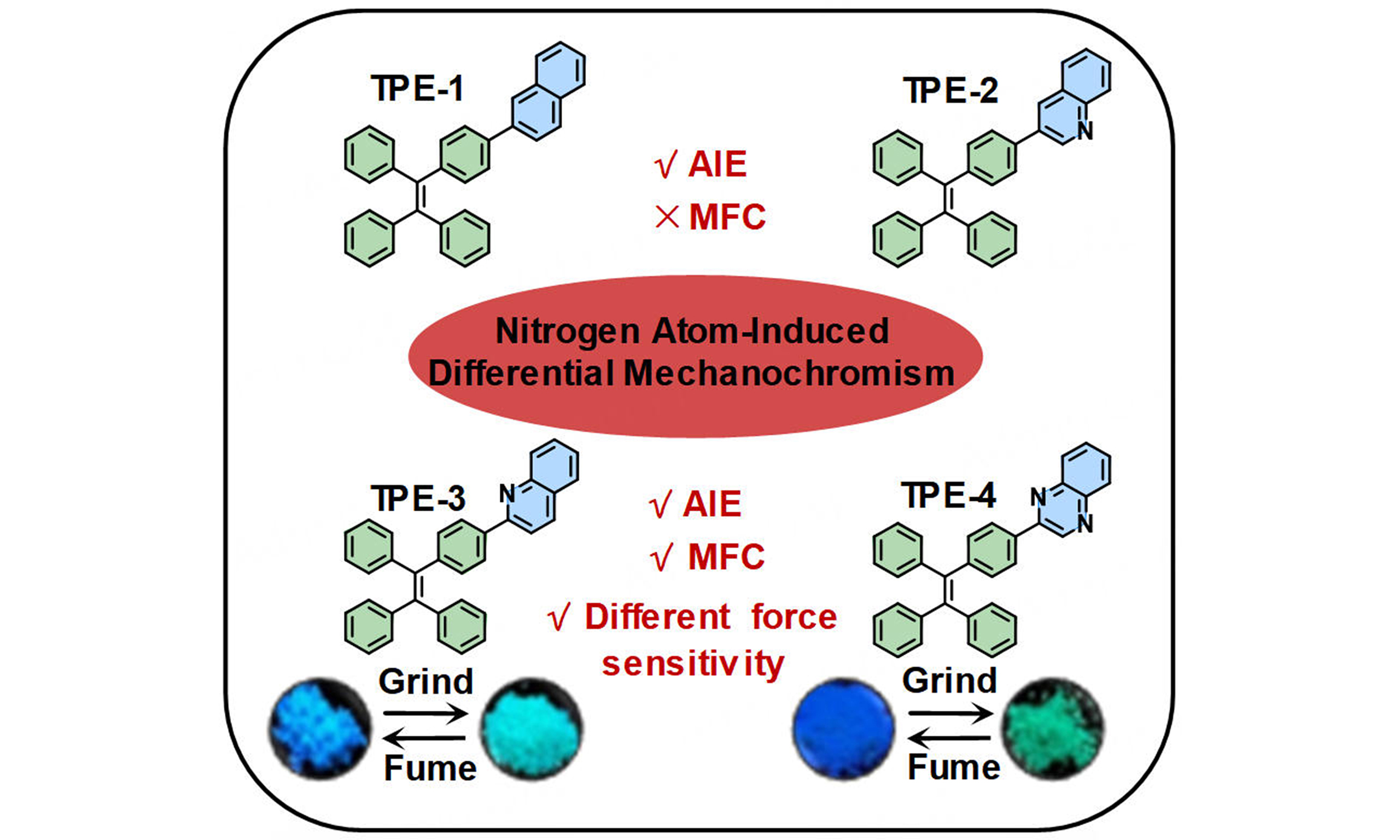
Nitrogen atom modulation enables high-sensitive mechanofluorochromism of tetraphenylethylene-based luminescent materials
Abstract
Mechanofluorochromic (MFC) materials, capable of undergoing color changes in response to external forces, hold vast potential for a wide range of applications. Here, we designed and synthesized four compounds based on the tetraphenylethylene (TPE) unit, denoted as TPE-1, TPE-2, TPE-3, and TPE-4. The TPE moiety serves as the common core among these compounds, while nitrogen atom fine-tuning is employed on the modifying groups of the four compounds to explore its influence on their photophysical properties and MFC performance. These compounds display significant variations in their solid-state photophysical properties and MFC characteristics. Especially, TPE-3 and TPE-4 exhibit excellent MFC properties and different sensitivity to external mechanical stimuli. These research findings not only offer a fresh perspective on the MFC mechanism of TPE-based compounds but also provide valuable insights for the design and development of novel MFC molecules.
Keywords
INTRODUCTION
With the continuous development of materials science and technology, traditional materials have struggled to meet the ever-growing demands of society. Therefore, the emergence and rapid advancement of smart materials have become an inevitable trend. Among these, materials capable of reversible changes in optical properties under external stimuli such as light, electricity, heat, and force are referred to as chromogenic materials. As an important category of smart materials, chromogenic materials exhibit high sensitivity, rapid response, and visualizability of optical signals, thus becoming a long-standing research focus for scientists[1-4]. Compared to other chromogenic materials, mechanofluorochromic (MFC) materials do not require external light sources, electric fields, or heat sources; they only necessitate simple mechanical force for significant fluorescence color changes[5-16]. The simplicity and relatively easier achievement of fluorescence color changes make them widely applicable in various fields such as sensors[17-19], data storage[20-22], and anti-counterfeiting materials[23-25].
