Lithium: from mood stabilizer to pivotal regulator of health - recent advances in lithium-based biomedicine
Abstract
Lithium, the lightest metallic element, has transcended its role as a classic mood stabilizer, with recent breakthroughs underscoring its profound significance in human health. Clinically, it remains the “gold standard” for bipolar disorder, while new findings on serum lithium isotopic signatures now enable the differentiation of psychiatric conditions, opening diagnostic frontiers. In neurodegeneration, cutting-edge research links lithium deficiency to early Alzheimer’s disease pathogenesis, with amyloid-evasive lithium formulations offering novel preventive strategies. As a regulator of metal homeostasis, lithium stabilizes Mg/Fe metabolism to protect neurons. Immunologically, recent discoveries - from reversing pulmonary fibrosis via myofibroblast necroptosis to revitalizing of CD8+ T cells for tumor immunotherapy - expand its therapeutic scope. Despite its broad therapeutic potential, the adverse effects of lithium have also drawn sustained attention from researchers. Recent animal study has demonstrated that excessive lithium exposure can induce reproductive toxicity, likely through accumulation-triggered cuproptosis in placental trophoblasts. These converging advances elevate lithium from a single-disease drug to a linchpin of elemental biomedicine, urging a broader focus on its role in health maintenance and chronic disease management.
Keywords: lithium, lithium stable isotope, metal homeostasis, neuroprotection, immune regulation, reproduction toxicity
Highlights
1. Lithium isotopic enable psychiatric differentiation and reveal dysregulated Mg metabolism in pathological states.
2. Lithium broadens therapeutic horizons by mitigating Alzheimer’s pathogenesis, reversing pulmonary fibrosis, and augmenting antitumor immunity.
3. Excessive lithium exposure triggers reproductive toxicity by inducing accumulation-dependent cuproptosis in placental trophoblasts.
4. As a multi-level regulator, lithium orchestrates neuro-immune-microbial systems by stabilizing essential trace element homeostasis (Mg, Fe, Cu).
INTRODUCTION
Lithium has gained increasing attention due to its extensive applications in emerging energy industries and the resulting environmental impacts[1].Besides, lithium also played a remarkable role in life science. Since its discovery as an effective mood stabilizer for bipolar disorder (BD), lithium has fascinated scientists across disciplines due to its paradoxical nature - a simple alkali metal capable of modulating complex neurobiological systems. Increasing evidence now indicates that lithium’s biological significance extends well beyond psychiatry. In this perspective, we highlight recent advances in elucidating lithium’s role in mental health, neurodegeneration, and immune regulation. While paving the way for a new frontier in lithium-based elemental biomedicine, we also emphasize the need for careful consideration of lithium’s potential toxicity,particularly in later clinical trials.
