Efficient photocatalytic hydrogen production under visible-light irradiation with 2D Molybdenum nitride cocatalyst
Abstract
Efficient visible-light-driven hydrogen production in the absence of noble metal is one of the major challenges for the application of photocatalytic water splitting. Herein, using cadmium sulfide (CdS) as a typical photocatalyst, we report on the synthesis of composite photocatalyst (CdS/MN) with CdS nanoparticles attached on two-dimensional (2D) layered molybdenum nitride (MN). The introduction of MN brings drastically enhanced photocatalytic activity of CdS by more than eight times, with an optimized hydrogen-production rate of
Keywords
INTRODUCTION
Photocatalytic water splitting offers a sustainable approach to convert and store solar energy in the form of hydrogen[1-3]. Since the initial discovery in 1972[4], extensive research has been conducted on various photocatalysts for hydrogen production, such as TiO2[5,6], SrTiO3[7,8], C3N4[9,10], cadmium sulfide (CdS)[11,12], etc. CdS, in particular, has attracted significant research interest due to the narrow band gap for visible-light utilization and the abundant elemental source for low-cost synthesis[13]. Nevertheless, as a typical direct bandgap semiconductor, CdS suffers from the fast recombination of photogenerated electrons and holes that limits the photocatalytic efficiency. Therefore, charge separation and surface reaction of CdS photocatalyst have been extensively investigated for improved hydrogen production. Strategies such as coupling CdS with other semiconductor photocatalysts, including TiO2, to form heterojunctions[14,15], establishing homojunctions of CdS with different crystal facets or phases to facilitate charge transfer via suitable band alignment[16,17], and fabricating one-dimensional (1D) or two-dimensional (2D) structures to reduce the distance of charge transfer from the bulk to the surface[18,19], have been developed.
Modification with cocatalysts provides an effective way to simultaneously accelerate charge separation and boost surface reaction, especially for metal cocatalysts[20]. Since the fermi level of metal is generally lower than that of semiconductor photocatalyst, photogenerated electrons could be efficiently extracted from the bulk to the surface of photocatalyst, and then to the surface of metal cocatalyst, thus facilitating charge separation[21]. Additionally, the Gibbs free energy variation for hydrogen adsorption and desorption is relatively low for metal cocatalyst, enabling easier proton reduction reaction and enhancing hydrogen production compared to typical semiconductors[22]. Noble metals, such as Pt and Au, have been reported as highly effective cocatalysts on CdS for photocatalytic hydrogen production[23,24]. Considering the cost implications for the future large-scale application of photocatalytic hydrogen production, there is a pressing need to explore efficient noble-metal free cocatalysts. Among them, 2D materials have garnered substantial interest in catalysis due to their unique properties[25], including abundant catalytic active sites from high specific surface area and effective charge transfer and separation via minimized thickness. Recently, 2D metal nitrides have been reported as efficient catalysts for hydrogen evolution reaction (HER)[26], demonstrating great potential as cocatalysts in photocatalytic water splitting.
Herein, we report on the fabrication of a novel photocatalyst composed of CdS and molybdenum nitride (MN). Detailed characterization and theoretical analysis reveal that the introduction of 2D metallic MN not only accelerates charge separation in CdS but also provides abundant reactive sites and lowers the overpotential for HER. Given the photocatalytic performance of highly efficient hydrogen production, MN is demonstrated as an excellent cocatalyst, with the corresponding mechanism elucidated. This work could inspire the development of low-cost photocatalytic systems for efficient energy conversion.
EXPERIMENTAL
Chemicals
Sodium hydroxide, cadmium acetate, thioacetamide, MoO3, Na2MoO4, and lactic acid were analytical grade, purchased from Sinopharm Chemical Reagent Co., Ltd, and were used without further purification. The deionized water was used in experiments with a resistivity of 18.2 MΩ·cm.
Synthesis of photocatalysts
CdS was synthesized by hydrothermal method. Sodium hydroxide, cadmium acetate, and thioacetamide were dissolved in water and stirred for ten minutes. The resulting mixture was then transferred into a Teflon-lined stainless autoclave and heated at 180 °C for 24 h. After naturally cooling to room temperature, the as-obtained precipitate was collected and rinsed with water and ethanol by centrifugation. The sample was subsequently dried in a vacuum oven and designated as CdS.
MN was synthesized by modified nitride treatment of the ball-milled mixture of MoO3 and Na2MoO4 powders with a molar ratio of 1:1[26]. The nitride treatment was conducted at 650 °C for 15 h with NH3 gas. The product was washed with deionized water by ultrasonication, then dried in a vacuum oven, and designated as MN.
The composites of CdS and MN were synthesized by mixing a certain amount of MN aqueous suspension and CdS aqueous suspension by ultrasonication. The resulting products were then subjected to centrifugation, and dried in a vacuum oven. Composite photocatalysts of CdS with different weight percentages of MN were fabricated, in which CdS/MN refers to that with 20 wt.% of MN during later discussion.
Characterizations
The characterization details were provided in the Supplementary Materials.
Photocatalytic tests
Photocatalytic H2 production experiments were conducted in a Pyrex glass cell with a side window for light irradiation. A 300W Xenon lamp equipped with a cutoff filter (λ > 420 nm) was used as the light source. Typically, 4 mg of the photocatalyst was dispersed into 80 mL of lactic acid aqueous solution (20 v%), where lactic acid served as the hole scavenger. After purging with argon gas to eliminate air, the dispersion was irradiated under light with a constant stirring velocity at 25 °C. The evolved H2 was analyzed by gas chromatography with Ar as carrier gas, a 5A molecular sieve column and a thermal conductivity detector (TCD).
The apparent quantum yield (AQY) was calculated according to:
Where n(H2) is the amount of H2 produced, NA is Avogadro’s constant, h is Planck’s constant, c is the speed of light, t is the irradiation time, λ is the wavelength, I is the light intensity, and A is the irradiated area.
