Advancing sustainability: Green chemistry applications of heterocyclic compounds for waste remediation
Abstract
This review examines eco-friendly strategies for the synthesis of heterocyclic compounds through the application of green chemistry principles. Traditional synthetic routes often rely on hazardous reagents, toxic solvents, and energy-intensive processes, leading to environmental and safety concerns. In contrast, green approaches emphasize mild reaction conditions, non-toxic catalysts, renewable feedstocks, and energy-efficient techniques to reduce waste and enhance sustainability. Key methods discussed include catalysis, solvent-free and green solvent systems, biocatalysis, and the use of biomass-derived starting materials. The review also highlights applications of green heterocyclic synthesis in pharmaceuticals, materials science, and environmental remediation. By integrating these sustainable strategies, heterocyclic chemistry can advance toward safer, more efficient, and environmentally responsible production, providing a roadmap for future research and industrial practice.
Keywords
INTRODUCTION
Sustainability has become a key focus in modern chemistry, guiding efforts toward eco-friendly methods and practices[1]. Among chemical synthesis areas, heterocyclic compounds are particularly important due to their roles in pharmaceuticals, agrochemicals, and materials science[2]. Developing sustainable approaches for heterocyclic synthesis has driven interest in applying green chemistry principles, which aim to reduce environmental impact and improve resource efficiency[3,4]. Green chemistry strategies in heterocyclic synthesis include maximizing atom economy, using renewable feedstocks, minimizing hazardous substances, and designing benign reaction conditions. Environmentally friendly solvents, such as water, ionic liquids (ILs), supercritical fluids, or other green media, can replace traditional organic solvents, reducing toxicity and waste[5]. Moreover, catalysis has emerged as a cornerstone in the effort to achieve sustainable heterocyclic synthesis[6]. Catalytic processes, ranging from organocatalysis to heterogeneous catalysis and enzymatic transformations, hold promise for enhancing reaction efficiency and selectivity, reducing energy consumption, and mitigating the generation of harmful byproducts[7]. Incorporating alternative energy sources, such as microwave irradiation and ultrasound, represents another dimension of sustainable approaches in heterocyclic synthesis[8]. These energy-efficient methods show notable promise in accelerating reactions, reducing byproducts, and improving overall yields, while decreasing the environmental impact of chemical processes[5].
Additionally, the exploration of renewable feedstocks as viable starting materials in heterocyclic synthesis represents a crucial aspect of sustainability. Harnessing biomass-derived or other renewable resources as precursors for heterocyclic compounds not only mitigates reliance on finite petrochemical resources but also contributes to the development of a circular economy model[9].
The drive to reduce waste and achieve high atom economy in heterocyclic synthesis underscores a commitment to sustainable practices[4]. Green chemistry principles encourage the design of synthetic routes that incorporate most of the starting materials into the final product, reducing waste and supporting efficient, sustainable synthesis[10]. In this pursuit, bioinspired approaches have also garnered attention, drawing on nature’s efficient processes to devise sustainable synthetic routes to heterocyclic compounds[11].
Advancing sustainable chemical synthesis in this field requires collaborative research, creative approaches, and input from multiple disciplines to meet the increasing demand for diverse and functional heterocyclic compounds[12]. In the following sections, this analysis further explores approaches, case studies, challenges, and future developments in sustainable heterocyclic synthesis[13]. This review explores various strategies, highlights successful examples, and assesses their environmental impact to identify more sustainable and eco-friendly approaches for synthesizing heterocyclic compounds. Sustainable heterocyclic synthesis benefits from contributions across multiple scientific disciplines, each offering unique perspectives and technologies to advance greener methods. Green chemistry provides the foundation for developing environmentally friendly processes, focusing on principles such as atom economy, the use of renewable feedstocks, and minimizing hazardous substances. Catalysis, a critical subfield of chemistry, provides more efficient reaction pathways, reducing the need for excess reagents and energy input. Materials science contributes through the design of novel, reusable catalysts and environmentally benign solvents, such as ILs or supercritical fluids, which minimize waste and toxicity[8]. Biotechnology offers significant promise through the use of biocatalysts (enzymes or whole cells) that enable selective, mild reactions, thereby reducing reliance on toxic reagents or extreme reaction conditions. Engineering plays a vital role in optimising reaction conditions through techniques such as flow chemistry and microwave-assisted synthesis, enhancing reaction efficiency while reducing energy consumption[2]. Computational chemistry and machine learning help make synthesis more sustainable by predicting and optimizing reaction pathways, reducing experimental waste and the need for trial-and-error approaches. Moreover, environmental science complements these efforts by evaluating the life-cycle impacts of heterocyclic compounds, ensuring that synthetic routes are both efficient and environmentally responsible. Collaboration across these disciplines can speed up the development of scalable, sustainable methods, tackling both the technical and environmental challenges in heterocyclic synthesis.
