Remission and low disease activity in systemic lupus erythematosus
Abstract
The treat-to-target strategy has significantly improved patient outcomes in several non-rheumatological disorders, such as diabetes mellitus, hypercholesterolemia, and arterial hypertension. This approach has also been successfully applied to certain rheumatological conditions, including rheumatoid arthritis, psoriatic arthritis, and spondyloarthropathies, and it has been proposed for systemic lupus erythematosus (SLE). However, identifying treatment targets in SLE remains challenging. The 2023 European Alliance of Associations for Rheumatology (EULAR) recommendations for SLE management emphasize remission or low disease activity (LDA) as the ideal treatment goals. Within this framework, the Definitions of Remission in SLE (DORIS) and Lupus Low Disease Activity State (LLDAS) are used to define remission and LDA, respectively, and also serve as secondary endpoints in an increasing number of randomized controlled trials. Achieving DORIS or LLDAS is associated with reduced mortality and improved
Keywords
INTRODUCTION
Despite significant advances in understanding the pathophysiology of systemic lupus erythematosus (SLE) and the development of novel therapies, patients with SLE continue to experience progressive organ damage, reduced health-related quality of life (HRQoL), and markedly increased mortality[1]. The
This unmet need has led to the development of multiple definitions of remission and, when remission is not feasible, of LDA, with the Definitions of Remission in SLE (DORIS) and Lupus Low Disease Activity State (LLDAS) being widely accepted by the international community, respectively[5,6].
This review aims to clarify the concepts of remission and LDA, examining their various definitions to guide clinical decision-making and their use as outcome measures in randomized controlled trials (RCTs). It will also discuss future directions for implementing these definitions within the T2T strategy for SLE management.
METHODS
This work focuses on the various definitions of remission and LDA and their associations with damage accrual, disease flares, and mortality.
The literature search was conducted in PubMed, Embase, and Scopus using a set of predefined keywords combined with SLE.
We considered all the papers published between June 2014 and December 2024. Keyword strategies were discussed and agreed upon collectively to ensure consistency throughout the manuscript and included key terms as “remission”, “DORIS”, “low disease activity”, “LLDAS”, “quality of life”, “HRQoL”, “patient reported outcomes”, “disease activity”, “damage”, “Systemic Lupus International Collaborating Clinics - Damage index (SLICC-DI)”, “mortality”, “disease flares”, “treat to target”.
Additionally, the snowballing technique was allowed, in order to identify other relevant studies.
We prioritized high-quality evidence, including RCTs, prospective cohort studies, large case-control studies, and meta-analyses. Grey literature, such as expert consensus statements and conference abstracts, was excluded from this study.
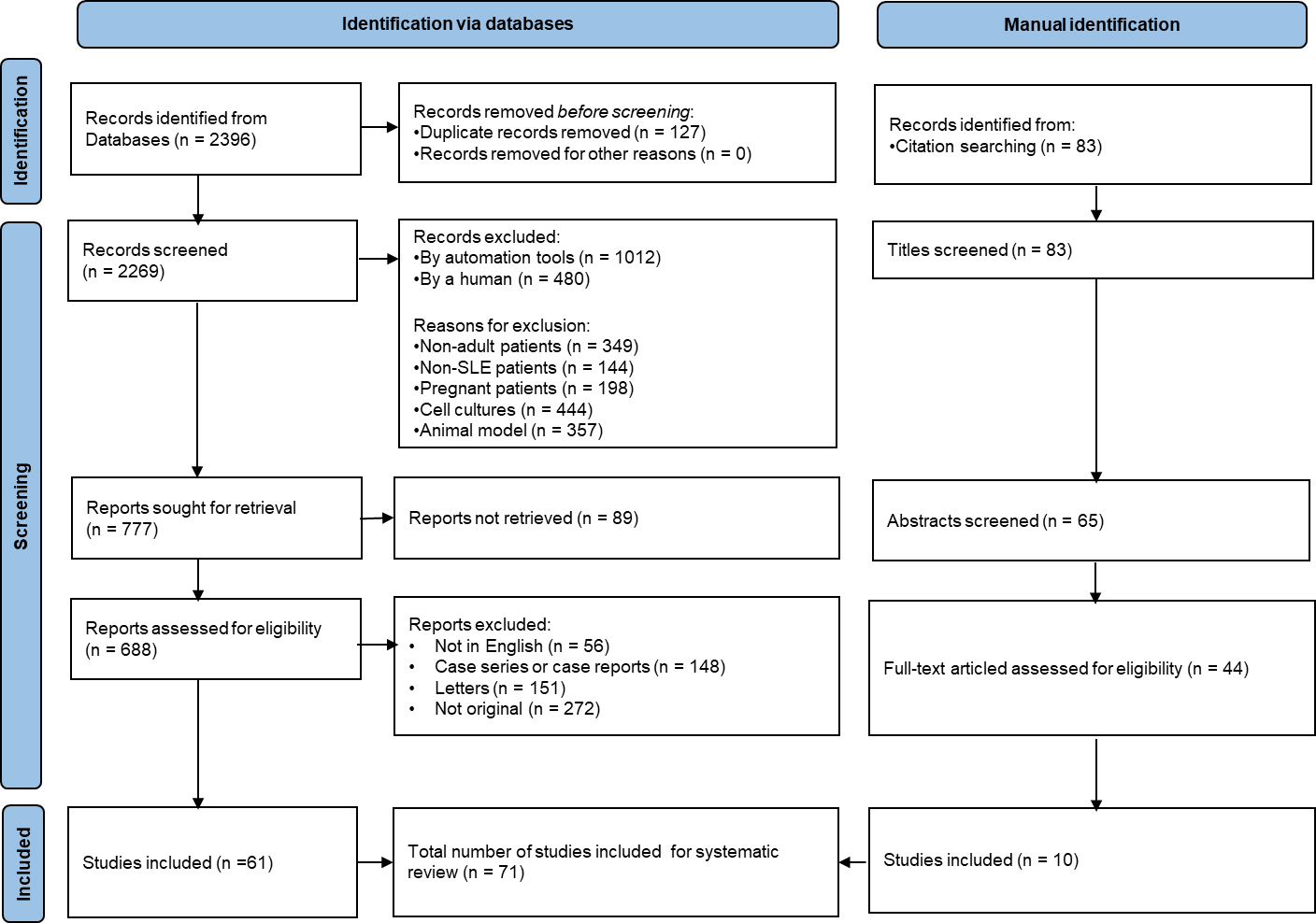
A Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram illustrating the study selection process is reported in Figure 1.
