | 1.7% |
| Myocardial infarction at 30 days | 1% | 0.9% |
| Myocardial infarction at 1 year | 1.2% | 1.7% |
| Major stroke at 30 days | 0.6% | 3.4% |
| Major stroke at 1 year | 1.2% | 4.1% |
| Major bleeding events at 30 days | 3.6% | 2.4% |
| Major bleeding events at 1 year | 7.7% | 3.2% |
REVIEW OF CURRENT EVIDENCE
The first recommendation to administer a DAPT with ASA plus clopidogrel for 3-6 months after TAVR was extrapolated from the experience with coronary stents. Multiple subsequent studies and metanalyses have questioned this approach due to the increased bleeding risk associated with the use of DAPT[16-18].
However, as many patients undergoing TAVR present a concomitant AF or received a prior percutaneous coronary intervention (PCI)[19] and are already receiving a tailored SAPT, DAPT, or OAC before a procedure, any further choice about antithrombotic therapy is challenging. Several trials have been designed to evaluate the best treatment regimen in these different settings.
Patients without indication for long-term OAC [Table 3]
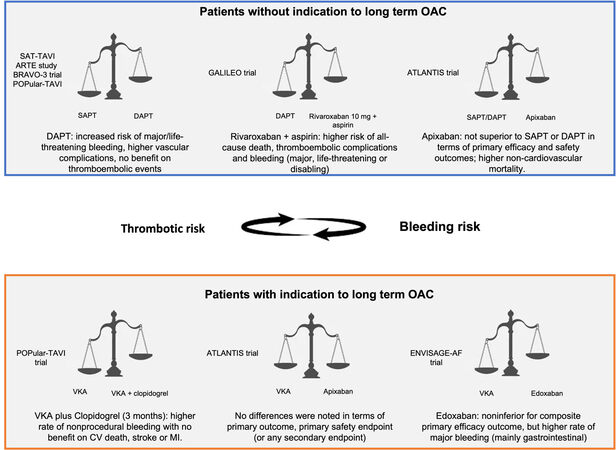
Ussia et al. first questioned the risk-benefit ratio of DAPT after TAVR[20]. Two small following randomized trials, the SAT-TAVI (single antiplatelet therapy for transcatheter aortic valve implantation) and ARTE studies (aspirin versus aspirin + clopidogrel following transcatheter aortic valve implantation), failed to show the benefit of DAPT compared to SAPT; furthermore, the use of DAPT was associated with an increased risk of major or life-threatening bleeding. Similar results have been obtained in subsequent meta-analyses[21], and in one of them, DAPT was associated with increased mortality at 30 days (RR: 0.57; P = 0.014)[22].
Main trials investigating the best antithrombotic regimen after TAVR in patients without indication for long-term oral anticoagulant therapy
| Incidence (%) | |||
| SAT-TAVI trial | ASA | DAPT | p |
| Life-threatening bleeding at 30 days | 5% | 6.7% | n.s. |
| Major bleeding at 30 days | 3% | 3% | n.s. |
| Major Stroke at 30 days | 1.7% | 1.7% | n.s. |
| Cardiovascular Death at 30 days | 3.3% | 1.7% | n.s. |
| Major and minor vascular complications at 30 days | 5% | 13.3% | < 0.05 |
| ARTE trial | ASA | Aspirin + clopidogrel | p |
| Life-threatening/major bleeding at 90 days | 3.7% | 10.9% | 0.040 |
| Myocardial infarction at 90 days | 0.9% | 3.6% | 0.178 |
| Stroke/TIA at 90 days | 0.9% | 2.7% | 0.317 |
| Death at 90 days | 3.7% | 6.4% | 0.381 |
| Combined endpoint at 90 days | 7.3% | 15.5% | 0.060 |
| POPular TAVI trial (cohort A) | ASA | ASA + clopidogrel | p |
| All bleeding at 12 months | 15.1% | 26.6% | 0.001 |
| Non-procedure-related bleeding at 12 months | 15.1% | 24.9% | 0.005 |
| Cardiovascular death at 12 months | 4.2% | 3.9% | |
| Death from any cause at 12 months | 6.3% | 5.7% | |
| Stroke at 12 months | 5.1% | 5.7% | |
| Myocardial infarction at 12 months | 1.2% | 1.8% | |
| Major, life-threatening, or disabling bleeding at 12 months | 5.1% | 10.8% | |
| First composite secondary outcome - Noninferiority analysis | 23% | 31.1% | < 0.001 |
| First composite secondary outcome - Superiority analysis Composite of bleeding, death from cardiovascular causes, non-procedure-related bleeding, stroke, MI | 23% | 31.1% | 0.04 |
