Recent advances in non-precious metal-based carbon materials for enhanced oxygen reduction and evolution reactions in rechargeable zinc-air batteries
Abstract
The quest for energy storage systems that are both sustainable and efficient has generated growing attention toward rechargeable zinc-air batteries (ZABs), known for their elevated theoretical specific energy, affordability, and eco-friendliness. Nevertheless, the effective application of ZABs faces challenges due to the slow kinetics associated with the oxygen reduction reaction and the oxygen evolution reaction. Traditionally, the preferred catalysts for these reactions have been platinum-group metals because of their remarkable catalytic activity and stability, but their prohibitive cost and scarcity have driven the search for cost-effective, non-precious metal (NPM)-based alternatives. NPM-based carbon materials, including metal-organic framework derivatives, metal-doped carbons, carbon nitrides, and heteroatom-doped carbons, have emerged as promising candidates for replacing platinum-group metals in ZABs. These materials offer high specific surface areas, tunable morphologies, and the ability to incorporate multiple active sites through doping with elements such as nitrogen (N), sulfur (S), phosphorus (P), and boron. The enhanced transfer of electrons and mass transport is facilitated by these attributes, resulting in better catalytic performance for both the oxygen reduction reaction and oxygen evolution reaction. This review highlights recent advancements in the design and synthesis of NPM-based carbon catalysts, detailing strategies to enhance their performance and providing examples of high-performance catalysts. These catalysts, especially when applied in solid-state ZABs, offer significant improvements in terms of efficiency and stability, making them promising candidates for next-generation energy storage systems. The future outlook includes the optimization of synthesis parameters and exploration of wider applications for these advanced electrocatalysts.
Keywords
INTRODUCTION
The increasing fascination with rechargeable zinc-air batteries (ZABs) has been driven by the demand for eco-friendly and efficient energy storage options, recognized for their impressive theoretical specific energy, affordability, and minimal environmental impact. However, the real-world use of ZABs is limited by the sluggish kinetics of both the oxygen reduction reaction (ORR) and oxygen evolution reaction (OER), which play crucial roles in the battery’s performance and overall efficiency[1-7]. Traditionally, platinum-group metals (PGMs) have been the preferred catalysts for these reactions because of their outstanding catalytic activity and stability. However, the prohibitive cost and scarcity of PGMs have spurred the search for cost-effective, non-precious metal (NPM)-based alternatives[8-10].
NPM-based carbon materials have risen as viable alternatives to PGMs in ZABs[7,10-12]. These materials, including metal-organic framework (MOF) derivatives[13-16], metal-doped carbons[4,5,17-19], carbon nitrides[20-23], heteroatom-doped carbons[24-27], and MXenes[23], offer a plethora of advantages. They boast high specific surface areas, tunable morphologies, and the ability to incorporate multiple active sites through doping with elements such as nitrogen (N), sulfur (S), phosphorus (P), and boron[28,29]. These characteristics facilitate more efficient electron transfer and improved mass transport, leading to enhanced catalytic efficiency for both the ORR and OER.
Moreover, the design of NPM-based carbon materials has been enriched by advancements in synthetic methodologies[9,16,22,25,30-32]. Techniques such as hydrothermal synthesis, pyrolysis, and electrospinning have enabled the creation of hierarchical porous structures, which are integral to achieving excellent catalytic activity and stability. The incorporation of metal nanoparticles, such as those of iron, cobalt, nickel, and copper, within these carbon matrices has further augmented the catalytic efficiency, particularly when these metals are dispersed as single atoms or clusters[1-3,11,19,21,26,33,34].
Research into NPM-based carbon materials has focused not only on enhancing catalytic activity but also on addressing issues such as methanol crossover and durability. The development of quasi-solid-state and all-solid-state ZABs, utilizing organohydrogel electrolytes and solid electrolytes, respectively, has opened new avenues for improving the safety and operational stability of these batteries[32]. Furthermore, the integration of these advanced materials into flexible and wearable electronic devices has been a significant breakthrough, expanding the potential applications of ZABs.
Despite the progress, challenges remain in terms of achieving optimal activity, stability, and cost-effectiveness in NPM-based catalysts. The precise control of active site density, the understanding of catalytic mechanisms, and the scalability of synthesis methods are areas that require further investigation. This review aims to consolidate the latest developments in NPM-based carbon materials for ZABs, highlighting key advances, ongoing challenges, and future directions for the field. By doing so, we aspire to contribute to the acceleration of ZABs toward commercial viability and widespread adoption as a cornerstone technology in the realm of sustainable energy storage.
