A contemporary treatment regimen normalizes muscle mass and adult height in children with Prader-Willi syndrome - a longitudinal analysis
Abstract
Aim: Prader-Willi syndrome (PWS) is a rare genetic disorder characterized by intellectual disability, behavioral problems, and hypothalamic dysfunction combined with specific dysmorphisms. PWS is associated with limited adult height and an unfavorable body composition. Premature pubarche is frequent. Data on adult height after long-term growth hormone therapy are limited, as are data on the development of body composition during growth hormone therapy and the role of premature pubarche. This study aimed to explore the effect of long-term growth hormone therapy on adult height and body composition at adult height in children with PWS.
Methods: This was a single-center, retrospective descriptive study involving 24 boys and 20 girls with genetically confirmed PWS. All participants began growth hormone therapy in early childhood until they reached adult height (AH). The children received internationally standardized growth hormone therapy. The primary outcome measures were AH, lean mass (LM), and fat mass (FM), as determined by dual-energy X-ray absorptiometry.
Results: Adult height was 172.4 cm (-0.87 SD) in males and 160 cm (-0.90 SD) in females. There was no difference between children with or without premature pubarche. At adult height, LM was normal, but FM was increased in both males and females.
Conclusion: Boys with PWS reached a normal height compared to their families; in girls, AH was just below the target height. AH did not differ between children with or without premature pubarche. LM was normal but should probably be higher, given the overweight, and FM was still increased after long-term growth hormone therapy.
Keywords
INTRODUCTION
Prader-Labhart-Willi syndrome (PWS) is a rare genetic disorder resulting from the lack of expression of the paternally derived chromosome 15q11-q13. PWS is characterized by intellectual disability, behavioral problems, and hypothalamic dysfunction combined with specific dysmorphisms[1,2]. Over the past three decades, the phenotype of children with PWS has changed significantly. Early growth hormone therapy, rigorous nutritional restriction, appropriate substitution of sex steroids, and enhanced physical activity have contributed to improvements in stature, body composition, and motor strength[3-5]. Among these, improvements in body composition and motor strength are the main reasons for initiating growth hormone therapy in children with PWS[4]. In contrast, untreated children with PWS exhibit increased fat mass and reduced lean mass compared to peers with non-syndromic obesity[6].
Thus, improving body composition is the main goal of growth hormone therapy in PWS. Reports on the development of body composition mostly do not account for changes in body size and are, therefore, not reliable. Of particular interest are changes in muscle mass, as peak lean body mass and peak bone mass are typically achieved during growth. Achieving normal levels of these parameters is important to help prevent osteoporosis and promote cardiovascular health[7,8]. Therefore, reliable data on the development of lean body mass, fat mass and bone mass during growth hormone treatment - up to adult height - are needed, along with an understanding of the factors that influence these outcomes. The presumed factors influencing adult height and body composition, e.g., premature adrenarche, puberty, obesity, and accelerated bone maturation, have not been studied systematically. Mean (near) adult height has been reported between -0.3 and -1.13 SD[5,9-11]. Mean BMI SDS was in the upper normal range and fat-free mass in the lower normal range[5,9,10]. Gaston[12] reported on the acceleration of growth and mid-childhood bone maturation in children with PWS and found an association of these phenomena with premature adrenarche but no effect of BMI. Very recently, Griffing reported on the effect of premature pubarche on adult height[11]. There was no difference in adult height between children with or without premature pubarche. They also found that obesity was not related to premature pubarche. Based on these existing data, it seems that with growth hormone therapy, adult height is in the normal range, fat-free mass in the lower normal range, and fat mass in the upper range. It seems that premature adrenarche does not have a negative effect on adult height.
Our investigated cohort contributes to the understanding of the effects of long-term growth hormone therapy on adult height and body composition with a longitudinal analysis of the data, as study data are limited. In particular, the influence of premature pubarche on adult height and body composition has only been seldom investigated. Due to the standardized procedure in our center, a uniform approach can be assumed, which certainly contributes to the quality of the analysis. This study, therefore, contributes to improving patient care management and optimizing treatment in PWS.
METHODS
Design
This study was approved by the local ethics committee (ethics committee of the Canton Zurich: BASEC 2024-02310). Parents or caregivers of patients provided written consent. For this retrospective study, we reviewed the medical records of patients with PWS who were treated at our institution. The diagnosis of PWS was genetically confirmed in all patients. The main inclusion criteria were treatment at our institution during childhood, growth hormone therapy in a dosage of 0.85 mg/m2/day for at least five years in the period from 1992 until 2023. Pubertal induction was standardized. We induced puberty at around 13 years in boys and around 11-14 years in girls or when, especially in boys, pubertal arrest became evident. In the period before 2010, in boys, we administered 500 to 1,500 IU human chorionic gonadotropin (hCG) twice weekly. Since 2010, we have administered testosterone enanthate with a starting dosage of 100 mg/4 weeks in this situation. After 15 years, the dosage is then increased to 250 mg/4 weeks. In girls, we used 17-beta-estradiol in increasing doses from a starting dosage of 0.5 or 1 mg to a final dosage of 2 mg or a combination of 2 mg 17-beta-estradiol and cyclical norethisterone acetate 1 mg. The patients had reached adult height if height velocity was < 1 cm/year and bone age > 16 years in girls and > 18 years in boys.