| GALILEO trial | Rivaroxaban 10 mg (+ ASA for 3 months) | ASA (+ clopidogrel for 3 months) | HR (95%CI) |
| Primary safety outcome Composite of VARC life-threatening, disabling, or major bleeding | 5.6% | 3.8% | 1.50 (0.95 to 2.37) |
| Primary efficacy outcome Composite of death, stroke, myocardial infarction, symptomatic valve thrombosis, pulmonary embolism, deep-vein thrombosis, or systemic embolism | 12.7% | 9.5% | 1.35 (1.01 to 1.81) |
| Net clinical benefit Composite of the primary efficacy and primary safety outcomes | 16.6% | 12.2% | 1.39 (1.08 to 1.80) |
| ATLANTIS trial - stratum 2 | Apixaban 5 mg bid | Standard of care | HR (95%CI) |
| Primary outcome Death, stroke, MI, systemic emboli, intracardiac or valve thrombosis, DVT/PE, major bleedings | 16.9% | 19.3% | 0.88 (0.66-1.17) |
| Primary safety endpoint Life-threatening (including fatal) or disabling or major bleeding (BARC 4, 3a, b and 3c), as defined by Valve Academic Research Consortium-2 (VARC-2) | 7.8% | 7.3% | 1.09 (0.69-1.69) |
| Low risk TAVR 2.0 trial | ASA | Warfarin + ASA | p |
| All-cause death at 30 days | 0% | 0% | - |
| VARC 2 life-threatening or major bleeding at 30 days | 4% | 2.3% | 0.64 |
| All stroke and TIA at 30 days | 4% | 0% | 0.18 |
| Myocardial infarction at 30 days | 0% | 0% | - |
Moreover, the BRAVO-3 trial (bivalirudin versus heparin anticoagulation in transcatheter aortic valve replacement) showed no benefit on thromboembolic events in patients undergoing TAVR with the administration of clopidogrel before or after the procedure; furthermore, pretreatment was associated with more vascular complications[23].
Recently, the randomized clinical trial POPular-TAVI (aspirin with or without clopidogrel after transcatheter aortic valve implantation - Cohort A) confirmed that aspirin alone reduced bleeding compared with aspirin plus clopidogrel in 665 patients not requiring OAC (RR: 0.57; 95%CI: 0.42-0.77; P = 0.001)[24]. In addition, SAPT was non-inferior to DAPT with respect to the composite of cardiovascular death, ischemic stroke or MI (RR: 0.98; 95%CI for noninferiority, -4.7 to 4.3; P = 0.004). These data support guideline indications about post-TAVR SAPT[1-5].
The GALILEO (global study comparing a rivaroxaban-based antithrombotic strategy to an antiplatelet-based strategy after transcatheter aortic valve replacement to optimize clinical outcomes) trial investigated the use of low-dose rivaroxaban (10 mg) plus aspirin vs. DAPT for three months in patients without indication to OAC undergoing TAVR. The study was prematurely terminated due to the higher risk of all-cause death, thromboembolic complications, and bleeding (major, life-threatening, or disabling) in patients receiving rivaroxaban plus aspirin[25].
The ATLANTIS (anti-thrombotic strategy to lower all cardiovascular and neurologic ischemic and hemorrhagic events after trans-aortic valve implantation for aortic stenosis) trial investigated apixaban in 1510 patients undergoing TAVR: comparators were VKA in patients with indication to long-term OAC (Stratum 1749 patients) and SAPT or DAPT in patients without indication to long-term OAC (Stratum 2751 patients). At one-year follow-up, in Stratum 2, apixaban was not superior to SAPT or DAPT in terms of primary efficacy and safety outcomes and resulted in higher non-cardiovascular mortality (HR: 0.88)[26,27].
