Magnetic sphincter for anal incontinence: an update
Abstract
The idea of using magnets to control the esophago-gastro-intestinal flow of contents dates back almost 20 years, from the first bench experiment in 2003, published in 2006, while the first clinical application at the anal level to prevent fecal incontinence took place in 2010 by means of a device called FENIX magnetic anal sphincter augmentation (MAS). The clinical experiences with MAS ranged from satisfactory success to partial failure depending on the various studies. The nonrandomized comparisons of MAS with sacral nerve stimulation (SNS) and artificial bowel sphincter (ABS) showed a similar effectiveness in fecal continence and quality of life, whereas the adverse events were more frequent and severe with MAS compared to SNS. ABS either failed to work or required an explantation for infection in 40% of patients, whereas MAS showed these adverse events in only 20% of cases. The comparison of MAS with anal slings and bulking infiltrations provided similar continence results, although with a shorter duration, whereas MAS showed more adverse events. Recently MAS has been withdrawn from the market, creating major inconveniences for surgeons and patients. Nevertheless, this can represent an opportunity for a system that reinforces the anal sphincter with “two magnetic plaques” to be finally implemented for use in patients after completing animal experimentation. This system offers various advantages compared with MAS: it has simpler operational activity, easier surgical implanting procedure, the possibility of “tailored” sphincter augmentation, and should turn out to cost less.
Keywords
INTRODUCTION
Fecal incontinence (FI) is the involuntary loss of feces, with a prevalence in the general population that can reach up to 15%[1], especially in elderly people living in communities[2]. The key mechanism of this embarrassing disease, whatever may be the cause, is the decrease or absence of tonic contraction of the anal sphincter. Consequently, both surgical and non-surgical treatment is aimed at strengthening the sphincter. The surgical techniques are based on creating an extrinsic constriction of the sphincter, which should keep the lumen sufficiently closed. However, to allow feces and gas passage, the endorectal pressure must overcome that of the extrinsic constriction, which is rather inelastic and yields only partially. Therefore, the constriction can neither be too tight, because it may cause difficulty in evacuation, nor too yielding, because it would not assure an effective anal closure. Thus, a closing device capable of obviating this drawback, opening the lumen to allow the fecal transit and automatically closing immediately thereafter, is necessary. This goal was reached with the use of magnets.
Before updating the events regarding the clinical experience with anal magnets, it could be interesting, and perhaps “instructive” to know how this application of the magnetic force against FI arose, becoming the only real novelty of the last 15 years in the field of surgical therapy of FI, and to assess its contribution to the solution of the FI problem until it was utilized. I had the idea of using magnetic force by observing the application of magnetic devices in gut surgery for a variety of purposes, as recently reviewed by Gagner[3], and in particular the creation of gastrointestinal anastomoses[4-6]. These are carried out by a couple of small magnetic disks applied face-to-face inside the walls of two adjacent portions of the gut. The magnets, attracting each other, cause necrosis of the compressed tissues of the two adjacent walls, creating a “passage” through them, with the aim of bypassing a distal lumen occlusion caused by scarring stenosis or an inoperable cancer. This application of the magnetic force made me think that a couple of magnets with a less powerful attraction force placed face-to-face outside the opposite walls of a sphincter, by attracting each other, could close the lumen without damaging the tissues.
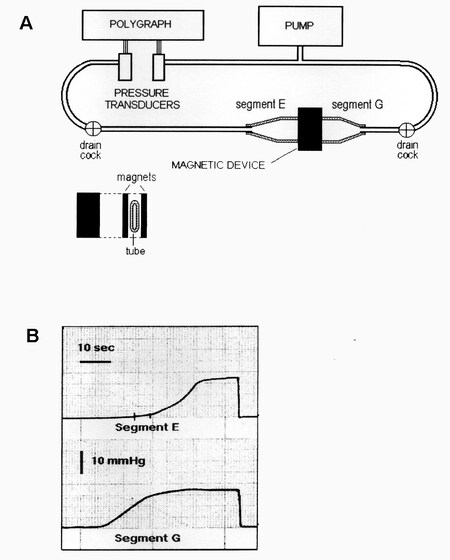
Therefore, in July 2003, I sent to the Journal of Biomechanics[7] a description of a bench experiment
Figure 1. (A) Schematic illustration of the bench model used to study the new antireflux device based on magnets. On the right, there is a flaccid polyethylene tube 2.8 cm in diameter, mimicking the gastroesophageal junction. It is squeezed perpendicularly by two rectangular magnets made of plastoferrite (Flexo) 2 cm × 4 cm × 0.5 cm with an attraction force of 0.36 N/cm2 when put in contact and 0.16 N/cm2 at 7 mm distance. It creates a high-pressure zone 2 cm wide that divides the tube into Segments E (esophagus ) and G (stomach). The tube is perfused with water by a pump, and the pressure variations of each segment are detected with two pressure transducers and recorded by a polygraph. (B) Intraluminal pressure variations in Segments G (bottom) and E (top). The pressure of Segment G (stomach) was progressively increased by the pump, and, when it reached the value of about 11.5 mmHg, the magnets, simulating the sphincter, were detached, so that the pressure in Segment E (esophagus) started to increase, mimicking gastroesophageal reflux and reaching the level of Segment G. Once the pump stopped, the pressure dropped and the magnets adhered again, closing the passage. Exchanging the Segment E for G and Segment G for E, this sequence of events may represent the passage of a bolus through the zone squeezed by the magnets. From: Ref.[7] (Reprinted with permission).
