Uniform mesoporous Nb2O5 microspheres with controlled porosity for efficient lithium storage
Abstract
Mesoporous metal oxides with both extraordinary porosity and functionality have been receiving increasing research attention in catalysis, sensing and various energy-related applications. However, progress in the rational and reliable syntheses of many components is quite alleviated, which severely restricts further potential explorations. Here, we present a facile template-free approach to synthesize a type of uniform mesoporous Nb2O5 microspheres with tunable grain and pore sizes. Such a method conducted in hydrothermal conditions undergoes a simple precursor hydrolysis and crystallite aggregation process, which enables flexible parameter control and is easy for large-scale production. In consequence, the obtained mesoporous Nb2O5 microspheres possess excellent and highly controlled mesoporosity and crystallinity, including large grain sizes (33.5 to 61.7 nm) and uniform pore sizes (7.7 to 45.0 nm). Due to the unique structural features for desirable mass diffusion and electrolyte access, the lithium-ion battery anode utilizing the mesoscopic Nb2O5 materials enables highly reversible pseudocapacitive charge storage, delivering a high specific capacity of 181 mAh·g-1 at 0.05 mA·cm-2 and volumetric capacity of
Keywords
INTRODUCTION
Mesoporous materials have the characteristics of high specific surface area, large pore capacity, adjustable pore structure, and nano-effects on mesochannels and pore walls, thereby attracting widespread attention from researchers in the fields of catalysis, biomedicine, gas sensors, supercapacitors, energy storage and conversion[1-9]. In terms of the components, mesoporous metal oxides have been emerging in a broad scope of applications owing to their high crystallinity and semiconducting properties and excellent porosities[10-14]. However, the synthesis of mesoporous crystalline oxides remains an exciting challenge. For mesoporous materials with crystalline skeletons, most of metal sources for building mesoporous frameworks are highly reactive and sensitive to moisture[15], which is hard to precisely control structural parameters both at the atomic- and nanoscale. In addition, despite rapid hydrolysis, the relatively weak interaction between metal precursors and surfactants also leads to severe phase separation[16], while the mesostructural collapse during crystallization is another problem due to the low thermal stability of metal oxides[17-20]. At present, the mesoscopic control of amorphous states, such as silica and carbon-based nanostructures, is very mature, but the synthesis of other elements or compounds still requires further development and investigation[21,22].
With the deepening of research, transition metal oxides have been gradually explored and utilized in various fields[23], such as WO3[24,25], MoO3[26-28], Co3O4[29,30], ZnO[31], and TiO2[32,33]. Among various transition metal oxides, Nb2O5 has attracted much attention in energy storage because of its “intercalation pseudocapacitance” behavior, which has been proven to be a type of high-rate electrochemical energy storage material. Therefore, incorporating mesopores into Nb2O5 materials would enable fascinating properties for better electrochemical storage[34,35]. Currently, quite a few reports have developed strategies, including soft-templating and hard-templating routes to synthesize mesoporous Nb2O5 materials. For example, Kim et al. synthesized a type of mesoporous Nb2O5 film by an evaporation-induced self-assembly (EISA) method[36]. Luo et al. proposed a resol-assisted co-assembly approach to fabricate ordered mesoporous Nb2O5 nanospheres[37]. Such a soft-templating method enables facile synthesis, but a composite instead of pure Nb2O5 is formed to inhibit grain growth and prevent structural collapse during annealing, thus the crystallinity is unsatisfactory. On the other hand, the utilization of hard templates can synthesize products with reverse mesostructure and high crystallinity[38-40]; however, the process is complicated, low-yield, and lacks reproducibility. In this regard, the development for the facile and reliable synthesis of highly crystalline mesoporous Nb2O5 materials is quite imperative for exploring advanced applications.
In this work, we propose a kind of uniform mesoporous Nb2O5 microspheres with controlled porosity and high crystallinity through a simple hydrothermal synthesis strategy. And the facile synthesis of mesoporous Nb2O5 with controlled parameters is the focus of the work. Such a template-free method in an autoclave is simple and convenient, which involves uniform nucleation and growth of nanocrystals from niobium chloride into accumulated mesoporous spheres. The sealed atmosphere that prevents access to moisture allows for uniform structural growth, and large mesopores from nanocrystal accumulation without usage of templates can endure high temperatures to achieve enhanced crystallinity and also be easily extended for gram-level production. The synthesized mesoporous Nb2O5 microspheres after annealing possess a uniform diameter of ~800 nm, a controllable pore size from 7.7 to 45 nm and grain size from 33.5 to 61.7 nm, as well as high crystallinity. Moreover, owing to high surface area and the large pore size for accelerating the electrolyte access and Li+ diffusion, the mesoporous Nb2O5 microspheres exhibit a highly reversible capacity of 181 mAh·g-1 at 0.05 mA·cm-2, good rate capability, and long cyclability at 2.5 mA·cm-2 as a lithium-ion battery (LIB) anode.
EXPERIMENTAL
Chemicals
Niobium pentachloride (NbCl5) was acquired from Sigma-Aldrich. Sinopharm Chemical Reagent Co., Ltd. (China) supplied ammonia solution (25-28 wt.%) and anhydrous ethanol. Graphene oxide (GO, multiple layers, 98%) was obtained from Yuan Ye. Oxalic acid (C2H2O4, 99 %) came from Macklin. Carboxyl methyl cellulose (CMC), Ketjen black (KJB), and styrene butadiene rubber (SBR) were sourced from Shenzhen Kejing Star Technology Co., Ltd. (China). All reagents were purchased from commercial sources and used without further purification. Deionized water was employed for all experiments.
Synthesis of mesoporous Nb2O5 microspheres
For a characteristic synthesis, NbCl5 (1.0 g) was dissolved in an ethanol solution (25 mL). After stirring for
Synthesis of loose Nb2O5 nanoparticles
NbCl5 (0.5 g) was dissolved in 10 mL of ethanol solution, and 50 mL of oxalic acid (4 wt.%) was added to the mixture under continuous agitation. After adjusting the pH value to 9.0 using ammonia, the solution was transferred to a 100 mL Teflon-lined autoclave, sealed and heated for 12 h at 180 °C. After standing and cooling down to room temperature, the precipitates were collected after centrifugation, and the precipitates were washed with water. Finally, after being annealed under air atmosphere for 2 h at 450 °C, the loose
Material characterization
A field-emission scanning electron microscope (FESEM, Regulus 8100) was used to observe the morphology and structure of the samples. Transmission electron microscopy (TEM) with a Tecnai F20 transmission electron microscope (200 kV) allows observation of nanostructures of samples. The composition and structure of the samples were analyzed by powder X-ray diffraction (XRD) using a PANalytical Empyrean diffractometer with Cu Kα radiation (λ = 1.5406 Å). The mesoporosity of samples was determined by nitrogen sorption isotherms at 77 K. The surface areas were calculated using the Brunauer-Emmett-Teller (BET) method, and pore volumes and sizes from adsorption branches can be calculated using the Barrett-Joyner-Halenda (BJH) model. X-ray photoelectron spectra (XPS) were gathered using a Thermo Scientific ESCALAB Xi+ using Al Kα as the excitation source. Raman spectra were conducted on a LabRAM HR 800 instrument with 532 nm excitations.
