Cyanide-free leaching of gold from used Au/MOx catalysts assist by oxidative carbonylation
Abstract
Recycling gold from used catalysts is significant for economic and environmental sustainability. Conventional methods, which heavily rely on the cyanide process, have posed significant environmental, health, and safety challenges. Herein, this study presents a novel approach for gold leaching from spent Au/metal oxide (Au/MOx) catalysts coupled with an oxidative carbonylation reaction. This tandem process not only efficiently dissolves Au nanoparticles from used heterogeneous Au catalysts into soluble AuI2- complex without using toxic cyanide, strong acids or oxidants, but also produces useful nitrogen-containing carbonyl compounds. Mechanistically, the leaching process is initiated by the in-situ generation of hydroiodic acid during the oxidative carbonylation reaction. This new approach has potential guide to the metal recovery and the design of stable heterogeneous catalysts.
Keywords
INTRODUCTION
Since the breakthrough in heterogeneous gold (Au) catalysis by Hutchings and Haruta, there has been a dramatic increase of papers and patents focusing on gold catalysis[1-3]. However, recycling gold from used Au catalysis has received limited attention. As a rare and precious metal, Au in spent catalysts is recognized as an attractive secondary resource. With typical Au content exceeding 0.1 wt%, these catalysts contain gold concentrations hundreds of times higher than natural ores or electronic waste[4,5]. Therefore, recovering Au from a spent catalyst is of great significance for economic and environmental sustainability. Currently, over 90% of gold refining from the ores and secondary resources relies on the cyanidation process, which needs stoichiometric and highly toxic cyanide salts as lixiviants. However, the use of cyanide salts posed significant challenges in terms of safety and environmental aspects. To this end, developing cyanide-free alternatives are essential to supersede the cyanidation process and meet the regulatory and safety standards. Alternative lixiviants, such as sulfides, organic aqua regia, pyridinethiols, and halide (chloride, bromide and iodide), have been widely investigated as non-cyanide options for gold recovery[6-9].
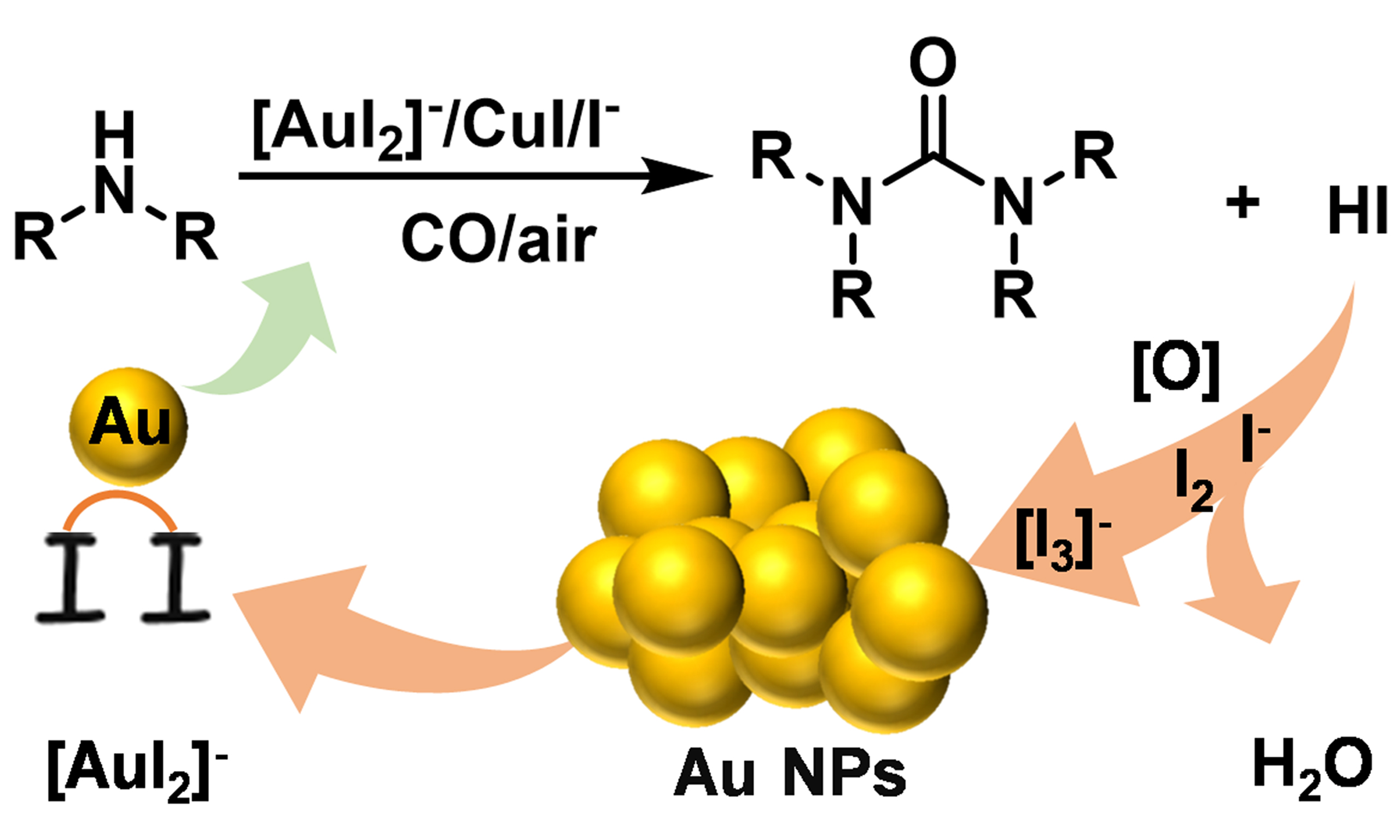
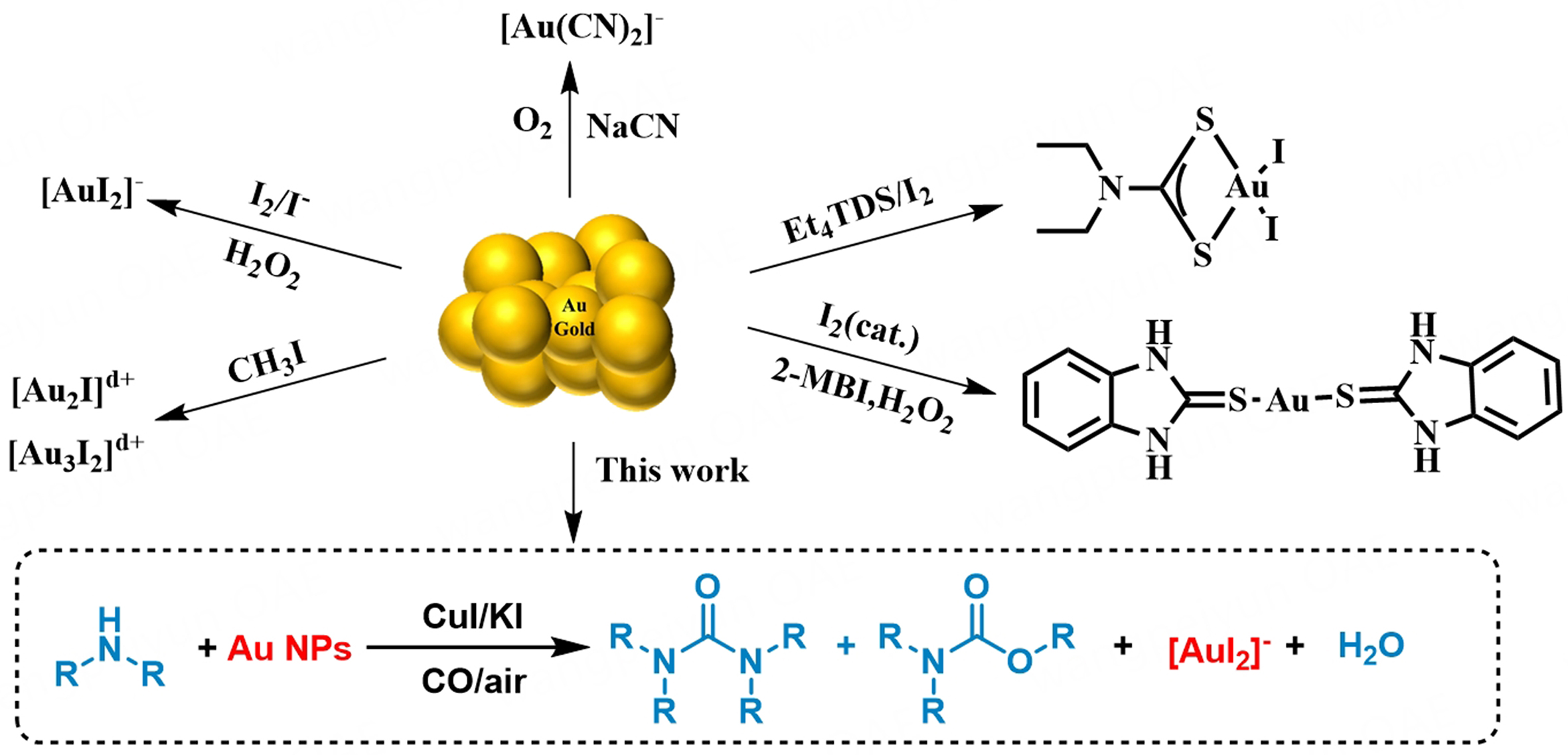
Therein, the iodine-iodide system (I2/I-/H2O2) stands out as a promising approach, forming stable gold-iodide complexes [AuI2]- as the stable forms. Moreover, a similar gold dissolution phenomenon is also