Meanwhile, a sophisticated magnetic device to reinforce the lower esophageal sphincter based on the same mechanism was manufactured, which appeared on the clinical scene in 2008. It was experimented on patients with gastroesophageal reflux by Bonavina et al., under the name of LINX magnetic sphincter augmentation (MSA), produced by TORAX Medical, Inc. Share View, Minnesota, USA[8]. Subsequently, in 2010, Lehur et al. published an article concerning the treatment of patients with fecal incontinence by means of a magnetic device named FENIX magnetic anal sphincter augmentation (MAS) produced by the same company, which was similar to the previous one[9].
In the years following, many other studies with MAS were performed, and today the time has come to assess the successes and failures of this device, as well as its drawbacks and complications, before considering the present perspective.
THE MAS “MAGNETIC COLLAR” ANTI-INCONTINENCE SYSTEM
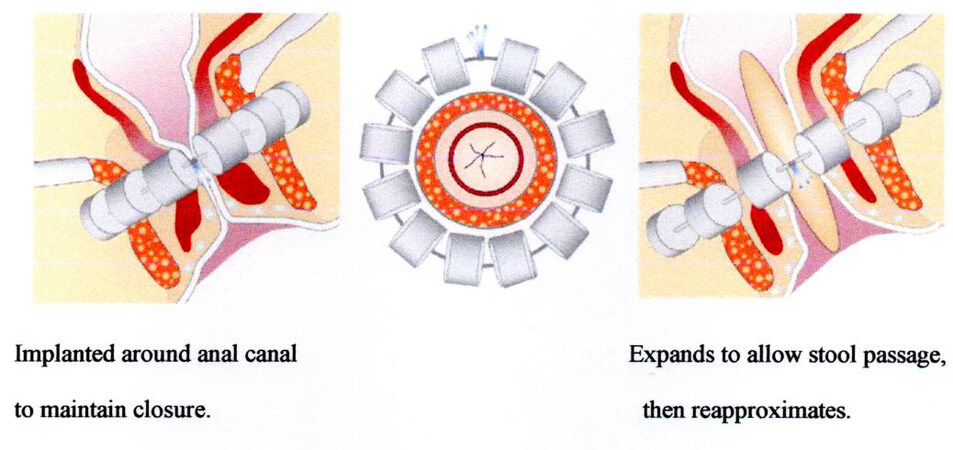
The magnetic anal sphincter[10] is made up of a series of titanium beads with magnetic cores connected with a flexible titanium wire along which they may slide one against the other, being attracted by their magnetic force [Figure 2][9]. This string of beads, also called a “magnetic collar”, is surgically placed in a tunnel around the anal canal, adapting the number of magnets to the previously measured circumference to surround and tighten[11]. The increase in pressure inside the anal lumen during the evacuation moves the beads away along the wire, widening the collar and consequently the anal lumen, thus enabling the passage of feces. After evacuation, the endoluminal pressure decreases and the collar tightens again, closing the lumen.
Figure 2. Magnetic anal sphincter augmentation device: closed (left); and open (right). From: Ref.[9] (Reprinted with permission).
Effectiveness of the MAS device in preventing fecal incontinence
The first report of the effects of this “magnetic collar” on fecal incontinence[9] was not very satisfactory, as it showed an improvement at six months in only 5 of 14 patients (all females). In these patients, the number of FI episodes/week decreased from 7.2 to 0.7 and the Wexner Continence Score from 17.2 to 7.8, whereas the FI Quality of Life Score (FIQoL) improved in all domains. After the first slightly disappointing study, in the subsequent years, there were others [Table 1], some of which with satisfactory results[12,13] and others completely negative[14], whereas another with favorable results[15] was demoted by numerous adverse events. More recently, a single-center study was published by Kim et al. collecting 45 patients (43 females) with a mean follow-up of 36 months (range 6-84 months), some of whom had been included in other publications and the MOS-STIC trial (MOS, Magnets Or Stimulation; STIC, “Soutien aux Technologies Innovantes et Coûteuses”)[9,15-17]. This study did not add significantly better results regarding Cleveland Clinic Incontinence Score (CCIS) and FIQoL with respect to those of the previous studies, but it established an interesting correlation between patient satisfaction and a reduction of FI episodes of ≥ 50% or a postoperative decrease by ≥ 5.5 points of CCIS. In this study, 48% of patients declared they were satisfied in correlation with the postoperative decrease of CCIS by ≥ 5.5 points. Furthermore, the authors performed an analysis of the causes of success or failure in patients implanted with MAS. They concluded that factors such as the origin of FI, previous damage to the sphincter, and its manometric values did not influence the outcome, whereas the only independent predictive factor for success after MAS implantation was no previous FI surgery. To better evaluate the results of MAS treatment, it was deemed necessary to compare them to those of other surgical therapies for FI.