RESULTS AND DISCUSSION
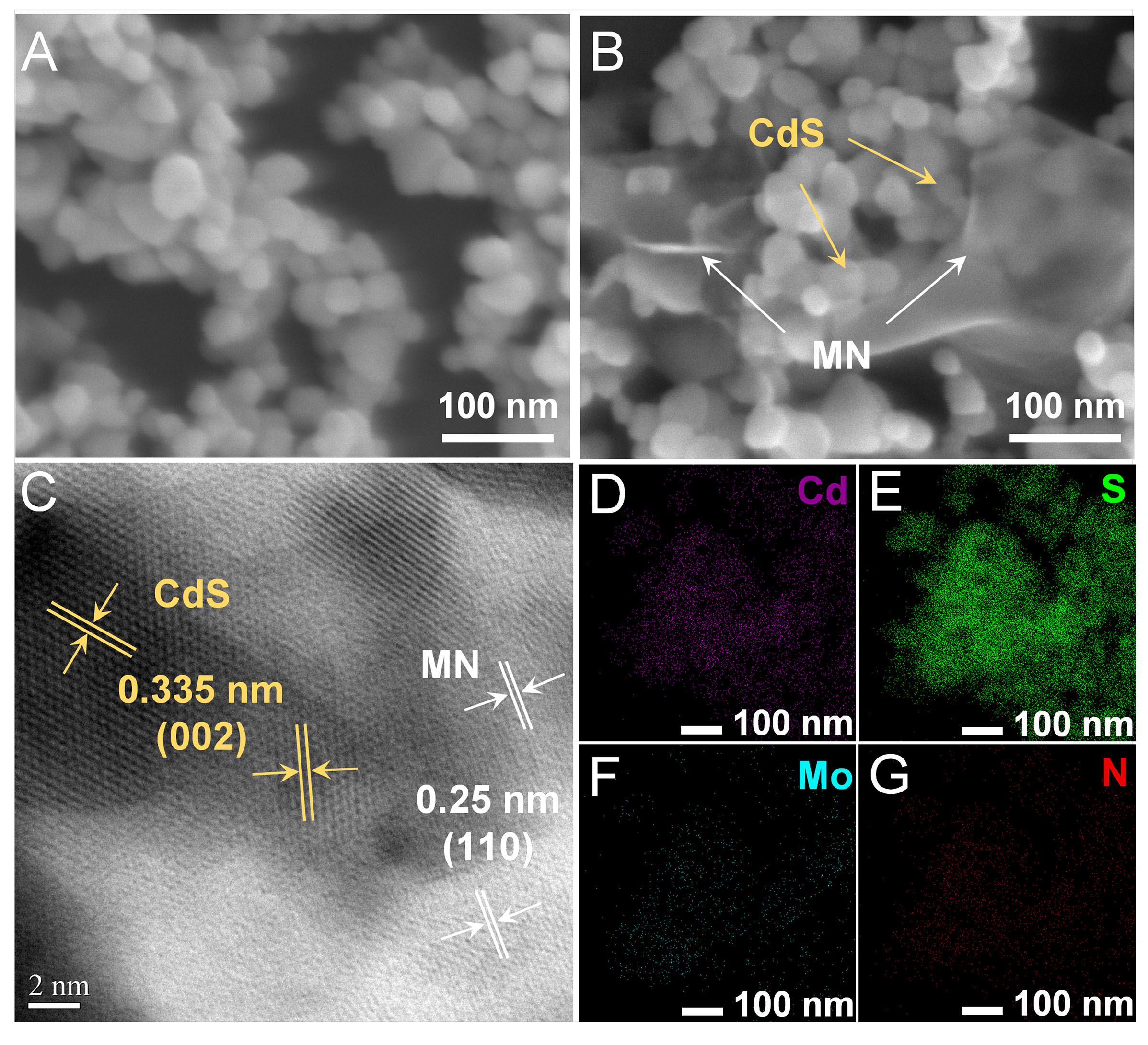
Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) images of CdS, MN and CdS/MN are shown in Figure 1, Supplementary Figures 1 and 2. It could be observed from Figure 1A that CdS is nanoparticles with size of 30~80 nm, while the morphology of as-prepared MN shown in Supplementary Figure 1 is predominantly 2D layered nanosheet with hundreds of nanometers in size. TEM and high-resolution TEM (HRTEM) images in Supplementary Figure 2 further support the layered structure of as-prepared MN. Figure 1B reveals that both CdS and MN are present in the SEM image of CdS/MN, in which CdS particles are tightly attached to the surface of 2D MN, with some CdS particles wrapped inside the layers of MN. It should be noted that since only 20 wt% of MN is utilized in the CdS/MN composite, self-aggregation of some CdS particles also occurs. A HRTEM image in Figure 1C provides further evidence of the combination of CdS and MN. Lattice fringes with a distance of 0.25 nm corresponding to the (110) facet of MoN1.2[26], and the (002) facet of hexagonal CdS with a lattice distance of 0.335 nm[27] offer a clearer view of the intimate contact between CdS and MN for potential charge transfer. Additionally, elemental mapping images in Figure 1D-G demonstrate uniform dispersion of Cd, S, Mo, and N, respectively, further confirming the good combination of CdS and MN.
Figure 1. SEM images of (A) CdS, and (B) CdS/MN composite; (C) HRTEM and (D-G) elemental mapping images of CdS/MN. SEM: Scanning electron microscopy; CdS/MN: cadmium sulfide/molybdenum nitride; HRTEM: high-resolution transmission electron microscopy.