observed in the iodide-promoted gold-catalyzed carbonylation reaction. For example, Hardacre demonstrated that gold nanoparticles (Au NPs) re-dispersed to the [Au2I]δ+ or [Au3I2]δ+ with methyl iodide during the methanol carbonylation[10]. We have successfully developed Au/CuI as synergistic catalysts for oxidative carbonylation of amines and proved that the iodide and copper were indispensable promoters to stabilize gold in an ionic state[11,12]. Inspired by the gold leaching via the iodine-iodide system and Au NP redispersion with iodide, we report the leaching of Au from used gold catalysts during the oxidative carbonylation of amines [Scheme 1]. This approach achieves a “killing two birds with one stone” goal - simultaneously recovering gold and producing valuable nitrogen-containing carbonyl compounds.
RESULTS AND DISCUSSION
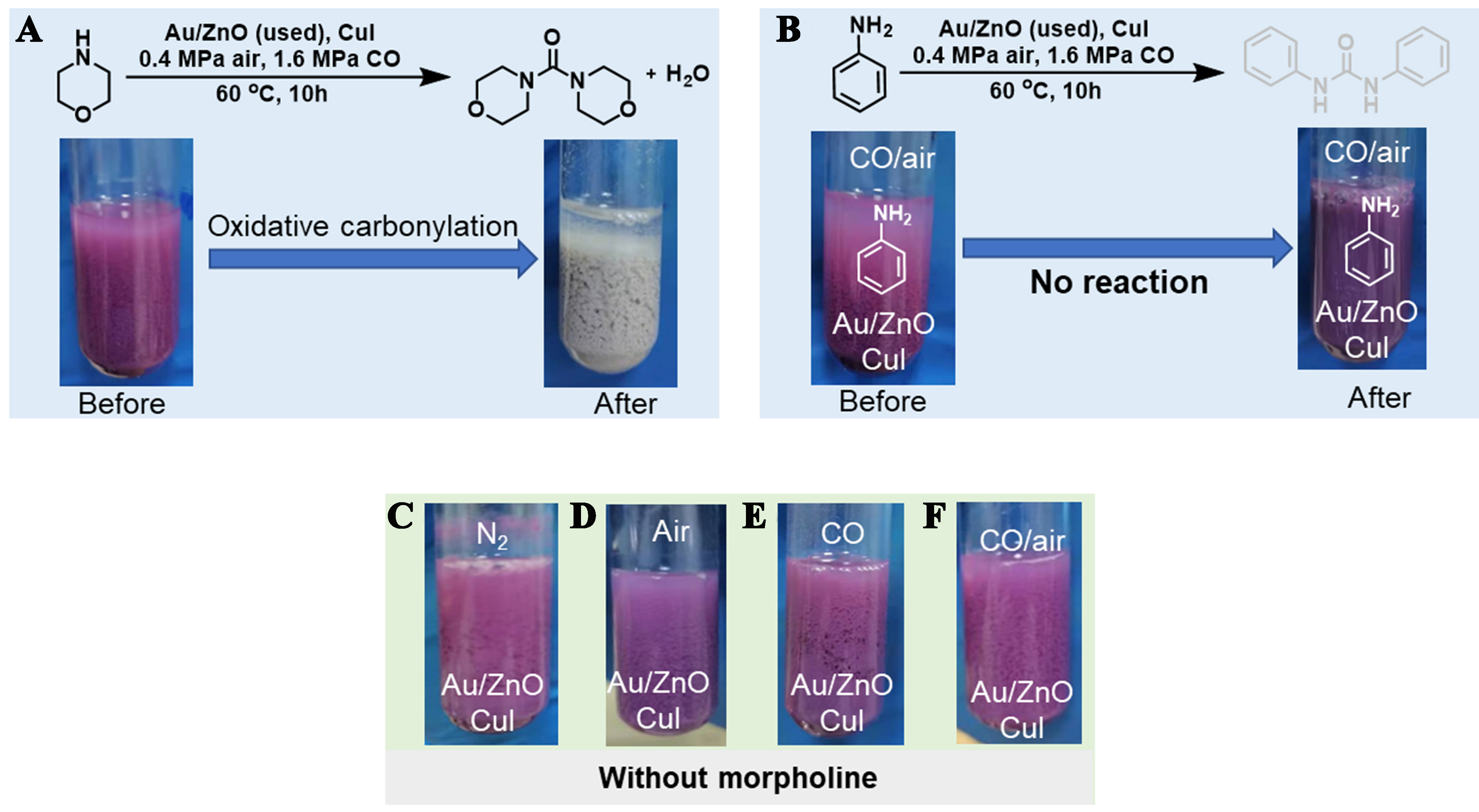
We first examined the leaching of the gold from used Au/ZnO (0.79 wt%)[13]. Remarkably, after oxidative carbonylation of morpholine in the presence of CuI, the catalyst changed color to light gray [Figure 1A], and ~6.02 mg/mL of Au ion was detected in the liquid phase [Supplementary Table 1]. To confirm that oxidative carbonylation of amine is the key factor for Au leaching from the Au/ZnO, we replaced