In a recent study, 94 low-risk patients treated with TAVR and not requiring long-term OAC were randomized to SAPT or aspirin plus VKA. The primary composite endpoint (HALT, moderately RLM, valve hemodynamic dysfunction with mean aortic valve gradient ≥ 20 mm Hg, effective orifice area ≤ 1.0 cm2, dimensionless valve index < 0.35, moderate-severe aortic regurgitation, stroke, or TIA) was significantly higher in patients receiving only aspirin (26.5% vs. 7.0%; OR: 4.8; P = 0.014), with no differences in terms of bleeding. These data suggest a potential benefit of OAC in patients at lower risk[28], but they need to be confirmed in larger studies.
In summary, current evidence does not support the use of OAC (DOAC or VKA) in patients undergoing TAVR with no preexisting indication.
Patients with indications for long-term OAC
Based on their comorbidities, most patients with AF undergoing TAVR have an indication to receive an OAC for the value of CHA2DS2-VASc score. The beneficial effect on reduction of peri-procedural thromboembolic events of OAC is still unknown; however, the continuation of OAC (VKA or DOAC) before TAVR was found to be safe in terms of bleeding and vascular complications[29].
In patients with a pre-procedural indication for OAC, the addition of antiplatelet therapy offers the theoretical advantage of preventing thrombus formation on struts of bioprosthesis, but several observational studies showed the safety and efficacy of an OAC-alone strategy, with both VKA and DOAC [Table 4][30-32].
Main trials investigating the best antithrombotic regimen after TAVR in patients with indication to long-term oral anticoagulant therapy
| Incidence (%) | |||
| POPular TAVI trial (cohort B) | VKA | VKA + clopidogrel | p |
| All bleeding at 12 months | 21.7% | 34.6% | 0.01 |
| Non-procedure-related bleeding at 12 months | 21.7% | 34.0% | 0.02 |
| Cardiovascular death at 12 months | 8.3% | 12.8% | |
| Death from any cause at 12 months | 13.4% | 15.4% | |
| Stroke at 12 months | 5.7% | 5.8% | |
| Myocardial infarction at 12 months | 0.6% | 0.6% | |
| Major, life-threatening, or disabling bleeding at 12 months | 8.9% | 16.7% | |
| Secondary composite 1 event- Noninferiority analysis | 31.2% | 45.5% | |
| Secondary composite 1 event - Superiority analysis Composite of death from cardiovascular causes, non-procedure-related bleeding, stroke from any cause, or MI | 31.2% | 45.5% | |
| ATLANTIS trial - stratum 1 | Apixaban | VKA | HR (95%CI) |
| Primary outcome Death, stroke, MI, systemic emboli, intracardiac or valve thrombosis, DVT/PE, major bleedings | 21.9% | 21.9% | 1.02 (0.68 to 1.91) |
| Primary safety endpoint Life-threatening (including fatal) or disabling or major bleeding (BARC 4, 3a, b and 3c), as defined by Valve Academic Research Consortium-2 (VARC-2) | 10.3% | 11.4% | 0.92 (0.52 to 1.60) |
| ENVISAGE-AF | Edoxaban | VKA | HR (95%CI) |
| Primary efficacy outcome Net adverse clinical events (death from any cause, MI, ischemic stroke, systemic thromboembolic event, valve thrombosis, or major bleeding) | 17.3% | 16.5% | 1.05 (0.85 to 1.31) |
| Primary safety outcome Major bleeding (ISTH definition) | 9.7% | 7.0% | 1.40 (1.03 to 1.91) |
Furthermore, the randomized POPular-TAVI trial (Cohort B) that investigated the safety and efficacy of VKA plus clopidogrel (for three months) versus VKA alone showed a higher rate of nonprocedural bleeding in the first group (34% vs. 21.7%; RR: 0.63; P = 0.01) with no benefit on CV death, stroke, or MI[33].
Data obtained from the France-TAVI and FRANCE-2 registries, linked with the nationwide administrative databases and analyzed after a propensity score matching, report that the use of DOAC was associated with lower mortality and major bleeding compared to VKA at three years with no difference in terms of ischemic stroke and acute coronary syndromes[34].