PSYCHIATRIC CLINICAL BENCHMARKS AND ISOTOPE DIAGNOSTIC ADVANCES
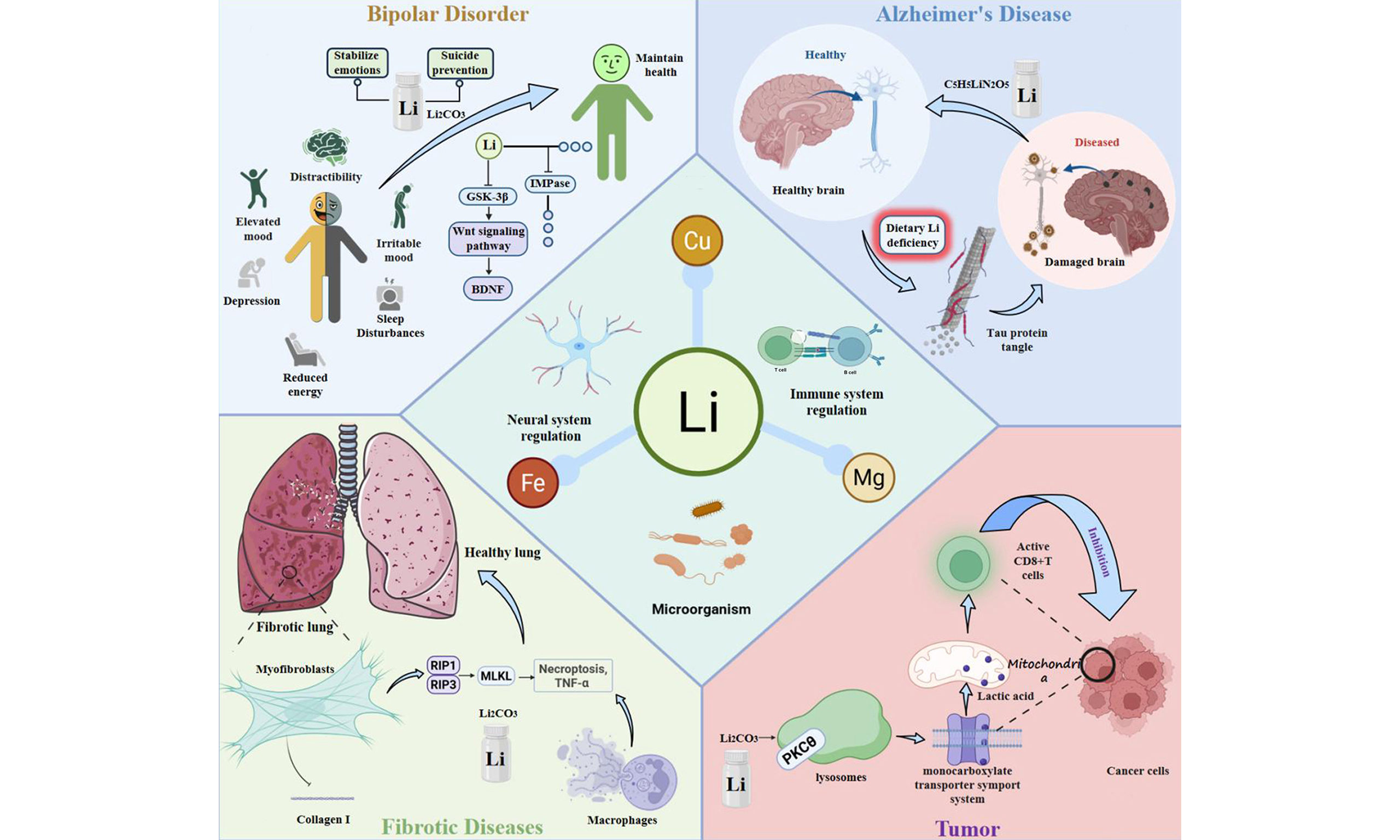
For more than half a century, lithium has served as the gold-standard treatment for BD, demonstrating unmatched efficacy in mood stabilization[2]. Beyond its clinical applications, epidemiological studies have also revealed a potential inverse correlation between environmental lithium levels and the prevalence of psychiatric disorders, highlighting its broader biological relevance[3]. These studies stimulated extensive research into the molecular mechanisms underlying the biological effects of lithium.
At the cellular level, lithium exerts pleiotropic effects by modulating multiple intracellular signaling pathways. It inhibits glycogen synthase kinase-3β (GSK-3β), stabilizes β-catenin in the wingless-related integration site (Wnt) signaling pathway, enhances the expression of brain-derived neurotrophic factor (BDNF), regulates inositol phosphate metabolism, improves mitochondrial function, and reduces reactive oxygen species (ROS). Collectively, these actions promote neuronal survival, synaptic plasticity, and resilience to stress, thereby conferring both neuroprotective and mood-stabilizing effects. Notably, studies on the molecular pathology of psychiatric disorders and pharmacodynamics have revealed that lithium primarily acts through reversible competition with Mg2+ at specific catalytic protein domains, thereby modulating the activity of key enzymes across these signaling pathways[4].
Recent advances in isotopic research have opened a new dimension in understanding the role of lithium. The substantial mass difference between the two stable isotopes of lithium results in notable isotopic fractionation during biological transport and metabolism, leading to measurable variations in isotopic composition (δ7Li) across different physiological states. Remarkably, clinical isotopic analyses have revealed that the serum lithium isotopic compositions (δ7Liserum) of patients with BD and schizophrenia (SZ) after lithium dosing exhibit striking and reproducible distinctions[5]. These isotopic signatures can reliably differentiate between the two psychiatric conditions, suggesting that δ7Liserum may serve as an intrinsic molecular fingerprint of distinct neurochemical states. Complementary cellular studies suggest that the differential isotopic behaviors reflect variations in Li+-Mg2+ competitive interactions, implying that subtle differences in Mg-dependent enzymatic systems may contribute to the distinct pathophysiology. This finding further strengthens the mechanistic link between lithium pharmacodynamics and Mg dysregulation. Notably, the human proteome harbors over 3,000 Mg2+-dependent proteins across a broad spectrum of physiological pathways, indicating that lithium’s pharmacological effects extend far beyond its conventional use in psychiatry.
