Recent progress in ammonia production via plasma-electrocatalysis cascade system
Abstract
Ammonia (NH3) is widely utilized in agriculture to manufacture fertilizers and is an important feedstock in the pharmaceutical, metallurgical, and textile industries. Owing to its highly compressible nature, NH3 is also regarded as an ideal carbon-free hydrogen carrier for the next-generation fuel cells. The Haber-Bosch process is currently the main method for NH3 production but suffers from high energy consumption and intensive carbon emission. Recently, the plasma-electrochemical cascade pathway - plasma nitrogen fixation from air in tandem with electrocatalytic synthesis of NH3 - has emerged as an alternative by the simplified process, low carbon emission, and high NH3 yield. However, the research in this area has not been summarized timely. This review systematically summarizes the research progress of plasma-electrochemical cascade pathway for the first time. Through performance and energy consumption analysis, key issues that need to be addressed have been identified: improving the efficiency of plasma nitrogen fixation and NH3 electrosynthesis. In response to these challenges, corresponding optimization strategies are provided in the end, pointing out the direction for the subsequent research on this emerging field.
Keywords
INTRODUCTION
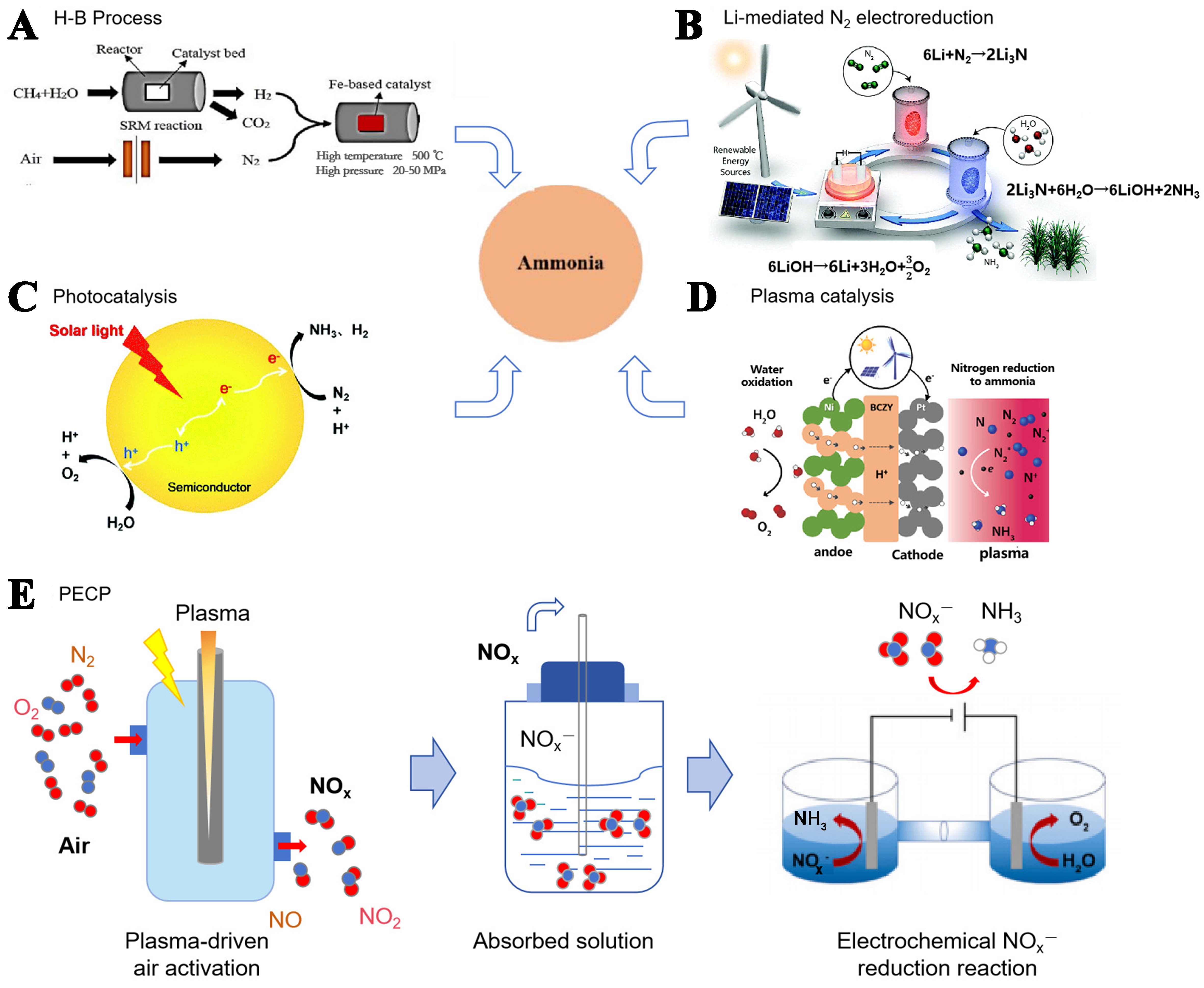
Ammonia (NH3), an essential chemical product in modern society with a global demand of over 200 million tons annually, is a crucial feedstock in industries such as fertilizer, textile, and pharmaceutical, and is considered a carbon-neutral energy carrier due to its high energy density (4.25 kW·h/L)[1-3]. Artificial NH3 synthesis was not realized until Haber proposed a chemical method for NH3 synthesis from atmospheric nitrogen (N2) in the 20th century, which converts N2 and H2 into NH3 at high temperatures (400-500 °C) and high pressures (100-200 kPa) by using iron [Figure 1A], where H2 is generally obtained from steam methane reforming (SMR) process [Equation (1)].
Figure 1. General NH3 synthesis processes. (A) H-B process: NH3 is prepared from H2 and N2 under high temperature (500 °C) and high pressure (20-50 MPa) over a Fe-based catalyst[18]; (B) Li-mediated three-step electrocatalytic cycling method for NH3 synthesis[11]; (C) Photocatalytic electron - excited N2 to NH3[13]; (D) Plasma directly dissociates N2 and then bonds with H+ to generate NH3[16]; (E) NH3 synthesis by PECP pathway. H-B: Haber-Bosch; PECP: plasma-electrocatalysis cascade pathway.
However, the SMR process consumes fossil energy and produces a large amount of greenhouse gases, accounting for 2% of the world’s total carbon emissions[4,5], accompanied by the energy consumption of
The electrochemical route, such as N2 electro-reduction, offers the potential to reduce carbon emissions which employs H2O as a clean hydrogen source and ideally can be driven by renewable electricity
Another pathway is photocatalysis [Figure 1C][13]. Under light irradiation, the adsorbed N2 and H2O are catalyzed into NH3 and O2 by electrons and holes, respectively. Photocatalytic NH3 synthesis can utilize solar energy directly, reduce energy consumption, and have no carbon pollution during the reaction. Yet, the photocatalytic reaction suffers from low NH3 yield for large-scale production.
Plasma NH3 synthesis could afford a higher reaction efficiency than the traditional thermal catalytic approach due to the readily activation of inert N≡N by high-temperature electrons [Figure 1D][14-16]. The N radical species can react with H+ to generate NH3, N2H4, H2, and other products[17]. This path uses H2 or H2O as the hydrogen source and does not produce carbon pollutant gases. Nevertheless, the interconversion between NH3 and N2 leads to low NH3 selectivity.
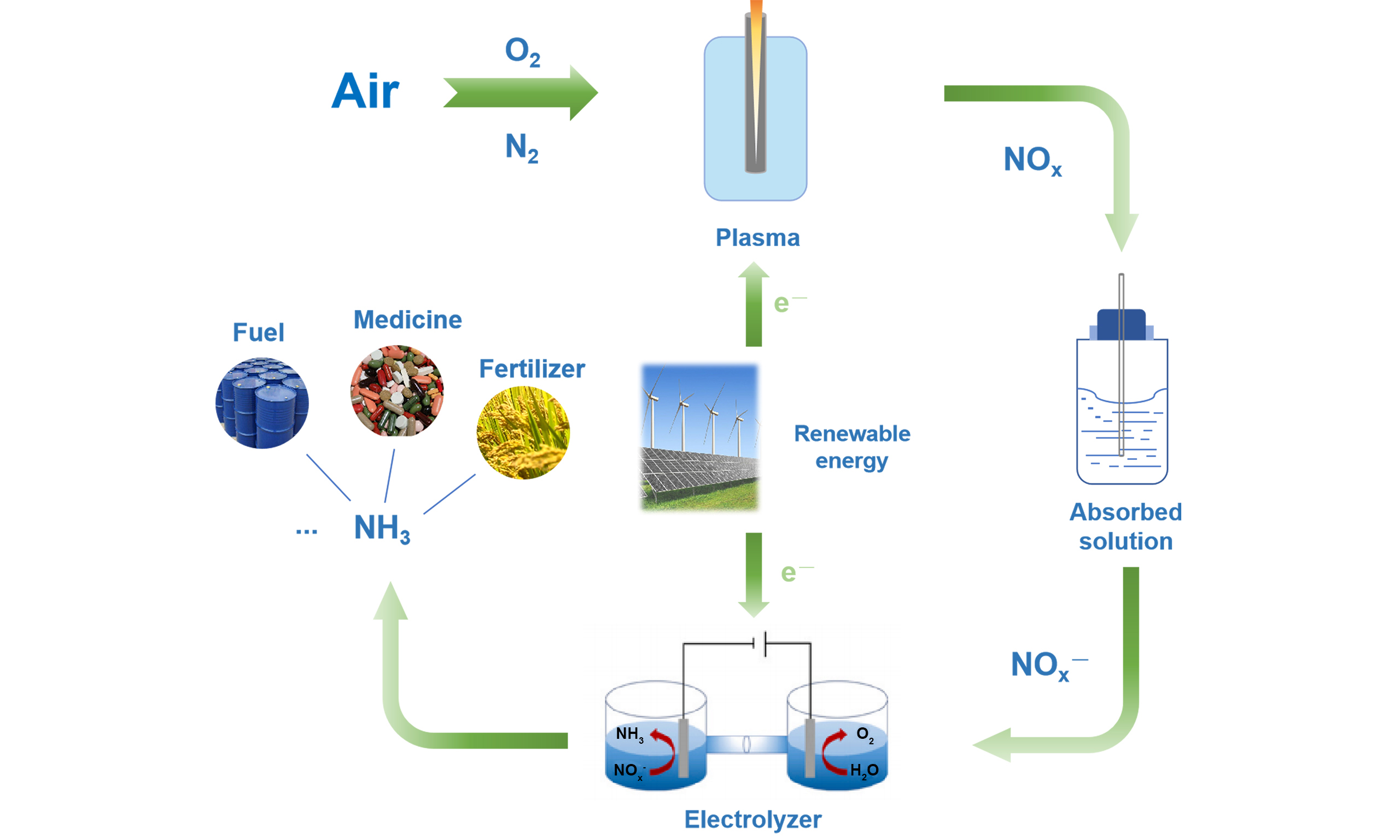
In recent years, inspired by natural lightning N2 fixation and the rapid development of electrocatalytic NOx electroreduction technology, plasma N2 fixation coupled with electrocatalysis has gradually entered the public’s vision for NH3 synthesis, i.e., plasma-electrocatalysis cascade pathway (PECP). The method integrates the advantages of both plasma thermal catalysis and electrocatalysis air and water as raw materials. N2 and O2 are readily activated into NOx- intermediates via plasma technology under ambient temperature and pressure. Subsequently, these intermediates are electro-reduced to produce NH3. The entire process typically involves two steps: plasma activation and electrocatalytic reduction [Figure 1E].
The novelty of the PECP strategy lies in overcoming the challenges of low N2 activation efficiency and poor yield in traditional electrocatalytic nitrogen reduction reactions, while also achieving continuous and efficient energy conversion through an integrated device design. Moreover, the technology operates under ambient conditions, avoiding the high temperature and pressure requirements of the traditional H-B process, thereby significantly reducing energy consumption and carbon emissions. Its compatibility with renewable energy sources and its potential to generate high-value byproducts further enhance its economic viability and environmental sustainability.
