A review of the relationship between the SLC6A4 gene and mental disorders
Abstract
The SLC6A4 gene, which encodes the serotonin (5-hydroxytryptamine; 5-HT) transporter, plays an important role in the pathogenesis of mental disorders by regulating serotonin reuptake in the synaptic cleft. This review summarizes current evidence on the associations of SLC6A4 polymorphisms, epigenetic modifications, and neuroimaging findings with schizophrenia (SCZ), major depressive disorder (MDD), bipolar disorder (BD), and other psychiatric conditions. Studies have shown that the impact of SLC6A4 polymorphisms varies across ethnic groups and populations. Epigenetic studies indicate that DNA methylation in the promoter and exon regions of SLC6A4 can inhibit gene expression and exacerbate imbalances in the 5-HT signaling pathway, which are closely related to negative symptoms in SCZ, childhood trauma, and gender-specific risks for MDD. Neuroimaging evidence further suggests that SLC6A4 polymorphisms and methylation status are significantly associated with brain structural and functional abnormalities, pointing to a multidimensional mechanism involving ontogeny, epigenetics, and neural networks. Moreover, alterations in SLC6A4 have also been implicated in BD and attention-deficit/hyperactivity disorder. Despite significant progress, challenges remain, including ethnic biases in study populations, discrepancies between epigenetic patterns in peripheral and central nervous systems, and unclear mechanisms underlying gene-environment interactions. Future research should integrate multi-omics approaches, large cross-ethnic cohorts, and gender-stratified analyses to elucidate the precise regulatory network of SLC6A4 in mental disorders.
Keywords
INTRODUCTION
According to the latest World Mental Health Report by the World Health Organization (WHO), nearly 1 billion people worldwide suffer from mental disorders, representing more than 13% of the global population. These disorders are characterized by high incidence, substantial resource utilization, and significant disability rates[1,2]. They frequently co-occur, which increases the complexity of diagnosis and treatment[3-5]. Strong genetic correlations between psychiatric traits have also been reported, with multiple neurotransmitters, hormones, and neurotrophic factors directly implicated in the development of these conditions[6,7]. Research exploring the pathological mechanisms of mental disorders - spanning social, psychological, biochemical, genetic, and neuroimaging perspectives - has consistently concluded that they are multifactorial in origin, involving both genetic and environmental influences[2].
As research has progressed, several hypotheses have been proposed to explain the development of psychiatric disorders[8-10], among which the serotonin (5-hydroxytryptamine; 5-HT) hypothesis remains the most prominent. The 5-HT system, a core neuromodulatory network, regulates a wide range of behaviors including sleep, memory, emotion, and aggression. Dysregulation of this system has been linked to various psychiatric disorders[11], such as schizophrenia as well as alcohol and drug dependence[12-15].
Within the serotonergic system, the 5-hydroxytryptamine transporter (5-HTT) plays a critical role in controlling the intensity and duration of neural signaling by regulating the reuptake of 5-HT in the synaptic cleft. Reduced 5-HTT activity results in abnormally elevated 5-HT concentrations in the synaptic cleft and diminished neurotransmitter storage within neurons. This imbalance may lead to receptor desensitization or impair synaptic plasticity, ultimately contributing to the emergence of psychiatric symptoms[16].
The SLC6A4 gene, located on chromosome 17q11.2, encodes the 5-HTT protein[17]. In recent years, numerous studies have investigated the relationship between SLC6A4 and psychiatric disorders, focusing on its role in disease pathogenesis and clinical manifestations. Evidence suggests that SLC6A4 is associated with several major psychiatric disorders, including schizophrenia, depression, and antisocial personality disorder[18-20]. Accordingly, this review synthesizes current evidence on the diverse roles of SLC6A4 genetic variation and epigenetic regulation in the pathophysiology of major psychiatric disorders, highlighting insights from genetic, epigenetic, and neuroimaging studies.
METHODOLOGY
The literature search was conducted in PubMed (https://pubmed.ncbi.nlm.nih.gov/), PsycINFO (https://www.apa.org/pubs/databases/psycinfo/) and Web of Science (https://www.webofscience.com/). The search terms included "SLC6A4", "5-HTT", "serotonin transporter", "polymorphism", "methylation", "schizophrenia", "depression", and "bipolar disorder", among others. The search was limited to publications between January 1, 2000, and August 25, 2025.
A total of 278 records were identified in the initial search. After removing duplicates, 230 articles remained. Screening by title and abstract excluded 129 articles that did not meet the inclusion criteria. The full texts of the remaining 101 articles were reviewed for eligibility. Of these, 20 were excluded for the following reasons: non-human studies (n = 5), non-original research (e.g., reviews, case reports; n = 9), and duplicate publications (n = 6). Thus, 81 articles were included in the final review. An updated search in 2025 identified 15 additional records. After duplicate removal and title/abstract screening, 5 full-text articles were assessed, of which 3 met the inclusion criteria and were added to the review, bringing the total number of included studies to 84.
Inclusion criteria: (1) case-control or cohort studies; (2) patients diagnosed with confirmed mental disorders (e.g., schizophrenia, depression, bipolar disorder); and (3) studies analyzing the association between SLC6A4 gene polymorphisms or methylation status and mental disorders. Exclusion criteria: (1) non-human studies; (2) non-original studies such as reviews or case reports; (3) duplicate publications. Based on these criteria, articles that did not meet the inclusion criteria were excluded from the initial 278 papers. The final 81 studies included in the review were published between 2000 and 2025 (see Tables 1-5 for details).
Studies on SLC6A4 gene polymorphisms and SCZ
| Author | Year | Journal | Biological material | Sample size | SLC6A4 region | Main findings |
| Zainullina et al.[24] | 2003 | Molekulyarnaya Biologiya | Human peripheral blood cells | Nscz = 214, NNC = 197 | 5-HTTLPR | Association of SLC6A4 polymorphisms with SCZ differs significantly between Tatar and Russian populations |
| Ikeda et al.[31] | 2006 | Journal of Neural Transmission | Human peripheral blood cells | Nscz = 383, NNC = 351 | All exons, introns, promoter regions, 3' UTR | No significant association between SLC6A4 polymorphisms and SCZ in Japanese patients |
| Pae et al.[32] | 2006 | Journal of Neural Transmission | Human peripheral blood cells | Nscz = 152, NNC = 152 | 5-HTTLPR | No significant association between SLC6A4 polymorphisms and SCZ in Korean patients |
| Güzey et al.[103] | 2007 | European Journal of Clinical Pharmacology | Human peripheral blood cells | Nscz = 119, NNC = 56 | 5-HTTLPR | No significant association between SLC6A4 polymorphisms and antipsychotic-induced extrapyramidal symptoms |
| Vijayan et al.[27] | 2009 | Journal of Human Genetics | Human peripheral blood cells | Nscz = 243, NNC = 243 | 5'end region | Polymorphisms in the 5' end region of SLC6A4 are significantly associated with SCZ in South Indian populations |
| Lin et al.[28] | 2009 | Neuroscience Letters | Human peripheral blood cells | Nscz = 329, NNC = 288 | Intron 2 STin2 VNTR, three intron-tagged SNPs (rs2054847, rs140700, rs2020942) | Two haplotypes (A-G-10, A-G-12) containing STin2 VNTR are significantly associated with SCZ |