Clinical assessment
Further variables extracted from the medical records for the time interval of interest were: height, sitting height, foot length and weight measured using standard techniques[13], age, bone age[14], height velocity per year calculated from 6-monthly interval measurements, testicular volume expressed as the maximum of both sides using a Prader orchidometer, pubic hair according to Tanner stages, concentrations of dehydroepiandrosterone sulfate (DHEAS) from morning serum. Furthermore, data on gestational age, birth weight, birth length, and parental height were extracted from the medical records. Premature pubarche was defined as pubarche before the age of 8 years in girls and 9 years in boys.
Hormone measurements
DHEAS was determined using Alinity i DHEA-S Reagent Kit (Abbott Cat# 9P3720) with a detection limit of 0.13 µmol/L and intra- and inter-assay coefficients of variation of less than 8%.
dual-energy X-ray absorptiometry measurements
Our primary outcome is adult height and the slope of course of the lean mass (LM) and fat mass (FM) and LM and FM at adult height. We also calculated the lean mass index (LMI), where LMI is the ratio of LBM in kilograms (kg) to height in square meters (m2). The fat mass index (FMI) was calculated accordingly. LM, which closely approximates muscle mass, was derived from dual-energy X-ray absorptiometry (DXA) measurements. We exported all available DXA measurements, including LM, FM, and bone mass, within the age interval of interest from the electronic patient files. DXA measurements were, in principle, routinely made at our institution during the yearly standard consultations using Hologic QDR 2000 and later using Hologic Discovery Wi machines (Hologic, Bedford, MA). Array modes were used on both machines. As recommended, we determined regression equations for body composition parameters during the transition phase to the new machine using 51 test subjects. We found that the effect of transforming values from the old to the new device was negligible.
Statistical methods
We were interested in adult height and the average effect of time on FM and LM. To model adult height, we used generalized additive models (GAM) to account for potential non-linear effects. In the first model, we explained adult height (AH in cm) using variables such as target height, premature pubarche, age at puberty, GH duration, age at the start of growth hormone therapy, and sex, using restricted maximum likelihood (REML) estimation to fit the model. For the second model, random effects were included to account for participant-level variability, particularly in models predicting adult height from repeated visits between ages 8 and 18. The models were checked for diagnostic accuracy using residual plots and concurvity checks, and model comparisons were conducted using the Chi-squared difference test to evaluate fit across models. LM and FM from longitudinal data: we analyzed longitudinal data to explore the factors associated with LM and FM in boys and girls using GAM. GAM allows for modeling potential non-linear relationships between LM and the predictors (age, FM, and DHEAS) and between FM and the predictors (age, LM, and DHEAS). Separate models were built for boys and girls, using restricted maximum likelihood (REML) estimation to fit the models. Smooth terms were applied to age, FM, and LM, respectively, and DHEAS to capture non-linear effects. Diagnostics for each model were examined, including checking residuals and concurvity, and the models were visualized to assess the fit and relationship between predictors and the response variable. This approach enabled us to assess both linear and non-linear associations, while accounting for the longitudinal structure of the data. Longitudinal change of LM and FM was visualized for individual patients over time. Moreover, we expressed the LM and FM as Z-scores for age and corrected for height compared to healthy European boys and girls[15].
We also investigated the differences in DHEAS values between children under the age of 10 with normal and premature pubarche. We used linear mixed-effects models to account for the longitudinal structure of the data, as each participant had multiple observations over time. Age was included as a fixed linear effect, and participant ID was modeled as a random intercept to account for individual variability. The primary predictors in the model were age and pubarche status (normal vs. premature). The mixed model approach allowed us to assess the association between DHEAS values and these predictors while controlling for within-subject variability. Model assumptions were checked using diagnostic plots, and significance was determined using t-tests with Satterthwaite's approximation for degrees of freedom.
Descriptive statistics were used for patient characteristics, growth hormone therapy, body composition at adult height, and adult height data, and Confidence intervals were calculated at the 95% level. Differences were tested using the Wilcoxon rank sum test, Fisher's exact test, and Wilcoxon rank sum exact test. Analyses were conducted using R statistical language (version 4.0.3)[16] and the packages lme4 (version 1.1.27)[17] and lmerTest (version 3.1.3)[18].