morpholine with aniline, the molecule with low reactivity in Au/Cu-catalyzed oxidative carbonylation reactions. Following the reaction with aniline, the catalyst color showed no significant change [Figure 1B], and the Au ion concentration in the liquid phase was below the detection limit of the instrument. In the absence of morpholine, similarly, the color of the catalyst remains unchanged in different atmospheres
Figure 1. Leaching of gold from used Au/ZnO catalysts assisted by oxidative carbonylation of amines. (A) Oxidative carbonylation of morpholine; (B) The photo of used Au/ZnO catalysts under different treatment atmospheres; Oxidative carbonylation of aniline, the photo of used Au/ZnO catalysts under different treatment atmospheres: (C) N2, (D) air, (E) CO, (F) CO/air.
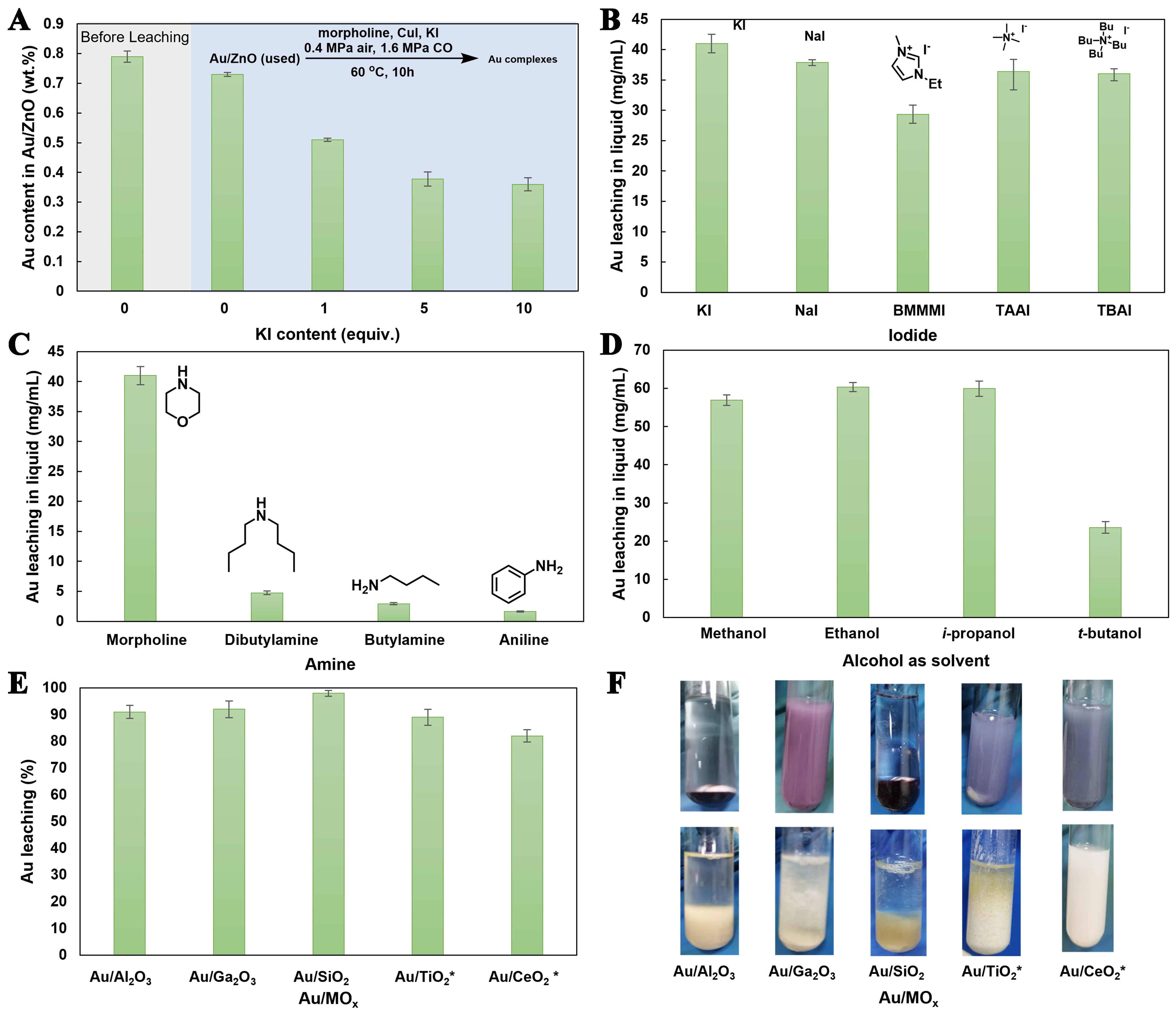
Then, the reaction conditions were optimized to further improve the leaching efficiency of Au from used Au/MOx. Firstly, drawing on previous findings regarding the promotional effect of KI on oxidative carbonylation of amines[14,15], we investigated its impact on gold leaching. Notably, the leaching efficiency of Au from used Au/ZnO catalysts was increased significantly with the addition of KI [Figure 2A and Supplementary Figure 1A]. While the amount