| Vázquez-Bourgon et al.[39] | 2010 | Psychiatry Research | Human peripheral blood cells | Nscz = 147 | 5-HTTLPR | L/S polymorphisms are significantly associated with early negative symptoms in first-episode psychosis |
| Kohlrausch et al.[37] | 2010 | Journal of Psychiatric Research | Human peripheral blood cells | Nscz = 116 | 5-HTTLPR, Intron 2 STin2 VNTR | The rs25531 locus of SLC6A4 may influence clozapine efficacy |
| Hung et al.[34] | 2011 | Neuroscience Letters | Human peripheral blood cells | Nscz = 168, NNC = 302 | 5-HTTLPR | The LA allele of 5-HTTLPR is significantly associated with suicide attempts in Chinese Han Chinese SCZ patients, particularly males |
| Božina et al.[104] | 2012 | Journal of Psychiatric Research | Human peripheral blood cells | Nscz = 519, NNC = 184 | 5-HTTLPR, Intron 2 VNTR | Suicide Attempters and Suicidal Ideators Differ from Controls in Depressive Symptoms and 5-HTTLPR/rs25531 Haplotype Distribution |
| Li et al.[29] | 2013 | Progress in Neuro-Psychopharmacology & Biological Psychiatry | Human peripheral blood cells | Nscz = 528, NNC = 528 | All exons, introns, regulatory regions | The rs140700 G/A polymorphism is significantly associated with SCZ susceptibility and strongly linked to negative and depressive/anxiety symptoms |
| Bilic et al.[38] | 2014 | Gene | Human peripheral blood cells | Nscz = 173 | SERT-PR, SERT-in2 | TRS is associated with the SERT-PR polymorphism of SLC6A4 |
| Alfimova et al.[36] | 2015 | Neuroscience and Behavioral Physiology | Human peripheral blood cells | Nscz = 299, NNC = 232 | 5-HTTLPR | The 5-HTTLPR polymorphism is significantly associated with emotion recognition in SCZ patients |
| Jing et al.[25] | 2016 | Journal of Shanghai Jiao Tong University | Human peripheral blood cells | Nscz = 624, NNC = 683 | 5-HTTLPR | Association of SLC6A4 promoter polymorphisms with SCZ susceptibility and atypical antipsychotic efficacy in the Chinese Han population |
Studies on SLC6A4 gene methylation in SCZ
| Author | Year | Journal | Biological material | Sample size | SLC6A4 region | Main findings |
| Philibert et al.[44] | 2007 | American Journal of Medical Genetics Part B: Neuropsychiatric Genetics | Human lymphoblastoid cell lines | Nscz = 49 | CpG island near exon 1A (799 bp) | Significant correlation between mean methylation and mRNA levels in CpG islands |
| Wang et al.[41] | 2010 | Schizophrenia Research | Human peripheral blood cells | Nscz = 46, NNC = 44 | Region encoding 5-HTT mRNA | In male SCZ patients, 5-HTT mRNA expression was significantly increased in peripheral blood leukocytes |
| Wockner et al.[40] | 2014 | Translational Psychiatry | Human postmortem prefrontal cortex tissue | Nscz = 24, NNC = 24 | genome-wide | In SCZ patients, 4641 probes corresponding to 2929 unique genes were differentially methylated |
| Beach et al.[48] | 2014 | Biological Psychology | Human peripheral blood cells | Nscz = 388 | CpG islands in exon 1 and 5'UTR | Under high SES risk, women carrying the S allele showed higher methylation levels |
| Ikegame et al.[45] | 2020 | Schizophrenia Bulletin | Human peripheral blood cells | Nscz = 440, NNC = 488 | CpG islands in promoter region | Male SCZ patients exhibited significantly higher methylation levels at the CpG3 locus than controls |
| Shen et al.[49] | 2021 | EBioMedicine | Human peripheral blood cells | Nscz = 106 | genome-wide | DNA methylation patterns tended to cluster within families |
| Ikegame et al.[45] | 2022 | Neuroscience Letters | Human peripheral blood cells | Nscz = 70, NNC = 68 | CpG islands downstream of exon 1 | DNA methylation levels were significantly higher in SCZ patients, with levels notably higher in females than males |
| Koning et al.[50] | 2024 | Psychoneuroendocrinology | Human saliva samples | Nscz = 384 | CpG islands in promoter region | Early life adversity was significantly associated with promoter methylation of SLC6A4 |
Studies on SLC6A4 gene polymorphisms and MDD
| Author | Year | Journal | Biological material | Sample size | SLC6A4 region | Main findings |
| Caspi et al.[59] | 2003 | Science | Human peripheral blood cells | NMDD = 847 | 5-HTTLPR | Polymorphisms in the 5-HTTLPR region significantly influence individual sensitivity to life stress |
| Tsang et al.[61] | 2017 | Neuroscience & Biobehavioral Reviews | Human peripheral blood cells | NLLD = 406, NNC = 688 | 5-HTTLPR | The 5-HTTLPR polymorphism is significantly associated with an increased risk of late-life depression |
| Mendonça et al.[62] | 2019 | Journal of Affective Disorders | Human oral epithelial cells | NMDD = 30, NNC = 30 | 5-HTTLPR | No statistically significant difference between S allele carriers and depression diagnosis |
| Ren et al.[65] | 2020 | Journal of Affective Disorders | Human peripheral blood cells | NMDD = 6,644 | 5-HTTLPR | The 5-HTTLPR polymorphism is significantly associated with antidepressant response and tolerance, especially in White patients |
| Jang et al.[68] | 2021 | Pharmacopsychiatry | Human peripheral blood cells | NMDD = 464 | 5-HTTLPR | The SS genotype of 5-HTTLPR is significantly associated with better antidepressant response and remission rates |
| Ugartemendia et al.[66] | 2021 | Clinical Nutrition | Human peripheral blood cells, urine | NMDD = 218 | 5-HTTLPR | 5-HTTLPR polymorphisms significantly influence the beneficial effects of tryptophan supplementation on age-related depression and social cognition |
| Hasan et al.[69] | 2021 | Heliyon | Database analysis | NMDD = 360 | SNPs | Five high-risk mutant SNPs (R104C, Y121C, W82R, R79W, W151R) were identified through computational simulations |
| Alshogran et al.[63] | 2021 | PLOS ONE | Human peripheral blood cells | NMDD = 265 | 5-HTTLPR VNTR | Polymorphisms in 5-HTTLPR and rs25531 were not significantly associated with anxiety or depressive symptoms |
| Garvert et al.[72] | 2022 | Progress in Neuro-Psychopharmacology and Biological Psychiatry | Human peripheral blood cells | NMDD = 44,586, NNC = 229,407 | 5-HTTLPR | Interaction between rs8105676 and 5-HTTLPR polymorphisms had a significant effect on lifetime depression |
Studies on SLC6A4 gene methylation and MDD
| Author | Year | Journal | Biological material | Sample size | SLC6A4 region | Main findings |
| Kim et al.[85] | 2013 | Journal of Psychiatric Research | Human peripheral blood cells | NPSD = 133, NNC = 475 | CpG island in promoter region | Hypermethylation of the promoter region of the SLC6A4 gene is independently associated with the development of post-stroke depression |
| Kang et al.[74] | 2013 | Progress in Neuro-Psychopharmacology and Biological Psychiatry | Human peripheral blood leukocytes | NMDD = 108 | Promoter CpG-enriched region | Methylation status of the promoter region of the SLC6A4 gene is significantly associated with certain clinical features of depression |
| Wankerl et al.[79] | 2014 | Translational Psychiatry | Human peripheral blood cells | NMDD = 133 | Promoter region | Both the S allele of 5-HTTLPR and early early-life stress (prenatal stress and childhood trauma) are associated with reduced SERT gene expression |
| Okada et al.[84] | 2014 | Journal of Psychiatric Research | Human peripheral blood cells | NMDD = 50, NNC = 50 | CpG island in promoter region | DNA methylation of the SLC6A4 promoter CpG island is associated with symptom severity, early adversity, and treatment response |
| Frodl et al.[76] | 2015 | Journal of Psychiatry and Neuroscience | Human peripheral blood cells | NMDD = 25, NNC = 35 | Promoter region (CpG5-15) | Methylation status of the SLC6A4 gene is significantly associated with functional brain changes during emotional processing |
| Schneider et al.[82] | 2018 | Neuropsychopharmacology | Human peripheral blood cells | NMDD = 122, NNC = 176 | AluJb elements in promoter region | The methylation rate of the AluJb element in the SLC6A4 promoter is significantly lower in patients with depression |