Electrochemical measurements
All the working electrodes were manufactured with the active material, KJB, CMC and SBR in a mass ratio of 80:10:5:5 using deionized water as the solvent and copper foil as the current collector. The coated electrodes were cut into 12 mm diameter slices after overnight drying at 80 °C under vacuum. The mass loading of thin-film electrodes was controlled at 2.5 mg·cm-2. To conduct lithium-ion storage measurements, the working electrodes and lithium metal discs, which served as both the count and reference electrodes, were assembled into coin cells (CR2032) within a glove box filled with Ar. The separator used was the Celgard-2325 membrane. The electrolyte was 1 M LiPF6 dissolved in a solution of ethylene carbonate (EC), dimethyl carbonate (DMC), and ethylmethyl carbonate (EMC) in a 1:1:1 volume ratio. Cyclic voltammetry (CV) tests were performed on an electrochemical workstation (Bio-Logic VSP). The galvanostatic charge and discharge (GCD) tests at various specific currents were conducted on NEWARE Battery Test System (CT-4008T). Galvanostatic intermittent titration technique (GITT) measurements were conducted by pulsing for 10 min at 0.01 A·g-1 followed by a 1 h rest. The potential range was set in 1.1-3 V vs. Li+/Li.
RESULTS AND DISCUSSION
Synthesis and characterization of mesoporous Nb2O5 microspheres
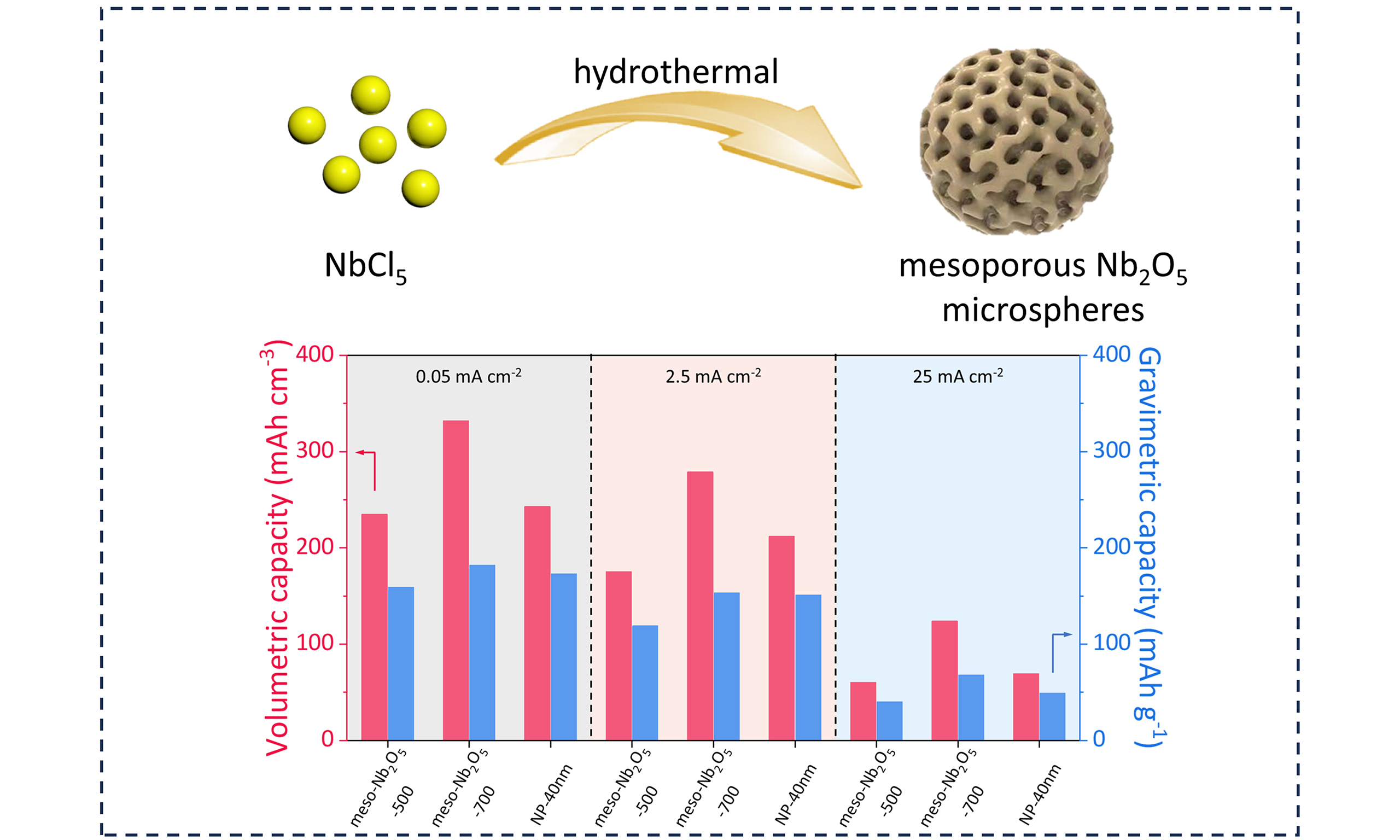
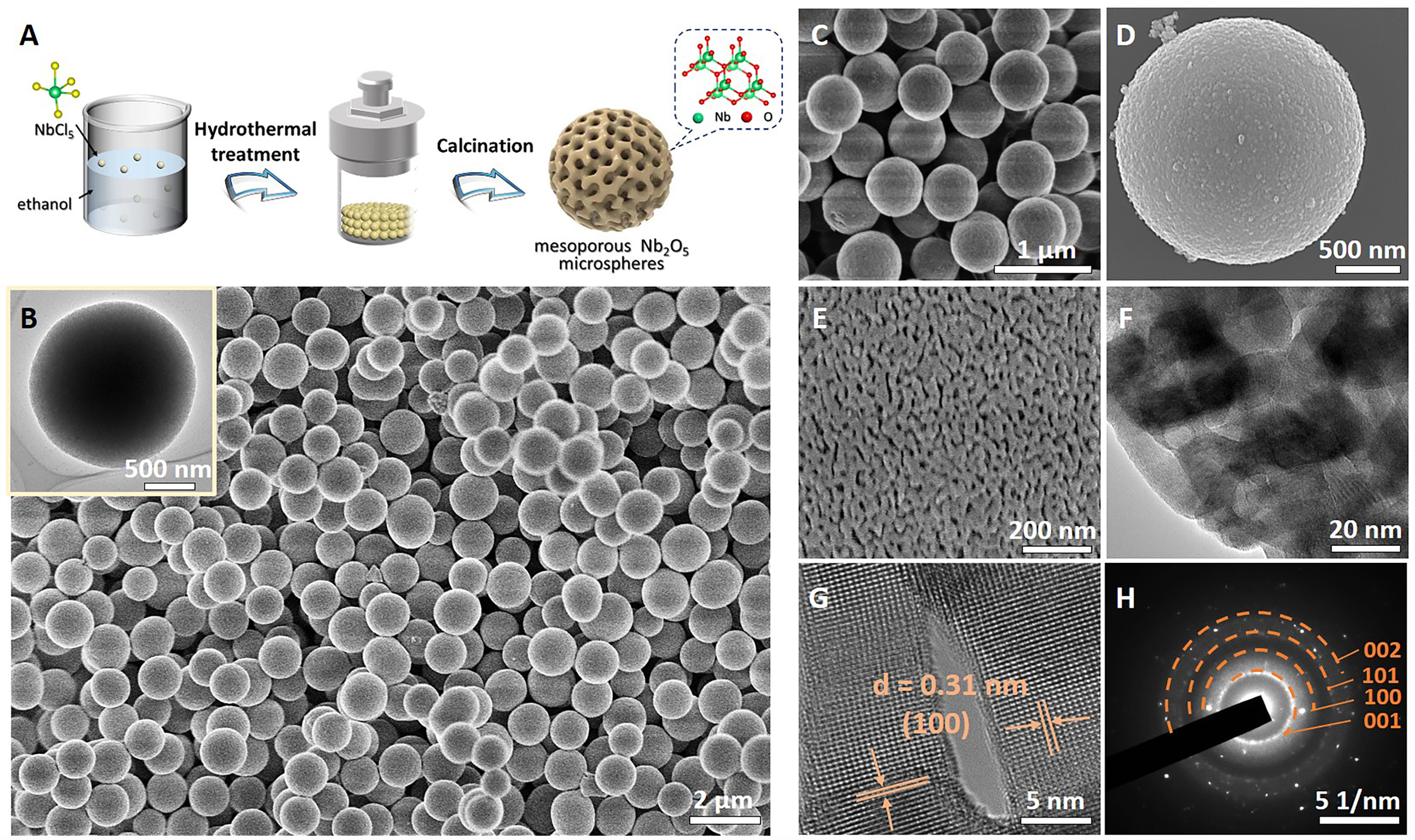
As shown in Figure 1A, a simple hydrothermal synthesis method was employed to synthesize mesoporous Nb2O5 microspheres. The template-free process consists of three main steps: Firstly, a precursor solution was prepared containing NbCl5 as the metal source and ethanol as the solvent. Afterward, the subsequent hydrothermal treatment in an autoclave at 150 °C for 24 h produced uniform niobium oxide microspheres with abundant mesopores from stacked nanocrystals. The final product can be obtained after calcination in air at 500 °C for 2 h to improve crystallinity. Notably, such a facile approach guarantees both good mesoporosity and crystallinity in comparison to those template methods, since the composed large nanocrystals can ensure steady accumulated mesopores for high-temperature crystallization.