RESULTS
In our center, 27 boys and 28 girls were eligible for the study. In 1 boy and 3 girls, the treatment duration in our institution was less than 5 years and they were excluded from the study. In 2 boys and 5 girls, adult height data were missing because they moved and were lost to follow-up. Thus, 44 children (24 boys and 20 girls) were included in the study. Patient characteristics are summarized in Table 1. Genetic findings: deletion was found in 28 children and uniparental disomy in 16 children. Eight out of 44 children were born small for gestational age. Growth hormone therapy was started at a mean age of 3.9 years and continued for a mean duration of 13.5 years. Premature pubarche was noted in 57 % of the children (52% in boys and 63% in girls). We compared the characteristics of the children with and without premature pubarche. Of the different variables, only the age of the start of growth hormone therapy was significantly lower in the girls who developed premature pubarche. In these girls, growth hormone therapy was started at a mean age of 2.2 years, compared to 5.75 years in girls without premature pubarche (P = 0.004). Results at adult height are shown in Tables 2 and 3 for boys and girls separately. Mean adult height was 172.4 cm (-0.87 SD) in boys and 160 cm (-0.90 SD) in girls. The difference between men and women is about 13 cm, like in the general population. The mean difference of adult height expressed as SDS to target height SDS was small - nonsignificant in boys and just significantly different from zero in girls. Adult height did not differ significantly between the children with or without premature pubarche.
Patient characteristics
| Sex | ||||
| Characteristic | Overall N = 441 | Boys N = 241 | Girls N = 201 | P-value2 |
| Birth weight (kg) | 2.56 (0.51) | 2.65 (0.51) | 2.46 (0.49) | 0.117 |
| Birth length (cm) | 47.19 (3.33) | 47.41 (3.73) | 46.93 (2.88) | 0.580 |
| Unknown | 1 | 1 | 0 | |
| Gestational age (weeks) | 39.15 (2.67) | 39.47 (2.77) | 38.76 (2.56) | 0.275 |
| Unknown | 2 | 1 | 1 | |
| SGA | 8/44 (18%) | 6/24 (25%) | 2/20 (10%) | 0.259 |
| Premature pubarche | 0.474 | |||
| Normal | 18/42 (43%) | 11/23 (48%) | 7/19 (37%) | |
| Premature | 24/42 (57%) | 12/23 (52%) | 12/19 (63%) | |
| Unknown | 2 | 1 | 1 | |
| Start of GHT (Age in years) Range | 3.92 (3.41) (0.35-12.69) | 3.87 (3.22) (0.35-10.53) | 3.98 (3.70) (0.37-12.69) | 0.825 |
| End of GHT (Age in years) | 17.45 (2.93) | 17.40 (2.01) | 17.51 (3.81) | 0.494 |
| Duration of GHT (years) | 13.54 (4.29) | 13.54 (3.92) | 13.54 (4.81) | 0.954 |
Adult height after long-term GH therapy in boys
| | Pubarche | | ||
| Characteristic | Overall N = 231 | normal N = 111 | premature N = 121 | P-value2 |
| Adult height (cm) | 172.40 (166.80, 177.50) | 172.50 (165.50, 177.00) | 172.20 (167.55, 177.75) | 0.651 |
| Age at adult height (yrs) | 18.70 (17.36, 19.44) | 18.21 (17.41, 19.72) | 18.83 (17.21, 19.41) | 0.880 |
| Adult height (SDS) | -0.87 (-1.53, -0.12) | -0.90 (-1.56, -0.14) | -0.84 (-1.52, 0.25) | 0.525 |
| Target height (cm) | 176.00 (173.50, 179.00) | 176.00 (173.55, 179.00) | 176.55 (171.75, 179.25) | 0.975 |
| Adult height minus Target height (SDS) | -0.12 (-0.88, 0.20) | -0.63 (-1.11, 0.20) | -0.08 (-0.46, 0.19) | 0.449 |
Adult height after long-term GH therapy in girls
| Pubarche | ||||
| Characteristic | Overall N = 191 | Normal N = 71 | Premature N = 121 | P-value2 |
| Adult height (cm) | 160.00 (157.60, 163.60) | 159.40 (157.60, 163.70) | 160.00 (157.50, 162.30) | 0.704 |
| Age at adult height (yrs) | 17.73 (16.08, 19.06) | 17.51 (16.08, 19.80) | 18.05 (16.12, 18.73) | 0.967 |
| Adult height (SDS) | -0.90 (-1.33, -0.54) | -1.04 (-1.24, -0.14) | -0.88 (-1.36, -0.72) | 0.711 |
| Target height (cm) | 165.00 (161.00, 167.00) | 162.50 (159.25, 169.00) | 165.00 (162.25, 166.50) | 0.799 |
| Adult height minus Target height (SDS) | -0.74 (-1.35, -0.02) | -0.40 (-1.35, 0.97) | -0.74 (-1.26, -0.30) | 0.482 |
Mean DHEAS levels were 1.3 μmol/L higher in boys with premature pubarche than in those without (not significantly different, P = 0.06). In girls, mean DHEAS levels were 1.4 μmol/L higher in those with premature pubarche compared to those without (not significantly different, P > 0.10).