Clinical effectiveness against FI and adverse events reported in a series of the most representative single- and multicenter studies after MAS implantation
| Barussaud et al., 2013[12] | Bridoux et al., 2014[14] | Pakravan et al., 2015[13] | Sugrue et al., 2017[15] | |
| Patients number and mean age | 23 (all females) 64 years (35-78) | 7 (6 females) 57 years (31-65) | 18 (15 females) 69 years (31-91) | 35 (34 females) |
| Mean follow-up with range (months) | 17.6 (6-36) | 9 (1-20) | 12-24.6 | 60 (6-72) |
| CCF-IS (average score) | From 15.2 to 6.9a,* | From 17.5 to 7.3a | From 15.7 to 7* | |
| FIQoL median index (average of 4 scales) | From 1.97 to 3.19a,* | No significant improvement in all 4 domainsa | Significant improvement in all 4 domains | From 8.2 to 12.8* |
| Wexner score | From 16 to 14.2a NS None reached > 50% reduction | |||
| Patient satisfaction | 69% | none | all | 53% |
| Device explantation or expulsion | 13% | 71% | 0 | 20% |
| Stoma creation | 1 | 1 | ||
| Difficulty in evacuation | 17% | 20% | ||
| Rectal perforation during surgery | 1 | 1 | ||
| Local pain | 14% | 29% | 29% | 14% |
| Infection | 43% | 5 | 11% | |
| Bleeding | 9% |
The comparison of MAS with Acticon Neosphincter to artificial bowel sphincter (ABS) and sacral nerve stimulation (SNS), albeit carried out in two small nonrandomized studies by Wong et al. [Table 2], showed that MAS did not obtain significantly better improvements in continence and quality of life as compared with the two other groups[18,19]. The only differences of MAS with ABS were in the lower number of devices “gone out of action” and in the fewer cases with fecal impaction and constipation. In the comparison with SNS, similar adverse events were observed in both groups. However, the follow-up of patients with ABS was about three times longer than those with MAS. Furthermore, it is important to observe that continence results similar to those of MAS with fewer adverse events have also been obtained by some anal canal narrowing techniques, such as anal slings[20] and bulking infiltrations[21] [Table 3]. However, most of these studies were characterized by follow-ups shorter than those of MAS and by patients with less severe FI. Consequently, these anal canal narrowing techniques require other prospective, randomized controlled studies to be considered as an alternative to MAS. Finally, a comparison between the two FI treatments MAS and SNS was the target of two multicenter, prospective, randomized, interventional, controlled trials announced in 2016, the French MOS-STIC[17] and the English SaFaRI[22]. The English SaFaRI trial in 2021 concluded that the success of FENIX was lower than previously reported, with high postoperative morbidity[23], whereas I have not been able to find the outcome of the other trial.
Comparison between the clinical outcomes of patients undergoing magnetic anal sphincter and artificial bowel sphincter implantations drawn from the study by Wong et al. (2011)[18] and a comparison between those of magnetic anal sphincter and sacral nerve stimulation from the study by Wong et al. (2012)[19]
| Magnetic anal sphincter | Artificial bowel sphincter | Magnetic anal sphincter | Sacral nerve stimulation | |
| Number of patients | 10 (10 women) | 10 (10 women) | 12 | 16 |
| Mean follow-up (months) | 8 (range, 6-13) | 22.5 (range, 6-72) | 18 (range, 8-30) | 22 (range, 10-28) |
| Jorge Waxner median score | From 17 to 6* | From 16 to 4* | From 16.5 to 6* | From 15 to 11.5* |
| FIQoL median score | From 2.03 to 3.51* | From 1.80 to 3.63* | Significant improvement in all 4 components | Significant improvement in all 4 components |
| Resting anal pressure cm H2O (median) | From 35 to 58.5* | From 34 to 75* | From 42.5 to 54* | From 34 to 33 |
| Device explantation, extrusion, or stopped working | 1 extrusion (spontaneous) 1 stopped working | 4 revisions 2 explantations 2 stopped working | 1 extrusion (spontaneous) | 1 explantation |
| Fecal impaction, constipation, or anti-diarrheal | 1 impaction 1 constipation | 2 impactions 4 constipations | 1 impaction 1 constipation 2 antidiarrheals | 1 constipation 6 antidiarrheals |
| Infections | 1 | 1 | ||
| Bleeding | 2 | 2 | ||
| Pain | 1 |
Comparison of the clinical outcomes after MAS device implantation from the study by Sugrue[15], the insertion of perianal elastic band from the study by Devesa et al.[20], the intra-anal injection of collagen from the study by Maslekar et al.[21]
| Magnetic anal sphincter | Elastic band perianal sling | Collagen intra-anal injection | |
| Number of patients | 35 (34 women) | 33 (20 women) | 100 (70 women) |
| Mean follow-up (months) | 60 | 65 | 36 |
| Symptom subjective improvement | 53% | 97% | 68% |
| Incontinence severity (average scores) | From 15.7 to 7* (Cleveland Clinic Incontinence Score) | From 15 to 7* (Jorge-Wexner Score) | From 14 to 8* (Cleveland Clinic Incontinence Score) |
| FIQoL median index (average of the 4 scales) | From 8.2 to 12.8* | From 7.8 to 14.3* | Not done |
| Device explantation or reoperation | 7 explantations (infections, erosions, and ineffectiveness) 1 creation of stoma for fecal impaction | 13 sling removals (sling breakage, infections, and erosions), with 10 reinsertions and further removals in 3 | 38%: 2nd injection 15%: 3rd injection |
| Adverse events (total) | 30 | 13 | None |
| Difficulty in evacuation | 20% | None | None |
| Pain | 14% | None | None |
| Erosion | 11% | 6% | None |
| Infection | 11% | 12% | None |
