Surface oxidation promotes the flotation of ilmenite: a critical review
Abstract
Due to its high efficiency, ease of operation, and superior selectivity, flotation separation has emerged as a promising technique for the extraction of ilmenite from natural resources. In light of the solution chemistry of ilmenite, it is widely accepted that ferrous ions and ferrous hydroxy compounds serve as the primary active sites for collector adsorption across a broad range of slurry pH values. The commonly used collectors like sodium oleate and hydroxamic acid are capable of chemical bonding with Fe2+ to form complexes and then enhance the floatability of ilmenite. However, Fe3+ ions perform a higher affinity to both collectors rather than Fe2+, the formed stronger complexes are advantageous for enhancing the hydrophobicity of ilmenite and increasing the probability for air bubble attachment, resulting in an improved ilmenite flotation recovery. Consequently, how to maximize the conversion efficiency of Fe2+ to Fe3+ and provide additional Fe3+ active sites on ilmenite surface for collector attachment have become the hot spot. Herein, this review aims to firstly analyze the crystal structure and solution chemistry of ilmenite and then provide a concise summary of recent advances in different oxidation technologies for promoting the conversion of Fe2+ to Fe3+, including hydroxyl radicals oxidation, direct chemical oxidation, and thermal oxidation, and the in-depth activation mechanisms are well illustrated. Also, current challenges and perspectives in this field are discussed. This review would benefit the development of next-generation flotation techniques for earth-abundant titanium resources.
Keywords
INTRODUCTION
Titanium is one of the most valuable strategic resources owing to its several desirable properties, such as high resistance to heat and corrosion, superior strength-to-weight ratio, and biocompatibility, all of which allow for diversified applications in various fields[1,2]. The three most prominent titanium minerals are ilmenite (FeTiO3), rutile (TiO2), and titanite (CaTiSiO5). In particular, ilmenite is considered the most promising titanium-bearing mineral for extracting titanium due to its much higher abundance[3]. As a result of ilmenite's high density, paramagnetism, and electrical conductivity, the most frequent technologies for ilmenite recovery are gravity separation, high-intensity magnetic separation, and electrostatic separation. In spite of this, the aforementioned technologies are rapidly losing their efficacy as readily processed ores are depleted. The ilmenite-bearing ores now for exploitation become more complex in their geological origins, severely complicating any attempts for further extraction[4-6].
Because of its high selectivity, ease of operation, and good efficiency, flotation separation techniques[7-12] have been steadily gaining favor to extract ilmenite from its associated gangue minerals[13-15]. Given the modest inherent floatability of ilmenite, the development of various strategies to enhance its flotation efficiency has become a topic of significant interest[16-18]. Surface dissolution, microwave oxidation, ultrasonic pretreatment, oxidant activation, Fenton oxidation, roasting oxidation, and ion activation are among the most promising techniques for improving the floatability of ilmenite. These methods can be broadly classified into two categories based on their intrinsic characteristics: the first category involves introducing additional active elements that exhibit a stronger affinity for collector species, while the second category involves altering the existing valence state or atomic structure of the ilmenite surface[19-21]. Specifically, the predominant approach investigated within the first category involves the introduction of supplementary active compounds onto the ilmenite surface, such as Pb2+ and Cu2+[22,23]. The addition of Pb2+ to the ilmenite surface can result in the formation of Pb(OH)+ and Pb2+ species, generating durable active sites for collector adsorption. Furthermore, the newly created Fe-O-Pb complexes on the ilmenite surface demonstrate a heightened propensity to bind to collectors, further augmenting the selectivity of the separation process. Analogous to the role of lead, the inclusion of copper ions performs a similar function. Although the introduction of Pb2+ and Cu2+ can effectively improve the recovery of ilmenite and yield satisfactory results in practical applications, it is imperative to note that these heavy metal ions can persist in the liquid phase. If the subsequent solution is not appropriately treated, the release of such ions can lead to serious environmental damage, which may include soil and water pollution and even negative impacts on human health.
The second category may alternatively be referred to as surface oxidation since it encompasses modifications to the valence state of iron ions. The associated techniques possess the capability to facilitate the conversion of Fe2+ to Fe3+, consequently enhancing the affinity between ilmenite and collectors. The currently reported methods for ilmenite oxidation can be classified based on different oxidation characteristics, which include hydroxyl radical oxidation, direct chemical oxidation, and thermal oxidation. For example, Miao et al. and Yu et al. attempted to enhance ilmenite recovery by utilizing Fenton oxidation pretreatment to generate reactive hydroxyl radicals that facilitate the conversion of Fe2+ to Fe3+. Subsequently, a significant improvement in ilmenite recovery was observed[23-25]. Similarly, Shu et al.,
Obviously, the surface oxidation methods and the regulation of valence state are gradually attracting popularity in ilmenite flotation. Of particular significance, certain surface oxidation methods are more environmentally sustainable and energy-efficient compared to modification through the addition of heavy metal ions. To date, no comprehensive review has addressed the role of surface oxidation in promoting the flotation of ilmenite. The oxidation mechanisms of different methods, strategies for enhancing ilmenite floatability, comparative advantages and limitations of various approaches, and prospects for future research directions remain largely unexplored in the literature.
In this context, we would like to provide an introduction to the role that surface oxidation plays in controlling the valence state or atomic structure of ilmenite and, therefore, in enhancing ilmenite recovery. The crystal structure and solution chemistry of ilmenite are firstly introduced. Then, we review the most common technologies of surface oxidation, including hydroxyl radicals oxidation, direct chemical oxidation, and thermal oxidation. Finally, some solutions to the problems encountered by present surface oxidation methods and suggestions for their future development are provided.