EXAMPLES OF GREEN CHEMISTRY STRATEGIES IN HETEROCYCLIC SYNTHESIS
Microwave-assisted synthesis
The synthesis of quinolines (a class of nitrogen-containing heterocycles) via microwave-assisted methods is a notable example. Traditionally, quinoline synthesis involves harsh reagents and high temperatures, leading to long reaction times and high energy consumption. By employing microwave irradiation, reaction times can be drastically reduced (often from several hours to a few minutes), resulting in higher yields and reduced energy consumption. This method increases reaction rates, enhances selectivity, and requires less solvent, thereby reducing environmental impact.
Biocatalysis
Enzymatic methods for the synthesis of pyrroles, which are essential building blocks in pharmaceuticals, can be carried out under mild conditions. Enzyme-catalysed processes offer high selectivity and efficiency without requiring toxic solvents or reagents. One example is the enzymatic reduction of nitrobenzene derivatives to form pyrroles in an aqueous medium, thereby avoiding the use of harmful solvents such as DMF (dimethylformamide). The use of biocatalysts ensures green processes by operating at ambient temperatures and mild pH, often using water as a non-toxic, environmentally benign solvent.
Green solvents
In the synthesis of imidazoles, a common heterocyclic scaffold in pharmaceuticals, traditional solvents such as dichloromethane or toluene are often used. However, ILs, which are liquid salts at room temperature, have been proposed as greener alternatives. ILs provide a stable and non-volatile reaction medium, thereby preventing the release of harmful volatile organic compounds (VOCs) into the atmosphere. They are reusable, non-flammable, and can dissolve a wide range of organic and inorganic compounds, enhancing the sustainability of the process.
Solvent-free synthesis
The Bohlmann-Rahtz cyclization, used to form indoles (another important heterocyclic structure), is commonly performed under solvent-free conditions. Such solvent-free synthesis reduces solvent waste and improves reaction efficiency.
Flow chemistry
Continuous flow reactors have been successfully used for heteroaryl coupling reactions, such as Suzuki-Miyaura cross-couplings, to synthesize various heterocyclic compounds, including benzothiophenes. In a continuous flow system, reagents pass through a reaction zone, where they are rapidly mixed and heated, leading to faster reactions, reduced waste, and improved control over reaction conditions. Flow chemistry offers enhanced safety, better heat and mass transfer, and greater scalability compared with batch processes.