The best model to predict adult height included target height, age, sex, and bone age, but excluded DHEAS, FM, or FMI. The model explains 87% of the variance. Compared to the boys without premature pubarche, the boys with premature pubarche were taller when they were between 5 and 13-14 years old. From the pubertal age, this changed, and at adult height, there was no difference anymore [Figure 1A and B]. In girls, no height difference was observed between those with and without premature pubarche [Figure 1C and D]. Body composition did not differ significantly between children with or without premature pubarche
Figure 1. Height standard deviation score (SDS) for chronological age in boys with (A) and without (B) premature pubarche and in girls with (C) and without (D) premature pubarche. Colored dotted lines: growth without growth hormone therapy; colored solid lines: growth with growth hormone therapy.
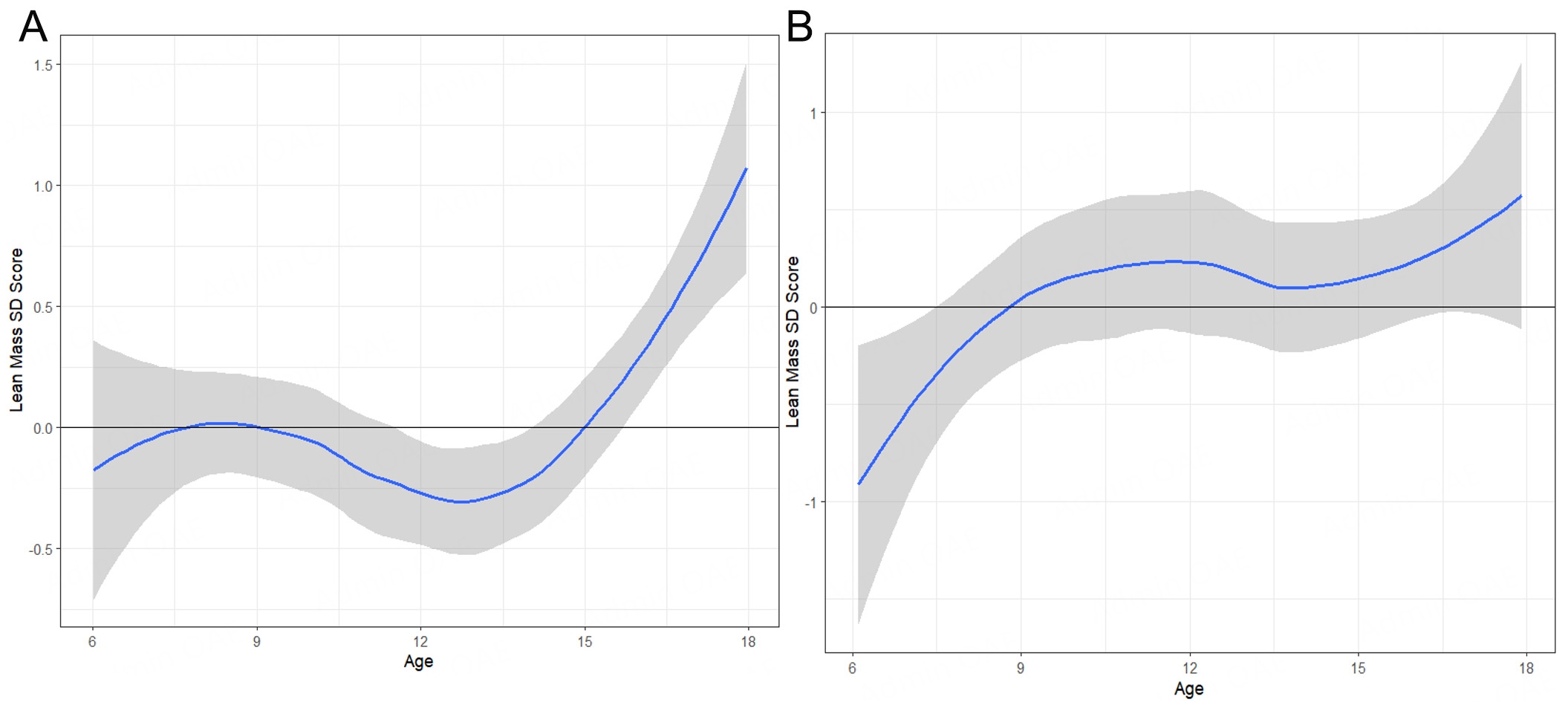
Figure 2. Lean mass standard deviation score (SDS) corrected for height longitudinal in Boys (A) and Girls (B). Smooth solid line: average trend SDS with standard error in grey (LOESS smoothing).