In MFC materials, high fluorescence emission intensity in the solid state and significant color changes before and after external force stimulation are two crucial aspects[26,27]. As the most representative aggregation-induced emission (AIE) fluorophore, tetraphenylethylene (TPE) and its derivatives have attracted widespread attention due to their simple molecular structure and excellent optical properties[28-36]. The TPE unit has also become one of the commonly used moieties for constructing MFC materials. Researchers have created numerous high-performance MFC materials by modifying various aromatic heterocycles on TPE[37-44] [Supplementary Figure 1]. Among them, the incorporation of heteroatoms significantly influences the photophysical properties and MFC behavior of these materials, with nitrogen being particularly prevalent in the design of MFC materials. Nonetheless, there has been limited exploration into the effects of nitrogen atom modifications on the MFC and photophysical properties of TPE-based compounds, leading to a lack of reference for subsequent TPE-based MFC molecule design.
Based on this, we embarked on the design and synthesis of four novel TPE-based compounds, namely TPE-1, TPE-2, TPE-3, and TPE-4 [Figure 1]. In these compounds, TPE moieties served as the common structural motif, while precise adjustments were made to the nitrogen atoms in the other moieties. By altering the presence, position, and number of nitrogen atoms, our aim is to investigate the impact of these microstructural changes on the MFC and photophysical properties. This endeavor provides new insights into the structure-property relationships of TPE-based compounds and serves as a significant reference for the design of superior-performing MFC materials.
RESULTS AND DISCUSSION
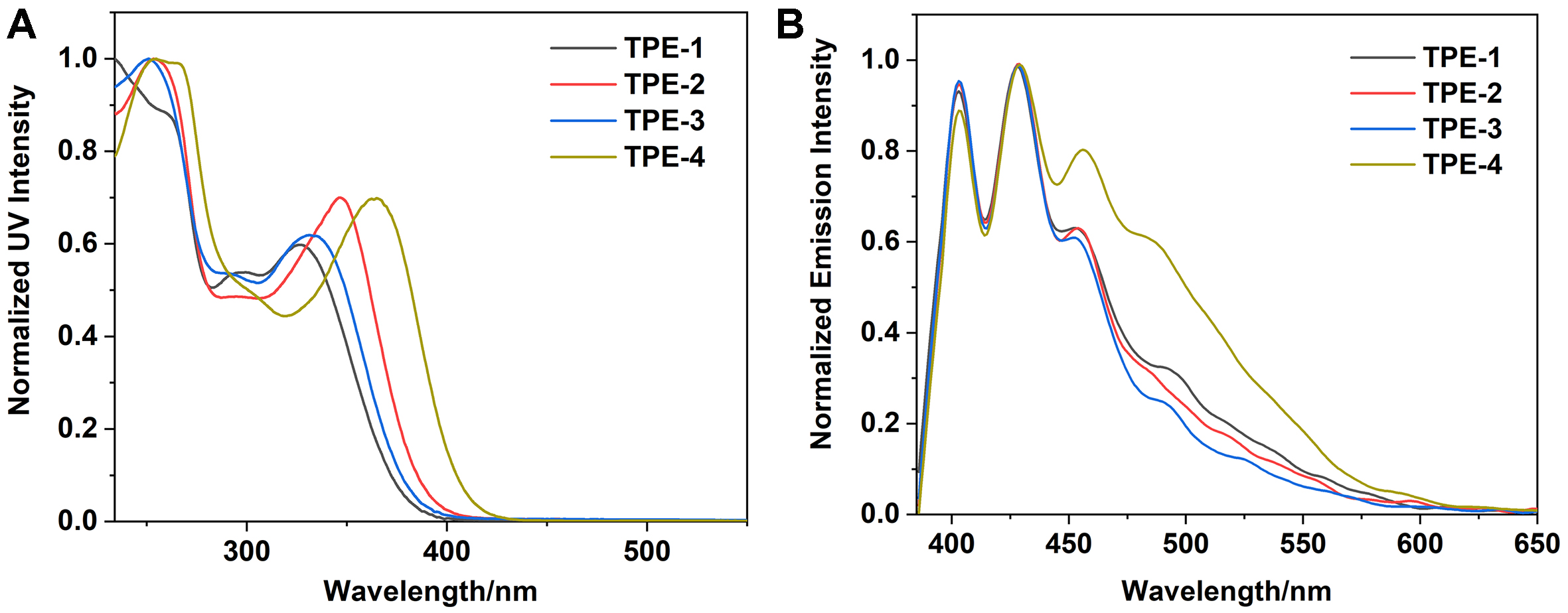
Compounds TPE-1, TPE-2, TPE-3, and TPE-4 were synthesized via highly efficient Suzuki coupling reactions. The detailed synthetic procedures and characterizations were provided in Sections 3 and 4 of the Supplementary Materials. Initially, ultraviolet-visible (UV-Vis) absorption tests were conducted on these four compounds in tetrahydrofuran (THF) solutions (10-5 M). As shown in Figure 2A, all four compounds exhibited absorption peaks around 250 nm, with broader absorption peaks observed in the range of 320 to 420 nm, centered at 327, 346, 332, and 362 nm, respectively [Table 1]. Compared with TPE-1, compounds TPE-3, TPE-2, and TPE-4 showed sequential redshifts in absorption. This phenomenon is attributed to the presence of nitrogen heterocycles in TPE-2, TPE-3, and TPE-4 induced intramolecular charge transfer from the TPE moiety to the nitrogen heterocycles, where TPE acts as the electron donor and the nitrogen heterocycles serve as the electron acceptor. Notably, TPE-4, containing two nitrogen atoms, exhibited the strongest electron-withdrawing capability and the most significant redshift in the maximum absorption peak. Subsequently, we further tested the solution-state emission of four compounds under 365 nm excitation. In dilute solutions, the four compounds exhibited similar fluorescence emissions [Figure 2B], with extremely weak fluorescence intensity. This is primarily due to the large free rotation and vibration of the TPE groups in solution, which increases intramolecular motion and enhances non-radiative energy loss. Additionally, the nitrogen in the molecule facilitates photo-induced electron transfer to the ground state of the excited TPE, potentially reducing the quantum yield. Upon the introduction of a poor solvent, the fluorescence intensity significantly increased, displaying typical AIE characteristics [Supplementary Figures 16-21]. Through this investigation, we found that the presence, position, and number of nitrogen atoms have some influence on the solution-state photophysical properties of four TPE-based compounds, although the extent of impact is not significant.