The ATLANTIS trial (Stratum 1) randomized 451 patients with OAC indication to apixaban or VKA. In this setting, no differences were noted in terms of primary outcome (21.9% vs. 21.9%; HR: 1.02), primary safety endpoint (10.3% vs. 11.4%; HR: 0.92), or any secondary endpoint[27].
Recently, the ENVISAGE-AF trial (edoxaban compared to standard care after heart valve replacement using a catheter in patients with atrial fibrillation) compared edoxaban vs. VKA in patients requiring long-term OAC after TAVR. Edoxaban was noninferior to VKA for composite primary efficacy outcome, but it was associated with a higher rate of major bleeding (mainly gastrointestinal bleeding)[35].
Observational data show neutral results regarding the thromboembolic risk associated with DOAC post-TAVR, but a German registry demonstrated higher all-cause mortality, MI, and cerebrovascular events at one year compared to VKA[36].
Therefore, evidence supporting DOAC over VKA in AF patients undergoing TAVR is still lacking. As expected, in patients with AF and recent PCI undergoing TAVI, the choice of optimal antithrombotic regimen is even more complex. In the absence of direct evidence, the duration of dual therapy should follow post-PCI recommendations. Based on individual thrombotic and bleeding risk, current guidelines recommend the combination of DOAC plus clopidogrel with a very short period of aspirin (maximum six months, only in patients with high thrombotic risk)[37].
The evidence of the best antithrombotic regimen in patients with or without indication for OAC is summarized in Figure 2.
Figure 2. Central illustration: the best antithrombotic regimen in patients with or without indication to long-term oral anticoagulant therapy. OAC: Oral anticoagulant; SAPT: single antiplatelet therapy; DAPT: dual antiplatelet therapy; VKA: vitamin K antagonist; CV: cardiovascular; MI: myocardial infarction.
Valve deterioration or leaflet thrombosis
Thrombosis contributes to the deterioration of bioprosthetic valves after both SAVR and TAVR[38,39], with the incidence increasing over the years.
Based on the available data, a subclinical leaflet thrombosis of the bioprosthesis was detected with CT scan in about 20%-30% at one year from TAVR, and its association with an increase in cerebrovascular events remains controversial[10,40].
Even if the clinical impact of HALT and RLM is questionable, selective use of oral anticoagulants should be considered[1], as a lack of OAC prescription at hospital discharge after TAVR was an independent predictor of bioprosthetic valve deterioration detected with echocardiogram[41].
In the French TAVI registry, a prescription of OAC at hospital discharge was associated with a reduction of dysfunction of the bioprosthesis[42].
Although the prescription of OAC after TAVR seemed to improve the motion of the leaflets and reduce their thrombosis, the controversial data for clinical events associated with this therapy, especially regarding DOAC, require further investigation.
In a 4D-CT analysis of the ATLANTIS study presented at American College of Cardiology 2021, HALT and RLM were lower with apixaban compared to with antiplatelets in patients without indication for OAC, but this was not associated with better clinical outcomes[43]. Low-dose rivaroxaban achieved similar results in the GALILEO 4D substudy[44].
FUTURE DIRECTIONS
As yet explained, in some patient subsets, robust clinical trial evidence is still lacking and actual recommendations are guided by expert opinion or findings derived from observational or small randomized studies. Although it might be supposed that in some settings such as valve-in-valve an aggressive antithrombotic therapy including OAC is required, it should be demonstrated in a specific randomized study.
Furthermore, no evidence on different antithrombotic therapies based on the type of implanted bioprosthesis (balloon or self-expandable) is available.
Several other randomized trials examining the safety and efficacy of various antithrombotic regimens are ongoing. For instance, TICTAVI (NCT02817789) and PTOLEMAIOS (NCT02989558) are randomized trials comparing ticagrelor (with or without aspirin) versus standard DAPT in TAVR patients.
Further data on valve thrombosis prevention will soon be available from the ongoing ADAPT-TAVR trial (anticoagulant versus dual antiplatelet therapy for preventing leaflet thrombosis and cerebral embolization after transcatheter aortic valve replacement) comparing edoxaban versus DAPT with aspirin and clopidogrel for six-month incidence of leaflet thrombosis and cerebrovascular events in patients without indication for OAC[45].