of KI was 5 equivalents, the content of Au in used Au/ZnO catalysts decreased to 0.38 wt%, ~52% of Au was dissolved from used Au/ZnO catalysts. Further increasing the amount of KI to 10 equivalents, the leaching efficiency of Au from used Au/ZnO catalysts reached ~54.4% without noticeably increasing [Figure 2A]. To further optimize gold leaching in the presence of iodide, the effect of cations in iodide was screened [Figure 2B and Supplementary Figure 1B]. In addition to potassium, sodium, imidazole, and quaternary ammonium-based iodide were suitable for the leaching of Au, but the efficiency of imidazole iodide was relatively poor (29.3 mg/mL).
Figure 2. Leaching of gold from used Au/MOx catalysts. (A) Au source: used Au/ZnO, effect of content of KI equivalent, I- mol/Au mol; (B) Au source: used Au/ZnO, the effect of cations in iodide, 10 equiv; (C) Au source: used Au/ZnO, the effect of amines, KI, 10 equiv; (D) Au source: used Au/ZnO, effect of alcohol solvents, KI, 10 equiv.; (E) Effect of various used Au/MOx catalysts, KI, 10 equiv; (F) The photo of used Au/MOx catalysts before and after leaching process. Oxidative carbonylation reaction condition: amine (1 mmol), CuI (0.02 mmol) solvent 5 mL, CO 1.6 MPa, air 0.4 MPa, T = 60 oC, t = 10 h. Au/MOx: Au/metal oxide.