Figure 2. (A) The UV-Vis absorption and (B) fluorescence emission spectra of TPE-1, TPE-2, TPE-3, and TPE-4 (c = 10-5 M). UV-Vis: Ultraviolet-visible; TPE: tetraphenylethylene.
Optical and electronic properties of TPE-1, TPE-2, TPE-3, and TPE-4
| λabs (nm) | λem (nm) | soln (ΦF) | aggr (ΦF) | LUMO (eV) | HOMO (eV) | |
| TPE-1 | 327 | 431 | ~0 | 19% | -0.053 | -0.190 |
| TPE-2 | 346 | 428 | ~0 | 2% | -0.060 | -0.195 |
| TPE-3 | 332 | 428 | ~0 | 11% | -0.061 | -0.192 |
| TPE-4 | 362 | 431 | ~0 | 19% | -0.073 | -0.195 |
To further investigate the intramolecular charge transfer in these compounds, density functional theory (DFT) calculations were conducted. Using Gaussian 09 software, the geometries of these four compounds were optimized at the B3LYP/6-31G(d,p) level [Supplementary Figure 31]. The computational results revealed that the lowest unoccupied molecular orbital (LUMO) and the highest occupied molecular orbital (HOMO) of TPE-1 were distributed throughout the entire molecule, indicating the absence of intramolecular charge transfer. In contrast, the LUMOs of TPE-2, TPE-3, and TPE-4 were predominantly located on the acceptor groups, while the HOMOs were mainly distributed on the TPE moieties, indicating intramolecular charge transfer in these three compounds. Particularly, TPE-4, containing two nitrogen atoms, exhibits the most pronounced charge separation. These results underscore the critical influence of nitrogen atoms on the intramolecular charge transfer of TPE-based compounds.
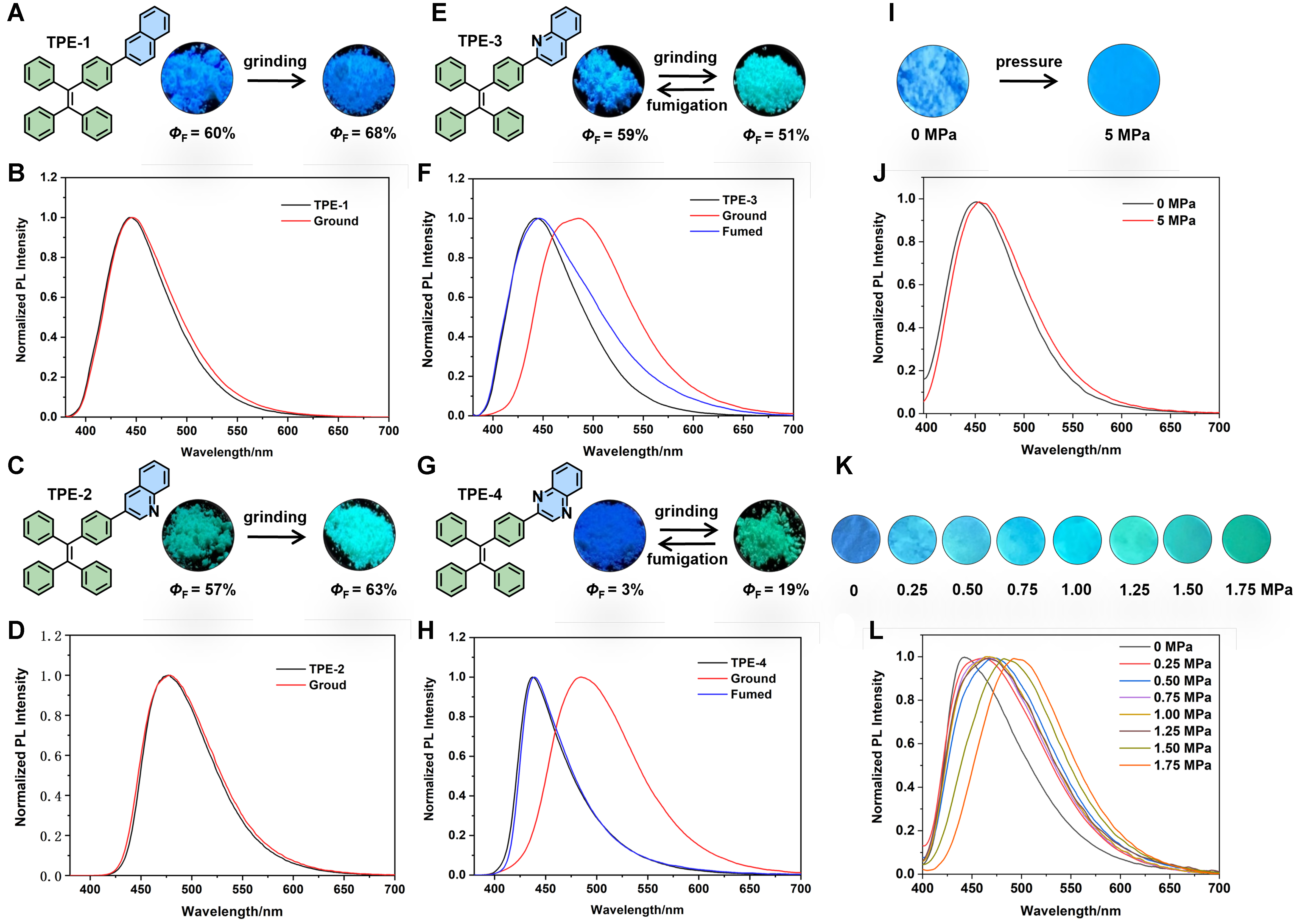
After detailed characterization of the photophysical properties of the four compounds in solution, the photophysical properties in solid-state and MFC behavior of these compounds were further characterized. We thoroughly ground each compound and characterized the solid powders before and after grinding. As shown in Supplementary Figure 22, the grinding process did not significantly alter the UV-Vis absorption of these compounds. Their luminescent properties in solid state and MFC behaviors were shown in Table 2 and Figure 3. The experimental results revealed distinct fluorescence response behaviors of these compounds after thorough grinding. For TPE-1 without N atoms, the fluorescence emission peak showed minimal change after grinding, indicating no MFC properties. Both the initial and post-grinding fluorescence emission peak centers were near 445 nm, displaying blue fluorescence [Figure 3A and B]. Similarly, for TPE-2, with a 3-quinoline acceptor moiety, the fluorescence emission peak remained unchanged after grinding, also showing no MFC properties. The initial and post-grinding fluorescence emission peak centers were both near 479 nm, exhibiting cyan fluorescence [Figure 3C and D]. It is noteworthy that although there was no significant shift, mechanical force exerted some influence on the fluorescence intensity of these two compounds. After grinding, the fluorescence quantum efficiency of
Figure 3. Fluorescence pictures and absolute quantum efficiency of (A) TPE-1, (C) TPE-2, (E) TPE-3 and (G) TPE-4 before and after grinding; solid-state fluorescence emission spectra of (B) TPE-1, (D) TPE-2 at initial, post-grinding and (F) TPE-3, (H) TPE-4 at initial, post-grinding and solvent fumigation; fluorescence images of (I) TPE-3 and (K) TPE-4 under different pressures; fluorescence emission spectra of (J) TPE-3 and (L) TPE-4 under different pressures. TPE: Tetraphenylethylene.