CRYSTAL STRUCTURE OF ILMENITE
Ilmenite mineral, which has the structural formula of Fe2+Ti4+O3, is relatively comparable to that of hematite, although the oxygen layers in ilmenite are somewhat distorted. In the direction of the triad axis, pairs of Ti4+ ions alternate with pairs of Fe2+ ions; as a result, each cation layer is a combination of Fe2+ and Ti4+[34], as shown in Figure 1A and B. More precisely, the formula of ilmenite may be fully expressed as (Fe, Mg, Mn) TiO3 with only a limited amount of Mg and Mn. The elements like Mn, Mg and Cr have the potential to replace the Fe or Ti in the original ilmenite structure, while elements like Al, Si, Th, P, V and Cr are commonly incorporated into the ilmenite grains during chemical weathering[35]. The partial replacement of Fe2+ or Ti4+ in the original ilmenite lattice by Mg2+, Mn2+ and V4+ has a remarkable effect on the surface isoelectric points (IEPs) of ilmenite, therefore affecting the flotation process. In other words, the IEPs varied from 4.2 to 6.25 based on the degree of substitution. The increase of Mn content in an ilmenite sample was found to increase its lattice constants, unit cell volume and IEPs, while Mg and V content decreased them. The IEP has a good positive correlation with Ti content in the ilmenite crystal structure and a good negative correlation with Fe3+ content. At pH values lower than the IEPs, ilmenite is predisposed to attract negatively charged collector species. Conversely, at pH values higher than the IEPs, positively charged collector species can readily adsorb onto the surface of ilmenite[36,37]. In the context of ilmenite flotation, the optimum pH range for effective adsorption of commonly used collectors is typically observed between 4 and 10, implying that the predominant mechanism governing the interaction between the ilmenite surface and collectors is chemical adsorption, rather than electrostatic adsorption[36,37].
SOLUTION CHEMISTRY OF ILMENITE
As the primary interaction between ilmenite and frequently utilized collectors is via chemical adsorption, a comprehensive understanding of the solution chemistry of ilmenite is critical for the development of more selective collectors and the design of efficient flotation strategies.
Before the flotation process, crushing and grinding are two vital steps to obtain the completely liberated particles for subsequent effective recovery. The Ti-O and Fe-O bonds are susceptible to being broken when external forces are introduced, which enables the Fe2+ and Ti4+ ions on the ilmenite surface to be in strongly unsaturated states [Figure 2A][38-40]. The unsaturated Fe and Ti atoms have the potential to interact with OH- or H2O in an aqueous medium to produce distinct active forms for reagent attachment[39]. Additionally, the stability and prevalence of active forms are significantly impacted by the pH value, which exerts a decisive influence on the dominant species involved in subsequent interactions. In other words, the activity of the ilmenite surface is contingent upon the formation of Fe2+ and Ti4+ hydroxy compounds, as well as the extent of their dissolution in water[41,42].
Figure 2A illustrates the solution chemistry of ilmenite with regards to pH. It can be observed that when the pH value is less than 3, the majority of ferrous ions are dissociated from the ilmenite surface. The residual metallic ions predominantly consist of titanium in the form of Ti(OH)3+ and Ti(OH)22+, indicating that the primary active sites for reagent adsorption are titanium complexes in a highly acidic environment. When the pH value is greater than 4, the titanium ions predominate as Ti(OH)4, whereas ferrous ions present as
FeO·TiO2 + 3H2O 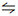 Fe2+ + Ti4+ + 6OH- (1)
Fe2+ + Ti4+ + 6OH- (1)
Fe2+ + Ti4+ + (m+n)OH-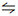 Fe(OH)m2-m + Ti(OH)n4-n (2)
Fe(OH)m2-m + Ti(OH)n4-n (2)
At present, the collectors employed for the recovery of ilmenite can be broadly classified into six categories: oxidized paraffin soap, tall oil, arsenic acid, oleic acid and its derivatives, phosphoric acid, and hydroxamic acid[38,43,44]. It is noteworthy that the most favorable pH range for ilmenite flotation using these collectors falls within 4-10[3]. In highly alkaline environments, ilmenite surfaces may undergo intense hydroxylation reactions, which can lead to competitive adsorption between hydroxyl ions and negatively charged collector species, thereby contributing to the undesirably poor flotation behavior of ilmenite[4]. As a result, the primary active sites for collector attachment are supposed to be ferrous ions and ferrous hydroxy compounds.
Of the aforementioned collectors, sodium oleate and hydroxamic acid have demonstrated the most favorable performance in the treatment of single ilmenite mineral. Although both collectors are capable of chemical bonding with Fe2+ to form complexes to make ilmenite more hydrophobic, the floatability of ilmenite still has much room for improvement. Fe3+ ions are supposed to have a higher affinity to sodium oleate and hydroxamic acid; when the pH value is less than 10, oleate ions prefer to interact with Fe3+ rather than Fe2+ due to the more negative value of ΔGθFe3+ [Figure 2B]; surface formed Fe(OL)3 (Ksp = 10-29.7) precipitates on ilmenite surface are claimed to be substantially less soluble than that of Fe(OL)2 (Ksp = 10-15.5), which is advantageous for enhancing the hydrophobicity of ilmenite and increasing the probability for air bubble attachment, resulting in an improved ilmenite flotation recovery. Consequently, how to maximize the oxidative efficiency of Fe2+ to Fe3+ and provide additional Fe3+ active sites on ilmenite surface for collector attachment have become the hot spot for ilmenite recovery improvement.
SURFACE OXIDATION
Based on the distinctive oxidative properties, surface oxidation techniques utilized to promote ilmenite recovery can be broadly classified into three categories: hydroxyl radical oxidation, direct chemical oxidation, and thermal oxidation. The subsequent sections will elaborate on the detailed oxidation mechanism and elucidate how surface oxidation promotes ilmenite flotation.