| Bleeding | 9% | None | None |
Adverse events and malfunctions of MAS
The assessment of the causes of adverse events and the analysis of malfunctioning mechanisms of MAS may be useful, not only to characterize this device but also to conceive an alternative to this magnetic sphincter devoid of its flaws. The rate of major complications that led to explantations and extrusions of the device and stoma creations reported in various studies[9,12-16,18,19] may reach a mean value of 18.3%, which is higher than that after SNS (5%)[24] but less than after ABS (24%)[25]. Patients with previous anorectal surgery may have a higher incidence of adverse events, for which Kim et al. suggested that these patients should be excluded from the MAS implantation by protocol[16]. During the creation of the tunnel around the anal sphincter, there is a risk of rectal perforation, which prevents device implantation[12,13], whereas after explantation, the defecatory function is compromised and a stoma creation is sometimes necessary[9,15]. In some cases, the useless device was left “in situ” and a stoma was created to avoid further complications[15]. Another complication is represented by the erosions of the anorectal wall, whose frequency is much higher than that after the “magnetic collar” LINX implantation for GER[26]. These erosions are likely caused by the padding of the “magnetic collar” FENIX against the rectal wall, together with the constriction due to the attraction of the magnets. In some cases, the deepening of the erosion up to the mucosa may cause frequent bleedings and can lead to the device penetrating into the rectal lumen, followed in rare cases by its spontaneous expulsion[9,12]. The device sometimes may harm the tissues so much that not only it is impossible to insert into another one, but it becomes necessary to make a stoma[9,12,15].
The appearance or worsening of incontinence and constipation, up to fecal obstruction, which begins to manifest itself some time after insertion, is likely due to an intervening malfunctioning of the device. In fact, although the working mechanism of MAS for closing and opening the anal canal denotes a high engineering skill and the device on the bench works perfectly, once implanted in an organism, it has to deal with the biological reaction of the tissues. Fibrotic production takes place around the device, as demonstrated by the necropsy carried out after 44 weeks in pigs with a similar “magnetic collar” implanted, which appeared encapsulated in fibrous tissue[27]. This phenomenon was also confirmed in some patients in whom the LINX device for LES was explanted for serious complications[28,29]. The fibrosis around the device could likely, in some way, hamper the detachment and reattachment of the magnetic beads, which must slip along the wires to open and close the rectal lumen[30]. The anal “magnetic collar” also inevitably follows the same destiny of being encapsulated in fibrous tissue, as observed in a series of studies in dogs[9]. Furthermore, the fibrous tissue becomes increasingly hard and rigid over time, and it is reasonable to suppose that in some cases, it could interfere with the movements of the magnetic beads of the device in an open or closed position, leading to incontinence or defecation difficulty, respectively. This phenomenon could explain the cases of worsening incontinence as well as the appearance of defecatory dysfunctions complained of by some patients. However, if, by hypothesis, the MAS device is completely blocked in the opening state by fibrosis, it could still be able to prevent the loss of feces. In fact, the “magnetic collar” could pad the wall of the anal canal, mimicking the anti-incontinence effect of a sling or a bulking agent. On the other hand, if this padding against the canal wall becomes too strong, an impairment of the evacuation and other complications described above could take place. A defecatory dysfunction or incontinence could also occur as a result of an incorrect length of the “magnetic collar” placed inside the tunnel around the anal sphincter. If the device is too tight, an obstruction or a difficult defecation could take place, whereas if it is too large, a liquid leakage may occur. The same phenomenon could also occur during device insertion. When adding a bead, the collar comes out too wide, giving rise to leakage; conversely, by not adding it, the collar becomes too tight, causing difficulty in evacuation. The decision can be taken based on the consistency of the stool: adding the bead with a solid stool and avoiding it with soft stool[9,12].
Comment on the results of MAS treatment
As previously reported, the clinical effectiveness of MAS is comparable to that of other methods of treatment, such as ABS[18] and SNS[19]. All these techniques, however, are unable to prevent minimal loss of stool, especially liquid stool, and even flatus[13,16]. Considering the causes of success or failure after MAS implantation, only previous surgical interventions, such as those of SNS, ABS, and injection of bulking agents, may prognosticate a negative result[13,16]. Furthermore, Pakravan et al. argued that implantation of MAS in patients, e.g., those of Bridoux et al., who are younger and more dynamic, with respect to those of their own study, may lead to worst results[13,14]. They concluded that better results with MAS implantation are obtained with older patients and those with a sedentary lifestyle.