Compared to primary amines (butylamine) or aromatic amines (aniline), secondary amines (morpholine) exhibited better efficiency [Figure 2C, Supplementary Figure 1C, Supplementary Scheme 1]. However, a poor efficiency (Au 4.7 mg/mL) in dibutylamine was obtained due to the strong steric effect. More than 50% of butylamine was converted to the corresponding urea with 99% selectivity, but only ~2.93 mg/mL of Au ions was detected. Alcohol solvents, such as methanol, ethanol, i-propanol, and t-butanol, are both reactants and solvents for the oxidative carbonylation of amines to different carbamates. As shown in Figure 2D, methanol (56.9 mg/mL), ethanol (60.3 mg/mL), and i-propanol (59.9 mg/mL) exhibited better leaching efficiency of Au than t-butanol (23.6 mg/mL) [Figure 2D, Supplementary Figure 1D, Supplementary Scheme 2].
Encouraged by the above results, we extended the study to other Au/MOx catalysts, including Au/Al2O3, Au/Ga2O3, Au/SiO2, Au/TiO2, and Au/CeO2, to evaluate the generality of gold leaching assisted by the oxidative carbonylation reaction [Figure 2E]. 91% of Au on Au/Al2O3 was dissolved, while the leaching efficiencies for Au/Ga2O3 and Au/SiO2 catalysts reached ~92% and ~98%, respectively. In contrast, Au/TiO2 and Au/CeO2 exhibited lower initial leaching efficiency of 47% and 32%, which may be due to the strong metal-support interaction [Supplementary Figure 2]. However, after the secondary leaching operation, the overall leaching efficiency reached 89% and 82%[3]. Apparently, the solid residues after leaching displayed colors similar to their original supports [Figure 2F], further indicating that most of the gold had been successfully leached from the used Au/MOx catalysts.
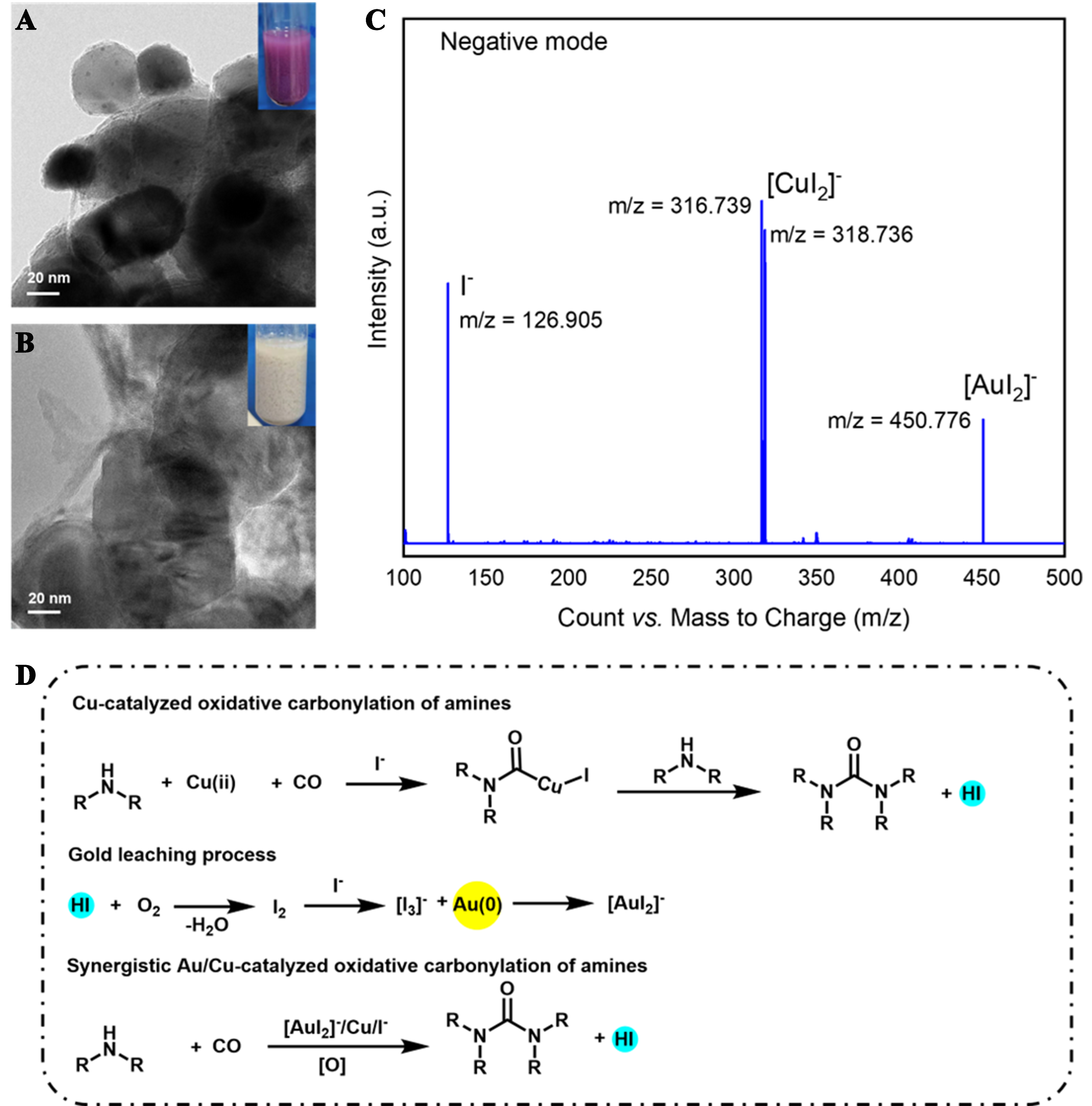
The transmission electron microscopy (TEM) patterns of the used Au/ZnO catalysts before and after leaching were collected to more intuitively observe the Au leaching from the used catalysts. As shown in Figure 3, Au NPs were easily observed on the ZnO surface at a 20 nm scale, and then disappeared after the leaching process. Then, to understand which gold complexes existed in the liquid during the leaching process, the high-resolution electrospray-ionization mass spectra (HRMS) of the liquid after the leaching process were collected [Figure 3C]. In the negative ion mode, four obvious molecular ion peaks were observed at m/z = 126.905, m/z = 316.739, m/z = 126.905, and m/z = 450.776, which can be attributed to the I-, [CuI2]-, and [AuI2]-. It is therefore evident that Au NPs on ZnO were dissolved, and formed a stable