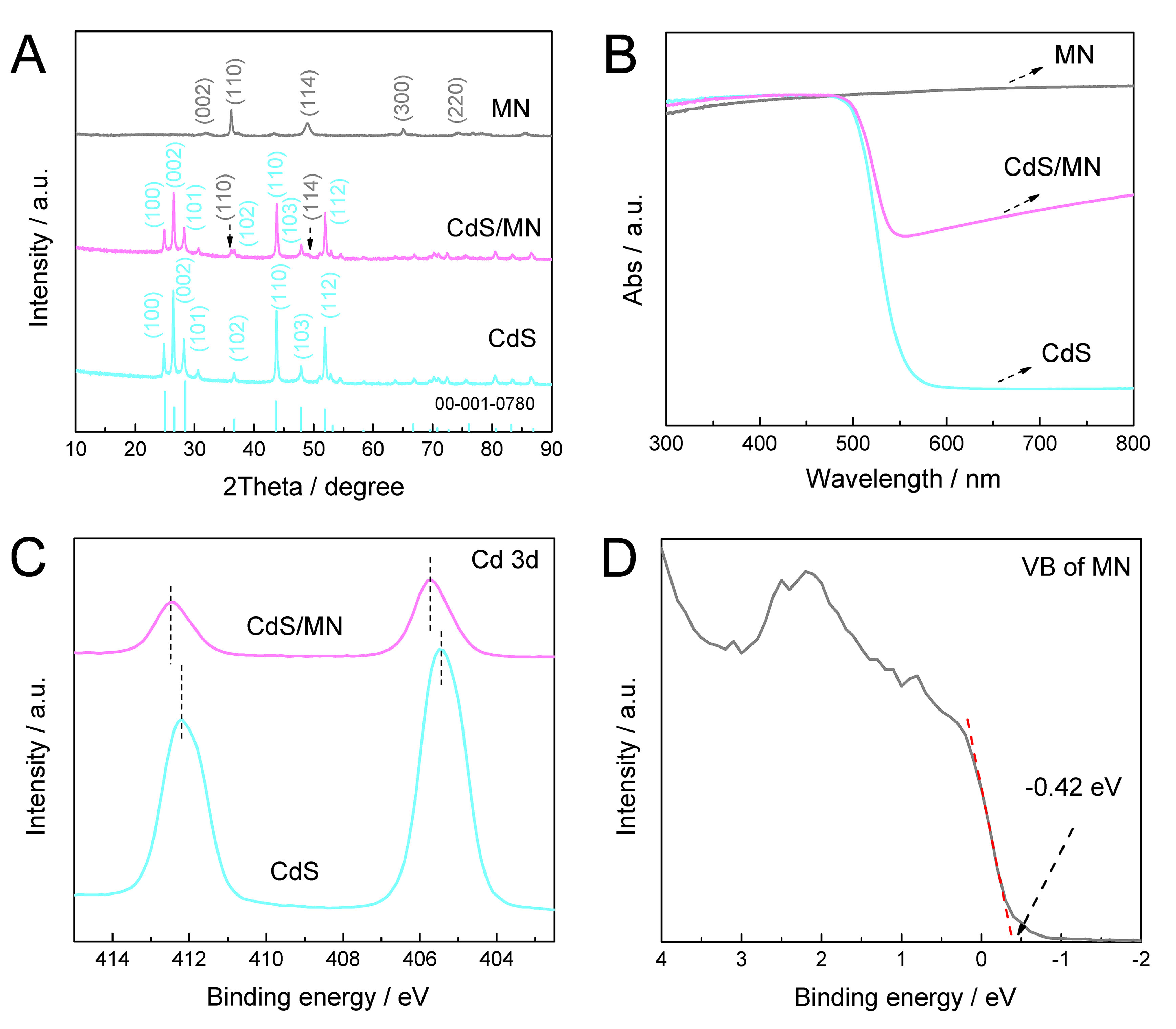
Figure 2A and Supplementary Figure 3 show the X-ray diffraction (XRD) patterns of as-prepared CdS, MN and CdS/MN. The diffraction pattern of CdS could be identified as hexagonal structure[27], and MN exhibits a similar XRD pattern with hexagonal Mo5N6. It is worth noting that the diffraction peaks of as-prepared MN are shifted towards low angles and the calculated lattice constants are significantly different when compared with standard Mo5N6, indicating the characteristics of 2D layered structure identified as
Figure 2. (A) XRD patterns and (B) UV-vis spectra of as-prepared CdS, MN and CdS/MN composite; (C) XPS spectra of Cd 3d orbitals of CdS and CdS/MN; (D) XPS VB spectrum of MN. XRD: X-ray diffraction; UV-vis: ultraviolet-visible; CdS/MN: cadmium sulfide/molybdenum nitride; XPS: X-ray photoelectron spectra; VB: valence band.
Figure 2B presents the light absorption of as-prepared samples determined by ultraviolet-visible (UV-vis) spectra. CdS exhibits a distinct absorption edge at ~550 nm, with an estimated band gap of ~2.3 eV (via Kubelka-Munk method, as shown in Supplementary Figure 4)[28]. MN exhibits high absorption across the wavelength region from 300 to 800 nm, originating from its metallic property[26]. Consequently, the light absorption in the long-wavelength region of CdS/MN is elevated, attributed to the presence of MN in the composite.
X-ray photoelectron spectra (XPS) were recorded to investigate the surface chemical state of as-prepared photocatalysts, and the results are shown in Figure 2C and D, and Supplementary Figure 5. As shown in Figure 2C, two peaks located at 405.4 and 412.2 eV correspond to the 3d5/2 and 3d3/2 orbitals of Cd2+, respectively[29], which are typical results for CdS photocatalyst. It is worth noting that these two peaks exhibit a significant shift toward higher binding energy in CdS/MN composite compared to CdS, and a similar shift is observed in S 2p orbitals [Supplementary Figure 5A]. Correspondingly, the peaks of Mo 3d orbitals in Supplementary Figure 5B exhibit a shift toward lower binding energy in CdS/MN composite compared to