| Peng et al.[80] | 2018 | Psychosomatic Medicine | Human peripheral blood cells | NEG = 126, NUG = 112 | CpG island in promoter region | DNA methylation levels of the SLC6A4 gene are significantly associated with depressive symptoms in two independent studies |
| Consoloni et al.[86] | 2018 | European Neuropsychopharmacology | Human peripheral blood mononuclear cells | NMDD = 103, NNC = 100 | SLC6A4 mRNA expression | Changes in SLC6A4 mRNA expression from baseline to week 8 predict suicide attempts |
| Palma-Gudiel et al.[83] | 2019 | Progress in Neuro-Psychopharmacology and Biological Psychiatry | Human peripheral blood cells, postmortem brain tissue | NMDD = 43, NNC = 105 | CpG island in promoter region | Methylation levels of the SLC6A4 promoter are significantly associated with somatization symptoms |
| Mendonça et al.[62] | 2019 | Journal of Affective Disorders | Human oral epithelial cells | NMDD = 30, NNC = 30 | CpG island in promoter region | DNA methylation of the AluJb repetitive element in the SLC6A4 promoter is significantly reduced in depression |
| Webb et al.[102] | 2020 | International Journal of Molecular Sciences | Human peripheral blood mononuclear cells, saliva, brain tissue | NMDD = 313, NNC = 115 | Promoter region | Methylation levels of the SLC6A4 promoter are significantly associated with antidepressant treatment response |
| Zhu et al.[77] | 2023 | Journal of Affective Disorders | Human peripheral blood cells, saliva | NMDD = 3,965 | Promoter region | Hypermethylation of the SLC6A4 gene was not significantly associated with depression risk in the overall analysis |
| Comtois-Cabana et al.[81] | 2023 | PLOS ONE | Human Saliva | NMDD = 156 | Specific CpG sites | DNA methylation of the SLC6A4 gene may mediate the relationship between childhood maltreatment and depressive symptoms in early adulthood |
| Bruzzone et al.[78] | 2024 | Clinical Epigenetics | Human peripheral blood cells | NMDD = 90, NNC = 254 | Specific CpG sites in promoter region | No significant association between SLC6A4 methylation and symptoms of depression, anxiety, or early-life stress |
| Bruzzone et al.[75] | 2025 | Progress in Neuro-Psychopharmacology and Biological Psychiatry | Human peripheral blood cells | NMDD = 90 | CpG island in promoter region | No significant association between SLC6A4 methylation at baseline and clinical response or symptom change after 8 weeks of treatment (ΔHAMD6) |
Studies on SLC6A4 gene polymorphisms, methylation and other psychiatric disorders
| Author | Year | Journal | Biological material | Sample size | SLC6A4 region | Main findings | |
| Ikeda et al.[31] | 2006 | Journal of Neural Transmission | Human peripheral blood cells | NBP = 109, NNC = 288 | All exons, promoter, introns | Neither common nor rare SLC6A4 variants showed significant association with BP in the Japanese population | |
| Sugawara et al.[19] | 2011 | Translational Psychiatry | Human lymphoblastoid cell lines, peripheral blood cells, postmortem brain tissue | NBD = 53, NNC = 54 | Promoter region | Hypermethylation of the SLC6A4 promoter is significantly associated with bipolar disorder | |
| Hesse et al.[105] | 2014 | European Journal of Nuclear Medicine and Molecular Imaging | Human peripheral blood cells, brain tissue | NMS = 23, NNC = 22 | Promoter, STin2-VNTR | SERT-LPR and STin2-VNTR polymorphisms in SLC6A4 do not differ significantly between MS patients and controls | |
| Dukal et al.[98] | 2015 | Borderline Personality Disorder and Emotion Dysregulation | Human neonatal cord blood cells | NELS = 45, NNC = 45 | Promoter CpG islands (CpG1-4) | Female newborns exhibited significantly higher SLC6A4 methylation than males | |
| Park et al.[94] | 2015 | Psychological Medicine | Human peripheral blood cells | NADHD = 102 | Promoter region | Hypermethylation of the SLC6A4 promoter is significantly associated with ADHD symptom presentation in children | |
| Schiele et al.[95] | 2019 | European Neuropsychopharmacology | Human peripheral blood cells | NPD = 119, NNC = 119 | Promoter region | Hypermethylation of the SLC6A4 promoter is significantly associated with comorbid depression (MDD) in PD patients | |
| Calabrò et al.[97] | 2020 | Molecular Biology Reports | Human peripheral blood cells | NP = 786, NNC = 539 | SNPs in promoter, introns, coding regions (rs2066713, rs4251417, rs6354, rs7224199) | Polymorphisms in SLC6A4 are significantly associated with alcohol dependence and Alzheimer’s disease | |
| Cheng et al.[96] | 2021 | Acta Neurologica Belgica | Human peripheral blood cells | NPD = 466, NC = 504 | 5-HTTLPR | 5-HTTLPR genotypes (especially the S allele) are significantly associated with depression risk in Parkinson's disease (PD) patients | |
Data extraction included the following: study characteristics (author, year, country), participant characteristics (sample size, diagnosis), genetic variation data (polymorphic loci, genotype frequencies), methylation data (CpG sites), and statistical results (e.g., odds ratios, P values).
RESULTS
The SLC6A4 gene and schizophrenia
Schizophrenia (SCZ) is a chronic mental disorder affecting approximately 1% of the global population[21]. Its symptoms can be divided into three categories: positive symptoms, including hallucinations, delusions, and speech disorders; negative symptoms, such as social withdrawal, affective flattening, lack of initiative, and anergia; and cognitive deficits[22]. The pathophysiology mechanisms of schizophrenia may involve genetic and epigenetic abnormalities, as well as changes in neurotransmitter structure or function[23].
In recent years, numerous studies have investigated the correlation between SLC6A4 and SCZ, examining its association with schizophrenia pathogenesis, clinical manifestations, and treatment response. Key findings are summarized below.
SLC6A4 gene polymorphism and schizophrenia
Polymorphisms in the SLC6A4 gene, particularly the linked polymorphic region in the promoter (5-HTTLPR) and the intron 2 variable number tandem repeat sequence (Intron 2 VNTR), have received widespread attention for their potential role in SCZ susceptibility. Several studies indicate that SLC6A4 polymorphisms are differentially associated with SCZ risk across different populations. For example, studies in Tatar and Russian populations have found that the association of the 5-HTTLPR polymorphism with SCZ varies significantly by ethnicity: the L/L genotype is strongly associated with persistent schizophrenia in Russians, whereas the S/S genotype is less frequent among individuals with intermittent schizophrenia in Tatars. These findings suggest that genetic background may modulate disease susceptibility[24]. A study of outpatients and inpatients at Shanghai Mental Health Center in China showed that polymorphisms in the SLC6A4 promoter region and the rs25531 locus were associated with SCZ susceptibility. Specifically, the
Several studies have also linked SLC6A4 polymorphisms to an increased risk of suicide in SCZ patients[31,34,35]. Additionally, certain polymorphisms (e.g., rs140700, rs2054847, and rs140700) have been associated with negative symptoms, cognitive deficits (such as fear recognition), and aggressive behavior[26,28,29,36].
SLC6A4 polymorphisms may also influence response to antipsychotic medications. For example, the S allele may be associated with a poorer response to clozapine[37], while other genotypes may predict treatment-resistant schizophrenia or the extent of improvement in negative symptoms[38,39].
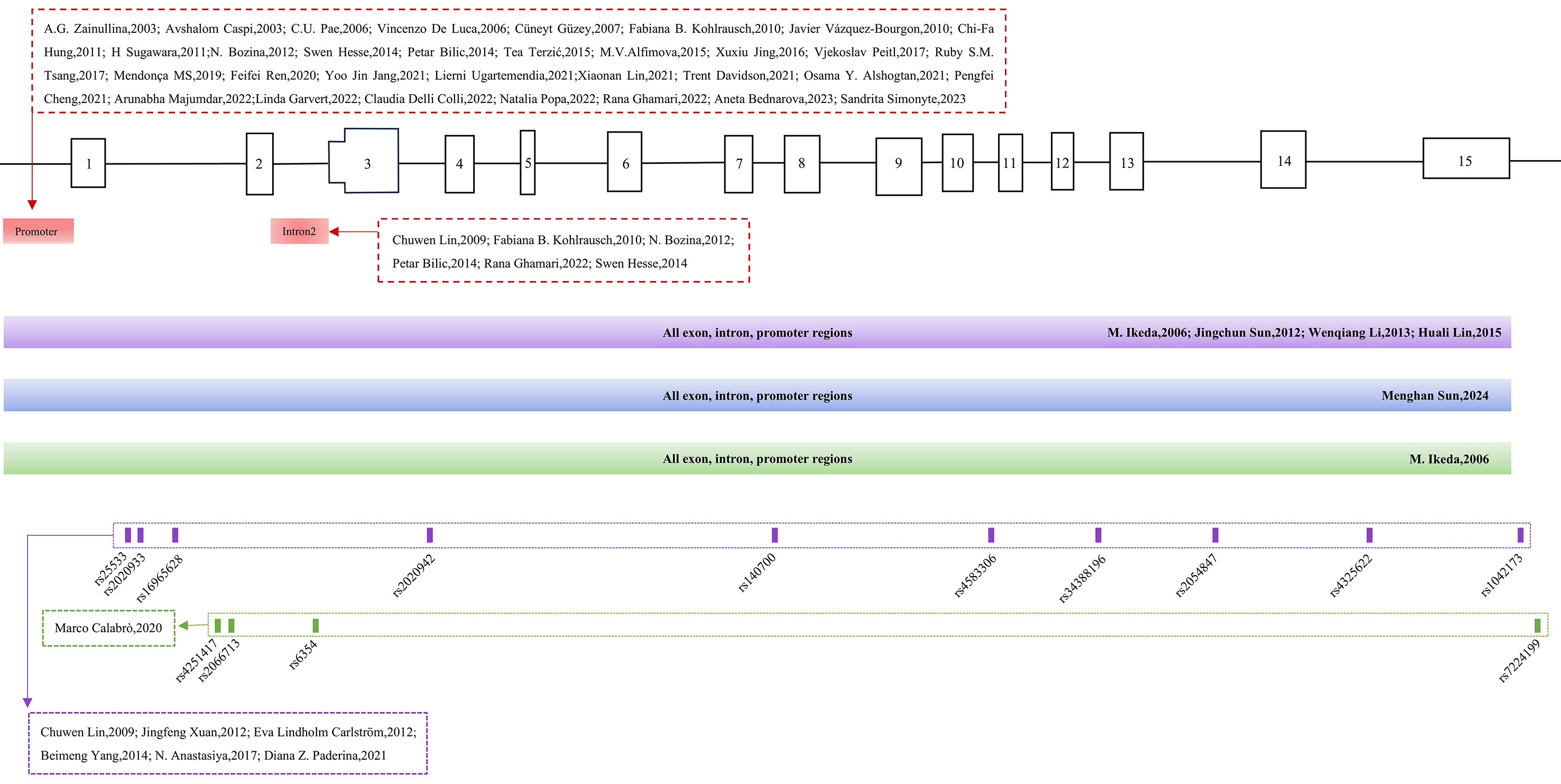
Overall, SLC6A4 polymorphisms appear to contribute to SCZ susceptibility, clinical presentation, and drug response, although their effects vary across populations, highlighting the gene’s multidimensional role in schizophrenia pathophysiology [Table 1, Figure 1].
