Environmental management of microplastics and additives: a critical review of treatment technologies and their impact
Abstract
Microplastics (MPs) and their associated chemical additives, such as bisphenol A (BPA), nonylphenol (NP), nonylphenol ethoxylate (NEPO), and tetrabromobisphenol (TBBPA), are widely recognized as significant environmental contaminants due to their widespread presence in ecosystems and their potential impact on human health. This review evaluates advanced treatment technologies, such as membrane filtration and oxidation processes, for mitigating these risks and highlights gaps in sustainability and efficiency. Actionable strategies for improving the removal of MPs and MP additives were presented in the assessment of innovative hybrid treatment tailored to different water matrices. The importance of anti-fouling technologies, comprehensive life cycle assessments (LCAs), and standardizing treatment methods to enhance the sustainability and applicability of these technologies were further highlighted. To further improve and optimize treatment processes and ensure sustainability, existing knowledge gaps must be addressed by framing a comprehensive understanding of the long-term ecological effects of MPs and their additives.
Keywords
INTRODUCTION
Plastics began gaining popularity in the 1940s and have since diversified into many types. Recently, annual global plastic production has increased to 359 million tons, with China accounting for 30% of this output[1]. The widespread use of plastics can be attributed to their convenience, affordability, light weight, and durability[1]. They are utilized in numerous sectors, including containers for food and beverages, thermal insulation materials, furniture for homes and offices, electronic gadgets, vehicle interiors, children’s toys, textiles, surface coatings, and medical equipment such as prosthetic joints, incubators, intravenous (IV) fluid bags, and drug delivery mechanisms[2]. However, managing plastic waste is a growing concern, as only about 15% of the plastics produced annually are recycled[3]. Thus, the main challenge with plastics is their long-lasting nature, with some lasting hundreds of years before breaking down. This durability contributes to plastic pollution in both land and water environments. Studies show that every year, between 1.15 and 2.41 million metric tons of plastic enter the ocean, leading to large areas of floating debris like the Great Pacific Garbage Patch, which covers about 1.6 million square kilometers[4]. Haque et al. (2023) reported that global plastic production increased from 1.3 million tons in 1950 to 359 million tons in 2018, expected to reach 634 million tons by 2025, highlighting the massive global increase in plastic production[5]. Among the various forms of plastic pollution, microplastics (MPs), and MP additives such as bisphenol A (BPA), nonylphenol (NP), and tetrabromobisphenol (TBBPA), have emerged as significant environmental contaminants. These particles are widespread in many ecosystems and pose a serious risk to human health, as well as terrestrial and aquatic environments, due to their persistence and ability to bioaccumulate[6].
Even though the treatment of MPs and MP additives is gaining momentum with new advancements and processes, several significant knowledge gaps persist[7]. The long-term ecological effects of MPs and their additives are not well understood[8]. Additionally, present treatment methods often fall short of complete removal and health effect mitigation of these contaminants, particularly in diverse environmental settings. Furthermore, the lack of standard operating procedures for the analysis and treatment of MPs in different water matrices complicates the evaluation and comparison of treatment performance[9].
In this study, a thorough review of the spread of MPs in the environment and their impacts on health, aquatic systems, and terrestrial systems will be presented. This work also aims to address the current gaps by providing a detailed assessment of advanced treatment technologies, including the innovative integration of membrane filtration with advanced oxidation processes (AOPs). The paper is structured as follows: an overview of the sources and types of MPs, followed by discussions on their environmental and health impacts. The bulk of the review evaluates several treatment techniques, emphasizing advanced oxidation and membrane filtration, with conclusions focusing on sustainability and the need for further research.
SOURCES AND TYPES OF MPS
MPs are small plastic particles, typically less than 5 mm in size, which can exist in various forms such as fibers, films, fragments, beads, and foams[10]. These particles are widespread in the environment due to their resistance to degradation and their persistent nature. MPs are classified into two primary types based on their origin: primary MPs, which are intentionally manufactured for specific purposes, and secondary MPs, which result from the breakdown of larger plastic materials[11].
Primary MPs
Primary MPs, like glitter and microbeads, are intentionally manufactured particles found in personal care products and industrial processes, contributing significantly to environmental contamination due to their small size and persistence[12].
Microbeads are another common form of MPs, and they are small spherical particles often found in personal care products such as exfoliants, cleansers, and toothpaste. These microbeads are added for their abrasive properties, aiding in the removal of dead skin cells or dirt. However, due to their tiny size and inability to be captured by wastewater treatment plants, they easily enter aquatic systems, contributing to widespread pollution[13]. Despite increasing regulatory efforts to ban microbeads in many countries, they remain a persistent problem in regions where regulations are not yet enforced or where existing products containing microbeads are still in use.
Secondary MPs
Secondary MPs are those that result from the breakdown of larger plastics under environmental stressors like UV radiation and mechanical abrasion into smaller particles over time[13-16]. This degradation occurs when macroplastics are exposed to prolonged environmental factors such as sunlight, weather, and moisture[17]. Research by Bajt highlights UV sunlight as the primary factor in this degradation process[18]. UV light breaks chemical bonds in the macroplastics, producing plastic fragments, fibers, or films. This process, known as photodegradation, increases the number of secondary MPs in the environment, contributing to their growing presence in various ecosystems. Mechanical abrasion and chemical interactions further accelerate plastic fragmentation. The continuous production of secondary MPs from plastic waste contributes to their persistent accumulation in marine and terrestrial environments, posing significant ecological challenges[19]. Figure 1 depicts the sources of MPs in the environment.
ENVIRONMENTAL IMPACT OF MPS
MPs have accumulated in significant quantities in both aquatic and terrestrial environments due to inadequate plastic waste management[20]. They often carry various micropollutants that can be harmful to the environment and human health, especially when they enter the food chain. The persistence of MPs in the environment makes understanding their full environmental impact essential for developing effective strategies to mitigate their consequences on both land and water ecosystems, as well as human health.
Impact on aquatic system
MPs in aquatic systems primarily originate from terrestrial environments due to industrial, agricultural, and domestic activities. These insoluble particles enter water bodies through various routes, including wastewater treatment plants, sewer floods, soil erosion, and precipitation[21]. Once in water bodies, MPs attract and accumulate organic and inorganic pollutants from their surroundings, facilitating the spread of contaminants across ecosystems. This contamination not only deteriorates water quality but also disrupts natural habitats and biodiversity. For instance, studies have shown that MPs can inhibit algae’s photosynthetic activity, leading to reduced growth rates, which has cascading effects throughout aquatic food webs[22]. Canniff et al. deduced that MPs can inhibit the photosynthetic activity of algae. For instance, the paper reports a 30% decline in the growth rate of the algae species Raphidocelis subcapitata due to exposure to MPs[23]. These findings illustrate that even at low concentrations, MPs can have cascading effects on aquatic food webs, ultimately impacting higher trophic levels, including humans.
Impact on terrestrial system
MPs are transported to the soil through natural soil processes like biopores and cracking, as well as through agricultural activities such as harvesting and plowing[24]. Their presence in the soil can significantly damage soil health by harming its microorganisms, as documented by various studies[25-27]. Additionally, improper disposal of plastic products and the accumulation of plastics in landfills pose major environmental challenges. Additionally, MPs block water drainage systems, contributing to flooding and mosquito breeding, which poses public health risks[28,29]. Furthermore, biodegradable MPs contribute to increased production of nitrous oxide (N2O), a strong greenhouse gas, thereby potentially intensifying global warming[30]. The accumulation of MPs in terrestrial environments not only threatens ecosystem health but also contributes to broader environmental issues such as climate change and water management challenges[31].
Impact on human health
MPs can enter the human body through inhalation, ingestion of contaminated food and water, or skin contact[32-34]. Their small size allows them to accumulate in human tissues, leading to oxidative stress, inflammation, and immune system disruptions[30,35]. Prolonged exposure has been linked to chronic conditions such as cardiovascular disease, respiratory issues, and even cancer[36,37]. MPs can also interfere with the human microbiome[28,38,39], potentially leading to metabolic disorders or immune dysfunction[40-43]. The long-term presence of MPs in human tissues underscores their potential for causing cumulative damage, raising serious concerns about public health implications, especially in highly exposed populations. Figure 2 illustrates the environmental impact and sources of MPs.
MP ADDITIVES
MP Additives are chemicals incorporated during the manufacturing process to enhance the physical and chemical properties of the plastics. These additives improve color, transparency, and resistance to temperature, light radiation, moisture, and humidity. Despite their utility in enhancing plastic properties, they pose significant environmental contamination risks, affecting soil, water, air, and human health, similar to what was discussed earlier[44]. Life cycle assessments (LCAs) of these additives have shown that current plastic recycling processes are not capable of effectively removing these harmful substances[45]. Common chemical additives used in the production of MPs include BPA, NP, nonylphenol ethoxylate (NEPO), and tetrabromobisphenol A (TTBA). Given their persistence in the environment, these additives continue to accumulate in ecosystems, compounding the challenges associated with plastic pollution and requiring comprehensive strategies for mitigation.
BPA
BPA, also known as 4,4’-(propane-2,2-diyl) diphenol, is a synthetic organic compound widely used in the manufacture of certain plastics and epoxy resins. Its properties enhance the transparency, durability, and heat resistance of these materials, making it a fundamental ingredient in various consumer products. BPA is primarily found in polycarbonate (PC) plastics, which are used to produce items such as water bottles, food storage containers, eyeglass lenses, and dental sealants. It is also used in epoxy resins, which coat food and beverage cans and line water pipes to prevent corrosion. The European Union Water Framework Directive has classified BPA as a priority pollutant due to its environmental impact. It enters the environment through sources such as wastewater treatment plants, runoff, landfill leachate, and industrial discharges. BPA is also classified as one of the endocrine-disrupting compounds (EDCs)[46,47]. Santoro et al. argued that BPA possesses estrogen-like and anti-androgenic properties, leading to damage in various tissues and organs, including those of the reproductive, immune, and neuroendocrine systems[48]. Similarly, research conducted by Seachrist et al. concluded that early-life exposure to BPA is a risk factor for breast and prostate cancer[49]. The widespread use of BPA in consumer products and its presence in various environmental mediums underscores the need for more stringent regulations and safer alternatives[50].
NP
NP encompasses a group of isomeric compounds, including 4-NP, phenol, p-nonyl-, 4-p-NP, and 4-nonyl-, para nonyl. NP, known as a major environmental contaminant, is present in different environmental mediums, including soil, air, bodies of water, and potable water systems. It is mainly utilized in producing antioxidants, lubricant oil additives, and NEPOs surfactants. As an endocrine disruptor, NP has been extensively studied for its effects on the growth, development, reproduction, and behavior of organisms, including impacts on future generations[51]. NP mimics the natural hormone 17β-estradiol, binding competitively to estrogen receptors and disrupting the endocrine systems of higher organisms[52,53]. Additionally, studies have shown that NP can induce the proliferation of breast tumor cells[54]. The ubiquitous presence, persistence, and resistance to degradation of NP in the environment pose serious concerns[51,55], highlighting the urgent need for rigorous monitoring and effective mitigation strategies to remove NP from water and wastewater systems. Failure to address NP contamination may lead to long-term ecological and human health risks, emphasizing the importance of identifying alternative chemicals and developing more efficient removal technologies[56]. The potential for long-term environmental and public health harm due to NP contamination highlights the need for developing more potent removal techniques and alternative chemical sources.
TBBPA
TBBPA is commonly used as a reagent in the production of epoxy and PC resins, which are commonly found in electronic devices, furniture, and various types of equipment[57,58]. It is extensively present in both abiotic and biotic environments, including air, water, soil, indoor dust, sediments, sewage sludge, and the food chain. Human exposure to TBBPA primarily occurs through dietary intake, ingestion of dust, and dermal contact[59]. Notably, TBBPA has been detected in human breast milk and maternal/cord serum[60], indicating its presence in the blood serum of infants and cord blood[61]. Research has shown that TBBPA can significantly impact growth and development, induce oxidative stress, generate reactive oxygen species (ROS), and affect antioxidant defense systems[62]. Furthermore, TBBPA acts as a thyroid hormone antagonist[63,64], impacting the hypothalamic-pituitary-thyroid axis and related gene expression[62,65]. TBBPA is widely used, and its discovery in human biological fluids raises major concerns about the substance’s long-term health impacts, particularly for vulnerable groups, including expecting mothers and newborns[66].
ADVANCED TREATMENT METHODS FOR MPS AND ADDITIVES
The presence of MPs and additives in both terrestrial and aquatic ecosystems has raised significant concerns regarding their potential impacts on environmental and human health. Addressing these emerging concerns necessitates the implementation of advanced treatment methods that can effectively remove MPs and additives from water. Membrane filtration is a technique used for removing many types of MPs, as it filters out particles larger than its pore size. However, it is not capable of removing dissolved additives associated with MPs. To overcome this limitation, AOPs have been developed to degrade these chemical additives[67].