Hydroxyl radical oxidation
The rapid advancement of contemporary technology has brought with it a host of new challenges for the field of environmental protection, chief among which is the perennial problem of organic pollution, which must be addressed as quickly as humanly feasible. As a result, advanced oxidation processes (AOPs) - which use reactive oxygen species like hydroxyl (•OH)[45], superoxide (•O2-) radicals[46], and sulphates[47] to degrade organic substrates - have been actively supported and encouraged due to their high efficiency and ecologically favorable characteristics. The AOPs could be mainly classified into six types, including Fenton oxidation, ozone oxidation, ultrasonic oxidation, photocatalytic oxidation, wet air oxidation, and supercritical fluid oxidation[45,48]. The expanding use of AOPs in mineral processing engineering is being encouraged by the superior properties of AOPs, which include low cost, powerful oxidizing property, high efficiency, and environmental friendliness. The application of AOPs in mineral flotation is capable of modifying the surface properties of targeted minerals, which in turn affects the interaction between collectors and mineral surfaces. To date, the most widely used techniques for modifying the surface characteristics of ilmenite to accurately regulate its floatability are Fenton oxidation and ultrasonic oxidation, both of which involve the production of reactive hydroxyl radicals (•OH). In this section, we would like to provide a brief overview of how reactive hydroxyl radicals have helped to boost ilmenite recovery and a more in-depth description of the intricate interaction mechanism behind this increase. Furthermore, the merits and weaknesses of both methods for promoting ilmenite recovery will be discussed.
Fenton oxidation
Fenton oxidation has been demonstrated as a potent technique for the degradation of persistent organic pollutants. According to Equation (3)[49], Fenton oxidation includes the catalytic breakdown of hydrogen peroxide (H2O2) by ferrous iron (Fe2+) to create HO• radicals. The produced HO• radicals then oxidize the organic contaminant of interest [Equation (4)]. The first step of the process involves the rapid breakdown of H2O2 by Fe2+, which produces a significant quantity of HO• and then converts Fe2+ to Fe3+
H2O2 + Fe2+ → Fe3+ + HO• + OH- (3)
HO• + organic → Products (4)
HO• + Fe2+ → Fe3+ + OH- (5)
Miao et al. have employed Fenton oxidation as a pretreatment to facilitate the adsorption of collectors on the surface of ilmenite by promoting the transformation of Fe2+ into Fe3+[23,25]. The reagents including salicylhydroxamic acid and sodium oleate were employed as collectors in their work and they separately investigated the effects of Fenton oxidation pretreatment on the ilmenite flotation when using different types of collectors.
According to their findings, the effect of Fenton oxidation pretreatment on the enhancement of ilmenite recovery is primarily attributed to two facts: (1) the increase of Fe3+ active sites on the ilmenite surface reinforces the adsorption of collector on the ilmenite surface, as salicylhydroxamic acid and sodium oleate are vulnerable to forming much stronger complexes with Fe3+ than with Fe2+; (2) the existence of Fe3+ ions can move the isoelectric point of ilmenite in the direction of a more positive orientation, which in turn improves the adsorption between collectors and the surface of ilmenite. The main reactions could be described as the following equations:
Fe2+ + HO• → Fe3+ +(OH)- (6)
3OL- + Fe(OH)3 + OH- = Fe(OL)3 + 3OH- (7)
Furthermore, the decreased recovery of ilmenite when SHA is used as a collector may be related to the textural features of ilmenite as well as the collecting capacity of SHA.
Along the same lines as Miao et al.[23,25], Yu et al.[24] sought to modify the ilmenite surface by using Fenton chemicals. The extra copper-ammonia ions were introduced as supplementary active sites for collector adhesion. Based on Table 1, it should be noted that the introduction of extra copper-ammonia when using Fenton reagents is beneficial in further enhancing the ilmenite recovery and the potential activation mechanism is demonstrated in Figure 3.
Figure 3. Potential activation mechanism of H2O2 + [Cu(NH3)4]2+ on ilmenite recovery[24].
Ilmenite recovery under different use of Fenton reagents
| Chemicals | Collector | Concentration | pH value | Recovery | Increment of Fe3+ amounts | Ref. |
| H2O2 | NaOL | NaOL = 1.75 × 10-4 mol/L H2O2 = 1.0 × 10-4 mol/L | 6 | 85.5% | 20.16% | [23] |
| H2O2 | SHA | SHA = 5 × 10-4 mol/L H2O2 = 2.5 × 10-4 mol/L | 7 | 80% | 20.2% | [25] |
| H2O2 + [Cu(NH3)4]2+ | NaOL | NaOL = 1.0 × 10-4 mol/L H2O2 = 1.0 × 10-4 mol/L [Cu(NH3)4]2+ = 1.0 × 10-4 mol/L | 5.5 | 90.4% | 21.43% | [24] |
According to the data presented in Table 1, it is possible to draw a conclusion that when NaOL is served as a collector, the addition of extra copper-ammonia ions can provide more active sites for collector adsorption. This is supported by the fact that the increment of Fe3+ amounts is 21.43% when copper-ammonia is present, while it is only 20.16% when it is absent and extra Cu(II) active sites are also available for collector adsorption. More importantly, the incorporation of copper-ammonia has the potential to bring about an increase in ilmenite recovery of 90.4% while also causing a minor reduction in the dosage of NaOL from 1.75 × 10-4 mol/L to 1.0 × 10-4 mol/L.
In the context of ilmenite flotation, the Fenton oxidation pretreatment was implemented by directly introducing the Fenton reagents following pH adjustment and stirring the slurry for a duration of 2-3 min. Subsequently, the collectors were added and stirred for a specific time to facilitate the flotation process, as depicted in Figure 4. It is important to note that although the Fenton oxidation pretreatment is known to enhance the conversion efficiency of Fe2+ to Fe3+, the HO• radicals produced as a byproduct may also have an impact on the organic collectors. However, there is a lack of research investigating the potential effects of Fenton oxidation on the amounts of collectors and their subsequent adsorption onto the ilmenite surface.