Figure 3. TEM pattern of the used Au/ZnO catalysts (A) before leaching and (B) after leaching; (C) Negative ion HRMS of the toluene solution after leaching; (D) Proposed mechanism for the gold leaching assisting by oxidative carbonylation of amines. TEM: Transmission electron microscopy; HRMS: high-resolution electrospray-ionization mass spectra.
With references to the previous reports on the possible mechanism of PdI2-catalyzing oxidative carbonylation and experiment results obtained in this work [Supplementary Schemes 3 and 4][14-17], the possible mechanism of gold leaching during the oxidative carbonylation is proposed [Figure 3D]. The re-oxidization of Cu or Au during the oxidative carbonylation reaction is omitted for clarity. Firstly, the leaching process may start with the formation of a carbamoylcopper iodide intermediate, from the reaction between Cu (ii) complex with iodide ligands, morpholine (amines), and CO. Then, the direct nucleophilic displacement by the amine leads to the formation of the urea and HI. Subsequently, the HI species was oxidized into I2 with active oxygen and further reacted with I- to produce [I3]-. Finally, Au NPs were oxidized by [I3]- to [AuI2]-. At this time, an efficient bimetallic catalysis for oxidative carbonylation is formed by a combination of [AuI2]- and CuI, which will generate more HI and [I3]-, thus realizing an efficient gold leaching process.
CONCLUSIONS
By integrating the hydroiodic acid (HI) generated in the oxidative carbonylation of amines, we realized a cyanide-free process for the Au recovery from spent Au/MOx catalysis. This tandem approach achieved a maximum leaching efficiency of 98%. Importantly, unlike the classical gold leaching method, our process avoids the use of cyanide salts, strong acids, or strong oxidants. Meanwhile, useful nitrogen-containing chemicals are produced during the leaching process, further highlighting the economic and practical value. In a wider context, this leaching strategy holds potential for application in the leaching of other metals, such as Pd, Rh, and Cu, from spent catalysts, ores, and electronic wastes.
