How did we move from the initial concept 24 years ago to the current understanding of treating patients with Fabry Disease? A narrative review and personal perspective
Abstract
Fabry disease is a complex, devastating, progressive, hereditary X-linked lysosomal storage disorder. Females were once thought to be only carriers, but 25 years ago it became clear that heterozygous females can be as symptomatic as males, depending on their X-chromosome inactivation pattern and other factors. Although females are generally less affected, some are as severely ill as males. Patients with classical mutations show different disease progression from those with later-onset mutations. In most cases, Fabry disease progresses slowly, which limits the ability to demonstrate strong evidence for the efficacy of approved treatments. The best current evidence for treatment effects comes from registries and real-world data, since controlled clinical trials are no longer feasible after drug approval. Patients with classical mutations, especially males, should start treatment early to prevent disease progression. Clinicians should avoid overtreatment in patients with late-onset mutations, where general organ-protective rather than Fabry-specific treatment may be sufficient. Attention to each patient’s psychological response to the disease is essential, and patient involvement in decisions is paramount for adherence. Regional and international collaborations are crucial to advancing individualized management of Fabry disease.
Keywords
INTRODUCTION
Fabry disease, also known as Andersen-Fabry disease, is a rare, complex X-linked lysosomal storage disorder caused by over 1,000 mutations in the galactosidase alpha (GLA) gene, resulting in reduced or absent α-galactosidase A (α-Gal A) activity. The diminished enzyme activity leads to the accumulation of globotriaosylceramide (Gb3)[1,2-8], causing progressive cellular, tissue, and organ damage. The clinical presentation includes severe pain, ocular, dermatologic, gastrointestinal, and neuro-otologic manifestations, and depression; severe cases may progress to renal failure, cardiovascular dysfunction, cardiomyopathy, cerebrovascular events, and premature death[9-12]. Milder forms of the disease also occur, particularly in females and in later-onset gene variants. Estimates of Fabry disease prevalence range from approximately 1 in 117,000 to 1 in 37,000 live male births for classic Fabry, and up to 1 in 1,400 in some newborn screening projects when atypical Fabry gene variants are included[1,13-16]. As an X-linked disease, Fabry disease was initially thought to affect mainly or only male patients, with females considered carriers of the abnormal gene variants; however, heterozygous female patients can also be affected, typically with later onset and more variable phenotypes[3,10]. Many factors are responsible for the variable clinical manifestations in female patients, including skewed X-inactivation; genetic variants; clonal expansion (which produces mature α-Gal A enzyme that cells do not readily take up), and somatic mosaicism, which generates α-Gal A enzyme more susceptible to plasma dephosphorylation[17].
Much of the information about Fabry disease above has accumulated since 2001, when two recombinant α-Gal A enzyme replacement therapies became available[18-20]. It was also the year when I became aware of, and responsible for, the Fabry patients in Denmark. The Institute of Genetics, headed by Professor Sven Asger Sørensen at the University of Copenhagen, previously conducted research on Fabry disease, identifying several affected families[21-28]. However, the first description of Fabry disease in Denmark was published much earlier[29], providing a pivotal contribution to the pathological characterization of the disease at the cellular level.
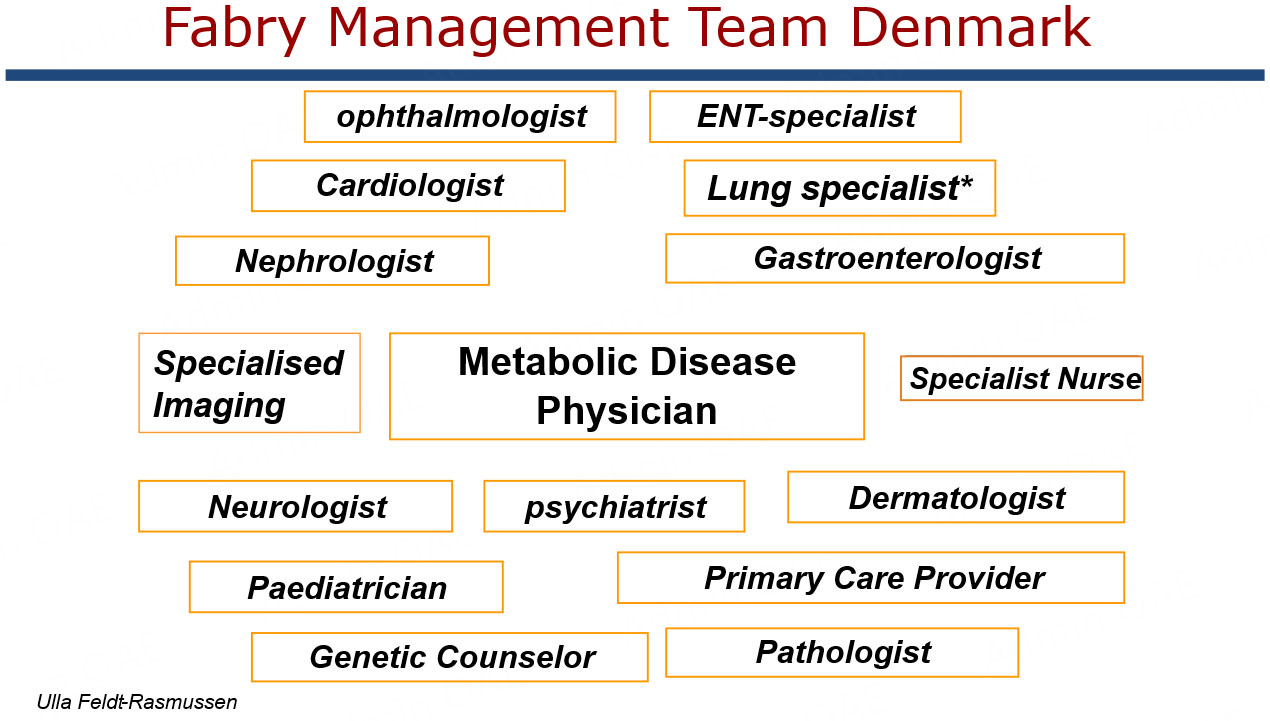
Patients with Fabry disease were scattered throughout Denmark, and at that time, none had a stable connection with a relevant clinical department, except for those with significant organ involvement, such as neurological, renal, cardiac, or other Fabry-related symptoms. As chief of the Department of Endocrinology and Metabolism at Rigshospitalet, Copenhagen, I was tasked with assuming clinical responsibility for these patients. The management of this group resembled that of other endocrine/metabolic disorders with multiorgan involvement, such as diabetes mellitus, and pituitary, adrenal, and thyroid diseases[30,31]. With experience in genetic counceling of families with inherited endocrine disorders such as multiple endocrine neoplasia[32,33], I accepted the task on behalf of my treatment team[30]. However, I was not familiar with the disease, which at that time was mentioned only sparsely in textbooks of internal medicine, paediatrics, and even genetics. After reviewing the limited literature, I decided, together with my coworkers, to organize an informational meeting with known family members in Denmark to explain the new treatment option of enzyme replacement. We also invited them to participate in a comprehensive multiorgan assessment to establish a proper baseline before making treatment decisions. Due to limited knowledge of phenotypic disease penetration, we offered complete baseline evaluations to both male and female Fabry patients with a confirmed disease variant, regardless of the presence of symptoms. Baseline assessments were conducted in collaboration with highly dedicated and skilled organ-specific specialists, who subsequently formed the National Fabry Team at Rigshospitalet. This team later expanded to the Capital Region following restructuring of the hospital system concerning specialty fields in Copenhagen [Figure 1]. We also initiated systematic cascade screening of families to identify all patients with potentially treatable phenotypes[34].