Figure 1. PRISMA Flow Diagram illustrating the study selection process for the literature review. PRISMA: Preferred reporting items for systematic reviews and meta-analyses; SLE: systemic lupus erythematosus.
Figure 1 outlines the number of records identified, screened, assessed for eligibility, and included in the final synthesis, along with reasons for exclusion at each stage.
REMISSION
The T2T paradigm has been proposed for patients with SLE; however, no consensus exists on the definition of remission, resulting in multiple interpretations over the past decade [Table 1].
Definitions and attainability of SLE remission according to the studies published in the last 10 years
| Definition of remission | Disease activity | Therapy allowed | Attainability |
| Zen et al., 2015 - (Padua remission)[7]-complete remission -Clinical remission off steroids -Clinical remission on steroids | SLEDAI-2k = 0 cSLEDAI-2k = 0 cSLEDAI-2k = 0 | AMA only AMA, PDN 0 mg, table IS, bDMARDs AMA, PDN ≤ 5 mg/day, stable IS, bDMARDs | 7.1% of patients, at least 5 years long 14.7% of patients, at least 5 years long 15.6% of patients, at least 5 years long |
| Ugarte-Gil et al., 2017[8] | SLEDAI = 0 | AMA, PDN ≤ 5 mg/day, stable IS | 20.5% of patients, at least once |
| Polachek et al., 2017[9] | cSLEDAI-2k = 0 | AMA | 30.8% of patients, at least one year long |
| Tselios et al., 2019[10] | cSLEDAI-2k = 0 | Unlimited | 10.1% of patients, at least 10 year long |
| Alarcón et al., 2019[11] | SLAM = 0 | AMA, PDN ≤ 5 mg/day | 1.8% of all visits |
| Jesus et al., 2021[12] | SLEDAS ≤ 2.08 | AMA, PDN ≤ 5 mg/day, stable IS, bDMARDs | Not investigated, (100% concordance with DORIS) |
| van Vollenhoven et al., 2021 - (DORIS)[5] | cSLEDAI-2k=0, PGA< 0.5 | AMA, PDN ≤ 5 mg/day, stable IS, bDMARDs | 67.9% of patients, no specific duration |
| Ugarte-Gil et al., 2022[13] | cSLEDAI-2k = 0 | AMA, PDN ≤ 5 mg/day, stable IS | 40.7% of patients, no specific duration |
This prompted the formation of a task force of 60 SLE experts in 2014 to agree on a potential definition of remission in SLE[14]. They defined remission as a desirable outcome for SLE patients, characterized by a durable state with at least the absence of major symptoms and signs of SLE. This collective effort culminated in 2021 with the proposal of a final DORIS definition[5].
The DORIS definition of remission includes a Clinical Systemic Lupus Erythematosus Disease Activity Index (cSLEDAI) of 0, a Physician Global Assessment (PGA) < 0.5, and a glucocorticoid ceiling of 5 mg/day prednisone equivalent. Permitted treatments include antimalarial agents and stable maintenance doses of immunosuppressive or biologic agents (see Table 2).
The 2021 DORIS definition of SLE
| Clinical SLEDAI = 0 |
| Physician global assessment < 0.5 |
| Antimalarials, prednisone ≤ 5 mg/day and/or stable immunosuppressants, including biological DMARDs are allowed |
| Serology not included |
The inclusion of the PGA is intended to compensate for the known limitations of the Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2k). As a matter of fact, the SLEDAI-2k score has been demonstrated in some studies to be only a modest predictor of organ damage accrual[15,16]. Moreover, the dichotomous nature of the SLEDAI-2k impairs its sensitivity to partial changes in disease activity[17], unlike semiquantitative indices such as systemic lupus erythematosus disease activity state (SLEDAS)[18,19]. For example, Jesus et al. showed that a change of ≥ 4 points in the SLEDAI-2k score between visits failed to detect almost two-thirds of clinically relevant changes in disease activity as judged by the physician[20]. The addition of PGA also aids in assessing some manifestations not included in the SLEDAI-2k score, such as hemolytic anemia, gastrointestinal disease, myelitis, and pneumonitis[21]. Nonetheless, as shown in Table 1, DORIS is the only definition of remission featuring the inclusion of the PGA which has been debated due to its low inter-rater and intra-rater reliability in a systematic review[22], prompting international efforts to reach a standardization consensus [Physician Global Assessment International Standardization COnsensus in Systemic Lupus Erythematosus (PISCOS)][23]. Therein, it was stated that only experienced physicians should be rating the PGA, preferably the same rater at each visit, making its use particularly challenging in multicenter and longitudinal studies. Moreover, another review by Ugarte et al. has shown that remission, regardless of definition, was protective against damage accrual, even in the absence of the PGA, suggesting that its addition may not add significant value[24].
Despite this, the PGA remains a part of the DORIS definition due to its independent association with adverse outcomes in some studies[22].
The DORIS definition deliberately excludes serological activity as no unequivocal linear correlation has been found between serological markers (complement and anti-dsDNA levels) and disease activity in SLE. In fact, recent literature suggests that abnormal serology may be only modestly associated with increased risk of future flares, especially renal flares[25] and it does not consistently correlate with organ damage in patients who are otherwise clinically inactive[26]. Moreover, requiring serological normalization would render remission unattainable for many, thereby reducing its applicability in both clinical trials and routine care. This exclusion also reflects the significant heterogeneity in laboratory techniques, with a lack of technical standardization across assays that compromises the reliability and reproducibility of serological measurements. Additionally, the interpretation of serological activity may benefit from a more nuanced, semiquantitative framework: subtle deviations from normal levels may carry limited clinical significance, whereas more pronounced abnormalities are more likely to correlate with adverse outcomes, including disease relapse.
Although the DORIS task force acknowledged the importance of duration in defining remission, it refrained from specifying precise time windows, fearing that additional restrictions would reduce attainability. Robust evidence from RCTs, with the post-hoc analysis of the TULIP-1 trial serving as a prominent example, reported a DORIS attainment rate of merely 15.3% at one year of treatment with anifrolumab[27] which increased up to 30.3% after 4 years[28]. The following real-world studies[11,29-39], while limited by their observational design and absence of controlled conditions, reveal considerable variability in attainability, which is strongly influenced by the duration of follow-up [Table 1].