GREEN SYNTHESIS STRATEGIES IN HETEROCYCLIC COMPOUNDS
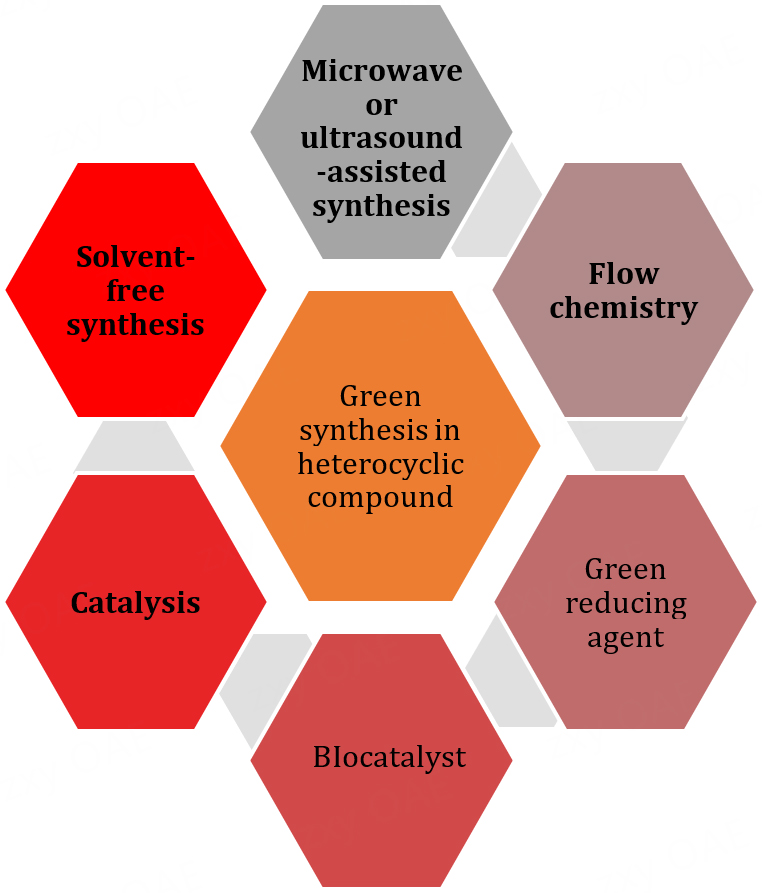
Green synthesis of heterocyclic compounds involves the design and development of environmentally friendly methods to produce these compounds[14]. Heterocyclic compounds are organic compounds containing atoms other than carbon in a cyclic structure, such as nitrogen, oxygen, and sulfur. Green synthesis aims to minimize or eliminate the use of hazardous reagents, solvents, and energy-intensive processes[15]. Some strategies used in the green synthesis of heterocyclic compounds are shown in Figure 1:
1. Catalysis: Catalysts reduce the amount of reagents needed, speed up reactions, and often increase selectivity. Catalysts such as metal complexes, organocatalysts, or biocatalysts are used in green synthesis methods.
2. Microwave- or Ultrasound-Assisted Synthesis: These techniques utilize microwave or ultrasound energy to facilitate reactions, reducing reaction times and energy consumption, thereby making the synthesis process more sustainable.
3. Solvent-Free Synthesis: Using solvent-free or minimal-solvent conditions reduces environmental impact by minimizing solvent waste and disposal. Techniques such as ball milling or solid-phase synthesis fall under this category.
4. Bio-Catalysis: Utilizing enzymes or whole-cell biocatalysts enables selective synthesis routes and reduces the need for harsh chemicals.
5. Green Reducing Agents: Employing green reducing agents such as vitamin C, sodium borohydride, or ascorbic acid instead of toxic or hazardous agents such as tin-, lead-, or mercury-based reagents.
6. Flow Chemistry: Continuous flow systems provide precise control of reactions, reduce waste, and improve safety.
These approaches are designed to make the production of heterocyclic compounds more sustainable, cost-effective, and eco-friendly by minimizing waste, reducing energy use, and curtailing the use of hazardous substances[16]. It is critical to recognize that selecting a green synthesis approach depends on multiple factors, including the specific heterocyclic compound desired, the required reaction conditions, scalability, and economic viability. Scientists are continually investigating and developing new techniques to improve the eco-sustainability of heterocyclic compound synthesis[17].