Figure 1. Composition of the National Fabry management team at Rigshospitalet/Capital Region, Denmark. The metabolic disease physician was in this case specialist in Medical Endocrinology with strong collaboration with all other organ specialists. The Paediatrician was instrumental for managing the children concerning diagnosis, treatment, growth and reproduction.
This review provides a personal account of my experience with this unique patient group over the past 20 years or more. The journey has involved gaining close insight into individuals affected by a devastating
THE FIRST ENCOUNTERS WITH FAMILIES WITH AN X-LINKED GENETIC CHRONIC DISEASE
The patients and their family members were initially very willing to accept our invitation to undergo a comprehensive baseline evaluation [Table 1], followed by individual discussions about initiating enzyme replacement therapy. The preparation, first approved by the European Medicines Agency (EMA) in Europe, was α-galactosidase-α. The males among members of the seven families were all seriously affected and carried what is now recognized as classical gene variants[35]. We had therefore expected to offer treatment to all hemizygous males, but not to females, who were considered heterozygous carriers and not phenotypically affected. Surprisingly, many heterozygous women exhibited typical Fabry-related symptoms[36]. These symptoms had been previously overlooked as Fabry-related and were attributed to other diagnoses to align the patient's symptoms with an established diagnosis. For example, acroparesthesia in some Fabry female patients was labeled as rheumatoid factor-negative rheumatoid arthritis and treated with prednisolone for decades. The steroid did not improve the symptoms but caused the usual side effects of long-term, high-dose glucocorticoid therapy, including central obesity, diabetes, hypertension, osteoporosis, muscle wasting, moon face, increased cardiovascular risk of strokes, and endogenous cortisol deficiency.
All patients in Denmark since 2001 have been referred to one National Expert Fabry Center, where evaluation of organ manifestations in both male and female adult patients has been conducted according to the schedule below (paediatric patients follow a modified schedule). All were offered subsequent regular follow-up depending on individual affections
| Organ assessment at baseline | Tests to be performed | Supplementary tests |
| Cardiac | Echocardiography ECG | MRI Troponin Brain natriuretic peptide Myocardial biopsy |
| Neurologic | Peripheral nerve assessment Tilt test Pain assessment incl. temperature Pain score | Skin biopsy |
| Renal | Creatinine mGFR (eGFR) Urinary albumin and protein Ultrasound kidney size | Kidney biopsy |
| Cerebral | Brain MRI General neurological assessment Cognitive function tests if needed | CT scan if MRI contraindicated |
| Dermatology | Visual skin inspection for angiokeratomas Sweat test pilocarpine | Biopsy |
| Audiology | Hearing test Tests for dizziness | Questionnaires |
| Ophthalmology | Retinal photo Vision, visual field Slit lamp examination for cornea verticillata detection | |
| Bones | DXA-scan of bones Bone markers, vitamin D | |
| Biomarkers | U-Gb3/plasma lyso-Gb3 | |
| Vascular | Brachial artery dilatory responses to increased flow and to nitroglycerin Neck ultrasound for carotid artery plaques | |
| Abdominal | PRO questionnaires | Referral to gastroenterologist |
| Psychology | Referral to psychiatrist or psychologist | |
| Quality of Life | Questionnaire PROs: SF-36; depression, Life situation form | |
| Blood tests | Measurement of all other major organ related blood tests including endocrine testing | |
| Lungs* | Lung function tests |
Furthermore, before we had completed the baseline assessments for all family members, a 21-year-old female from a family with a severe gene variant and many early male deaths was admitted as an emergency to the local hospital with a severe stroke and complete hemiparesis (see later regarding treatment).
The struggle to obtain approval from the Danish medicines authorities
Faced with the emergent situation of an acute event in a young female officially considered only a carrier and not having the disease, I sought permission from the Danish Health Authorities to use the EMA-approved drug, which would entail reimbursement from the Danish public healthcare system. At the time, this medication was by far the most expensive for any disease, and my request sparked a broad discussion on the prioritization of medical resources. The existing literature had not yet established when treatment should begin to be effective, and uncertainty remained regarding its efficacy in females. Nevertheless, drawing on data from initial clinical trials - although these were brief, involved small cohorts, and included only males - it was possible to obtain approval for the first patient. We eventually received general approval for the condition, with the stipulation that prescriptions be limited to a single national center [Figure 1]. Another requirement was close follow-up to monitor drug efficacy in terms of disease progression or improvement. With the introduction of additional treatment options, this approach has generally persisted. We were required to adhere to international guidelines and not commence therapy in patients who did not meet global and European consensus criteria[37]. Regular updated reports on treatment efficacy and outcomes were also mandated, which has been challenging due to the extremely slow progression of disease in each organ. Evidence regarding improvements in clinical events and hard endpoints, such as mortality, has generally remained weak.
We initially involved pediatricians who were also clinical geneticists in planning the diagnostic and follow-up program for children carrying the family Fabry gene variant [Figure 1]. The goal was to assess the necessary organ measurements while adapting the program for children, minimizing burden and potential side effects from scans or other assessments involving radioactivity. My team handled the planning, and my laboratory technician collected all blood and urine samples from the children simultaneously with those from adult family members. This approach allowed us, as adult physicians, and the rest of our staff to become acquainted with the children and adolescents from their first visit, facilitating a smooth transition from pediatric to adult care and enabling collaborative publications along the way[38].