Optical properties of TPE-1, TPE-2, TPE-3, and TPE-4 in the solid state
| Original powder [λem(nm)] | Original powder (ΦF) | Ground powder [λem(nm)] | Ground powder (ΦF) | |
| TPE-1 | 445 | 60% | 445 | 68% |
| TPE-2 | 479 | 57% | 479 | 63% |
| TPE-3 | 443 | 59% | 485 | 51% |
| TPE-4 | 437 | 3% | 485 | 19% |
During the grinding process of TPE-3 and TPE-4, we observed starkly different responses to external force between the two compounds. TPE-3 required a prolonged grinding time to exhibit noticeable changes in fluorescence color, while TPE-4 showed an immediate fluorescence color change upon grinding. This sparked our interest in investigating this phenomenon further. Therefore, we studied the fluorescence changes of TPE-3 and TPE-4 under various pressures to gain deeper insights into the sensitivity of these compounds to external forces. The experimental results revealed that TPE-4 exhibited excellent sensitivity to pressure. When the pressure increased from 0 to 0.25 MPa, the fluorescence color changed immediately, consistent with the grinding-induced color change phenomenon. With further pressure increase, the fluorescence emission peak continued to redshift, transitioning from blue to green fluorescence, and eventually stabilized under a pressure of 1.75 MPa [Figure 3K and L]. In contrast, TPE-3 showed poor sensitivity to pressure, exhibiting good stability in fluorescence emission under different pressures. Even with pressure increasing from 0 to 5 MPa, only minor changes were observed in its fluorescence emission
To gain deeper insights into the MFC properties of the four compounds, we conducted powder X-ray diffraction (PXRD) characterization [Supplementary Figure 25]. In the initial solid state of all four compounds, sharp and intense reflections were observed, indicating an ordered microcrystalline structure. After grinding, the PXRD patterns of TPE-1 and TPE-2 showed almost no changes, whereas the peak intensities of TPE-3 and TPE-4 decreased, accompanied by the disappearance of some peaks. We believe this indicates that during the grinding process, TPE-3 and TPE-4 underwent partial transformation from an ordered microcrystalline state to an amorphous state. Subsequently, we analyzed the thermal behavior of the four compounds using thermogravimetric analysis (TGA). The TGA analysis revealed that all four compounds underwent thermal decomposition around 300 °C [Supplementary Figure 26], indicating their stability at elevated temperatures. Additionally, we performed differential scanning calorimetry (DSC) analysis on all four compounds before and after grinding [Supplementary Figure 27]. For TPE-3 and TPE-4, the ground samples exhibited additional exothermic peaks at 77 and 85 °C, respectively, compared to the original samples, indicating possible crystal phase transitions between microcrystals for these two compounds after grinding. However, for TPE-1 and TPE-2, no additional endothermic or exothermic peaks were observed in two states (before or after grinding).