For instance, in the longitudinal Hopkins Lupus Cohort, patients spent 27% and 13% of their follow-up time in DORIS remission on-treatment and off-treatment, respectively[29]. In a multinational longitudinal cohort study of the Asia Pacific region, patients were in DORIS remission for 36.1% of the time while on treatment and 10.8% of the time while off treatment[30].
However, durability is a key aspect of remission. A growing body of evidence has shown that prolonged remission may significantly influence patient outcomes, describing a positive association between the cumulative time spent in remission and mitigation of damage accrual[11,31,32]. More specifically, spending longer than five years in remission in the Amsterdam cohort[31] and longer than two years in the Padova cohort[33,34] was associated with protection against damage accumulation (see Table 3). Interestingly, a recent paper from Golder et al.[35] illustrated that remission intervals as short as 3 months may already yield tangible benefits in terms of damage accrual [hazard ratio (HR) 0.66; 95% confidence interval (95%CI)
Impact of various remission definitions on damage accrual and relapse rate
| Outcome | Study | Design | N. patients | Definition and duration of remission | Mean FU | RE pooled estimates | P |
| Damage accrual ^ | Zen et al., 2015[7] | Retrospective | 224 | Padua, ≥ 5 years | 5.0 years | RR 0.40 (0.20-0.78) | 0.008 |
| Tsang-A-Sjoe et al., 2017[31] | Retrospective | 183 | Padua, ≥ 5 years | 5.0 (3.1-5.3) years | OR 0.20 (0.07-0.53) | 0.001 | |
| Ugarte-Gil et al., 2017[8] | Prospective | 1,480 | SLEDAI = 0 off-therapy* | 2.4 (0.7-5.6) years | HR 0.51 (0.35-0.75) | 0.0006 | |
| Petri et al., 2018[29] | Prospective | 1,356 | DORIS, < 25% of FU | 46 (1-292) person-months | RR 0.54 (0.44-0.67) | < 0.0001 | |
| Golder et al., 2019[30] | Prospective | 1,707 | DORIS, ≥ 50% of FU | 2.19 (1.51-2.99) years | HR 0.49 (0.38-0.65) | < 0.0001 | |
| Saccon et al., 2020[34] | Retrospective | 646 | DORIS, ≥ 2 years | 5.0 years | OR 0.48 (0.33-0.70) | < 0.0001 | |
| Golder et al., 2024[35] | Prospective | 3,449 | DORIS, > 3 months | 2.8 (1.1-5.6) years | HR 0.66 (0.57-0.76) | < 0.0001 | |
| Pitsigavdaki et al., 2024[39] | Retrospective | 348 | DORIS, ≥ 6 months | 54 ± 18 months | HR 0.58 (0.36-0.93) | < 0.05 | |
| Relapse | Golder et al., 2019[30] | Prospective | 1,707 | DORIS, ≥ 50% of FU | 2.19 (1.51-2.99) years | HR 0.54 (0.46-0.63) | < 0.0001 |
| Golder et al., 2024[35] | Prospective | 3,449 | DORIS, > 3 months | 2.8 (1.1-5.6) years | HR 0.60 (0.51-0.71) | < 0.0001 | |
| Pitsigavdaki et al., 2024[39] | Retrospective | 348 | DORIS, ≥ 6 months | 54 ± 18 months | HR 0.14 (0.08-0.27) | < 0.001 |
Remarkably, not only the time intervals but also the proportion of follow-up spent in remission is seemingly tied to positive clinical outcomes: attaining DORIS remission for < 25% of the observed follow-up was associated with approximately a 50% reduction in organ damage by SLICC-DI (see Table 3)[29]. Moreover, being in DORIS for longer than 50% of the time was able to predict about a 40% reduction in damage accrual and a 60% reduction in severe flares with high specificity (85.2% and 95.1%, respectively)[39]. A comparison of the effects of various remission criteria and their duration on long-term outcomes is reported in Table 3. It is important to note that wide variation in follow-up duration limits comparability across the studies reported hereafter, as short observation periods may underestimate time-dependent outcomes such as relapse and organ damage, which often emerge in the long term.
Glucocorticoid-free remission
In SLE, the pursuit of glucocorticoid-free remission reflects a broader shift toward minimizing long-term treatment toxicity while preserving disease control. Although sustained remission off-treatment remains relatively uncommon, emerging evidence suggests that this goal may be more attainable than previously assumed in the pre-biologic era.
The feasibility of glucocorticoid-free remission varies widely across patient populations depending on baseline disease activity and treatment status. In an active SLE cohort described by Oiwa et al. (2024)[40], only 28.3% of patients were able to discontinue glucocorticoids over five years whereas withdrawal rates reached 84.6% among patients already in clinical or complete remission on low-dose prednisone
As expected, the duration of follow-up appears to be a critical factor as well. Long-term data from the belimumab trial extension[42] showed a 15% increase in glucocorticoid withdrawal over seven years, indicating that glucocorticoid tapering and discontinuation often require considerable time.
Recent real-world data underscore the survival advantage of achieving remission without glucocorticoids. Patients who maintained DORIS-defined remission for ≥ 50% of the observed time had a HR for mortality of 0.52 (0.29-0.93), while those in glucocorticoid-free DORIS remission for the same duration exhibited an even more profound reduction in mortality risk (HR 0.13; 95%CI 0.02-0.96)[43].
Moreover, long-term (≥ 5 years) remission on glucocorticoids has been associated with increased
Thus, the notion that remission on-treatment and off-treatment yield equivalent outcomes remains highly controversial, as glucocorticoid withdrawal may confer additional long-term benefits in both disease control and treatment toxicity. Nevertheless, the DORIS definition remains intentionally more lenient regarding permitted therapies compared to other remission definitions, out of concern that stricter criteria could encourage overly aggressive withdrawal of necessary treatments with potentially harmful consequences. Cautious tapering therefore remains essential.
LOW DISEASE ACTIVITY
When remission cannot be achieved, LDA may serve as a pragmatic treatment goal. Although LDA represents an intermediate state between remission and high disease activity, its exact boundaries are difficult to define. As a result, multiple definitions of LDA have been proposed over the years [Table 4].