Figure 3. Fat mass standard deviation score (SDs) corrected for height longitudinal in boys (A) and girls (B). Smooth solid line: average trend SDS with standard error in grey (LOESS smoothing).
Body composition at adult height in boys (n = 12 at AH visit, n = 4 within 1 year after AH, n = 3 within 1 year before AH)
| Pubarche | ||||
| Characteristic | Overall N = 191 | Normal N = 81 | Premature N = 111 | P-value2 |
| LMI (kg/m2) | 17.01 (2.31), +0.06 | 16.84 (1.52) | 18.27 (3.25) | 0.206 |
| FMI (kg/m2) | 10.97 (4.63) +1.76 | 10.44 (4.87) | 11.00 (3.55) | 0.840 |
| LM (kg) | 49.47 (7.60), -0.42 | 49.43 (3.81), -0.42 | 53.27 (12.90), +0.19 | 0.272 |
| FM (kg) | 32.50 (13.02), +1.58 | 33.46 (14.03), +1.68 | 32.46 (13.40), +1.58 | 0.968 |
| BMC (gr) | 2,321.99 (255.03) | 2,381.53 (235.06) | 2,262.91 (324.69) | 0.717 |
| Age at adult height (years) | 18.95 (1.87) | 19.16 (1.66) | 18.70 (2.04) | 0.442 |
| Adult height (cm) | 172.00 (8.95) | 171.70 (9.30) | 172.00 (8.95) | 0.600 |
| Weight at adult height (kg) | 86.50 (16.50) | 86.30 (17.43) | 88.70 (29.50) | 0.442 |
Body composition at adult height in girls (n = 5 at AH visit, n = 6 within 1 year after AH, n = 3 within 1 year before AH)
| Pubarche | ||||
| Characteristic | Overall N = 141 | Normal N = 61 | Premature N = 81 | P-value2 |
| LMI (kg/m2) | 14.76 (1.84), +0.64 | 14.73 (3.60) | 15.00 (1.88) | 0.282 |
| FMI (kg/m2) | 11.89 (4.72), +1.67 | 11.89 (2.96) | 11.31 (5.79) | 0.852 |
| LM (kg) | 37.49 (3.42), -0.13 | 37.87 (10.46) -0.05 | 37.31 (2.39) -0.16 | > 0.999 |
| FM (kg) | 32.92 (12.79) +2.08 | 32.92 (7.59) +2.08 | 28.51 (16.64) +1.37 | 0.755 |
| BMC (gr) | 1,885.83 (323.43) | 1,995.77 (302.45) | 1,789.03 (335.78) | 0.950 |
| Age at adult height (year) | 17.36 (2.27) | 17.22 (1.53) | 17.78 (2.42) | 0.755 |
| Adult height (cm) | 159.70 (6.20) | 161.15 (5.45) | 159.00 (5.00) | 0.414 |
| Weight at adult height (kg) | 71.35 (21.20) | 73.70 (22.18) | 68.30 (18.40) | 0.950 |
DISCUSSION
The most important findings of our study are: (1) normal linear growth with normal adult height can be achieved with moderate doses of GH; (2) premature pubarche has no influence on adult height; (3) under our current treatment regimen with growth hormone therapy, substitution of sex steroids at a normal age and measures to influence eating behavior and exercise, LM (muscle mass) develops normally; and (4) the FM (fat mass) develops on higher levels up to a certain degree of maturity and then increases significantly.
Our analysis of adult height clearly shows normalization of height outcomes. Gamage et al. recently reported long-term adult height results in individuals with PWS, comparing those with and without growth hormone therapy[19]. They found that the adult or near-adult height SDS was -1.04 for boys and -1.58 for girls. In contrast, our cohort achieved heights closer to the normal range, with mean adult height SDS values of -0.87 for boys and -0.90 for girls. This corresponded to an average adult height of 172.4 cm for boys and 160.0 cm for girls in our study. A likely key factor for this difference is the shorter duration of treatment in the Gamage cohort, which ranged from 3.32 to 6.61 years, depending on the year group. In comparison, our participants began GH therapy at an average age of 3.58 years and received treatment for a significantly longer duration of 13.84 years. In our study, the age at adult height was about the same for boys and girls, although typically girls achieve adult height at a younger age. Additionally, the age range at adult height was wider in girls, which may reflect greater variability in the timing of puberty induction. This is consistent with the fact that hypogonadism is less frequent and more difficult to diagnose in girls with PWS than in boys[20]. As reported in literature and observed in our patients, hypogonadism manifested as hypogonadotropic, hypergonadotropic, or a combination of both forms. Furthermore, there is generally less urgency among families to start puberty induction in girls, partly because estrogens, produced through the aromatization of androgens, often lead to some degree of spontaneous breast development.