HISTORY AND OUTCOME OF APPROVED FABRY DISEASE-SPECIFIC TREATMENTS
As mentioned above, the EMA approved enzyme replacement therapy (ERT) with α-Gal A (Replagal) for the treatment of Andersen-Fabry disease in August 2001, and soon after, they approved a second ERT, α-galactosidase Beta (Fabrazyme). In the USA, the Food and Drug Administration (FDA) initially hesitated to endorse ERTs. The FDA ultimately approved α-galactosidase Beta, not Alpha, and in 2021, Beta received full FDA approval.
Two companies marketed ERT in Europe: Genzyme (later Sanofi) with Fabrazyme and TKT Europe 5S with Replagal (later Shire, subsequently Takeda). Fabrazyme is produced in Chinese hamster ovary cells, whereas Replagal is manufactured using genetically engineered human cell lines. Whether the biological properties of the two drugs differ in detail remains unclear[39]. Findings from the two short-term randomized, placebo-controlled, double-masked studies, which enrolled few patients[18,19], were encouraging but inconclusive. The studies differed in patient entry criteria, administered dose, and primary and secondary endpoints. ERT was initially, and remains, extremely expensive, with an annual cost per patient of approximately €150,000. The annual cost was nearly identical for both drugs, even though the recommended dose of Replagal is only 20% of that for Fabrazyme (0.2 vs. 1.0 mg/kg every other week). We commented that the limited evidence suggested moderate efficacy and urged clinical and pharmaceutical societies to consider ERT for Fabry disease as experimental therapy. Larger trials were needed to demonstrate potential benefits, as well as dose-finding and comparative studies to identify the most cost-effective dose and drug[39]. Although formal trials did not occur, several investigator-initiated (often single-center) studies have contributed to some clarification of dose comparisons, highlighted potential antidrug antibody formation, and examined organ-specific efficacy. A Canadian attempt to conduct a well-powered head-to-head comparison was disrupted by an unexpected manufacturing shortage of one ERT. Another attempt used the shortage period to compare efficacy but was limited by small cohort sizes. A retrospective comparison showed that treatment with agalsidase Beta at a higher dose versus agalsidase Alpha did not result in differences in clinical events, primarily occurring in patients with advanced disease[40]. Other underpowered studies from the same period also reported no efficacy differences, though a type 2 error could not be excluded. Since most experimental evidence indicated similar bioavailability of the two ERTs, the Danish Medicines Agency requested that we primarily use Fabrazyme. Nevertheless, Replagal has been used in cases of Fabrazyme intolerance (e.g., antidrug antibodies[41]) or when patients preferred Replagal for convenience (shorter infusion duration) after the shortage period. Some patients with severe classical GLA gene variants later chose to return to Fabrazyme.
We have advised patients to switch ERT products in cases of frequent infusion reactions that could not be managed by other means. Antidrug antibodies have been measured annually since their availability, but we have not observed a strong correlation with infusion reactions. We have therefore continually assessed other potential causes, including infections (e.g., from a Port-A-Cath), intolerance to the cannula, syringes, or tubing, and have addressed these by changing equipment as needed. We have also tried treatments with antihistamines, nonsteroidal anti-inflammatory drugs, and/or glucocorticoids[42]. In some cases, blood samples were taken before and after infusion to measure eosinophils, other white blood cells, CRP, and immunoglobulin E. These investigations have occasionally identified causes, such as a fungal infection in a Port-A-Cath, which was subsequently treated with a favorable outcome. An alternative approach in the presence of antidrug antibodies could be increasing the ERT dose[42], though we have never attempted this.
Finally, we are left with long-term information from single-center real-world evidence, small multi-center studies, or pharmaceutical company-based follow-up via post-marketing patient registries, such as the Fabry Registry for Fabrazyme and FOS for Replagal (both recently reporting 20-year results)[43-45]. These sources form the basis for decisions on whether the drugs are equipotent and which to use in individual cases.
The EMA and FDA have approved two additional products for treating Fabry disease. First, the Amicus-produced chaperone migalastat (Galafold®) was approved as an oral therapy suitable for specific amenable GLA gene variants[46]. We were fortunate to have several Fabry patients with amenable mutations willing to participate in some of the initial trials, which allowed us to become familiar with the drug early[47,48]. Patients could subsequently join the patient-involving Amicus Registry for Migalastat, FollowME[49]. Large randomized clinical trials have not been performed; instead, a short-term placebo-controlled trial[47,50] and a head-to-head comparison with ERT[51] were conducted. Subsequent studies have been either trial extensions, registry-based, or real-world evidence studies. Overall, the Migalastat studies indicated a stabilizing effect on Fabry disease-induced organ damage[52-54]. However, demonstrating an overall beneficial impact of Migalastat, as with other Fabry treatment modalities, still requires more substantial evidence.
Furthermore, another approved ERT, pegunigalsidase Alpha, produced in plants and with a prolonged half-life[55], was designed with two specific goals: (1) to reduce immune reactivity by minimizing antidrug antibody formation; and (2) to extend product efficacy through pegylation. Despite its approval, it has not been widely adopted. Initial results on immune reactivity appear favorable[55]. Recently, the Danish Medicines Agency recommended pegunigalsidase Alpha as the first-choice ERT for Fabry disease in Denmark[56], a recommendation based entirely on financial considerations despite limited data compared with agalsidase Alpha and Beta.
Several other treatment approaches are under investigation, including substrate-reducing agents and mRNA- or gene-based therapies, the latter being considered the potential ultimate cure for the disease[57-60]. None of these approaches is yet close to routine use in general patient management.
Finally, clinicians and researchers should remember that general supportive therapies for organ-damaging effects were initiated in most Fabry disease patients at the same time Fabry-specific treatments were approved and started[53]. Consequently, the results of clinical trials are also influenced by these supportive therapies[53]. Such therapies include angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) to protect renal function in patients with proteinuria or microalbuminuria. Recently, SGLT2 inhibitors have been added as kidney and heart protectors, demonstrating beneficial effects on microalbuminuria[61]; further studies are needed to assess their full potential in Fabry disease. Additional supportive measures include antiplatelet drugs (e.g., clopidogrel or acetylsalicylic acid) to prevent strokes, antiarrhythmic drugs or implantable cardioverter-defibrillators (ICDs) to reduce death from severe cardiac antiarrhytmia drugs, smoking cessation, antihypertensive and antiarrhytmia drugs, and pain management for small-fibre neuropathy (e.g., carbamazepine). Paracetamol may also be used during infections to reduce fever crises[53].