Despite the lackluster performance of MAS treatment and the not negligible number and severity of adverse events, the MAS system was approved by US Food and Drug Administration (FDA) as a Humanitarian Device Exemption for its use in patients with FI who do not respond to SNS. Consequently, the implantation of MAS should not precede that of SNS, whereas the latter has been proposed as a surgical treatment in selected patients with end-stage FI[9,12,13]. However, the indication for patients with idiopathic moderate to severe FI remained uncertain, also because other surgical managements with fairly good effectiveness and a low or null risk of serious complications present themselves as valid candidates. The insertion of silastic slings[20] or the local infiltration of bulking agents, such as collagen[21] and Gatekeeper[31], proved to be valid in the comparison with MAS [Table 3], not only for anti-incontinence efficacy, whose shorter duration in the long term is offset by easy repeatability, but also for the scarcity of adverse events, simplicity of realization, and low cost. Bulking agent injection or perianal elastic slings could compete with MAS in the treatment of borderline severe FI, whereas MAS was considered for patients with severe incontinence refractory to other major surgical treatments[16].
However, while the indications for MAS were being discussed and other studies were being conducted and planned to compare MAS and SNS, as mentioned above, bombshell news arrived that took everyone aback and disrupted those programs. Torax Medical, part of the group Ethicon Johnson and Johnson, decided to discontinue sales and clinical studies on the FENIX continence restoration system, i.e., MAS. A severe protest was raised by Lehur et al. in an Editorial published in 2020[32], complaining that the industry would, practically speaking, deprive all incontinent patients worldwide of an effective possibility to efficaciously fight severe fecal incontinence, being the result of the ABS device lower than expectations, mostly due to a high rate of complications. He concluded that, given the fact that the artificial anal sphincter is practically in a deadlock, it is time to (re)develop a reliable artificial anal sphincter device. On the grounds of the decision to suspend the FENIX availability, one may wonder if the magnetic solution for FI has reached a dead-end. I do not believe that, but I am certain that MAS was only an inadequate realization of a good idea, as I wrote in 2015[33], and that another type of magnetic device, more efficient and with a lower risk of complications, could be realized, as explained below.
THE NEW ERA OF THE “TWO PLAQUES” ANTI-INCONTINENCE
For the reasons expressed above, I believe that this is the moment to develop another anti-incontinence magnetic device following the original idea with two magnetic plaques to reinforce the incontinent anal sphincter.
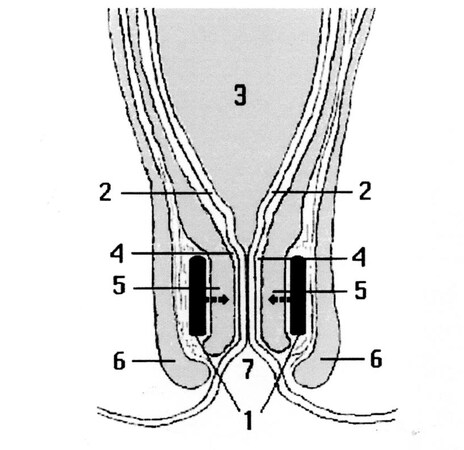
This new anti-incontinence system consists of two small plaques, which are to be placed between the external and internal muscle bundles [Figure 3][34], or outside them, positioned on the antero-posterior and longitudinal plan, with the opposite polarities face to face in order to attract each other, squeezing the anal canal as pliers. These two plaques covered by a biocompatible and soft material must be fixed to the surrounding tissues with suture thread passing through appropriate holes of the plaques. When the endoluminal pressure increase induced by defecation exceeds the attraction force of the magnets, they become detached, thereby opening the anal lumen. When the stools have been expelled, the endoluminal pressure reduces to a level lower than the force of attraction of the two plaques, which approach again, closing the lumen.
Figure 3. Schematic section following a vertical frontal plane of the recto-anal region showing the pair of magnets in profile (1) inserted between the muscular bundles of the internal (5) and external (6) anal sphincters, with the opposite polarities face to face attracting each other. Note: (2) mucosa; (3) rectal ampulla; (4) submucosa; (7) anus. From: Ref.[34] (Reprinted with permission).
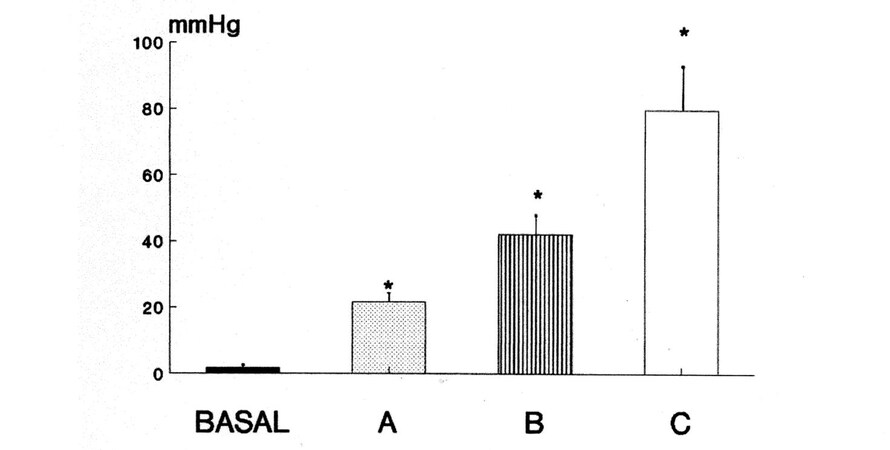
The force of this magnetic closure was evaluated in a pilot study[34] in a series of porcine anatomical preparations weighing from 25 to 35 kg. The three pairs of magnets tested in the experiment were ovoidal in shape and showed a diameter of 20-30 mm, a thickness of 1.5-2.5 mm, and were made of different magnetic materials that had different forces of attraction: one was neodymium, which is stronger than ferrite and more powerful than plastoferrite, the other two materials. The endoanal pressure was measured manometrically, averaging the values of three pull-throughs of a slight side-hole catheter perfused by means of a pump and joined to a Statham P23 Db pressure transducer and a Beckman R 612 polygraph to record the pressure values. The mean endoanal pressures obtained before and after implantation of each magnetic couple were compared with the basal value by means of the Student t-test. The results are reported in Figure 4. The pair of neodymium magnets showed an endoanal pressure of 79.7 ± 13.1 mmHg (mean ± SD), whereas those of ferrite and plastoferrite displayed values of 42.1 ± 5.6 and 21.6 ± 4.6 mmHg, respectively. All of these values of endoanal pressure were significantly higher than the basal value of 1.72 ± 0.71 mmHg. The results of this experiment demonstrate that we may choose a pair of magnets that, when implanted outside the anal canal, creates a local zone of high pressure with values higher than the previously measured value of the patient with fecal incontinence.