Ultrasonic oxidation
Both Fenton oxidation and ultrasonic oxidation have the ability to create HO• radicals, and the difference is that there are no extra chemical reagents required for the latter one.
Ultrasonic oxidation may directly cause water to dissociate according to the following equations[26,52], releasing active radicals that can aid in the oxidation of Fe2+ to Fe3+. Some studies related to ultrasound oxidation are summarized in Table 2.
Effect of ultrasonic oxidation on the recovery of ilmenite
| Parameters | pH value | Collector | Concentration | Recovery | Increment of Fe3+ amounts | Ref. |
| Time: 300 s Power: 500W | 4-5 | NaOL | 2 × 10-4 mol/L | 89.54% | 24.97% | [26,27] |
| Time: 300 s Power: 500W | 6 ± 0.1 | NaOL | 2 × 10-4 mol/L | ~80% | 9.86% | [28] |
| Time: 300 s Power: 500W | 5 ± 0.1 | NaOL | 2 × 10-4 mol/L | 86.89% | 6.92% | [29] |
| Time: 450 s Power: 800W | 6 | NaOL | 2 × 10-4 mol/L | 75% | 41% | [30] |
H2O → H+ + OH- (8)
H+ + H+→ H2 (9)
OH- + OH- → H2O2 (10)
H+ + O2 → HO2 (11)
HO2 + HO2 → H2O2 + O2 (12)
OH- + H2O → H2O2 + H+ (13)
H2O2 + 2Fe2+ + 2H + = 2H2O + 2Fe3+ (14)
Ultrasonic pretreatment, as shown by Shu et al., significantly enhances ilmenite recovery by partly converting surface Fe2+ ions to Fe3+ ions, which may form strong complexes with NaOL in a mildly acidic environment. Furthermore, the IEP of the ilmenite treated by ultrasonic can be shifted from ~6.2 to ~4.2, which is advantageous for strengthening the chemisorption of NaOL on ilmenite surface[27]. More importantly, from their research work, it can also be found that under an acidic environment (pH 4-5), Ca2+ and Mg2+ atoms in the crystal structure of ilmenite tend to be dissolved off, and under a basic environment (pH 8-9), they are susceptible to re-adsorbing back to the ilmenite surface in the form of hydroxy complexes of CaOH(I) and MgOH(I) and precipitates of Ca(OH)2 and Mg(OH)2, subsequently helping to form a stable layer for collector adsorption[26,28]. Similarly, Wu et al.[29] and Luo et al.[30] attempted to evaluate the adaptability and feasibility of ultrasonic pretreatment on the flotation separation of ilmenite and titanaugite. Both studies demonstrated that the pretreatment by ultrasonic is helpful in promoting the conversion of Fe2+ to Fe3+ and thus enhancing the ilmenite recovery. Ultrasonic pretreatment is able to change the surface morphology of ilmenite and titanaugite and cause a severe dissolution of Ca2+ and Mg2+ ions from titanaugite to affect its subsequent recovery.
Similar to the Fenton oxidation pretreatment, the ultrasonic oxidation pretreatment was conducted prior to the flotation process. The ultrasonic probe was introduced into the flotation cell to activate the ilmenite surface. After that, the slurry pH and collectors were regulated and added successively. Unlike Fenton oxidation, researchers investigated that the ultrasonic oxidation ptreatment could significantly improve the -Qads values, demonstrating that the adsorption of collectors on activated ilmenite surface was more intensive than that of untreated surface. But the effect of reactive hydroxyl radicals on the collectors has not been provided as well.
Compared to the Fenton oxidation pretreatment, the advantages of ultrasonic oxidation are that: (1) there are no extra chemicals required, which can reduce the cost; (2) ultrasonic oxidation has the ability to regulate the morphology of ilmenite and can further affect the dissolution of associated ions, thus affecting the forms of the active sites on ilmenite surface.
Direct chemical oxidation
Oxidants like sodium hypochlorite (NaClO), potassium permanganate (KMnO4), sodium chlorite (NaClO2), and sodium ferrate (Na2FeO4) have been directly applied to accelerate the conversion of Fe2+ to Fe3+ on ilmenite surface and then strengthen the adsorption of sodium oleate on the newly provided active sites.
Cai et al. have investigated the potential of strong oxidants like NaClO and KMnO4 for altering ilmenite’s surface characteristics[22,31]. They demonstrated that both oxidants had the ability to boost the number of active sites for collector attachment by encouraging the conversion of Fe2+ to Fe3+. Additionally, the pretreatment by NaClO showed a more pronounced favorable impact than that by KMnO4 in terms of enhanced recovery. Under the optimal parameters, the recovery of ilmenite could be increased from 85% to 95% and 90% when NaClO and KMnO4 were used respectively. The primary reactions could be described as the following equations:
4FeO·TiO2 + O2 = 2Fe2O3 + 4TiO2 (15)
3Fe2+ + MnO4- + 4H+ = 3Fe3+ + MnO2 + 2H2O (16)
6Fe2+ + 3ClO- + 3H2O = 2Fe(OH)3 + 3Cl- + 4Fe3+ (17)
Fe3+ + 3OL- = Fe(OL)3 (18)
The transformation of Fe2+ into Fe3+ is beneficial to the improvement of the ilmenite recovery when sodium oleate is served as the collector. The fundamental mechanism of activation is described in the following ways: (1) partial conversion of Fe2+ to Fe3+ may lead to the formation of Fe(OH)3 precipitates on the ilmenite surface, and these precipitates can then react with oleate ions (OL-) through an ion-exchange process to yield Fe(OL)3; (2) Fe2+ and Fe3+ ions both can serve as active sites and may spontaneously react with oleate ions to generate Fe(OL)2 and Fe(OL)3, respectively; (3) among the slurry pH, Fe3+ can interact with OL- to form stronger complexes and this process can proceed readily than that of Fe2+.