As a limitation of our effort, a critical approach of different treatments within each trial is lacking; however, it appears very difficult to carry out, because all included patients have been stratified based on requirement or not of long-term OAC.
In our opinion, future clinical trials should be focused on both baseline patients’ risk profiles (thrombotic and hemorrhagic, as done for DAPT duration after coronary stent implantation) and specific procedural settings, such as valve-in-valve.
CONCLUSION
The optimal antithrombotic therapy after TAVR is still a matter of debate. However, based on current evidence, a single antiplatelet therapy can be considered the first-line treatment in patients not requiring long-term OAC and without recent coronary stent implantation. Conversely, VKA or DOAC alone should be continued without antiplatelet therapy after the procedure when there is an indication for OAC.
DECLARATIONS
Authors’ contributionsMade a substantial contribution to present paper: Mauri S, Lanzillo G, Ferlini M
Availability of data and materialsNot applicable.
Financial support and sponsorshipNone.
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2022.
REFERENCES
1. Vahanian A, Beyersdorf F, Praz F, et al. ESC/EACTS Scientific Document Group. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J 2022;43:561-632.
2. Yadgir S, Johnson CO, Aboyans V, et al. Global Burden of Disease Study 2017 Nonrheumatic Valve Disease Collaborators. Global, regional, and national burden of calcific aortic valve and degenerative mitral valve diseases, 1990-2017. Circulation 2020;141:1670-80.
3. Mangieri A, Montalto C, Poletti E, et al. Thrombotic versus bleeding risk after transcatheter aortic valve replacement: JACC review topic of the week. J Am Coll Cardiol 2019;74:2088-101.
4. Ten Berg J, Sibbing D, Rocca B, et al. Management of antithrombotic therapy in patients undergoing transcatheter aortic valve implantation: a consensus document of the ESC Working Group on Thrombosis and the European Association of Percutaneous Cardiovascular Interventions (EAPCI), in collaboration with the ESC Council on Valvular Heart Disease. Eur Heart J 2021;42:2265-9.
5. Otto CM, Nishimura RA, Bonow RO, et al. Writing Committee Members. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. J Am Coll Cardiol 2021;77:e25-e197.
6. Mastoris I, Schoos MM, Dangas GD, Mehran R. Stroke after transcatheter aortic valve replacement: incidence, risk factors, prognosis, and preventive strategies. Clin Cardiol 2014;37:756-64.
7. Stortecky S, Windecker S. Stroke: an infrequent but devastating complication in cardiovascular interventions. Circulation 2012;126:2921-4.
8. Van Mieghem NM, Schipper ME, Ladich E, et al. Histopathology of embolic debris captured during transcatheter aortic valve replacement. Circulation 2013;127:2194-201.
9. Faroux L, Guimaraes L, Wintzer-Wehekind J, et al. Coronary artery disease and transcatheter aortic valve replacement: JACK state-of-the-art review. J Am Coll Cardiol 2019;74:362-72.
10. Blanke P, Leipsic JA, Popma JJ, et al. Evolut Low Risk LTI Substudy Investigators. Bioprosthetic aortic valve leaflet thickening in the EVOLUT low risk sub-study. J Am Coll Cardiol 2020;75:2430-42.
11. Chakravarty T, Søndergaard L, Friedman J, et al. Subclinical leaflet thrombosis in surgical and transcatheter bioprosthetic aortic valves: an observational study. The Lancet 2017;389:2383-92.
12. Généreux P, Cohen DJ, Mack M, et al. Incidence, predictors, and prognostic impact of late bleeding complications after transcatheter aortic valve replacement. J Am Coll Cardiol 2014;64:2605-15.
13. Makkar RR, Fontana GP, Jilaihawi H, et al. PARTNER Trial Investigators. Transcatheter aortic-valve replacement for inoperable severe aortic stenosis. N Engl J Med 2012;366:1696-704.
14. Mack MJ, Leon MB, Thourani VH, et al. PARTNER 3 Investigators. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med 2019;380:1695-705.
15. Piccolo R, Pilgrim T, Franzone A, et al. Frequency, timing, and impact of access-site and non-access-site bleeding on mortality among patients undergoing transcatheter aortic valve replacement. JACC Cardiovasc Interv 2017;10:1436-46.