Membrane filtration
Membrane filtration is a commonly used technology in water treatment because it physically blocks particles larger than the membrane’s pore size, successfully removing MPs from water. Membranes are comprised of a variety of polymers that offer durability and chemical resistance, making them suitable for use in a wide range of municipal, industrial, and residential water treatment systems.
Microfiltration
Microfiltration (MF) consists of a semipermeable membrane with pore sizes typically ranging from 0.1 to 10 micrometers[68]. They are made from various polymers, including polyvinylidene fluoride (PVDF), cellulose acetate (CA), polytetrafluoroethylene (PTFE), olefins, and PC. These polymers are characterized by their excellent film-forming capabilities, mechanical strength, and thermal stability. Additionally, they maintain physical and chemical stability across a broad range of pH levels[69]. The required pressure to force the contaminated water through the membrane is relatively low due to the large pore sizes, allowing for high water flux with minimal resistance[70].
Yahyanezhad et al. conducted a study exploring the use of a PVDF membrane with a 0.1 μm pore size for MF[71]. The study utilized an aeration system to prevent membrane fouling and examined three samples with varying concentrations of MPs in wastewater. The MP concentrations in the samples were 220 ± 23, 216 ± 19, and 184 ± 26 MPs/L, with corresponding removal efficiencies of 98%, 100%, and 98.9%, respectively. The results indicated that even at higher concentrations of MPs and their additives, a removal efficiency of 98% can be obtained.
While the study reports a high removal efficiency in removing MPs and their additives, several critical aspects are inadequately addressed, diminishing the robustness of the findings. The paper emphasizes the removal efficiency without sufficiently exploring the long-term sustainability of the MF process, particularly the significant issue of membrane fouling, which can degrade performance over time and increase operational costs. The brief mention of post-treatment needs is insufficient, as the paper does not specify viable methods to address the MPs that still pass through the system, which would have strengthened their conclusions. The experimental design also has limitations, particularly regarding the seasonal variability of samples, yet the study fails to conduct a robust statistical analysis to determine whether these differences are statistically significant. Moreover, the results are based on a specific wastewater treatment plant and the paper does not adequately discuss the limitations of generalizing these findings to other regions with different wastewater compositions or treatment processes.
Expanding on the examination of different membrane materials and their effectiveness, Pizzichetti et al. investigated the effectiveness of three different MF PC, CA, and PTFE for removing MPs from tap water[69]. All membranes showed high mass removal efficiencies above 94% for polyamide and polystyrene MPs. Among these, the study found that CA membranes provided an optimal balance between water flux, transmembrane pressure (TMP), and MP removal efficiency, making them suitable for household water filtration. While the authors report high removal efficiencies of above 94% for all tested membranes, they do not adequately address how different membrane materials interact with MPs and their additives, which could affect the generalizability of the findings. The paper also discusses the issue of MPs breaking down into smaller particles during filtration but does not sufficiently explore the implications of this phenomenon, particularly the potential formation of nanoplastics, which could pose even greater environmental risks. Overall, the discussion could be strengthened by addressing these limitations and providing a more critical and comprehensive analysis of the experimental results and their implications. The materials used in MF membranes contribute to the durability and reliability of the system under various operating conditions, maintaining consistent performance over time, thus resulting in reduced maintenance costs and an extended lifespan of the filtration system[72].
Nevertheless, MF encounters several notable challenges in the removal of MPs from water. MF is less effective for removing smaller particles, necessitating supplementary treatment processes for comprehensive removal, leading to increased time and operational costs and increased energy consumption[73]. The efficiency of MF in removing MPs is significantly affected by the density of the particles. High-density MPs, such as polyethylene terephthalate (PET) (1.37 g/cm3), are more effectively captured by sedimentation and MF processes compared to lower-density MPs like polyethylene (PE) and polypropylene (PP) (0.91-
Ultrafiltration
Ultrafiltration (UF) is an effective membrane filtration technique that utilizes small pore sizes, typically ranging from 0.001 to 0.1 microns, to selectively retain MPs[75]. The process of filtration begins by applying TMP, which forces water through the membrane and ensures that contaminants, including MPs, are retained on the membrane surface[76]. UF typically operates under a TMP range of 1 to 10 bar (14.5 to 145 psi)[77]. During the UF process, viscosity and total resistance are monitored as they are critical factors affecting water flux. Increased viscosity and resistance decrease the flow rate, thereby reducing flux. While viscosity can be improved by selecting low-resistance membranes and implementing anti-fouling strategies such as regular cleaning, it can also be managed by pre-treating the feed water[78,79].
Tadsuwan and Babel evaluated a pilot-scale UF system within conventional wastewater treatment plants[77]. The goal was to assess the potential of UF as a tertiary treatment stage for removing MPs. The study found that traditional treatment systems removed 86.14% of MPs from the influent. When UF was introduced, the removal efficiency increased significantly to 96.97%. Similarly, a study conducted by Magni et al. examined the effectiveness of UF membranes in removing MPs from coastal waters[80]. The study utilized UF membranes with a pore size of 0.04 microns to filter water samples collected from various coastal locations known for high levels of MP pollution. The findings revealed that the UF membranes were highly efficient, achieving a removal efficiency of up to 98% for MPs and additives. It also highlighted that UF could effectively capture a wide range of MP sizes, including those as small as 0.1 microns.
However, the discussion does not sufficiently address the limitations and potential challenges associated with this approach. For instance, while the UF system significantly enhances MP removal, the study does not delve into the operational challenges such as membrane fouling, which could impair long-term efficiency and increase maintenance costs. Additionally, the study’s reliance on grab sampling may introduce variability and limit the generalizability of the findings, as this method may not capture the full range of MP sizes in the wastewater. Moreover, the study overlooks the broader environmental implications of the retained MPs in the sludge, which, if not properly managed, could contribute to secondary pollution when the sludge is used in agriculture or disposed of in the environment.
To examine the efficiency of the UF technique under different conditions, UF was evaluated in various applications: wastewater treatment, industrial processes, and drinking water purification. In drinking water purification, UF reaches up to 99% removal efficiency, as the feed water typically has lower concentrations of contaminants, allowing more effective removal of MPs. Pre-treatment processes such as coagulation and sedimentation, which often precede UF in drinking water systems, further reduce the burden on the membranes[81]. In wastewater treatment, UF achieves up to 97% removal efficiency. In industrial processes, UF achieves up to 98% removal efficiency, maintaining product purity and ensuring compliance with regulatory standards. Although UF is a highly effective filtration technology, its performance is heavily influenced by the quality of the feed water, the presence and effectiveness of pre-treatment processes, and the operational conditions in each specific application. A comprehensive evaluation of these factors is essential for optimizing UF performance and ensuring consistent, long-term efficiency across different applications.
UF presents high removal efficiency for MPs and additives, which can be attributed to the ultrafine pore sizes of UF membranes that enable them to effectively capture and eliminate contaminants. Its slightly lower removal efficiency in wastewater treatment compared to drinking water treatment is due to the higher and more varied contaminant loads, including organic matter and oils, which can interfere with the filtration process[82]. Similarly, variations in the efficiency of industrial effluent treatment are attributed to the specific contaminants and MPs[80]. Despite these challenges, UF demonstrates superior performance over MF by effectively handling high densities of contaminants.
In summary, UF demonstrates high removal efficiency for a wide range of MP sizes, making it a valuable tool in water treatment. However, the technique requires further optimization to address issues related to membrane fouling and the handling of MP-laden sludge to ensure long-term sustainability.
Nanofiltration
Nanofiltration (NF) is a membrane filtration process that operates between UF and RO. NF membranes typically have pore sizes ranging from 1 to 10 nanometers and operate at pressures between 4 and 10 bars[83]. As water passes through the NF membrane, MPs and other larger contaminants are retained on the membrane surface, reducing their concentration in the treated water. The shape of MPs can significantly influence their interaction with NF membranes, potentially affecting removal efficiency. A study conducted by Jang et al. shows that fibrous MPs can form a dense network on the membrane, leading to more significant fouling and reduced water flux compared to spherical MPs, which tend to form more uniform layers[84]. Furthermore, the density of MPs influences their interaction with the membrane. Higher-density particles are more likely to settle and cause fouling, leading to the formation of a cake layer, which provides additional hydraulic resistance and further reduces the membrane’s performance[85].
NF has demonstrated impressive performance in removing MPs from both drinking water and wastewater. Research conducted by Ziajahromi et al. indicated that NF membranes could achieve over 90% removal efficiency of MPs from treated wastewater, highlighting their potential to reduce environmental pollution and improve water quality[86]. In this study, wastewater samples were first subjected to pre-treatment processes to remove larger debris and organic matter. The treated wastewater, containing MPs of varying sizes, was then passed through NF membranes under controlled conditions[86,87]. Moreover, Ziajahromi et al. conducted an in-depth study focused on the effect of operational pressure on the efficiency of NF membranes in removing MPs from wastewater[86]. The treated wastewater, containing MPs of varying sizes, was then filtered through NF membranes under controlled pressures of 5, 10, and 15 bar. At low pressure (5 bar), larger MPs were effectively removed, but a significant flux decline occurred due to fouling. Medium pressure (10 bar) provided an optimal balance, achieving over 90% removal efficiency for all MP sizes with moderate fouling. High pressure (15 bar) resulted in the highest flux but slightly reduced removal efficiency for smaller MPs and increased fouling due to forceful deposition.
While the study successfully introduced a new method for sampling and processing MPs in wastewater, there are several experimental limitations that warrant critical consideration. The experiment was conducted using a single sampling campaign over a short period, which may not fully capture the temporal variability in MP concentrations within wastewater treatment plants. This limitation raises concerns about the representativeness of the results, as different environmental conditions, such as seasonal changes and varying wastewater compositions, could significantly influence MP levels. Furthermore, the validation of the sampling device was performed using polystyrene MPs in a controlled setting, which does not fully reflect the diversity and complexity of MPs present in actual wastewater samples. This could lead to an overestimation of the method’s efficiency in capturing MPs of different compositions and sizes under real-world conditions. Addressing these experimental limitations would strengthen the study’s conclusions and provide a more accurate assessment of the effectiveness of the proposed method in diverse wastewater treatment scenarios.
A comprehensive study by Khoo et al. examined the fouling behavior of NF membranes compared to UF and MF membranes, specifically focusing on the presence of MPs[88]. Using laboratory-scale filtration units, the researchers maintained consistent operational parameters across all tests and monitored the flux decline and fouling extent over time. Scanning electron microscopy (SEM) and atomic force microscopy (AFM) analyses showed that NF membranes had smoother surfaces, reducing the adhesion of MPs and their additives, which led to lower fouling rates.
The discussion and experiment conducted in the study regarding NF for the removal of micropollutants (MPs) reveal several areas that could benefit from more rigorous analysis and critical reflection.
While the study successfully demonstrates the potential of NF for removing various MPs, the discussion does not adequately address the variability in removal efficiency based on the physicochemical properties of the MPs. The rejection mechanisms, including size exclusion, adsorption, and electrostatic interactions, are complex and can vary significantly depending on the specific characteristics of each pollutant, such as molecular weight, hydrophobicity, and charge. However, the study does not sufficiently explore how these factors influence the performance of the NF membranes. A deeper analysis of these variables and their impact on the efficiency of MP removal would have strengthened the study’s conclusions. Compared to other filtration technologies such as MF and UF, NF offers superior performance in removing MPs due to its diffusion mechanism. NF, with its pressure-driven filtration, ensures that even the smallest particles are filtered out, providing a high level of purification. Furthermore, NF is less prone to fouling due to the pressure exerted, which can generate shear forces that are strong enough to effectively mitigate particle adhesion.
While NF has a lower risk of fouling compared to other types of membrane filtration, it can still be affected by organic matter and biofilms commonly found with MPs in wastewater. This fouling can significantly decrease membrane efficiency and lifespan, necessitating frequent cleaning and maintenance, which in turn increases operational costs[87]. Additionally, NF systems have a high initial cost, including the expense of the membranes and the associated infrastructure, which can be prohibitive for smaller or budget-constrained projects[86]. These disadvantages highlight the need for careful consideration and optimization when implementing NF technology for MPs removal in water treatment systems. It could be deduced that despite its high removal efficiency, NF’s performance can be significantly impacted by membrane fouling and the variability in MP properties. Further research is needed to optimize NF membranes for broader applications, including handling a wider range of MP shapes and sizes.