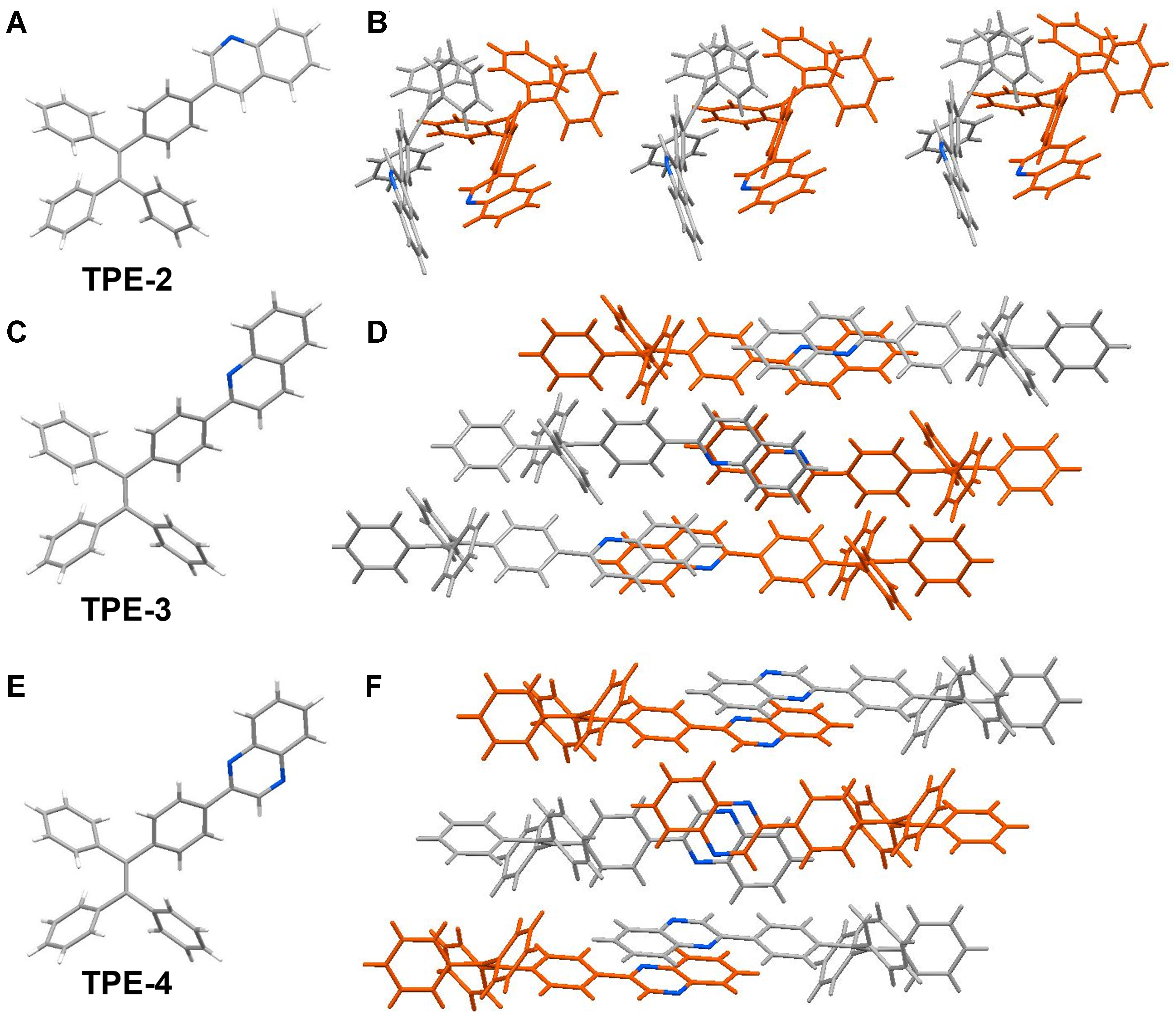
To delve deeper into the inherent relationship between the distinct MFC properties exhibited by the four compounds and their corresponding molecular structures, single-crystal characterization was employed to investigate their molecular aggregation states. By vapor-diffusing isopropyl ether into chloroform solutions containing TPE-2, TPE-3 and TPE-4, we successfully obtained crystals of these three compounds. Their crystal structures and molecular packing patterns are illustrated in Figure 4, with detailed crystallographic information provided in Supplementary Tables 1-3. The molecular conformations of all three compounds demonstrated highly distorted characteristics due to the presence of TPE. Within the molecular packing of these three compounds, numerous weak intermolecular interactions were observed [Table 3]. The highly distorted molecular structures and various weak interactions contribute to the formation of loose intermolecular packing modes for these three compounds. However, while TPE-3 and TPE-4 exhibit MFC properties, TPE-2 does not display such behavior. We speculate that this discrepancy is closely related to the intermolecular packing modes of the three compounds. TPE-3 and TPE-4 share a similar molecular stacking pattern, with their nitrogen heterocycles adopting a face-to-face parallel stacking mode, thereby forming strong π-π interactions [Figure 4D and F]. In contrast, the nitrogen heterocycles in TPE-2 are arranged in an approximate perpendicular stacking mode [Figure 4B]. We hypothesize that when subjected to external force, the nitrogen heterocycles in TPE-3 and TPE-4 adopt a face-to-face parallel stacking, facilitating intermolecular slip, and increasing π-π interactions between the nitrogen heterocycles upon grinding, resulting in redshifted emission. On the other hand, in TPE-2, the nitrogen heterocycles are arranged in an approximately perpendicular stacking mode, lacking the face-to-face stacking necessary for enhanced π-π interactions. Therefore, TPE-2 does not exhibit MFC properties. Overall, the position and number of nitrogen atoms influence the solid-state packing arrangement of TPE-based compounds, leading to different MFC properties among these compounds.
Figure 4. Single crystal structures of (A) TPE-2, (C) TPE-3 and (E) TPE-4; molecular packing diagrams of (B) TPE-2, (D) TPE-3 and (F) TPE-4. TPE: Tetraphenylethylene.
The list of various intermolecular interactions for TPE-2, TPE-3 and TPE-4
| TPE-2 (14 interactions) | TPE-3 (18 interactions) | TPE-4 (16 interactions) | |
| π…π | 2*3.291 Å 1*3.363 Å | 2*3.342 Å 1*3.784 Å 1*3.219 Å | |