MECHANISM OF SYNTHESIS
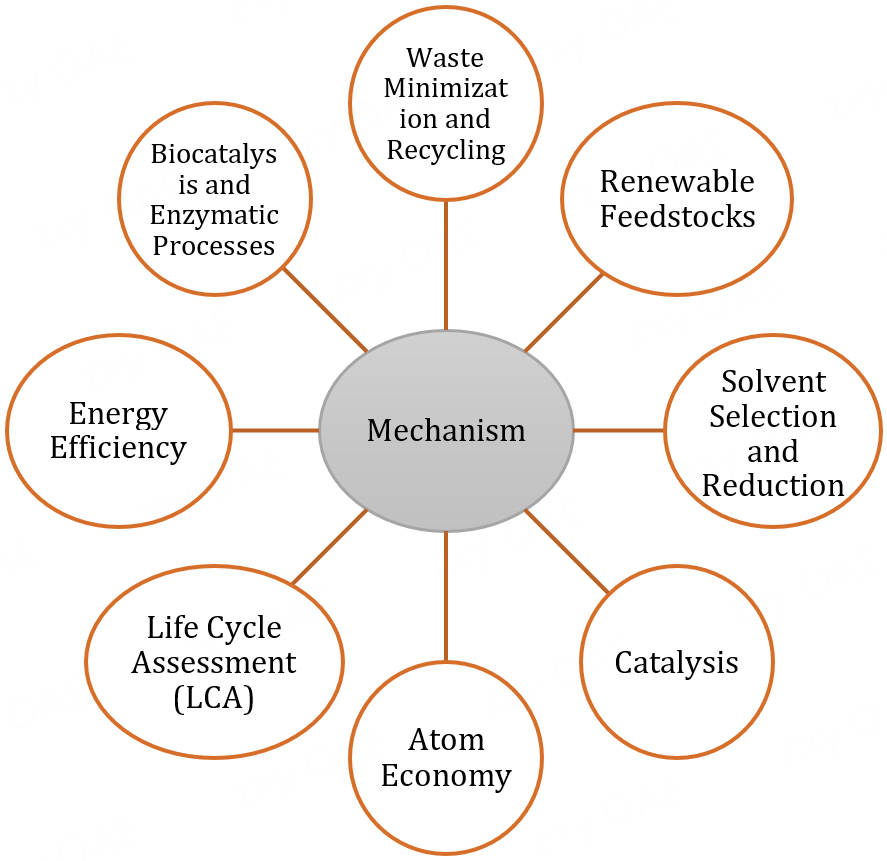
The advancement of sustainability through green chemistry in the synthesis of heterocyclic compounds involves several key mechanisms and principles [Figure 2]:
1. Atom Economy: Green chemistry emphasizes maximizing the incorporation of all starting materials into the final product. Methods with high atom economy reduce waste by utilizing the majority of atoms from reactants in the desired product, thereby promoting sustainability[18].
2. Catalysis: Implementation of catalytic processes is a fundamental strategy in green chemistry. Catalysts accelerate reactions, enhance selectivity, and enable milder reaction conditions, reducing energy consumption and the need for harsh reagents.
3. Solvent Selection and Reduction: Green chemistry advocates using safer, less hazardous solvents or, ideally, solvent-free conditions. Minimizing or eliminating traditional organic solvents decreases environmental impact and simplifies product isolation and purification.
4. Renewable Feedstocks: Utilizing renewable feedstocks or biomass-derived raw materials in heterocyclic compound synthesis reduces dependence on fossil-based resources and contributes to sustainability.
5. Energy Efficiency: Employing energy-efficient methods, such as microwave- or ultrasound-assisted reactions, reduces reaction times, energy consumption, and overall environmental footprint.
6. Bio-catalysis and Enzymatic Processes: Incorporating biocatalysts or enzymes in synthetic routes for heterocyclic compounds enables highly selective reactions under mild conditions, reducing reliance on harsh chemicals and promoting sustainability.
7. Waste Minimization and Recycling: Green chemistry aims to minimize waste generation during synthesis. Methods involving recyclable catalysts, reagents, or processes that produce minimal byproducts contribute to sustainability.
8. Life Cycle Assessment (LCA): Considering the environmental impact of a compound or process throughout its life cycle helps identify opportunities to improve sustainability, guiding the development of more eco-friendly synthetic routes for heterocyclic compounds.
By integrating these mechanisms and principles into the synthesis of heterocyclic compounds, green chemistry aims to advance sustainability by reducing the environmental footprint, minimizing waste, and promoting more efficient and environmentally friendly synthetic methodologies[19].