Reverse osmosis
Reverse osmosis (RO) is a highly effective water purification method that utilizes a semipermeable membrane to remove MPs from water. The process involves applying pressure to the water, forcing it through the membrane[89]. The applied pressure causes the water molecules to diffuse in the opposite direction - from the contaminated side to the purified side - through the semipermeable membrane. By doing so, contaminants, including MPs, are left behind, and clean water is collected on the other side of the membrane. Notably, the pressure must be high enough to overcome the natural osmotic pressure[89].
Additionally, temperature needs to be controlled during the process since it affects the viscosity of water, thereby impacting the movement of water molecules. At higher temperatures, the viscosity of water decreases, allowing water molecules to move more freely and pass through the RO membrane more easily[90].
Harharah et al. assessed the impact of flow rate on the efficiency of RO in removing MPs using artificial wastewater contaminated with MPs of known sizes and concentrations[91]. The researchers varied the flow rates (e.g., 3, 6, 9, and 12 L/min) and monitored the permeate flux and removal efficiency of MPs. The results showed that higher flow rates improved the removal efficiency due to enhanced shear forces, which reduced membrane fouling. At an optimal flow rate of 12 L/min, the RO system achieved up to 99.9% removal efficiency of MPs. Multiple studies investigated the impact of pressure on the efficiency of RO systems for removing MPs. Water samples contaminated with MPs were subjected to RO filtration under varying pressures, ranging from 10 to 20 bar. It was deduced that increasing the pressure improved the removal efficiency from 95% to 99.9%. Researchers used fluorescence microscopy and SEM to observe MP behavior[92-94].
While the study presents promising findings, a more comprehensive analysis of the experimental limitations and practical challenges would be addressed. The reliance on artificial wastewater with predefined MP sizes and concentrations limits the applicability of the findings to real-world situations, where wastewater composition is more complex and variable. Additionally, while the study explores the effect of varying pressures on MP removal, it does not adequately consider the potential consequences, such as increased energy consumption and operational costs, that come with maintaining higher pressures and flow rates. The discussion would be strengthened by a deeper examination of these practical implications, as well as by exploring how these findings could be applied to various wastewater treatment systems.
A case study conducted by researchers at the Guangdong Key Laboratory of Membrane Materials and Separation Technologies was designed to evaluate the impact of MP density and concentration on fouling in RO systems[95]. The researchers used water samples artificially contaminated with MPs of two different sizes: 100 nanometers (nm) and 1 micrometer (μm). These samples were processed through the RO system under controlled conditions. The findings indicated that higher concentrations of MPs led to increased fouling, forming dense particulate layers that significantly reduced membrane performance. Despite these important findings, this study does not explore potential mitigation strategies or how these findings might influence the design and operation of RO systems in practical applications. A more comprehensive analysis that includes diverse MP types, real wastewater conditions, and possible solutions to fouling would provide a more robust understanding of the issue and its implications for RO system performance. RO systems significantly improve water quality by effectively removing a wide range of contaminants, up to 99.9% of MPs, including heavy metals, bacteria, and dissolved solids. This high level of purification results in water that is typically free from unpleasant tastes and odors, providing cleaner and safer drinking water.
ROs have one of the highest removal efficiencies of MPs, but their application is often limited by high running costs and membrane fouling risk. Further research is necessary to adjust operating parameters, like pressure and flow rate, to reduce maintenance costs and strike a balance between efficiency and energy consumption.
Membrane bioreactors
Membrane bioreactors (MBRs) effectively remove MPs from wastewater by integrating biological treatment with membrane filtration. In the biological treatment phase, MPs are reduced through biodegradation and adsorption as microorganisms break them down and produce extracellular polymeric substances (EPS). The EPS form a sticky matrix that facilitates the adsorption of MPs onto microbial floc, a process driven by hydrophobic interactions and van der Waals forces, thereby improving the removal of MPs from aqueous environments in water treatment processes. The filtration process, driven by an exerted pressure or pressure gradient, involves forcing water through the membrane pores, effectively trapping solids, including MPs, on the membrane surface. This combination of biological and physical processes ensures the efficient removal of MPs from wastewater[96-98].
The Gaobeidian Wastewater Treatment Plant in Beijing, China, implemented an MBR system to enhance its wastewater treatment capabilities, focusing on the removal of MPs. The membrane filtration phase employs MF membranes with a pore size of 0.1 μm to retain MPs , and other suspended solids. The implementation of the MBR system resulted in a significant improvement in MP removal efficiency, reducing the concentration of MPs and in the influent from approximately 300 particles per liter to around 1-2 particles per liter in the effluent, achieving an overall removal efficiency of over 99%[96].
The study has several important limitations that need to be addressed. One major issue is the inconsistency in how MPs were detected across different wastewater treatment plants. Additionally, the study relies heavily on visual identification of MPs, which can lead to errors and inaccuracies. Although the authors mention the need for standardized methods, this issue remains unresolved, which limits the study’s impact. The discussion also falls short of addressing how these methodological problems could influence the overall conclusions, which might lead to an overestimation of the effectiveness of current wastewater treatment plant (WWTP) processes.
However, Ma et al. conducted a comprehensive study tackling the adsorption and absorption mechanisms of MPs in MBR systems and their impact on overall removal efficiency[99]. The study utilized a pilot-scale MBR system combining biological treatment with UF membranes, operating with high mixed liquor suspended solids (MLSS) concentrations ranging from 10,000 to 12,000 mg/L and employing membranes with a pore size of 0.04 micrometers. The study revealed that the adsorption process significantly reduced the concentration of free-floating MPs in the mixed liquor, enhancing their removal efficiency during the membrane filtration phase.
While the discussion effectively summarizes the experimental findings, it lacks a critical analysis of the limitations of the study. The study’s reliance on controlled laboratory settings may not accurately reflect the complexity and variability of real-world water treatment environments, which could influence the effectiveness of the coagulation and UF processes. Moreover, the exclusive focus on PE limits the applicability of the findings to a broader range of MP pollutants that vary in size, shape, and chemical composition. This limitation is acknowledged briefly but not critically evaluated in the context of the study’s conclusions. Additionally, the discussion does not sufficiently consider the long-term operational challenges associated with membrane fouling, particularly in scenarios involving varying concentrations and types of MPs.
The combination of biological treatment and membrane filtration enhances the overall removal efficiency of MPs. This dual action ensures that a significant portion of MPs will be captured and removed from the treated water before even reaching the membrane filtration stage[100].
Despite the high removal efficiencies of membrane filtration, all technologies face similar challenges, including membrane fouling, high operational pressures, and increased energy consumption[72,101]. Fouling occurs when MPs and organic matter accumulate on the membrane surface, reducing filtration efficiency and requiring frequent cleaning and maintenance. For example, in UF systems, fouling can result in a reduction in permeate flux by up to 50% and can decrease membrane lifespan by 30%-50%, necessitating costly maintenance and downtime[78]. UF systems also require higher operational pressures compared to MF, which increases energy consumption. Despite achieving higher removal efficiencies, UF’s need for higher pressures can lead to a 20%-30% increase in energy costs compared to MF systems[72]. The cost of replacing membranes, combined with the energy required to maintain high-pressure systems (especially in NF and RO), increases the overall operational costs. Addressing fouling with anti-fouling technologies and developing self-cleaning membranes could enhance the long-term sustainability of these systems. Studies have shown that the presence of MPs can increase the rate of RO membrane fouling by up to 25% compared to systems without MPs, due to the formation of a dense fouling layer on the membrane surface[102]. For instance, an experiment demonstrated a flux decline of 30% when the concentration of MPs in the feed water was increased from 1 to 10 mg/L[103]. The cake layer formed by MPs on the membrane surface increases the operational pressure required to maintain the desired water flux. This additional pressure can lead to higher energy consumption and operational costs. Golgoli et al. reported that the pressure increase could be as much as 20% to 30% in the presence of high concentrations of MPs[104]. Fouling is also a significant threat to the effectiveness of MBR systems where MPs can disrupt microbial activity in the biological treatment phase by providing surfaces for harmful bacteria to grow, leading to imbalances in the microbial community and reducing the degradation of organic pollutants[100]. Additionally, MPs can carry toxic chemicals and heavy metals, further harming microbial communities and compromising the overall treatment efficiency of MBR systems[96].
AOPs
MPs and their chemical additives are effectively broken down by AOPs, which generate reactive radicals that break down complex organic contaminants. AOPs are commonly used in water treatment to remove impurities that are not entirely removed by conventional filtration systems. Fenton reactions, electro-oxidation, and photocatalysis are a few examples of these processes[105,106].
Photocatalysis
Photocatalysis is a chemical process that occurs in the presence of light and a photocatalyst, typically a semiconductor such as titanium dioxide (TiO2). The process begins with the absorption of photons by the photocatalyst, which creates electron-hole pairs[107]. These pairs generate highly reactive species, such as hydroxyl radicals and superoxide anions, that degrade organic pollutants. Photocatalysis has been particularly effective in removing MP additives like BPA, NP, and TBBPA. For instance, Tang et al. reported a 100% removal of NP using hydrophobic TiO2 nanotubes within 40 min[108].
The study presents promising results in the advanced photocatalytic oxidation of NP using hydrophobic titanium dioxide nanotubes (H-TiO2NTs), yet several aspects of the experimental design and discussion require further consideration. The hydrophobic modification of TiO2 nanotubes, while effective in improving NP degradation, suffers from long-term stability issues, as the hydrophobic surface reverts to hydrophilicity under prolonged irradiation, significantly reducing photocatalytic efficiency. This raises concerns about the practicality of using H-TiO2NTs in continuous or long-term applications, a challenge that the experiment did not fully address. While the study highlights the selective oxidation of NP in the presence of other hydrophilic contaminants, it does not critically evaluate how this selectivity might be affected by the presence of other hydrophobic pollutants, nor does it propose clear future research directions to address these limitations.
Acarer[68] investigates the effectiveness of zinc oxide nanoparticles (ZnO NPs) in promoting the photodegradation of MPs under simulated sunlight. The study extracted MPs from commercial sunscreens and exposed them to ZnO NPs under UV light for 12 h. The results showed a significant increase in the carbonyl index of the MPs, indicating surface oxidation and fragmentation, with a 2.5-fold rise in the carbonyl index reflecting the degree of degradation. Additionally, the study highlighted the enhanced cytotoxicity of the degraded MPs, finding that these transformed particles caused significant increases in lysosomal accumulation and mitochondrial damage. While the study effectively demonstrates the potential of ZnO NPs in accelerating MPs degradation, it does not provide a direct percentage of removal efficiency, focusing instead on the structural changes and biological impacts of the degraded products.
Fenton process
The Fenton process is an advanced oxidation process involving the reaction of H2O2 with ferrous iron (Fe2+) to produce hydroxyl radicals, which are potent oxidizing agents. These radicals can degrade a wide range of organic pollutants. The Fenton process is particularly noted for its effectiveness under acidic conditions and its ability to achieve high removal efficiencies.
He et al. (2017) discuss the synthesis and application of a bifunctional hollow mesoporous catalyst for the degradation of TBBPA. The Fe0CMnFe2O4 catalyst was used in combination with hydrogen peroxide (H2O2) and hydroxylamine (NH2OH) to degrade TBBPA in aqueous solutions. The experiments demonstrated that the catalyst could achieve a removal efficiency of up to 90% within 120 min under optimal conditions, particularly at a neutral pH and with a catalyst dosage of 0.3 g/L. The degradation mechanism involved both reductive debromination and oxidative mineralization processes, facilitated by the generation of hydroxyl radicals (•OH) from H2O2 activated by the catalyst. Additionally, the Fe0CMnFe2O4 catalyst showed good stability and reusability, retaining nearly 80% of its removal efficiency after 10 cycles of use. These findings suggest that the Fe0CMnFe2O4 catalyst is a promising candidate for the effective degradation of persistent organic pollutants like TBBPA, with potential applications in environmental remediation[109]. While the results are promising, the study focuses on optimal conditions without testing the catalyst’s robustness in varied environmental scenarios, limiting its real-world applicability. Additionally, the paper lacks an analysis of potential byproducts and long-term catalyst performance in complex wastewater conditions.
To demonstrate the potential of the thermal Fenton reaction for effective environmental remediation of MPs, Hu et al. showed that the hydrothermal Fenton system achieved a 95.9% weight loss of ultrahigh-molecular-weight polyethylene (UHMWPE) MPs within 16 h and 75.6% mineralization in 12 h[110]. This efficiency is due to the synergistic effects of hydrothermal hydrolysis and hydroxyl radical production, which break down polymer chains through a two-stage process of chain unfolding and oxidation. The system effectively degrades various plastics and maintains its performance in real-world water samples, including tap, river, and seawater. Toxicity tests with Escherichia coli confirmed that the degradation intermediates are non-toxic, highlighting the system’s environmental safety.