Figure 1. Structural characterizations. (A) Schematic illustration for the formation of mesoporous Nb2O5 microspheres; (B and C) SEM images with different magnifications and TEM image (Inset) of the as-made Nb2O5 microspheres; (D and E) SEM images, (F) TEM image, (G) HRTEM image and (H) corresponding SAED pattern of the mesoporous Nb2O5 microsphere after calcination in air. SEM: Scanning electron microscopy; TEM: transmission electron microscopy; HRTEM: high-resolution TEM; SAED: selected area electron diffraction.
Scanning electron microscopy (SEM) images at low magnifications reveal a very uniform spherical morphology of approximately 0.8 μm in diameter with the presence of a smooth surface without any unassembled nanoparticles [Figure 1B and C, Supplementary Figure 1], demonstrating the high purity of the as-made sample. After calcination in air, SEM images show clearly enlarged grain size and mesoporous skeleton in the microsphere [Figure 1D and E]. TEM further illustrates the presence of mesopores of about 7.7 nm in diameter [Figure 1F and Supplementary Figure 2]. High-resolution TEM (HRTEM) allows the observation of lattice spacing of 0.39 nm, which is consistent with the (001) plane of the hexagonal phase of Nb2O5 [Figure 1G]. The selected area electron diffraction (SAED) pattern proves that the obtained mesoporous Nb2O5 microsphere after calcination has much improved crystallinity than as-made sample
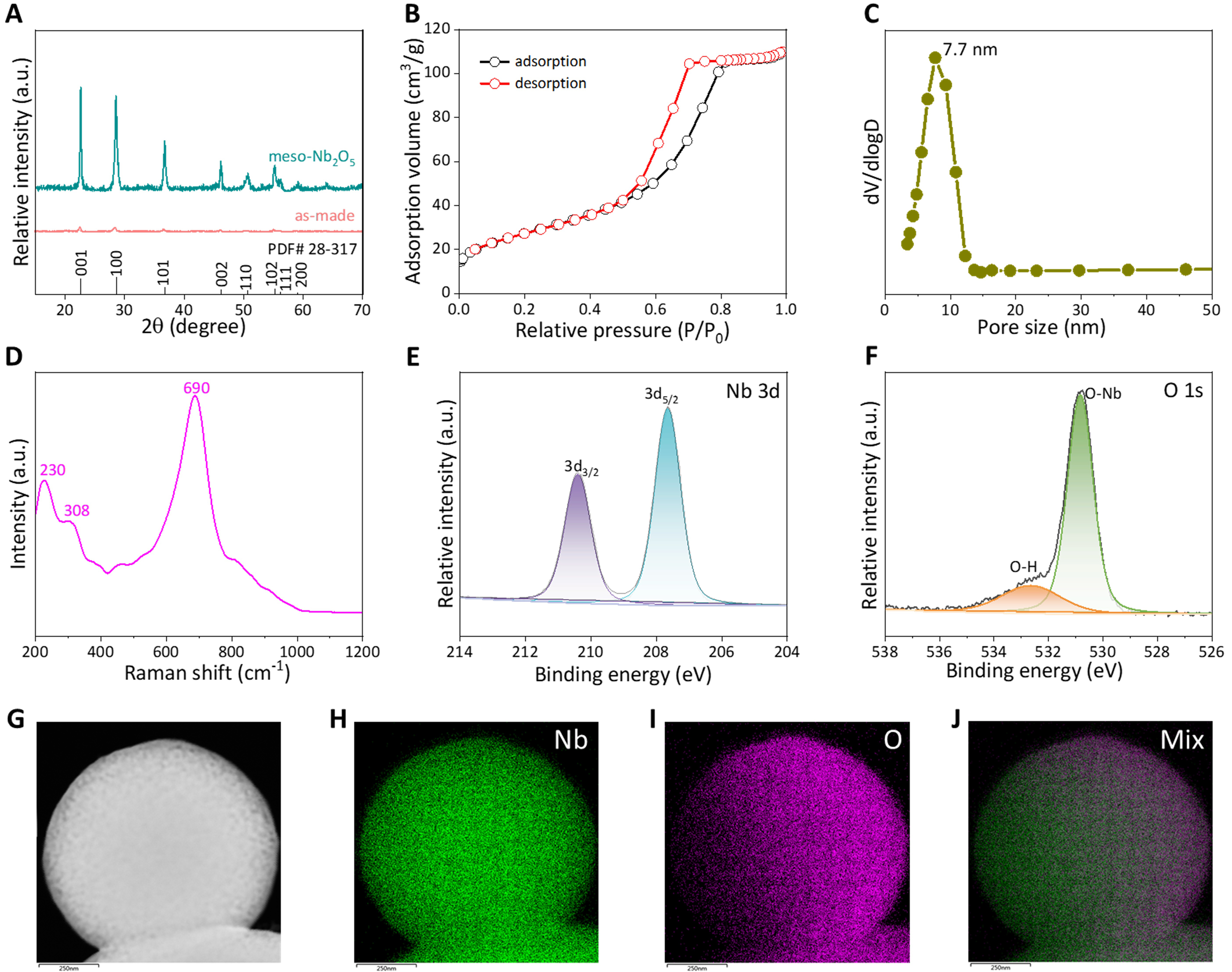
As shown in XRD patterns, the typical and sharp diffraction peaks are located at 22.6°, 28.6°, and 36.7°, which match with the (001), (100), and (101) planes, confirming that the mesoporous Nb2O5 is the high-crystalline hexagonal phase [Figure 2A][41,42]. Nitrogen adsorption and desorption curves exhibit a hysteresis loop at P/P0 = 0.5 to 0.8, suggesting the characteristic type IV curve [Figure 2B]. The well-defined mesoporous structure can also be evidenced by the pore size distribution curve centered at ~7.7 nm [Figure 2C]. The BET surface area and pore volume of meso-Nb2O5-500 are calculated to be 93 m2·g-1 and
Figure 2. Physicochemical characterizations. (A) XRD patterns of the mesoporous Nb2O5 microspheres before and after calcination; (B) Nitrogen adsorption-desorption isotherms; and (C) pore size distribution curve of the mesoporous Nb2O5 microspheres; (D) Raman spectrum of the mesoporous Nb2O5 microspheres; High-resolution XPS spectra of (E) Nb 3d, and (F) O 1s; (G-J) EDS images of the mesoporous Nb2O5 microspheres. XRD: X-ray diffraction; XPS: X-ray photoelectron spectra; EDS: energy dispersive X-ray spectrometry.