Angulo et al. were the first to show the results of long-term treatment (average treatment duration of
Adult height in our cohort could be predicted by factors that are well known to experts in the field: target height, age, and bone age. Growth hormone dosage and therapy duration were not included in the model, which is unsurprising given that both were relatively uniform across the cohort. However, it can be concluded that the growth hormone dosage was sufficient to reach normal height. Since the dosage used in PWS is approximately equivalent to the replacement dose for growth hormone deficiency, this suggests normal growth hormone sensitivity in individuals with PWS. Therefore, our data also support the approach with a rather low growth hormone dose, as this appears sufficient to normalize growth.
As premature pubarche is frequent in children with PWS, the relationship between premature androgen production/adrenarche and adult height warrants further investigation. In our cohort, more than half of the children had premature pubarche - a proportion higher than previously reported[21-24], but consistent with the findings of Griffing[11]. One possible explanation is the earlier start of growth hormone therapy over the years. Supporting this hypothesis, our data show that girls with premature pubarche began GH therapy earlier than those without. In PWS, elevated DHEAS and androgen levels could be detected from an early age, indicating premature adrenal cortex maturation[12,23]. However, unlike in children with obesity[12,22], no direct correlation was observed between increased fat mass and androgen levels in our cohort. Similarly, children with premature pubarche did not have higher fat mass compared to those without. This indicates that factors other than fat mass likely contribute to the early maturation of the adrenal cortex. We found no difference in adult height between children with and without premature pubarche, confirming Griffing’s findings[11]. Thus, premature pubarche does not appear to affect adult height. Nevertheless, boys with premature pubarche exhibited accelerated growth during childhood followed by reduced growth during puberty [Figure 1A and B]. These boys also showed accelerated bone maturation during childhood, presumably due to increased androgen levels (data not shown). Interestingly, this pattern was not observed in girls, and differences in androgen levels between girls with and without premature pubarche were less pronounced. The finding that premature pubarche does not impact adult height is reassuring. In the absence of central precocious puberty, there is no indication for therapeutical interventions aimed at slowing bone maturation. This approach is not standard practice and has not been systematically investigated.
Apart from promoting normal height, GH therapy in PWS is also aimed at improving body composition. Untreated individuals with PWS typically exhibit greater fat mass and lower muscle mass compared to non-syndromic individuals with obesity[6,25-27]. The positive influence of GH therapy - especially when combined with adjunctive treatment - on body composition has been well demonstrated[3-5,28,29]. However, most of these studies are limited by relatively short follow-up periods. Our Dutch colleagues[5,30], reporting on 8 years of GH treatment, demonstrated stabilization of percent fat SDS and BMI SDS in children with PWS. Nevertheless, full normalization was not achieved, and LM remained in the lower normal range.
In our cohort, LM [Figure 2] developed normally from age 6 until attainment of adult height, while FM
Nevertheless, challenges remain. FM remained within the upper normal range until around age 12, after which it increased to levels indicative of overweight. No clear sex differences were observed. A likely contributing factor is increased autonomy and reduced external control after this age, leading to higher caloric intake. Evidently, pharmacological intervention with growth hormone and the current treatment regimen are insufficient to normalize FM. Hypothalamic dysregulation seems to play a central role in the pathogenesis of obesity in PWS, and pharmacological interventions such as GLP-1 agonists have not yet been proven effective[36,37]. A multimodal, multidisciplinary approach remains essential in the treatment of PWS[38]. Recent studies have confirmed that care provided in specialized residential facilities, as opposed to living with parents or in non-specialized homes, yields better outcomes in weight development and prevention of morbid obesity[39,40].
The main limitations of our study include its single-center, retrospective design, and the relatively small cohort size. However, the standardized treatment approach enhances its relevance to current clinical practice in the Western world. A key strength is the longitudinal follow-up through to adult height in a consistently treated cohort.
In summary, our study demonstrated normalization of adult height following long-term growth hormone therapy in children with PWS. Premature pubarche did not negatively influence adult height. LM, and thus muscle mass, developed normally compared to the general population, although it may ideally need to be higher given the elevated FM. FM did not normalize. Future research should focus on optimizing the ratio of muscle mass to fat mass and investigating how this ratio affects long-term cardiovascular health.
DECLARATIONS
Acknowledgments
The authors would like to acknowledge Prof. Dr. Michael Hermanussen for the useful discussions on methodology.
Authors’ contributions
Made substantial contributions to the conception and design of the study and performed data analysis and interpretation: Noordam C, Eiholzer U
Performed data acquisition and analysis: Stephan A
Performed data interpretation and contributed to the writing and revision of the paper: Dubinski I
Wrote the first draft of the paper and finalized the paper: Noordam C
Contributed to the writing and revision of the paper: Eiholzer U
Availability of data and materials
The data supporting our findings are stored in accordance with the Declaration of Helsinki in Center for Paediatric Endocrinology Zurich (PEZZ) and will be available from the corresponding author upon reasonable request.