Electro-oxidation
Electro-oxidation is an electrochemical process where electrical energy is used to generate oxidizing agents directly at the anode surface or indirectly in the bulk solution. This method is effective in treating various organic pollutants, including MP additives.
Ji et al. investigated the impact of micelle formation on the electro-oxidation degradation of NEPOs using a Ti4O7 anode[111]. Micelle formation was found to significantly inhibit NEPO degradation by creating steric hindrance that prevents hydroxyl radicals from effectively attacking NEPOs. Despite this, the anode achieved 92% degradation within 1 h, with the primary degradation occurring through the cleavage of the Caryl-Oether bond, producing ethoxylated chains and polyethylene glycols (PEGs) as byproducts. Ning
The degradation rate achieved is relatively low, especially considering that it was obtained under optimal conditions, with careful adjustments made to key parameters like temperature, current density, pH, and electrolyte concentration. Furthermore, the time required to reach even this limited level of degradation is quite lengthy, which further diminishes the process’s practicality for large-scale applications.
Sonocatalysis
Sonocatalysis involves the use of ultrasonic waves to enhance the catalytic degradation of pollutants. The ultrasonic waves generate cavitation bubbles, which, upon collapse, produce localized high temperatures and pressures, leading to the formation of reactive radicals. This method has shown high efficacy in the degradation of organic pollutants.
A hybrid AOP was developed by combining persulfate (PS) activation with nano zero-valent iron (nZVI) supported on reduced graphene oxide (rGO), all enhanced by ultrasound (US). The experiment involved optimizing various parameters, including pH, reaction time, PS concentration, nZVI-rGO dosage, and US power, to maximize the degradation efficiency of NP.
The main findings of the study revealed that the optimized PS/US/nZVI-rGO system achieved a high NP removal efficiency of 98.2%. This result demonstrated a significant synergistic effect compared to other systems lacking one or more of these components. The research also identified the degradation pathway of NP, confirming that sulfate radicals (SO4•-) generated during the process played a predominant role in breaking down the NP molecules. The study concludes that the PS/US/nZVI-rGO system is a highly effective and promising method for treating organic pollutants in water, offering potential for environmental remediation applications[113]. While the study effectively demonstrates the potential of combining PS activation, nano nZVI supported on rGO, and US for the degradation of NP, it does not sufficiently explore the scalability of the process or the economic feasibility of implementing such a system on a larger scale, which are critical factors for practical environmental remediation. The potential environmental impact of the byproducts generated during the degradation process is also not thoroughly examined, particularly regarding the long-term safety of this approach.
Ioannidi et al. (2024) investigate the use of palladium-cerium oxide (Pd/CeO2) as a catalyst for the sonocatalytic degradation of BPA in water. The study focuses on understanding the degradation process, identifying the transformation products formed, and assessing the toxicity of these products. The main findings of the research indicate that the Pd/CeO2 catalyst significantly enhances the degradation of BPA when combined with US, achieving a degradation efficiency of 95% within 60 min. The process was influenced by various operational parameters, including US power and catalyst loading. The study also identified nine transformation products formed during the degradation process, some of which exhibited reduced toxicity compared to the parent compound BPA, although a few remained toxic[114].
This work highlights the potential of using sonocatalysis with Pd/CeO2 nanoparticles for the effective degradation of BPA, while also underscoring the importance of understanding the environmental impact of the resulting byproducts. However, while the focus on BPA is important, the study’s limited scope does not account for how this system might perform with other pollutants. Furthermore, while the optimization of operational parameters is discussed, the study does not fully examine the interactions between these parameters, which could provide valuable insights for improving process efficiency. Addressing these limitations would strengthen the study’s relevance and applicability for broader environmental remediation efforts. Anastopoulos et al. investigated the use of palladium-cerium oxide (Pd/CeO2) for the sonocatalytic degradation of BPA in water, achieving 95% degradation within 60 min[115]. The study identified nine transformation products, some with reduced toxicity. While the research demonstrates the effectiveness of Pd/CeO2 in degrading BPA, it lacks examination of other pollutants and the interactions between operational parameters, limiting broader environmental applicability.
Catalytic oxidation
Catalytic oxidation processes involve the use of catalysts to accelerate the oxidation of pollutants, often using agents such as oxygen, ozone, or hydrogen peroxide. These processes are highly effective in degrading various organic compounds.
A study conducted by Zanaty et al. (2024) explores the use of a novel nanocomposite for the catalytic oxidation and removal of MPs. The research focuses on the effectiveness of this material in breaking down pollutants, demonstrating high removal efficiencies with 97% degraded through catalytic oxidation. Additionally, the study highlights the nanocomposite’s recyclability, retaining 78% efficiency after six cycles, positioning it as a promising candidate for sustainable water treatment[116].
While the study presents promising results, the study does not provide a detailed analysis of the potential environmental impact of the degradation byproducts, which could be significant in real-world applications. Furthermore, the recyclability of the material does not examine the potential loss of catalytic activity over extended cycles, which could affect its practicality for large-scale use. Addressing these limitations would provide a more robust understanding of the nanocomposite’s applicability and effectiveness in real-world water treatment.
The use of catalysts in AOPs for the degradation of MPs in water, when enhanced with metals like Ag or Pt, is highly effective in generating reactive radicals such as hydroxyl radicals (•OH), which play a crucial role in breaking down MPs into smaller, less harmful fragments. The research highlights that photocatalytic systems such as TiO2 and ZnO can achieve near-complete degradation of certain MPs under optimal conditions, with reported efficiencies approaching 100%. The study also explores the potential of hybrid catalyst systems, which combine different catalytic materials to further enhance the degradation efficiency, making these processes promising for real-world water treatment applications[117]. However, while the catalytic approaches show great promise, the discussion also points to the need for further research to address challenges such as the potential formation of secondary pollutants and the economic feasibility of large-scale applications. Figure 3 summarizes the treatment methods employed for MPs and their additives.
Approach to combined treatment methods
In the effort to improve the efficiency of water treatment processes in removing MPs, it has become evident that no single treatment method can address all the challenges effectively. Therefore, implementing a combined treatment approach becomes crucial to enhance the removal of MPs and their additives, reduce operational costs, minimize environmental impact, and improve overall water quality. To provide a clear overview, Table 1 summarizes the advantages, disadvantages, and removal efficiencies of various membrane technologies employed in removing MPs, while Table 2 summarizes the methods of AOPs. Integrating UF with AOPs, specifically TiO2 Photocatalysis, offers an efficient solution. UF is well-regarded for its capability to remove a broad spectrum of MPs due to its diffusion mechanism. However, UF membranes suffer from fouling, where organic matter and other impurities accumulate on the membrane surface, thereby reducing their efficiency and lifespan. TiO2 photocatalysis can mitigate this issue by using titanium dioxide as a catalyst and it is also able to degrade the additives of MPs efficiently. This process prevents the accumulation of organic matter on UF membranes, enhancing the removal efficiency of both MPs and organic contaminants, while reducing the need for frequent membrane cleaning and maintenance. This combination results in a more comprehensive and efficient water treatment system.
Advantages and disadvantages of various membrane technologies employed in removing MPs
| Method | Removal efficiency | Advantages | Disadvantages |
| MF | 94% to 100% | - High removal efficiency for larger MPs - Low energy consumption due to low-pressure operation - Durable and stable membranes | - Less effective for smaller particles - Frequent cleaning and potential membrane replacement due to fouling - Efficiency affected by the density of MPs |
| UF | 96% to 99% | - High removal efficiency for a broad range of MPs - Longer membrane lifespan and resistance to fouling | - Higher energy requirements due to increased operational pressures - Significant fouling issues, particularly with organic matter - Increased operational costs due to energy and maintenance |
| NF | 90% | - Very high removal efficiency for smaller MPs - Lower fouling rates compared to MF and UF | - Susceptibility to fouling by organic matter and biofilms - High initial cost and infrastructure expenses - Limited removal efficiency for very small particles |
| RO | 95% to 99.9% | - Extremely high removal efficiency for all MPs - Comprehensive removal of various contaminants | - Significant membrane fouling issues - High operational pressures and energy consumption - High initial and operational costs |
| MBR | 99% | - Very high removal efficiency for MPs - Combines biological and physical treatment for comprehensive contaminant removal | - Significant membrane fouling - Potential disruption to biological processes - High operational complexity and costs |
Removal efficiencies of MPs and their additives using AOPs
| Treatment method | Pollutants | Removal efficiency | Limitations |
| Photocatalysis | NP | 100% for NP | Hydrophobic modification of TiO2 suffers from long-term stability issues |
| Fenton process | TBBPA | Up to 90% | Potential byproducts and their environmental impacts not thoroughly analyzed |
| UHMWPE | 95.9% | Long-term performance and environmental impact of degradation intermediates not fully assessed | |
| Electro-oxidation | NEPOs | Over 92% | Micelle formation inhibits degradation. Controlled lab conditions may not reflect real-world wastewater complexities. Lack of alternative mitigation strategies |
| MPs | 38.67% | Low efficiency and lengthy treatment time under optimal conditions | |
| Sonocatalysis | NP | 98.2% | Scalability and economic feasibility not explored. Environmental impact of byproducts not thoroughly examined |
| BPA | 95% | Interactions between operational parameters not fully explored. Broader applicability and environmental impact need further study | |
| Catalytic oxidation | MPs | 97% | Recyclability and loss of catalytic activity over cycles not thoroughly assessed |
Similarly, combining NF with MBR presents a synergistic approach to water treatment. NF is effective in removing smaller MPs and various other contaminants due to its diffusive characteristics. However, NF membranes are prone to fouling by organic matter and biofilms, which can decrease their effectiveness and increase operational costs. MBR systems, which integrate biological treatment with membrane filtration, can alleviate this issue. By integrating NF with MBR, the biological treatment reduces the organic load before it reaches the NF membrane, thus minimizing fouling and extending the membrane’s lifespan. This approach benefits from the high removal efficiency of NF while benefiting from the comprehensive treatment capabilities of MBR. Figure 4 depicts the hybrid MBR UF/MF system with percent removal of select MPs and MP additives.
Figure 4. Schematic of hybrid MBR-UF/MF treatment system. MBR: Membrane bioreactor; UF: ultrafiltration; MF: microfiltration.
RO and catalytic oxidation effectively eliminate organic pollutants and MP while resolving RO’s problems with fouling and high operating pressure. Catalytic oxidation enhances performance while consuming less energy and requiring less maintenance by decomposing chemical additives. This integration improves the therapy’s efficiency and cost-effectiveness. Table 3 summarizes the cost-benefit analysis by contrasting the initial, continuing, and maintenance costs of each strategy while emphasizing its overall efficacy and environmental impact[68,72,100,103,104,118].
Cost-benefit analysis for each combined treatment method
| Method | Initial cost | Operational cost | Maintenance cost | Efficiency | Environmental impact |
| Hybrid UF + AOP (TiO2 photocatalysis) | High | Moderate | Moderate | Extremely high for all particles and contaminants | Positive (less waste, moderate energy) |
| Hybrid NF + MBR | High | High | Moderate to high | Very high for smaller particles | Mixed (high resource use, extended lifespan) |
| Hybrid RO + catalytic oxidation | Very high | High | Moderate | Extremely high for all particles and contaminants | Mixed (high energy, reduced fouling) |
It can be deduced from Table 3 that although all three hybrid systems are effective, their costs vary from low to high; therefore, the optimum choice will depend on the type of water. Surface water, which is regularly contaminated by pollutants from runoff and organic debris, is the ideal application for the UF/AOP hybrid system. For wastewater treatment, the MBR/NF system is recommended since it combines biological degradation with fine filtration for comprehensive treatment. Because the RO/Catalytic Oxidation system efficiently removes salts and contaminants to produce high-purity water, it is ideal for brackish and seawater.
REMOVAL MECHANISMS
Understanding the mechanisms of removal of MPs and MP additives from water and wastewater is essential to ensuring the effectiveness of treatment methods and optimizing their performance. These systems can be broadly divided into two groups: physical processes that trap and retain pollutants, and chemical processes that convert pollutants into less recalcitrant forms.
Physical removal mechanisms are mainly highlighted using membrane treatment processes, where size exclusion, adsorption, and in the case of RO and NF, ionic diffusion through the membrane. On the other hand, the premise behind chemical removal in processes such as coagulation\flocculation involves the addition of a chemical that forms bonds with the MP surface and later combines multiple particles due to electrostatic interaction[Figure 5][119]. Guo et al. concluded that electrostatic interaction was the dominant force between surface functionalized 2-acrylamido-2-methylpropane sulfonic acid polymer nanoparticle and aluminum oxide particle in the flocculation process of MPs[120]. Furthermore, Khan et al. deduced that coagulation of MPs is highly dependent on optimizing various factors such as coagulant type, dosage, pH, and the physical characteristics of MPs[121].