Figure 4. Anal pressure measured manometrically in basal conditions and after the insertion of the magnets made of: plastoferrite (A); ferrite (B); and neodymium (C). * P < 0.05. The endoanal pressure after the insertion of neodymium magnets was 79.7 ± 13.1 mmHg (mean ± SD), after ferrite magnets 42.1 ± 5.6 mmHg, and after plastoferrite magnets 21.6 ± 4.6 mmHg, all of them significantly higher than the pressure recorded in basal conditions (1.72 ± 0.71 mmHg). From: Ref.[34] (Reprinted with permission).
Advantages of the “two-plaque” system
As explained above, unlike the other anti-incontinence systems that narrow more or less consistently and continuously the anal lumen and create an obstacle to the passage of stool during defecation, the “two-plaque” magnetic system realizes a “dynamic closure” of the anus. In the basal condition, the two plaques keep the anus closed, whereas during defecation, they detach, leaving an easy passage of feces. Furthermore, this system possesses several theoretical advantages over the MSA system consisting of a collar of magnets. As explained above, the latter device, albeit denoting a skillful engineering quality and operating perfectly at the workbench, once placed into an organism, is progressively wrapped by a coating of fibrous tissue, which with time becomes stiffer and could cause its dysfunction in some cases. On the contrary, the “two-plaque” system does not have mechanical sliding parts that could be blocked by fibrin deposition and, therefore, is not subjected to this drawback. In fact, the fibrin coating around each magnetic plaque does not hinder the attraction force that acts through the lumen of the anal canal, so that the magnetic plaques are free to approach and separate. Moreover, the fibrin encapsulation, instead of impeding their operative activity, may contribute to securing them in their cranny of the rectal wall, in addition to other fixing systems (hooks, anchors, suture stitches, biological glue, etc.), to avoid their expulsion.
Another advantage of this method, unlike the “magnetic collar”, lies in the possibility of adapting the force of closure to the local characteristics of each patient by choosing magnets with different attraction forces and sizes. In fact, the distance between the two plaques due to interposed tissues may vary from one patient to another, the force of attraction of the magnets varies with the square of the distance, and the weakness entity of the anal sphincter may also vary from one patient to another. Consequently, plaques with greater attraction force are required for greater distances and for weaker sphincters, and vice versa. The choice of magnets may be oriented by measuring with a manometric probe or other systems before implantation, as suggested by Bharucha et al., the anal sphincter tone, or better, during the magnet implantation, the endoanal pressure produced by magnets[35]. In this way, by choosing the magnets with the most suitable attraction force, a “tailored augmentation” of the anal sphincter can be obtained, on the one hand sufficiently high to prevent fecal incontinence and fluid leakage at rest[36] and on the other hand sufficiently low to be overcome by the endorectal pressure increase during defecation. This system of intraoperative manometric measurement and choice of the plaques more suitable by force of attraction, together with shapes that better fit their anatomical position and coverage with a soft bio-compatible material on the face towards the anal lumen, should avoid the complication of ischemia and erosions of the compressed tissues.
A further, not insignificant advantage is represented by the fact that the surgical procedure for implanting the plaques is presumably less complex with respect to that of the “magnetic collar”, which requires both the laborious creation of a tunnel around the anal canal, a procedure that may expose to rectal perforation[13], and the measure of its circumference with a sizing tool[11].
Furthermore, the plaques can be easily disinfected and sterilized, thus making the appearance of local infections more difficult, a not uncommon complication during this kind of operation for fecal incontinence.
Finally, the “two-plaque” system would cost less than the “magnetic collar” system, as regards both the device and the complexity and duration of the intervention.
This “two-plaque” system may be applied to reinforce any other gut sphincter that has lost its tone and function, for example, the LES, with the aim of preventing gastroesophageal reflux, as it has already been devised and experimented in ex vivo[37] and in vivo[38] animals.