Definitions and attainability of SLE LDA according to the studies published in the last 10 years
| Definition of LDA | Disease activity | Therapy allowed | Attainability |
| Franklyn et al., 2016 - (LLDAS)[6] | SLEDAI-2k ≤ 4, PGA ≤ 1.0 | AMA, PDN ≤ 7.5 mg/d, stable IS, bDMARDs | 88.5% of patients, at least once (including remission) |
| Ugarte-Gil et al., 2017[8] | SLEDAI ≤ 4 | AMA, PDN ≤ 7.5 mg/d, stable IS | 34.4% of patients, at least once (14.2% excluding remission) |
| Polachek et al., 2017 - (LDA-TC)[9] | cSLEDAI-2k < 3 | AMA | 43.7% of patients, at least one year long (12.9% excluding remission) |
| Tselios et al., 2019[10] | cSLEDAI-2k ≤ 2 | Unlimited | 28.1% of patients, at least 10 years long (18.0% excluding remission) |
| Alarcón et al., 2019[11] | SLAM ≤ 3 | AMA, PDN ≤ 7.5 mg/d | 16.9% of all visits, (15.1% excluding remission) |
| Tani et al., 2018 - (LLDAS5)[45] | SLEDAI-2k ≤ 4, PGA ≤ 1.0 | AMA, PDN ≤ 5 mg/d, stable IS, bDMARDs | 73.9% of patients through the follow-up (26.1% excluding remission) |
| Assunção et al., 2022[46] | SLEDAS ≤ 2.48 | AMA, PDN ≤ 7.5 mg/d, stable IS, bDMARDs | Not reported (~100% agreement with LLDAS) |
| Ugarte-Gil et al., 2022 - (mLLDAS)[13] | SLEDAI-2k ≤ 4 | AMA, PDN ≤ 7.5 mg/d, stable IS | 50.8% of all visits (5.6% excluding remission and LDA-TC) |
Among the various definitions of LDA, the Asia Pacific Lupus Collaboration (APLC) criteria-namely, the LLDAS-are the most widely applied in clinical studies of SLE[6]. Established in 2016, LLDAS allows a SLEDAI score of ≤ 4, provided it does not reflect major organ involvement, new features compared with the previous assessment, hemolytic anemia, or gastrointestinal activity. It also requires a PGA ≤ 1 and a prednisone (or equivalent) dose ≤ 7.5 mg/day. Conceptually, LLDAS was defined as a state which, if sustained, carries a low risk of adverse outcomes by balancing disease activity with medication safety. Although distinct from remission, this definition overlaps substantially with it. Indeed, Golder et al.[30,47] stated that up to 75% of visits classified as LLDAS were also in remission by the DORIS definition, while only 25% represented LLDAS without remission.
Understandably, such a consistent overlap has opened the avenue for speculation around the actual benefits conferred by attainment of LDA without remission, given the vast array of studies merging the two. Similarly, considering the often relapsing-remitting course of SLE, it has also been postulated that LDA could represent only a transient state of passage, a sort of dividing line, between disease activity and remission rather than an independent entity[21]. Such interpretations imply that most, if not any, improvement of patient outcomes tied to achievement of LDA may, thus, be spurious and only driven by its proximity to remission.
Nonetheless, a growing body of evidence indicates that LDA and remission may instead represent a spectrum of disease states, a clinical gradient associated with increasingly favorable patient prognoses[35]. Hence, continuous measures of disease activity such as SLEDAS which establish a clear cut-off between remission and LDA, may be more appropriate to capture the subtle nuances of disease activity without the ambiguity generated by potential overlaps[12,46].
Emerging evidence highlighted that LDA can be sustained for prolonged periods on end and yield tangible benefits independently of an overlap with remission. It was recently illustrated that even excluding remission, a short time spent in LLDAS (< 25% of the follow-up) was still associated with a substantial reduction in organ damage rates [adjusted rate ratio (aRR) 0.75 - 95%CI 0.61-0.91][48]. Longer durations in LDA (> 75% of follow-up) were proportional to organ damage reduction (aRR 0.35 - 95%CI 0.28-0.44) especially in the musculoskeletal, renal, and cardiovascular domain[48].
Additionally, it has been suggested that the time threshold necessary for LLDAS to yield any protective effect on damage accrual may be as low as 3 months (HR 0.60; 95%CI 0.51-0.71; P < 0.0001)[30]. A dose-effect of deepening protection was described with progressively longer durations of sustained LLDAS in a previous work as well[35]. This dose-dependent beneficial effect also seems to extend to the improvement of HRQoL and flare rates[38,39]. In fact, attainment of LLDAS for ≥ 60% of time or ≥ 36 months was found to be the time proportion threshold exhibiting the highest specificity (88.1%) for correlation with reduced severe flares [incident rate ratio (IRR) 0.71; 95%CI 0.57-0.88; P = 0.001], hospitalization (IRR 0.70; 95%CI 0.53-0.91; P = 0.007), and mortality rates (IRR 0.13; 95%CI 0.03-0.65; P = 0.013)[39]. An associated reduced mortality rate had already been described with the achievement of LLDAS ≥ 50% of the time (HR 0.31;
Nonetheless, it should be underscored that not only the duration but also the timing of its attainment is critically important. Indeed, the inability to attain LLDAS within 6 months from diagnosis was associated with early damage accumulation in a predominantly Caucasian cohort (see Table 5)[50]. A shorter disease duration from diagnosis was found to correlate with the probability and rapidity of achievement of LLDAS, i.e., ≤ 12 months (HR 1.37; 95%CI 1.16-1.61; P < 0.001)[51]. Further evidence suggests that early attainment of LLDAS (≤ 12 months from diagnosis) may be tied to an increase in the probability of attaining a prolonged LDA (LLDAS ≥ 50%) and demonstrates a potential advantage in glucocorticoid intake as well[52]. Unfortunately, only 7.6% and 51.9% of patients achieved LLDAS at 6 and 12 months in this same cohort, respectively.