WORKING MECHANISM FOR PECP
Mechanism of nitrogen fixation by plasma
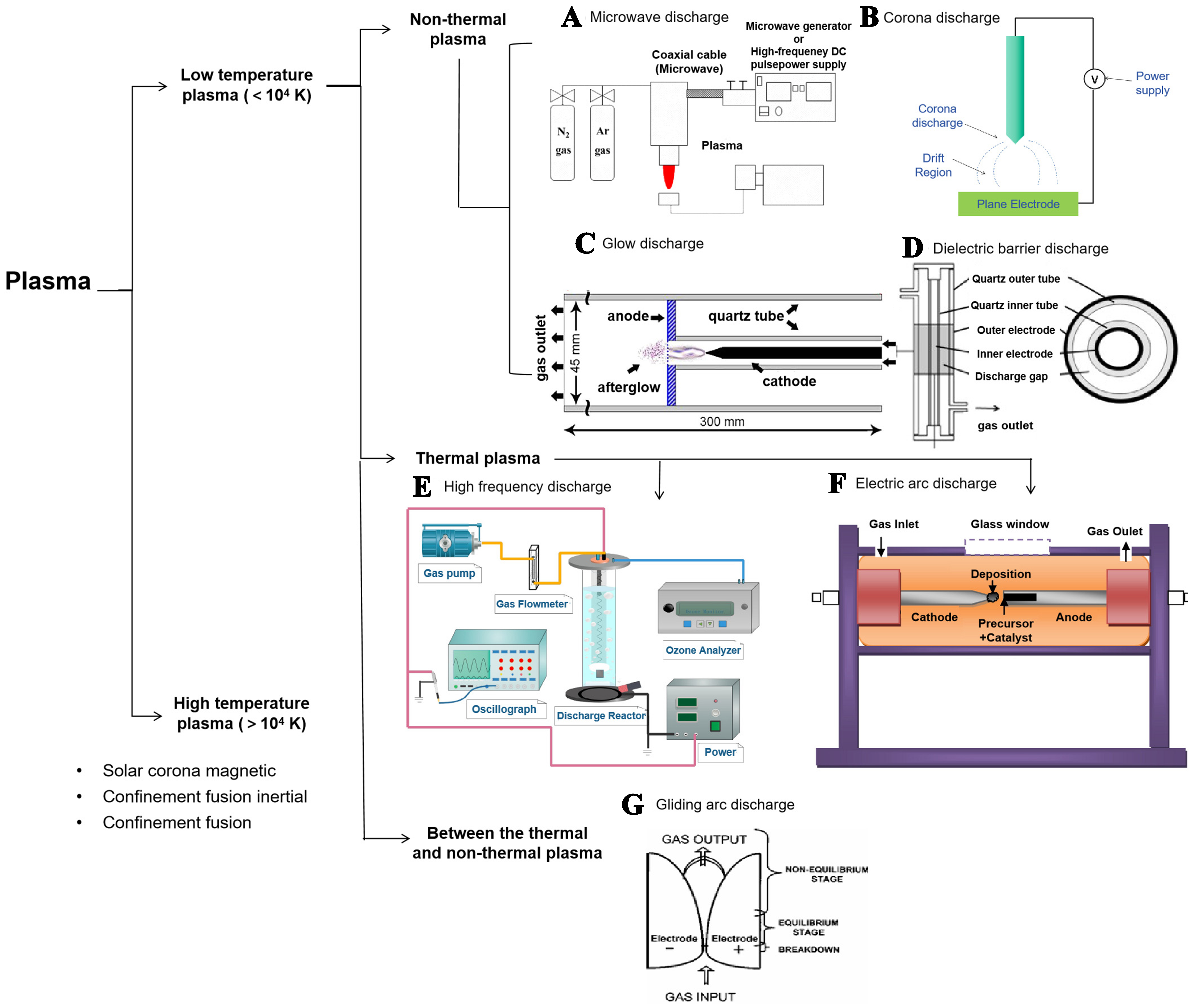
Plasma is considered to be the fourth state of matter, which consists of numerous positive ions, negative ions, electrons, and unionized neutral particles, but maintains overall electric neutrality[15]. Plasma can be classified into high-temperature plasma and low-temperature plasma according to the system temperature.
The low-temperature plasma is more widely used because of its lower energy consumption working below 104 K, which can be further divided into thermal plasma with an approximately balanced thermodynamic state and non-thermal plasma (or cold plasma) with an unbalanced thermodynamic state[18]. The high-temperature plasma generally has a system temperature > 104 K[19], such as solar corona, magnetic confinement fusion, and inertial confinement fusion. Non-thermal plasmas include microwave discharge
In the study of N2 fixation via plasma-generated nitrogen oxides, the reaction pathways of different discharge mechanisms show significant differences. These mechanisms can be broadly classified into three groups:
(1) High-energy electron-dominated discharge mechanisms: Microwave discharge generates high-energy electrons through the interaction of high-frequency electromagnetic wave energy with gas molecules. High-frequency discharge excites gas molecules via a high-frequency electromagnetic field. Arc discharge and gliding arc plasma achieve discharge through high current density and high-temperature environments.
(2) Discharge mechanisms involving synergistic effects of electric field and surface reactions: The reaction pathway of corona discharge begins with the ionization of gas molecules by electrons accelerated in the electric field near the electrode surface, producing free electrons and positive ions. DBD applies a high voltage between electrodes while restricting the discharge range with a dielectric barrier layer, forming microdischarge channels. Within these channels, high-energy electrons collide with gas molecules, triggering ionization and excitation reactions.
(3) Uniform discharge mechanisms at low pressure: In a glow discharge, high-energy electrons near the cathode generate free electrons and ions through collisional ionization. These electrons and ions then collide with N2 and O2 molecules to form excited-state gas molecules.
Despite differences in plasma discharge mechanisms, the N2 fixation mechanisms share a high degree of similarity in their core reaction pathways, which can be primarily summarized as follows[29]:
First, the generation of high-energy electrons: The essence of all plasma discharge mechanisms is the generation of high-energy electrons through various means. These high-energy electrons act to initiate subsequent chemical reactions. Then, the high-energy electrons collide with N2 and O2, causing ionization and excitation, and forming reactive intermediates.
Then, the next molecular bonding reaction depends on the vibrational energy of the molecule. The main chemical reaction for the generation of NOx in atmospheric pressure air plasma is carried out through the vibration-enhanced Zeldovich mechanism[30,31] which consists of the following reactions:
Last, the N atoms formed in Equation (7) can further react with the ground-state and vibrationally excited O2 molecules to produce an NO molecule. The reaction also produces an additional O atom, which can further react with the ground-state and vibrationally excited N2 molecules to form a tightly coupled reaction cycle. In addition, O3 is formed as a byproduct and can be formed by the reaction of O atoms with O2 molecules. The generated O3 may be directly discharged or oxidized NO to generate NO2[29].
To understand the specific differences between these plasmas intuitively, some examples of plasma N2 fixation are summarized in Table 1. The N2 fixation efficiency, energy consumption, and their advantages and disadvantages are presented and compared.
Comparison of N2 fixation performance of different plasma devices
| Discharge type | NOx volume fraction | Energy efficiency/(MJ·mol-1) | Advantages | Disadvantages | Ref. |
| Spark | 10.9% | ~9.82 | High yield Low energy consumption | Easy to lead to fire and explosion accidents | [32] |
| DBD | 0.3% | 56-140 | Easy to operate Easy to combine with the catalyst | Low yield High energy consumption | [33] |
| Magnetically confined microwave plasma | 14% | 0.28 | High yield Low energy consumption | Harsh operating conditions Industrialize difficulty | [34] |
| Gliding arc plasma | 1%-2% | 2.8-4.8 | Low energy consumption Good development prospect | The yield needs to be further improved | [14] |
| Propeller arc plasma | 0.4% | 4.2 | No need for gas flow Discharge frequency can be controlled | The yield needs to be further improved | [33] |
| γ-Al2O3 catalyze DBD | 0.5% | 18 | Low energy consumption Good development prospect | Need catalyst | [35] |
Table 1 shows that NOx yields vary by a few percentage points among different discharge types, but energy consumption for N2 fixation varies greatly, from 0.28 to 140 MJ/mol. The table indicates that microwave plasma is most effective for N2 fixation at low pressures, with a 6% NOx yield and 0.84 MJ/mol energy consumption for microwave plasma synergistic catalytic N2 fixation. The lowest energy consumption of
Overall, gliding arc plasma is considered suitable for N2 fixation due to its ability to oscillate and excite N2 molecules[27,36]. Similarly, DBD is regarded as a promising candidate because it operates at atmospheric pressure and is compatible with catalyst integration[37,38].
The mechanism of NOx reduction
There are two different pathways for the reduction of NO3-, including the indirect autocatalytic reduction pathway and the direct electrocatalytic reduction pathway[39,40]. In high acidity (pH < 0) and high concentration of NO3- (> 1 mol·L-1), there is a tendency for indirect autocatalytic reduction of NO3-, in which NO3- does not participate in electron transfer; the latter participates in the electron transfer process of NO3- at low concentrations (< 1 mol·L-1), which is mainly discussed in this section.
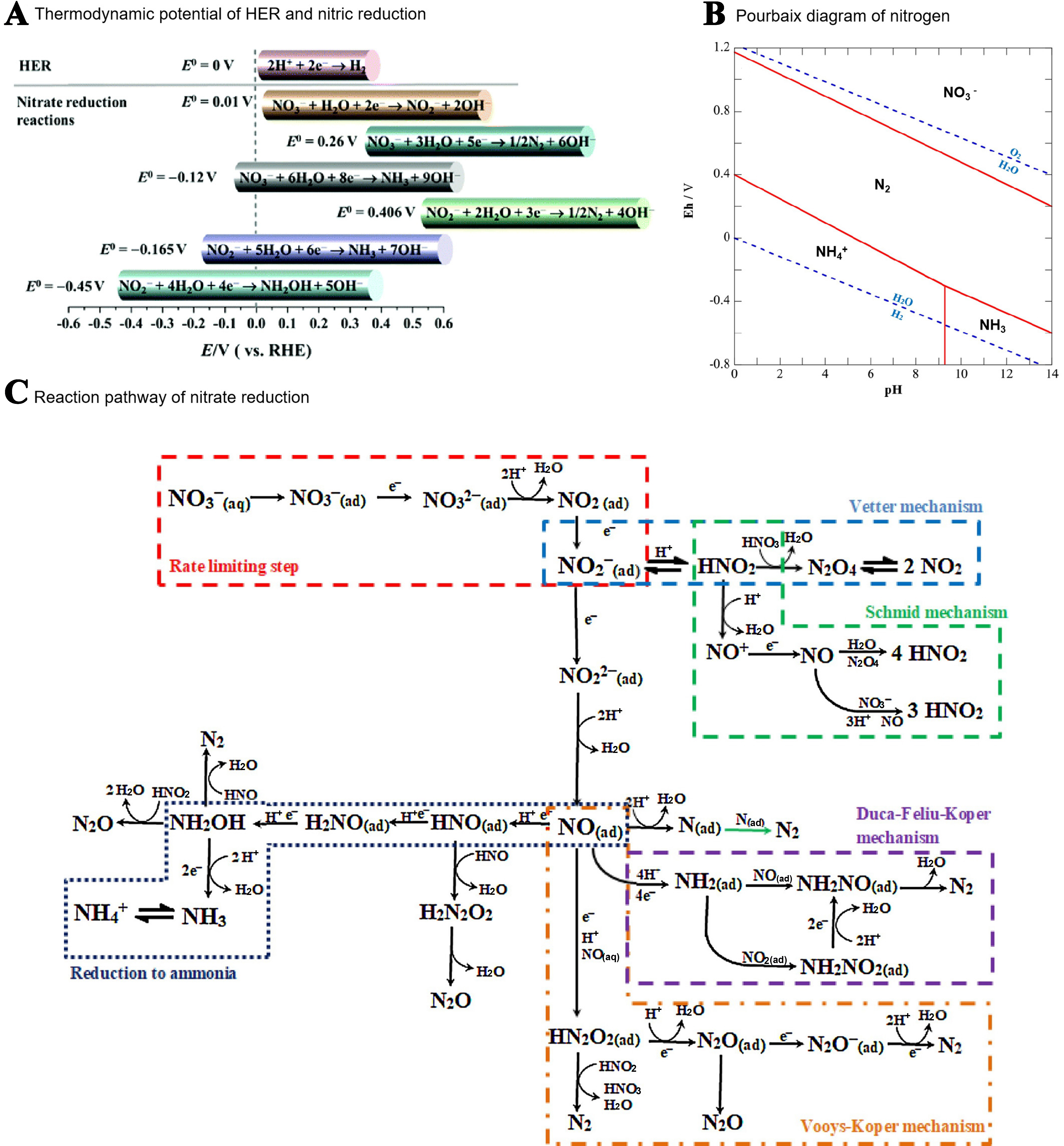
Nitrate reduction reaction (NO3RR) is a complex multi-electron transfer process with multiple possible reaction pathways[41]. The mechanism and products of NO3RR highly depend on the concentration of NO3-, the applied potential, and the pH of the electrolyte. Figure 3A shows the main reduction reactions and their thermodynamic potentials on the surface of the cathode electrode in alkaline or neutral solutions[42]. Figure 3B shows that NH3 and NH4+ are the most thermally stable products under negative electrode potentials[41]. The process of electrochemical conversion of NO3- to NH3 involves the transfer of eight electrons coupled with nine protons [Figure 3C].