NEURODEGENERATIVE PATHOGENESIS AND PREVENTIVE STRATEGIES
The claim that lithium can alleviate symptoms of Alzheimer’s disease (AD) has a long history. Recent experimental studies yielded groundbreaking mechanistic insights - amyloid-beta (Aβ) plaques can sequester lithium, reduce its non-plaque bioavailability, and exacerbate tau pathology. This phenomenon was further validated in a mouse model subjected to a lithium-deficient diet[6], which reduced cortical lithium levels by approximately 50%. This model successfully recapitulated human AD-like transcriptomic alterations - the overlapping differentially expressed genes (DEGs) are enriched in key signaling pathways, providing compelling evidence that disrupted “lithium homeostasis” is an early causal driver of AD. Building on this concept, the development of amyloid-evasive lithium orotate (LiO) has emerged as a promising AD therapy, offering a safer and more targeted approach for restoring lithium balance in the brain[6]. Accumulating evidence demonstrates that lithium exerts a protective effect against neuronal damage through several signaling pathways, including glycogen synthase kinase-3 (GSK-3), Wnt, mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK), BDNF, mammalian target of rapamycin (mTOR), and glutamate receptors. Collectively, these findings highlight the potential of lithium as both a therapeutic and preventive agent for neurodegenerative disorders, including AD and Parkinson’s disease.
ROLES IN REGULATING METAL HOMEOSTASIS (Mg, Fe, Cu)
The therapeutic effects of lithium likely arise largely from its competition with Mg. Emerging evidence further indicates that lithium also plays a broader role in regulating the homeostasis of Fe and Cu. Ferroptosis, a regulated form of Fe-dependent cell death, has been implicated in both neurodegeneration and psychiatric disorders. In AD, disrupted iron homeostasis contributes to neuronal injury by promoting lipid peroxidation, reducing glutathione peroxidase 4 (GPX4) activity, and impairing iron transport and storage, collectively exacerbating Aβ aggregation, tau pathology, and neuroinflammation[7]. Lithium may counteract these processes by stabilizing Fe-dependent enzymatic systems, lowering ROS, and supporting proper ferritin-mediated Fe storage. Similarly, BD is associated with ferroptosis-related pathology. Elevated brain and serum Fe interacts with dopamine and glutamate dysregulation to exacerbate oxidative stress and neuronal dysfunction[8]. Fe-dependent dopamine metabolism generates ROS and increases intracellular Fe, amplifying oxidative damage, while glutamate promotes Fe uptake via Divalent Metal Transporter 1 (DMT1) upregulation, creating a positive feedback loop. Lithium exerts neuroprotective effects in BD by modulating oxidative stress, inflammatory signaling [e.g., nuclear factor kappa B (NF-κB)], and immune responses, normalizing soluble interleukin (IL)-2 and IL-6 receptor levels, thereby mitigating ferroptosis-driven neuronal injury.
Beyond Fe, lithium also influences copper homeostasis. Cuproptosis, a newly reported form of Cu-dependent programmed cell death[9], has been linked to lithium-induced pregnancy miscarriage[10]. Mechanistically, lithium upregulates the transcription factor Forkhead Box O1 (FOXO1), which promotes expression of the downstream gene Six-Yransmembrane Epithelial Antigen of The Prostate 4 (STEAP4). The STEAP4 protein functions as a metal reductase that reduces extracellular Cu2+ to Cu+, the latter being transported into cells via SLC31A1 (Solute Carrier Family 31, Member 1), leading to intracellular Cu+ accumulation and subsequent cuproptosis. This process impairs the function of the trophoblast cell line Swan 71 and contributes to miscarriage. These findings have been corroborated in lithium-exposed mouse models - intervention with cuproptosis inhibitors, FOXO1 inhibitors, or STEAP4 inhibitors effectively alleviated lithium-induced cuproptosis and miscarriage phenotypes[11].