On the other hand, photocatalysis, Fenton reactions, and electro-oxidation involve the generation of reactive species, mainly hydroxyl radicals, that break down MPs into less harmful byproducts. Photocatalysis using TiO2 can effectively degrade NP and other additives, converting them into harmless byproducts. Figure 6 illustrates the breakdown of PVC using Fenton oxidation, where the hydroxyl radicals produced electrochemically enable the breakdown of polymer chains into smaller, less harmful molecules, ultimately converting them into CO2 and H2O. To improve MPs’ environmental management, increase treatment efficacy, and decrease the long-term impacts of MP-related pollution, further research into these mechanisms is required.
FUTURE NEEDS AND GAPS
Future research needs to address several critical gaps in our understanding of MPs and their environmental impact. Comprehensive studies are necessary to identify and quantify the primary sources of MPs and understand their transport mechanisms from these sources to aquatic and terrestrial environments. This requires robust models that simulate their movement through various environmental matrices, including soil, water, and air. Additionally, the long-term ecotoxicological impact of MPs on different levels in aquatic and terrestrial ecosystems needs further exploration, especially regarding chronic exposure effects on organisms and potential bioaccumulation and biomagnification in food webs. While MF and UF are effective in removing MPs, fouling remains a significant challenge, highlighting the need to develop anti-fouling coatings and self-cleaning membranes to enhance system longevity and efficiency. Integrating UF with AOPs has shown promise in degrading MPs into harmless byproducts, and future research should optimize these systems for large-scale, cost-effective, and environmentally sustainable applications. The development of mixed matrix membranes (MMMs) that incorporate adsorbents for enhanced MP capture represents a significant advancement, necessitating further research into novel materials that offer higher selectivity, durability, and resistance to fouling. Conducting pilot-scale studies in diverse environmental settings will provide valuable data on the practical feasibility, efficiency, scalability, and environmental sustainability of these hybrid systems, ensuring reliable implementation for comprehensive water treatment.
LCA OF MPS AND THEIR ADDITIVES
A substantial gap persists in the understanding of the full life cycle of MPs and their additives, from production to environmental degradation and remediation, necessitating LCA studies. At every stage of the MP life cycle, LCA provides a framework for evaluating the environmental costs of extracting and manufacturing raw materials, using them, disposing of them, and causing environmental degradation. MPs are a particularly serious environmental issue because they remain in ecosystems and emit toxic substances that may have negative effects on biodiversity and human health. Addressing this issue requires assessing the carbon footprint of MPs and their additives, including their production, usage, and disposal, which is crucial for quantifying associated greenhouse gas emissions and developing strategies to minimize their environmental impact. A novel method for incorporating patent literature into projected LCAs has been presented by Spreafico et al.[122]. Through the identification of innovative eco-design solutions and state-of-the-art technology in MP collection and treatment, this method provides a more forward-looking assessment of the environmental effects. By incorporating patent analysis into LCA, future advancements in MP remediation, such as membrane filtering and AOPs, can be evaluated for their potential to significantly reduce the environmental effect of MP-related technologies. This strategy helps close the gap between current practices and the possible environmental advantages of technological advancement.
Applying proper LCA to MPs is still difficult because of data shortages and difficulties in assessing long-term environmental repercussions, as many studies rely on imprecise data addressing MP release, degradation, and ecological effects. Jiao et al. claim that the lack of consistent reporting makes it difficult to conduct comprehensive assessments of the life cycle impacts of MPs[123]. The persistence of MPs in ecosystems and their capacity to generate toxic metabolites complicate evaluations. Future LCA research must use multi-scale data and advanced modeling to more precisely predict MP behavior across ecosystems. Policy frameworks that cover the whole lifespan of MPs from production to disposal will help foster innovation in treatment technologies and reduce the environmental impact of MPs by expanding knowledge and creating sustainable solutions.
CONCLUSION
In this study, we have comprehensively examined the issue of MPs and their additives, including their sources, impacts, and treatment methods, highlighting key strategies for improving removal efficiency. MPs and their additives pose significant ecological risks. Advanced treatment technologies, while promising, require optimization and further research, especially concerning fouling and byproduct management. AOPs and membrane filtration are two examples of treatment technologies that offer promising ways to mitigate the environmental risks that MPs generate. By addressing problems like membrane fouling and optimizing treatment processes, these technologies support sustainable water management techniques. However, further research into novel materials is required to improve their performance and resistance to fouling. The development of innovative materials and hybrid treatment approaches further enhances the practical applicability of these methods in diverse environmental settings. The paper highlights the need for LCA studies, which include carbon footprint analysis to develop mitigation plans, to assess the environmental impacts of MPs and their additives over the duration of their lifecycle. Standardization of sampling and reporting practices is necessary to ensure data consistency. Highlights of the paper include pointing out significant research gaps and offering a thorough analysis of MPs’ causes, effects, and treatment modalities. In conclusion, managing MPs necessitates advanced treatment technology, comprehensive environmental evaluations, and strong regulatory frameworks. Effective treatment options are essential to safeguarding both human and environmental health.
DECLARATIONS
Authors’ contributions
Worked on writing-original draft: Sawma MJ, Ghaddar R
Writing-review and editing: Zayyat RM
Validation, supervision, and conceptualization: Ayoub GM
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2024.
REFERENCES
1. Dissanayake PD, Kim S, Sarkar B, et al. Effects of microplastics on the terrestrial environment: a critical review. Environ Res. 2022;209:112734.
2. Hale RC, Seeley ME, La Guardia MJ, Mai L, Zeng EY. A global perspective on microplastics. JGR Oceans. 2020;125:e2018JC014719.
3. Shen L, Worrell E. Chapter 31 - Plastic recycling. In: Handbook of recycling. Elsevier; 2024. pp. 497-510.
4. Alhazmi H, Almansour FH, Aldhafeeri Z. Plastic waste management: a review of existing life cycle assessment studies. Sustainability 2021;13:5340.
5. Haque F, Fan C. Fate of microplastics under the influence of climate change. iScience. 2023;26:107649.
6. McMullen K, Vargas FH, Calle P, Alavarado-Cadena O, Pakhomov EA, Alava JJ. Modelling microplastic bioaccumulation and biomagnification potential in the Galápagos penguin ecosystem using Ecopath and Ecosim (EwE) with Ecotracer. PLoS One. 2024;19:e0296788.
7. Matavos-Aramyan S. Addressing the microplastic crisis: a multifaceted approach to removal and regulation. Environm Adv. 2024;17:100579.
8. Li XF, Mitch WA. Drinking water disinfection byproducts (DBPs) and human health effects: multidisciplinary challenges and opportunities. Environ Sci Technol. 2018;52:1681-9.
9. Sutkar PR, Gadewar RD, Dhulap VP. Recent trends in degradation of microplastics in the environment: a state-of-the-art review. J Hazard Mater Adv. 2023;11:100343.
10. Pothiraj C, Amutha Gokul T, Ramesh Kumar K, et al. Vulnerability of microplastics on marine environment: a review. Ecol Indic. 2023;155:111058.
11. Ziani K, Ioniță-Mîndrican CB, Mititelu M, et al. Microplastics: a real global threat for environment and food safety: a state of the art review. Nutrients. 2023;15:617.
12. An L, Liu Q, Deng Y, Wu W, Gao Y, Ling W. Sources of microplastic in the environment. In: He D, Luo Y, editors. Microplastics in terrestrial environments. Cham: Springer International Publishing; 2020. pp. 143-59.
13. Singh A, Mishra BK. Microbeads in personal care products: an overlooked environmental concern. J Clean Prod. 2023;427:139082.
14. Anbumani S, Kakkar P. Ecotoxicological effects of microplastics on biota: a review. Environ Sci Pollut Res Int. 2018;25:14373-96.
15. Nabi I, Bacha A, Zhang L. A review on microplastics separation techniques from environmental media. J Clean Prod. 2022;337:130458.
16. Rillig MC. Microplastic in terrestrial ecosystems and the soil? Environ Sci Technol. 2012;46:6453-4.
17. Yang Y, Jalalah M, Alsareii SA, et al. Plastic wastes (PWs) and microplastics (MPs) formation: management, migration, and environmental impact. J Environ Chem Eng. 2024;12:112926.
19. Dube E, Okuthe GE. Plastics and micro/nano-plastics (MNPs) in the environment: occurrence, impact, and toxicity. Int J Environ Res Public Health. 2023;20:6667.
20. Wu P, Huang J, Zheng Y, et al. Environmental occurrences, fate, and impacts of microplastics. Ecotoxicol Environ Saf. 2019;184:109612.
21. Rossatto A, Arlindo MZF, de Morais MS, de Souza TD, Ogrodowski CS. Microplastics in aquatic systems: a review of occurrence, monitoring and potential environmental risks. Environ Adv. 2023;13:100396.
22. Wright SL, Thompson RC, Galloway TS. The physical impacts of microplastics on marine organisms: a review. Environ Pollut. 2013;178:483-92.
23. Canniff PM, Hoang TC. Microplastic ingestion by daphnia magna and its enhancement on algal growth. Sci Total Environ. 2018;633:500-7.
24. Rillig MC, Ziersch L, Hempel S. Microplastic transport in soil by earthworms. Sci Rep. 2017;7:1362.
25. Huang Y, Liu Q, Jia W, Yan C, Wang J. Agricultural plastic mulching as a source of microplastics in the terrestrial environment. Environ Pollut. 2020;260:114096.
26. Kumar M, Xiong X, He M, et al. Microplastics as pollutants in agricultural soils. Environ Pollut. 2020;265:114980.
27. de Souza Machado AA, Kloas W, Zarfl C, Hempel S, Rillig MC. Microplastics as an emerging threat to terrestrial ecosystems. Glob Chang Biol. 2018;24:1405-16.
28. Dad FP, Khan WU, Kirkham MB, Bolan N, Tanveer M. Microplastics: a review of their impacts on different life forms and their removal methods. Environ Sci Pollut Res Int. 2023;30:86632-55.
29. Oliveira M, Almeida M, Miguel I. A micro(nano)plastic boomerang tale: a never ending story? TrAC Trend Anal Chem. 2019;112:196-200.
30. Chen G, Feng Q, Wang J. Mini-review of microplastics in the atmosphere and their risks to humans. Sci Total Environ. 2020;703:135504.
31. Conti G, Rapisarda P, Ferrante M. Relationship between climate change and environmental microplastics: a one health vision for the platysphere health. One Health Adv. 2024;2:17.
32. Chen C, Pan J, Xiao S, et al. Microplastics alter nitrous oxide production and pathways through affecting microbiome in estuarine sediments. Water Res. 2022;221:118733.
33. Prata JC, da Costa JP, Lopes I, Duarte AC, Rocha-Santos T. Environmental exposure to microplastics: an overview on possible human health effects. Sci Total Environ. 2020;702:134455.
34. Yu Y, Chen H, Hua X, et al. Polystyrene microplastics (PS-MPs) toxicity induced oxidative stress and intestinal injury in nematode Caenorhabditis elegans. Sci Total Environ. 2020;726:138679.
35. Persiani E, Cecchettini A, Ceccherini E, Gisone I, Morales MA, Vozzi F. Microplastics: a matter of the heart (and vascular system). Biomedicines. 2023;11:264.
36. Segovia-Mendoza M, Nava-Castro KE, Palacios-Arreola MI, Garay-Canales C, Morales-Montor J. How microplastic components influence the immune system and impact on children health: focus on cancer. Birth Defects Res. 2020;112:1341-61.
37. Sharma MD, Elanjickal AI, Mankar JS, Krupadam RJ. Assessment of cancer risk of microplastics enriched with polycyclic aromatic hydrocarbons. J Hazard Mater. 2020;398:122994.
38. Çobanoğlu H, Belivermiş M, Sıkdokur E, Kılıç Ö, Çayır A. Genotoxic and cytotoxic effects of polyethylene microplastics on human peripheral blood lymphocytes. Chemosphere. 2021;272:129805.
39. Kratina P, Watts TJ, Green DS, Kordas RL, O'Gorman EJ. Interactive effects of warming and microplastics on metabolism but not feeding rates of a key freshwater detritivore. Environ Pollut. 2019;255:113259.
40. Kannan K, Vimalkumar K. A review of human exposure to microplastics and insights into microplastics as obesogens. Front Endocrinol. 2021;12:724989.
41. Sheng Y, Ye X, Zhou Y, Li R. Microplastics (MPs) act as sources and vector of pollutants-impact hazards and preventive measures. Bull Environ Contam Toxicol. 2021;107:722-9.