Porosity regulation and formation study
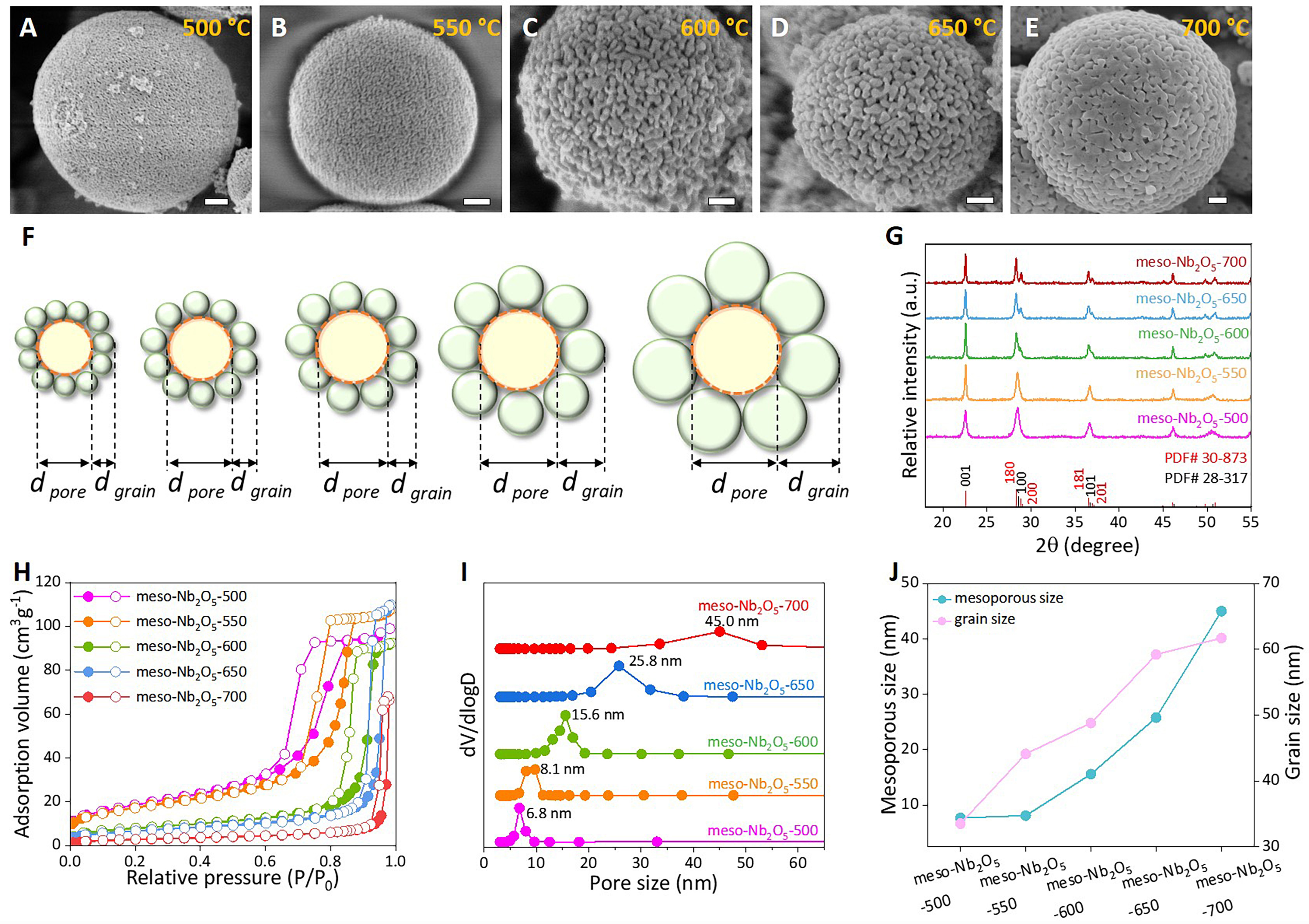
The structural parameters of the mesoporous Nb2O5 microspheres can be highly regulated by controlling synthetic conditions. A series of mesoporous Nb2O5 microspheres with tunable grain size and pore diameter can be obtained at different calcination temperatures. With the gradual increase of the calcination temperature, the spherical shape remains unchanged while the grain size shows a growing tendency
Figure 3. Porosity regulation. (A-E) SEM images of the mesoporous Nb2O5 microspheres after calcination at varied temperatures from 500 to 700 °C in air. Scale bars in (A-E) are all 200 nm; (F) Planar structural illustrations of the mesopores accumulated from Nb2O5 grains. The dpore stands for the mesopore size and the dgrain is grain size; (G) XRD patterns; (H) nitrogen adsorption-desorption isotherms and (I) pore size distribution curves of the mesoporous Nb2O5 samples calcined in air at different temperatures from 500 to 700 °C; (J) Plots of variations for mesopore and grain size versus calcination temperature. SEM: Scanning electron microscopy; XRD: X-ray diffraction.
The formation process was investigated by terminating the reaction at different time intervals. A TEM image [Figure 1F] shows the formation of mesoporous Nb2O5 microspheres with a uniform diameter after hydrothermal heating at 150 °C for 24 h. SEM images demonstrate the samples harvested at various hydrothermal time intervals. As illustrated in Supplementary Figure 4, smaller Nb2O5 particles can be generated after hydrothermal reaction for just 2 h, which implies the nucleation and growth of nanocrystals is very fast and hard to match with templates that require moderate assembly. As the hydrothermal reaction proceeds, the spherical size increases gradually to a micrometer scale, indicating a bottom-up progression from small grains to large microspheres. Additionally, the control experiment was conducted with 60 mL of ethanol, showing a constant spherical mesoporous Nb2O5 with slightly worse uniformity [Supplementary Figure 5], which suggests the minor effect of concentration on the nanostructural formation.
According to the above results, uniform mesoporous Nb2O5 microspheres can be basically formed through the following procedures: the hydrolysis and condensation of a niobium precursor, aggregation of tiny grains into a sphere, and crystallite enhancement [Supplementary Figure 6]. Initially, the metal source NbCl5 is hydrolyzed and condensed in ethanol solution to form hydrated niobium under the thermodynamic driving force. Afterward, these metal hydrates start to dehydrate and crystallize into massive tiny nanometer-sized oxide grains during the hydrothermal process. As the hydrothermal synthesis proceeds, the small microcrystalline units aggregate into larger nanospheres due to the lowest surface energy of spheres, which is followed by gradual growth of spherical size to a micrometer scale. After further high-temperature calcination to improve crystallinity, the mesoporous Nb2O5 microspheres with increased grain and pore size are finally generated. In summary, such a simple synthetic process is easy for manipulation over parameters, produces high crystallinity, and can be available for large-quantity preparation.