REPRESENTATIVE GREEN SYNTHETIC REACTIONS FOR HETEROCYCLIC COMPOUNDS
1. Cu(I)-Catalyzed Azide-Alkyne Cycloaddition
R–C=CH +R’N3
This reaction forms a 1,4-disubstituted 1,2,3-triazole with excellent atom economy under aqueous, mild conditions.
2. Mn(I)-Catalyzed Acceptorless Dehydrogenative Coupling
o-Phenylenediamine + HO–CH2–CH2–OH
This reaction produces quinoxaline while releasing hydrogen gas as the only byproduct, making it a green and atom-efficient method.
APPLICATIONS
Catalysis and reaction efficiency
The Backbone of Sustainable Synthesis. One of the most compelling aspects of advancing sustainability in heterocyclic synthesis is the role of catalysis. Traditional heterocyclic synthesis often relied on stoichiometric amounts of reagents, leading to significant waste and inefficient use of materials. The adoption of catalytic processes has transformed this approach. Catalysts - whether metal-based, organocatalysts, or biocatalysts - enable reactions that are faster, more selective, and typically require fewer resources, thereby improving atom economy.
In particular, biocatalysis offers a remarkable avenue for environmentally friendly synthesis. Enzymatic processes not only perform selective transformations under mild conditions but also operate without toxic reagents or harsh solvents, aligning closely with the principles of green chemistry. The incorporation of biocatalysts into synthetic strategies represents one of the most exciting developments in heterocyclic chemistry, potentially offering greener alternatives to conventional chemical processes.
Solvent-free and green solvents
The evolution of reaction media solvents has long been a source of environmental concern in chemical synthesis, often contributing to toxic waste and requiring energy-intensive recovery processes. The quest for sustainable solvent-free or green solvent alternatives has gained considerable momentum. Methods such as microwave-assisted and ultrasound-assisted synthesis have emerged as key approaches that minimize solvent use, reduce reaction time, and promote energy efficiency.
However, the push toward solvent-free reactions is not without challenges. For many heterocyclic compounds, traditional solvents are necessary to dissolve reactants and ensure uniform mixing. In such cases, the use of green solvents, such as water, ILs, or supercritical CO2, offers a practical solution[8]. These solvents are often safer, recyclable, and exhibit lower toxicity, making them attractive alternatives to conventional organic solvents, such as dichloromethane or acetone.
Renewable feedstocks and the circular economy
The use of renewable feedstocks represents another significant shift in heterocyclic synthesis, aligning with the principles of the circular economy. By moving away from petrochemical-based raw materials, researchers can utilize biomass-derived or waste-based resources, thereby reducing dependency on finite resources and curbing environmental pollution.
Energy efficiency: reducing the carbon footprint
An often-overlooked but essential aspect of sustainable heterocyclic synthesis is energy efficiency. Traditional synthetic routes can be energy-intensive, requiring high temperatures or pressures, which contribute significantly to environmental degradation. Microwave-assisted synthesis and continuous flow systems represent innovative approaches that reduce reaction times and energy requirements. These technologies not only improve reaction efficiency but also enhance sustainability by lowering the energy consumption associated with heterocyclic compound production[22].
By integrating such energy-efficient techniques, the carbon footprint of chemical manufacturing can be reduced, enabling the scale-up of production while adhering to sustainability standards.
Addressing waste and promoting atom economy
A crucial principle of green chemistry is atom economy, which encourages the development of synthetic routes that maximize the incorporation of starting materials into the final product. In heterocyclic synthesis, this principle is particularly important, as traditional routes often generate substantial waste in the form of byproducts, solvents, and unreacted materials. Approaches emphasizing high atom economy - such as one-pot or cascade reactions - can minimize waste production and improve overall sustainability[21].
Moreover, reducing byproducts is closely linked to waste minimization. Green chemistry promotes the adoption of methods that either avoid waste entirely or recycle it into the process. This is a critical step in fostering more sustainable and circular practices in industrial heterocyclic synthesis.
Regulatory and economic viability
Ensuring the widespread adoption of green chemistry requires prioritizing economic viability and regulatory compliance. While the scientific community may recognize the value of sustainable practices, industries must consider the cost-effectiveness of implementing such methods at scale. This includes factors such as the cost of green solvents, the availability of renewable feedstocks, and the economic scalability of energy-efficient technologies[23].