Compared to the above study, Liao et al. have used Na2FeO4 as the oxidant, which may not only enhance the conversion of Fe2+ to Fe3+ on the ilmenite surface, but also supply additional Fe3+ active sites due to the hydrolysis of itself[32]. The equations for the main reactions are presented as follows:
H3FeO4+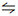 H+ + H2FeO4 (19)
H+ + H2FeO4 (19)
H2FeO4 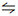 H+ + HFeO4- (20)
H+ + HFeO4- (20)
HFeO4- 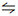 H+ + FeO42- (21)
H+ + FeO42- (21)
4FeO42- + 10H2O → 4Fe(OH)2+ + 3O2 ↑ + 4OH- (22)
4HFeO4- + 6H2O → 4Fe(OH)2+ + 3O2 ↑ (23)
FeO42- + 3Fe2+ + 4H2O → 4Fe(OH)2+ (24)
HFeO4- + 3Fe2+ + OH- + 3H2O → 4Fe(OH)2+ (25)
4Fe2+ + O2 + 4 OH- + 2H2O → 4Fe(OH)2+ (26)
It is possible to draw the following conclusion from these equations: the addition of Na2FeO4 is likely to result in the production of a significant number of Fe3+ active sites on the surface of ilmenite. The hydrolyzed compounds, such as HFeO4- and FeO42-, have a powerful oxidation effect on Fe2+. Additionally, both compounds are capable of undergoing a hydrolysis reaction to produce Fe3+ species, which provides a dual effect on the increment of active sites for collector attachment. The potential activation mechanism is depicted in Figure 5.
Figure 5. Potential activation mechanism of Na2FeO4 on ilmenite recovery[32].
The specific outcomes of the flotation experiments using four different chemical oxidants are summarized in Table 3 for comparison. Three oxidants (NaClO, KMnO4, NaClO2) all produce identical results since their function is to expedite the transformation of Fe2+ into Fe3+, but the fourth oxidant (Na2FeO4) is unique in that its hydrolysis may produce extra Fe3+ active sites for collector attachment.
Ilmenite recovery under different use of oxidants
| Oxidants | pH value | Oxidants dosage | Time | Collector concentration | Ilmenite recovery | Increment of Fe3+ amounts | Ref. |
| NaClO | 8 | 5 × 10-4 mol/L | 3 min | 1.75 × 10-4 mol/L | 95% | 21.64% | [22] |
| KMnO4 | 8 | 1 × 10-4 mol/L | / | 1.75 × 10-4 mol/L | 90% | 20.1% | [31] |
| NaClO2 | 8 | 80 mg/L | 3 min | 1.5 × 10-4 mol/L | 83.18% | 14.28% | [53] |
| Na2FeO4 | 6 | 2 × 10-5 mol/L | 3 min | 1.75 × 10-4 mol/L | 90% | 13.53% | [32] |
Thermal oxidation
Apart from the hydroxyl radicals oxidation and direct chemical oxidation, thermal oxidation is another alternative to realize the oxidation of Fe2+ to Fe3+ through thermal transmission. Roasting oxidation and microwave irradiation are thermal oxidation techniques based on two different heating ways. The improvement of ilmenite recovery and the difference between roasting oxidation and microwave irradiation will be discussed in this section.
Roasting oxidation
Roasting oxidation is a traditional heating method in which minerals are directly put into the heating equipment and heated under a desired temperature. The roasting process can accelerate the phase transition of ilmenite to rutile, hematite, and pseudo brookite by carefully adjusting the reaction temperature. This facilitates the conversion of Fe2+ to Fe3+ and changes the surface characteristics of ilmenite for subsequent recovery.
Here are some of the key oxidation processes that take place during the roasting process[34]:
2FeTiO3 + 1/2O2 → 2Fe2O3·TiO2 (27)
Fe2O3·TiO2 → Fe2O3 + 2TiO2 (28)
Fe2O3 + TiO2 → Fe2TiO5 (29)
When temperature ranges from 600 to 750 °C (45 min), Fe2O3·TiO2 phase particles are generated as an intermediate along with the formation of rutile and hematite [Equations (27) and (28)] and a further increase in temperature may stimulate the production of Fe2TiO5 [Equation(29)]. Under 600 °C, the capacity of ilmenite to chemically bond with oleate ions is greatly improved by the extensive coverage of iron(III) oxide small particles over almost all of its surface. As temperature rises, ilmenite’s structure is altered and the arrangement of cations on the surface of the particles is disrupted, leading to a decrease in flotation recovery. It was established by Mehdilo et al.[34] that an ilmenite recovery of 91% could be achieved with a NaOL dose of 3.65 × 10-4 mol/L, slurry pH of 6.3, and roasting oxidation temperature of 600 °C.
Microwave irradiation oxidation
Microwave irradiation offers superior heat retention compared to other heating methods due to its ability to heat materials uniformly throughout their volume at a consistent rate, whereas traditional heating methods rely on gradual surface heat transmission[54]. The ilmenite mineral samples employed for flotation were firstly pretreated through microwave irradiation with the help of a microwave oven under a certain power and time.
Mehdilo et al.[33] established that pretreatment of ilmenite by microwave irradiation is helpful for producing a superior flotation result under the same flotation scheme compared to that by roasting oxidation (the ilmenite recoveries are 91% and 94% when roasting oxidation and microwave irradiation are employed, respectively). Furthermore, the adsorption of oleate ions on ilmenite surface could be greatly enhanced under a relatively short time of microwave irradiation pretreatment. The flotation recovery of the rough concentrate could be increased from 84.6% to 92.2% after 1 min of microwave irradiation pretreatment and the value can be further increased to 92.7% with increasing microwave irradiation time. This is mainly due to the fact that most of the ferrous ions on the ilmenite surface were oxidized to ferric ions[55]. It should also be noted that the microwave irradiation pretreatment can reduce the adsorption capacity of collectors on the gangue mineral (titanaugite) as the active Ca and Mg sites on its surface would fall off, thereby realizing the selective recovery of ilmenite[30].