MN (the intensity of Mo 3d signals from CdS/MN were multiplied by 5 for better comparison). These results indicate possible electron transfer from CdS to MN within CdS/MN composite[30]. The valence band (VB) spectrum analysis of MN in Figure 2D further supports this phenomenon, as the minimum VB band position of MN from XPS test is below zero (estimated as
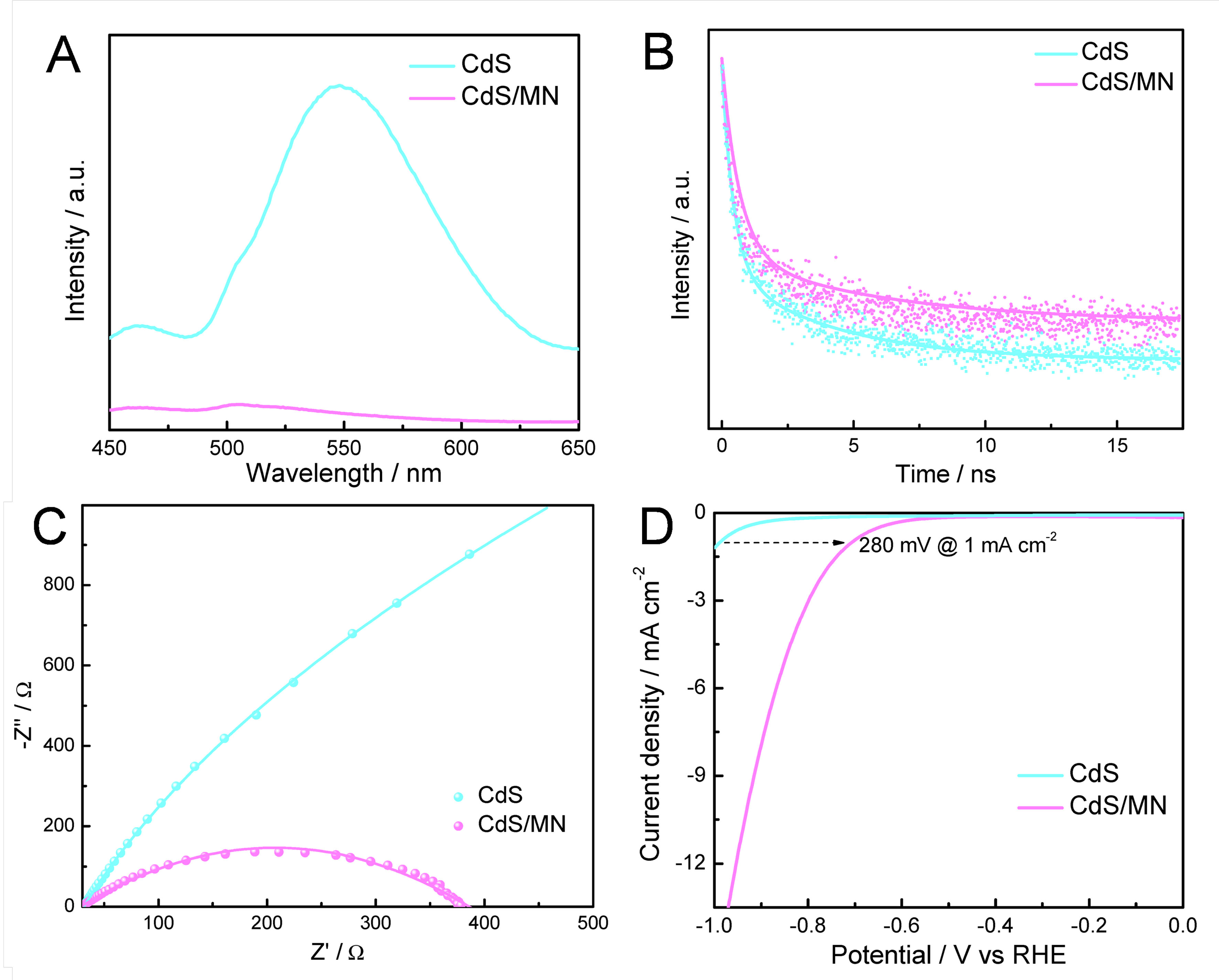
Photoluminescence (PL) spectra were recorded and the results are presented in Figure 3A. CdS exhibits an intense PL emission peak, and significantly lowered emission is observed on CdS/MN, indicating that the recombination of photogenerated charge carriers in CdS is greatly suppressed upon the introduction of MN[32]. Time-resolved PL (TRPL) was further tested to investigate the charge-transfer kinetics of CdS/MN composite. As shown in Figure 3B and Supplementary Table 2, the average lifetime of photogenerated charge carriers in CdS and CdS/MN is 3.40 and 4.63 ns, which demonstrates that the formation of CdS/MN composite promotes more efficient utilization of long-lived photogenerated electrons for HER[33].
Figure 3. (A) PL spectra, (B) TRPL spectra, (C) EIS spectra and (D) LSV curves of CdS and CdS/MN composite. PL: Photoluminescence; TRPL: time-resolved photoluminescence; EIS: electrochemical impedance spectroscopy; LSV: linear sweep voltammetry; CdS/MN: cadmium sulfide/molybdenum nitride; RHE: reversible hydrogen electrode.
Electrochemical impedance spectroscopy (EIS) curves in Figure 3C, represented by the Nyquist plot of CdS and CdS/MN, reveal a smaller arc radius for CdS/MN compared to CdS (fitted parameters in Supplementary Table 3). This further indicates the reduced charge transfer resistance and efficient charge separation in the CdS/MN composite photocatalyst[34]. Linear sweep voltammetry (LSV) curves in Figure 3D demonstrate a shift of onset potentials toward lower values for CdS/MN compared to CdS, indicating that the hydrogen production reaction could occur at relatively lower overpotentials on CdS/MN[35,36]. Therefore, it is revealed that MN could serve as cocatalyst in CdS/MN composite photocatalyst during photocatalytic reaction for hydrogen production.