Electrochemical performances
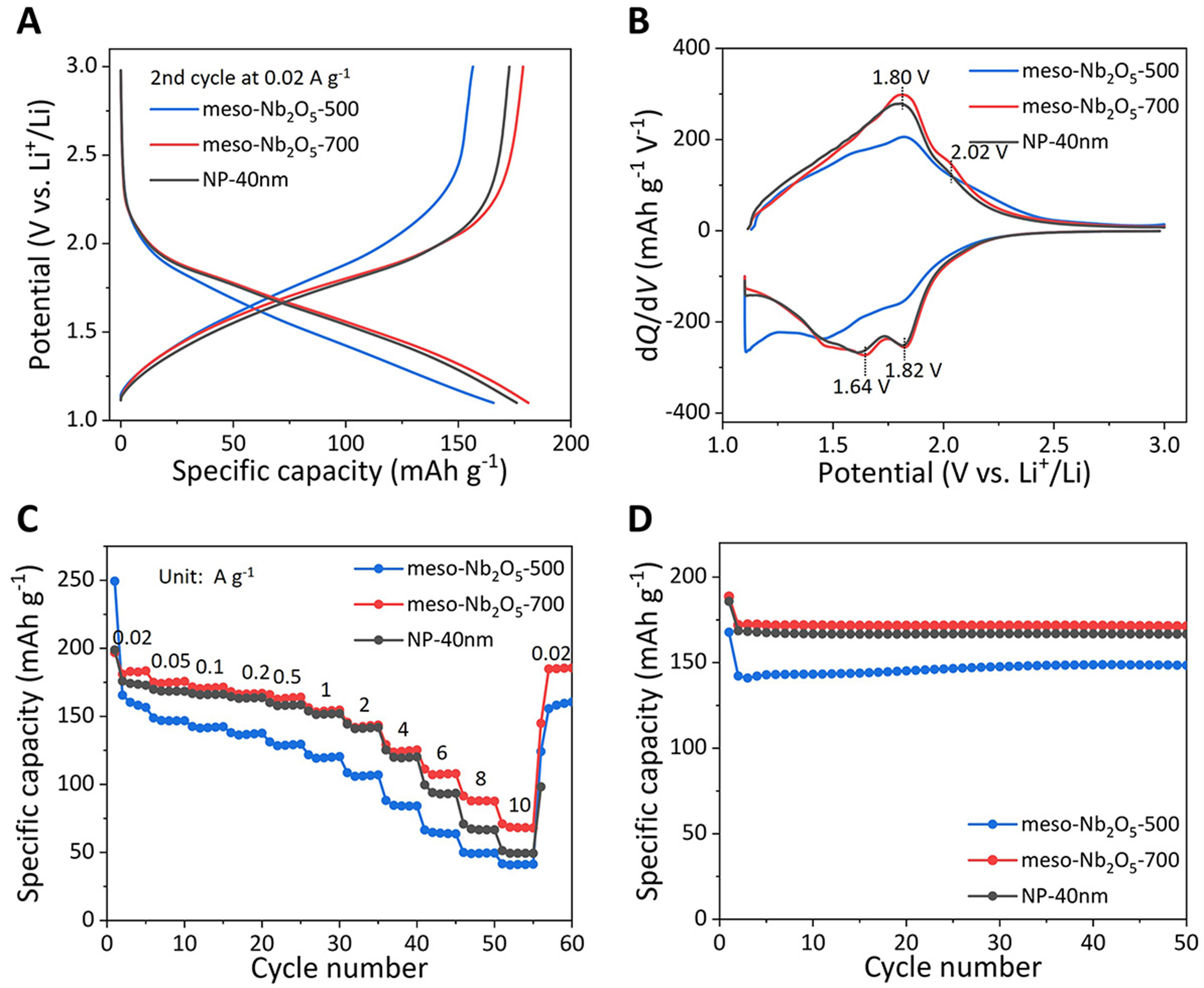
The electrochemical lithium-ion storage properties of the Nb2O5 mesoporous microspheres and loose nanoparticles [Supplementary Figure 7] were tested in half cells. We measured the electrochemical properties of single-phase meso-Nb2O5-500, meso-Nb2O5-700 and NP-40 nm electrodes, aiming to explore the potential of the mesoporous spheres compared to loose Nb2O5 nanoparticles. The electrolyte decomposed and formed a solid electrolyte interface in 1-3 V. Thus, we set the potential range between 1.1-3 V to avoid adverse reactions [Supplementary Figure 8]. As shown in Figure 4A and Supplementary Figure 9, the meso-Nb2O5-500, meso-Nb2O5-700, and NP-40 nm electrodes all exhibit sloping GCD curves, which correspond to the typical pseudocapacitive lithium-ion storage reactions of Nb2O5. These three electrodes provide initial discharge capacities of 249.3, 196.9, and 198.9 mAh·g-1 at 0.02 A·g-1, and the irreversible capacity contribution during the 1st cycle is mainly ascribed to the trapping of lithium ions by defects and the formation of solid electrolyte interface[46]. The meso-Nb2O5-700 provides a higher reversible capacity than the meso-Nb2O5-500, due to more suitable Li+ accommodation of T-Nb2O5 than the TT-Nb2O5[47]. The peaks situated at 1.82/2.02 V and 1.64/1.80 V correspond to the Li+ intercalation and deintercalation with redox reactions involving the couples of Nb5+/Nb4+ and Nb4+/Nb3+ [Figure 4B][48]. The rate performances
Figure 4. Electrochemical properties for lithium-ion storage. (A) GCD curves and (B) corresponding dQ/dV plots of the
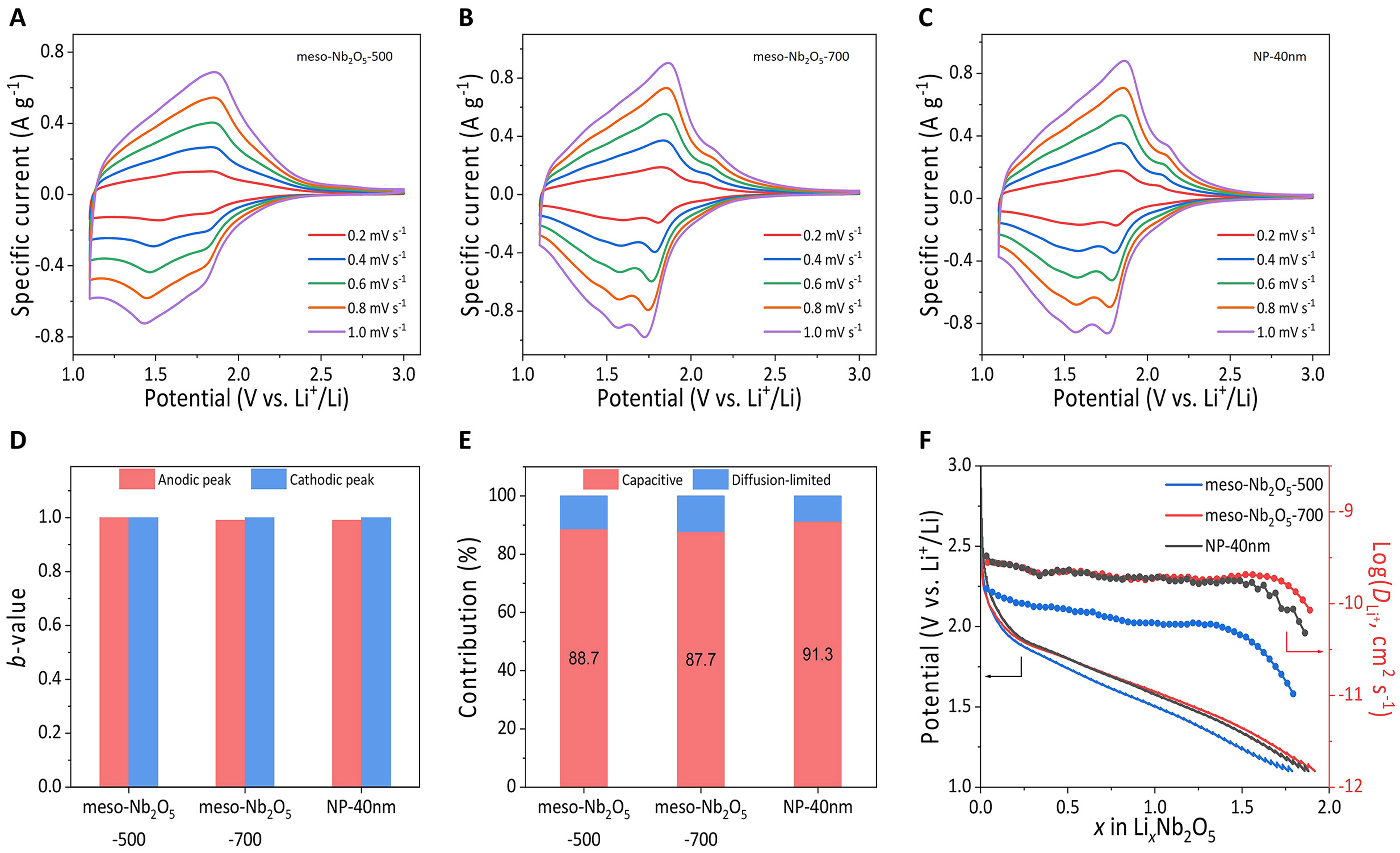
To analyze the electrochemical kinetics, CV curves at different sweep rates were performed. All three electrodes exhibit broad Li+ intercalation/deintercalation peaks, consistent with the dQ/dV plots [Figure 5A-C]. With the increase of sweep rates, the response currents from the three electrodes all rise linearly. The response peak current (i) vs. sweep rate (v) meets the rule[47]: i = avb, where the b-value of 1.0 represents a capacitance-dominated process and 0.5 indicates diffusion-controlled. By employing a linear fitting on the slope of log(i) vs. log(v) plots [Supplementary Figure 12], it was found that