| CH…π | 2*2.848 Å 2*2.731 Å 1*2.751 Å 1*2.866 Å 1*2.784 Å 1*2.811 Å | 2*2.771 Å 2*2.798 Å 1*2.867 Å 1*2.840 Å 1*2.790 Å 1*2.813 Å 1*2.759 Å 1*2.807 Å 1*2.897 Å 1*2.761 Å 1*2.799 Å 1*2.757 Å 1*2.571 Å | 2*2.818 Å 1*2.839 Å 1*2.863 Å 1*2.889 Å 1*2.841 Å 1*2.824 Å 1*2.778 Å 1*2.759 Å 1*2.746 Å 1*2.707 Å 1*2.663 Å |
| CH…N | 2*2.635 Å 2*2.730 Å | ||
| C…N | 1*3.249 Å | ||
| H…H | 1*2.374 Å |
CONCLUSIONS
In summary, this study designed and synthesized four TPE-based compounds to investigate the impact of nitrogen atom introduction, position, and number on their photophysical properties and MFC behavior. These compounds exhibited similar photophysical properties in solution. However, significant differences were observed in their photophysical properties and MFC behavior in the solid state. Specifically, TPE-1 and TPE-2 showed no MFC behavior, with their solid-state fluorescence colors being blue and cyan, respectively. On the other hand, TPE-3 and TPE-4 exhibited notable MFC phenomena, with both initially displaying blue fluorescence, which changed to cyan and green, respectively, under external mechanical stimuli. Notably, TPE-3 exhibited lower sensitivity to external stimuli for solid-state fluorescence under mechanical force, whereas TPE-4 displayed higher sensitivity. This study highlights the significant influence of nitrogen atoms on the solid-state photophysical properties and MFC behavior of TPE-based compounds, providing valuable insights for the molecular design of future MFC materials.
DECLARATIONS
Authors’ contributions
Conceived and designed the experiments: Wang, M.; Yu, H.
Performed the synthesis, NMR, MS, single crystal X-ray diffraction characterization and DFT calculations: Yu, H.; Chen, X.
Performed relevant characterizations of optical analysis and thermal analysis testing: Li, M.; Chen, X.; Han, N.; Gao, Z.; Guo, Z.; Zhang, X.; Wei, Y.; Xu, Z.
Availability of data and materials
The authors confirm that the detailed experimental and data supporting the findings of this study are available within its Supplementary Materials.
Financial support and sponsorship
This work was supported by the National Natural Science Foundation of China (22271116 and 22071079 for Wang, M.), the fellowship of China Postdoctoral Science Foundation (2023M741338 for Yu, H.), and the Postdoctoral Fellowship Program of CPSF (GZC20230940 for Yu, H.).
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2026.
Supplementary Materials
REFERENCES
1. Fihey, A.; Perrier, A.; Browne, W. R.; Jacquemin, D. Multiphotochromic molecular systems. Chem. Soc. Rev. 2015, 44, 3719-59.
2. Cai, G.; Wang, J.; Lee, P. S. Next-generation multifunctional electrochromic devices. Acc. Chem. Res. 2016, 49, 1469-76.