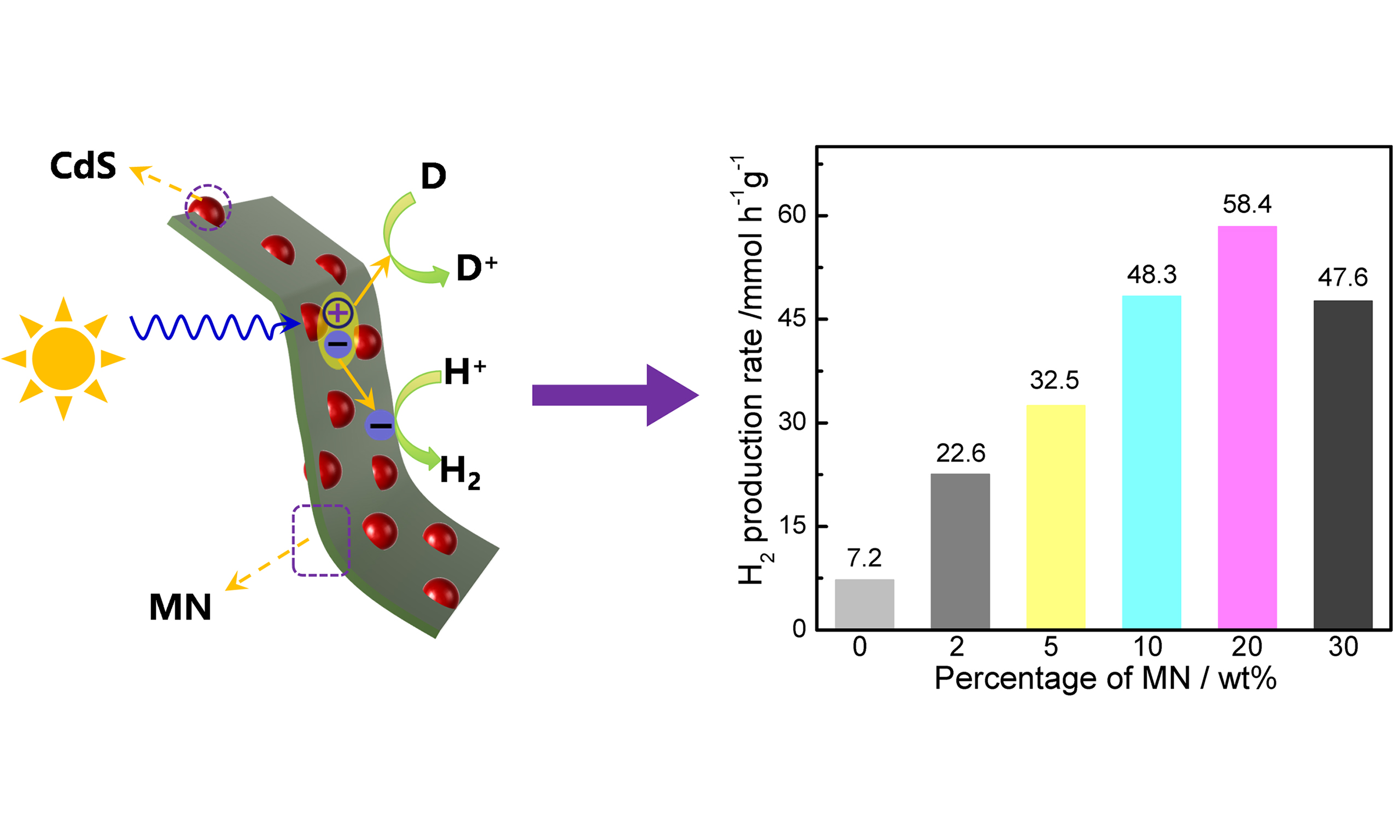
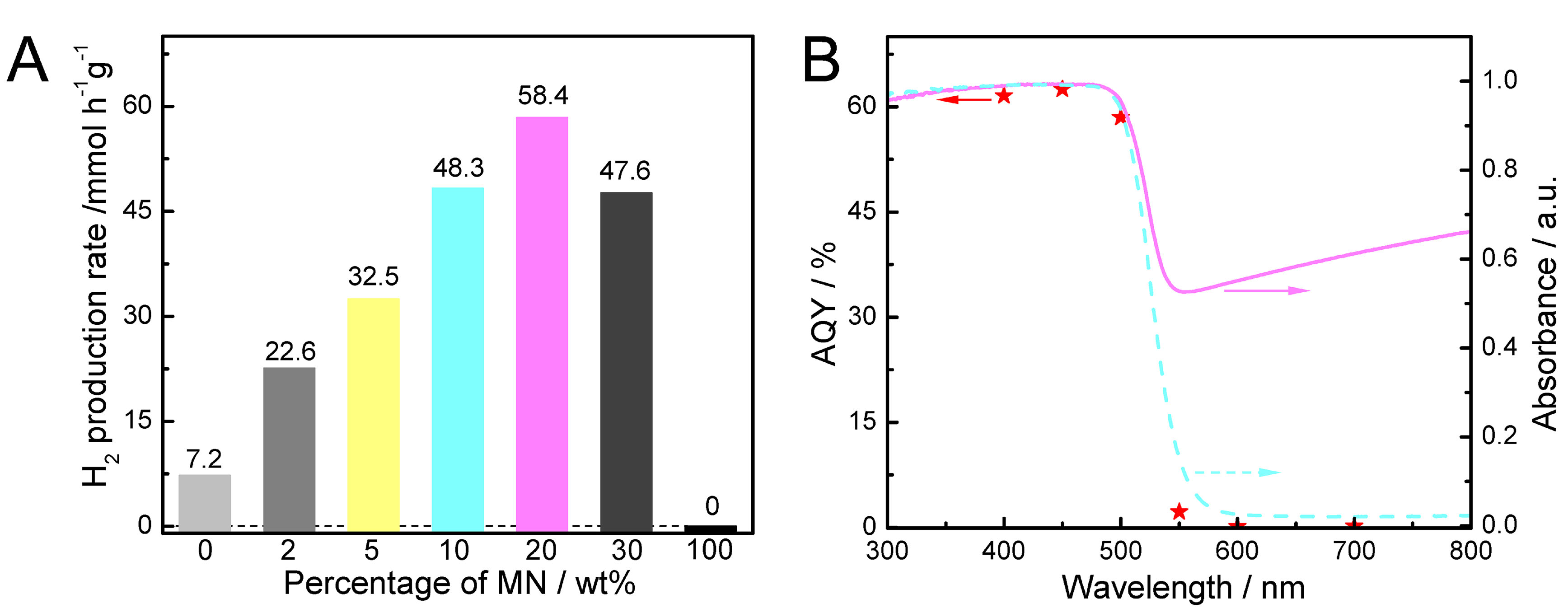
The photocatalytic activity of as-prepared samples was evaluated by hydrogen production test, and the results are displayed in Figure 4A. Pure CdS exhibits relatively low activity, with a hydrogen production rate of ~7.2 mmol h-1·g-1 under illumination of visible light (λ > 420 nm). Significantly, the introduction of MN could bring dramatic enhancement in photocatalytic activity. The optimized hydrogen production performance of CdS/MN reaches ~58.4 mmol·h-1·g-1 by loading 20 wt% MN, which is more than eight times that of pure CdS. Further increment of MN content leads to a slight decrease in photocatalytic hydrogen production, which could be ascribed to the less photogenerated charge carriers resulting from the potential shielding effect of MN on the light absorption of CdS and the further decreased proportion of CdS content in CdS/MN composite[37]. It is worth noting that no activity is detected for MN, hence demonstrating that MN serves as effective cocatalyst in the CdS/MN composite photocatalyst. Figure 4B illustrates the AQY of CdS/MN for photocatalytic hydrogen production along with the light absorption of CdS and CdS/MN. The trends of AQYs for different wavelengths closely align with the absorption curve of CdS. Although MN exhibits light absorption beyond 550 nm, it does not provide electrons that drive photocatalytic reaction, further confirming the cocatalytic effect of MN in CdS/MN composite photocatalyst. Notably, the highest AQY of CdS/MN is determined as ~62.5% @450 nm (see Supplementary Table 4 for details), demonstrating efficient photocatalytic hydrogen that surpasses most reported AQY values from CdS modified with earth-abundant cocatalyst [Supplementary Table 5] and CdS composited with 2D metal oxides [Supplementary Table 6]. Besides, cyclic test for photocatalytic hydrogen production [Supplementary Figure 6] and SEM images after photocatalytic reaction [Supplementary Figure 7] demonstrate the excellent stability of as-prepared CdS/MN composite photocatalyst.