IMMUNOTHERAPEUTIC ROLES IN GUT, FIBROSIS AND TUMOR
Lithium also exerts profound immunomodulatory effects across a wide spectrum of immune-related diseases. Over the past decades, studies have consistently demonstrated that lithium salts regulate the activity of lymphocytes and polymorphonuclear leukocytes (PMNs) in both in vitro and in vivo biological models. Lithium also influences T lymphocyte differentiation, modulating T helper 17 (Th17)/regulatory T cell (Treg) ratios, which helps constrain excessive pro-inflammatory responses. Beyond these cellular effects, lithium profoundly modulates the gut–immune–brain axis. It reshapes gut microbial composition and metabolite profiles, increasing short-chain fatty acids (SCFAs) and neurotransmitter precursors, promoting Treg cell activation, and suppressing neurotoxic indole derivatives. Moreover, lithium attenuates hyperactivation of the hypothalamic-pituitary-adrenal (HPA) axis, thereby lowering glucocorticoid release[12]. By mitigating HPA overactivity, lithium indirectly supports a balanced gut microbiome, closing the feedback loop between stress, immunity, and neuroimmune regulation. Notably, Huang et al. identified a key role of lithium in anti-fibrotic immunity: lithium carbonate (Li2CO3) reverses pulmonary fibrosis by inducing myofibroblast necroptosis[12]. Li2CO3 promotes alveolar macrophages to secrete TNF-α and directly binds pro-caspase-8 to block its activity - redirecting cell death to the RIP1/RIP3/MLKL-dependent (RIP1 = receptor-interacting serine/threonine-protein kinase 1; RIP3 = receptor-interacting serine/threonine-protein kinase 3; MLKL = mixed lineage kinase domain-like protein) necroptotic pathway. This finding extends the immunomodulatory role of lithium to fibrotic diseases[13]. Recent evidence has expanded the immunological relevance of lithium to cancer immunometabolism. Lactic acid is known to inhibit CD8+ T cell activation in peripheral blood following anti-CD3/CD28 stimulation. Remarkably, lithium carbonate can counteract this suppression by modulating the “lysosome-lactate-mitochondria metabolic axis,” increasing cytoplasmic Ca2+ levels, and thereby significantly enhancing antitumor immunity[14]. Additionally, lithium can sensitize cancer cells to ferroptosis by downregulating ferritin heavy chain (Fth1) and disrupting iron homeostasis. This pro-ferroptotic effect synergizes with lithium’s capacity to augment CD8+ T cell infiltration, collectively bolstering the immune-mediated suppression of tumor growth[15]. This mechanism has been demonstrated in lung and colon cancer models, where lithium exerts its effects, in part, through the TGF-β-Smad3 (TGF-β = transforming growth factor-beta) signaling pathway. Epidemiological evidence further substantiates these mechanistic insights. Observational studies have reported an inverse association between lithium levels in drinking water and the incidence of cancer[16]. Individuals receiving Li2CO3 treatment exhibited a more favorable gut microbiome, characterized by increased Akkermansia muciniphila[12]. Collectively, these findings underscore the role of lithium as a multifaceted immunomodulatory agent, exhibiting a promising candidate for the prevention and treatment of immune-related and neuroinflammatory disorders.
TOXIC EFFECTS AND MOLECULAR MECHANISMS ON FEMALE REPRODUCTION
The toxicity of lithium has long been recognized, necessitating strict dosage control in clinical applications. For BD patients, serum lithium concentrations must be meticulously maintained within a narrow therapeutic window, typically ranging from 0.4 to 1.0 mmol L−1, to balance therapeutic efficacy with systemic safety. Even within this range, lithium may cause various side effects, and excessive intake can damage organs such as kidneys. Recent mouse models demonstrated that exposure to lithium at 5.6-fold the environmental dose is linked to structural and functional abnormalities in the ovaries and placenta. When exposure reaches 28 times the environmental level, it significantly increases the rate of miscarriage and leads to fetal developmental restriction. A systematic investigation by Zhang et al. characterized the dynamic distribution of lithium, revealing its preferential enrichment in reproductive organs, especially in the ovaries and uterus. This accumulation interferes with key hormone levels and affects the expression of genes related to placental development [such as Pgr (progesterone receptor), Ptch2 (patched homolog 2), and Nr2f2 (Nuclear Receptor Subfamily 2, Group F, Member 2)][9].