Simultaneously, regulatory support is vital to drive the adoption of green synthesis methods. Governments and environmental agencies are increasingly mandating lower carbon emissions and reduced chemical waste in industrial processes. Regulatory frameworks and incentives that encourage the application of green chemistry principles will help make these methods more commercially viable, accelerating their integration into mainstream heterocyclic compound production[24].
TYPES OF HETEROCYCLIC COMPOUNDS
A variety of heterocyclic compounds containing heteroatoms such as N, S, and O are reported in the literature. These compounds have applications in waste treatment, green synthesis, and other environmentally beneficial processes, though certain limitations exist, as summarized in Tables 1 and 2.
Different heterocyclic compounds and their applications
| Compound name | Example compounds | Use in waste treatment | Green synthesis method | Environmental benefits |
| Pyridine | Pyridine, picoline | Metal ion chelation, dye degradation | Biomass-based synthesis, water as solvent | Less toxic, biodegradable |
| Imidazole | Imidazole, benzimidazole | Catalyst in wastewater treatment | Solvent-free & microwave-assisted synthesis | Energy saving, less waste |
| Furan | Furfural, furfuryl alcohol | Dye adsorption & degradation | Renewable feedstock (biomass) | Eco-friendly, renewable source |
| Thiophene | Thiophene derivatives | Photocatalytic degradation of dyes | Ultrasound-assisted synthesis | Low energy & high efficiency |
| Indole | Indole, tryptophan derivatives | Degradation of pharmaceutical & dye pollutants | Catalytic reactions with green solvents | Biodegradable, low-harmful by-products |
Challenges and limitations in applying heterocyclic compounds for green waste remediation
| Aspect | Advantages | Challenges/limitations |
| Heterocyclic adsorbents/catalysts | High selectivity, tunable functional groups for metal/dye binding | Catalyst fouling reduced reusability after the cycle |
| Green solvents & media | Water-based or solvent-free processes reduce toxicity | Limited compatibility with all heterocycles or reactions |
| Bio-based or mild catalysis | Eco-friendly, regioselective, lower energy requirement | Slow reaction rates, sensitivity to environmental conditions |
| Photocatalytic degradation | Uses UV/solar light for efficient degradation | Needs specific materials and light sources; scalability issue |
| Scalability & resource use | Potential for sustainable large-scale application | High cost of some catalysts; industrial scaling is difficult |
| Pollutant specificity | Effective for many dyes and heavy metals | Limited affinity for some emerging contaminants |
| Process efficiency | Can integrate adsorption + degradation | Byproduct formation, difficulty in achieving high selectivity |
FUTURE SCOPE
Future research should explore sustainable synthetic routes via organocatalysis, biocatalysis, and photocatalysis for compounds such as pyridine, imidazole, and furan, while addressing challenges such as high cost, limited scalability, and catalyst recovery. The use of renewable feedstocks, such as biomass-derived furfural for furan synthesis, should be explored, along with low-cost purification and conversion technologies to enable practical large-scale production[25]. Research on green solvents, such as ILs and supercritical CO2, can also be included, highlighting existing challenges in their recycling and potential toxicity. Combining computational approaches with experimental validation can help optimize reaction pathways and design efficient synthetic methods. Regarding applications, emphasis should be placed on heterocyclic catalysts for dye degradation and metal ion removal, while improving their stability and reusability under real wastewater conditions. Finally, future work should encourage the adoption of circular economy principles and stronger collaboration among researchers, industry, and policymakers to make these approaches feasible at the industrial level[26].
CONCLUSION
Green chemistry aims to reduce or eliminate hazardous substances in chemical processes and products[27]. Applying these principles to heterocyclic compounds - organic molecules with carbon rings containing at least one heteroatom such as nitrogen, oxygen, or sulfur - can improve sustainability. Atom economy is important, as it maximizes the use of starting materials and minimizes waste[20]. Choosing solvent alternatives, such as water or bio-based solvents, reduces toxicity and improves process safety[28]. Using renewable feedstocks further decreases reliance on fossil resources and supports circular chemical practices. Energy-efficient methods, including reactions under mild conditions and lower temperatures, help reduce the carbon footprint. Careful attention to toxicity, biodegradability, and environmentally safe degradation ensures that compounds break down without causing long-term pollution. Process safety remains essential, particularly for large-scale production.