FINAL CONSIDERATIONS
Nowadays, the disappearance from the market of the FENIX magnetic anal sphincter has left a void that must be filled with another device capable of adequately treating patients with severe FI, once an SNS or sacral nerve modulation (SNM)[39] attempt has failed. Indeed, the Acticon Neosphincter ABS, although it works satisfactorily, has some limitations and has turned out to be over time below expectations, especially as regards the adverse events[40]. Thus, it is desirable to develop a more reliable device whose performance must closely resemble that of a natural sphincter. To achieve this, researchers have to consider the development of a device based on magnetic activity. However, it is not worthwhile to waste time and money to restore a device similar to MAS after the negative conclusion of the English SaFaRI trial[23] and in view of the assessment of its mediocre effectiveness with too many adverse events, as highlighted in the present study. Considering the good results of the magnetic plaques, which have proved capable of augmenting LES pressure in animals, both ex vivo and in vivo, it would be right to move forward in this direction with experiments in vivo also for the anal sphincter, given the good results already obtained in animals ex vivo[33]. In addition, as described above, this magnetic system presents some plausible advantages as compared with the MAS system. It has simpler and presumably more reliable operational activity with an easier surgical procedure for implanting the plaques; it provides the chance to choose the most suitable plaques for each patient regarding the force of attraction, shape, and dimensions, guaranteeing the possibility of a higher level of sterilization; and it should be less expensive.
The development of this kind of magnetic device requires cooperation between researchers and a manufacturing company eager to fill this gap in the market while being aware that this new road is a long and arduous one, but worthwhile undertakingas, already claimed in a previous article[41].
DECLARATIONS
AcknowledgementThe author thanks Henry Monaco BA (Hons) for reviewing the English language.
Authors’ contributionsThe author contributed solely to the article.
Availability of data and materialsNot Applicable.
Financial support and sponsorshipNone.
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2022.
REFERENCES
2. Macmillan AK, Merrie AE, Marshall RJ, Parry BR. The prevalence of fecal incontinence in community-dwelling adults: a systematic review of the literature. Dis Colon Rectum 2004;47:1341-9.
3. Gagner M. History of Magnets Used in Surgery. In: Gagner M, editor. Magnetic Surgery. Cham: Springer International Publishing; 2021. pp. 19-25.
4. Jansen A, Brummelkamp WH, Davies GA, KlopperPJ, Keeman JN. Clinical applications of magneticrings in colorectal anastomosis. Surg Gynecol Obstet 1981;153:537-45.
5. Cope C, Clark TW, Ginsberg G, Habecker P. Stent placement of gastroenteric anastomoses formed by magnetic compression. J Vasc Interv Radiol 1999;10:1379-86.
6. Cope C, Ginsberg GG. Long-term patency of experimental magnetic compression gastroenteric anastomoses achieved with covered stents. Gastrointest Endosc 2001;53:780-4.
8. Bonavina L, Saino GI, Bona D, et al. Magnetic augmentation of the lower esophageal sphincter: results of a feasibility clinical trial. J Gastrointest Surg 2008;12:2133-40.
9. Lehur PA, McNevin S, Buntzen S, Mellgren AF, Laurberg S, Madoff RD. Magnetic anal sphincter augmentation for the treatment of fecal incontinence: a preliminary report from a feasibility study. Dis Colon Rectum 2010;53:1604-10.
10. Mantoo S, Meurette G, Podevin J, Lehur PA. The magnetic anal sphincter: a new device in the management of severe fecal incontinence. Expert Rev Med Devices 2012;9:483-90.
11. Kim M, Lehur PA. How to size the anal canal circumference when implanting a magnetic anal sphincter for fecal incontinence. Evolution and update of a new surgical technique. Dis Colon Rectum 2016;59:901-3.
12. Barussaud ML, Mantoo S, Wyart V, Meurette G, Lehur PA. The magnetic anal sphincter in faecal incontinence: is initial success sustained over time? Colorectal Dis 2013;15:1499-503.
13. Pakravan F, Helmes C. Magnetic anal sphincter augmentation in patients with severe fecal incontinence. Dis Colon Rectum 2015;58:109-14.
14. Bridoux V, Gourcerol G, Leroi AM, Ducrotte P, Michot F, Tuech JJ. Response to Barussaud et al.: the magnetic anal sphincter in faecal incontinence, is initial success sustained over time? Colorectal Dis 2014;16:145-6.
15. Sugrue J, Lehur PA, Madoff RD, et al. Long-term experience of magnetic anal sphincter augmentation in patients with fecal incontinence. Dis Colon Rectum 2017;60:87-95.
16. Kim M, Meurette G, Ragu R, Wyart V, Lehur PA. Functional results and quality of life following magnetic anal sphincter augmentation in severely incontinent patients. Ann Surg 2019;269:310-4.
17. Lehur PA, Wyart V, Riche VP. SaFaRI: sacral nerve stimulation versus the Fenix® magnetic sphincter augmentation for adult faecal incontinence: a randomised investigation. Int J Colorectal Dis 2016;31:1505.
18. Wong MT, Meurette G, Stangherlin P, Lehur PA. The magnetic anal sphincter versus the artificial bowel sphincter: a comparison of 2 treatments for fecal incontinence. Dis Colon Rectum 2011;54:773-9.
19. Wong MT, Meurette G, Wyart V, Lehur PA. Does the magnetic anal sphincter device compare favourably with sacral nerve stimulation in the management of faecal incontinence? Colorectal Dis 2012;14:e323-9.
20. Devesa JM, Vicente R. The use of a simple anal sling in the management of anal incontinence. Gastroenterol Rep (Oxf) 2014;2:136-9.