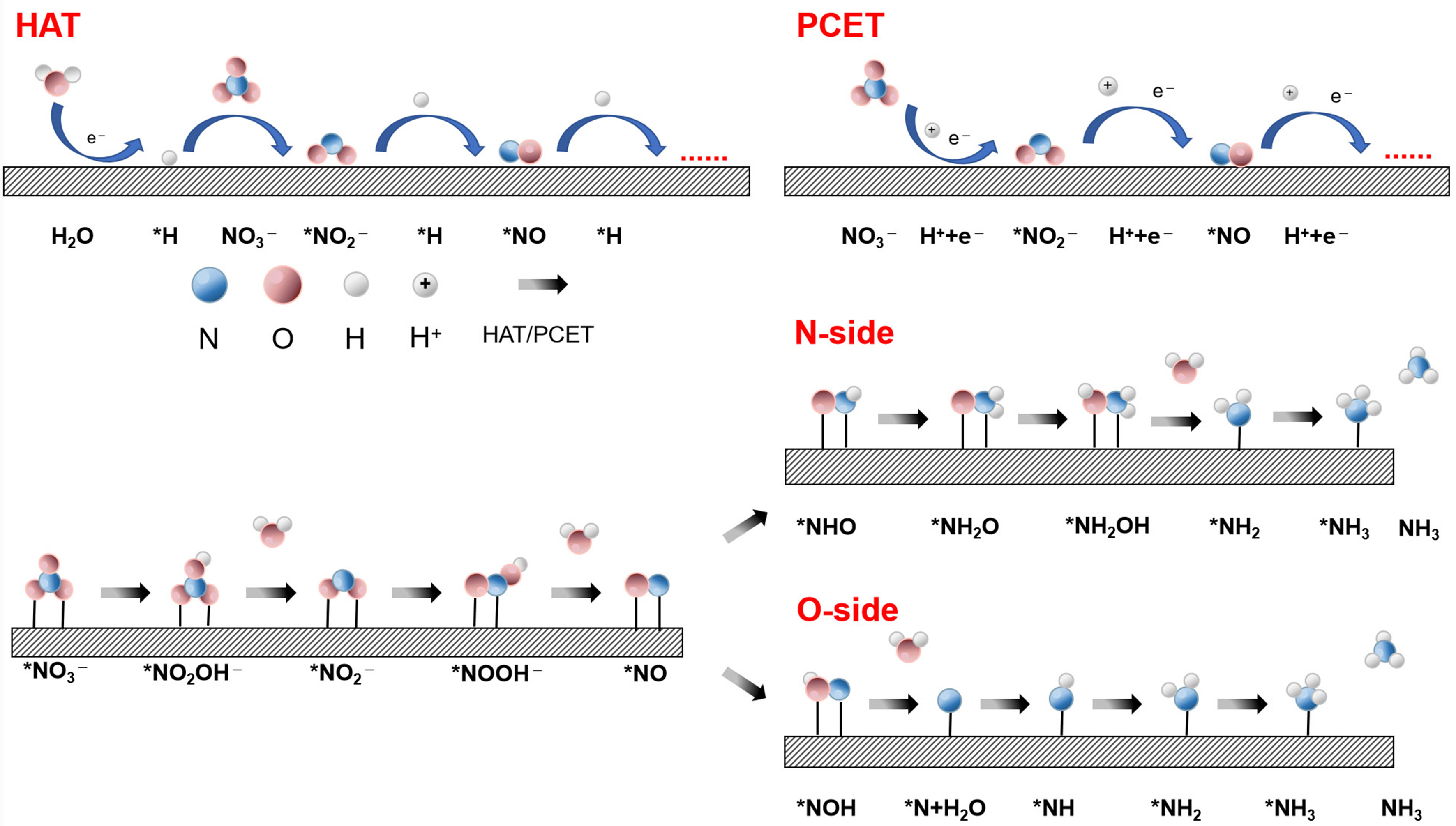
The direct electro-reduction of NO3- to NH3 mainly includes two mechanisms. One is proton-coupled electron transfer (PCET), in which protons and electrons often appear in pairs to complete the reduction, and the other is hydrogen atom transfer (HAT), in which the reduction is carried out by directly adding active H* from water reduction.
The specific path for the reduction of NO2- to NH3 is as follows [Figure 4]: the NO3- in the bulk solution first adsorbs on the catalyst to form *NO3-, and further transforms *NO2- and *NO intermediates via PCET or HAT process. After the formation of *NO intermediate, there will be two different reaction paths toward NH3. One is the N-side pathway: *H is first added to the N atom to form the *NHO intermediate, and then hydrogenated twice to form the hydroxylamine intermediate (*NH2OH). Moreover, it is noted that the *NH2OH may be dissociated to NH3 + *OH or *NH2 + *OH, which subsequently undergo a hydrogenation process for the formation of NH3. The other way is the O-side pathway, which is different from the previous path:
The mechanism of electrocatalytic NH3 synthesis involves a multi-electron transfer process that reduces NO3- or NO2- to NH3 via PCET and HAT. The complex reaction process and multiple intermediates pose significant challenges to efficient electrochemical NH3 synthesis. In recent years, strategies such as catalyst design and interfacial engineering have significantly enhanced reaction efficiency and selectivity.
RESEARCH PROGRESS ON NH3 SYNTHESIS USING PECP SYSTEMS
In parallel to the mechanism, experimental developments and system optimization of the PECP have played an important role in ambient NH3 synthesis. Great efforts have led to significant progress in recent years [Table 2]. By comparing the NH3 synthesis performance and energy consumption of different systems, a systematic reference can be provided for the design and performance optimization of PECP. Specifically, since plasma nitrogen fixation accounts for the majority of the total energy consumption in PECP, the following discussion on energy consumption will primarily focus on plasma N2 fixation.
PECP systems and their performance
| Plasma device | Catalyst | NH3 yield | FE of NH3 | Applied potential vs. RHE | Energy consumption (kWh·kg-1 NH3) | Ref. |
| Non-thermal plasma bubble column reactor | CuNWs | 23.2 mg·h-1 | ~100% | -0.50 V | 253 | [45] |
| DBD | Co nanosheets | 16.21 mg·h-1 | > 90% | -0.70 V | 78.9 | [46] |
| Gliding arc plasma | Co SAs/N-C | ~3 mg·h-1 | 62% | -0.63 V | 51.8 | [47] |
| Spark discharge plasma | Ni(OH)x/Cu | 3 mmol·h-1·cm-2 | 92% | -0.25 V | 295.8 | [48] |
| Nanosecond pulsed spark discharge | Ni3B@NiB2.74 | 3.37 mg·cm-2·h-1 | ~100% | -0.30 V | N.A. | [49] |
| RF | N-MoS2/VGs | 7.30 mg·cm-2·h-1 | 38.1% | -0.53 V | 39.2 | [50] |
| High-voltage electric spark | Cu10Fe1 | 190.46 μmol·h-1·cm-2 | 93.74% | -0.35 V | N.A. | [51] |
| Spark discharge plasma | Cu nanoparticles | ~40 nmol·s-1·cm-2 | ~90% | -0.9 V | N.A. | [52] |
| Nanosecond-pulsed plasma | Cu nanoparticles | 30 nmol·s-1·cm-2 | 97% | -0.5 V | 188.2 | [29] |
| Gliding arc-microwave conjunction plasma | Cu2Pd/CBC | 1,956.65 μg·h-1·mg-1 | 93.79% | -0.2 V | 177.1 | [53] |
| Warm GAD plasma | Perovskite oxide catalyst | 8.55 mmol·cm-2·h-1 | ~100% | -0.9 V | 52.78 | [54] |
| Cascade jet plasma | PdNi/N-CNTs | 34.96 mg·h-1·mgcat-1 | 98.21% | -0.38 V | 190.1 | [55] |
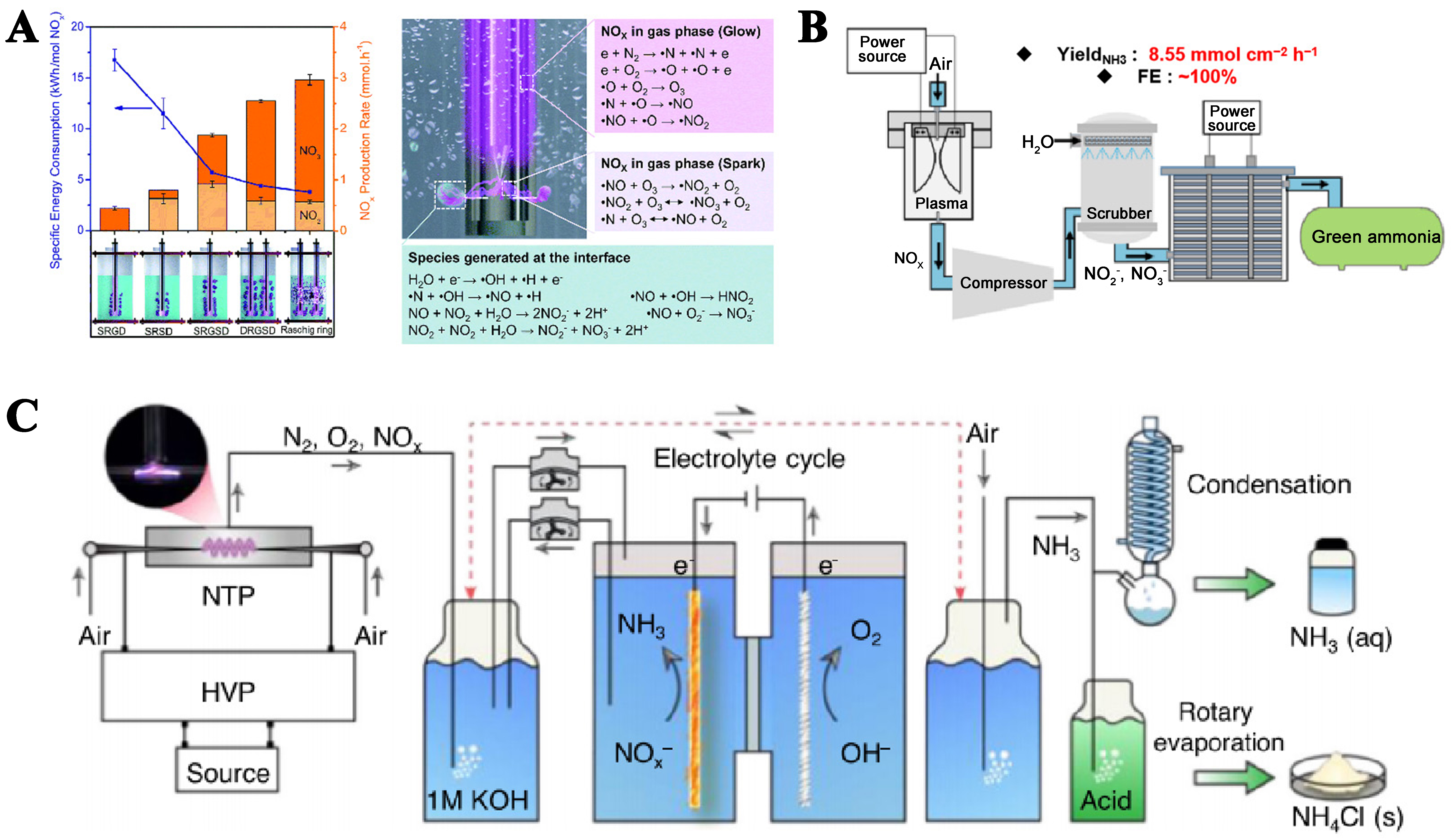
The pioneering study of NH3 production by the PECP system was reported by Sun et al. in 2021. They developed a continuous and scalable non-thermal bubble column reactor electrochemical hybrid approach for NH3 production and investigated the NOx production efficiency from N2 and O2 by using different kinds of plasmas [Figure 5A][45]. Although this NH3 synthesis strategy is emerging and promising, there is substantial room for improvement in terms of energy consumption (253 kWh·kg-1 NH3).