DECLARATIONS
Authors’ contributions
Supervised the project and revised the manuscript: He, L.
Responsible for data collection, data analysis, and wrote the original manuscript: Cao, Y.
Availability of data and materials
Supplementary Materials are available online for this paper. Other raw data that support the findings of this study are available from the corresponding author upon reasonable request.
Financial support and sponsorship
The work was supported by the National Key Research and Development Program of Ministry of Science and Technology (2022YFA1504602), National Natural Science Foundation of China (U22B20137, U24A20494, 22302214), Major Science and Technology Projects in Gansu Province (22ZD6GA003, 23ZDFA016), and Gansu Province Basic Innovation Group (24JRRA043).
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
Supplementary Materials
REFERENCES
2. Ciriminna, R.; Falletta, E.; Della Pina, C.; Teles, J. H.; Pagliaro, M. Industrial applications of gold catalysis. Angew. Chem. Int. Ed. Engl. 2016, 55, 14210-7.
3. Wang, C.; Yang, M.; Flytzani-Stephanopoulos, M. Single gold atoms stabilized on nanoscale metal oxide supports are catalytic active centers for various reactions. AIChE. J. 2016, 62, 429-39.
5. Chen, Y.; Xu, M.; Wen, J.; et al. Selective recovery of precious metals through photocatalysis. Nat. Sustain. 2021, 4, 618-26.
6. Zupanc, A.; Install, J.; Jereb, M.; Repo, T. Sustainable and selective modern methods of noble metal recycling. Angew. Chem. Int. Ed. Engl. 2023, 62, e202214453.
7. Liang, C. J.; Li, J. Y. Recovery of gold in iodine-iodide system - a review. Sep. Sci. Technol. 2019, 54, 1055-66.
8. Billy, E.; Dourdain, S.; Legeai, S.; et al. Process for the selective recovery of gold by means of green chemistry from an element containing gold and a platinoid. 2023. https://patents.google.com/patent/FR3126892A1/en?oq=FR3126892. (accessed 2025-04-02)
9. Jalbout, A. F.; Elentriecy, H. Recovery of gold and silver from precious metals-containing solids. 2017. https://patents.google.com/patent/US20190368060A1/en. (accessed 2025-04-02).
10. Goguet, A.; Hardacre, C.; Harvey, I.; Narasimharao, K.; Saih, Y.; Sa, J. Increased dispersion of supported gold during methanol carbonylation conditions. J. Am. Chem. Soc. 2009, 131, 6973-5.
11. Cao, Y.; Yang, J. G.; Deng, Y.; et al. Amine-responsive disassembly of AuI-CuI double salts for oxidative carbonylation. Angew. Chem. Int. Ed. Engl. 2020, 59, 2080-4.
12. Cao, Y.; Huang, Y.; He, L. Sustainable route toward N-Boc amines: AuCl3/CuI-catalyzed N-tert-butyloxycarbonylation of amines at room temperature. ChemSusChem 2022, 15, e202102400.
13. Cao, Y.; Peng, Y.; Cheng, D.; et al. Room-temperature CO oxidative coupling for oxamide production over interfacial Au/ZnO catalysts. ACS. Catal. 2023, 13, 735-43.
14. Mancuso, R.; Della Ca’, N.; Veltri, L.; Ziccarelli, I.; Gabriele, B. PdI2-based catalysis for carbonylation reactions: a personal account. Catalysts 2019, 9, 610.
15. Gabriele, B.; Salerno, G.; Mancuso, R.; Costa, M. Efficient synthesis of ureas by direct palladium-catalyzed oxidative carbonylation of amines. J. Org. Chem. 2004, 69, 4741-50.
16. Wu, X. F.; Neumann, H.; Beller, M. Palladium-catalyzed oxidative carbonylation reactions. ChemSusChem 2013, 6, 229-41.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Special Issue
Copyright
Data & Comments
Data


















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].