Future research should explore catalytic systems that improve reaction selectivity and efficiency while using less energy. Renewable raw materials, bio-derived catalysts, and environmentally friendly reagents should be prioritized. Scalable synthetic routes are needed to translate laboratory methods to industrial production without losing environmental benefits. Evaluating each process with life-cycle assessments can prevent unintended impacts at different stages of synthesis. By integrating these strategies, heterocyclic synthesis can become more efficient, sustainable, and suitable for large-scale chemical manufacturing[29].
DECLARATIONS
Acknowledgment
The authors would like to thank K. R. Mangalam University, Gurugram, India for providing necessary support.
Author’s contributions
Conceptualization, data curation, investigation, methodology, visualization, writing - original draft: Sharma, B.
Conceptualization, formal analysis, methodology, writing - review and editing: Kumari, S.
Visualization, writing - review and editing, project administration, resources: Mohan, C.
Resources, review: Zaidi, N.
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2026.
REFERENCES
1. Singh R, Sangwan S, Gulati S. Green methods for synthesis of various Heterocycles: sustainable approach. Int. J. Chem. Stud. 2017, 5, 479-85. https://www.researchgate.net/publication/337592277 (accessed 2025-12-24).
2. Sharma, S.; Gangal, S.; Rauf, A. Green chemistry approach to the sustainable advancement of the synthesis of heterocyclic chemistry. Rasayan J. Chem. 2008, 1, 693-717. https://www.rasayanjournal.co.in/vol-1/issue-4/1.pdf (accessed 2025-12-24).
3. Majee, S.; Shilpa,
4. Zeleke, D.; Damena, T. Advance in green synthesis of pharmacological important heterocycles using multicomponent reactions and magnetic nanocatalysts (MNCs). Results Chem. 2024, 7, 101283.
5. D’Souza, A.; D’Souza, A. S.; Mendonca, A. P.; et al. A review on green multicomponent synthesis of heterocyclic compounds. Int. J. Health Sci. 2022, 6, 4601-23.
6. Daştan, A.; Kulkarni, A.; Török, B. Environmentally benign synthesis of heterocyclic compounds by combined microwave-assisted heterogeneous catalytic approaches. Green Chem. 2012, 14, 17-37.
7. Asif, M.; Imran, M. A chapter on synthesis of various heterocyclic compounds by environmentally friendly green chemistry technologies. In Handbook of Greener Synthesis of Nanomaterials and Compounds; Synthesis at the Macroscale and Nanoscale, Vol. 2; Elsevier, 2021; pp 69-108.
8. Parvez, S.; Kaur, P.; Sharma, R.; et al. Green chemistry: recent advancements and future perspectives. Eur. Chem. Bull. 2023, 12, 14188-97.
9. Jiang, A.; Li, J.; Shah, K. J.; You, Z. Implementing green chemistry principles for pollution control to achieve environmental sustainability - a review. In Advances in Green Chemistry; Green Chemistry for Environmental Sustainability - Prevention-Assurance-Sustainability (P-A-S) Approach; IntechOpen, 2023.
10. Robinson, J.; Kumari, N.; Srivastava, V.; Taskaeva, N.; Mohan, C. Sustainable and environmental friendly energy materials. Mater. Today Proc. 2022, 69, 494-8.
12. Feng, L.; Zheng, S.; Li, J.; et al. Highly efficient eco-friendly drug inhibitors for Q235 steel in 1 M HCl: experimental & theoretical study. Arab. J. Chem. 2024, 17, 105535.
13. Kumar, R.; Garima, K.; Srivastava, V.; Singh, P. P.; Singh, P. K. Visible light mediated eosin-Y catalysed synthesis of benzylidene-methylisoxazolone. Tetrahedron Lett. 2023, 133, 154841.