Impact of various LDA definitions on damage accrual and relapse rate
| Outcome | Study | Design | N. patients | Definition and duration of LDA | Mean FU | RE pooled estimates | P |
| Damage accrual ^ | Ugarte-Gil et al., 2017[8] | Prospective | 1,480 | SLEDAI ≤ 4, off-glucocorticoids* | 2.4 (0.7-5.6) years | HR 0.62 (0.43-0.87) | 0.007 |
| Piga et al., 2017[50] | Retrospective | 107 | LLDAS, ≤ 6 months from diagnosis | 18.0 months | OR 0.20 (0.06-0.67) | 0.009 | |
| Tsang-A-Sjoe et al. 2017[31] | Retrospective | 183 | LLDAS ≥ 50% of FU | 5.0 (3.1-5.3) years | OR 0.52 (0.28-0.99) | 0.046 | |
| Petri et al., 2018[29] | Prospective | 1,356 | LLDAS, 25%-49% of FU | 46 (1-292) person-months | RR 0.63 (0.48-0.84) | 0.0012 | |
| Golder et al., 2019[30] | Prospective | 1,707 | LLDAS ≥ 50% of FU | 2.19 (1.51-2.99) years | HR 0.54 (0.42-0.70) | < 0.0001 | |
| Alarcón et al., 2019[11] | Prospective | 558 | SLAM ≤ 3, PDN ≤ 7.5 mg/d | 6 (4-6) visits | RR 0.18 (0.12-0.26) | < 0.0001 | |
| Golder et al., 2024[35] | Prospective | 3,449 | LLDAS > 3 months | 2.8 (1.1-5.6) years | HR 0.60 (0.51-0.71) | < 0.0001 | |
| Pitsigavdaki et al., 2024[39] | Retrospective | 348 | LLDAS ≥ 6 months | 54 ± 18 months | HR 0.61 (0.43-0.86) | < 0.01 | |
| Relapse | Golder et al., 2019[30] | Prospective | 1,707 | LLDAS ≥ 50% of FU | 2.19 (1.51-2.99) years | HR 0.41 (0.35-0.48) | < 0.0001 |
| Golder et al., 2024[35] | Prospective | 1,356 | LLDAS, 25%-49% of FU | 2.8 (1.1-5.6) years | HR 0.56 (0.51-0.63) | < 0.0001 | |
| Pitsigavdaki et al., 2024[39] | Retrospective | 1,707 | LLDAS ≥ 50% of FU | 54 ± 18 months | HR 0.19 (0.13-0.27) | < 0.001 |
Table 5 summarizes the main clinical outcomes associated with different definitions of LDA of varying durations. Herein, meaningful differences in follow-up duration across studies hinder comparability, particularly in assessing the temporal evolution of organ damage and relapses.
Interestingly, ethnic differences also appear to influence the time required to achieve LDA[53]: Multiethnic cohorts have shown that African American patients may take nearly twice as long as Caucasian patients to reach comparable rates of LLDAS (Caucasians: 52% at one year, 76% at two years; African Americans: 36% at one year, 58% at two years)[54]. This finding is consistent with previous reports of higher disease activity and greater organ damage among African American patients, even after adjustment for socioeconomic factors[29].
Potential predictors of LDA were identified as the lack of articular, mucocutaneous, renal and hematological involvement[39,44,55], use of immunosuppressive drugs, lower disease activity early in the disease course[56] and older age[57].
Intriguingly, meaningful differences exist within the LDA spectrum itself as the absence of serological activity may identify a subset of LDA patients with better patient outcomes than their serologically active counterparts, in line with a recent study reporting that LLDAS with neither clinical nor serological activity bears the strongest protective association with severe flares (clinically and serologically quiescence: HR 0.21; 95%CI 0.14-0.33; P < 0.001 vs. serological activity: HR 0.33; 95%CI 0.26-0.42; P < 0.001)[58].
However, it is important to emphasize that most of the evidence supporting the association between LDA and favorable outcomes comes from observational cohort studies[10,13,29,35,38,39,43,48-52]. While these studies provide valuable insights, they are inherently limited by potential confounding and the inability to establish causality. In contrast, fewer but more methodologically rigorous data are available from RCTs[59], notably BLISS-52 and BLISS-76, in which SLEDAS LDA successfully discriminated between treatment and placebo arms (OR 1.53; 95%CI 1.07-2.20; P = 0.019)[60].
TREAT TO TARGET STRATEGY IN SLE
The T2T strategy involves adjusting treatments at predetermined intervals to achieve specific, clinically relevant goals. This approach has been successfully applied to chronic conditions such as diabetes, hypertension, and hyperlipidemia, using evidence-based targets such as glycated hemoglobin, blood pressure, and cholesterol levels. Large RCTs have shown that T2T yields superior outcomes compared to standard care, even without new therapeutic agents[61].
In rheumatology, T2T is more complex due to the need to normalize multiple parameters simultaneously, often represented in a composite score. This complexity can lead to misclassification of treatment responses, especially in chronic conditions with irreversible damage. The T2T concept was first explored in rheumatoid arthritis through the Tight Control in Rheumatoid Arthritis (RA) (TICORA) trial, which revealed that T2T resulted in better treatment responses, higher remission rates, and less radiographic damage compared to standard of care[62]. Subsequent studies confirmed T2T efficacy in RA, exhibiting improvements in physical function and HRQoL. T2T is now firmly embedded in The European Alliance of Associations for Rheumatology (EULAR) treatment recommendations for RA. The benefits of T2T have also been demonstrated in other rheumatological diseases, such as psoriatic arthritis and gout[63-64].
Although an international task force formulated recommendations for implementing T2T in SLE (DORIS) in 2014, more than a decade after its initial theoretical proposal, an RCT formally comparing this approach with standard of care in SLE remains unpublished. This delay may suggest that while a rigid framework is suitable for RCTs, it may be inadequate for a complex and heterogeneous disease such as SLE. More flexible and personalized strategies, rather than a “one size fits all” approach, may be required to achieve remission or LDA in routine clinical practice[65,66].
The T2T approach should be regarded as a stepwise process: the initial target is the induction of remission or, alternatively, LDA; the second target is the maintenance of these states; and ultimately, the tapering or withdrawal of glucocorticoids and immunosuppressive therapies[3]. Unfortunately, relapses are common in SLE, particularly after discontinuation of glucocorticoids or immunosuppressants, making it extremely challenging to balance damage related to disease activity with that related to treatment. Notably, most studies investigating relapse risk during treatment tapering or withdrawal have not included patients receiving biologic disease-modifying antirheumatic drugs (bDMARDs)[67,68].
In line with the paradigm shift promoted by the latest EULAR treatment recommendations, biologics may aid in decreasing disease activity and facilitate the delicate transition to treatment withdrawal and
FUTURE DIRECTIONS
Although DORIS and LLDAS have been extensively validated in numerous real-world studies, the search for improved definitions of remission and LDA should continue. Reliance on the SLEDAI-2k score and the PGA carries intrinsic limitations in assessing disease activity, highlighting the need for more sensitive, continuous, and inter-rater reliability indices. Additionally, because certain types of disease activity are more strongly associated with damage accrual, new target state definitions should consider using appropriately weighted scoring for these manifestations.