I usually advise patients to take 1 g of paracetamol as empirical treatment before intense exercise to prevent heat accumulation caused by the reduced sweating ability present in many patients. The mechanisms behind this effect are not well understood and have not been specifically studied in Fabry patients. Paracetamol (acetaminophen) can act at the hypothalamus, modulating serotonin and other neuroregulatory substances to influence thermoregulation[62]. It may also affect the immune system through cytokines, which can, in turn, impact the hypothalamic thermoregulatory center, and influence the autonomic nervous system via central effects in the brain and cerebellum. Acetaminophen may also modulate neurotransmission in the medulla oblongata, primarily by inhibiting COX-3 and altering prostaglandins. These mechanisms could be relevant for sweat gland modulation in patients with hypohidrosis, as opposed to complete anhidrosis.
For comparison, in another chronic disease with a tendency to overheat during exercise (multiple sclerosis), a recent randomized placebo-controlled trial demonstrated a significant protective effect of paracetamol over placebo[63], supporting my experience with Fabry patients. A controlled trial in Fabry patients would be valuable to provide robust evidence. Patients generally appreciate this advice because it enhances their physical performance and ability to maintain high levels of fitness. Reduced sweating was recently identified as the most common skin manifestation in Fabry disease, affecting 57.6% of patients, according to a systematic review and meta-analysis[64].
BACK TO THE PATIENTS
Our first encounters with patients were marked by enthusiasm at finally having a treatment option (ERT). However, shortly after individual conversations, this excitement often began to wane. Patients exhibited a wide range of psychological reactions to coping with the disease while considering a complex treatment. Their perspectives on the potential benefits of therapy were highly individual, making clinical discussions challenging. Some patients were relieved that treatment was available and looked forward to starting. Others denied being ill despite organ involvement or symptoms. Notably, several significantly affected young males with classical variants initially refused treatment, even when we demonstrated organ involvement and explained the potential to slow disease progression. Many expressed a preference to “live fast and die young”, attributing their outlook to family history - growing up without adult males at family gatherings because of early deaths. These powerful experiences appeared to strongly shape their views on life and family. In such cases, our approach required patience and understanding, aiming to persuade them to at least accept annual follow-ups to monitor disease progression. Unfortunately, at least one young male ultimately died early, having completely denied the disease and remained beyond our reach.
Many patients still believed that only males could be affected, particularly females with a verified Fabry gene variant. Many women carried a sense of family guilt for being “responsible” for the disease. They rarely considered that they themselves might have Fabry-related symptoms. In fact, many did, but because it was commonly believed at the time that females were merely carriers, neither they, their general practitioners, nor other physicians considered a connection to the family disease. As a result, many affected females were misdiagnosed with other conditions, such as rheumatoid arthritis, and treated accordingly, while still believing they were “only” carriers. Accepting that they had actually experienced Fabry symptoms and organ damage for decades proved to be a complicated process. Some were still eligible for treatment, which we offered. However, many had minimal symptoms and declined therapy, and some continue to do so even 24 years later. When we later had the opportunity to study Gb3 and lyso-Gb3 analogue profiles in plasma and urine (in collaboration with Professor Christiane Auray-Blais, Canada), their decision appeared justified[65]. Another study assessing similar measurements in males with Fabry gene variants is nearing completion (unpublished; presented at WORLDSymposium 2024).
Both males and females also bore the heavy family burden of knowing that, at a certain time, it had become customary to abort male fetuses after gender determination via chromosome tests. There are no publications documenting this practice. Today, this approach has been replaced by modern fertility techniques, which allow sorting of in vitro fertilized eggs after testing for the presence of the GLA gene variant before implantation. Several of our young female patients have undergone this procedure following thorough genetic counseling[59,66].
We made every effort to maintain close contact with the families and individual patients and assisted them in establishing a Danish Fabry patient organization (Fabry.dk). This organization remains highly active and communicates with both Nordic (Iceland, Norway, Denmark, Sweden, and Finland) and international Fabry patient organizations, such as the Fabry International Network (FIN). They have also formed a subgroup of young Fabry patients who provide mutual support in many aspects of life.
In 2014, we established a stronger Nordic Fabry Expert Group, holding biannual meetings to foster collaboration in conducting joint clinical trials and combining Nordic data, given our similar healthcare systems and practices. While we successfully hosted several scientifically productive meetings, joint studies proved more complex than anticipated due to legal obstacles, even between Nordic countries. Hopefully, Nordic colleagues can improve this situation in the future.