Collectively, these experimental studies demonstrate that while lithium research continues to pave the way for new frontiers in lithium biomedicine, particular attention must be paid to its potential toxicity. Crucially, recent advances suggest several potential strategies to mitigate lithium-induced toxicity. For example, alternative salts such as LiO can promote neuro-targeted uptake, minimizing systemic exposure. Clinically, while lithium avoidance during pregnancy remains ideal, intercepting the STEAP4-mediated cuproptosis pathway via inhibitors offer a promising means to counteract lithium toxicity when treatment is unavoidable. Furthermore, precision biomarkers, such as lithium isotopes, facilitate high-fidelity monitoring and proactive dose optimization, effectively preempting the progression from subclinical alterations to overt adverse outcomes.
KEY TRANSDISCIPLINARY QUESTIONS SHAPING GLOBAL LITHIUM RESEARCH
The relationship between lithium and human health has long been characterized by fascination, misconception, and repeated rediscovery. The 19th-century “lithium craze”, driven by the uric acid hypothesis, gradually waned under scientific scrutiny, followed by nearly half a century of relative obscurity. It was not until John Cade’s pioneering studies led to the U.S. Food and Drug Administration (FDA) approval of lithium for BD that this simple element regained scientific and clinical credibility. Subsequent advancements in molecular biology have systematically unveiled lithium’s extensive biological significance reaching far beyond its traditional psychiatric applications.
As lithium’s value spans human health, chemistry, materials science, environment, and pharmacology, addressing the following critical questions will define the future trajectory of global lithium research:
(1) Does lithium deficiency-driven pathogenesis (e.g., Alzheimer’s) validate its status as an essential human trace element?
(2) Can δ7Li serve as a universal diagnostic for cross-category diseases?
(3) What are the interaction effects and intrinsic mechanisms of lithium with other metal ions?
(4) What molecular drivers underpin lithium’s synergism with biological carriers (e.g., orotate)?
(5) How can a precise balance between lithium’s therapeutic efficacy and toxicity be achieved, and can advanced pharmaceutical materials optimize delivery to broaden its narrow therapeutic window?
(6) How do gut microbiota mediate lithium’s systemic regulatory functions in vivo?
(7) How can analytical frameworks linking lithium’s dynamics to phenotypes be developed and standardized?
(8) What are the cumulative effects of lithium on the female reproductive system and its impacts on gestational toxicity?
In summary, lithium has evolved from a classic psychiatric drug to a multifaceted agent with key biological functions. By competitively modulating the metabolism of essential metal ions such as Mg2+, Fe2+ and Cu2+, it influences critical processes including neuronal survival, ferroptosis, cuproptosis, the immune microenvironment, reproductive toxicity, and the gut microbiome [Figure 1]. Its therapeutic potential now extends to early intervention in major chronic diseases such as AD. These diverse functions suggest that lithium is not merely a therapeutic agent but may, in fact, constitute a potentially essential trace element for maintaining physiological balance - a hypothesis that warrants further clinical investigations to conclusively validate its biological necessity.
Figure 1. Schematic illustration of the lithium’s role in biological systems, highlighting its involvement in brain function, mood regulation, nerve activity, energy metabolism, anti-fibrotic therapy, microbial regulation, and coordinated control of essential metal ions (Mg, Fe, and Cu). (Generated by OpenAI DALL-E 3 and post-edited by the authors for technical accuracy and labeling).
Progress toward fully elucidating lithium’s mechanisms - and translating this knowledge into precision medicine - depends critically on two converging fronts: deeper interdisciplinary integration and advanced analytical innovation. Only by combining insights from analytical chemistry, molecular biology, clinical medicine, and computational science can we systematically decode lithium’s dynamic behavior across cellular, tissue, and organismal scales. Crucially, a deeper understanding of its physiological significance has become achievable through cutting-edge metallomics technologies. The integrated application of stable isotope tracing, high-resolution elemental mapping, and single-cell analysis is revitalizing lithium research, enabling scientists to directly correlate its spatial distribution and chemical speciation with pharmacological activity and pathological states. Together, interdisciplinary collaboration, coupled with these sophisticated tools, will unlock lithium’s full biological value.
DECLARATIONS
Acknowledgments
The Graphical Abstract Created in BioRender. Zhang, P. (2026) https://BioRender.com/9jgax73.
We acknowledge the foundational and seminal publications in lithium biology, and we thank the authors of recent exceptional studies in this field. We apologize that space constraints prevent citing all of these impactful works, and we thank the scientific community for advancing lithium research and elemental biomedicine.