14. Quintero, W. J.; Ávila, M. C.; Ochoa-puentes, C. Essential oils and eutectic solvents in the povarov reaction for the synthesis of tetrahydroquinolines: a lesson of cycloadditions, stereochemistry, and green chemistry. J. Chem. Educ. 2023, 100, 4763-71.
15. Deshmukh, A. G.; Rathod, H. B.; Patel, P. N. Fungus reinforced sustainable gold nanoparticles: an efficient heterogeneous catalyst for reduction of nitro aliphatic, aromatic and heterocyclic scaffolds. Results Chem. 2023, 6, 101199.
16. Brahmachari, G. Green synthetic approaches for biologically relevant heterocycles: advanced synthetic techniques - an overview. In Green Synthetic Approaches for Biologically Relevant Heterocycles, 2nd ed.; Advanced Synthetic Techniques, Vol. 1; Elsevier, 2021; pp 1-8.
17. Marotta, L.; Rossi, S.; Ibba, R.; et al. The green chemistry of chalcones: valuable sources of privileged core structures for drug discovery. Front. Chem. 2022, 10, 988376.
18. Asif, M. Green synthesis of benzimidazole derivatives: an overview on green chemistry and its applications. Chem. Methodol. 2019, 3, 620-31.
19. Rizwan, K.; Khan, A.; Asiri, A. M. A. Handbook of Functionalized Nanostructured MXenes: Synthetic Strategies and Applications from Energy to Environment Sustainability; Springer, 2023.
20. Dhadda, S.; Guleria, A. Recent green synthesis and biological applications of 4H-pyrimidobenzothiazole derivatives. In Fundamentals of Medical Biotechnology; Recent Trends in Biotechnology; Nova Science Publishers, 2021; pp 75-90. https://www.researchgate.net/publication/350591329 (accessed 2025-12-24).
21. Rezaeifard, A.; Bakherad, M.; Navidpour, L.; Abedi, Bajestani. M.; Onagh, G. Furo, pyrano, and pyrido[2,3-d]pyrimidines: a comprehensive review of synthesis and medicinal applications. ChemistrySelect 2024, 9, e202302345.
22. Yadav, S.; Yadav, A.; Mohan, C.; Garg, V. K.; Kumari, N. Introduction to environmental and green chemistry. In Green Chemistry Approaches to Environmental Sustainability; Advances in Green and Sustainable Chemistry; Elsevier, 2024; pp 1-22.
23. Gogula, S. S.; Prasanna, D. V.; Thumma, V.; et al. Efficient green synthesis, anticancer activity, and molecular docking studies of indolemethanes using a bioglycerol-based carbon sulfonic acid catalyst. ACS Omega 2023, 8, 36401-11.
24. Reddy, S. R.; Jayakumar, J. Cu-catalysed tandem reactions for building poly hetero atom heterocycles-green chemistry tool. Phys. Sci. Rev. 2023, 8, 3859-83.
25. Tiris, G.; Genc, A. A.; Oven, E.; Erk, N. A green approach for the analysis of emtricitabine bictegravir and tenofovir in a pharmaceutical preparation using novel HPLC and spectrophotometric methods. Biomed. Chromatogr. 2023, 37, e5712.
26. Patel, K.; Patel, H.; Shah, D.; et al. Unlocking the potential: a comprehensive review for the synthesis of benzofuran derivatives. Curr. Green Chem. 2024, 11, 12-36.
27. Banik, B. K.; Sahoo, B. M.; Kumar, B. V. V. R.; et al. Green synthetic approach: an efficient eco-friendly tool for synthesis of biologically active oxadiazole derivatives. Molecules 2021, 26, 1163.
28. Tripathi, G.; Kumar, A.; Rajkhowa, S.; Tiwari, V. K. Synthesis of biologically relevant heterocyclic skeletons under solvent-free condition. In Green Synthetic Approaches for Biologically Relevant Heterocycles, 2nd ed.; Advanced Synthetic Techniques, Vol. 1; Elsevier, 2021; pp 421-59.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Copyright
Data & Comments
Data















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].