21. Maslekar S, Smith K, Harji D, Griffiths B, Sagar PM. Injectable collagen for the treatment of fecal incontinence: long-term results. Dis Colon Rectum 2013;56:354-9.
22. Williams AE, Croft J, Napp V, et al. SaFaRI: sacral nerve stimulation versus the FENIX magnetic sphincter augmentation for adult faecal incontinence: a randomised investigation. Int J Colorectal Dis 2016;31:465-72.
23. Jayne DG, Williams AE, Corrigan N, et al. Sacral nerve stimulation versus the magnetic sphincter augmentation device for adult faecal incontinence: the SaFaRI RCT. Health Technol Assess 2021;25:1-96.
24. Mellgren A, Wexner SD, Coller JA, et al. SNS Study Group. Long-term efficacy and safety of sacral nerve stimulation for fecal incontinence. Dis Colon Rectum 2011;54:1065-75.
25. Hong KD, Dasilva G, Kalaskar SN, Chong Y, Wexner SD. Long-term outcomes of artificial bowel sphincter for fecal incontinence: a systematic review and meta-analysis. J Am Coll Surg 2013;217:718-25.
26. Faucheron JL, Sage PY, Trilling B. Erosion rate of the magnetic sphincter augmentation device is much higher for anal incontinence than for antireflux. J Gastrointest Surg 2019;23:389-90.
27. Ganz RA, Gostout CJ, Grudem J, Swanson W, Berg T, DeMeester TR. Use of a magnetic sphincter for the treatment of GERD: a feasibility study. Gastrointest Endosc 2008;67:287-94.
28. Asti E, Siboni S, Lazzari V, Bonitta G, Sironi A, Bonavina L. Removal of the magnetic sphincter augmentation device: surgical technique and results of a single-center cohort study. Ann Surg 2017;265:941-5.
29. Harnsberger CR, Broderick RC, Fuchs HF, et al. Magnetic lower esophageal sphincter augmentation device removal. Surg Endosc 2015;29:984-6.
30. Bortolotti M. The “magnetic collar”: the ultimate solution for gastroesophageal reflux? Scand J Gastroenterol 2014;49:511-2.
31. Brusciano L, Tolone S, Del Genio G, et al. Middle-term outcomes of gatekeeper implantation for fecal incontinence. Dis Colon Rectum 2020;63:514-9.
32. Lehur PA, Christoforidis D, Meurette G. Artificial sphincters to treat severe fecal incontinence: currently in a deadlock. Dis Colon Rectum 2020;63:1017-9.
33. Bortolotti M. The magnetic anal sphincter: a seductive promise still not kept. Colorectal Dis 2015;17:824.
34. Bortolotti M, Ugolini G, Grandis A, Montroni I, Mazzero G. A novel magnetic device to prevent fecal incontinence (preliminary study). Int J Colorectal Dis 2008;23:499-501.
35. Bharucha AE. Pro: anorectal testing is useful in fecal incontinence. Am J Gastroenterol 2006;101:2679-81.
36. Hill J, Corson RJ, Brandon H, Redford J, Faragher EB, Kiff ES. History and examination in the assessment of patients with idiopathic fecal incontinence. Dis Colon Rectum 1994;37:473-7.
37. Bortolotti M, Grandis A, Mazzero G. A novel endoesophageal magnetic device to prevent gastroesophageal reflux. Surg Endosc 2009;23:885-9.
38. Dobashi A, Wu SW, Deters JL, et al. Endoscopic magnet placement into subadventitial tunnels for augmenting the lower esophageal sphincter using submucosal endoscopy: ex vivo and in vivo study in a porcine model (with video). Gastrointest Endosc 2019;89:422-8.
39. Jottard K, Van den Broeck S, Komen N, Bruyninx L, De Wachter S. Treatment Of Fecal Incontinence With A Rechargeable Sacral Neuromodulation System: Efficacy, Clinical Outcome, And Ease Of Use-Six-Month Follow-Up. Neuromodulation 2021;24:1284-8.
40. der Wilt AA, Breukink SO, Sturkenboom R, Stassen LP, Baeten CG, Melenhorst J. The artificial bowel sphincter in the treatment of fecal incontinence, long-term complications. Dis Colon Rectum 2020;63:1134-41.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Bortolotti M. Magnetic sphincter for anal incontinence: an update. Mini-invasive Surg 2022;6:45. http://dx.doi.org/10.20517/2574-1225.2022.33
AMA Style
Bortolotti M. Magnetic sphincter for anal incontinence: an update. Mini-invasive Surgery. 2022; 6: 45. http://dx.doi.org/10.20517/2574-1225.2022.33
Chicago/Turabian Style
Bortolotti, Mauro. 2022. "Magnetic sphincter for anal incontinence: an update" Mini-invasive Surgery. 6: 45. http://dx.doi.org/10.20517/2574-1225.2022.33
ACS Style
Bortolotti, M. Magnetic sphincter for anal incontinence: an update. Mini-invasive. Surg. 2022, 6, 45. http://dx.doi.org/10.20517/2574-1225.2022.33
About This Article
Special Issue
Copyright
Data & Comments
Data
 Cite This Article 3 clicks
Cite This Article 3 clicks











Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.