42. Shruti VC, Pérez-Guevara F, Elizalde-Martínez I, Kutralam-Muniasamy G. First study of its kind on the microplastic contamination of soft drinks, cold tea and energy drinks - future research and environmental considerations. Sci Total Environ. 2020;726:138580.
43. Watts AJR, Lewis C, Goodhead RM, et al. Uptake and retention of microplastics by the shore crab Carcinus maenas. Environ Sci Technol. 2014;48:8823-30.
44. Costa JPD, Avellan A, Mouneyrac C, Duarte A, Rocha-Santos T. Plastic additives and microplastics as emerging contaminants: mechanisms and analytical assessment. TrAC Trend Ana Chem. 2023;158:116898.
45. Hahladakis JN, Velis CA, Weber R, Iacovidou E, Purnell P. An overview of chemical additives present in plastics: migration, release, fate and environmental impact during their use, disposal and recycling. J Hazard Mater. 2018;344:179-99.
46. Kitamura S, Suzuki T, Sanoh S, et al. Comparative study of the endocrine-disrupting activity of bisphenol A and 19 related compounds. Toxicol Sci. 2005;84:249-59.
47. Zielińska M, Bułkowska K, Cydzik-Kwiatkowska A, Bernat K, Wojnowska-Baryła I. Removal of bisphenol A (BPA) from biologically treated wastewater by microfiltration and nanofiltration. Int J Environ Sci Technol. 2016;13:2239-48.
48. Santoro A, Chianese R, Troisi J, et al. Neuro-toxic and reproductive effects of BPA. Curr Neuropharmacol. 2019;17:1109-32.
49. Seachrist DD, Bonk KW, Ho SM, Prins GS, Soto AM, Keri RA. A review of the carcinogenic potential of bisphenol A. Reprod Toxicol. 2016;59:167-82.
50. Adamovsky O, Groh KJ, Białk-Bielińska A, et al. Exploring BPA alternatives - environmental levels and toxicity review. Environ Int. 2024;189:108728.
51. Yang W, Gao X, Wu Y, et al. The combined toxicity influence of microplastics and nonylphenol on microalgae Chlorella pyrenoidosa. Ecotoxicol Environ Saf. 2020;195:110484.
52. Brix R, Hvidt S, Carlsen L. Solubility of nonylphenol and nonylphenol ethoxylates. On the possible role of micelles. Chemosphere. 2001;44:759-63.
53. Soares A, Guieysse B, Jefferson B, Cartmell E, Lester JN. Nonylphenol in the environment: a critical review on occurrence, fate, toxicity and treatment in wastewaters. Environ Int. 2008;34:1033-49.
54. Noorimotlagh Z, Mirzaee SA, Martinez SS, Rachoń D, Hoseinzadeh M, Jaafarzadeh N. Environmental exposure to nonylphenol and cancer progression Risk-A systematic review. Environ Res. 2020;184:109263.
55. Ömeroğlu S, Murdoch FK, Sanin FD. Investigation of nonylphenol and nonylphenol ethoxylates in sewage sludge samples from a metropolitan wastewater treatment plant in Turkey. Talanta. 2015;131:650-5.
56. Wang F, Xiang L, Sze-Yin Leung K, et al. Emerging contaminants: a one health perspective. Innovation. 2024;5:100612.
57. Covaci A, Voorspoels S, Abdallah MA, Geens T, Harrad S, Law RJ. Analytical and environmental aspects of the flame retardant tetrabromobisphenol-A and its derivatives. J Chromatogr A. 2009;1216:346-63.
58. Yu Y, Yu Z, Chen H, et al. Tetrabromobisphenol A: disposition, kinetics and toxicity in animals and humans. Environ Pollut. 2019;253:909-17.
59. Barghi M, Shin ES, Kim JC, Choi SD, Chang YS. Human exposure to HBCD and TBBPA via indoor dust in Korea: estimation of external exposure and body burden. Sci Total Environ. 2017;593-4:779-86.
60. Cariou R, Antignac JP, Zalko D, et al. Exposure assessment of French women and their newborns to tetrabromobisphenol-A: occurrence measurements in maternal adipose tissue, serum, breast milk and cord serum. Chemosphere. 2008;73:1036-41.
61. Kim UJ, Oh JE. Tetrabromobisphenol A and hexabromocyclododecane flame retardants in infant-mother paired serum samples, and their relationships with thyroid hormones and environmental factors. Environ Pollut. 2014;184:193-200.
62. Parsons A, Lange A, Hutchinson TH, et al. Molecular mechanisms and tissue targets of brominated flame retardants, BDE-47 and TBBPA, in embryo-larval life stages of zebrafish (danio rerio). Aquat Toxicol. 2019;209:99-112.
63. Kitamura S, Kato T, Iida M, et al. Anti-thyroid hormonal activity of tetrabromobisphenol A, a flame retardant, and related compounds: affinity to the mammalian thyroid hormone receptor, and effect on tadpole metamorphosis. Life Sci. 2005;76:1589-601.
64. Zhang YF, Xu W, Lou QQ, et al. Tetrabromobisphenol A disrupts vertebrate development via thyroid hormone signaling pathway in a developmental stage-dependent manner. Environ Sci Technol. 2014;48:8227-34.
65. Zhu B, Zhao G, Yang L, Zhou B. Tetrabromobisphenol A caused neurodevelopmental toxicity via disrupting thyroid hormones in zebrafish larvae. Chemosphere. 2018;197:353-61.
66. Miao B, Yakubu S, Zhu Q, Issaka E, Zhang Y, Adams M. A review on tetrabromobisphenol A: human biomonitoring, toxicity, detection and treatment in the environment. Molecules. 2023;28:2505.
67. Sturm MT, Myers E, Schober D, et al. Comparison of AOP, GAC, and novel organosilane-based process for the removal of microplastics at a municipal wastewater treatment plant. Water. 2023;15:1164.
68. Acarer S. A review of microplastic removal from water and wastewater by membrane technologies. Water Sci Technol. 2023;88:199-219.
69. Pizzichetti ARP, Pablos C, Álvarez-Fernández C, Reynolds K, Stanley S, Marugán J. Evaluation of membranes performance for microplastic removal in a simple and low-cost filtration system. Case Stud Chem Environ Eng. 2021;3:100075.
70. Malucelli LC, Ozeri I, Matos M, Magalhães WLE, Filho MASC, Eisen MS. High-flux, porous and homogeneous PVDF/cellulose microfiltration membranes. Cellulose. 2022;29:1943-53.
71. Yahyanezhad N, Bardi MJ, Aminirad H. An evaluation of microplastics fate in the wastewater treatment plants: frequency and removal of microplastics by microfiltration membrane. Water Pract Technol. 2021;16:782-92.
72. Gul A, Hruza J, Yalcinkaya F. Fouling and chemical cleaning of microfiltration membranes: a mini-review. Polymers. 2021;13:846.
73. Singh S, Kalyanasundaram M, Diwan V. Removal of microplastics from wastewater: available techniques and way forward. Water Sci Technol. 2021;84:3689-704.
74. Long Z, Pan Z, Wang W, et al. Microplastic abundance, characteristics, and removal in wastewater treatment plants in a coastal city of China. Water Res. 2019;155:255-65.
75. Zhang Y, Diehl A, Lewandowski A, Gopalakrishnan K, Baker T. Removal efficiency of micro- and nanoplastics (180 nm-125 μm) during drinking water treatment. Sci Total Environ. 2020;720:137383.
76. Zhang Y, Jiang H, Wang H, Wang C. Removal strategies for aquatic microplastics. In: Khan A, Wang C, Asiri AM, editors. Microplastic sources, fate and solution. Singapore: Springer Nature Singapore; 2023. pp.71-88.
77. Tadsuwan K, Babel S. Microplastic abundance and removal via an ultrafiltration system coupled to a conventional municipal wastewater treatment plant in Thailand. J Environ Chem Eng. 2022;10:107142.
78. Zhang J, Li G, Yuan X, et al. Reduction of ultrafiltration membrane fouling by the pretreatment removal of emerging pollutants: a review. Membranes. 2023;13:77.
79. Xiao T, Zhu Z, Li L, Shi J, Li Z, Zuo X. Membrane fouling and cleaning strategies in microfiltration/ultrafiltration and dynamic membrane. Sep Purif Technol. 2023;318:123977.
80. Magni S, Gagné F, André C, et al. Evaluation of uptake and chronic toxicity of virgin polystyrene microbeads in freshwater zebra mussel Dreissena polymorpha (Mollusca: Bivalvia). Sci Total Environ. 2018;631-2:778-88.
81. Le LT, Bui XB, Tran CS, Chiemchaisri C, Pandey A. Chapter 9 - Membrane and filtration processes for microplastic removal. In: Current developments in biotechnology and bioengineering. Elsevier; 2023. pp.203-20.
82. Michielssen MR, Michielssen ER, Ni J, Duhaime MB. Fate of microplastics and other small anthropogenic litter (SAL) in wastewater treatment plants depends on unit processes employed. Environ Sci Water Res Technol. 2016;2:1064-73.
83. Oatley-Radcliffe DL, Walters M, Ainscough TJ, Williams PM, Mohammad AW, Hilal N. Nanofiltration membranes and processes: a review of research trends over the past decade. J Water Process Eng. 2017;19:164-71.
84. Jang H, Kang S, Kim J. Identification of membrane fouling with greywater filtration by porous membranes: combined effect of membrane pore size and applied pressure. Membranes. 2024;14:46.
85. Fang Y, Duranceau SJ. Study of the effect of nanoparticles and surface morphology on reverse osmosis and nanofiltration membrane productivity. Membranes. 2013;3:196-225.
86. Ziajahromi S, Neale PA, Rintoul L, Leusch FD. Wastewater treatment plants as a pathway for microplastics: development of a new approach to sample wastewater-based microplastics. Water Res. 2017;112:93-9.
87. Pivokonsky M, Cermakova L, Novotna K, Peer P, Cajthaml T, Janda V. Occurrence of microplastics in raw and treated drinking water. Sci Total Environ. 2018;643:1644-51.
88. Khoo YS, Goh PS, Lau WJ, et al. Removal of emerging organic micropollutants via modified-reverse osmosis/nanofiltration membranes: a review. Chemosphere. 2022;305:135151.
89. Sturm MT, Schuhen K, Horn H. Method for rapid biofilm cultivation on microplastics and investigation of its effect on the agglomeration and removal of microplastics using organosilanes. Sci Total Environ. 2022;806:151388.
90. Wang L, He J, Heiranian M, et al. Water transport in reverse osmosis membranes is governed by pore flow, not a solution-diffusion mechanism. Sci Adv. 2023;9:eadf8488.
91. Harharah RH, Abdalla GMT, Elkhaleefa A, Shigidi I, Harharah HN. A study of copper (II) ions removal by reverse osmosis under various operating conditions. Separations. 2022;9:155.
92. Popov K, Oshchepkov M, Pervov A, et al. A case study of calcium carbonate crystallization during reverse osmosis water desalination in presence of novel fluorescent-tagged antiscalants. Membranes. 2022;12:194.
93. Yu YH, Jenne D. Numerical modeling and dynamic analysis of a wave-powered reverse-osmosis system. J Mar Sci Eng. 2018;6:132.
94. Sheriff I, Awang NA, Halim HB, et al. Extraction and analytical methods of microplastics in wastewater treatment plants: isolation patterns, quantification, and size characterization techniques. Desalin Water Treat. 2024;318:100399.
95. Wang Z, Liu K, Gao Y, et al. Removal and fouling influence of microplastics in fertilizer driven forward osmosis for wastewater reclamation. Membranes. 2021;11:845.
96. Sun J, Dai X, Wang Q, van Loosdrecht MCM, Ni BJ. Microplastics in wastewater treatment plants: detection, occurrence and removal. Water Res. 2019;152:21-37.
97. Kraatz S, Libra JA, Drastig K, Hunstock U, Zare M, Jacobs H. Water use indicators at farm scale - an agro-hydrological software solution. Sci Total Environ. 2019;678:133-45.
98. Wang Z, Lin T, Chen W. Occurrence and removal of microplastics in an advanced drinking water treatment plant (ADWTP). Sci Total Environ. 2020;700:134520.
99. Ma B, Xue W, Hu C, Liu H, Qu J, Li L. Characteristics of microplastic removal via coagulation and ultrafiltration during drinking water treatment. Chem Eng J. 2019;359:159-67.
100. Zhang J, Xue Y, Eshtiaghi N, Dai X, Tao W, Li Z. Evaluation of thermal hydrolysis efficiency of mechanically dewatered sewage sludge via rheological measurement. Water Res. 2017;116:34-43.