Financial support and sponsorship
This study was supported by the Foundation Growth Puberty Adolescence, Zurich, Switzerland.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
This study was approved by the Ethics Committee of the Canton Zurich (BASEC 2024-02310). Parents or caregivers of patients provided written consent.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Prader A, Labhart A, Willi H. Ein syndrom von adipositas, kleinwuchs, kryptorchismus und oligophrenie nach myatonieartigem zustand im neugeborenenalter. Schweiz Med Wochenschr. 1956;86:1260-1. Available from: https://www.scienceopen.com/document?vid=a8320db5-9d18-41e2-8d53-491fc0e120ae [Last accessed on 3 Jun 2025].
2. Tauber M, Hoybye C. Endocrine disorders in Prader-Willi syndrome: a model to understand and treat hypothalamic dysfunction. Lancet Diabetes Endocrinol. 2021;9:235-46.
3. Eiholzer U, Gisin R, Weinmann C, et al. Treatment with human growth hormone in patients with Prader-Labhart-Willi syndrome reduces body fat and increases muscle mass and physical performance. Eur J Pediatr. 1998;157:368-77.
4. Carrel AL, Myers SE, Whitman BY, Eickhoff J, Allen DB. Long-term growth hormone therapy changes the natural history of body composition and motor function in children with prader-willi syndrome. J Clin Endocrinol Metab. 2010;95:1131-6.
5. Bakker NE, Kuppens RJ, Siemensma EPC, et al. Eight years of growth hormone treatment in children with Prader-Willi syndrome: maintaining the positive effects. J Clin Endocrinol Metab. 2013;98:4013-22.
6. von Mil EG, Westerterp KR, Gerver WJ, Van Marken Lichtenbelt WD, Kester AD, Saris WH. Body composition in Prader-Willi syndrome compared with nonsyndromal obesity: relationship to physical activity and growth hormone function. J Pediatr. 2001;139:708-14.
7. Boot AM, de Ridder MA, van der Sluis IM, van Slobbe I, Krenning EP, Keizer-Schrama SM. Peak bone mineral density, lean body mass and fractures. Bone. 2010;46:336-41.
8. Gordon CM, Zemel BS, Wren TA, et al. The determinants of peak bone mass. J Pediatr. 2017;180:261-9.
9. Angulo MA, Castro-Magana M, Lamerson M, Arguello R, Accacha S, Khan A. Final adult height in children with Prader-Willi syndrome with and without human growth hormone treatment. Am J Med Genet A. 2007;143A:1456-61.
10. Lindgren AC, Lindberg A. Growth hormone treatment completely normalizes adult height and improves body composition in Prader-Willi syndrome: experience from KIGS (Pfizer International Growth Database). Horm Res. 2008;70:182-7.
11. Griffing E, Halpin K, Lee BR, Paprocki E. Premature pubarche in Prader-Willi syndrome: risk factors and consequences. Clin Endocrinol. 2024;101:162-9.
12. Gaston LS, Stafford DE. Premature adrenarche in Prader-Willi syndrome is associated with accelerated pre-pubertal growth and advanced bone age. J Pediatr Endocrinol Metab. 2023;36:185-94.
13. Prader A, Largo RH, Molinari L, Issler C. Physical growth of Swiss children from birth to 20 years of age. First Zurich longitudinal study of growth and development. Helv Paediatr Acta Suppl. 1989;52:1-125.
14. Greulich WW, Pyle SI. Radiographic atlas of skeletal development of the hand and wrist. University Press; 1959, p. 256.
15. Ofenheimer A, Breyer-Kohansal R, Hartl S, et al. Reference charts for body composition parameters by dual-energy X-ray absorptiometry in European children and adolescents aged 6 to 18 years-Results from the Austrian LEAD (Lung, hEart, sociAl, boDy) cohort. Pediatr Obes. 2021;16:e12695.
16. R Core Team. The R project for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. Available from: https://www.R-project.org/ [Last accessed on 3 Jun 2025].
17. Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Soft. 2015;67:1-48.
18. Kuznetsova A, Brockhoff PB, Christensen RHB. lmerTest package: tests in linear mixed effects models. J Stat Soft. 2017;82:1-26.
19. Gamage DS, Ambler G, Chan A, Srinivasan S, Maguire AM, Cho YH. Outcomes of growth hormone treatment in children with Prader-Willi syndrome over a 30-year period: a single tertiary center experience. J Pediatr Endocrinol Metab. 2024;37:680-5.
20. Noordam C, Höybye C, Eiholzer U. Prader-Willi Syndrome and hypogonadism: a review article. Int J Mol Sci. 2021;22:2705.