The first families we referred for organ assessment and commencement of treatment all carried classical gene variants, identified based on the phenotype of a proband and subsequent genetic screening
Illustration of the affected individuals of the Danish Fabry cohort at diagnosis and/or baseline assessment from 2001 onwards and at the end of the study period
| Amino acid change | Nucleotide change | Exon | Likely phaenotype | |
| Missense | ||||
| p.Asn34Ser | N34S | c.101A>G | 1 | Classic |
| p.Gly85Asn | G85N | c.254G>A | 2 | Classic |
| p.Arg112Cys | R112C | c.334C>T | 2 | Classic |
| p.Ala156Thr | A156T | c.466G>A | 3 | Classic |
| p.Gly171Ser | G171S | c.511G>A | 2 | Late-onset |
| p.Ile232Thr | I232T | c.695T>C | 5 | Likely classic |
| p.Trp236Cys | W236C | c.708G>C | 5 | Classic |
| p.Gly261Asp | G261D | c.782G>A | 5 | Classic |
| p.Gly271Ser | G271S | c.811G>A | 6 | Classic |
| p.Ser276Asn | S276N | c.827G>A | 6 | Classic |
| p.Gln279Arg | Q279R | c.836A>G | 6 | Classic |
| p.Ser297Phe | S297F | c.890C>T | 6 | Classic |
| p.Arg301Gln | R301Q | c.902G>A | 6 | Late-onset†† |
| p.Ile317Thr | I317T | c.950T>C | 6 | Classic |
| p.Asn355Lys | N355K | c.1065C>A | 7 | Classic |
| Nonsense | ||||
| p.Lys168Ter | K168* | c.502A>T | 3 | Not investigated† |
| p.Arg227Ter | R227* | c.679C>T | 5 | Classic |
| p.Arg301Ter | R301* | c.901C>T | 6 | Classic |
| p.Arg342Ter | R342* | c.1024C>T | 7 | Classic |
| Other | ||||
| (large deletion) | c.195-?_801 + ?del | 2-5 | Not investigated† | |
| (deletion-insertion) | c.369+3_c.547+954del4096insT | 3-4 | Classic | |
| p.Phe295Leufs*22 (deletion-frameshift) | c.885del | 6 | Not investigated† | |
| (splice site deletion exon 6 skipping) | c.999+1del* | Not investigated† | ||
| p.Ile289Tyrfs*10 (duplication-frameshift) | c.864dup | 6 | Not investigated† |
The 26 families, their 115 Fabry disease patients and their corresponding GLA-gene variants of the Danish Fabry Disease Register
| Family | Amino acid change | Nucleotide change | N (deceased) | Index-cases | ||||
| ID | All | Male | Female | Sex (age) | First manifestation | |||
| Missense | ||||||||
| 1 | p.Asn34Ser | N34S | c.101A>G | 4 | 2 | 2 | F (27) | neuropathic pain |
| 2 | p.Asn34Ser | N34S | c.101A>G | 4(1) | 2 (1) | 2 | M (?) | N/A deceased |
| 3 | p.Gly85Asn | G85N | c.254G>A | 26(7) | 8 (2) | 18 (5) | F (?) | N/A deceased |
| 4 | p.Arg112Cys | R112C | c.334C>T | 15 | 3 | 12 | M (12) | neuropathic pain |
| 5 | p.Ala156Thr | A156T | c.466G>A | 13(3) | 3 (1) | 10 (2) | M (33) | N/A deceased |
| 6 | p.Gly171Ser | G171S | c.511G>A | 2 | 1 | 1 | M (28) | nephropathy |
| 7 | p.Ile232Thr | I232T | c.695T>C | 10(1) | 4 | 6 (1) | M (36) | proteinuria |
| 8 | p.Trp236Cys | W236C | c.708G>C | 3 | 2 | 1 | F (51) | cardiomyopathy |
| 9 | p.Gly261Asp | G261D | c.782G>A | 6 | 2 | 4 | F (68) | cardiomyopathy |
| 10 | p.Gly271Ser | G271S | c.811G>A | 4(1) | 1 | 3 (1) | F (66) | cardiomyopathy |
| 11 | p.Ser276Asn | S276N | c.827G>A | 1 | 1 | - | F (?)† | cardiomyopathy |
| 12 | p.Gln279Arg | Q279R | c.836A>G | 1 | 1 | - | M (54) | cardiomyopathy |
| 13 | p.Ser297Phe | S297F | c.890C>T | 2 | 1 | 1 | F (73) | cardiomyopathy |
| 14 | p.Arg301Gln | R301Q | c.902G>A | 2 | - | 2 | N/A | |
| 15 | p.Ile317Thr | I317T | c.950T>C | 2 | 1 | 1 | M (40) | cardiomyopathy, nephropathy, neuropathic pain |
| 16 | p.Asn355Lys | N355K | c.1065C>A | 2 | 1 | 1 | M (36) | neuropathic pain |
| Nonsense | ||||||||
| 17 | p.Lys168Ter | K168X | c.502A>T | 1 | - | 1 | F (63)††† | cardiomyopathy |
| 18 | p.Arg227Ter | R227X | c.679C>T | 2 | - | 2 | M (?) | N/A deceased |
| 19 | p.Arg301Ter | R301X | c.901C>T | 3 | - | 3 | F (53) | cardiomyopathy |
| 20 | p.Arg301Ter | R301X | c.901C>T | 1 | - | 1 | M†† | non-Danish |
| 21 | p.Arg342Ter | R342X | c.1024C>T | 4 | 1 | 3 | M (33) | neuropathic pain |
| Other | ||||||||
| 22 | (Large deletion) | c.195-?_801 + ?del | 1 | 1 | - | M (47) | cardiomyopathy | |
| 23 | (Deletion-insertion) | c.369+3_c.547+954del4096insT | 1 | - | 1 | F (16) | migraine | |
| 24 | p.Phe295Leufs*22 (deletion-frameshift) | c.885del | 3(1) | 2 (1) | 1 | M (10) | neuropathic pain | |
| 25 | (Splice site deletion exon 6 skipping) | c.999+1del* | 1 | 1 | - | M†† | non-Danish | |
| 26 | p.Ile289Tyrfs*10 (duplication-frameshift) | c.864dup | 1 | 1 | - | M†† | non-Danish | |
| Total | 115 | 39 | 76 | |||||
Referral of Fabry patients to our center has changed markedly over the past several years, with many now referred following the screening of various patient groups or the incidental detection of a Fabry gene variant. A large proportion are referred by cardiologists who screen for hereditary causes of hypertrophic cardiomyopathy when no apparent cause is identified, with GLA gene testing included in their diagnostic panel[67]. As a result, the diagnostic landscape for Fabry disease has become increasingly varied and complex. Most probands are now adults, often of relatively advanced age, and many incidental findings prove to be either non-pathogenic or variants of unknown significance (VUS). Some represent late-onset variants, associated with milder or even absent symptoms apart from cardiomyopathy - or, when screened for other conditions, limited to manifestations such as acroparaesthesia, kidney failure, stroke, or angiokeratomas. Classical gene variants are now detected only rarely.
A new challenge has emerged, as patients referred from colleagues are often informed that a definitive diagnosis has been established and treatment is available. The first step, however, is to explain that Fabry disease may not be present and that confirmation depends on diagnostic testing. At our center, this involves retesting for the Fabry genetic variant and measuring the biomarker lyso-Gb3[20,68,69]. In female patients, it is essential, if possible, to test a male family member to determine whether a variant is pathogenic[70]. The next step is to decide whether, and to what extent, family screening should be pursued. In some cases, results ultimately exclude Fabry disease, which requires informing the patient. Although one might expect this to bring relief, it often provokes anger and disappointment, amounting to a psychological crisis. In patients where a pathogenic Fabry variant is confirmed, a full organ assessment should be performed, along with family screening and genetic counseling. Based on age, sex, and phenotype, the next step is to decide whether the patient qualifies for Fabry-specific therapy, supportive or organ-preserving treatment, or no treatment. In younger patients, early treatment may be indicated[71,72], but not always.
Using recently published consensus guidelines[73,74], a more uniform and evidence-based approach across patient groups may be achievable. The “PRoposing Early Disease Indicators for Clinical Tracking in Fabry Disease (PREDICT-FD)” initiative identified expert consensus on 27 early indicators of disease progression, as well as on factors influencing treatment initiation in Fabry disease. This framework may support the development and international adoption of clearer, more consistent guidance for treatment initiation in Fabry disease[67,75].