Figure 4. (A) Photocatalytic hydrogen production rates of CdS/MN composites with different weight percentages of MN, in which
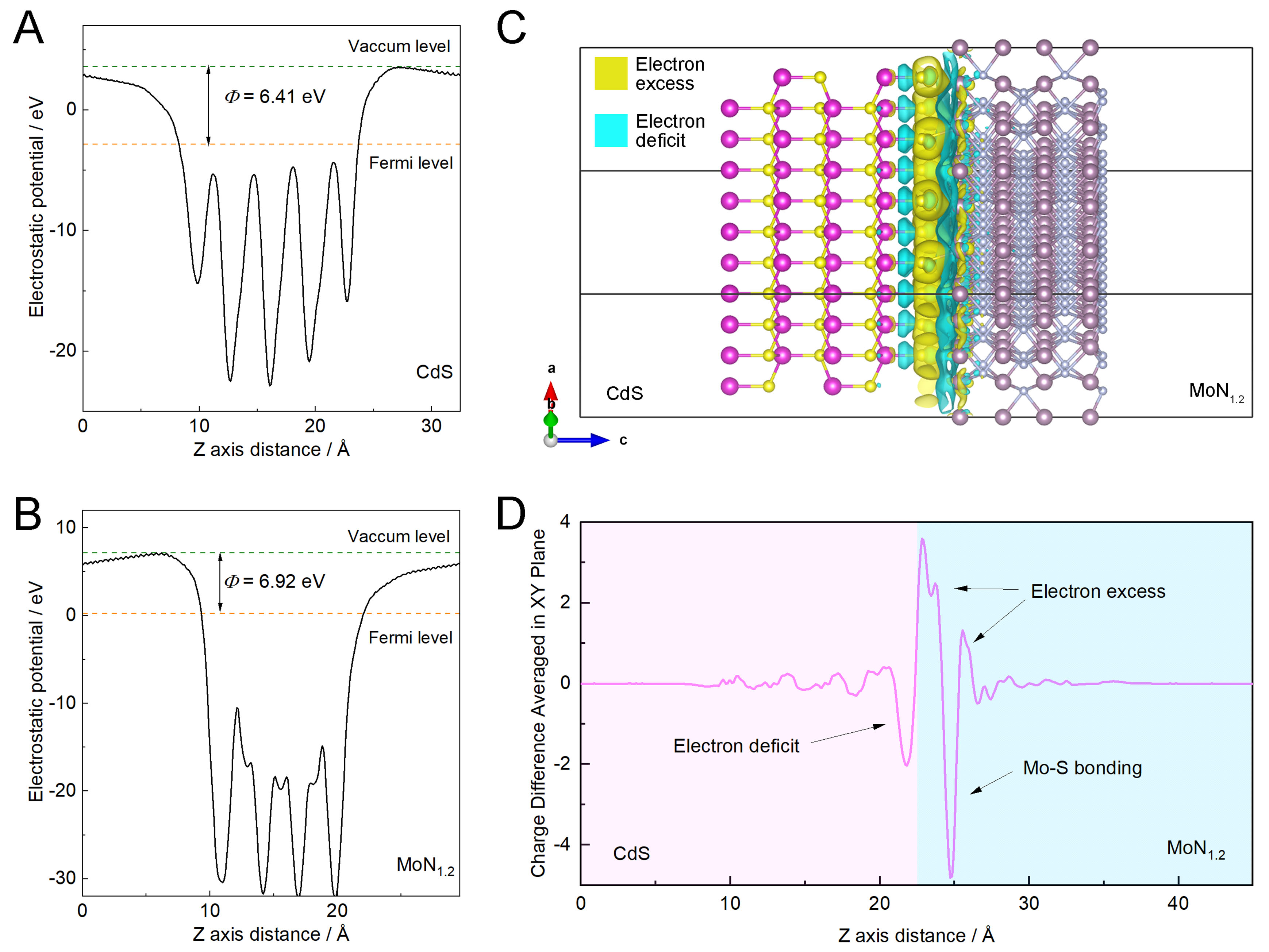
Calculation based on density functional theory (DFT) was conducted and the results are shown in Figure 5, Supplementary Figures 8 and 9, and Supplementary Table 7. As shown in Figure 5A and B, the work functions of CdS and MN are calculated as 6.41 and 6.92 eV, respectively, which also supports the electron extraction from CdS and MN as discussed above. The spatial differential charge distribution at the interface of CdS and MN in Figure 5C also suggests the interfacial charge transfer from CdS to MN. The charge redistribution is clarified via the plane-averaged charge potential along the direction parallel to the interface of CdS and MN [Figure 5D], indicating the electron deficit on the Cd atom layer of CdS near the interface while electron excess on the Mo atoms on the MN side, with the generation of Mo-S bond when heterojunction is constructed. The theoretical analysis further supports the experimental results demonstrating the cocatalytic effect of MN for photocatalytic hydrogen production[38].
Figure 5. (A) Average potential profiles of (A) CdS and (B) MN; (C) charge density difference and (D) planar average potential along Z direction of CdS and MN interfacial heterojunction. CdS: Cadmium sulfide; MN: molybdenum nitride.
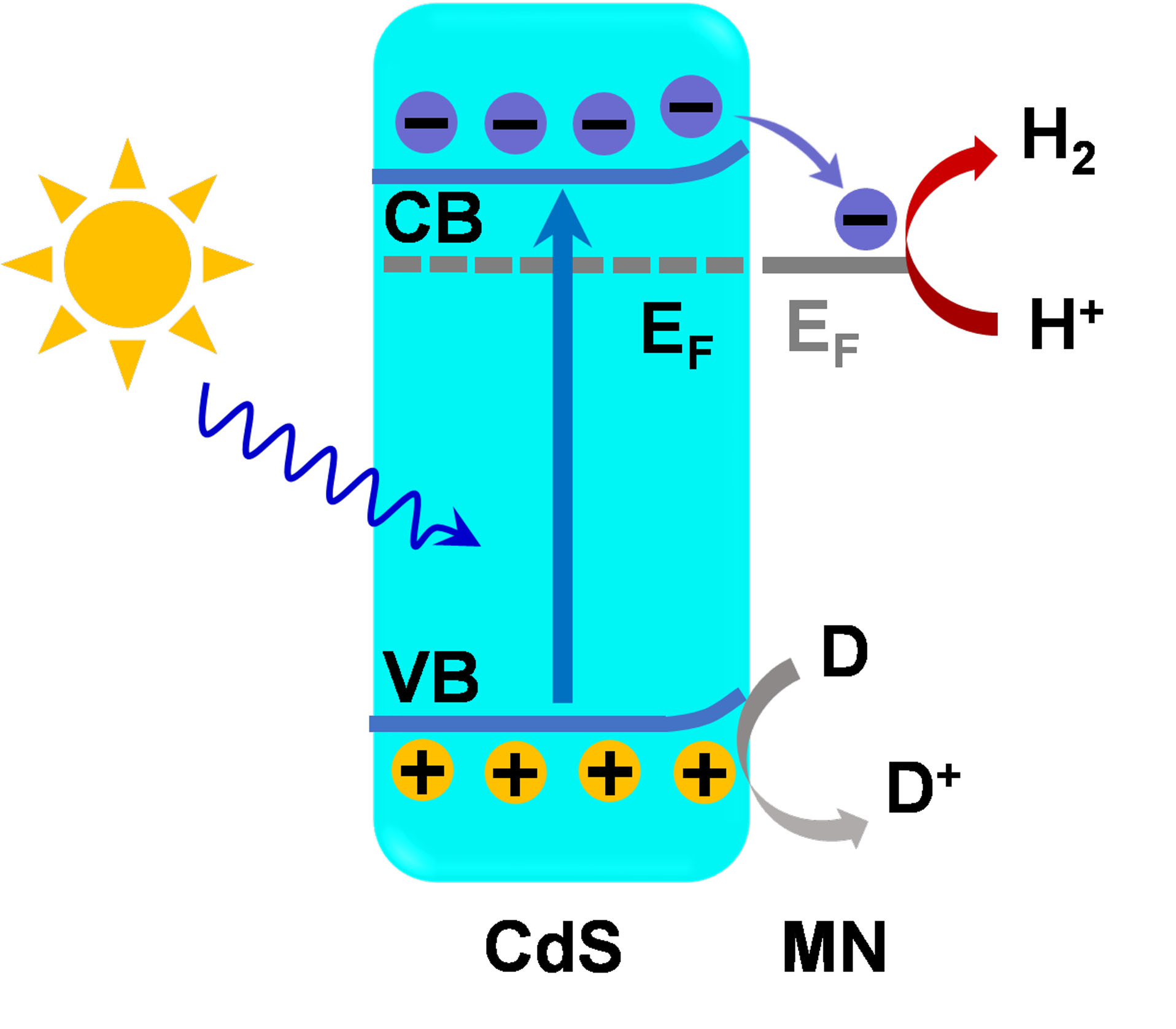
The Mott-Schottky curve [Supplementary Figure 10] and ultraviolet photoelectron spectrum (UPS, Supplementary Figure 11) were recorded, and the band diagram of CdS and MN [Supplementary Figure 12] indicates the lower fermi level of MN than CdS, which is consistent with the as-calculated results of work functions. Based on the photocatalytic hydrogen-production activity and the previous characterization and analysis, a possible mechanism has been proposed and is depicted in Figure 6. Due to the metallic character of MN, a Schottky junction is formed between CdS and MN with intimate contact. Upon light irradiation, photogenerated charge carriers are excited in CdS photocatalyst, and the electrons in the conduction band (CB) of CdS are extracted and transferred to MN with a lower Fermi level. In this way, the recombination of electrons and holes in CdS is inhibited, thus facilitating charge separation and lengthening the lifetime of carriers. Additionally, the introduction of MN helps to reduce the overpotential of hydrogen production reaction. The electrons transferred to MN could rapidly reduce the protons in the reaction solution, promoting efficient hydrogen production. As such, excellent photocatalytic hydrogen activity could be achieved with the assistance of cocatalytic effect provided by MN.
CONCLUSIONS