b-values of the anodic and cathodic peaks for meso-Nb2O5-500, meso-Nb2O5-700 and NP-40 nm are all close to 1.0 [Figure 5D], indicating the pseudocapacitive processes with fast reaction kinetics, consistent with reported results[50-52]. Furthermore, the current responses of the lithium-ion storage process consist mainly of the contribution from capacitance (k1v) and diffusion (k2v1/2)[53]: i = k1v + k2v1/2. The capacities of three electrodes are overwhelmingly dominated by pseudocapacitance with contributions over 85% at 0.2 mV·s-1 [Figure 5E and Supplementary Figure 13]. From GITT measurements, Li+ diffusion coefficient (
Figure 5. Charge storage kinetics. CV curves of the (A) meso-Nb2O5-500, (B) meso-Nb2O5-700, and (C) NP-40 nm electrodes at sweep rates varied from 0.2 to 1.0 mV·s-1; The fitted b-values of (D) anodic and cathodic peaks, along with the (E) simulated contributions from capacitance and diffusion at 0.2 mV·s-1 for the three electrodes; (F) GITT curves and
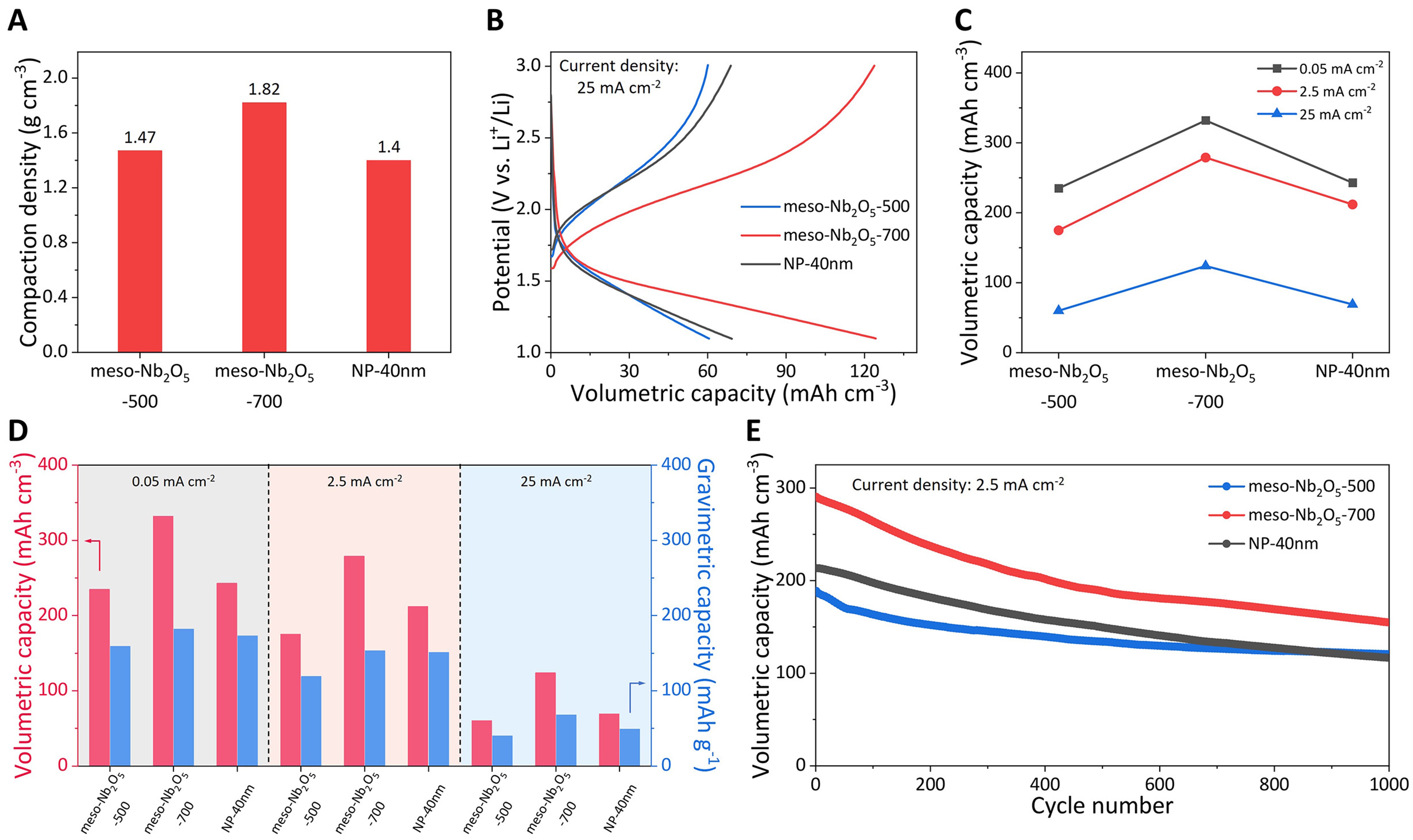
Nanomaterials used in electrode materials shorten the cation diffusion distance, but a large number of voids lead to low compaction density, which compromises the volumetric capacity. We further explored the potential of mesoporous materials in volumetric capacity utilization. The cross-section SEM images show the thinner thickness of meso-Nb2O5-700 electrodes [Supplementary Figure 14] due to the spatially packed mesoporous structure. The electrode compaction densities of the meso-Nb2O5-500, meso-Nb2O5-700 and NP-40 nm electrodes are 1.47, 1.82, and 1.4 g·cm-3, respectively [Figure 6A and Supplementary Table 2][45]. At 0.05 mA·cm-2, the meso-Nb2O5-700 achieves a volumetric capacity of as high as 333 mAh·cm-3 [Supplementary Figure 15A]. The meso-Nb2O5-700 electrode still provides a volumetric capacity of
Figure 6. Volumetric capacity utilization. (A) Electrode compaction density of the meso-Nb2O5-500, meso-Nb2O5-700 and NP-40 nm electrodes; (B) GCD curves of the three electrodes at 25 mA·cm-2; (C) The volumetric capacities of the three electrodes at different current densities; (D) The gravimetric and volumetric capacities of the three electrodes; (E) Cycling performance at 2.5 mA·cm-2 of the three electrodes. GCD: Galvanostatic charge and discharge.