KEY CONCLUSIONS
Fabry disease is a complex, devastating, progressive, X-linked lysosomal storage disorder. Historically, females were considered only carriers; however, it was established more than 25 years ago that heterozygous females can be as symptomatic as males, depending on their X-chromosome inactivation pattern. In general, females are less severely affected and present later in life, but some are as seriously ill as males and require the same management. Disease progression differs between patients with classical mutations and those with later-onset variants. In most cases, Fabry disease progresses slowly, which has contributed to the difficulty in demonstrating strong evidence for the efficacy of approved Fabry-specific therapies.
Nevertheless, treatment with either alpha-galactosidase beta (Fabrazyme) or alpha-galactosidase alpha (Replagal) has shown convincing effects, supported by robust long-term data from the Fabry and FOS registries. Patients or families with milder mutations than the classical type, as well as females with fewer or milder symptoms, may benefit from alternative therapies and still achieve favorable outcomes. In addition to Fabry-specific therapy, appropriate management of affected organs is essential, following general clinical guidelines - for example, kidney protection in microalbuminuria with ACE inhibitors or ARBs, pain management for acroparaesthesia, and treatment of gastrointestinal symptoms.
The best current evidence for treatment efficacy is derived from registries and real-world data, as controlled clinical trials are generally not feasible after drug approval, except for studies of new formulations or treatment strategies. Small molecules targeting substrate reduction, for example, are currently under investigation. In patients with classical mutations, early initiation of therapy is essential to prevent disease progression, particularly in males. Early treatment is also indicated in females with a clear Fabry phenotype. For late-onset mutations, overtreatment should be avoided, and in many cases, general organ-protective therapy is sufficient rather than Fabry-specific treatment. Psychological aspects of disease perception must be carefully considered, as they significantly affect adherence, and patient involvement in decision-making is equally crucial. Regional and international collaboration is strongly recommended to enable evidence-based progress in managing Fabry disease, as no single center can generate sufficient data for this rare condition. Emerging therapies, such as gene transfer or stem cell therapy, hold promise for more durable effects and reduced frequency of hospital visits, although their widespread application remains distant.
DECLARATIONS
Acknowlegements
UFR’s research salary was sponsored by the Kirsten and Freddy Johansen Fund. I am deeply grateful to my national and international colleagues for many fruitful discussions on this topic over the years. I especially thank the Nordic Fabry Expert Group for their invaluable inspiration, which fostered a Nordic collaboration based on our shared registries and exchange of ideas across countries. I also extend my thanks to the National Danish Fabry Team and the Capital Region Fabry Team for their excellent collaboration in data collection during patient follow-up programs. Finally, I wish to thank the Danish Fabry patients and the Fabry patient organization for their dedicated participation in the follow-up programs, contributing essential data for future clinical management despite the challenges this imposed on their daily lives.
Authors’ contributions
The author contributed solely to the article.
Availability of data and materials
Not applicable.
Financial support and sponsorship
The author has received travel grants, and speaker honoraria from Chiesi, Protalix, Shire/Takeda, Freeline, and Sanofi/Genzyme, and she has received research grants from Takeda/Shire and Sanofi/Genzyme.
Conflicts of interest
The author declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Giugliani R, Niu DM, Ramaswami U, et al. A 15-year perspective of the Fabry outcome survey. J Inborn Errors Metab Screen. 2016;4:232640981666629.
2. Brady RO, Gal AE, Bradley RM, Martensson E, Warshaw AL, Laster L. Enzymatic defect in Fabry's disease. Ceramidetrihexosidase deficiency. N Engl J Med. 1967;276:1163-7.
3. Mehta A, Beck M, Linhart A, Sunder-Plassmann G, Widmer U. History of lysosomal storage diseases: an overview. In: Mehta A, Beck M, Sunder-Plassmann G, editors. Fabry disease: perspectives from 5 years of FOS. Oxford: Oxford PharmaGenesis; 2006.
4. Eng CM, Niehaus DJ, Enriquez AL, Burgert TS, Ludman MD, Desnick RJ. Fabry disease: twenty-three mutations including sense and antisense CpG alterations and identification of a deletional hot-spot in the alpha-galactosidase A gene. Hum Mol Genet. 1994;3:1795-9.
5. Terryn W, Vanholder R, Hemelsoet D, et al. Questioning the pathogenic role of the GLA p.Ala143Thr “mutation” in Fabry disease: implications for screening studies and ERT. JIMD Rep. 2013;8:101-8.
6. Hsu TR, Chang FP, Chu TH, et al. Correlations between endomyocardial biopsies and cardiac manifestations in Taiwanese patients with the Chinese hotspot IVS4+919G>A mutation: data from the fabry outcome survey. Int J Mol Sci. 2017;18:119.
7. Liu H, Perrin A, Hsu T, et al. Age at first cardiac symptoms in Fabry disease: association with a Chinese hotspot Fabry mutation (IVS4+919G>A), classical Fabry mutations, and sex in a Taiwanese population from the Fabry outcome survey (FOS). JIMD Rep. 2015;22:107-13.
8. Stenson PD, Mort M, Ball EV, et al. The human gene mutation database: towards a comprehensive repository of inherited mutation data for medical research, genetic diagnosis and next-generation sequencing studies. Hum Genet. 2017;136:665-77.
9. Mehta A, Ricci R, Widmer U, et al. Fabry disease defined: baseline clinical manifestations of 366 patients in the Fabry outcome survey. Eur J Clin Invest. 2004;34:236-42.
10. MacDermot KD, Holmes A, Miners AH. Anderson-Fabry disease: clinical manifestations and impact of disease in a cohort of 60 obligate carrier females. J Med Genet. 2001;38:769-75.
11. MacDermot KD, Holmes A, Miners AH. Anderson-Fabry disease: clinical manifestations and impact of disease in a cohort of 98 hemizygous males. J Med Genet. 2001;38:750-60.
12. Politei JM, Solar B. Gastrointestinal involvement in Fabry disease. Rare Dis Orphan Drugs J. 2024;3:10.
13. Spada M, Pagliardini S, Yasuda M, et al. High incidence of later-onset fabry disease revealed by newborn screening. Am J Hum Genet. 2006;79:31-40.