Authors’ contributions
Writing - original draft: Dong, J.; Yue, W.; Xing, S.; Huang, B.; Min, J.
Writing - review and editing: Dong, J.; Wang, F.; Zhu, Z.
Visualization: Dong, J.; Yue, W.; Liu, Y.
Conceptualization: Wang, F.; Zhu, Z.
Supervision: Zhu, Z.
Funding acquisition: Zhu, Z.
Dong, J., Yue, W., and Xing, S. contributed equally to the article.
Availability of data and materials
Not applicable.
AI and AI-assisted tools statement
Declaration on the use of AI: The authors used OpenAI’s DALL-E 3 to generate conceptual visualization in Figure 1. The authors have reviewed and edited the content as needed and took full responsibility for the scientific accuracy and integrity of the figure in this publication.
Financial support and sponsorship
This work is supported by the Natural Science Foundation of Hubei Province (2025AFA048) and the National Natural Science Foundation of China (22274145).
Conflicts of interest
Wang, F. is the Editor-in-Chief of the journal Element. Min, J. and Zhu, Z. are the members of the Editorial Board of the journal Element. Wang, F., Min, J. and Zhu, Z. were not involved in any steps of the editorial process, including reviewer selection, manuscript handling, or decision making. All other authors declare no competing interests.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2026.
REFERENCES
1. Yang, X. et al. Emerging research needs for characterizing the risks of global lithium pollution under carbon neutrality strategies. Environ. Sci. Technol. 57, 5103-6 (2023).
3. Liew, Z. et al. Association between estimated geocoded residential maternal exposure to lithium in drinking water and risk for autism spectrum disorder in offspring in Denmark. JAMA Pediatr. 177, 617-24 (2023).
4. Dudev, T.; Mazmanian, K.; Weng, W. H.; Grauffel, C.; Lim, C. Free and bound therapeutic lithium in brain signaling. Acc. Chem. Res. 52, 2960-70 (2019).
5. Dong, J. et al. Natural lithium isotope variations in serum after lithium administration as a novel biomarker for differentiating schizophrenia and bipolar disorder. Transl. Psychiatry 15, 386 (2025).
6. Aron, L. et al. Lithium deficiency and the onset of Alzheimer’s disease. Nature 645, 712-21 (2025).
7. Wang, Y. et al. Lithium chloride inhibits iron dysregulation and ferroptosis in induced pluripotent stem cells with ApoE4/E4 from a sporadic Alzheimer’s disease patient. [Preprint]. bioRxiv 2025.08.28.672956 (2025).
8. Yehia, A.; Melhuish Beaupre, L. M.; Ho, M. C.; Biernnacka, J. M.; Frye, M. A.; Abulseoud, O. A. Ferroptosis as a potential molecular mechanism of bipolar disorder. Transl. Psychiatry 15, 205 (2025).
9. Fan, Q. et al. Organ-specific lithium accumulation and its toxic effects on female reproduction. J. Hazard. Mater. 494, 138516 (2025).
10. Chen, L.; Min, J.; Wang, F. Copper homeostasis and cuproptosis in health and disease. Signal Transduct. Target. Ther. 7, 378 (2022).
11. Xing, S. et al. Lithium exposure causes trophoblast cuproptosis by upregulating FOXO1/STEAP4 axis in unexplained miscarriage. Adv. Sci. 12, e02139 (2025).
12. Huang, S. et al. Lithium carbonate alleviates colon inflammation through modulating gut microbiota and Treg cells in a GPR43-dependent manner. Pharmacol. Res. 175, 105992 (2022).
13. Wei, K. et al. Lithium carbonate induces myofibroblast necroptosis to reverse pulmonary fibrosis. Immun. Inflamm. 1, 3 (2025).
14. Ma, J. et al. Lithium carbonate revitalizes tumor-reactive CD8+ T cells by shunting lactic acid into mitochondria. Nat. Immunol. 25, 552-61 (2024).
15. Zhu, B. et al. Lithium enhances ferroptosis sensitivity in melanoma cells and promotes CD8+ T cell infiltration and differentiation. Free Radical Biol. Med. 227, 233-45 (2025).
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
Article Notes
About This Article
Copyright


Data & Comments
Data












Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].