When the ilmenite ore is processed by microwave irradiation instead of roasting oxidation, the grinding process can release a greater quantity of ilmenite particles than roasting oxidation. This is because of the growth of microscopic fissures along the border between ilmenite and gangue minerals, as well as inside the ilmenite itself, as a result of heat moving from the interior to the outside. Furthermore, when the ilmenite was pretreated by microwave irradiation as opposed to roasting oxidation, a higher proportion of Fe2+ ions were transferred to Fe3+, and the density of Fe(OL)3 on ilmenite surface was more intense, thereby increasing the probability for air bubble attachment and resulting in an enhanced ilmenite flotation. Figure 6 illustrates the possible activation mechanism that is brought about by microwave irradiation and roasting oxidation.
Figure 6. Potential activation mechanism of microwave irradiation and roasting oxidation[33].
Although roasting oxidation performs the same effect as microwave irradiation oxidation, the pretreatment time and energy cost are not satisfactory and there is a phase transition during this process, which is not favorable for ilmenite recovery. Furthermore, microwave irradiation prior to grinding process can also promote the liberation of valuable particles during grinding, which can largely reduce the cost input and energy in the grinding process.
CONCLUSIONS AND FUTURE PERSPECTIVES
As a consequence of excessive mining and inefficient use of titanium resources, there is a growing need for answers to the issue of how ilmenite might be extracted from its associated gangue minerals in a manner that is both more effective and less harmful to the surrounding ecosystem. Surface oxidation technologies have been getting a lot of attention lately, and they are now seen as the most promising candidates to substitute the conventional addition of heavy metals like Pb2+ and Cu2+. The main conclusions of this review can be drawn as below:
(1) Mg2+, Mn2+ and V4+ have the ability to replace the Fe2+ and Ti4+ in the original ilmenite structure, which can change the IEPs of the ilmenite and the IEPs varied from 4.2 to 6.25 based on the degree of substitution. The optimum pH range for effective adsorption of commonly used collectors is typically observed between 4 and 10, implying that the predominant mechanism governing the interaction between the ilmenite surface and collectors is chemical adsorption rather than electrostatic adsorption.
(2) When the pH value is greater than 4, the titanium ions predominate as Ti(OH)4, whereas ferrous ions present as Fe2+, Fe(OH)3-, Fe(OH)2, and Fe(OH)+. The Ti(OH)4 compounds are relatively stable (KTi(OH)4 = 10-58.3), making them less likely to serve as active sites for reagents adsorption, which demonstrates that only ferrous ions perform as active sites in a weak acid or a weak base environment.
(3) The commonly used collectors like sodium oleate and hydroxamic acid are capable of chemical bonding with Fe2+ to form complexes to make ilmenite more hydrophobic. Fe3+ ions are supposed to have a higher affinity to sodium oleate and hydroxamic acid; when the pH value is less than 10, oleate ions prefer to interact with Fe3+ rather than Fe2+ due to the more negative value of ΔGθFe3+; surface formed Fe(OL)3 (Ksp = 10-29.7) precipitates on ilmenite surface are claimed to be substantially less soluble than that of Fe(OL)2 (Ksp = 10-15.5), which is advantageous for enhancing the hydrophobicity of ilmenite and increasing the probability for air bubble attachment, resulting in an improved ilmenite flotation recovery.
(4) Fenton oxidation and ultrasonic oxidation pretreatment contributes favorably to the enhancement of ilmenite recovery. The produced HO• radicals are able to encourage the conversion of Fe2+ to Fe3+ on the surface of ilmenite, and the SHA and NaOL collectors preferred to engage with Fe3+ rather than Fe2+ in order to form stronger complexes. In addition, the transformation of Fe2+ into Fe3+ has the ability to alter the zeta potential of ilmenite, which in turn has an effect on the collector attachment. The extra addition of ions like copper-ammonia in conjunction with Fenton oxidation can provide supplementary active sites for collector attachment.
(5) Direct chemical oxidation has the potential to accelerate the conversion of Fe2+ to Fe3+ on ilmenite surface and the increase of active sites is highly up to the oxidizability of their respective ability. Furthermore, the harvesting effect of collectors is also responsible for the ilmenite recovery.
(6) Both microwave irradiation oxidation and roasting oxidation can promote the conversion of Fe2+ to Fe3+. The microwave irradiation oxidation is susceptible to converting more Fe2+ to Fe3+ and forming a more intense Fe(OL)3 layer on ilmenite surface.
Notwithstanding current achievements, there are some crucial issues that need further exploration. The greatest difficulty of current surface oxidation technologies is that most of the reseaches have only been concentrated on the study of single minerals. The researches of reactive hydroxyl radicals oxidation and direct chemical oxidation only remain at the laboratory scale. Their applicability in real ores has not been systematically demonstrated yet. The reactive hydroxyl radicals also have the ability to degrade the organic collectors, but their effect on the collector has not been explained yet. Aside from reactive hydroxyl radicals oxidation, there are still other types of reactive radicals available for promoting the conversion of Fe2+ to Fe3+, such as superoxide (•O2-) radicals and sulphates. Furthermore, the effect of oxidative degree on the solubility of ilmenite is of significant importance and needs to be explored further. The economic value needs to be evaluated as well to verify their industrial feasibility. There is a pressing need for research into the use of surface oxidation technologies for the recovery of ilmenite from real ores, specifically addressing the challenges posed by gangue minerals and developing methods to increase flotation efficiency. Lastly, further studies are suggested to integrate experimental and computational tools to gain in-depth insights into the oxidation and flotation mechanisms, which would guide the design of novel oxidation processes and flotation reagents.