16. Maes F, Stabile E, Ussia GP, et al. Meta-analysis comparing single versus dual antiplatelet therapy following transcatheter aortic valve implantation. Am J Cardiol 2018;122:310-5.
17. Rodés-Cabau J, Masson JB, Welsh RC, et al. Aspirin versus aspirin plus clopidogrel as antithrombotic treatment following transcatheter aortic valve replacement with a balloon-expandable valve: the ARTE (Aspirin Versus Aspirin + Clopidogrel Following Transcatheter Aortic Valve Implantation) randomized clinical trial. JACC Cardiovasc Interv 2017;10:1357-65.
18. Raheja H, Garg A, Goel S, et al. Comparison of single versus dual antiplatelet therapy after TAVR: a systematic review and meta-analysis. Catheter Cardiovasc Interv 2018;92:783-91.
19. Sherwood MW, Xiang K, Matsouaka R, et al. Incidence, temporal trends, and associated outcomes of vascular and bleeding complications in patients undergoing transfemoral transcatheter aortic valve replacement: insights from the society of thoracic surgeons/American college of cardiology transcatheter valve therapies registry. Circ Cardiovasc Interv 2020;13:e008227.
20. Ussia GP, Scarabelli M, Mulè M, et al. Dual antiplatelet therapy versus aspirin alone in patients undergoing transcatheter aortic valve implantation. Am J Cardiol 2011;108:1772-6.
21. Siddamsetti S, Balasubramanian S, Yandrapalli S, et al. Meta-analysis comparing dual antiplatelet therapy versus single antiplatelet therapy following transcatheter aortic valve implantation. Am J Cardiol 2018;122:1401-8.
22. Al Halabi S, Newman J, Farkouh ME, et al. Meta-analysis of studies comparing dual- versus mono-antiplatelet therapy following transcatheter aortic valve implantation. Am J Cardiol 2018;122:141-8.
23. Nijenhuis VJ, Ten Berg JM, Hengstenberg C, et al. Usefulness of clopidogrel loading in patients who underwent transcatheter aortic valve implantation (from the BRAVO-3 Randomized Trial). Am J Cardiol 2019;123:1494-500.
24. Brouwer J, Nijenhuis VJ, Delewi R, et al. Aspirin with or without clopidogrel after transcatheter aortic-valve implantation. N Engl J Med 2020;383:1447-57.
25. Dangas GD, Tijssen JGP, Wöhrle J, et al. GALILEO Investigators. A controlled trial of rivaroxaban after transcatheter aortic-valve replacement. N Engl J Med 2020;382:120-9.
26. Collet JP, Berti S, Cequier A, et al. Oral anti-Xa anticoagulation after trans-aortic valve implantation for aortic stenosis: the randomized ATLANTIS trial. Am Heart J 2018;200:44-50.
27. Collet JP. Oral anti-Xa anticoagulation after trans-aortic valve implantation for aortic stenosis: the randomized ATLANTIS trial. Am Heart J 2018;200:44-50.
28. Rogers T, Shults C, Torguson R, et al. Randomized trial of aspirin versus warfarin after transcatheter aortic valve replacement in low-risk patients. Circ Cardiovasc Interv 2021;14:e009983.
29. Brinkert M, Mangner N, Moriyama N, et al. Safety and efficacy of transcatheter aortic valve replacement with continuation of vitamin k antagonists or direct oral anticoagulants. JACC Cardiovasc Interv 2021;14:135-44.
30. Abdul-Jawad Altisent O, Durand E, Muñoz-García AJ, et al. Warfarin and antiplatelet therapy versus warfarin alone for treating patients with atrial fibrillation undergoing transcatheter aortic valve replacement. JACC Cardiovasc Interv 2016;9:1706-17.
31. Geis NA, Kiriakou C, Chorianopoulos E, Pleger ST, Katus HA, Bekeredjian R. Feasibility and safety of vitamin K antagonist monotherapy in atrial fibrillation patients undergoing transcatheter aortic valve implantation. EuroIntervention 2017;12:2058-66.