In this work, composite photocatalysts consisting of CdS and 2D MN were prepared for photocatalytic hydrogen production under visible-light irradiation. The 2D layered MN is found to serve as a cocatalyst that helps to facilitate charge separation and provides reactive sites for promoting hydrogen production reaction. Consequently, the composite photocatalysts exhibit greatly improved photocatalytic activity, with a hydrogen production rate of 58.4 mmol·h-1·g-1 and AQY of 62.5%. The cocatalytic effect of 2D MN surpasses most reported noble-metal-free cocatalysts; hence, this work provides useful guidance on the designing of photocatalytic systems with low-cost and earth-abundant elements. Future advancement based on this research could be expected by utilizing the infrared-light absorption capability of MN via photothermal catalysis for efficient hydrogen production.
DECLARATIONS
Acknowledgments
We would like to acknowledge the help from Ms. Xiaojing Zhang, School of Physics, for HRTEM analysis.
Authors’ contributions
Made substantial contributions to conception and design of the study and performed data analysis and interpretation: Guan, X.; Huang, Z.
Performed data acquisition and wrote the draft of the manuscript: Guan, X.; Yuan, Y.; Tian, L.; Huang, Z.
Provided technical and material support: Ye, X.; Zhang, T.; Zhu, B.; Zhou, Z.
Discussed and revised the manuscript: Guan, X.; Yuan, Y.; Tian, L.; Huang, Z.; Ye, X.; Zhang, T.; Zhu, B.; Zhou, Z.
Availability of data and materials
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author(s).
Financial support and sponsorship
This work was supported by the National Key Research and Development Program of China (No. 2022YFB3803600), the National Natural Science Foundation of China (No. 52376209), China Postdoctoral Science Foundation (Nos. 2020T130503 and 2020M673386), and China Fundamental Research Funds for the Central Universities. Huang, Z. would like to thank the support from Jiangxi Academy of Science Foundation (Nos. 2022YYB05 and 2022YSBG21014).
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
Supplementary Materials
REFERENCES
1. Nishioka, S.; Osterloh, F. E.; Wang, X.; Mallouk, T. E.; Maeda, K. Photocatalytic water splitting. Nat. Rev. Methods. Primers. 2023, 3, 42.
2. Song, H.; Luo, S.; Huang, H.; Deng, B.; Ye, J. Solar-driven hydrogen production: recent advances, challenges, and future perspectives. ACS. Energy. Lett. 2022, 7, 1043-65.
3. Jiang, Z.; Ye, Z.; Shangguan, W. Recent advances of hydrogen production through particulate semiconductor photocatalytic overall water splitting. Front. Energy. 2022, 16, 49-63.
4. Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37-8.
5. Schneider, J.; Matsuoka, M.; Takeuchi, M.; et al. Understanding TiO2 photocatalysis: mechanisms and materials. Chem. Rev. 2014, 114, 9919-86.
6. Chen, X.; Liu, L.; Yu, P. Y.; Mao, S. S. Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science 2011, 331, 746-50.
7. Takata, T.; Jiang, J.; Sakata, Y.; et al. Photocatalytic water splitting with a quantum efficiency of almost unity. Nature 2020, 581, 411-4.
8. Tian, L.; Guan, X.; Dong, Y.; et al. Improved overall water splitting for hydrogen production on aluminium-doped SrTiO3 photocatalyst via tuned surface band bending. Environ. Chem. Lett. 2023, 21, 1257-64.
9. Wang, X.; Maeda, K.; Thomas, A.; et al. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76-80.
10. Zhao, D.; Guan, X.; Shen, S. Design of polymeric carbon nitride-based heterojunctions for photocatalytic water splitting: a review. Environ. Chem. Lett. 2022, 20, 3505-23.