DECLARATIONS
AcknowledgementThe authors acknowledge the financial support provided by China Scholarship Council (CSC).
Authors’ contributionsMade substantial contributions to the conception and design of the study and performed data analysis and interpretation: Chen Q, Chen Z
Performed data acquisition, as well as provided writing review: Kasomo RM
Availability of data and materialsNot applicable.
Financial support and sponsorshipNot applicable.
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2023.
REFERENCES
1. Chen Q, Kasomo RM, Li H, et al. Froth flotation of rutile - an overview. Miner Eng 2021;163:106797.
2. Chen P, Zhai J, Sun W, Hu Y, Yin Z, Lai X. Adsorption mechanism of lead ions at ilmenite/water interface and its influence on ilmenite flotability. J Ind Eng Chem 2017;53:285-93.
3. Cai J, Deng J, Wang L, et al. Reagent types and action mechanisms in ilmenite flotation: a review. Int J Miner Metall Mater 2022;29:1656-69.
4. Zhai J, Chen P, Sun W, Chen W, Wan S. A review of mineral processing of ilmenite by flotation. Miner Eng 2020;157:106558.
5. Mehdilo A, Irannajad M, Rezai B. Chemical and mineralogical composition of ilmenite: Effects on physical and surface properties. Miner Eng 2015;70:64-76.
6. Mehdilo A, Irannajad M, Rezai B. Effect of crystal chemistry and surface properties on ilmenite flotation behavior. Int J Miner Process 2015;137:71-81.
7. Chen Z, Ren Z, Gao H, Lu J, Jin J, Min F. The effects of calcium ions on the flotation of sillimanite using dodecylammonium chloride. Minerals 2017;7:28.
8. Jin J, Gao H, Ren Z, Chen Z. The flotation of kyanite and sillimanite with sodium oleate as the collector. Minerals 2016;6:90.
9. Ren Z, Yu F, Gao H, Chen Z, Peng Y, Liu L. Selective separation of fluorite, barite and calcite with valonea extract and sodium fluosilicate as depressants. Minerals 2017;7:24.
10. Cheng G, Zhang M, Li Y, Lau E. Improving micro-fine mineral flotation via micro/nano technologies. Sep Sci Technol 2023;58:520-37.
11. Cheng G, Zhang M, Zhang Y, Lin B, Zhan H, Zhang H. A novel renewable collector from waste fried oil and its application in coal combustion residuals decarbonization. Fuel 2022;323:124388.
12. Cheng G, Zhang J, Su H, Zhang Z. A novel collector for high-sulfur bauxite flotation desulfurization. Sep Sci Technol 2023;58:86-100.
13. Li H, Chen Y, Zheng H, et al. Effect of geological origin of apatite on reverse flotation separation of phosphate ores using phosphoric acid as depressant. Miner Eng 2021;172:107182.
14. Chen Q, Tian M, Kasomo RM, et al. Depression effect of Al(III) and Fe(III) on rutile flotation using dodecylamine polyxyethylene ether as collector. Colloids Surfaces A Physicochem Eng Asp 2020;603:125269.
15. Li H, Mao Y, Zheng H, et al. Impact of geological origin on flotation separation of apatite from dolomite using β-naphthyl sulfonate formaldehyde condensate as depressant. Miner Eng 2022;176:107323.
16. Chen Z, Ren Z, Gao H, Qian Y, Zheng R. Effect of modified starch on separation of fluorite from barite using sodium oleate. Available from: http://hdl.handle.net/10453/134186 [Last accessed on 29 Mar 2023].
17. Zheng R, Ren Z, Gao H, Chen Z, Qian Y, Li Y. Effects of crystal chemistry on sodium oleate adsorption on fluorite surface investigated by molecular dynamics simulation. Miner Eng 2018;124:77-85.
18. Chen Z, Ren Z, Gao H, Zheng R, Jin Y, Niu C. Flotation studies of fluorite and barite with sodium petroleum sulfonate and sodium hexametaphosphate. J Mater Res Technol 2019;8:1267-73.
19. Li F, Zhong H, Wang S, Liu G. The activation mechanism of Cu(II) to ilmenite and subsequent flotation response to α-hydroxyoctyl phosphinic acid. J Ind Eng Chem 2016;37:123-30.
20. Zeng Y, Liu J, Ru S, Wen S, Wang Y. DFT study the adsorption of ethyl xanthate on the S-site of Cu-activated sphalerite (1 1 0) surface in the presence of water molecule. Results Phys 2019;13:102271.
21. Liu J, Zeng Y, Luo D, Wen S, Wang Y. Ab initio molecule dynamic simulation of Cu(OH)2 interaction with sphalerite (1 1 0) surface. Miner Eng 2018;122:176-8.
22. Cai J, Deng J, Wen S, et al. Surface modification and flotation improvement of ilmenite by using sodium hypochlorite as oxidant and activator. J Mater Res Technol 2020;9:3368-77.
23. Miao Y, Wen S, Feng Q, Liao R. Surface modification of ilmenite and flotation improvement using Fenton as an oxidant. Appl Surf Sci 2021;555:149749.
24. Yu P, Ding Z, Bi Y, Li J, Wen S, Bai S. Surface modification of ilmenite by introducing copper-ammonia ion and its response to flotation in H2SO4-H2O2 system. Miner Eng 2021;171:107102.
25. Miao Y, Wen S, Feng Q, Liao R. Enhanced adsorption of salicylhydroxamic acid on ilmenite surfaces modified by Fenton and its effect on floatability. Colloids Surfaces A Physicochem Eng Asp 2021;626:127057.