MECHANISM AND COMPOSITION OF ZABS
Mechanism of ZABs
ZABs operate on the principle of the redox reactions between zinc metal and atmospheric oxygen, making them a promising energy storage system because of the high theoretical energy density, affordability, and environmental sustainability[24]. The electrochemical processes in ZABs mainly revolve around the ORR during discharge and the OER during charge, both taking place at the air electrode. Understanding the mechanisms of the ORR and OER is fundamental to the development of efficient electrocatalysts for rechargeable metal-air batteries, particularly for ZABs.
Electrode composition of ZABs
Discharge process (ORR): ORR is a complex multi-electron process that involves the reduction of oxygen molecules to water or hydroxide ions in alkaline media. During the discharge phase of a ZAB, the following reactions take place[30]:
1. Zinc anode oxidation: Metallic zinc at the anode is oxidized to zinc ions (Zn2+), releasing electrons in the process.
Zn → Zn2+ + 2e-
2. Oxygen reduction at the air cathode: Oxygen from the air is reduced at the air cathode in the presence of a catalyst, typically absorbing water from the electrolyte to form hydroxide ions (OH-).
O2 + 2H2O + 4e- → 4OH-
3. Formation of zinc hydroxide complex: The zinc ions generated at the anode react with the hydroxide ions produced at the cathode to form a zinc hydroxide complex [Zn(OH)42-], which eventually precipitates out as zinc oxide (ZnO) upon saturation in the electrolyte.
Zn2+ + 4OH- → Zn(OH)42-
Zn(OH)42- → ZnO + 2H2O + 2OH-
The overall reaction during the discharge phase is:
2Zn + O2 → 2ZnO
with a theoretical open circuit voltage of approximately 1.65 V.
However, the reaction can proceed via several pathways, including a two-electron and a four-electron process. The two-electron pathway results in the formation of hydrogen peroxide (H2O2) as an intermediate, which is undesirable due to its instability and propensity to decompose into water and oxygen, leading to efficiency losses. The four-electron pathway, on the other hand, directly reduces oxygen to water or hydroxide ions, which is more efficient and preferred for battery applications.
The four-electron pathway can be broken down into individual steps, where the oxygen molecule is initially adsorbed onto the active site of the catalyst (M):
(1). Adsorption of O2: O2 + M → O2*
(2). First protonation and electron transfer: O2* + H2O + 2e- → OOH* + OH-
(3). Second protonation and electron transfer: OOH* + H2O + 2e- → 2OH-
Charge process (OER): OER is a multistep reaction that proceeds slowly and requires a high overpotential to occur. During the charging phase, the reactions proceed in reverse:
1. Decomposition of zinc oxide: The zinc oxide formed during the discharge phase is decomposed back into zinc and hydroxide ions.
ZnO + 2H2O + 2e- → Zn2+ + 4OH-
2. Oxygen evolution at the air cathode: Hydroxide ions are oxidized at the air cathode, releasing oxygen back into the air.
4OH- → O2 + 2H2O + 4e-
3. Deposition of zinc at the anode: The zinc ions produced during the decomposition of zinc oxide migrate to the anode and deposit as metallic zinc.
Zn2+ + 2e- → Zn
The overall reaction during the charging phase is the reverse of the discharge phase, effectively restoring the original state of the battery components.
The mechanism of OER is not yet fully understood, but it is generally accepted that it involves the following steps:
(1). Dehydration of OH-: OH- + M → OH*
(2). First electron transfer and dehydration: OH* + OH- → O* + H2O + e-
(3). Second electron transfer and dehydration: O* + OH- → OOH* + e-
(4). Final electron transfer and oxygen evolution: OOH* + OH- → O2 + H2O + 2e-
LIMITING FACTORS FOR THE APPLICATION OF ZABS
Both ORR and OER involve the adsorption and desorption of oxygen species, along with the transfer of protons and electrons. The adsorption energy of oxygen intermediates (*OOH, *O, *OH) is critical in determining the catalytic activity and selectivity of the electrocatalyst. A volcano-type relationship is often observed between the activity of an electrocatalyst and the binding energy of the oxygen intermediates, indicating that optimal adsorption energy is necessary for achieving high catalytic activity.