3. Aburas, M.; Soebarto, V.; Williamson, T.; Liang, R.; Ebendorff-Heidepriem, H.; Wu, Y. Thermochromic smart window technologies for building application: a review. Appl. Energy. 2019, 255, 113522.
4. Di, B. H.; Chen, Y. L. Recent progress in organic mechanoluminescent materials. Chin. Chem. Lett. 2018, 29, 245-51.
5. Bhuin, S.; Chakraborty, P.; Sivasakthi, P.; Samanta, P. K.; Yogeeswari, P.; Chakravarty, M. Asymmetrical organic D–π–A conjugate with ‘V’-shaped crystal packing: quest to transcend the limits of photophysical properties and applications. J. Mater. Chem. C. 2023, 11, 11270-82.
6. Zhao, H.; Huang, L.; Wang, Y.; et al. Mechanochromic luminescence of 2,6-bis(4-biphenyl)isonicotinic acid via interconversion of classical/frustrated Brönsted pair. J. Org. Chem. 2021, 86, 12591-6.
7. Zhang, Y.; Han, T.; Gu, S.; et al. Mechanochromic behavior of aryl-substituted buta-1,3-diene derivatives with aggregation enhanced emission. Chemistry 2014, 20, 8856-61.
8. Hirai, Y.; Wrona-Piotrowicz, A.; Zakrzewski, J.; et al. Mechanofluorochromism and self-recovery of alkylsilylpyrene-1-carboxamides. J. Mater. Chem. C. Mater. 2024, 12, 1952-7.
9. Yu, H.; Tian, P.; Han, N.; Li, M.; Wang, M. Nitrogen atom induced contrast effect on the mechanofluorochromic characteristics of anthracene-based acceptor-donor-acceptor fluorescent molecules. Chem. Asian. J. 2023, 18, e202300712.
10. Mullin, W. J.; Müller, P.; Schaefer, A. J.; Guzman, E.; Wheeler, S. E.; Thomas, I. S. W. Crystal engineering of heterocyclic arylene(ethynylene) oligomers through programmed aromatic stacking. J. Mater. Chem. C. 2022, 10, 11199-210.
11. Li, G.; Xu, Y.; Zhuang, W.; Wang, Y. Preparation of organic mechanochromic fluorophores with simple structures and promising mechanochromic luminescence properties. RSC. Adv. 2016, 6, 84787-93.
12. Wang, L.; Ye, K. Q.; Zhang, H. Y. Organic materials with hydrostatic pressure induced mechanochromic properties. Chin. Chem. Lett. 2016, 27, 1367-75.
13. Huang, Z.; Ding, A.; Yang, J.; Wang, C.; Tang, F. Conjugating coumarin with tetraphenylethylene to achieve dual-state emission for reversible mechanofluorochromism and live cell imaging. Chemistry 2023, 29, e202203628.
14. Yin, Y.; Ding, A.; Yang, L.; Kong, L.; Yang, J. Fusing rigid planar units to engineer twisting molecules as dual-state emitters. Mater. Chem. Front. 2022, 6, 1261-8.
15. Zhang, X.; Wang, D.; Shen, H.; et al. 3,6-Diamino-7,8-dihydroisoquinoline-4-carbonitrile derivatives: unexpected facile synthesis, full-color-tunable solid-state emissions and mechanofluorochromic activities. Org. Chem. Front. 2021, 8, 856-67.
16. Afrin, A.; Chinna, A. S. P. Symphony of light: AIE and MFC in carbazole-based cyanostilbenes. J. Mater. Chem. C. 2024, 12, 1923-44.
17. Wen, T.; Zhang, D. X.; Liu, J.; Lin, R.; Zhang, J. A multifunctional helical Cu(I) coordination polymer with mechanochromic, sensing and photocatalytic properties. Chem. Commun. 2013, 49, 5660-2.
18. Raisch, M.; Genovese, D.; Zaccheroni, N.; et al. Highly sensitive, anisotropic, and reversible stress/strain-sensors from mechanochromic nanofiber composites. Adv. Mater. 2018, 30, e1802813.
19. Zhu, Q.; Van, V. K.; Holten-Andersen, N.; Miserez, A. A double-layer mechanochromic hydrogel with multidirectional force sensing and encryption capability. Adv. Funct. Mater. 2019, 29, 1808191.
20. Hou, Y.; Du, J.; Hou, J.; et al. Rewritable optical data storage based on mechanochromic fluorescence materials with aggregation-induced emission. Dyes. Pigments. 2019, 160, 830-8.
21. Shi, P.; Zhao, R.; Zhang, M.; Lin, M.; Duan, Y.; Han, T. An information carrier based on turn-on type mechanochromic luminescent material: application for rewritable binary data storage. Mater. Lett. 2019, 243, 38-41.
22. Han, J.; Sun, J.; Li, Y.; Duan, Y.; Han, T. One-pot synthesis of a mechanochromic AIE luminogen: implication for rewritable optical data storage. J. Mater. Chem. C. 2016, 4, 9287-93.
23. Guo, Y.; Wu, A.; Qiu, C.; et al. Force-induced molecular isomerization for the construction of multicolor luminescent segmented molecular crystals. Adv. Opt. Mater. 2022, 10, 2101794.