Figure 1. Polymorphic sites in the SLC6A4 gene in studies of SCZ, MDD and other psychiatric disorders. The red sections indicate study sites in SCZ, MDD, and other disorders; purple indicates SCZ-specific sites; blue indicates MDD-specific sites; green indicates sites related to other psychiatric disorders.
Methylation of the SLC6A4 gene and schizophrenia
Recent studies indicate that epigenetic modifications, particularly DNA methylation, play a crucial role in mediating the interactions between genetic and environmental factors. The methylation status of the SLC6A4 gene may be involved in the pathophysiology of SCZ by regulating its expression and influencing the 5-HT signaling pathway.
Several studies have demonstrated a significant association between SLC6A4 methylation and both the incidence and severity of SCZ. For instance, a genome-wide methylation analysis of prefrontal cortex tissue from SCZ patients conducted by Wockner identified 2,929 genes, including SLC6A4, with abnormal methylation patterns[40]. In a study of an East Chinese population, the expression of 5-HTT mRNA in peripheral blood leukocytes was significantly increased in male SCZ patients[41]. Although some studies reported no significant differences[42], subsequent research by Abdolmaleky and Philibert revealed that DNA methylation levels in the promoter region of SLC6A4 were significantly increased in postmortem brain tissue, saliva samples of SCZ patients, and human lymphoblastic cell lines, correlating with decreased gene expression[43,44]. These findings suggest that promoter hypermethylation may impair 5-HTT function by inhibiting transcriptional activity, thereby exacerbating SCZ pathology. Methylation of SLC6A4 not only appears linked to disease pathogenesis but may also influence core symptoms. In male SCZ patients, the CpG3 locus in the promoter region exhibited significantly higher methylation than in controls[45]. Our team pre-analyzed 17 CpG islands downstream of exon 1 of SLC6A4 and found that methylation levels upstream of exon 1 were significantly higher in female patients than in males, indicating a potential sex-specific role of methylation in regulating gene expression and contributing to SCZ development[46].
Environmental factors, particularly early-life adversity, have been proposed to increase the risk of SCZ by altering SLC6A4 methylation[47-50].
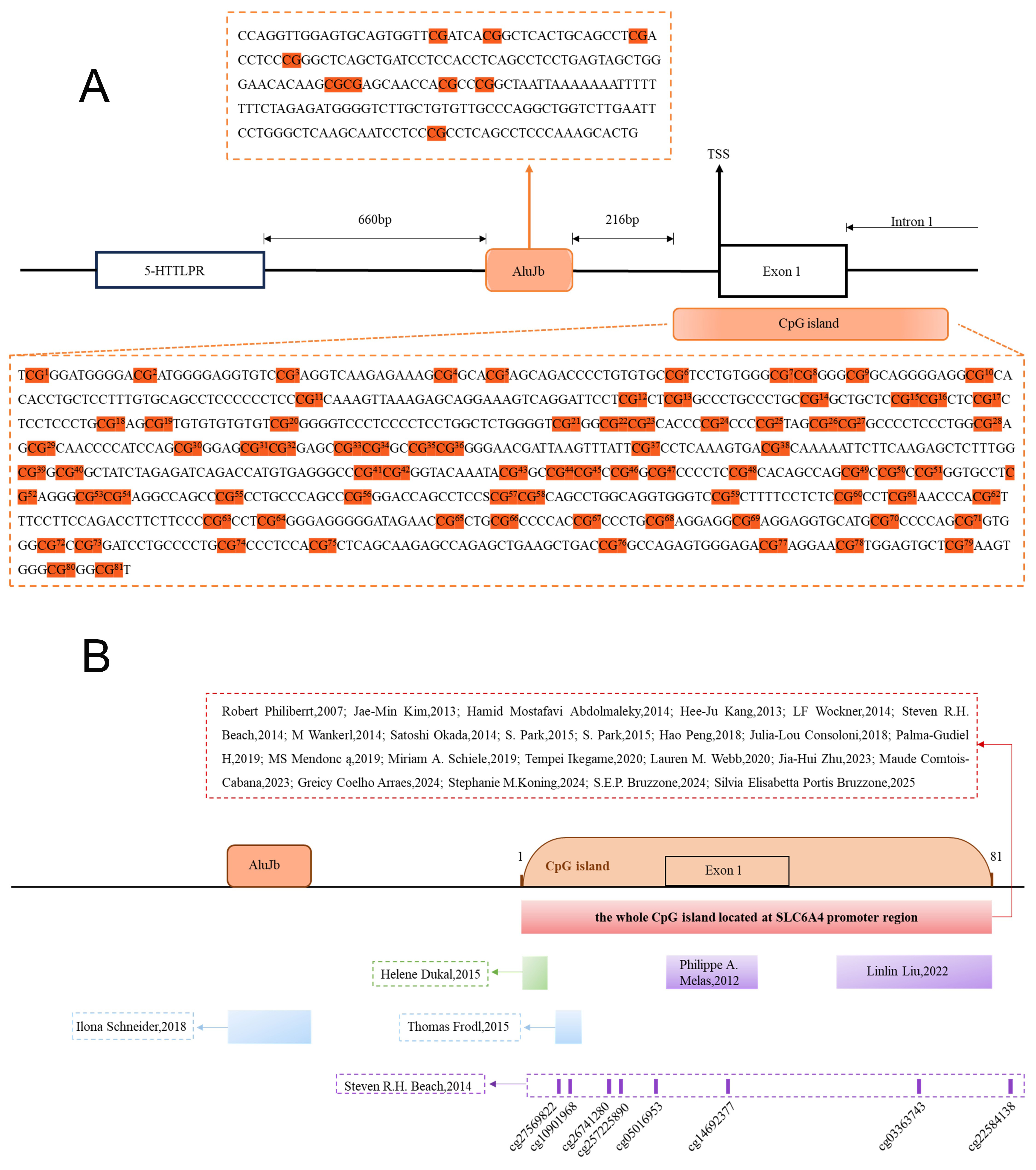
Emerging evidence also suggests that SLC6A4 methylation may influence the response to antipsychotic treatment. One study found that hypermethylation of the SLC6A4 promoter was significantly higher in SCZ patients who had not received antipsychotic treatment compared with controls, and this hypermethylation was closely correlated with reduced 5-HTT expression[43]. Overall, the methylation status of SLC6A4 appears to affect both disease onset and severity, highlighting its potential as a focus for future research [Table 2, Figure 2].
Figure 2. Methylated CpG sites in the SLC6A4 gene from studies on SCZ, MDD, and other psychiatric disorders. (A) CpG sites examined in studies included in this review. (B) Red indicates study sites in SCZ, MDD, and other disorders; purple indicates SCZ-specific sites; blue indicates MDD-specific sites; green indicates sites related to other psychiatric disorders.
SLC6A4 and imaging changes in schizophrenia
Recent advances in molecular genetics and neuroimaging have increasingly highlighted the potential role of SLC6A4 in SCZ.
Alterations in SLC6A4 expression during brain development may significantly contribute to the pathogenesis of SCZ. Evidence from animal models suggests that developmental changes in SLC6A4 expression are associated with structural abnormalities in brain regions implicated in SCZ[51].
Beyond structural effects, SLC6A4 also influences brain function by regulating 5-HT signaling. Neuroimaging studies have revealed that SLC6A4 availability is closely related to the functioning of brain regions such as the thalamus and prefrontal cortex, as well as to negative symptoms and insight deficits in patients with SCZ[52-54].
SLC6A4 gene and depression
Depression (or major depressive disorder; MDD) is one of the most common mental disorders worldwide, affecting approximately 3.8% of the global population in 2023[55]. The World Health Organization predicts that depression will be one of the two leading causes of global health burden and disability, and the largest contributor to the global disease burden by 2030[56,57]. Its pathogenesis involves multiple biological factors, including genetics, epigenetics, and interactions with environmental factors[58].
In recent years, numerous studies have investigated the correlation between the SLC6A4 gene and MDD, examining its role in the pathogenesis, clinical manifestations, and treatment response. The following section provides a review of the current understanding of the relationship between the SLC6A4 gene and MDD.
Polymorphisms of the SLC6A4 gene and depression
A growing body of evidence suggests that the 5-HTTLPR polymorphism plays a major role in depression. This polymorphism affects the risk of developing depression in several ways, including altering individual susceptibility to life stress, influencing antidepressant response, and interacting with other genes. Studies indicate that carriers of the S allele are more likely to develop depression under stressful conditions[59], and in high-stress environments, individuals with certain genetic polymorphisms show more severe depressive symptoms[60]. These findings suggest that 5-HTTLPR not only modulates stress sensitivity but also influences depressive symptom severity in high-stress contexts. Furthermore, the risk of late-life depression appears to depend not solely on the 5-HTTLPR genotype but on its interaction with stressful life events. Higher frequency or intensity of stress is associated with a dose-dependent increase in depression risk among S allele carriers[61-64]. Thus, SLC6A4 gene polymorphisms (particularly the S allele), may elevate depression risk by increasing stress susceptibility, highlighting the central role of gene-environment interactions in MDD pathogenesis.