101. Tang N, Zhang S, Si Y, Yu J, Ding B. An ultrathin bacterial cellulose membrane with a Voronoi-net structure for low pressure and high flux microfiltration. Nanoscale. 2019;11:17851-9.
102. Ozden S, Monti S, Tozzini V, et al. Egg protein derived ultralightweight hybrid monolithic aerogel for water purification. Mater Today. 2022;59:46-55.
103. Okamoto Y, Lienhard JH. How RO membrane permeability and other performance factors affect process cost and energy use: a review. Desalination. 2019;470:114064.
104. Golgoli M, Khiadani M, Shafieian A, et al. Microplastics fouling and interaction with polymeric membranes: a review. Chemosphere. 2021;283:131185.
105. Wang J, Wang S. Reactive species in advanced oxidation processes: formation, identification and reaction mechanism. Chem Eng J. 2020;401:126158.
106. Hübner U, Spahr S, Lutze H, et al. Advanced oxidation processes for water and wastewater treatment - guidance for systematic future research. Heliyon. 2024;10:e30402.
107. Ameta R, Solanki MS, Benjamin S, Ameta SC. Chapter 6 - Photocatalysis. In: Advanced oxidation processes for waste water treatment. Academic Press; 2018. pp.135-75.
108. Tang C, Huang X, Wang H, Shi H, Zhao G. Mechanism investigation on the enhanced photocatalytic oxidation of nonylphenol on hydrophobic TiO2 nanotubes. J Hazard Mater. 2020;382:121017.
109. He F, Ji Y, Wang Y, Zhang Y. Preparation of bifunctional hollow mesoporous Fe0@C@MnFe2O4 as Fenton-like catalyst for degradation of tetrabromobisphenol A. J Taiwan Inst Chem Eng. 2017;80:553-62.
110. Hu K, Zhou P, Yang Y, et al. Degradation of microplastics by a thermal fenton reaction. ACS EST Eng. 2022;2:110-20.
111. Ji Y, Niu J, Fang Y, Tan Nou A, Warsinger DM. Micelles inhibit electro-oxidation degradation of nonylphenol ethoxylates. Chem Eng J. 2022;430:133167.
112. Ning Z, Duan X, Li Y, Zhao X, Chang L. Degradation of polyvinyl chloride microplastics via electrochemical oxidation with a CeO2–PbO2 anode. J Clean Prod. 2023;432:139668.
113. Rahmani AR, Salari M, Shabanloo A, Shabanloo N, Bajalan S, Vaziri Y. Sono-catalytic activation of persulfate by nZVI-reduced graphene oxide for degradation of nonylphenol in aqueous solution: process optimization, synergistic effect and degradation pathway. J Environ Chem Eng. 2020;8:104202.