In ZABs, the efficiency of electrode reactions is directly affected by the stability of the catalysts. During the reaction, catalysts may experience degradation or deactivation, which can lead to diminished discharge and charge performance. For instance, structural changes in the catalyst can reduce the number of reaction sites, thereby decreasing the reaction rate. Stable catalysts maintain the number and distribution of active sites, ensuring efficient reactions. Conversely, unstable catalysts may lose active sites over repeated cycles, reducing the reaction rate and affecting the battery’s power output. Meanwhile, the cycle life of ZABs is closely tied to the stability of the catalysts. After multiple charge-discharge cycles, unstable catalysts may undergo morphological and compositional changes, affecting catalytic activity and potentially leading to a decline in overall battery performance. Stable catalysts, however, can retain their performance over extended periods, extending the battery’s lifespan.
Selecting stable NPM catalysts helps reduce the production costs and environmental impact of ZABs. Due to the low cost and good stability of NPM materials, they become more economically viable for large-scale applications. Stable catalysts can minimize material consumption and replacement frequency, thereby lowering maintenance costs. Transition metals and their oxides/hydroxides, carbon-based materials, and MOFs have been actively explored for this purpose[35-43]. The design and optimization of these catalysts often involve strategies such as surface engineering, defect engineering, heteroatom doping, and the creation of active sites with selective adsorption properties to improve the catalytic activity and stability, thereby enhancing the overall efficiency and longevity of ZABs.
TYPES, SYNTHESIS METHODS AND ELECTROCATALYTIC PERFORMANCES OF DOPED CARBON MATERIALS
NPM-based carbon materials refer to carbon-based materials that are treated by physical or chemical methods to incorporate NPM elements. These materials encompass a wide range including graphdiyne[27,34], carbon nanotubes (CNTs)[44-55], graphene[56-60], carbon nanofibers (CNFs)[17,45,61-66], porous carbons[67-76], carbon aerogels[77-80], mesoporous carbons[37,81-83], and carbon materials derived from MOFs[18]. Through doping with elements such as N[31,84-88], S[89-91], and P[92], they form specific active sites.
N doping
N doping enhances the reactivity of ZABs significantly by altering the electronic properties and structural characteristics of the catalyst. Specifically, N atoms introduce additional electron density, thereby improving the conductivity and oxygen adsorption capacity of the catalyst. These attributes are closely related to the electrochemical activity of the catalyst.
Research indicates that the degree of N doping significantly affects the electrochemical performance of the catalyst[61]. An appropriate level of N doping can optimize the catalyst’s activity, whereas excessive doping may lead to a decline in performance. The incorporation of N into a carbon framework, exemplified by the CNT-graphene-like architecture found in NiFeCo-NC materials, can enhance both the electronic characteristics and catalytic efficacy of the material. By doping with N, defects can be introduced into the carbon matrix, which are advantageous for increasing the number of active sites involved in ORR and OER. Furthermore, N serves as an electron donor, capable of modifying the electronic structure of nearby atoms, thus improving the material’s capacity to promote these reactions. This is supported by the reduced potential gap observed between the half-wave potential of ORR and the oxidation potential at a current density of
Figure 1. (A) Graphical abstract of FeACs-VNNCs (1:1)/NFC; (B) Calculated d orbital centers of FeNCs/NFC, VNNCs/NFC and FeACs-VNNCs (1:1)/NFC[68]; (C) XPS survey spectrum of Zn 2p, N 1s of ZnCo-Te@NC, and Co 2p XPS spectrum of ZnCo-Te@NC and ZnCo@NC[69]; (D) Performance of zinc-air batteries assembled by FeCo@CNTs-60; (E) Stabilized adsorption configurations of ORR/OER intermediates[46]; (F) TEM and HRTEM (inset) images of CoN/UNG[57]; (G) HRTEM images of CoFe-NCNFs[81]; (H) Illustration of Fe3C@NPW[94]. NFC: N-doped carbon framework; FeACs: iron-based activated carbons; VNNCs: vanadium-based nanostructured catalysts; XPS: X-ray photoelectron spectroscopy; CNTs: carbon nanotubes; ORR: oxygen reduction reaction; OER: oxygen evolution reaction; TEM: transmission electron microscopy; HRTEM: high-resolution transmission electron microscopy; NCNFs: N-doped carbon nanoflowers.