It was mentioned that different plasmas have varying N2 fixation efficiency in the previous chapter. Among them, the spark discharge is used in PECP by some researchers because of its high power and high N2 fixation. For example, Ren et al. designed a spark plasma system with the same electrocatalyst for NH3 synthesis[52]. The NH3 yield rate of ~40 nmol·s-1·cm-2 and 90% Faradaic efficiency (FE) was achieved. While spark discharge can efficiently generate NOx, its high energy consumption (295 kWh·kg-1 NH3) cannot be ignored. Therefore, optimizing the N2 fixation performance of plasma and reducing reaction energy consumption is key to achieving efficient NH3 synthesis. Liu et al. further designed the PECP using Cu10Fe1 nanocatalyst[51]. The yield of NH3 (190.46 μmol·cm-2·h-1) and FE (93.74%) is improved together by using CuFe alloy compared with bare Cu due to the enhanced NO3- adsorption. In addition, Li et al. employed the improved Ni3B@NiB2.74 electrocatalyst[49]. The system exhibits 100% FE of NH3 with a yield of
Gliding arc plasma could effectively use vibrationally excited N2 particles to facilitate the N2 fixation reaction at atmospheric pressure. Based on this, Wu et al. combined the gliding arc plasma and Co-SAC/N-C producing 3 mg·h-1 NH3 and 62% FE[47]. The distance between the plasma and the electrolyte was optimized in this study to maximize the absorption of generated NOx by the electrolyte, thereby further conserving energy (51.8 kWh·kg-1 NH3). Li et al. combined two plasma devices to improve the N2 fixation efficiency using the gliding arc-microwave plasma and developed the Cu2Pd/CBC electrocatalyst[53]. The NH3 yield reached 1,756.65 μg·h-1·mg-1 and the FE improved to 93.7%. Comparative studies have demonstrated that the synergistic action of plasma significantly enhances the yield of NOx, though it also increases energy consumption (177.1 kWh·kg-1 NH3). This finding indicates that plasma-assisted N2 fixation is both feasible and challenging. Guo et al. developed a perovskite oxide catalyst La1.5Sr0.5Ni0.5Fe0.5O4 for NOx reduction reaction (NOxRR) under strongly acidic conditions with pH = 0[54]. Synergies between many metals in the electrocatalyst combined with gliding arc plasma result in the best performance of PECP ever achieved. The FE of NH3 is close to 100%, obtaining an NH3 yield of 8.55 mmol·cm-2·h-1 [Figure 5B]. Owing to the low energy consumption characteristic of gliding arc plasma, the system’s energy consumption is correspondingly low (52.78 kWh·kg-1 NH3). This study can serve as a valuable reference for the future design and performance optimization of PECP.
There are many other kinds of plasma, such as pulsed plasma, radio frequency (RF) plasma, jet plasma, etc. They are also used for NOx production in the PECP. Sun et al. developed a plasma bubble reactor driven by nanosecond pulses and used Cu nanoparticles to obtain an NH3 yield of 30 nmol·s-1·cm-2 and 97% FE[29]. The energy consumption of this process is 188.2 kWh·kg-1 NH3. When comparing the NOx production rate and energy consumption, the system still has significant potential for further improvement. Zheng et al. designed the RF plasma couple with an N-doped molybdenum sulfide nanosheet (N-MoS2/VGs)[50]. At
With the improvement of technology, PECP is no longer limited to small-scale production. In 2023, Gao
To date, PECP has been able to devise a relatively comprehensive system. However, achieving a complete large-scale production chain requires further work. In the field of plasma N2 fixation, it is already understood that different plasmas or a combination of multiple plasmas can be employed for N2 fixation, yet the low N2 fixation efficiency and high energy consumption for N2 fixation remain the primary challenges. Energy consumption is also a key factor in plasma N2 fixation. Currently, gliding arc plasma demonstrates good performance in NOx synthesis with relatively low energy consumption. In conclusion, there is significant potential in optimizing PECP by enhancing N2 fixation efficiency. On the other hand, in the electrocatalytic synthesis of NH3 from NO3-, researchers have primarily focused on catalysts, investigating the enhancement of NH3 synthesis efficiency through doping, nanostructure engineering, and other catalyst modification strategies. Currently, FE has seen considerable progress, with the vast majority exceeding 90%, and there is still potential for further improvement in NH3 yield. Catalyst modification and N2 fixation efficiency improvement will become an important research method for enhancing the efficiency of PECP.
STRATEGIES TO IMPROVE PECP
In light of the two-step operation process of PECP, two paths for optimizing PECP can be given accordingly. This section will mainly explain the strategy of promoting NH3 synthesis by improving the efficiency of N2 fixation and electrocatalytic NH3 synthesis from NO3-.
Optimization of nitrogen fixation efficiency
Improvement of plasma discharge device
Increasing the N2 fixation efficiency can effectively increase the concentration of NOx- in the electrolyte, thus promoting the synthesis of NH3. High energy consumption and low NOx yield are the main problems faced by plasma N2 fixation. The current energy consumption optimal value is 2.4 MJ/mol, which is still higher than the traditional H-B process and cannot meet the needs of industrial applications. To become fully competitive, it needs to be further reduced to 0.7 MJ/mol[57]. In addition, improving the NOx yield by improving the plasma discharge device is also an important means to improve the efficiency of N2 fixation.
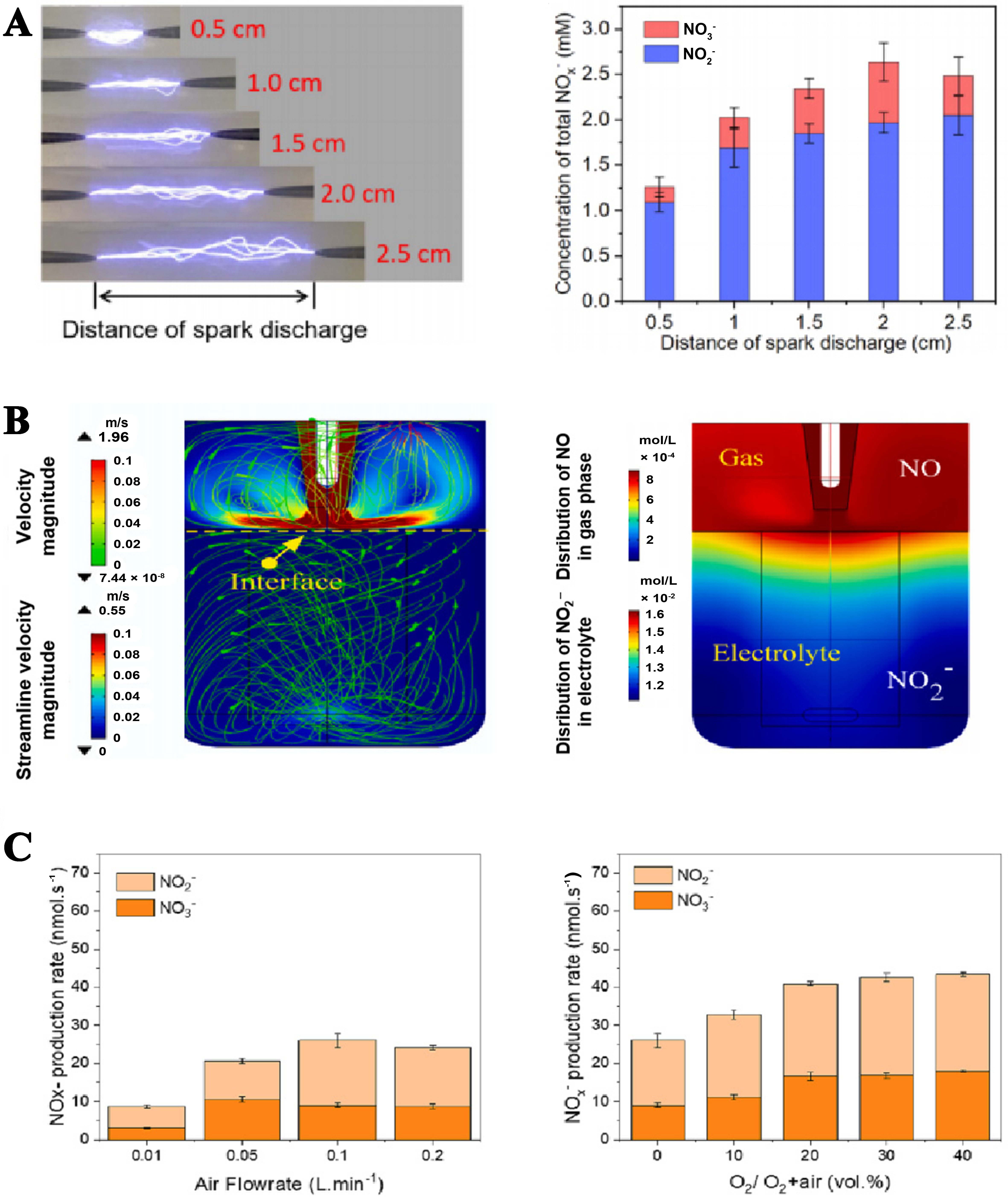
For example, Ren et al. researched the effect of spark discharge distance on NOx generation[52]. As shown in Figure 6A, as the spark distance increases (0.5-2.0 cm), the residence times of reactants in the discharge zone would augment, increasing the concentration of NOx. However, further increasing the spark distance (2-2.5 cm), the electric breakdown of gaseous reactants would consume more energy and ultimately decrease the concentration of NOx to some degree. In addition, Wu et al. discovered the distance between plasma and electrolytes was also a key parameter for the formed NOx[47]. The velocity field distribution was determined by plasma-electrolyte distance and gas flow rate, which in turn affected the air discharge and transportation of plasma-induced species [Figure 6B]. The closer the electrolyte solution is to the plasma, the higher the concentration of NOx that accumulates, and as the distance increases, the concentration of NOx decreases. Starting from nitrogen-fixing raw materials, Sun et al. adjusted the feed ratio of N2 and O2 and found that 36% was the best proportion of O2[58]. Meanwhile, it was found that changing the gas injection speed would also affect the yield of NOx [Figure 6C].
Spark distance, plasma-electrolyte distance, gas feed ratio, and injection speed all affect NOx concentration. Optimizing these parameters can enhance nitrogen fixation efficiency and NOx yield. Current research is predominantly focused on electrocatalysts for NH3 electrosynthesis, yet there is substantial potential for enhancing the efficiency of plasma N2 fixation. Future research should focus on optimizing discharge parameters and developing scalable reactor designs.
Catalyst promoted nitrogen fixation
In addition to improving the plasma device to optimize the nitrogen fixation efficiency, the collaborative nitrogen fixation of plasma and catalyst can also effectively improve the nitrogen fixation efficiency. This is mainly because the catalyst (TiO2, Al2O3, Fe2O3, etc.) can adsorb NO, N2O, and other substances, increase the reaction time, and increase the yield of NOx.
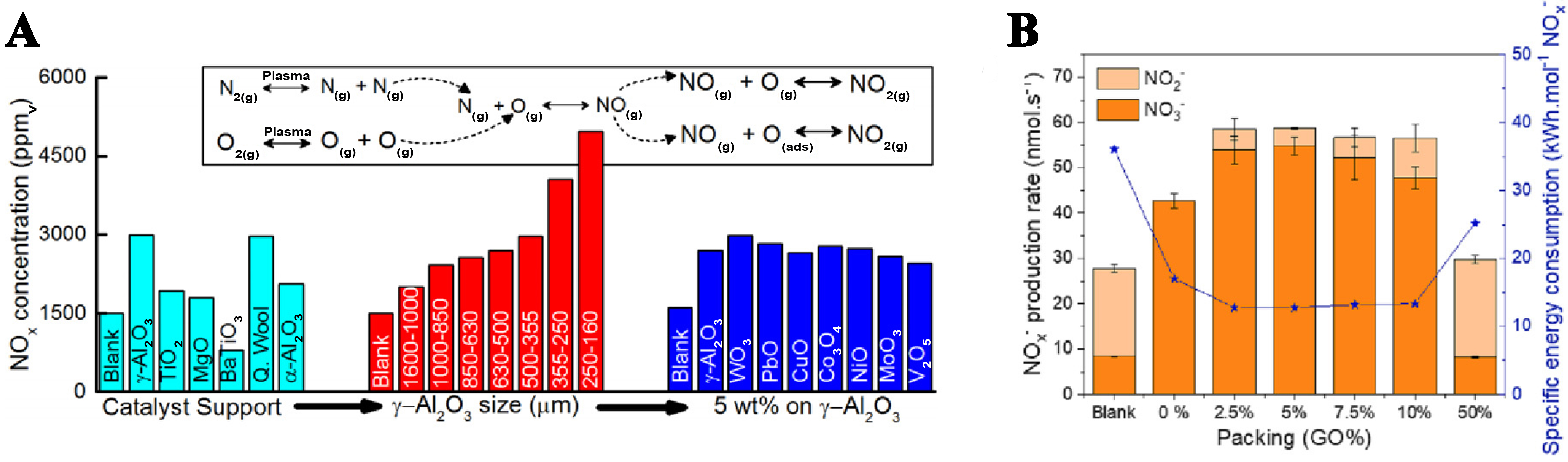
Patil et al. introduced the direct synthesis of NOx from N2 and O2 under atmospheric pressure and low-temperature conditions by non-thermal plasma[59]. In a DBD reactor, the direct synthesis of NOx was studied by filling different catalyst support materials [Figure 7A]. By changing the type and size of catalysts, the NOx yield can be continuously optimized.