The 5-HTTLPR polymorphism also affects antidepressant efficacy and tolerability, with the L allele generally associated with a better treatment response[65-68].
Beyond 5-HTTLPR, other single-nucleotide polymorphisms (SNPs) in SLC6A4 have been implicated in depression. These SNPs may influence depression risk by altering the expression, function, or stability of 5-HT, thereby affecting receptor activity. Functional prediction studies and animal models support the central role of SLC6A4 dysfunction in MDD[69,70].
Depression arises not solely from genetic or environmental factors in isolation but from their complex interplay. Recent studies have highlighted the importance of dynamic interactions between genetic polymorphisms and environmental stressors, such as peer bullying, in the onset of MDD[71-73]. In summary, interactions between SLC6A4 polymorphisms and environmental factors are critical to depression pathogenesis.
Overall, the 5-HTTLPR polymorphism significantly influences depression risk and is closely linked to antidepressant response and tolerance. These findings highlight the need to explore depression mechanisms from multiple angles, considering additional SLC6A4 variants and environmental influences [Table 3].
Methylation of the SLC6A4 gene and depression
Studies examining the relationship between SLC6A4 methylation and antidepressant efficacy have yielded inconsistent results[74,75]. These discrepancies indicate that future research should integrate additional epigenetic markers and clinical characteristics to improve the accuracy of predicting treatment response in depression. Thomas Frodl reported that SLC6A4 methylation may contribute to the onset of depression by affecting insular and pontine emotional processing[76]. However, not all studies support a link between SLC6A4 polymorphisms and susceptibility to MDD, with several meta-analyses and individual studies failing to find a significant correlation with MDD risk or symptoms[77,78].
Early-life environmental stressors, such as prenatal stress and childhood trauma, are closely related to the methylation levels of the SLC6A4 gene. Numerous studies have confirmed significant correlations between childhood trauma and changes in SLC6A4 methylation, suggesting a potential role in mediating the risk of depression in adulthood[79-81]. These findings imply that childhood trauma may increase the likelihood of adult depression by modifying SLC6A4 methylation patterns. The methylation status of SLC6A4 is also influenced by gene-environment interactions. For instance, the methylation rate of the AluJb element in the SLC6A4 promoter is significantly reduced in patients with depression, and this reduction shows a notable interaction with the 5-HTTLPR/rs25531 polymorphism. This indicates that genetic variants and environmental stressors may jointly affect depression risk through changes in methylation[82]. Gender differences have also been observed: SLC6A4 promoter methylation is related to somatization symptoms, with higher methylation levels found in females, suggesting that sex may be an important moderating factor[83].
MS Mendonca examined DNA methylation of the AluJb repeat element in the SLC6A4 promoter and found that methylation levels were significantly reduced in patients with SCZ[62]. Additionally, methylation at specific CpG sites of SLC6A4 has been linked to symptom severity and treatment response[84,85]. For example, hypermethylation in the promoter region may correlate with better antidepressant efficacy[85] and with the development of post-stroke depression[86], while alterations in SLC6A4 mRNA expression can predict changes in suicide risk during treatment[87]. In summary, the methylation status of the SLC6A4 gene holds potential as a biomarker for both the diagnosis and treatment of depression [Table 4].
SLC6A4 gene and imaging changes in depression
Numerous studies have shown that the 5-HTTLPR polymorphism and DNA methylation levels of the SLC6A4 gene are closely related to structural brain changes, white matter integrity abnormalities, and dysfunction in emotion-related brain regions in MDD patients.
The 5-HTTLPR polymorphism of the SLC6A4 gene has a significant impact on hippocampal volume. Specifically, carrying different alleles (S or L) is associated with heterogeneous reductions in hippocampal volume, and these effects vary between genders[87,88].
Abnormal amygdala function, a core component of the brain's emotion-processing network, is strongly linked to MDD susceptibility. Epigenetic mechanisms play a crucial role; environmental stressors, such as low socioeconomic status, can increase the methylation of the SLC6A4 gene, leading to heightened amygdala responsiveness to negative stimuli and an elevated risk of depression[89,90].
White matter integrity abnormalities constitute another important neuroimaging finding. Patients with depression often exhibit reduced integrity in the corpus callosum and other white matter tracts, which is associated with both the 5-HTTLPR genotype and elevated SLC6A4 methylation levels[91,92]. Additionally, evidence suggests that SLC6A4 may interact synergistically with other risk genes (such as BDNF and COMT) to exacerbate prefrontal cortical thinning and white matter damage[93].
SLC6A4 gene and other psychiatric disorders
Dysfunction of the SLC6A4 gene has also been implicated in other psychiatric disorders. In bipolar disorder (BD), evidence for an association with SLC6A4 gene polymorphisms is weak, but epigenetic studies suggest that hypermethylation of its promoter region may be linked to the disease[19,31]. In children with attention-deficit/hyperactivity disorder (ADHD), hypermethylation in the SLC6A4 promoter region correlates with clinical symptoms[94]. Parkinson's disease (PD) patients frequently experience depression, potentially due to increased methylation of the SLC6A4 promoter and the presence of the 5-HTTLPR S allele[95,96]. Furthermore, polymorphisms in this gene have been linked to the risk or progression of alcohol dependence and Alzheimer's disease[97].
Sex differences have also been reported in epigenetic modifications, with higher SLC6A4 promoter methylation observed in female neonates, which partially explains the increased risk of depression in females later in life[98]. Animal studies further support a regulatory role of SLC6A4 genetic variants in anxiety-like behaviors[99]. In summary, SLC6A4 contributes to the pathophysiology of multiple neuropsychiatric diseases through epigenetic regulation and gene-environment interactions, although findings are heterogeneous across populations [Table 5].
DISCUSSION
As the key gene encoding 5-HTT, SLC6A4 has been extensively studied for its association with polymorphisms, epigenetic modifications, and dysfunction in a variety of mental disorders. This review highlights the crucial role of SLC6A4 in schizophrenia (SCZ), major depressive disorder (MDD), bipolar disorder (BD), attention-deficit/hyperactivity disorder (ADHD), and other psychiatric conditions. Its mechanisms involve genetic variation, epigenetic regulation, and neuroimaging changes, profoundly influencing clinical manifestations by modulating 5-HT reuptake efficiency and synaptic plasticity.
Polymorphisms in SLC6A4, such as 5-HTTLPR and the intron 2 VNTR region, exhibit heterogeneous associations across psychiatric disorders. For example, the 5-HTTLPR S allele is linked to negative SCZ symptoms[29], suicidal behavior[35], and altered drug response[67], while the L allele is often associated with better antidepressant efficacy in MDD[65]. These polymorphisms influence not only transcriptional activity and mRNA stability but also directly affect 5-HTT kinetics at the protein level: the S allele reduces 5-HTT expression on the cell membrane and increases 5-HTT internalization, impairing 5-HT reuptake, prolonging synaptic 5-HT retention, and affecting neurotransmission efficiency and receptor desensitization[100]. Additionally, some SNPs (e.g., rs25531) may further modulate 5-HTT transport activity and stability through changes in mRNA secondary structure or post-translational modifications[69]. These molecular and cellular alterations ultimately lead to abnormal expression of synaptic plasticity-related proteins (e.g., BDNF, PSD-95), which are closely related to clinical symptoms such as cognitive deficits in SCZ and emotional processing disorders in MDD.
Epigenetic modifications, especially DNA methylation, play a key role in regulating SLC6A4 expression. Promoter and exon hypermethylation recruit methyl-binding proteins (e.g., MeCP2) and histone deacetylases (HDACs) to form condensed chromatin structure, inhibiting transcription factor binding and downregulating SLC6A4 expression. In SCZ, this mechanism is associated with reduced synaptic 5-HT reuptake, impaired signal transduction, and severity of negative symptoms[45]. In MDD, environmental stressors such as childhood trauma induce hypermethylation at specific CpG sites of SLC6A4 via the glucocorticoid receptor-DNMT signaling axis. This not only decreases 5-HTT expression but also inhibits neurotrophic signaling (e.g., BDNF transcription) through epigenetic cross-talk, impairing neuroplasticity in the hippocampus and prefrontal cortex, and aggravating cognitive and emotional symptoms[81,101].