14. Meikle PJ, Hopwood JJ, Clague AE, Carey WF. Prevalence of lysosomal storage disorders. JAMA. 1999;281:249-54.
15. Lin HY, Chong KW, Hsu JH, et al. High incidence of the cardiac variant of Fabry disease revealed by newborn screening in the Taiwan Chinese population. Circ Cardiovasc Genet. 2009;2:450-6.
16. Germain DP, Levade T, Hachulla E, et al. Challenging the traditional approach for interpreting genetic variants: lessons from Fabry disease. Clin Genet. 2022;101:390-402.
17. Beck M, Cox TM. Comment: why are females with Fabry disease affected? Mol Genet Metab Rep. 2019;21:100529.
18. Schiffmann R, Kopp JB, Austin HA 3rd, et al. Enzyme replacement therapy in Fabry disease: a randomized controlled trial. JAMA. 2001;285:2743-9.
19. Eng CM, Guffon N, Wilcox WR, et al. Safety and efficacy of recombinant human alpha-galactosidase A replacement therapy in Fabry's disease. N Engl J Med. 2001;345:9-16.
20. Schiffman R. Investigating Fabry disease - some lessons learned. Rare Dis Orphan Drugs J. 2024;3:4.
21. Hasholt L, Wandall A, Sørensen SA. Enzyme replacement in Fabry endothelial cells and fibroblasts: uptake experiments and electron microscopical studies. Clin Genet. 1988;33:360-71.
23. Hasholt L, Sørensen SA, Wandall A, Andersen EB, Arlien-Søborg P. A Fabry's disease heterozygote with a new mutation: biochemical, ultrastructural, and clinical investigations. J Med Genet. 1990;27:303-6.
24. Andersen MV, Dahl H, Fledelius H, Nielsen NV. Central retinal artery occlusion in a patient with Fabry's disease documented by scanning laser ophthalmoscopy. Acta Ophthalmol. 1994;72:635-8.
25. Madsen KM, Hasholt L, Sørensen SA, Fermér ML, Dahl N. Two novel mutations (L32P) and (G85N) among five different missense mutations in six Danish families with Fabry's disease. Hum Mutat. 1995;5:277-8.
26. Madsen KM, Hasholt L, Sørensen SA, van Loo A, Vanholder R. The utility of single-strand conformation polymorphism (SSCP) analysis: results obtained in families with Fabry's disease. Scand J Clin Lab Invest. 1996;56:177-82.
27. Madsen KM, Hasholt L, Berger J, Sørensen SA. SSCP analysis of paraffin wax embedded tissues in a family with an atypical form of Fabry disease. Clin Mol Pathol. 1996;49:M310-2.
28. Rosenberg KM, Schiffmann R, Kaneski C, Brady RO, Sorensen SA, Hasholt L. Five novel mutations in fourteen patients with Fabry disease. Hum Mutat. 2000;15:207-8.
29. Jensen E. On the pathology of angiokeratoma corporis diffusum (Fabry). Acta Pathol Microbiol Scand. 1966;68:313-31.
30. Feldt-Rasmussen U, Rasmussen AK, Mersebach H, Rosenberg KM, Hasholt L, Sorensen SA. Fabry disease: a new challenge in endocrinology and metabolism? Eur J Endocrinol. 2002;146:741-2.
31. Eng CM, Germain DP, Banikazemi M, et al. Fabry disease: guidelines for the evaluation and management of multi-organ system involvement. Genet Med. 2006;8:539-48.
32. Chandrasekharappa SC, Guru SC, Manickam P, et al. Positional cloning of the gene for multiple endocrine neoplasia-type 1. Science. 1997;276:404-7.
33. Jäger AC, Friis-Hansen L, Hansen TV, et al. Characteristics of the Danish families with multiple endocrine neoplasia type 1. Mol Cell Endocrinol. 2006;249:123-32.
34. Effraimidis G, Rasmussen ÅK, Dunoe M, et al. Systematic cascade screening in the Danish Fabry Disease Centre: 20 years of a national single-centre experience. PLoS One. 2022;17:e0277767.
35. Ortiz A, Germain DP, Desnick RJ, et al. Fabry disease revisited: management and treatment recommendations for adult patients. Mol Genet Metab. 2018;123:416-27.
36. Wilcox WR, Oliveira JP, Hopkin RJ, et al. Females with Fabry disease frequently have major organ involvement: lessons from the Fabry Registry. Mol Genet Metab. 2008;93:112-28.
37. Biegstraaten M, Arngrímsson R, Barbey F, et al. Recommendations for initiation and cessation of enzyme replacement therapy in patients with Fabry disease: the European Fabry Working Group consensus document. Orphanet J Rare Dis. 2015;10:36.
38. Borgwardt L, Feldt-Rasmussen U, Rasmussen AK, Ballegaard M, Lund AM. Fabry disease in children: agalsidase-beta enzyme replacement therapy. Clin Genet. 2013;83:432-8.
39. Bengtsson BA, Johansson JO, Hollak C, Linthorst G, FeldtRasmussen U. Enzyme replacement in Anderson-Fabry disease. Lancet. 2003;361:352.
40. Arends M, Biegstraaten M, Wanner C, et al. Agalsidase alfa versus agalsidase beta for the treatment of Fabry disease: an international cohort study. J Med Genet. 2018;55:351-8.
41. Wilcox WR, Linthorst GE, Germain DP, et al. Anti-α-galactosidase A antibody response to agalsidase beta treatment: data from the Fabry Registry. Mol Genet Metab. 2012;105:443-9.
42. Lenders M, Brand E. Precision medicine in Fabry disease. Nephrol Dial Transplant. 2021;36:14-23.
43. Wanner C, Ortiz A, Wilcox WR, et al. Global reach of over 20 years of experience in the patient-centered Fabry Registry: advancement of Fabry disease expertise and dissemination of real-world evidence to the Fabry community. Mol Genet Metab. 2023;139:107603.
44. Beck M, Ramaswami U, Hernberg-Ståhl E, et al. Twenty years of the Fabry Outcome Survey (FOS): insights, achievements, and lessons learned from a global patient registry. Orphanet J Rare Dis. 2022;17:238.
45. Ramaswami U, Pintos-Morell G, Kampmann C, et al. Two decades of experience of the Fabry Outcome Survey provides further confirmation of the long-term effectiveness of agalsidase alfa enzyme replacement therapy. Mol Genet Metab Rep. 2025;43:101215.
47. Schiffmann R, Bichet DG, Jovanovic A, et al. Migalastat improves diarrhea in patients with Fabry disease: clinical-biomarker correlations from the phase 3 FACETS trial. Orphanet J Rare Dis. 2018;13:68.