Sun et al. also added different proportions of TiO2/[graphene oxide (GO)] to the plasma reactor to explore the differences in N2 fixation conversion performance[58]. In Figure 7B, when the GO content in the TiO2@GO catalyst is 2.5 wt% to 5 wt%, the generation rate of NOx- production and the reduction of energy consumption. However, a higher GO ratio (> 10 wt%) reduces the amount of catalyst available for the reaction, thus significantly negatively affecting catalytic performance. This indicates that an appropriate amount of GO doping is beneficial to the formation of NOx.
Adding catalysts is an effective way to enhance N2 fixation efficiency. However, the addition of a catalyst may affect the emission of NOx because the catalyst has the potential to clog the gas outlet. The development of highly efficient and porous catalysts, which improve gas flow and facilitate plasma permeation, is likely to become a popular research focus in the future.
Improvement of NH3 electrosynthesis efficiency
Optimizing the structure of the cathode catalyst is one of the powerful strategies to improve the electrocatalytic synthesis of NH3. The reduction of NO3- is accelerated by increasing the active site on the catalyst. It can also improve the catalytic activity of the catalyst site and directly improve the efficiency of NH3 synthesis. Thus, exploring suitable catalysts and catalyst structures is an important strategy to promote the efficiency of the electrocatalytic NH3 synthesis from NOx.
Catalyst nanostructure engineering
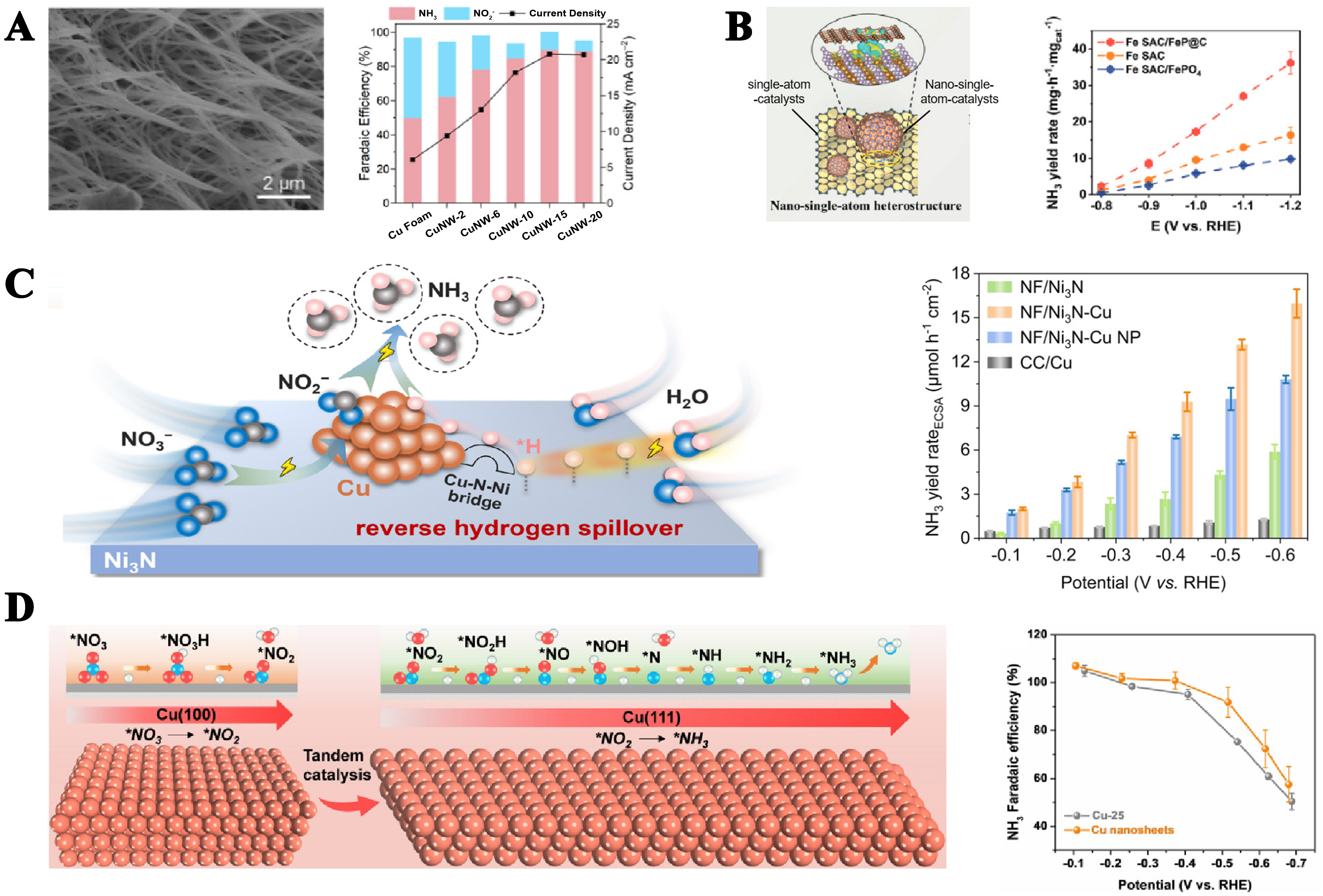
Electrode nanostructuring has been demonstrated as a promising way to realize high-performance NH3 electrosynthesis. The high surface-to-volume ratios of nanostructures give large electrochemical surface areas with much more exposed atoms and hence improve the performance per unit electrode area and/or material mass[60]. Shi et al. reported that a Cu nanowire electrocatalyst was prepared for NO3- reduction[61]. As a result, the optimized Cu nanowire electrode showed a 3-fold increase in NO3- reduction activity with a 90% FE for NH3 production at a low overpotential of -0.1 V vs. RHE. Among the copper nanowires (CuNW)-X (X = copper foam oxidation time) variants, CuNW-15 had the highest FE and current density [Figure 8A]. Increasing the number of active sites is a fundamental strategy for enhancing reaction performance. Another approach is to augment the catalytic strength of these sites.
Figure 8. (A) Array: schematic diagram of the array; NH3 yield of arrays formed at different oxidation times[60]; (B) Heterojunction: schematic diagram of heterojunction; NH3 yield rate of Fe SAC, Fe SAC/FeP@C, and FeSAC/FePO4[62]; (C) Schematic diagram of Ni3N/Cu; NH3 yield rate of NF/Ni3N, NF/Ni3N-Cu, NF/Ni3N-Cu NP[63]; (D) Tandem interaction of Cu(100) and Cu(111) facets[64]; the FE of NO2- to NH3 on Cu(111) at low concentration[64]. NP: Nanoparticle; FE: Faradaic efficiency.
Different from the array, the heterogeneous interface formed by the heterojunction can enhance the catalyst interaction and increase the reaction activity. For instance, Song et al. demonstrated an unprecedented nano-single-atom heterointerface composed of Fe single-atoms and carbon-shell-coated FeP nanoparticles (Fe SAC/FeP@C) was an efficient electrocatalyst[62]. The unique redistribution of charge at the nano-single-atom heterointerface and the regulation of the electronic structure of the Fe active center enabled Fe SAC/FeP@C to exhibit favorable adsorption of NO3- and enhanced activity of NO3- reduction [Figure 8B]. Similarly, Ouyang et al. proposed a Ni3N nanosheet array decorated with Cu nanoclusters to enhance
The adsorption energy of different crystal faces for species is different, making it difficult to complete the step with a high energy barrier when reducing NO3-. By adjusting the crystal surface and improving the adsorption state, the reaction can be carried out more smoothly and the yield of synthetic NH3 can be increased. Thus, Fu et al. reported a high-performance Cu nanosheet catalyst that has the tandem catalysis of Cu(100) and Cu(111) crystal planes[64]. Among them, Cu(100) is more likely to adsorb NO3- and promote its conversion to NO2-. The generated NO2- then migrates to Cu(111) for further reduction, thereby promoting the generation of NH3 [Figure 8D]. Electron transfer and reactant migration between crystal facets not only optimize reaction pathways but also significantly enhance reaction selectivity and efficiency. Analogously, Lu et al. prepared Co3O4 hexagonal nanosheets with exposed different facets. They discovered the (111) facet exhibited stronger adsorption of *H, facilitating the hydrogenation of NOx nd NHx intermediates and inhibiting the HER[65]. Co3O4 (111) exhibited the highest NH3 yield rate
Catalyst nanostructure engineering focuses on the microscale regulation of catalysts. By exposing more active surface areas and modulating electron interactions, it enhances reactant adsorption, lowers reaction energy barriers, and promotes reaction progress. Additionally, grain boundary engineering enhances the catalytic activity and stability of noble metal catalysts by increasing the density of active sites.
Doping
Doping refers to mixing multiple substances, such as alloys, single atoms doped with heteroatoms, etc. This strategy can enhance the activity and stability of catalysts through interatomic interactions and synergistic catalysis.
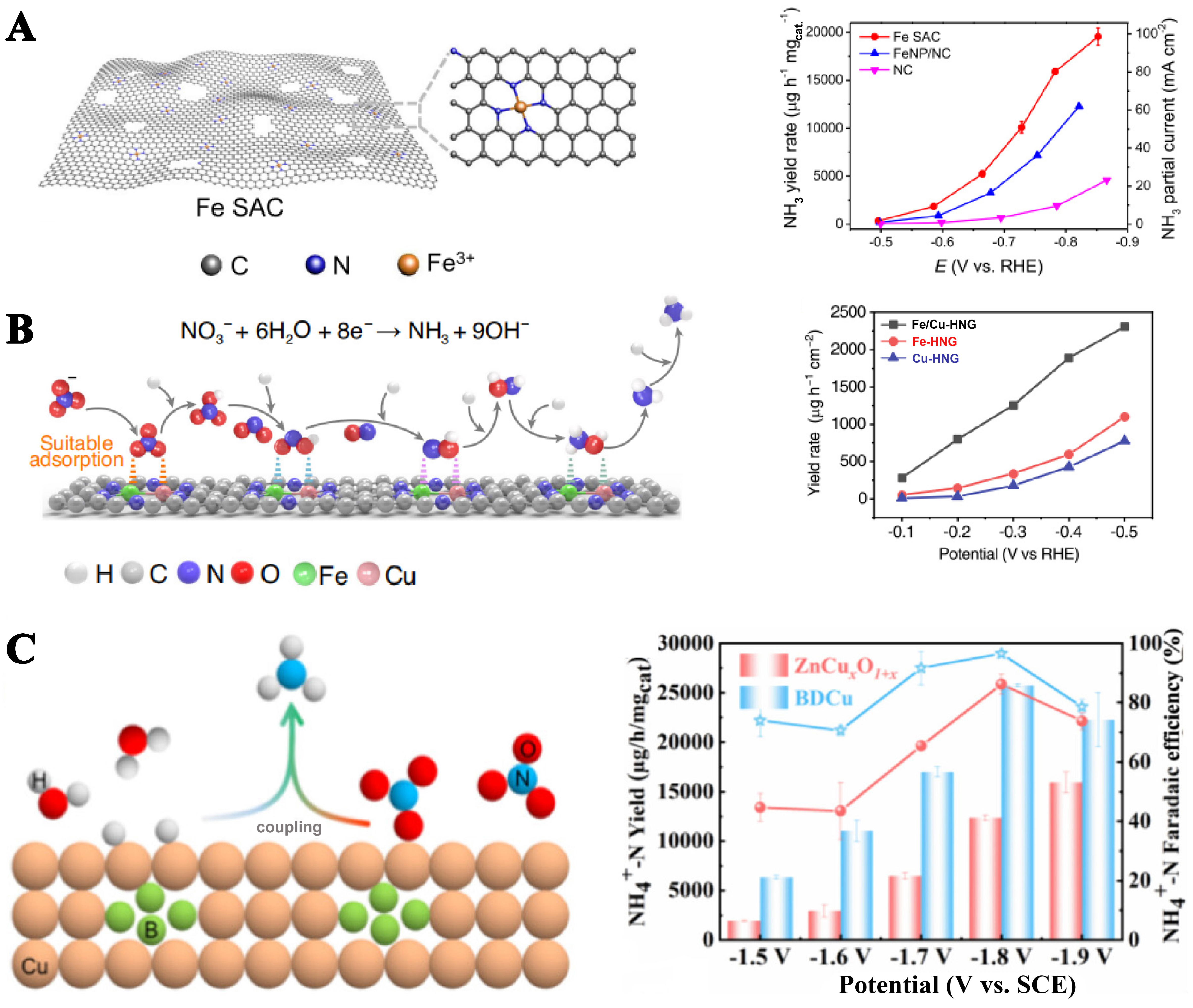
Wu et al. reported the selective active NO3- reduction to NH3 on an iron single-atom catalyst, which was mainly connected to the surface of the support by bonding with N, with an NH3 yield as high as
Figure 9. (A) Single atom: Schematic illustration of the synthesis of Fe SAC; NH3 yield rate and partial current density of Fe SAC, FeNP/NC, and NC[66]; (B) Alloy: Catalytic conversion steps from NO3- to NH3 on Fe-Cu alloy; NH3 yield rate of Fe/Cu-HNG, Fe-HNG, Cu-HNG[67]; (C) Heteroatom: Schematic illustration of the synthesis of BDCu; Comparison of properties of BDCu and ZnCuxO1+x at different potentials[68]. FeNP/NC: Fe nanoparticles in the N-doped carbon matrix; HNG: holey edge sites of nitrogen-dopedgraphene.