CONCLUSIONS
In summary, a simple template-free method is presented to synthesize uniform mesoporous Nb2O5 microspheres. The prepared mesoporous Nb2O5 microspheres have controllable porosity and crystallinity, such as adjustable large grain size (33.5 to 61.7 nm) and pore size (7.7 to 45.0 nm). The LIB anode utilizing this mesoporous Nb2O5 microspheres enables highly reversible pseudocapacitive charge storage, including a reversible capacity of 181 mAh·g-1 at 0.05 mA·cm-2, volumetric capacity of 280 mAh·cm-3 at 2.5 mA·cm-2, rate capability and 1,000 cycles at 2.5 mA·cm-2. Our study presents a pathway for the facile synthesis of high-crystalline mesoporous metal oxides, which could have implications in preparing inorganic materials for advanced potential applications.
DECLARATIONS
Authors’ contributions
Made substantial contributions to conception and design of the study, performed data analysis and interpretation, and wrote the original draft: Yang, L.; Tang, D.
Performed data acquisition and provided administrative, technical, and material support: Li, R.; Zhang, J.; Wang, W.; Li, J.; He, Y.; Wen, X.
Discussed and revised the manuscript and provided administrative support: Lan, K.; Wei, Q.
Availability of data and materials
The original contributions presented in this study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author(s).
Financial support and sponsorship
Lan, K. acknowledges the support by the National Natural Science Foundation of China (22205118 and 22375108), National Key R&D Program of China (2024YFE0101100), Junma Program of Inner Mongolia University, Grassland Talent Program of Inner Mongolia, Young Talents of Science and Technology of Inner Mongolia (NJYT23037), and Natural Science Foundation of Inner Mongolia (2023JQ06). Wei, Q. appreciates the support from the National Natural Science Foundation of China (22179113) and the Fundamental Research Funds for the Central Universities (20720230028).
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
The Author(s) 2026.
Supplementary Materials
REFERENCES
1. Li, W.; Liu, J.; Zhao, D. Mesoporous materials for energy conversion and storage devices. Nat. Rev. Mater. 2016, 1, 16023.
2. Duan, L.; Wang, C.; Zhang, W.; et al. Interfacial assembly and applications of functional mesoporous materials. Chem. Rev. 2021, 121, 14349-429.
3. Zu, L.; Zhang, W.; Qu, L.; et al. Mesoporous materials for electrochemical energy storage and conversion. Adv. Energy. Mater. 2020, 10, 2002152.
4. Kresge, C. T.; Leonowicz, M. E.; Roth, W. J.; Vartuli, J. C.; Beck, J. S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710-2.
5. Zhao, D.; Feng, J.; Huo, Q.; et al. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 1998, 279, 548-52.
6. Prabhu, P.; Lee, J. M. Metallenes as functional materials in electrocatalysis. Chem. Soc. Rev. 2021, 50, 6700-19.
7. Yue, Q.; Sun, J.; Kang, Y.; Deng, Y. Advances in the interfacial assembly of mesoporous silica on magnetite particles. Angew. Chem. Int. Ed. Engl. 2020, 59, 15804-17.
8. Harper, G.; Sommerville, R.; Kendrick, E.; et al. Recycling lithium-ion batteries from electric vehicles. Nature 2019, 575, 75-86.
9. Zhang, X.; Li, X.; Zhang, Y.; et al. Accelerated Li+ desolvation for diffusion booster enabling low-temperature sulfur redox kinetics via electrocatalytic carbon-grazfted-CoP porous nanosheets. Adv. Funct. Mater. 2023, 33, 2302624.
10. Qin, J.; Yang, Z.; Xing, F.; Zhang, L.; Zhang, H.; Wu, Z. Two-dimensional mesoporous materials for energy storage and conversion: current status, chemical synthesis and challenging perspectives. Electrochem. Energy. Rev. 2023, 6, 177.
11. Yang, X.; Shi, Y.; Xie, K.; Fang, S.; Zhang, Y.; Deng, Y. Cocrystallization enabled spatial self-confinement approach to synthesize crystalline porous metal oxide nanosheets for gas sensing. Angew. Chem. Int. Ed. Engl. 2022, 61, e202207816.
12. Xiong, H.; Zhou, H.; Sun, G.; et al. Solvent-free self-assembly for scalable preparation of highly crystalline mesoporous metal oxides. Angew. Chem. Int. Ed. Engl. 2020, 59, 11053-60.
13. Xiong, H.; Zhou, H.; Qi, C.; et al. Polymer-oriented evaporation induced self-assembly strategy to synthesize highly crystalline mesoporous metal oxides. Chem. Eng. J. 2020, 398, 125527.
14. Hu, M.; Yang, W.; Tan, H.; et al. Template-free synthesis of mesoporous and crystalline transition metal oxide nanoplates with abundant surface defects. Matter 2020, 2, 1244-59.
15. Zhang, H.; Banfield, J. F. Structural characteristics and mechanical and thermodynamic properties of nanocrystalline TiO2. Chem. Rev. 2014, 114, 9613-44.
16. Li, W.; Wu, Z.; Wang, J.; Elzatahry, A. A.; Zhao, D. A perspective on mesoporous TiO2 materials. Chem. Mater. 2014, 26, 287-98.
17. Lan, K.; Zhao, D. Functional ordered mesoporous materials: present and future. Nano. Lett. 2022, 22, 3177-9.
18. Lan, K.; Liu, L.; Yu, J.; et al. Stepwise monomicelle assembly for highly ordered mesoporous TiO2 membranes with precisely tailored mesophase and porosity. JACS. Au. 2023, 3, 1141-50.
19. Jiang, Y.; Jiang, N.; Liang, K.; Yuan, C.; Fang, X.; Xu, A. A simple and general route to prepare functional mesoporous double-metal oxy(hydroxide). J. Mater. Chem. A. 2019, 7, 7932-8.
20. Cölfen, H.; Antonietti, M. Mesocrystals: inorganic superstructures made by highly parallel crystallization and controlled alignment. Angew. Chem. Int. Ed. Engl. 2005, 44, 5576-91.
21. Zhao, X.; Kalidas, N.; Lehto, V. Self-standing mesoporous Si films as anodes for lithium-ion microbatteries. J. Power. Sources. 2022, 529, 231269.