Neuroimaging studies provide system-level evidence supporting these mechanisms. SLC6A4 polymorphisms and methylation status are closely related to structural abnormalities (e.g., hippocampal atrophy, reduced amygdala volume), functional alterations (e.g., reduced SERT availability in the prefrontal cortex), synaptic plasticity markers, and clinical behavioral phenotypes. For example, in SCZ patients, S allele carriers exhibit thinner prefrontal cortices and reduced SERT binding in the thalamo-cortical pathway, correlating with negative symptoms and executive dysfunction[52]. In MDD patients, individuals with hypermethylation show enhanced amygdala responses to negative stimuli and abnormal default mode network connectivity, which are directly associated with core symptoms such as anhedonia and cognitive delays. Notably, decreased white matter integrity (e.g., reduced fractional anisotropy in the corpus callosum) interacts with SLC6A4 hypermethylation and the 5-HTTLPR S allele, reflecting disrupted myelination and synaptic microstructure, thereby affecting network synchronization and information processing efficiency[92]. Collectively, these findings indicate that SLC6A4 variants influence neural circuit development and function by regulating 5-HT reuptake and synaptic plasticity, ultimately contributing to specific clinical symptoms.
Despite significant progress, several limitations and points of contention remain. First, most studies focused on specific ethnic or regional populations (e.g., East Asian or European), limiting generalizability. For example, the association between 5-HTTLPR and SCZ was significant in Tatars[24] and Chinese Han populations[25] but not replicated in Japanese[31] or Korean cohorts[32]. These discrepancies may arise from genetic background differences (e.g., haplotype structure), environmental exposures (e.g., cultural stressors), or statistical limitations (e.g., small sample size). Second, gender differences are often overlooked, and most studies do not perform stratified analyses, despite females showing distinct methylation patterns and disease risks. Third, methylation studies largely rely on peripheral blood or saliva, with limited evidence from brain tissue. For example, SLC6A4 promoter hypermethylation was observed in postmortem SCZ brains[43], whereas peripheral studies were inconsistent, suggesting differences between central and peripheral epigenetic regulation. Fourth, while associations between early adversity (e.g., childhood trauma[79]) and SLC6A4 methylation are repeatedly reported, precise quantification of environmental factors (e.g., type, duration) and dynamics of interaction (timing-specific windows) remain underexplored. For example, the interaction between peer bullying and 5-HTTLPR was significant only in adolescents[73], whereas stress in adulthood may involve different mechanisms. Whether epigenetic modifications are reversible (e.g., through environmental interventions) remains unclear. Although SLC6A4 polymorphisms and methylation status are suggested as potential predictors of drug response or disease prognosis, existing results are often contradictory. For instance, associations between SLC6A4 methylation and antidepressant efficacy vary across cohorts: Kang reported hypermethylation linked to symptom severity[74], whereas Webb found it associated with better response[102]. Such discrepancies may stem from study design differences (baseline symptom heterogeneity, follow-up duration) or uncontrolled confounders (co-medication, comorbidities). Future studies should integrate multi-omics approaches (e.g., epigenome-transcriptome analysis), large cross-ethnic cohorts, and longitudinal designs to elucidate the precise regulatory networks of SLC6A4 in psychiatric disorders and to explore its potential as a biomarker or therapeutic target.
CONCLUSION
As a core regulator of the 5-HT system, SLC6A4 is susceptible to genetic and epigenetic alterations, which can disrupt 5-HTT expression, impair serotonergic signaling, and contribute to the development of psychiatric disorders. This highlights its key role in the pathophysiology of mental illnesses and underscores its potential as a target for research. However, current studies face challenges including significant heterogeneity, technical limitations, and gaps in understanding disease mechanisms. Addressing these issues through research on population diversity, brain-specific regulation, and dynamic gene-environment interactions is crucial for advancing knowledge and identifying innovative therapeutic strategies.
DECLARATIONS
Authors’ contributions
Conceptualization, validation, writing-review and editing, supervision, project administration, funding acquisition: Nie S, Liu L
Conceptualization, supervision: Hu L
Software, writing-original draft preparation, writing-review and editing, visualization: Yuan H
Writing-original draft preparation, visualization, funding acquisition: Qing L
Software: Zou T
Methodology: Cheng T, Zhou C, Guo X, Li Y
Availability of data and materials
Not applicable.
Financial support and sponsorship
This work was supported by the Joint Special Funds for the Department of Science and Technology of Yunnan Province-Kunming Medical University (202301AY070001-232 and 202401AY070001-058), the Funds for the Department of Science and Technology of Yunnan Province (202501AS070018), the Scientific Research Fund Project of Education Department of Yunnan Province (2023Y0616), the Kunming Medical University Talent Introduction Research Project (K132310511), and the NHC Key Lab of Drug Addiction Medicine (Kunming Medical University) Open Projects Fund (KN202411 and KN202420). The funders had no role in the study design, data collection and analysis, decision to publish, or manuscript preparation.
Conflicts of interest
Nie S and Liu L are Junior Editorial Board members of Journal of Translational Genetics and Genomics. Nie S and Liu L were not involved in any steps of editorial processing, notably including reviewer selection, manuscript handling, or decision making, while the other authors have declared that they have no conflicts of interest.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204-22.
2. Cuijpers P, Javed A, Bhui K. The WHO world mental health report: a call for action. Br J Psychiatry. 2023;222:227-9.
3. Andrews G, Slade T, Issakidis C. Deconstructing current comorbidity: data from the Australian national survey of mental health and well-being. Br J Psychiatry. 2002;181:306-14.
4. Plana-Ripoll O, Pedersen CB, Holtz Y, et al. Exploring comorbidity within mental disorders among a danish national population. JAMA Psychiatry. 2019;76:259-70.
5. Bijl RV, Ravelli A, van Zessen G. Prevalence of psychiatric disorder in the general population: results of The Netherlands Mental Health Survey and Incidence Study (NEMESIS). Soc Psychiatry Psychiatr Epidemiol. 1998;33:587-95.
6. Martin J, Taylor MJ, Lichtenstein P. Assessing the evidence for shared genetic risks across psychiatric disorders and traits. Psychol Med. 2018;48:1759-74.
7. Anttila V, Bulik-Sullivan B, Finucane HK, et al. Analysis of shared heritability in common disorders of the brain. Science. 2018;360:eaap8757.
8. Menculini G, Chipi E, Paolini Paoletti F, et al. Insights into the pathophysiology of psychiatric symptoms in central nervous system disorders: implications for early and differential diagnosis. Int J Mol Sci. 2021;22:4440.
9. Zuo Y, Wei D, Zhu C, Naveed O, Hong W, Yang X. Unveiling the pathogenesis of psychiatric disorders using network models. Genes. 2021;12:1101.
10. Cui L, Li S, Wang S, et al. Major depressive disorder: hypothesis, mechanism, prevention and treatment. Signal Transduct Target Ther. 2024;9:30.
11. Okaty BW, Commons KG, Dymecki SM. Embracing diversity in the 5-HT neuronal system. Nat Rev Neurosci. 2019;20:397-424.
13. Palma-Gudiel H, Fañanás L. An integrative review of methylation at the serotonin transporter gene and its dialogue with environmental risk factors, psychopathology and 5-HTTLPR. Neurosci Biobehav Rev. 2017;72:190-209.
14. Khaliq S, Haider S, Ahmed SP, Perveen T, Haleem DJ. Relationship of brain tryptophan and serotonin in improving cognitive performance in rats. Pak J Pharm Sci. 2006;19:11-5.
15. Williams E, Stewart-Knox B, Helander A, McConville C, Bradbury I, Rowland I. Associations between whole-blood serotonin and subjective mood in healthy male volunteers. Biol Psychol. 2006;71:171-4.
16. Heiming RS, Mönning A, Jansen F, Kloke V, Lesch KP, Sachser N. To attack, or not to attack? The role of serotonin transporter genotype in the display of maternal aggression. Behav Brain Res. 2013;242:135-41.
17. Heils A, Teufel A, Petri S, et al. Allelic variation of human serotonin transporter gene expression. J Neurochem. 1996;66:2621-4.
18. Xu FL, Wang BJ, Yao J. Association between the SLC6A4 gene and schizophrenia: an updated meta-analysis. Neuropsychiatr Dis Treat. 2019;15:143-55.
19. Sugawara H, Iwamoto K, Bundo M, et al. Hypermethylation of serotonin transporter gene in bipolar disorder detected by epigenome analysis of discordant monozygotic twins. Transl Psychiatry. 2011;1:e24.
20. Booij L, Szyf M, Carballedo A, et al. DNA methylation of the serotonin transporter gene in peripheral cells and stress-related changes in hippocampal volume: a study in depressed patients and healthy controls. PLoS One. 2015;10:e0119061.
21. Solmi M, Seitidis G, Mavridis D, et al. Incidence, prevalence, and global burden of schizophrenia - data, with critical appraisal, from the Global Burden of Disease (GBD) 2019. Mol Psychiatry. 2023;28:5319-27.
23. Millan MJ, Andrieux A, Bartzokis G, et al. Altering the course of schizophrenia: progress and perspectives. Nat Rev Drug Discov. 2016;15:485-515.
24. Zainullina AG, Juriev EB, Bikbulatova SR, Khusnutdinova EK. [Association of the hSERT and SLC6A4 polymorphic markers of the serotonin transporter gene with schizophrenia in different ethnic groups]. Mol Biol. 2003;37:601-6.
25. Jing X, Zhang R, Gao K, Sun S, Qian Y, Yi Z. Study on the correlation between the polymorphism in promoter region of 5-hydroxytryptamine transporter gene and schizophrenia in Chinese Han population. J Shanghai Jiaotong Univ (Med Sci). 2016;36:537-41.