In conclusion, the stability of the catalyst is enhanced by the coordination ability of heteroatom-doped single atoms, and its utilization efficiency is greatly improved by the single-atom structure. The alloy enhances the adsorption and reactivity of the substance through synergistic effects and electronic structure changes, improving NH3 synthesis efficiency.
Relay catalysis
Due to the adsorption of different substances by various catalysts, the reaction activity is different, and the complex process of eight electrons and nine protons experienced by NO3RR is difficult to effectively reduce by a single catalyst. A relatively high overpotential is needed to overcome the kinetic barrier of the reaction, for the NO3--to-NO2- step which is often considered to be the rate-determining step[69-71]. The use of different catalysts for relay catalysis is also one of the measures to promote NH3 synthesis.
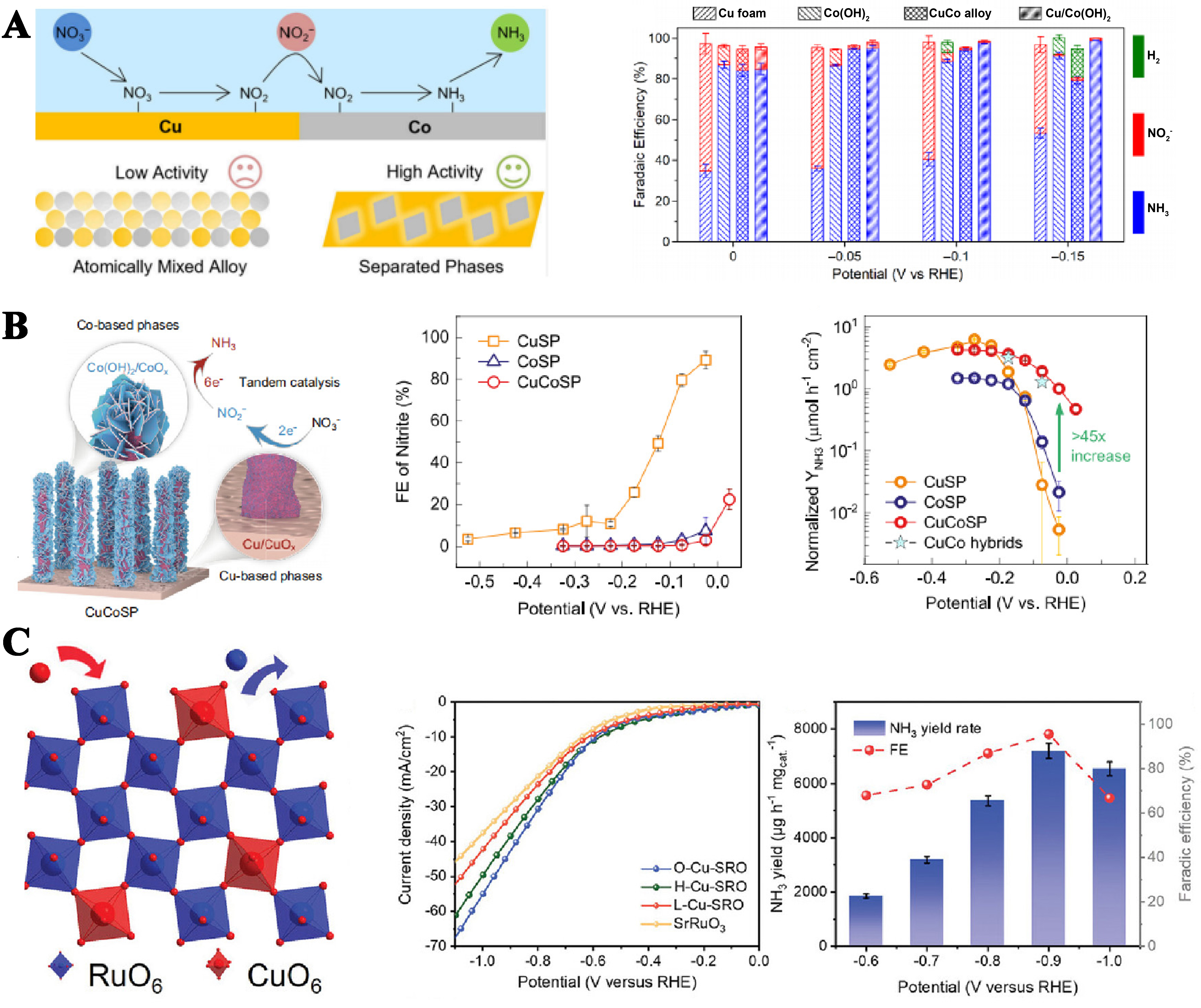
NO2- is a main side product that lowers the FE for NH3 production, while the intermediately produced NO2- can be further reduced to NH3 on the electrode, suggesting a unique dual role of NO2- in the NO3RR catalysis. Thus, Ren et al. proposed a tandem catalytic mechanism involving the reduction of NO3- on Cu to form NO2-. NO2- is transferred through the solution, and then further reduced to NH3 at the Co base site
Figure 10. (A) Cu/Co(OH)2 relay catalyst: the faradaic efficiency for the NO3RR on the Cu foam, Co(OH)2 sample, Cu–Co alloy, and Cu/Co(OH)2 tandem catalyst at selected potentials in H-cell[72]; (B) CuCoSP relay catalyst: the faradaic efficiency and the yield of NH3 on the CuSP, CoSP, CuCoSP, and CuCo hybrids[73]; (C) Cu-SrRuO3 relay catalyst: the faradaic efficiency and the yield of NH3 on the O-Cu-SRO on diffierent voltage[74]. NO3RR: Nitrate reduction reaction.
Leveraging the catalytic activities of different catalysts towards distinct reactive species, relay catalysis - employing multiple catalysts in sequence - emerges as an efficient strategy for complex catalytic processes. During relay catalysis, intermediates are promptly captured and converted into substrates for subsequent reaction steps, minimizing the likelihood of side reactions involving these intermediates. This significantly enhances overall reaction efficiency.
Pulse voltage
At present, most of the strategies to improve the efficiency of NH3 electrosynthesis are based on the design of catalysts and reactors, while the influence of electrolytic methods is usually ignored. Recently, pulse voltage has been used in electrocatalytic experiments because it can reconstruct the catalyst structure, change the local species concentration, and strengthen the mass transfer process[75-77].
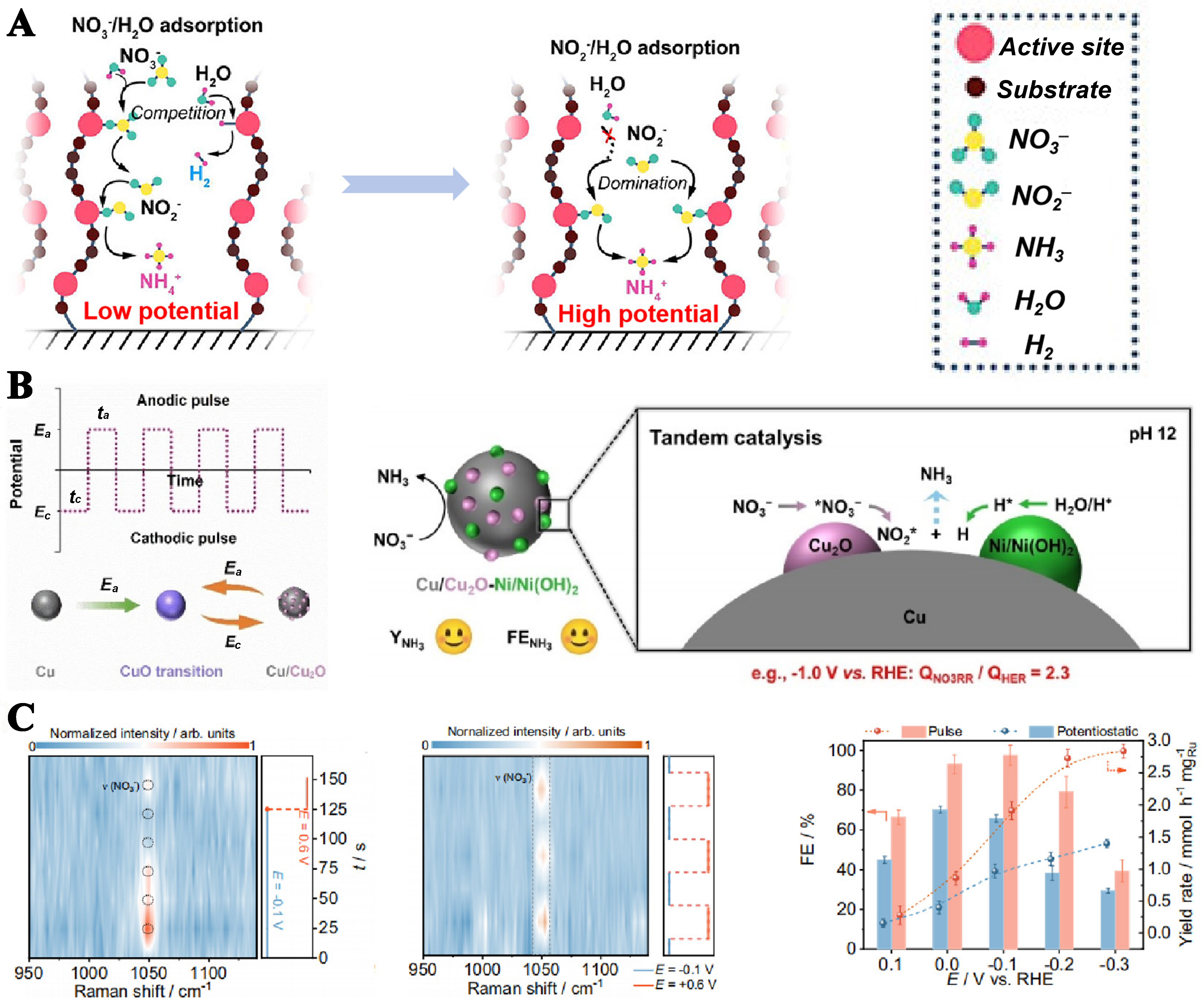
Li et al. employed Cu single-atom gel (SAG) under pulsed electrolysis to achieve highly efficient NO3- to NH3 conversion[78]. At low potentials, the primary process was NO2- reduction to NO2-. At higher potentials, accumulated NO2- facilitated NH3 formation while suppressing the HER [Figure 11A]. Compared to constant potential electrolysis, this approach enhanced the FE and NH3 yield while conserving electrical energy.
Figure 11. (A) Cu-sag led catalytic reaction at different potentials; (B) Oulse adjusts the catalyst structure: the pulsed electrolysis (upper panel); evolution of Cu valence state under pulse reaction conditions (lower panel)[79]; (C) Enrichment of NO3- by pulsed voltage: Comparison of NO3- concentration under constant potential and pulse potential; Comparison of FE and yield under constant potential and pulse potential[75]. FE: Faradaic efficiency.