21. Schmidt H, Schwarz HP. Premature adrenarche, increased growth velocity and accelerated bone age in male patients with Prader-Labhart-Willi syndrome. Eur J Pediatr. 2001;160:69-70.
22. L'Allemand D, Eiholzer U, Rousson V, et al. Increased adrenal androgen levels in patients with Prader-Willi syndrome are associated with insulin, IGF-I, and leptin, but not with measures of obesity. Horm Res. 2002;58:215-22.
23. Siemensma EP, de Lind van Wijngaarden RF, Otten BJ, de Jong FH, Hokken-Koelega AC. Pubarche and serum dehydroepiandrosterone sulphate levels in children with Prader-Willi syndrome. Clin Endocrinol. 2011;75:83-9.
24. Lecka-Ambroziak A, Wysocka-Mincewicz M, Marszałek-Dziuba K, Rudzka-Kocjan A, Szalecki M. Premature adrenarche in children with Prader-Willi syndrome treated with recombinant human growth hormone seems to not influence the course of central puberty and the efficacy and safety of the therapy. Life. 2020;10:237.
25. Brambilla P, Bosio L, Manzoni P, Pietrobelli A, Beccaria L, Chiumello G. Peculiar body composition in patients with Prader-Labhart-Willi syndrome. Am J Clin Nutr. 1997;65:1369-74.
26. Goldstone AP, Brynes AE, Thomas EL, et al. Resting metabolic rate, plasma leptin concentrations, leptin receptor expression, and adipose tissue measured by whole-body magnetic resonance imaging in women with Prader-Willi syndrome. Am J Clin Nutr. 2002;75:468-75.
27. Theodoro MF, Talebizadeh Z, Butler MG. Body composition and fatness patterns in Prader-Willi syndrome: comparison with simple obesity. Obesity. 2006;14:1685-90.
28. Carrel AL, Myers SE, Whitman BY, Allen DB. Benefits of long-term GH therapy in Prader-Willi syndrome: a 4-year study. J Clin Endocrinol Metab. 2002;87:1581-5.
29. Myers SE, Carrel AL, Whitman BY, Allen DB. Sustained benefit after 2 years of growth hormone on body composition, fat utilization, physical strength and agility, and growth in Prader-Willi syndrome. J Pediatr. 2000;137:42-9.
30. Grootjen LN, Trueba-Timmermans DJ, Damen L, Mahabier EF, Kerkhof GF, Hokken-Koelega ACS. Long-term growth hormone treatment of children with PWS: the earlier the start, the better the outcomes? J Clin Med. 2022;11:2496.
31. Plotkin LI, Bruzzaniti A, Pianeta R. Sexual dimorphism in the musculoskeletal system: sex hormones and beyond. J Endocr Soc. 2024;8:bvae153.
32. Donini LM, Busetto L, Bischoff SC, et al. Definition and diagnostic criteria for sarcopenic obesity: ESPEN and EASO consensus statement. Obes Facts. 2022;15:321-35.
33. Carbone S, Canada JM, Billingsley HE, Siddiqui MS, Elagizi A, Lavie CJ. Obesity paradox in cardiovascular disease: where do we stand? Vasc Health Risk Manag. 2019;15:89-100.
34. Eiholzer U, Stephan A, Fritz C, Katschnig C, Noordam C; Clinical and Scientific Advisory Board of the International Prader-Willi Syndrome Organisation (IPWSO). Gonadal hormone substitution in people with Prader-Labhart-Willi Syndrome: an international Prader-Willi Syndrome organisation survey. Horm Res Paediatr. 2021;94:176-85.
35. Eiholzer U, Nordmann Y, L'Allemand D, Schlumpf M, Schmid S, Kromeyer-Hauschild K. Improving body composition and physical activity in Prader-Willi Syndrome. J Pediatr. 2003;142:73-8.
36. Diene G, Angulo M, Hale PM, et al. Liraglutide for weight management in children and adolescents with Prader-Willi syndrome and obesity. J Clin Endocrinol Metab. 2022;108:4-12.
37. Ng NBH, Low YW, Rajgor DD, et al. The effects of glucagon-like peptide (GLP)-1 receptor agonists on weight and glycaemic control in Prader-Willi syndrome: a systematic review. Clin Endocrinol. 2022;96:144-54.
38. Eiholzer U, Whitman BY. A comprehensive team approach to the management of patients with Prader-Willi syndrome. J Pediatr Endocrinol Metab. 2004;17:1153-75.
39. Hirsch HJ, Benarroch F, Genstil L, et al. Long-term weight control in adults with Prader-Willi syndrome living in residential hostels. Am J Med Genet A. 2021;185:1175-81.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Special Issue
Copyright
Data & Comments
Data


















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].