24. Zhang, X.; Yuan, S.; Lu, Y.; Lan, H.; Xiao, S.; Yi, T. A temperature-dependent tricoloured mechanochromic fluorescence material with polymorphic structures. J. Mater. Chem. C. 2022, 10, 15920-8.
25. Zhang, H.; Wu, S.; Wang, Y.; et al. Mechanochromic luminescent property and anti-counterfeiting application of AIE-active cyclometalated platinum(II) complexes featuring a fused five-six-membered metallacycle. Dyes. Pigments. 2022, 197, 109857.
26. Ciardelli, F.; Ruggeri, G.; Pucci, A. Dye-containing polymers: methods for preparation of mechanochromic materials. Chem. Soc. Rev. 2013, 42, 857-70.
27. Chen, W.; Pan, Y.; Chen, J.; Ye, F.; Liu, S. H.; Yin, J. Stimuli-responsive organic chromic materials with near-infrared emission. Chin. Chem. Lett. 2018, 29, 1429-35.
28. Tian, H.; Lin, W.; Hu, X.; et al. Ratiometric sensing of β-galactosidase based on excited-state intramolecular proton transfer (ESIPT) and solid-state luminescence enhancement. Org. Chem. Front. 2023, 10, 2913-7.
29. Zeng, Y.; Shi, J.; Li, K.; et al. Coordination-driven [2+2] metallo-macrocycles isomers: conformational control and photophysical properties. Chem. Synth. 2022, 2, 12.
30. Feng, H. T.; Yuan, Y. X.; Xiong, J. B.; Zheng, Y. S.; Tang, B. Z. Macrocycles and cages based on tetraphenylethylene with aggregation-induced emission effect. Chem. Soc. Rev. 2018, 47, 7452-76.
31. Khan, F.; Urbonas, E.; Volyniuk, D.; Grazulevicius, J. V.; Mobin, S. M.; Misra, R. White hyperelectrofluorescence from solution-processable OLEDs based on phenothiazine substituted tetraphenylethylene derivatives. J. Mater. Chem. C. 2020, 8, 13375-88.
32. Han, N.; Ma, J.; Yu, H.; et al. Sandwich-like heterochromophore metallo-supramolecules based on dense chromophore arrangements with energy and chirality transfer properties. CCS. Chem. 2024, 6, 1264-77.
33. Tanaka, Y.; Machida, T.; Noumi, T.; Sada, K.; Kokado, K. Emissive tetraphenylethylene (TPE) derivatives in a dissolved state tightly fastened by a short oligo(ethylene glycol) chain. Org. Chem. Front. 2020, 7, 2649-56.
34. Ma, X.; Hu, L.; Han, X.; Yin, J. Vinylpyridine- and vinylnitrobenzene-coating tetraphenylethenes: aggregation-induced emission (AIE) behavior and mechanochromic property. Chin. Chem. Lett. 2018, 29, 1489-92.
35. Cheng, M.; Chen, D.; Zhang, L.; Xiao, T.; Jiang, J.; Wang, L. Chemical fuel-driven gelation with dissipative assembly-induced emission. Org. Chem. Front. 2023, 10, 1380-5.
36. Zhan, J.; Yao, C.; Han, Z.; Wu, Y.; Feng, H.; Qian, Z. Fluorescent photoswitches with improved emission efficiency based on aggregation-induced emission luminogens by eliminating the heavy-atom effect. J. Mater. Chem. C. 2024, 12, 3498-505.
37. Huang, G.; Jiang, Y.; Yang, S.; Li, B. S.; Tang, B. Z. Multistimuli response and polymorphism of a novel tetraphenylethylene derivative. Adv. Funct. Mater. 2019, 29, 1900516.
38. Yuan, W. Z.; Tan, Y.; Gong, Y.; et al. Synergy between twisted conformation and effective intermolecular interactions: strategy for efficient mechanochromic luminogens with high contrast. Adv. Mater. 2013, 25, 2837-43.
39. Huang, G.; Xia, Q.; Huang, W.; et al. Multiple anti-counterfeiting guarantees from a simple tetraphenylethylene derivative - high-contrasted and multi-state mechanochromism and photochromism. Angew. Chem. Int. Ed. Engl. 2019, 58, 17814-9.
40. Wang, J.; Mei, J.; Hu, R.; Sun, J. Z.; Qin, A.; Tang, B. Z. Click synthesis, aggregation-induced emission, E/Z isomerization, self-organization, and multiple chromisms of pure stereoisomers of a tetraphenylethene-cored luminogen. J. Am. Chem. Soc. 2012, 134, 9956-66.
41. Shen, X. Y.; Wang, Y. J.; Zhao, E.; et al. Effects of substitution with donor–acceptor groups on the properties of tetraphenylethene trimer: aggregation-induced emission, solvatochromism, and mechanochromism. J. Phys. Chem. C. 2013, 117, 7334-47.
42. Hu, T.; Yao, B.; Chen, X.; et al. Effect of ionic interaction on the mechanochromic properties of pyridinium modified tetraphenylethene. Chem. Commun. 2015, 51, 8849-52.
43. Yin, Y.; Guan, Q.; Chen, Z.; et al. Force-triggered hypso- and bathochromic bidirectional fluorescence switching beyond 120 nm and its anticounterfeiting applications. Sci. Adv. 2024, 10, eadk5444.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Copyright
Data & Comments
Data


















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].