Through the repeated application of positive and negative potentials, the catalyst can improve its structure and improve the efficiency of NH3 synthesis. Bu et al. reported a pulsed electrolysis strategy to achieve a reliable Cu/Cu2O structure. The Cu/Cu2O heterojunction exhibits high reactivity due to the strong electronic interactions at the Cu/Cu2O interface[76]. Moreover, the pulse voltage will not completely reduce CuxO, and the Cu/Cu2O structure is more stable[79]. Alloying with Ni further modulates hydrogen adsorption, promoting NH3 formation with a high NO3--to-NH3 FE (88.0% ± 1.6%, pH 12) and NH3 yield rate (583.6 ± 2.4 μmol·cm-2·h-1) under optimal pulsed conditions [Figure 11B].
The efficient reduction of NO3- can be achieved at a low concentration of NOx- by applying the pulse voltage of alternating positive and negative transformation. When a negative voltage is applied to the cathode, the reduction reaction proceeds normally. When converted to a positive voltage, NH4+ near the electrode is more likely to diffuse into the electrolyte, while negatively charged NOx- is attracted by the positive charge and accumulates around the electrode, preparing for subsequent reduction at negative potential. Constant potential reduction cannot achieve this effect. Furthermore, Huang et al. verified pulse voltage is beneficial to the enrichment of NO3-[75]. In particular, at low concentrations, the FE and yield of NH3 increased significantly due to the continuous supplementation of NO3- [Figure 11C].
In summary, the pulsed electrochemical process primarily serves two main functions. One is to enhance mass transfer: in pulsed electrochemistry, periodic voltage changes promote the enrichment of reactants and the desorption of products through electrostatic interactions. The other is catalyst reorganization: under constant current conditions, catalysts are prone to oxidation or reduction, losing their original structure and thereby reducing their performance. The pulsed electrochemical process, through parameter optimization, can maintain the catalyst structure or induce reorganization via alternating potentials, thereby enhancing catalytic efficiency.
SUMMARY AND OUTLOOK
The PECP is an emerging green technology. Compared with the traditional H-B process, it offers several advantages, including mild reaction conditions, low energy consumption, and no greenhouse gas emissions. To date, PECP has developed a relatively comprehensive system. The subsequent development and optimization of the PECP primarily focus on enhancing N2 fixation and electrocatalytic efficiency.
For N2 fixation efficiency, plasmas from spark discharge, gliding arc discharge, and DBD discharge have been explored. System parameters, such as discharge conditions and residence time, are crucial for developing efficient reactors and achieving cleaner NOx production with lower energy input. DBD and gliding arc plasmas are major future research directions for N2 fixation. Optimizing electrocatalytic efficiency primarily focuses on catalyst design. Providing an adequate supply of H* and suppressing the HER are key factors for designing efficient catalysts. Strategies such as nanostructural engineering and relay catalysis are emerging as major directions for future electrochemical NH3 synthesis. Additionally, modifying the electrolysis method, for example, using pulsed electrolysis, is a popular strategy for enhancing NH3 synthesis efficiency.
Despite some breakthroughs in this field, several key challenges and limitations must be overcome before industrial application can be realized:
(1) Energy consumption issues. Generating plasma requires significant electrical energy, particularly to maintain stable plasma conditions. Further optimization of overall energy consumption is needed; (2) Technical and equipment limitations. Most studies currently separate plasma discharge and electrocatalytic reduction into distinct steps, limiting continuous operation and restricting industrial-scale application; (3) Product collection and separation challenges. During NH3 synthesis, the low concentration of NH3 necessitates additional separation and purification steps.
Future research must address existing technological bottlenecks to advance this technology toward higher efficiency, sustainability, and industrial feasibility. The following section outlines potential research directions:
(I) Optimization of the PECP
(i) N2 fixation. Improving plasma discharge parameters, such as adjusting the discharge distance of the plasma, and controlling the gas flow rate, can not only enhance the efficiency of plasma N2 fixation but also reduce energy consumption. Additionally, employing catalysts in conjunction with plasma N2 fixation is another means to improve fixation efficiency. Developing efficient and breathable catalysts can be considered a future research direction.
(ii) NH3 electrosynthesis efficiency. Improving the efficiency of NH3 electrosynthesis involves not only the design of catalysts but also the alteration of electrolysis methods, such as pulsed and alternating current electrolysis. In the design of catalysts, many efficient catalysts have already been developed. Future research directions may focus on developing more economical and environmentally friendly catalysts. Strategies such as heteroatom doping and relay catalysis using different metals may become the main approaches to replace precious metals in catalysts.
(iii) Design integrated reactors. To enhance the efficiency of NH3 electrosynthesis, a device can be inserted between the plasma and electrocatalysis processes, allowing NOx generated by the plasma to be directly used for electro-reduction to produce NH3. For instance, Liu et al. designed an absorption pool that can absorb the generated NOx on one side and transfer the absorption liquid to the electrolysis pool for electrolysis on the other side, basically achieving the goal of integrating PECP[48].
(II) Industrialization and subsequent NH3 processes
(i) Industrial-relevant current density. High-current electrolysis is essential for industrialization. Utilizing membrane electrode assemblies, which feature efficient proton transport, allows the electrolysis current to reach the ampere level. For instance, Guo et al. designed a stacked membrane electrode assembly that integrates the catalyst, which can increase the current by nearly ten times[54].
(ii) Subsequent NH3 separation and purification processes. NH3 has a high solubility but is unstable. NH3 in the electrolyte can be blown out and absorbed, or the solution pH can be adjusted to weakly alkaline, slightly heated, and then NH3 is released and absorbed by the acid solution to evaporate and form ammonium chloride crystals. Su et al. achieved an NH3 recovery rate of over 85% using this method[80]. Developing advanced membrane separation technology or adsorbent materials can also efficiently separate low-concentration NH3. In addition to the recovery and utilization of NH3, the synthesis of subsequent chemical products based on NH3 may also become a future research direction, such as nitrogen fertilizers and MAP. The synthesis of these chemical products may bring greater economic and practical application value.
(III) Techno-economic assessments
Future research endeavors are suggested to incorporate comprehensive techno-economic assessments to evaluate the industrial feasibility of emerging technologies. This involves a detailed analysis of various cost components, such as material costs, the energy consumption of plasma and electrocatalyst reaction, the service life of a catalyst, equipment maintenance, and NH3 separation costs. By conducting such assessments, researchers can identify the most cost-effective and sustainable approaches, ensuring that the technology is not only scientifically sound but also economically viable for large-scale industrial applications.
In summary, the PECP holds significant importance in green chemistry and sustainable development. Future research will focus on addressing current challenges and advancing the industrial application of this technology.
DECLARATIONS
Authors’ contributions
Writing and revising the manuscript: Yang, C.; Xiao, X.
Discussing the manuscript and collecting data: Cen, W.
Co-directing this project and collecting data: Liu, J.
Directing this project and revising the manuscript: Li, J.
Availability of data and materials
Not applicable.
Financial support and sponsorship
This work is supported by the National Natural Science Foundation of China (grant No. 22402135), Natural Science Foundation of Sichuan Province (grant No. 2025ZNSFSC0896), Postdoctoral Fellowship Program of CPSF (GZB20240475, GZC20231777), and Fundamental Research Funds for the Central Universities.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. He, W.; Zhang, J.; Dieckhöfer, S.; et al. Splicing the active phases of copper/cobalt-based catalysts achieves high-rate tandem electroreduction of nitrate to ammonia. Nat. Commun. 2022, 13, 1129.
2. Ithisuphalap, K.; Zhang, H.; Guo, L.; Yang, Q.; Yang, H.; Wu, G. Photocatalysis and photoelectrocatalysis methods of nitrogen reduction for sustainable ammonia synthesis. Small. Methods. 2019, 3, 1800352.
3. Wu, T.; Fan, W.; Zhang, Y.; Zhang, F. Electrochemical synthesis of ammonia: progress and challenges. Mater. Today. Phys. 2021, 16, 100310.
4. Liu, H. Ammonia synthesis catalyst 100 years: practice, enlightenment and challenge. Chin. J. Catal. 2014, 35, 1619-40.
5. Wang, K.; Smith, D.; Zheng, Y. Electron-driven heterogeneous catalytic synthesis of ammonia: current states and perspective. Carbon. Resour. Convers. 2018, 1, 2-31.
6. Kyriakou, V.; Garagounis, I.; Vourros, A.; Vasileiou, E.; Stoukides, M. An electrochemical haber-bosch process. Joule 2020, 4, 142-58.
7. Martín, A. J.; Shinagawa, T.; Pérez-Ramírez, J. Electrocatalytic reduction of nitrogen: from Haber-Bosch to ammonia artificial leaf. Chem 2019, 5, 263-83.
8. Qing, G.; Ghazfar, R.; Jackowski, S. T.; et al. Recent advances and challenges of electrocatalytic N2 reduction to ammonia. Chem. Rev. 2020, 120, 5437-516.
9. Fu, X.; Pedersen, J. B.; Zhou, Y.; et al. Continuous-flow electrosynthesis of ammonia by nitrogen reduction and hydrogen oxidation. Science 2023, 379, 707-12.
10. Li, S.; Zhou, Y.; Fu, X.; et al. Long-term continuous ammonia electrosynthesis. Nature 2024, 629, 92-7.
11. Mcenaney, J. M.; Singh, A. R.; Schwalbe, J. A.; et al. Ammonia synthesis from N2 and H2O using a lithium cycling electrification strategy at atmospheric pressure. Energy. Environ. Sci. 2017, 10, 1621-30.
12. Xu, X.; Pan, Y.; Zhong, Y.; Ran, R.; Shao, Z. Ruddlesden–Popper perovskites in electrocatalysis. Mater. Horiz. 2020, 7, 2519-65.
13. Han, Q.; Jiao, H.; Xiong, L.; Tang, J. Progress and challenges in photocatalytic ammonia synthesis. Mater. Adv. 2021, 2, 564-81.
14. Gorbanev, Y.; Vervloessem, E.; Nikiforov, A.; Bogaerts, A. Nitrogen fixation with water vapor by nonequilibrium plasma: toward sustainable ammonia production. ACS. Sustain. Chem. Eng. 2020, 8, 2996-3004.
15. Conrads, H.; Schmidt, M. Plasma generation and plasma sources. Plasma. Sources. Sci. Technol. 2000, 9, 441-54.
16. Sharma, R. K.; Patel, H.; Mushtaq, U.; et al. Plasma activated electrochemical ammonia synthesis from nitrogen and water. ACS. Energy. Lett. 2021, 6, 313-9.
17. Wang, Y.; Craven, M.; Yu, X.; et al. Plasma-enhanced catalytic synthesis of ammonia over a Ni/Al2O3 catalyst at near-room temperature: insights into the importance of the catalyst surface on the reaction mechanism. ACS. Catal. 2019, 9, 10780-93.
18. Gharahshiran V, Zheng Y. Sustainable ammonia synthesis: an in-depth review of non-thermal plasma technologies. J. Energy. Chem. 2024, 96, 1-38.
19. Abe, R. Giant cluster expansion theory and its application to high temperature plasma. Prog. Theor. Phys. 1959, 22, 213-26.
20. Yuji, T.; Fujii, S.; Mungkung, N.; Akatsuka, H. Optical emission characteristics of atmospheric-pressure nonequilibrium microwave discharge and high-frequency DC pulse discharge plasma jets. IEEE. Trans. Plasma. Sci. 2009, 37, 839-45.
21. Schutze, A.; Jeong, J. Y.; Babayan, S. E.; Park, J.; Selwyn, G. S.; Hicks, R. F. The atmospheric-pressure plasma jet: a review and comparison to other plasma sources. IEEE. Trans. Plasma. Sci. 1998, 26, 1685-94.
22. Trenchev, G.; Nikiforov, A.; Wang, W.; Kolev, S.; Bogaerts, A. Atmospheric pressure glow discharge for CO2 conversion: model-based exploration of the optimum reactor configuration. Chem. Eng. J. 2019, 362, 830-41.
23. Gallon, H. J.; Tu, X.; Twigg, M. V.; Whitehead, J. C. Plasma-assisted methane reduction of a NiO catalyst - low temperature activation of methane and formation of carbon nanofibres. Appl. Catal. B. Environ. 2011, 106, 616-20.
24. Kan, H.; Wang, T.; Yang, Z.; et al. High frequency discharge plasma induced plasticizer elimination in water: removal performance and residual toxicity. J. Hazard. Mater. 2020, 383, 121185.
25. Arora, N.; Sharma, N. Arc discharge synthesis of carbon nanotubes: comprehensive review. Diam. Relat. Mater. 2014, 50, 135-50.
26. Mutaf-Yardimci, O.; Saveliev, A. V.; Fridman, A. A.; Kennedy, L. A. Thermal and nonthermal regimes of gliding arc discharge in air flow. J. Appl. Phys. 2000, 87, 1632-41.