Furthermore, the lack of alignment among these definitions, which often leads to overlaps between DORIS remission and LLDAS, represents a critical issue to be addressed in future research. Developing an enhanced and unified definition of remission and LDA is essential for their use as secondary endpoints in RCTs. At the same time, multiple definitions tailored to account for heterogeneity in ethnicity, demographics, and access to care may be required to better suit different patient cohorts in routine clinical practice. Similarly, the influence of ancestry, socioeconomic status, healthcare system, and access to care on achieving LLDAS (or remission) remains underexplored, despite these disparities being recognized as significant contributors to poor outcomes in SLE[70,71]. The impact of early diagnosis and timely therapy initiation is another relevant aspect that warrants further investigation.
Moreover, because most current data come from observational real-world studies, causality between attainment of these target states and improved patient outcomes cannot yet be inferred. Therefore,
Despite a strong theoretical basis and expert advocacy for T2T in SLE, the appropriate medication adjustments when targets are not met remain unclear.
Additionally, implementing the T2T approach necessitates more frequent patient evaluations, yet there is no consensus on the optimal interval between clinical assessments. This could potentially pose logistical challenges in real-world practice though novel digital technologies may assist in this regard. Moreover, this approach could prompt frequent therapy changes, affecting adherence and healthcare costs. Setting less stringent targets such as LDA at early stages may be beneficial to avoid abrupt and overly zealous therapeutic changes, considering the limited therapeutic armamentarium available.
As relapses are common in SLE, T2T targets should include a minimum duration in DORIS remission or LLDAS to ensure positive outcomes. Further research is needed to establish a consensus on the minimum protective duration. However, time proportion thresholds appear to be valuable surrogate measures for assessing positive outcomes without the need for longer waiting periods, making them particularly useful in the context of RCTs.
Moreover, further studies are needed to elucidate the role of serology in predicting flares and to determine whether it should be included in the definitions of remission or LDA.
Novel biologic therapies for SLE patients can help achieve the ambitious goals of DORIS remission and LLDAS, potentially reducing disease progression, improving HRQoL, lowering mortality, and reducing healthcare costs.
CONCLUSIONS
Remission and LDA represent contiguous yet clinically distinct target disease states, for which multiple definitions have been proposed over the years, with DORIS remission and LLDAS being the most widely accepted. Achieving these states is associated with significant reductions in adverse patient outcomes-such as damage accrual, disease flares, and mortality-while also improving HRQoL. However, both DORIS remission and LLDAS have intrinsic limitations, highlighting the need for better definitions. A unified definition, free from equivocal overlap between the two, could serve as a more reliable and discriminative endpoint in clinical trials. Personalized definitions tailored to individual patients may be more appropriate for routine clinical practice. Further research, alongside the development of new biological therapies, could enable the successful and widespread implementation of the T2T strategy in everyday care.
DECLARATIONS
Acknowledgement
The graphical abstract was created using BioRender and Microsoft Office, both accessed under institutional licenses provided by the University of Padova. (Created in BioRender. Bracalenti M (2025) https://BioRender.com/hqpnfie).
Authors’ contributions
Made substantial contributions to the conception and revision of the review: Doria A, Iaccarino L
Made substantial contributions to the drafting of the review: Bracalenti M
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Zen M, Salmaso L, Amidei CB, et al. Mortality and causes of death in systemic lupus erythematosus over the last decade: data from a large population-based study. Eur J Intern Med. 2023;112:45-51.
2. Eastman RC, Keen H. The impact of cardiovascular disease on people with diabetes: the potential for prevention. Lancet. 1997;350 Suppl 1:SI29-32.
3. Gatto M, Zen M, Iaccarino L, Doria A. New therapeutic strategies in systemic lupus erythematosus management. Nat Rev Rheumatol. 2019;15:30-48.
4. Doria A, Gatto M, Zen M, Iaccarino L, Punzi L. Optimizing outcome in SLE: treating-to-target and definition of treatment goals. Autoimmun Rev. 2014;13:770-7.
5. van Vollenhoven RF, Bertsias G, Doria A, et al. 2021 DORIS definition of remission in SLE: final recommendations from an international task force. Lupus Sci Med. 2021;8:e000538.
6. Franklyn K, Lau CS, Navarra SV, et al; Asia-Pacific Lupus Collaboration. Definition and initial validation of a lupus low disease activity state (LLDAS). Ann Rheum Dis. 2016;75:1615-21.
7. Zen M, Iaccarino L, Gatto M, et al. Prolonged remission in caucasian patients with SLE: prevalence and outcomes. Ann Rheum Dis. 2015;74:2117-22.
8. Ugarte-Gil MF, Wojdyla D, Pons-Estel GJ, et al; GLADEL. Remission and low disease activity status (LDAS) protect lupus patients from damage occurrence: data from a multiethnic, multinational Latin American Lupus Cohort (GLADEL). Ann Rheum Dis. 2017;76:2071-4.
9. Polachek A, Gladman DD, Su J, Urowitz MB. Defining low disease activity in systemic lupus erythematosus. Arthritis Care Res. 2017;69:997-1003.
10. Tselios K, Gladman DD, Touma Z, Su J, Anderson N, Urowitz MB. Clinical remission and low disease activity outcomes over 10 years in systemic lupus erythematosus. Arthritis Care Res. 2019;71:822-8.
11. Alarcón GS, Ugarte-Gil MF, Pons-Estel G, Vilá LM, Reveille JD, McGwin G Jr. Remission and low disease activity state (LDAS) are protective of intermediate and long-term outcomes in SLE patients. Results from LUMINA (LXXVIII), a multiethnic, multicenter US cohort. Lupus. 2019;28:423-6.
12. Jesus D, Larosa M, Henriques C, et al. Systemic lupus erythematosus disease activity score (SLE-DAS) enables accurate and user-friendly definitions of clinical remission and categories of disease activity. Ann Rheum Dis. 2021;80:1568-74.
13. Ugarte-Gil MF, Hanly J, Urowitz M, et al. Remission and low disease activity (LDA) prevent damage accrual in patients with systemic lupus erythematosus: results from the systemic lupus international collaborating clinics (SLICC) inception cohort. Ann Rheum Dis. 2022;81:1541-8.
14. van Vollenhoven RF, Mosca M, Bertsias G, et al. Treat-to-target in systemic lupus erythematosus: recommendations from an international task force. Ann Rheum Dis. 2014;73:958-67.
15. Gatto M, Saccon F, Zen M, et al. Early Disease and low baseline damage as predictors of response to belimumab in patients with systemic lupus erythematosus in a real-life setting. Arthritis Rheumatol. 2020;72:1314-24.