32. Geis NA, Kiriakou C, Chorianopoulos E, Uhlmann L, Katus HA, Bekeredjian R. NOAC monotherapy in patients with concomitant indications for oral anticoagulation undergoing transcatheter aortic valve implantation. Clin Res Cardiol 2018;107:799-806.
33. Nijenhuis VJ, Brouwer J, Delewi R, et al. Anticoagulation with or without clopidogrel after transcatheter aortic-valve implantation. N Engl J Med 2020;382:1696-707.
34. Didier R, Lhermusier T, Auffret V, et al. STOP-AS and France-TAVI. TAVR patients requiring anticoagulation: direct oral anticoagulant or vitamin k antagonist? JACC Cardiovasc Interv 2021;14:1704-13.
35. Van Mieghem NM, Unverdorben M, Hengstenberg C, et al. Edoxaban versus vitamin k antagonist for atrial fibrillation after TAVR. N Engl J Med 2021;385:2150-60.
36. Jochheim D, Barbanti M, Capretti G, et al. Oral anticoagulant type and outcomes after transcatheter aortic valve replacement. JACC Cardiovasc Interv 2019;12:1566-76.
37. Valgimigli M, Bueno H, Byrne RA, et al. ESC Scientific Document Group. , ESC Committee for Practice Guidelines (CPG)., ESC National Cardiac Societies. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2018;39:213-60.
38. Ruel M, Kulik A, Rubens FD, et al. Late incidence and determinants of reoperation in patients with prosthetic heart valves. Eur J Cardiothorac Surg 2004;25:364-70.
39. De Marchena E, Mesa J, Pomenti S, et al. Thrombus formation following transcatheter aortic valve replacement. JACC Cardiovasc Interv 2015;8:728-39.
40. Makkar RR, Fontana G, Jilaihawi H, et al. Possible subclinical leaflet thrombosis in bioprosthetic aortic valves. N Engl J Med 2015;373:2015-24.
41. Del Trigo M, Muñoz-Garcia AJ, Wijeysundera HC, et al. Incidence, timing, and predictors of valve hemodynamic deterioration after transcatheter aortic valve replacement: multicenter registry. J Am Coll Cardiol 2016;67:644-55.
42. Overtchouk P, Guedeney P, Rouanet S, et al. Long-term mortality and early valve dysfunction according to anticoagulation use: the FRANCE TAVI registry. J Am Coll Cardiol 2019;73:13-21.
43. Montalescot G. Apixaban and valve thrombosis after transcatheter aotic valve implantation: the ATLANTIS 4D-CT substudy. Available from: https://www.crtonline.org/presentation-detail/apixaban-vale-thrombosis-after-transcatheter-aorti [Last accessed on 8 Aug 2022].
44. Backer O, Dangas GD, Jilaihawi H, et al; GALILEO-4D investigators. Reduced leaflet motion after transcatheter aortic-valve replacement. N Engl J Med 2020;382:130-9.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Mauri S, Lanzillo G, Ferlini M. Antithrombotic therapy in patients undergoing transcatheter aortic valve replacement (TAVR): from current evidence to perspective. Mini-invasive Surg 2022;6:49. http://dx.doi.org/10.20517/2574-1225.2022.34
AMA Style
Mauri S, Lanzillo G, Ferlini M. Antithrombotic therapy in patients undergoing transcatheter aortic valve replacement (TAVR): from current evidence to perspective. Mini-invasive Surgery. 2022; 6: 49. http://dx.doi.org/10.20517/2574-1225.2022.34
Chicago/Turabian Style
Mauri, Silvia, Giuseppe Lanzillo, Marco Ferlini. 2022. "Antithrombotic therapy in patients undergoing transcatheter aortic valve replacement (TAVR): from current evidence to perspective" Mini-invasive Surgery. 6: 49. http://dx.doi.org/10.20517/2574-1225.2022.34
ACS Style
Mauri, S.; Lanzillo G.; Ferlini M. Antithrombotic therapy in patients undergoing transcatheter aortic valve replacement (TAVR): from current evidence to perspective. Mini-invasive. Surg. 2022, 6, 49. http://dx.doi.org/10.20517/2574-1225.2022.34
About This Article
Special Issue
Copyright
Data & Comments
Data

 Cite This Article 4 clicks
Cite This Article 4 clicks











Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.