27. Li, S.; Medrano, J.; Hessel, V.; Gallucci, F. Recent progress of plasma-assisted nitrogen fixation research: a review. Processes 2018, 6, 248.
28. Liu, J.; Li, X.; Liu, J.; Zhu, A. Understanding arc behaviors and achieving the optimal mode in a magnetically-driven gliding arc plasma. Plasma. Sources. Sci. Technol. 2020, 29, 015022.
29. Sun, J.; Zhou, R.; Hong, J.; et al. Sustainable ammonia production via nanosecond-pulsed plasma oxidation and electrocatalytic reduction. Appl. Catal. B. Environ. 2024, 342, 123426.
30. Glarborg, P.; Miller, J. A.; Kee, R. J. Kinetic modeling and sensitivity analysis of nitrogen oxide formation in well-stirred reactors. Combust. Flame. 1986, 65, 177-202.
31. Zeldovich, J. Acta physicochim. URSS.1946, 21, 577. https://cir.nii.ac.jp/crid/1572543023999418112. (accessed 22 Oct 2025)
32. Rouwenhorst, K. H. R.; Engelmann, Y.; van ‘t Veer, K.; Postma, R. S.; Bogaerts, A.; Lefferts, L. Plasma-driven catalysis: green ammonia synthesis with intermittent electricity. Green. Chem. 2020, 22, 6258-87.
33. Eyde, S. Oxidation of atmospheric nitrogen and development of resulting industries in Norway. J. Ind. Eng. Chem. 1912, 4, 771-4.
34. Patil, B. S.; Peeters, F. J. J.; van Rooij, G. J.; et al. Plasma assisted nitrogen oxide production from air: using pulsed powered gliding arc reactor for a containerized plant. AIChE. J. 2018, 64, 526-37.
35. Bian, W.; Shi, J.; Yin, X. Nitrogen fixation into water by pulsed high-voltage discharge. IEEE. Trans. Plasma. Sci. 2009, 37, 211-8.
36. Wang, W.; Patil, B.; Heijkers, S.; Hessel, V.; Bogaerts, A. Nitrogen fixation by gliding arc plasma: better insight by chemical kinetics modelling. ChemSusChem 2017, 10, 2145-57.
37. van ‘t Veer, K.; Engelmann, Y.; Reniers, F.; Bogaerts, A. Plasma-catalytic ammonia synthesis in a DBD plasma: role of microdischarges and their afterglows. J. Phys. Chem. C. 2020, 124, 22871-83.
38. Subedi, D. P.; Joshi, U. M.; Wong, C. S. Dielectric barrier discharge (DBD) plasmas and their applications. In: Rawat RS, editor. Plasma science and technology for emerging economies. Singapore: Springer; 2017. pp. 693-737.
39. de Groot, M.; Koper, M. The influence of nitrate concentration and acidity on the electrocatalytic reduction of nitrate on platinum. J. Electroanal. Chem. 2004, 562, 81-94.
40. Sicsic, D.; Balbaud-Célérier, F.; Tribollet, B. Mechanism of nitric acid reduction and kinetic modelling. Eur. J. Inorg. Chem. 2014, 2014, 6174-84.
41. Garcia-Segura, S.; Lanzarini-Lopes, M.; Hristovski, K.; Westerhoff, P. Electrocatalytic reduction of nitrate: fundamentals to full-scale water treatment applications. Appl. Catal. B. Environ. 2018, 236, 546-68.
42. Xu, H.; Ma, Y.; Chen, J.; Zhang, W. X.; Yang, J. Electrocatalytic reduction of nitrate - a step towards a sustainable nitrogen cycle. Chem. Soc. Rev. 2022, 51, 2710-58.
43. Kong, X.; Ni, J.; Song, Z.; et al. Synthesis of hydroxylamine from air and water via a plasma-electrochemical cascade pathway. Nat. Sustain. 2024, 7, 652-60.
44. Anastasiadou, D.; van Beek, Y.; Hensen, E. J. M.; Costa Figueiredo, M. Ammonia electrocatalytic synthesis from nitrate. Electrochem. Sci. Adv. 2023, 3, e2100220.
45. Sun, J.; Alam, D.; Daiyan, R.; et al. A hybrid plasma electrocatalytic process for sustainable ammonia production. Energy. Environ. Sci. 2021, 14, 865-72.
46. Liang, W. P.; Zhang, X. M.; Bai, P. W.; et al. Cascade N2 reduction process with DBD plasma oxidation and electrocatalytic reduction for continuous ammonia synthesis. Environ. Sci. Technol. 2023, 57, 14558-68.
47. Wu, A.; Yang, J.; Xu, B.; et al. Direct ammonia synthesis from the air via gliding arc plasma integrated with single atom electrocatalysis. Appl. Catal. B. Environ. 2021, 299, 120667.
48. Liu, W.; Xia, M.; Zhao, C.; et al. Efficient ammonia synthesis from the air using tandem non-thermal plasma and electrocatalysis at ambient conditions. Nat. Commun. 2024, 15, 3524.
49. Li, L.; Tang, C.; Cui, X.; et al. Efficient nitrogen fixation to ammonia through integration of plasma oxidation with electrocatalytic reduction. Angew. Chem. Int. Ed. Engl. 2021, 60, 14131-7.
50. Zheng, J.; Zhang, H.; Lv, J.; et al. Enhanced NH3 synthesis from air in a plasma tandem-electrocatalysis system using plasma-engraved N-doped defective MoS2. JACS. Au. 2023, 3, 1328-36.
51. Liu, Y.; Ma, J.; Huang, S.; Niu, S.; Gao, S. Highly dispersed copper-iron nanoalloy enhanced electrocatalytic reduction coupled with plasma oxidation for ammonia synthesis from ubiquitous air and water. Nano. Energy. 2023, 117, 108840.
52. Ren, Y.; Yu, C.; Wang, L.; et al. Microscopic-level insights into the mechanism of enhanced NH3 synthesis in plasma-enabled cascade N2 oxidation-electroreduction system. J. Am. Chem. Soc. 2022, 144, 10193-200.
53. Li, W.; Zhang, S.; Ding, J.; et al. Sustainable nitrogen fixation to produce ammonia by electroreduction of plasma-generated nitrite. ACS. Sustain. Chem. Eng. 2023, 11, 1168-77.
54. Guo, X.; Wang, Z.; Gao, Y.; et al. Highly stable perovskite oxides for electrocatalytic acidic NOx- reduction streamlining ammonia synthesis from air. Angew. Chem. Int. Ed. Engl. 2024, 63, e202410517.
55. Ding, J.; Li, W.; Zhang, H.; et al. A cascade jet plasma oxidation - electroreduction system using Pd-Ni dual-site catalyst for sustainable ammonia production from air. Adv. Funct. Mater. 2024, 34, 2410768.
56. Gao, R.; Dai, T. Y.; Meng, Z.; et al. A bifunctional catalyst for green ammonia synthesis from ubiquitous air and water. Adv. Mater. 2023, 35, e2303455.
57. Rouwenhorst, K. H. R.; Jardali, F.; Bogaerts, A.; Lefferts, L. From the Birkeland-Eyde process towards energy-efficient plasma-based NOX synthesis: a techno-economic analysis. Energy. Environ. Sci. 2021, 14, 2520-34.
58. Sun, J.; Zhang, T.; Hong, J.; et al. Insights into plasma-catalytic nitrogen fixation from catalyst microanalysis and chemical kinetics modelling. Chem. Eng. J. 2023, 469, 143841.
59. Patil, B.; Cherkasov, N.; Lang, J.; Ibhadon, A.; Hessel, V.; Wang, Q. Low temperature plasma-catalytic NOx synthesis in a packed DBD reactor: effect of support materials and supported active metal oxides. Appl. Catal. B. Environ. 2016, 194, 123-33.
60. Xu, R.; Du, L.; Adekoya, D.; et al. Well-defined nanostructures for electrochemical energy conversion and storage. Adv. Energy. Mater. 2021, 11, 2001537.
61. Shi, K.; Willis, M. D.; Ren, Z.; Feng, X. Efficient recycling of dilute nitrate to ammonia using Cu nanowire electrocatalyst. J. Phys. Chem. C. 2023, 127, 20710-7.
62. Song, J.; Qian, S.; Yang, W.; et al. Nano-single-atom heterointerface engineering for pH-universal electrochemical nitrate reduction to ammonia. Adv. Funct. Mater. 2024, 34, 2409089.
63. Ouyang, X.; Qiao, W.; Yang, Y.; et al. Intensifying interfacial reverse hydrogen spillover for boosted electrocatalytic nitrate reduction to ammonia. Angew. Chem. Int. Ed. Engl. 2025, 64, e202422585.
64. Fu, Y.; Wang, S.; Wang, Y.; et al. Enhancing electrochemical nitrate reduction to ammonia over Cu nanosheets via facet tandem catalysis. Angew. Chem. Int. Ed. Engl. 2023, 62, e202303327.
65. Lu, S.; Lin, G.; Yan, H.; et al. In situ facet transformation engineering over Co3O4 for highly efficient electroreduction of nitrate to ammonia. ACS. Catal. 2024, 14, 14887-94.
66. Wu, Z. Y.; Karamad, M.; Yong, X.; et al. Electrochemical ammonia synthesis via nitrate reduction on Fe single atom catalyst. Nat. Commun. 2021, 12, 2870.
67. Zhang, S.; Wu, J.; Zheng, M.; et al. Fe/Cu diatomic catalysts for electrochemical nitrate reduction to ammonia. Nat. Commun. 2023, 14, 3634.
68. Wang, J.; Ou, Z.; Dong, C.; et al. Electronic structure modulated by B-doped Cu promotes electrocatalytic nitrate reduction for ammonia production. ACS. Catal. 2025, 15, 156-66.
69. Ouyang, L.; Liang, J.; Luo, Y.; et al. Recent advances in electrocatalytic ammonia synthesis. Chin. J. Catal. 2023, 50, 6-44.
70. Zhang, J.; He, W.; Quast, T.; et al. Single-entity electrochemistry unveils dynamic transformation during tandem catalysis of Cu2O and Co3O4 for converting NO3- to NH3. Angew. Chem. Int. Ed. Engl. 2023, 62, e202214830.
71. Fan, Z.; Cao, C.; Yang, X.; et al. Interfacial electronic interactions promoted activation for nitrate electroreduction to ammonia over Ag-modified Co3O4. Angew. Chem. Int. Ed. Engl. 2024, 63, e202410356.
72. Ren, Z.; Shi, K.; Meng, Z.; Willis, M. D.; Feng, X. Complete single-pass conversion of dilute nitrate to ammonia using Cu/Co(OH)2 tandem electrocatalyst. ACS. Energy. Lett. 2024, 9, 3849-58.
73. Guo, Y.; Stroka, J. R.; Kandemir, B.; Dickerson, C. E.; Bren, K. L. Cobalt metallopeptide electrocatalyst for the selective reduction of nitrite to ammonium. J. Am. Chem. Soc. 2018, 140, 16888-92.
74. Lv, L.; Tan, H.; Liu, Y.; et al. Cu-Ru bicenter synergistically triggers tandem catalytic effect for electroreduction of nitrate to ammonium. Adv. Funct. Mater. 2025, 2423612.
75. Huang, Y.; He, C.; Cheng, C.; et al. Pulsed electroreduction of low-concentration nitrate to ammonia. Nat. Commun. 2023, 14, 7368.
76. Bu, Y.; Wang, C.; Zhang, W.; Yang, X.; Ding, J.; Gao, G. Electrical pulse-driven periodic self-repair of Cu-Ni tandem catalyst for efficient ammonia synthesis from nitrate. Angew. Chem. Int. Ed. Engl. 2023, 62, e202217337.
77. Yang, Q.; Bu, Y.; Pu, S.; et al. Matched kinetics process over Fe2O3-Co/NiO heterostructure enables highly efficient nitrate electroreduction to ammonia. Angew. Chem. Int. Ed. Engl. 2024, 63, e202400428.
78. Li, P.; Li, R.; Liu, Y.; Xie, M.; Jin, Z.; Yu, G. Pulsed nitrate-to-ammonia electroreduction facilitated by tandem catalysis of nitrite intermediates. J. Am. Chem. Soc. 2023, 145, 6471-9.
79. Wang, Y.; Zhou, W.; Jia, R.; Yu, Y.; Zhang, B. Unveiling the activity origin of a copper-based electrocatalyst for selective nitrate reduction to ammonia. Angew. Chem. Int. Ed. Engl. 2020, 59, 5350-4.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Copyright
Data & Comments
Data
















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].