48. Bichet DG, Torra R, Wallace E, et al. Long-term follow-up of renal function in patients treated with migalastat for Fabry disease. Mol Genet Metab Rep. 2021;28:100786.
49. Hughes DA, Sunder-Plassmann G, Jovanovic A, et al. Renal and multisystem effectiveness of 3.9 years of migalastat in a global real-world cohort: Results from the followME Fabry Pathfinders registry. J Inherit Metab Dis. 2025;48:e12771.
50. Germain DP, Hughes DA, Nicholls K, et al. Treatment of Fabry's disease with the pharmacologic chaperone migalastat. N Engl J Med. 2016;375:545-55.
51. Hughes DA, Nicholls K, Shankar SP, et al. Oral pharmacological chaperone migalastat compared with enzyme replacement therapy in Fabry disease: 18-month results from the randomised phase III ATTRACT study. J Med Genet. 2017;54:288-96.
52. Feldt-Rasmussen U, Hughes D, Sunder-Plassmann G, et al. Long-term efficacy and safety of migalastat treatment in Fabry disease: 30-month results from the open-label extension of the randomized, phase 3 ATTRACT study. Mol Genet Metab. 2020;131:219-28.
53. Hughes DA, Bichet DG, Giugliani R, et al. Long-term multisystemic efficacy of migalastat on Fabry-associated clinical events, including renal, cardiac and cerebrovascular outcomes. J Med Genet. 2023;60:722-31.
54. Perretta F, Jaurretche S. Fabry disease: switch from enzyme replacement therapy to oral chaperone migalastat: what do we know today? Healthcare. 2023;11:449.
55. Holida M, Linhart A, Pisani A, et al. A phase III, open-label clinical trial evaluating pegunigalsidase alfa administered every 4 weeks in adults with Fabry disease previously treated with other enzyme replacement therapies. J Inherit Metab Dis. 2025;48:e12795.
56. Fabrys sygdom. Available from: https://medicinraadet.dk/anbefalinger-og-vejledninger/behandlingsvejledninger-og-laegemiddelrekommandationer/fabrys-sygdom [Last accessed on 12 Sep 2025].
57. Jeyakumar JM, Kia A, Tam LCS, et al. Preclinical evaluation of FLT190, a liver-directed AAV gene therapy for Fabry disease. Gene Ther. 2023;30:487-502.
58. Rodríguez-Castejón J, Beraza-Millor M, Solinís MÁ, Rodríguez-Gascón A, Del Pozo-Rodríguez A. Targeting strategies with lipid vectors for nucleic acid supplementation therapy in Fabry disease: a systematic review. Drug Deliv Transl Res. 2024;14:2615-28.
59. Lenders M, Menke ER, Brand E. Progress and challenges in the treatment of Fabry disease. BioDrugs. 2025;39:517-35.
60. der Veen SJ, Hollak CEM, van Kuilenburg ABP, Langeveld M. Developments in the treatment of Fabry disease. J Inherit Metab Dis. 2020;43:908-21.
61. Tsatsaronis A, Tiong M, Nicholls K, Ruderman I. Sodium-glucose cotransporter 2 inhibitors reduce albuminuria in patients with Fabry disease: a real-world case series. Intern Med J. 2025;55:617-21.
62. Ghanem CI, Pérez MJ, Manautou JE, Mottino AD. Acetaminophen from liver to brain: New insights into drug pharmacological action and toxicity. Pharmacol Res. 2016;109:119-31.
63. Leavitt VM, Tozlu C, Nelson KE, et al. A randomized controlled trial of oral antipyretic treatment to reduce overheating during exercise in adults with multiple sclerosis. J Neurol. 2024;271:2207-15.
64. Al-Chaer RN, Folkmann M, Mårtensson NL, Feldt-Rasmussen U, Mogensen M. Cutaneous manifestations of Fabry disease: a systematic review. J Dermatol. 2025;52:571-82.
65. Effraimidis G, Feldt-Rasmussen U, Rasmussen ÅK, et al. Globotriaosylsphingosine (lyso-Gb3) and analogues in plasma and urine of patients with Fabry disease and correlations with long-term treatment and genotypes in a nationwide female Danish cohort. J Med Genet. 2021;58:692-700.
66. Madsen CV, Christensen EI, Nielsen R, Mogensen H, Rasmussen ÅK, Feldt-rasmussen U. Enzyme replacement therapy during pregnancy in Fabry patients: review of published cases of live Births and a new case of a severely affected female with Fabry Disease and pre-eclampsia complicating pregnancy. JIMD Rep. 2019;44:93-101.
67. Hespe S, Waddell A, Asatryan B, et al. Genes associated with hypertrophic cardiomyopathy: a reappraisal by the ClinGen hereditary cardiovascular disease gene curation expert panel. J Am Coll Cardiol. 2025;85:727-40.
68. Ramaswami U, West ML, Tylee K, et al. The use and performance of lyso-Gb3 for the diagnosis and monitoring of Fabry disease: a systematic literature review. Mol Genet Metab. 2025;145:109110.
69. Bichet DG, Aerts JM, Auray-Blais C, et al. Assessment of plasma lyso-Gb3 for clinical monitoring of treatment response in migalastat-treated patients with Fabry disease. Genet Med. 2021;23:192-201.
70. Effraimidis G, Rasmussen ÅK, Bundgaard H, Sørensen SS, Feldt-Rasmussen U. Is the alpha-galactosidase A variant p.Asp313Tyr
71. Hopkin RJ, Cabrera GH, Jefferies JL, et al. Clinical outcomes among young patients with Fabry disease who initiated agalsidase beta treatment before 30 years of age: an analysis from the Fabry Registry. Mol Genet Metab. 2023;138:106967.
72. Germain DP, Altarescu G, Barriales-Villa R, et al. An expert consensus on practical clinical recommendations and guidance for patients with classic Fabry disease. Mol Genet Metab. 2022;137:49-61.
73. Brand E, Linhart A, Deegan P, et al. Clinical management of female patients with Fabry disease based on expert consensus. Orphanet J Rare Dis. 2025;20:7.
74. Hughes DA, Aguiar P, Lidove O, et al. Do clinical guidelines facilitate or impede drivers of treatment in Fabry disease? Orphanet J Rare Dis. 2022;17:42.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Special Issue
Copyright
Data & Comments
Data

















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].