16. Apostolopoulos D, Kandane-Rathnayake R, Louthrenoo W, et al; Asia-Pacific Lupus Collaboration. Factors associated with damage accrual in patients with systemic lupus erythematosus with no clinical or serological disease activity: a multicentre cohort study. Lancet Rheumatol. 2020;2:e24-30.
17. Zen M, Gatto M, Doria A. Defining the targets in SLE management: insights and unmet gaps. Ann Rheum Dis. 2022;81:1483-5.
18. Cruciani C, Zen M, Gatto M, Morand E, Doria A. Assessment of disease activity and damage in SLE: are we there yet? Best Pract Res Clin Rheumatol. 2023;37:101896.
19. Jesus D, Matos A, Henriques C, et al. Derivation and validation of the SLE disease activity score (SLE-DAS): a new SLE continuous measure with high sensitivity for changes in disease activity. Ann Rheum Dis. 2019;78:365-71.
20. Jesus D, Rodrigues M, Matos A, Henriques C, Pereira da Silva JA, Inês LS. Performance of SLEDAI-2K to detect a clinically meaningful change in SLE disease activity: a 36-month prospective cohort study of 334 patients. Lupus. 2019;28:607-12.
21. Samões B, Zen M, Abelha-Aleixo J, Gatto M, Doria A. Caveats and pitfalls in defining low disease activity in systemic lupus erythematosus. Autoimmun Rev. 2022;21:103165.
22. Chessa E, Piga M, Floris A, Devilliers H, Cauli A, Arnaud L. Use of physician global assessment in systemic lupus erythematosus: a systematic review of its psychometric properties. Rheumatology. 2020;59:3622-32.
23. Piga M, Chessa E, Morand EF, et al; PISCOS Investigator Group. Physician global assessment international standardisation consensus in systemic lupus erythematosus: the PISCOS study. Lancet Rheumatol. 2022;4:e441-9.
24. Ugarte-Gil MF, Mendoza-Pinto C, Reátegui-Sokolova C, et al. Achieving remission or low disease activity is associated with better outcomes in patients with systemic lupus erythematosus: a systematic literature review. Lupus Sci Med. 2021;8:e000542.
25. Gomez A, Lindblom J, Parodis I, Bertsias G. Treat-to-target in SLE: is serology important? Results from an integrated analysis of five randomized clinical trials of belimumab. Rheumatology. 2025;64:3598-605.
26. Kostopoulou M, Ugarte-Gil MF, Pons-Estel B, van Vollenhoven RF, Bertsias G. The association between lupus serology and disease outcomes: a systematic literature review to inform the treat-to-target approach in systemic lupus erythematosus. Lupus. 2022;31:307-18.
27. Morand EF, Abreu G, Furie RA, Golder V, Tummala R. Lupus low disease activity state attainment in the phase 3 TULIP trials of anifrolumab in active systemic lupus erythematosus. Ann Rheum Dis. 2023;82:639-45.
28. Morand EF, van Vollenhoven R, Furie RA, et al. LLDAS and remission attainment with anifrolumab treatment in patients with systemic lupus erythematosus: results from the TULIP and long-term extension randomised controlled trials. Ann Rheum Dis. 2025;84:777-88.
29. Petri M, Magder LS. Comparison of remission and lupus low disease activity state in damage prevention in a United States systemic lupus erythematosus cohort. Arthritis Rheumatol. 2018;70:1790-5.
30. Golder V, Kandane-Rathnayake R, Huq M, et al; Asia Pacific Lupus Collaboration. Evaluation of remission definitions for systemic lupus erythematosus: a prospective cohort study. Lancet Rheumatol. 2019;1:e103-10.
31. Tsang-A-Sjoe MW, Bultink IE, Heslinga M, Voskuyl AE. Both prolonged remission and lupus low disease activity state are associated with reduced damage accrual in systemic lupus erythematosus. Rheumatology. 2017;56:121-8.
32. Gatto M, Frontini G, Calatroni M, et al. Effect of sustained clinical remission on the risk of lupus flares and impaired kidney function in patients with lupus nephritis. Kidney Int Rep. 2024;9:1047-56.
33. Zen M, Iaccarino L, Gatto M, et al. The effect of different durations of remission on damage accrual: results from a prospective monocentric cohort of Caucasian patients. Ann Rheum Dis. 2017;76:562-5.
34. Saccon F, Zen M, Gatto M, et al. Remission in systemic lupus erythematosus: testing different definitions in a large multicentre cohort. Ann Rheum Dis. 2020;79:943-50.
35. Golder V, Kandane-Rathnayake R, Li N, et al; Asia Pacific Lupus Collaboration. Association of sustained lupus low disease activity state with improved outcomes in systemic lupus erythematosus: a multinational prospective cohort study. Lancet Rheumatol. 2024;6:e528-36.
36. Ugarte-Gil MF, Pons-Estel GJ, Vila LM, McGwin G, Alarcón GS. Time in remission and low disease activity state (LDAS) are associated with a better quality of life in patients with systemic lupus erythematosus: results from LUMINA (LXXIX), a multiethnic, multicentre US cohort. RMD Open. 2019;5:e000955.
37. Tsang-A-Sjoe MWP, Bultink IEM, Heslinga M, van Tuyl LH, van Vollenhoven RF, Voskuyl AE. The relationship between remission and health-related quality of life in a cohort of SLE patients. Rheumatology. 2019;58:628-35.
38. Emamikia S, Oon S, Gomez A, et al. Impact of remission and low disease activity on health-related quality of life in patients with systemic lupus erythematosus. Rheumatology. 2022;61:4752-62.
39. Pitsigavdaki S, Nikoloudaki M, Garantziotis P, et al. Pragmatic targets for moderate/severe SLE and their implications for clinical care and trial design: sustained DORIS or LLDAS for at least 6 months is sufficient while their attainment for at least 24 months ensures high specificity for damage-free progression. Ann Rheum Dis. 2024;83:464-74.
40. Oiwa H, Suga T, Hosokawa Y, Araki K. Glucocorticoid-free remission in patients with SLE in the era of biologics: Immune complex disease is likely to benefit from current medications. Lupus. 2024;33:502-10.
41. Tani C, Elefante E, Signorini V, et al. Glucocorticoid withdrawal in systemic lupus erythematosus: are remission and low disease activity reliable starting points for stopping treatment? A real-life experience. RMD Open. 2019;5:e000916.