26. Ghamari R, Yazarlou F, Khosravizadeh Z, Moradkhani A, Abdollahi E, Alizadeh F. Serotonin transporter functional polymorphisms potentially increase risk of schizophrenia separately and as a haplotype. Sci Rep. 2022;12:1336.
27. Vijayan NN, Iwayama Y, Koshy LV, et al. Evidence of association of serotonin transporter gene polymorphisms with schizophrenia in a South Indian population. J Hum Genet. 2009;54:538-42.
28. Lin C, Tang W, Hu J, et al. Haplotype analysis confirms association of the serotonin transporter (5-HTT) gene with schizophrenia in the Han Chinese population. Neurosci Lett. 2009;453:210-3.
29. Li W, Yang Y, Lin J, et al. Association of serotonin transporter gene (SLC6A4) polymorphisms with schizophrenia susceptibility and symptoms in a Chinese-Han population. Prog Neuropsychopharmacol Biol Psychiatry. 2013;44:290-5.
30. Lin H, Lei Y, Zhang B, Dai Z, Lu X. Common variants of HTR1A and SLC6A4 confer the increasing risk of Schizophrenia susceptibility: A population-based association and epistasis analysis. Am J Med Genet B Neuropsychiatr Genet. 2015;168:749-55.
31. Ikeda M, Iwata N, Suzuki T, et al. No association of serotonin transporter gene (SLC6A4) with schizophrenia and bipolar disorder in Japanese patients: association analysis based on linkage disequilibrium. J Neural Transm. 2006;113:899-905.
32. Pae CU, Serretti A, Artioli P, et al. Interaction analysis between 5-HTTLPR and TNFA -238/-308 polymorphisms in schizophrenia. J Neural Transm. 2006;113:887-97.
33. Yang B, Huang X, Ruan L, et al. No association of SLC6A3 and SLC6A4 gene polymorphisms with schizophrenia in the Han Chinese population. Neurosci Lett. 2014;579:114-8.
34. Hung CF, Lung FW, Chen CH, et al. Association between suicide attempt and a tri-allelic functional polymorphism in serotonin transporter gene promoter in Chinese patients with schizophrenia. Neurosci Lett. 2011;504:242-6.
35. Lindholm Carlström E, Saetre P, Rosengren A, et al. Association between a genetic variant in the serotonin transporter gene (SLC6A4) and suicidal behavior in patients with schizophrenia. Behav Brain Funct. 2012;8:24.
36. Alfimova MV, Golimbet VE, Korovaitseva GI, et al. Effects of the 5-HTTLPR polymorphism of the serotonin transporter gene on the recognition of mimicked emotional expressions in schizophrenia. Neurosci Behav Physi. 2015;45:605-11.
37. Kohlrausch FB, Salatino-Oliveira A, Gama CS, Lobato MI, Belmonte-de-Abreu P, Hutz MH. Influence of serotonin transporter gene polymorphisms on clozapine response in Brazilian schizophrenics. J Psychiatr Res. 2010;44:1158-62.
38. Bilic P, Jukic V, Vilibic M, Savic A, Bozina N. Treatment-resistant schizophrenia and DAT and SERT polymorphisms. Gene. 2014;543:125-32.
39. Vázquez-Bourgon J, Arranz MJ, Mata I, et al. Serotonin transporter polymorphisms and early response to antipsychotic treatment in first episode of psychosis. Psychiatry Res. 2010;175:189-94.
40. Wockner LF, Noble EP, Lawford BR, et al. Genome-wide DNA methylation analysis of human brain tissue from schizophrenia patients. Transl Psychiatry. 2014;4:e339.
41. Wang G, Hu C, Jiang T, et al. Overexpression of serotonin receptor and transporter mRNA in blood leukocytes of antipsychotic-free and antipsychotic-naïve schizophrenic patients: gender differences. Schizophr Res. 2010;121:160-71.
42. Melas PA, Rogdaki M, Ösby U, Schalling M, Lavebratt C, Ekström TJ. Epigenetic aberrations in leukocytes of patients with schizophrenia: association of global DNA methylation with antipsychotic drug treatment and disease onset. FASEB J. 2012;26:2712-8.
43. Abdolmaleky HM, Nohesara S, Ghadirivasfi M, et al. DNA hypermethylation of serotonin transporter gene promoter in drug naïve patients with schizophrenia. Schizophr Res. 2014;152:373-80.
44. Philibert R, Madan A, Andersen A, Cadoret R, Packer H, Sandhu H. Serotonin transporter mRNA levels are associated with the methylation of an upstream CpG island. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:101-5.
45. Ikegame T, Bundo M, Okada N, et al. Promoter activity-based case-control association study on SLC6A4 highlighting hypermethylation and altered amygdala volume in male patients with schizophrenia. Schizophr Bull. 2020;46:1577-86.
46. Liu L, Hu Y, Lu Y, Hu L, Gao C, Nie S. Sex-dependent DNA hypermethylation of SLC6A4 in patients with schizophrenia. Neurosci Lett. 2022;769:136394.
47. Faulk C, Dolinoy DC. Timing is everything: the when and how of environmentally induced changes in the epigenome of animals. Epigenetics. 2011;6:791-7.
48. Beach SR, Dogan MV, Brody GH, Philibert RA. Differential impact of cumulative SES risk on methylation of protein-protein interaction pathways as a function of SLC6A4 genetic variation in African American young adults. Biol Psychol. 2014;96:28-34.
49. Shen L, Lv X, Huang H, et al. Genome-wide analysis of DNA methylation in 106 schizophrenia family trios in Han Chinese. EBioMedicine. 2021;72:103609.
50. Koning SM, Kessler CL, Canli T, et al. Early-life adversity severity, timing, and context type are associated with SLC6A4 methylation in emerging adults: Results from a prospective cohort study. Psychoneuroendocrinology. 2024;170:107181.
51. Favre G, Banta Lavenex P, Lavenex P. Developmental regulation of expression of schizophrenia susceptibility genes in the primate hippocampal formation. Transl Psychiatry. 2012;2:e173.
52. Kim JH, Son YD, Kim JH, et al. Serotonin transporter availability in thalamic subregions in schizophrenia: a study using 7.0-T MRI with [(11)C]DASB high-resolution PET. Psychiatry Res. 2015;231:50-7.
53. Kim JH, Kim JH, Son YD, et al. Altered interregional correlations between serotonin transporter availability and cerebral glucose metabolism in schizophrenia: a high-resolution PET study using [(11)C]DASB and [(18)F]FDG. Schizophr Res. 2017;182:55-65.
54. Kim JH, Son YD, Kim HK, Kim JH. Association between lack of insight and prefrontal serotonin transporter availability in antipsychotic-free patients with schizophrenia: a high-resolution PET study with [(11)C]DASB. Neuropsychiatr Dis Treat. 2021;17:3195-203.
55. Klinedinst NJ, Regenold WT. A mitochondrial bioenergetic basis of depression. J Bioenergy Biomembr. 2015;47:155-71.
56. Nalepa IF, Nielsen V, Wolf TE, et al. Sex differences in the murine HPA axis after acute and repeated restraint stress. Stress. 2025;28:2447079.
57. Depressive disorder (depression). Available from: https://www.who.int/news-room/fact-sheets/detail/depression [Last accessed on 19 Sep 2025].
58. Lolak S, Suwannarat P, Lipsky RH. Chapter Five - Epigenetics of Depression. In: Progress in molecular biology and translational science. Elsevier; 2014. pp. 103-37.
59. Caspi A, Sugden K, Moffitt TE, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386-9.
60. Davidson T, Braudt DB, Keers R, Assary E, Harris KM, Boardman JD. Genome-wide stress sensitivity moderates the stress-depression relationship in a nationally representative sample of adults. Sci Rep. 2021;11:20332.
61. Tsang RS, Mather KA, Sachdev PS, Reppermund S. Systematic review and meta-analysis of genetic studies of late-life depression. Neurosci Biobehav Rev. 2017;75:129-39.
62. Mendonça MS, Mangiavacchi PM, De Sousa PF, et al. Epigenetic variation at the SLC6A4 gene promoter in mother-child pairs with major depressive disorder. J Affect Disord. 2019;245:716-23.
63. Alshogran OY, Al-Eitan LN, Altawalbeh SM, Aman HA. Association of DRD4 exon III and 5-HTTLPR VNTR genetic polymorphisms with psychiatric symptoms in hemodialysis patients. PLoS One. 2021;16:e0249284.
64. Simonyte S, Grabauskyte I, Macijauskiene J, et al. Associations of the serotonin transporter gene polymorphism, 5-HTTLPR, and adverse life events with late life depression in the elderly Lithuanian population. Sci Rep. 2023;13:12920.
65. Ren F, Ma Y, Zhu X, Guo R, Wang J, He L. Pharmacogenetic association of bi- and triallelic polymorphisms of SLC6A4 with antidepressant response in major depressive disorder. J Affect Disord. 2020;273:254-64.