114. Ioannidi AA, Bampos G, Antonopoulou M, et al. Sonocatalytic degradation of bisphenol A from aquatic matrices over Pd/CeO2 nanoparticles: kinetics study, transformation products, and toxicity. Sci Total Environ. 2024;919:170820.
115. Anastopoulos I, Kyzas GZ. Are the thermodynamic parameters correctly estimated in liquid-phase adsorption phenomena? J Mol Liq. 2016;218:174-85.
116. Zanaty M, Zaki A, El-Dek S, Abdelhamid HN. Zeolitic imidazolate framework@hydrogen titanate nanotubes for efficient adsorption and catalytic oxidation of organic dyes and microplastics. J Environ Chem Eng. 2024;12:112547.
117. Zoppas F, Sacco N, Soffietti J, Devard A, Akhter F, Marchesini FA. Catalytic approaches for the removal of microplastics from water: recent advances and future opportunities. Chem Eng J Adv. 2023;16:100529.
118. Truppi A, Petronella F, Placido T, et al. Visible-light-active TiO2-based hybrid nanocatalysts for environmental applications. Catalysts. 2017;7:100.
119. Zhang Y, Wang X, Li Y, et al. Improving nanoplastic removal by coagulation: Impact mechanism of particle size and water chemical conditions. J Hazard Mater. 2022;425:127962.
120. Guo Y, Kong F, Fatehi P. Generation and use of lignin-g-AMPS in extended DLVO theory for evaluating the flocculation of colloidal particles. ACS Omega. 2020;5:21032-41.
121. Khan MT, Ahmad M, Hossain MF, et al. Microplastic removal by coagulation: a review of optimizing the reaction conditions and mechanisms. Water Emerg Contam Nanoplastics. 2023;2:22.
122. Spreafico C, Landi D, Russo D. A new method of patent analysis to support prospective life cycle assessment of eco-design solutions. Sustain Prod Consump. 2023;38:241-51.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.





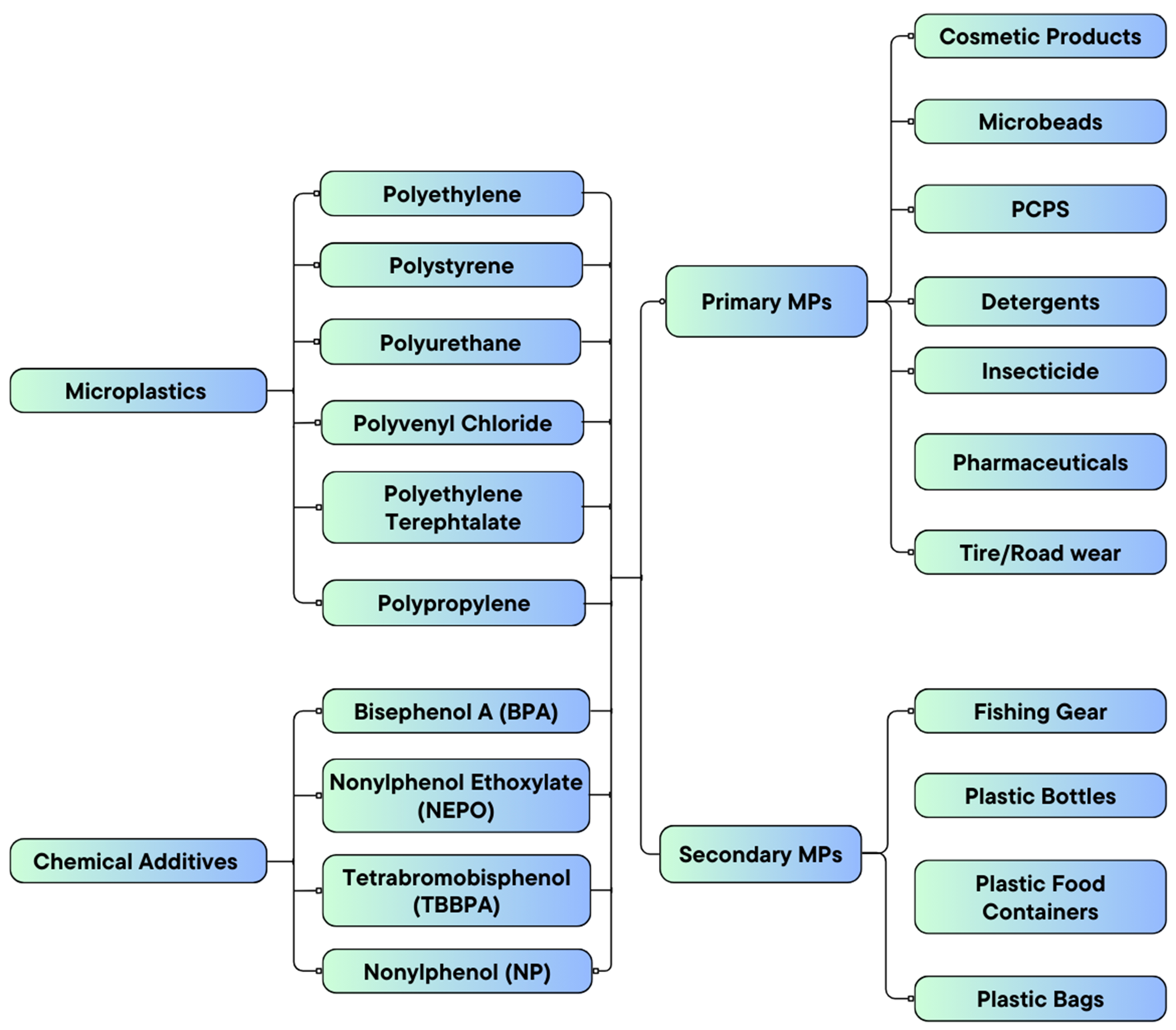
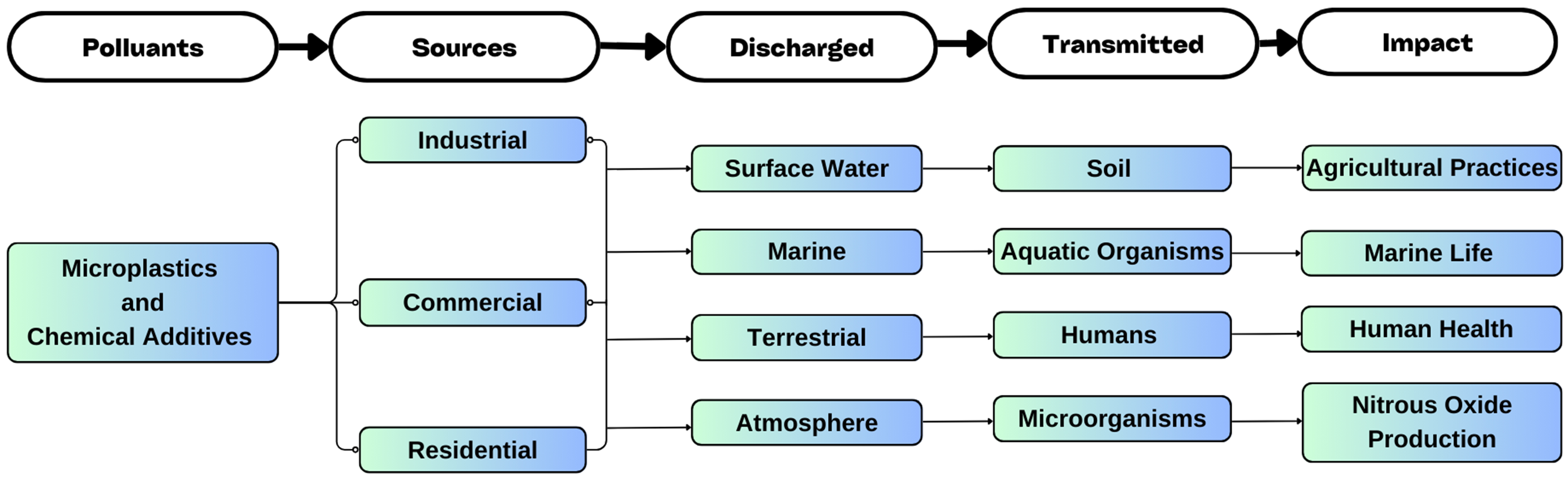
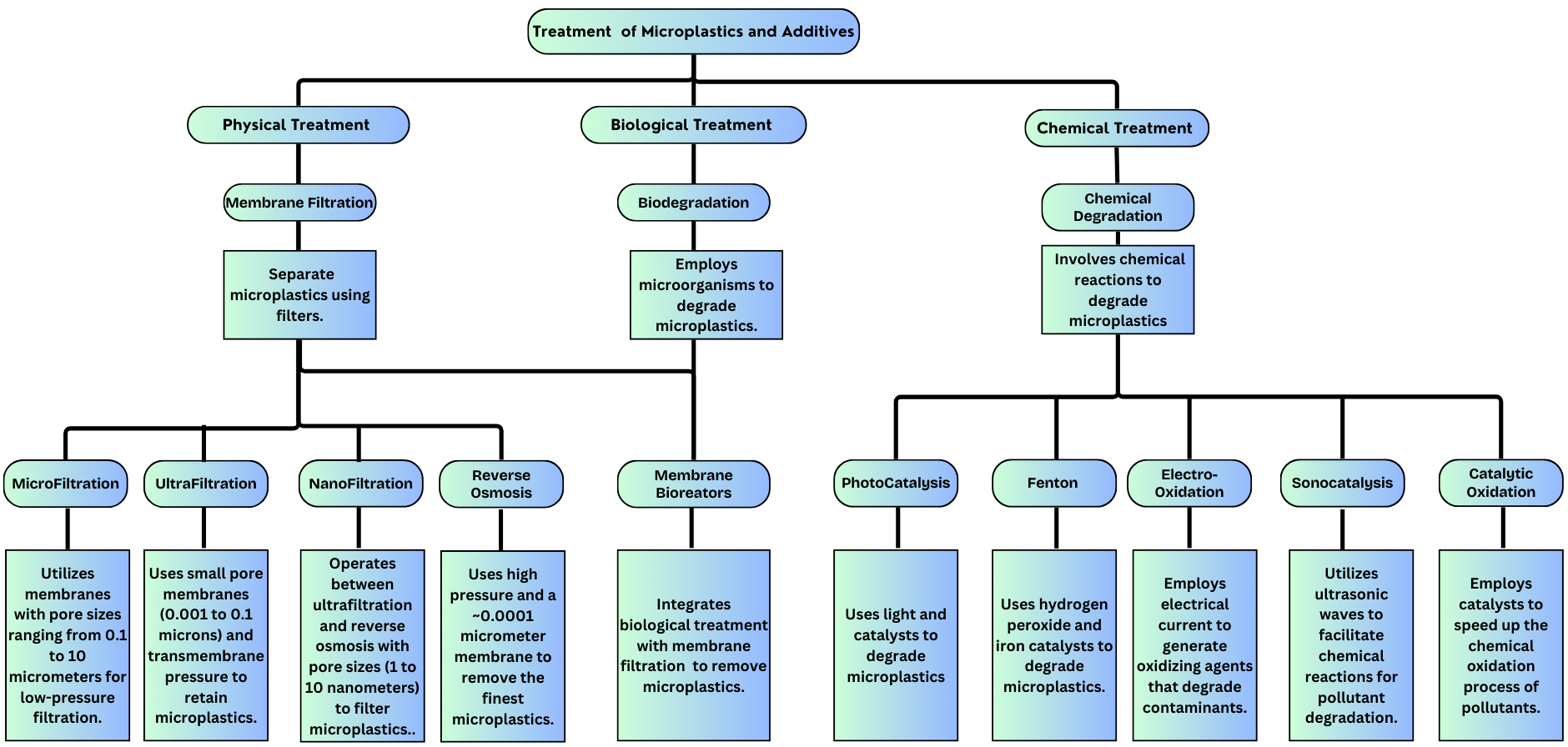
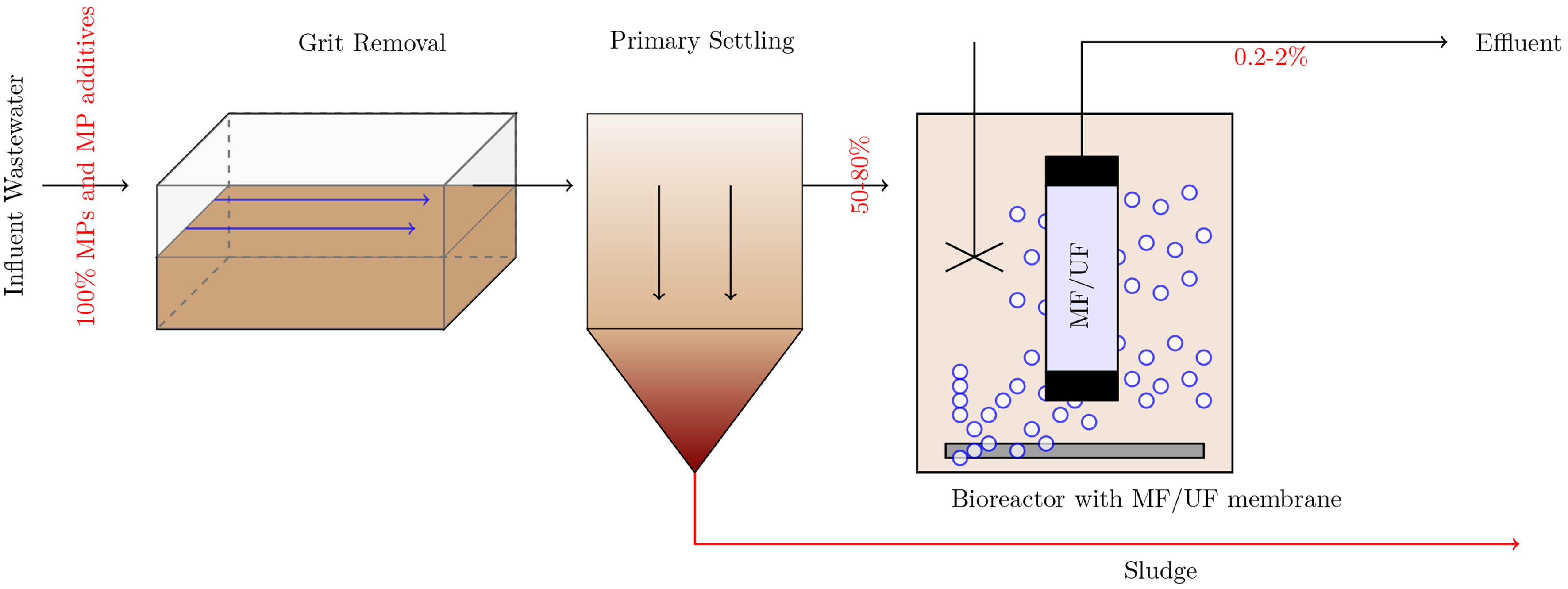
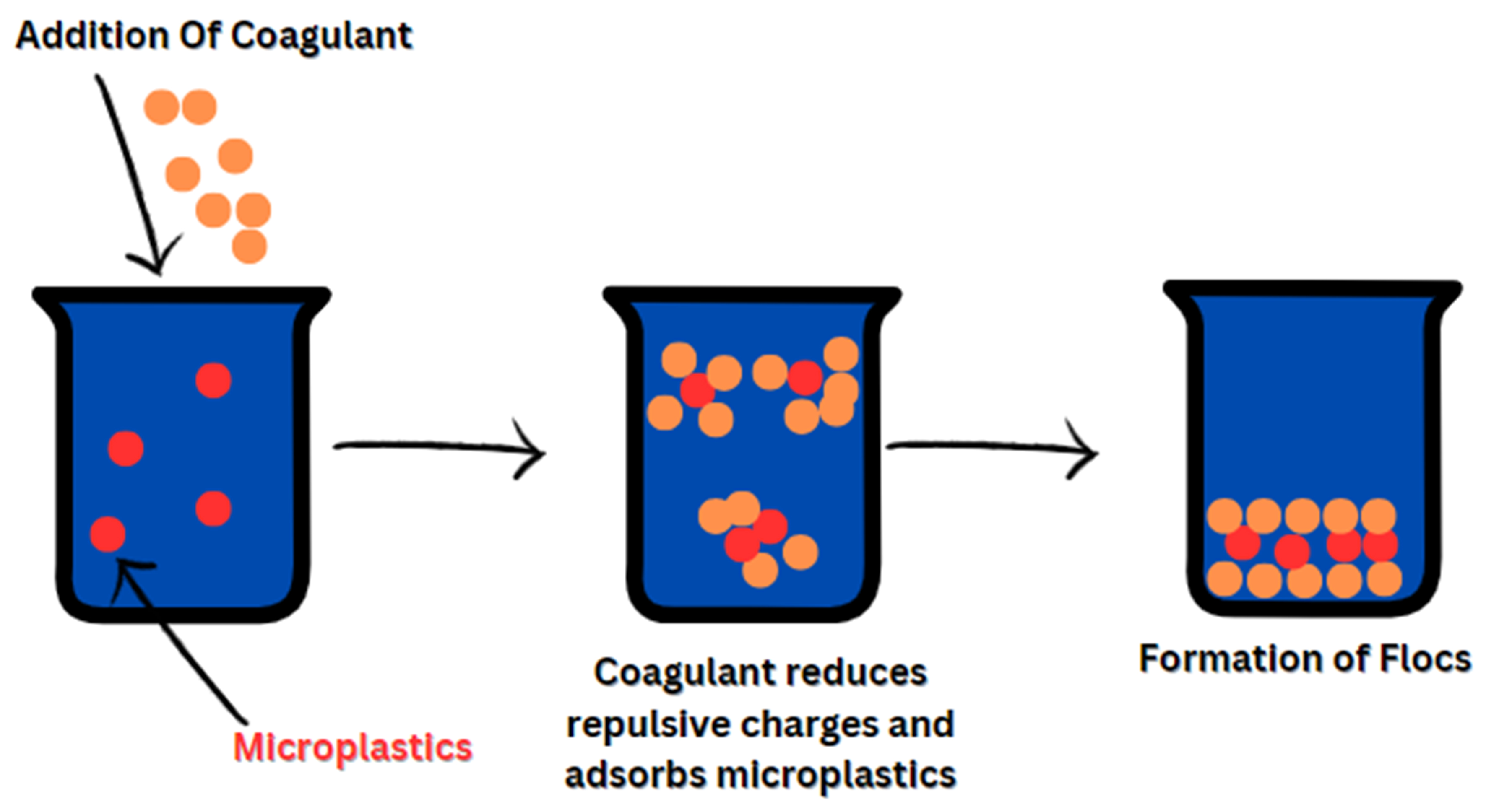
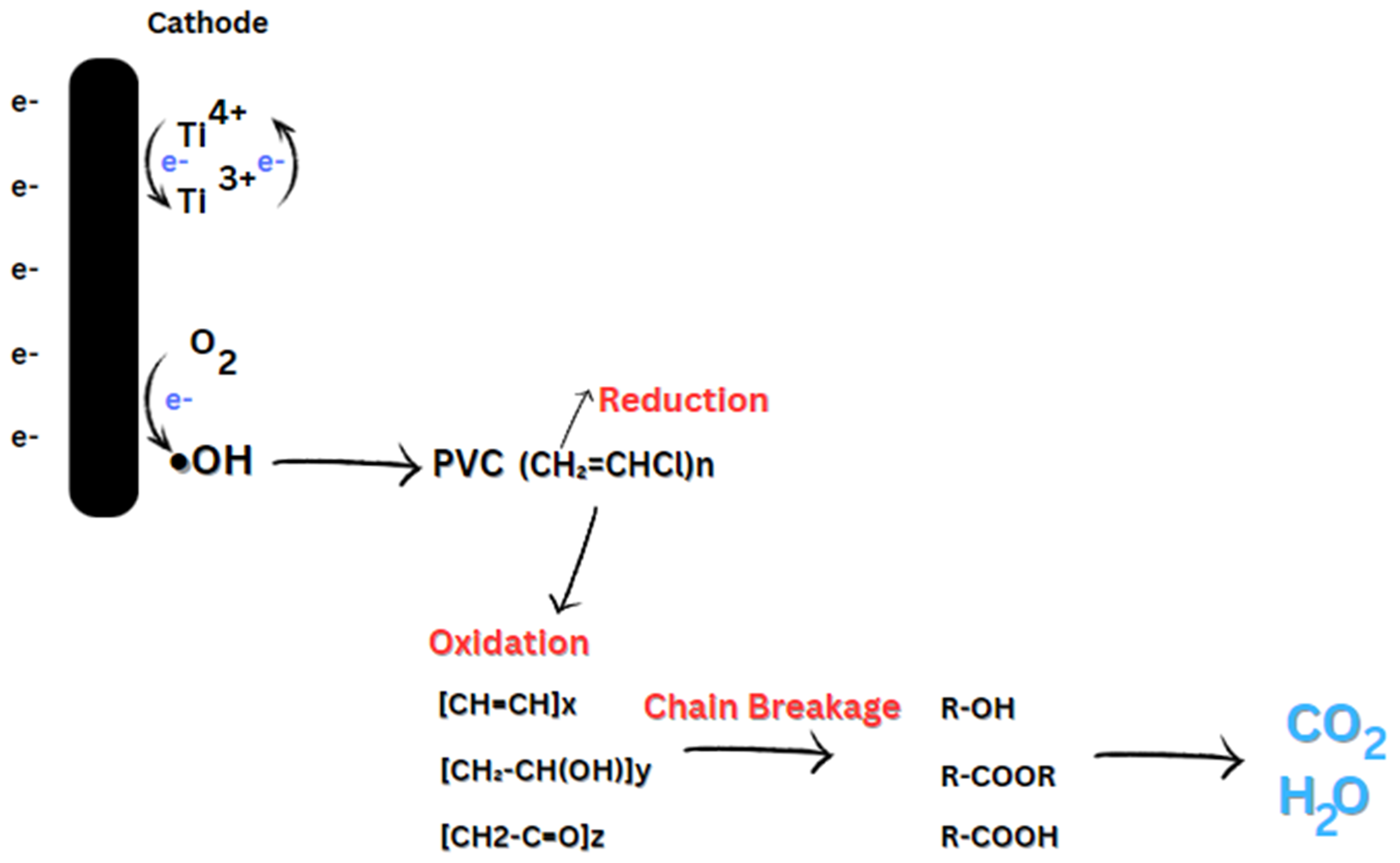













Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].