26. Shu K, Xu L, Wu H, Wang Z, Xu Y, Fang S. Influence of ultrasound pre-treatment on ilmenite surface chemical properties and collectors’ adsorption behaviour. Ultrason Sonochem 2019;57:98-107.
27. Shu K, Xu L, Wu H, et al. Effects of ultrasonic pre-treatment on the flotation of ilmenite and collector adsorption. Miner Eng 2019;137:124-32.
28. Fang S, Xu L, Wu H, et al. Influence of surface dissolution on sodium oleate adsorption on ilmenite and its gangue minerals by ultrasonic treatment. Appl Surf Sci 2020;500:144038.
29. Wu H, Fang S, Shu K, et al. Selective flotation and adsorption of ilmenite from titanaugite by a novel method: ultrasonic treatment. Powder Technol 2020;363:38-47.
30. Luo L, Wu H, Yang J, et al. Effects of microwave pre-treatment on the flotation of ilmenite and titanaugite. Miner Eng 2020;155:106452.
31. Cai J, Deng J, Yang H, et al. A novel activation for ilmenite using potassium permanganate and its effect on flotation response. Colloids Surfaces A Physicochem Eng Asp 2020;604:125323.
32. Liao R, Wen S, Liu J, Zuo Q, Zheng Y, Luo D. Flotation behavior and mechanism of ilmenite using ferrate as activator. Miner Eng 2022;178:107400.
33. Mehdilo A, Irannajad M. Comparison of microwave irradiation and oxidation roasting as pretreatment methods for modification of ilmenite physicochemical properties. J Ind Eng Chem 2016;33:59-72.
34. Mehdilo A, Irannajad M. Comparison of microwave irradiation and oxidation roasting as pretreatment methods for modification of ilmenite physicochemical properties. J Ind Eng Chem 2016;33:59-72.
35. Mehdilo A, Irannajad M, Rezai B. Effect of chemical composition and crystal chemistry on the zeta potential of ilmenite. Colloids Surfaces A Physicochem Eng Asp 2013;428:111-9.
36. Chernyshova IV, Ponnurangam S, Somasundaran P. Adsorption of fatty acids on iron (hydr)oxides from aqueous solutions. Langmuir 2011;27:10007-18.
38. Chen P, Zhai J, Sun W, Hu Y, Yin Z. The activation mechanism of lead ions in the flotation of ilmenite using sodium oleate as a collector. Miner Eng 2017;111:100-7.
39. Fan X, Waters KE, Rowson NA, Parker DJ. Modification of ilmenite surface chemistry for enhancing surfactants adsorption and bubble attachment. J Colloid Interface Sci 2009;329:167-72.
40. Fan X, Zhang Z, Li G, Rowson N. Attachment of solid particles to air bubbles in surfactant-free aqueous solutions. Chem Eng Sci 2004;59:2639-45.
41. Fan X, Rowson N. Surface modification and column flotation of a massive ilmenite ore. Can Ceram Q 2002;41:133-42.
42. Dyk JP, Vegter NM, Pistorius P. Kinetics of ilmenite dissolution in hydrochloric acid. Hydrometallurgy 2002;65:31-6.
43. Li F, Zhong H, Zhao G, Wang S, Liu G. Adsorption of α-hydroxyoctyl phosphonic acid to ilmenite/water interface and its application in flotation. Colloids Surfaces A Physicochem Eng Asp 2016;490:67-73.
44. Fang S, Xu L, Wu H, et al. Adsorption of Pb(II)/benzohydroxamic acid collector complexes for ilmenite flotation. Miner Eng 2018;126:16-23.
45. Mao Y, Zhang W, Li H, et al. Utilization of Fenton oxidation approach in wet phosphoric acid process for mitigating foam generation. Colloids Surfaces A Physicochem Eng Asp 2022;648:129297.
46. Yi H, Jiang M, Huang D, et al. Advanced photocatalytic Fenton-like process over biomimetic hemin-Bi2WO6 with enhanced pH. J Taiwan Inst Chem Eng 2018;93:184-92.
47. Mazivila SJ, Ricardo IA, Leitão JM, Esteves da Silva JC. A review on advanced oxidation processes: From classical to new perspectives coupled to two- and multi-way calibration strategies to monitor degradation of contaminants in environmental samples. Trends Environ Anal Chem 2019;24:e00072.
48. Liu X, Wu F, Qu G, et al. Application prospect of advanced oxidation technology in wet process phosphoric acid production. J Environ Chem Eng 2022;10:108868.
49. Alalm M, Tawfik A, Ookawara S. Comparison of solar TiO2 photocatalysis and solar photo-Fenton for treatment of pesticides industry wastewater: operational conditions, kinetics, and costs. J Water Process Eng 2015;8:55-63.
50. Munoz M, de Pedro ZM, Casas JA, Rodriguez JJ. Preparation of magnetite-based catalysts and their application in heterogeneous Fenton oxidation - a review. Appl Catal B Environ 2015;176-177:249-65.
51. Bello MM, Abdul Raman AA, Asghar A. A review on approaches for addressing the limitations of Fenton oxidation for recalcitrant wastewater treatment. Process Saf Environ Prot 2019;126:119-40.
52. Saikia BK, Dutta AM, Saikia L, Ahmed S, Baruah BP. Ultrasonic assisted cleaning of high sulphur Indian coals in water and mixed alkali. Fuel Process Technol 2014;123:107-13.
53. Hu Y, Ye G, Zuo Q, et al. Activation of ilmenite flotation by sodium chlorite in the sodium oleate system. Sep Purif Technol 2023;305:122506.
54. Stuerga, P. Gaillard, Microwave heating as a new way to induce localized enhancements of reaction rate. Non-isothermal and heterogeneous kinetics. Tetrahedron 1996;52:5505-10.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.




















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].