66. Ugartemendia L, Bravo R, Reuter M, et al. SLC6A4 polymorphisms modulate the efficacy of a tryptophan-enriched diet on age-related depression and social cognition. Clin Nutr. 2021;40:1487-94.
67. Stein K, Maruf AA, Müller DJ, Bishop JR, Bousman CA. Serotonin transporter genetic variation and antidepressant response and tolerability: a systematic review and meta-analysis. J Pers Med. 2021;11:1334.
68. Jang YJ, Lim SW, Moon YK, et al. 5-HTTLPR-rs25531 and antidepressant treatment outcomes in korean patients with major depression. Pharmacopsychiatry. 2021;54:269-78.
69. Hasan MA, Hakim FT, Islam Shovon MT, Islam MM, Islam MS, Islam MA. The investigation of nonsynonymous SNPs of human SLC6A4 gene associated with depression: an in silico approach. Heliyon. 2021;7:e07815.
70. Sun M, Brivio P, Shan L, et al. Offspring's own serotonin transporter genotype, independently from the maternal one, increases anxiety- and depression-like behavior and alters neuroplasticity markers in rats. J Affect Disord. 2024;350:89-101.
71. Delli Colli C, Borgi M, Poggini S, et al. Time moderates the interplay between 5-HTTLPR and stress on depression risk: gene x environment interaction as a dynamic process. Transl Psychiatry. 2022;12:274.
72. Garvert L, Kirchner K, Grabe HJ, Van der Auwera S. Genome-wide gene-gene interaction of the 5-HTTLPR promoter polymorphism emphasizes the important role of neuroplasticity in depression. Prog Neuropsychopharmacol Biol Psychiatry. 2022;119:110614.
73. Lin X, Cao Y, Ji L, Zhang W. Inhibitory control mediates the interaction between serotonin transporter gene (5-HTTLPR) and peer victimization on adolescent depressive symptoms. Sci Rep. 2021;11:14640.
74. Kang HJ, Kim JM, Stewart R, et al. Association of SLC6A4 methylation with early adversity, characteristics and outcomes in depression. Prog Neuropsychopharmacol Biol Psychiatry. 2013;44:23-8.
75. Bruzzone SEP, Ozenne B, Fisher PM, et al. DNA methylation of serotonin genes as predictive biomarkers of antidepressant treatment response. Prog Neuropsychopharmacol Biol Psychiatry. 2025;136:111160.
76. Frodl T, Szyf M, Carballedo A, et al. DNA methylation of the serotonin transporter gene (SLC6A4) is associated with brain function involved in processing emotional stimuli. J Psychiatry Neurosci. 2015;40:296-305.
77. Zhu JH, Bo HH, Liu BP, Jia CX. The associations between DNA methylation and depression: A systematic review and meta-analysis. J Affect Disord. 2023;327:439-50.
78. Bruzzone SEP, Ozenne B, Fisher PM, et al. No association between peripheral serotonin-gene-related DNA methylation and brain serotonin neurotransmission in the healthy and depressed state. Clin Epigenetics. 2024;16:71.
79. Wankerl M, Miller R, Kirschbaum C, Hennig J, Stalder T, Alexander N. Effects of genetic and early environmental risk factors for depression on serotonin transporter expression and methylation profiles. Transl Psychiatry. 2014;4:e402.
80. Peng H, Zhu Y, Strachan E, et al. Childhood trauma, DNA methylation of stress-related genes, and depression: findings from two monozygotic twin studies. Psychosom Med. 2018;80:599-608.
81. Comtois-Cabana M, Barr E, Provençal N, Ouellet-Morin I. Association between child maltreatment and depressive symptoms in emerging adulthood: The mediating and moderating roles of DNA methylation. PLoS One. 2023;18:e0280203.
82. Schneider I, Kugel H, Redlich R, et al. Association of serotonin transporter gene AluJb methylation with major depression, amygdala responsiveness, 5-HTTLPR/rs25531 polymorphism, and stress. Neuropsychopharmacology. 2018;43:1308-16.
83. Palma-Gudiel H, Peralta V, Deuschle M, Navarro V, Fañanás L. Epigenetics-by-sex interaction for somatization conferred by methylation at the promoter region of SLC6A4 gene. Prog Neuropsychopharmacol Biol Psychiatry. 2019;89:125-31.
84. Okada S, Morinobu S, Fuchikami M, et al. The potential of SLC6A4 gene methylation analysis for the diagnosis and treatment of major depression. J Psychiatr Res. 2014;53:47-53.
85. Kim JM, Stewart R, Kang HJ, et al. A longitudinal study of SLC6A4 DNA promoter methylation and poststroke depression. J Psychiatr Res. 2013;47:1222-7.
86. Consoloni JL, Ibrahim EC, Lefebvre MN, et al. Serotonin transporter gene expression predicts the worsening of suicidal ideation and suicide attempts along a long-term follow-up of a Major Depressive Episode. Eur Neuropsychopharmacol. 2018;28:401-14.
87. Frodl T, Möller HJ, Meisenzahl E. Neuroimaging genetics: new perspectives in research on major depression? Acta Psychiatr Scand. 2008;118:363-72.
88. Ancelin ML, Carrière I, Artero S, et al. Lifetime major depression and grey-matter volume. J Psychiatry Neurosci. 2019;44:45-53.
89. Swartz JR, Hariri AR, Williamson DE. An epigenetic mechanism links socioeconomic status to changes in depression-related brain function in high-risk adolescents. Mol Psychiatry. 2017;22:209-14.
90. Jaworska N, MacMaster FP, Foster J, Ramasubbu R. The influence of 5-HTTLPR and Val66Met polymorphisms on cortical thickness and volume in limbic and paralimbic regions in depression: a preliminary study. BMC Psychiatry. 2016;16:61.
91. Tatham EL, Ramasubbu R, Gaxiola-Valdez I, et al. White matter integrity in major depressive disorder: implications of childhood trauma, 5-HTTLPR and BDNF polymorphisms. Psychiatry Res Neuroimaging. 2016;253:15-25.
92. Won E, Choi S, Kang J, et al. Association between reduced white matter integrity in the corpus callosum and serotonin transporter gene DNA methylation in medication-naive patients with major depressive disorder. Transl Psychiatry. 2016;6:e866.
93. Kostic M, Canu E, Agosta F, et al. The cumulative effect of genetic polymorphisms on depression and brain structural integrity. Hum Brain Mapp. 2016;37:2173-84.
94. Park S, Lee JM, Kim JW, et al. Associations between serotonin transporter gene (SLC6A4) methylation and clinical characteristics and cortical thickness in children with ADHD. Psychol Med. 2015;45:3009-17.
95. Schiele MA, Kollert L, Lesch KP, et al. Hypermethylation of the serotonin transporter gene promoter in panic disorder-Epigenetic imprint of comorbid depression? Eur Neuropsychopharmacol. 2019;29:1161-7.
96. Cheng P, Zhang J, Wu Y, et al. 5-HTTLPR polymorphism and depression risk in Parkinson's disease: an updated meta-analysis. Acta Neurol Belg. 2021;121:933-40.
97. Calabrò M, Mandelli L, Crisafulli C, et al. Psychiatric disorders and SLC6A4 gene variants: possible effects on alcohol dependence and alzheimer's disease. Mol Biol Rep. 2020;47:191-200.
98. Dukal H, Frank J, Lang M, et al. New-born females show higher stress- and genotype-independent methylation of SLC6A4 than males. Borderline Personal Disord Emot Dysregul. 2015;2:8.
99. Popa N, Bachar D, Roberts AC, Santangelo AM, Gascon E. Region-specific microRNA alterations in marmosets carrying SLC6A4 polymorphisms are associated with anxiety-like behavior. EBioMedicine. 2022;82:104159.
100. Mihaljević-Peleš A, Šagud M, Božina N, Živković M, Jovanović N. Pharmacogenetics and antipsychotics in the light of personalized pharmacotherapy. Psychiatr Danub. 2010;22:335-7.
101. Chen Y, Wang P, Li Z. Exploring genetic and epigenetic markers for predicting or monitoring response to cognitive-behavioral therapy in obsessive-compulsive disorder: a systematic review. Neurosci Biobehav Rev. 2025;174:106192.
102. Webb LM, Phillips KE, Ho MC, Veldic M, Blacker CJ. The relationship between DNA methylation and antidepressant medications: a systematic review. Int J Mol Sci. 2020;21:826.
103. Güzey C, Scordo MG, Spina E, Landsem VM, Spigset O. Antipsychotic-induced extrapyramidal symptoms in patients with schizophrenia: associations with dopamine and serotonin receptor and transporter polymorphisms. Eur J Clin Pharmacol. 2007;63:233-41.
104. Božina N, Jovanović N, Podlesek A, et al. Suicide ideators and attempters with schizophrenia - The role of 5-HTTLPR, rs25531, and 5-HTT VNTR Intron 2 variants. J Psychiatr Res. 2012;46:767-73.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Copyright
Data & Comments
Data

















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].