23. Leng, H.; Zhang, P.; Wu, J.; et al. The elemental pegging effect in locally ordered nanocrystallites of high-entropy oxide enables superior lithium storage. Nanoscale 2023, 15, 19139-47.
24. Li, Y.; Luo, W.; Qin, N.; et al. Highly ordered mesoporous tungsten oxides with a large pore size and crystalline framework for H2S sensing. Angew. Chem. Int. Ed. 2014, 126, 9181-6.
25. Ma, J.; Ren, Y.; Zhou, X.; et al. Pt nanoparticles sensitized ordered mesoporous WO3 semiconductor: gas sensing performance and mechanism study. Adv. Funct. Mater. 2018, 28, 1705268.
26. Brezesinski, T.; Wang, J.; Tolbert, S. H.; Dunn, B. Ordered mesoporous α-MoO3 with iso-oriented nanocrystalline walls for thin-film pseudocapacitors. Nat. Mater. 2010, 9, 146-51.
27. Luo, Z.; Miao, R.; Huan, T. D.; et al. Mesoporous MoO3-x material as an efficient electrocatalyst for hydrogen evolution reactions. Adv. Energy. Mater. 2016, 6, 1600528.
28. Peng, L.; Peng, H.; Wang, S.; et al. One-dimensionally oriented self-assembly of ordered mesoporous nanofibers featuring tailorable mesophases via kinetic control. Nat. Commun. 2023, 14, 8148.
29. Chen, D.; Peng, L.; Yuan, Y.; et al. Two-dimensional holey Co3O4 nanosheets for high-rate alkali-ion batteries: from rational synthesis to in situ probing. Nano. Lett. 2017, 17, 3907-13.
30. Guan, M. H.; Dong, L. Y.; Wu, T.; Li, W. C.; Hao, G. P.; Lu, A. H. Boosting selective oxidation of ethylene to ethylene glycol assisted by in situ generated H2O2 from O2 electroreduction. Angew. Chem. Int. Ed. Engl. 2023, 62, e202302466.
31. Sun, Z.; Liao, T.; Dou, Y.; et al. Generalized self-assembly of scalable two-dimensional transition metal oxide nanosheets. Nat. Commun. 2014, 5, 3813.
32. Liu, Y.; Lan, K.; Bagabas, A. A.; et al. Ordered macro/mesoporous TiO2 hollow microspheres with highly crystalline thin shells for high-efficiency photoconversion. Small 2016, 12, 860-7.
33. Lan, K.; Wei, Q.; Zhao, D. Versatile synthesis of mesoporous crystalline TiO2 materials by monomicelle assembly. Angew. Chem. Int. Ed. Engl. 2022, 61, e202200777.
34. Yang, H.; Xu, R.; Gong, Y.; Yao, Y.; Gu, L.; Yu, Y. An interpenetrating 3D porous reticular Nb2O5@carbon thin film for superior sodium storage. Nano. Energy. 2018, 48, 448-55.
35. Kim, K.; Park, B.; Kim, J.; Jo, C.; Kim, J. K. Recent progress in block copolymer soft-template-assisted synthesis of versatile mesoporous materials for energy storage systems. J. Mater. Chem. A. 2023, 11, 7358-86.
36. Kim, H.; Lim, E.; Jo, C.; et al. Ordered-mesoporous Nb2O5/carbon composite as a sodium insertion material. Nano. Energy. 2015, 16, 62-70.
37. Luo, W.; Li, Y.; Dong, J.; et al. A resol-assisted co-assembly approach to crystalline mesoporous niobia spheres for electrochemical biosensing. Angew. Chem. Int. Ed. Engl. 2013, 52, 10505-10.
38. Brezesinski, K.; Wang, J.; Haetge, J.; et al. Pseudocapacitive contributions to charge storage in highly ordered mesoporous group V transition metal oxides with iso-oriented layered nanocrystalline domains. J. Am. Chem. Soc. 2010, 132, 6982-90.
39. Wang, L.; Bi, X.; Yang, S. Partially single-crystalline mesoporous Nb2O5 nanosheets in between graphene for ultrafast sodium storage. Adv. Mater. 2016, 28, 7672-9.
40. Zhou, H.; Zhang, D.; Xie, H.; et al. Modulating oxygen vacancies in lead chromate for photoelectrocatalytic water splitting. Adv. Mater. 2023, 35, e2300914.
41. Sun, L.; Sun, J.; Zhai, S.; et al. Nb2CTx MXene derived polymorphic Nb2O5. Small 2023, 19, e2300914.
42. Han, J.; Liu, Z.; Ma, Y.; et al. Ambient N2 fixation to NH3 at ambient conditions: using Nb2O5 nanofiber as a high-performance electrocatalyst. Nano. Energy. 2018, 52, 264-70.
43. Wang, X.; Li, Q.; Zhang, L.; et al. Caging Nb2O5 nanowires in PECVD-derived graphene capsules toward bendable sodium-ion hybrid supercapacitors. Adv. Mater. 2018, 30, e1800963.
44. Lin, X.; Xia, S.; Zhang, L.; et al. Fabrication of flexible mesoporous black Nb2O5 nanofiber films for visible-light-driven photocatalytic CO2 reduction into CH4. Adv. Mater. 2022, 34, e2200756.
45. Lan, K.; Liu, L.; Zhang, J. Y.; et al. Precisely designed mesoscopic titania for high-volumetric-density pseudocapacitance. J. Am. Chem. Soc. 2021, 143, 14097-105.
46. Cheong, J. Y.; Jung, J.; Youn, D.; et al. Mesoporous orthorhombic Nb2O5 nanofibers as pseudocapacitive electrodes with ultra-stable Li storage characteristics. J. Power. Sources. 2017, 360, 434-42.
47. Hu, Z.; He, Q.; Liu, Z.; et al. Facile formation of tetragonal-Nb2O5 microspheres for high-rate and stable lithium storage with high areal capacity. Sci. Bull. 2020, 65, 1154-62.
48. Jing, P.; Liu, K.; Soule, L.; et al. Engineering the architecture and oxygen deficiency of T-Nb2O5-carbon-graphene composite for high-rate lithium-ion batteries. Nano. Energy. 2021, 89, 106398.
49. Ding, X.; Lin, J.; Huang, H.; Zhao, B.; Xiong, X. Competitive redox chemistries in vanadium niobium oxide for ultrafast and durable lithium storage. Nanomicro. Lett. 2023, 15, 195.
50. Wei, Q.; Chang, X.; Butts, D.; et al. Surface-redox sodium-ion storage in anatase titanium oxide. Nat. Commun. 2023, 14, 7.
51. Meng, J.; He, Q.; Xu, L.; et al. Identification of phase control of carbon-confined Nb2O5 nanoparticles toward high-performance lithium storage. Adv. Energy. Mater. 2019, 9, 1802695.
52. Griffith, K. J.; Forse, A. C.; Griffin, J. M.; Grey, C. P. High-rate intercalation without nanostructuring in metastable Nb2O5 bronze phases. J. Am. Chem. Soc. 2016, 138, 8888-99.
53. Zheng, Y.; Qiu, W.; Wang, L.; Liu, J.; Chen, S.; Li, C. Triple conductive wiring by electron doping, chelation coating and electrochemical conversion in fluffy Nb2O5 anodes for fast-charging Li-ion batteries. Adv. Sci. 2022, 9, e2202201.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Copyright
Data & Comments
Data
















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].