42. Ginzler EM, Wallace DJ, Merrill JT, et al; LBSL02/99 Study Group. Disease control and safety of belimumab plus standard therapy over 7 years in patients with systemic lupus erythematosus. J Rheumatol. 2014;41:300-9.
43. Kandane-Rathnayake R, Golder V, Louthrenoo W, et al; Asia-Pacific Lupus Collaboration. Lupus low disease activity state and remission and risk of mortality in patients with systemic lupus erythematosus: a prospective, multinational, longitudinal cohort study. Lancet Rheumatol. 2022;4:e822-30.
44. Fasano S, Coscia MA, Pierro L, Ciccia F. Which patients with systemic lupus erythematosus in remission can withdraw low dose steroids? Results from a single inception cohort study. Lupus. 2021;30:991-7.
45. Tani C, Vagelli R, Stagnaro C, Carli L, Mosca M. Remission and low disease activity in systemic lupus erythematosus: an achievable goal even with fewer steroids? Real-life data from a monocentric cohort. Lupus Sci Med. 2018;5:e000234.
46. Assunção H, Jesus D, Larosa M, et al. Definition of low disease activity state based on the SLE-DAS: derivation and validation in a multicentre real-life cohort. Rheumatology. 2022;61:3309-16.
47. Golder V, Tsang-A-Sjoe MWP. Treatment targets in SLE: remission and low disease activity state. Rheumatology. 2020;59:v19-28.
48. Hunnicutt J, Georgiou ME, Richards A, et al. Patients achieving low lupus disease activity state, systemic lupus erythematosus disease control or remission showed lower rates of organ damage during longitudinal follow-up: analysis of the Hopkins Lupus Cohort. Lupus Sci Med. 2024;11:e001206.
49. Sharma C, Raymond W, Eilertsen G, Nossent J. Association of achieving lupus low disease activity state fifty percent of the time with both reduced damage accrual and mortality in patients with systemic lupus erythematosus. Arthritis Care Res. 2020;72:447-51.
50. Piga M, Floris A, Cappellazzo G, et al. Failure to achieve lupus low disease activity state (LLDAS) six months after diagnosis is associated with early damage accrual in Caucasian patients with systemic lupus erythematosus. Arthritis Res Ther. 2017;19:247.
51. Golder V, Kandane-Rathnayake R, Louthrenoo W, et al; Asia Pacific Lupus Collaboration. Comparison of attainment and protective effects of lupus low disease activity state in patients with newly diagnosed versus established systemic lupus erythematosus. J Rheumatol. 2024;51:790-7.
52. Kikuchi J, Hanaoka H, Saito S, et al. Lupus low disease activity state within 12 months is associated with favourable outcomes in severely active systemic lupus erythematosus. Rheumatology. 2022;61:3777-91.
53. Wilhelm TR, Magder LS, Petri M. Remission in systemic lupus erythematosus: durable remission is rare. Ann Rheum Dis. 2017;76:547-53.
54. Babaoğlu H, Li J, Goldman D, Magder LS, Petri M. Time to lupus low disease activity state in the Hopkins Lupus Cohort: role of African American ethnicity. Arthritis Care Res. 2020;72:225-32.
55. Zen M, Iaccarino L, Gatto M, et al. Lupus low disease activity state is associated with a decrease in damage progression in Caucasian patients with SLE, but overlaps with remission. Ann Rheum Dis. 2018;77:104-10.
56. Pawlak-Buś K, Schmidt W, Leszczyński P. Remission and low disease activity in Polish patients with systemic lupus erythematosus - real-life, five-year follow-up outcomes. Eur Rev Med Pharmacol Sci. 2023;27:949-59.
57. Ugarte-Gil MF, Wojdyla D, Pons-Estel GJ, et al. Predictors of remission and low disease activity state in systemic lupus erythematosus: data from a Multiethnic, Multinational Latin American Cohort. J Rheumatol. 2019;46:1299-308.
58. Hao Y, Hansen D, Louthrenoo W, et al. Characterisation and outcomes of different subsets of low disease activity states in patients with systemic lupus erythematosus. Lupus Sci Med. 2024;11:e001217.
59. Parodis I, Lindblom J, Levy RA, et al. Attainment of remission and low disease activity after treatment with belimumab in patients with systemic lupus erythematosus: a post-hoc analysis of pooled data from five randomised clinical trials. Lancet Rheumatol. 2024;6:e751-61.
60. Jesus D, Henriques C, Matos A, Doria A, Inês LS. Systemic lupus erythematosus disease activity score remission and low disease activity states discriminate drug from placebo and better health-related quality of life. Arthritis Care Res. 2024;76:788-95.
61. Patel A, MacMahon S, Chalmers J, et al; ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560-72.
62. Grigor C, Capell H, Stirling A, et al. Effect of a treatment strategy of tight control for rheumatoid arthritis (the TICORA study): a single-blind randomised controlled trial. Lancet. 2004;364:263-9.
63. Dures E, Shepperd S, Mukherjee S, et al. Treat-to-target in PsA: methods and necessity. RMD Open. 2020;6:e001083.
64. Kiltz U, Smolen J, Bardin T, et al. Treat-to-target (T2T) recommendations for gout. Ann Rheum Dis. 2017;76:632-8.
65. Doria A, Gatto M, Iaccarino L, Punzi L. Value and goals of treat-to-target in systemic lupus erythematosus: knowledge and foresight. Lupus. 2015;24:507-15.
66. Gatto M, Zen M, Cruciani C, Iaccarino L, Doria A. Navigating the landscape of SLE treatment: An expert viewpoint on the rationality and limitations of early biologic intervention. Autoimmun Rev. 2024;23:103612.
67. Zen M, Saccon F, Gatto M, et al. Prevalence and predictors of flare after immunosuppressant discontinuation in patients with systemic lupus erythematosus in remission. Rheumatology. 2020;59:1591-8.
68. Mathian A, Pha M, Haroche J, et al. Withdrawal of low-dose prednisone in SLE patients with a clinically quiescent disease for more than 1 year: a randomised clinical trial. Ann Rheum Dis. 2020;79:339-46.
69. Zen M, Fuzzi E, Loredo Martinez M, et al. Immunosuppressive therapy withdrawal after remission achievement in patients with lupus nephritis. Rheumatology. 2022;61:688-95.
70. Arora S, Yazdany J. Use of quality measures to identify disparities in health care for systemic lupus erythematosus. Rheum Dis Clin North Am. 2020;46:623-38.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Special Topic
Copyright
Data & Comments
Data

















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].