11. Zhang, K.; Guo, L. Metal sulphide semiconductors for photocatalytic hydrogen production. Catal. Sci. Technol. 2013, 3, 1672.
12. Yan, H.; Yang, J.; Ma, G.; et al. Visible-light-driven hydrogen production with extremely high quantum efficiency on Pt-PdS/CdS photocatalyst. J. Catal. 2009, 266, 165-8.
13. Cheng, L.; Xiang, Q.; Liao, Y.; Zhang, H. CdS-based photocatalysts. Energy. Environ. Sci. 2018, 11, 1362-91.
14. Ge, H.; Xu, F.; Cheng, B.; Yu, J.; Ho, W. S-scheme heterojunction TiO2/CdS nanocomposite nanofiber as H2-production photocatalyst. ChemCatChem 2019, 11, 6301-9.
15. Chen, Y.; Wang, L.; Lu, G. M.; Yao, X.; Guo, L. Nanoparticles enwrapped with nanotubes: a unique architecture of CdS/titanate nanotubes for efficient photocatalytic hydrogen production from water. J. Mater. Chem. 2011, 21, 5134-41.
16. Liu, M.; Jing, D.; Zhou, Z.; Guo, L. Twin-induced one-dimensional homojunctions yield high quantum efficiency for solar hydrogen generation. Nat. Commun. 2013, 4, 2278.
17. Hao, X.; Hu, Y.; Cui, Z.; Zhou, J.; Wang, Y.; Zou, Z. Self-constructed facet junctions on hexagonal CdS single crystals with high photoactivity and photostability for water splitting. Appl. Catal. B. Environ. 2019, 244, 694-703.
18. Jin, J.; Yu, J.; Liu, G.; Wong, P. K. Single crystal CdS nanowires with high visible-light photocatalytic H2-production performance. J. Mater. Chem. A. 2013, 1, 10927.
19. Xu, Y.; Zhao, W.; Xu, R.; Shi, Y.; Zhang, B. Synthesis of ultrathin CdS nanosheets as efficient visible-light-driven water splitting photocatalysts for hydrogen evolution. Chem. Commun. 2013, 49, 9803-5.
20. Yang, J.; Wang, D.; Han, H.; Li, C. Roles of cocatalysts in photocatalysis and photoelectrocatalysis. Acc. Chem. Res. 2013, 46, 1900-9.
21. Wang, H.; Zhang, L.; Chen, Z.; et al. Semiconductor heterojunction photocatalysts: design, construction, and photocatalytic performances. Chem. Soc. Rev. 2014, 43, 5234-44.
22. Ran, J.; Zhang, J.; Yu, J.; Jaroniec, M.; Qiao, S. Z. Earth-abundant cocatalysts for semiconductor-based photocatalytic water splitting. Chem. Soc. Rev. 2014, 43, 7787-812.
23. Dong, J.; Duan, L.; Wu, Q.; Yao, W. Facile preparation of Pt/CdS photocatalyst by a modified photoreduction method with efficient hydrogen production. International. J. Hydrogen. Energy. 2018, 43, 2139-47.
24. Xu, Z.; Yue, W.; Li, C.; et al. Rational synthesis of Au-CdS composite photocatalysts for broad-spectrum photocatalytic hydrogen evolution. ACS. Nano. 2023, 17, 11655-64.
25. Deng, D.; Novoselov, K. S.; Fu, Q.; Zheng, N.; Tian, Z.; Bao, X. Catalysis with two-dimensional materials and their heterostructures. Nat. Nanotechnol. 2016, 11, 218-30.
26. Jin, H.; Gu, Q.; Chen, B.; et al. Molten salt-directed catalytic synthesis of 2D layered transition-metal nitrides for efficient hydrogen evolution. Chem 2020, 6, 2382-94.
27. Dang, Y.; Luo, L.; Wang, W.; et al. Improving the photocatalytic H2 evolution of CdS by adjusting the (002) crystal facet. J. Phys. Chem. C. 2022, 126, 1346-55.
28. Guan, X.; Guo, L. Cocatalytic effect of SrTiO3 on Ag3PO4 toward enhanced photocatalytic water oxidation. ACS. Catal. 2014, 4, 3020-6.
29. Tang, Y.; Ye, F.; Li, B.; et al. Electronic structure modulation of oxygen-enriched defective CdS for efficient photocatalytic H2O2 production. Small 2024, 2400376.
30. Ye, X.; Dong, Y.; Zhang, Z.; et al. Mechanism insights for efficient photocatalytic reforming of formic acid with tunable selectivity: accelerated charges separation and spatially separated active sites. Appl. Catal. B. Environ. 2023, 338, 123073.
31. Ye, X.; Dong, Y.; Zhang, Z.; et al. Syngas production by photoreforming of formic acid with 2D VxW1-xN1.5 solid solution as an efficient cocatalyst. Front. Energy. 2024, 1-10.
32. Shi, J.; Cui, H.; Liang, Z.; et al. The roles of defect states in photoelectric and photocatalytic processes for ZnxCd1-xS. Energy. Environ. Sci. 2011, 4, 466-70.
33. Patriarchea, C.; Vamvasakis, I.; Koutsouroubi, E. D.; Armatas, G. S. Enhancing interfacial charge transfer in mesoporous MoS2/CdS nanojunction architectures for highly efficient visible-light photocatalytic water splitting. Inorg. Chem. Front. 2022, 9, 625-36.
34. Kannan, K.; Chanda, D.; Meshesha, M. M.; Yang, B. Impressive efficiency of zinc oxide-manganese oxide/MAX composite in two-electrode system for photovoltaic-electrolyzer water splitting. Colloids. Surf. A. Physicochem. Eng. Aspects. 2024, 689, 133599.
35. Zong, X.; Yan, H.; Wu, G.; et al. Enhancement of photocatalytic H2 evolution on CdS by loading MoS2 as cocatalyst under visible light irradiation. J. Am. Chem. Soc. 2008, 130, 7176-7.
36. Tian, L.; Min, S.; Wang, F. Integrating noble-metal-free metallic vanadium carbide cocatalyst with CdS for efficient visible-light-driven photocatalytic H2 evolution. Appl. Catal. B. Environ. 2019, 259, 118029.
37. Chen, H.; Jiang, D.; Sun, Z.; Irfan, R. M.; Zhang, L.; Du, P. Cobalt nitride as an efficient cocatalyst on CdS nanorods for enhanced photocatalytic hydrogen production in water. Catal. Sci. Technol. 2017, 7, 1515-22.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Copyright
Data & Comments
Data


















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].