By incorporating N into carbon-based materials, researchers have successfully tailored the electronic properties of these materials to improve their catalytic performance for ORR and OER in ZABs. The strategic use of N doping offers a promising avenue for enhancing the efficiency and durability of electrocatalysts, which is critical for the advancement of renewable energy technologies.
S doping
S-doped carbon materials, such as S-doped CNFs, through the introduction of S, improve the electron affinity of the catalyst and enhance the activation and desorption of oxygen intermediates, thus boosting catalytic performance[28,95]. Researchers developed ternary sulfides (MInS/NH2-CNT, M = Co, Fe, or Mn) based on super-tetrahedral metal sulfide clusters (T4 clusters) as efficient bifunctional electrocatalysts for OER and ORR[28]. Prepared through a mild self-assembly method using electrostatic interactions, CoInS/NH2-CNT showed notable electrocatalytic performance. This strategy extends to other cluster-based sulfide materials, offering NPM alternatives for energy applications. The synthesis involved mixing T4 crystals with NH2-CNT at room temperature, facilitated by ultrasonication and magnetic stirring. CoInS/NH2-CNT demonstrated excellent OER and ORR activity, highlighting the synergy between T4 clusters and NH2-CNT. This study provides an effective approach for constructing advanced sulfide materials for various applications. Transition metal sulfides, such as Co9S8 and FeNi sulfide nanoalloys, have proven to be efficient bifunctional electrocatalysts due to their unique electronic structures and high conductivity[96]. Encapsulated in S, N co-doped carbon, Co9S8 not only boosts the catalytic performance of ORR and OER but also exhibits good cycling stability and resistance to methanol poisoning. FeNi sulfide nanoalloys, through S modulation, achieve ORR performance comparable to Pt/C while maintaining excellent OER activity, making them ideal electrode materials for ZABs. As shown in Figure 2A, Zhang et al. highlights a cobalt sulfide/multi-heteroatom doped porous carbon composite, created through a single-step sulfidation method using ZIFs, which acts as a highly effective trifunctional electrocatalyst for ORR, OER, and HER[89]. Its three-dimensional (3D) architecture enhances mass transport and exposes abundant active sites. Performance-wise, it exhibits superior ORR and OER activities, with low overpotentials and high stability, rivaling commercial Pt/C and outperforming other samples. The catalyst’s stability is highlighted by negligible performance decay after thousands of cycles. Applied in ZABs, it delivers high power density and energy efficiency, exceeding commercial Pt/C-IrO2-based batteries. The achievement is ascribed to the distinct architecture that facilitates effective transport of electrons and ions, the presence of several active sites, and the cooperative influence of heteroatoms along with cobalt sulfide.
Figure 2. (A) Illustration of Co9S8/CoNSC[89]; (B) Illustration of Cu3P/MoP@C; (C) Structural characterization of Cu3P/MoP@C,
P doping
P-doped carbon materials, including P-doped CNFs, alter the electronic structure of carbon materials by incorporating P, further optimizing the kinetics of ORR[97]. For instance, as shown in Figure 2B, a nanostructured composite made of copper phosphide and molybdenum phosphide, doped with P (Cu3P/MoP), has been developed to serve as an efficient electrocatalyst for the ORR in ZABs[97]. Fabricated through an impregnation and high-temperature phosphidation process, Cu3P and MoP are co-anchored onto hollow porous carbon spheres, yielding the Cu3P/MoP@C catalyst. This distinctive architecture not only furnishes abundant active sites but also facilitates electronic and mass transport through its porosity. The Cu3P/MoP@C exhibits a half-wave potential of 0.90 V, surpassing the 0.84 V of commercial Pt/C. X-ray photoelectron spectroscopy (XPS) demonstrates that the significant electronic interactions occurring between Cu3P and MoP species are crucial for its excellent ORR performance. Moreover, the large specific surface area and mesoporous structure of Cu3P/MoP@C allow for enhanced contact with the electrolyte, accelerating ORR kinetics [Figure 2C]. This triumph is attributed to the synergistic effect between Cu3P and MoP, coupled with the porous structure that facilitates more efficient electron transfer, thus enhancing overall ZAB performance.
Multi-doping
Multi-doped carbon materials, such as N, S co-doped carbon and N, P co-doped carbon[67,77,98-105], through synergistic effects, not only increase catalytic activity but also enhance the stability of the catalyst. S, N
As shown in Figure 3A, an enhanced nanoemulsion method synthesized Co2P nanoparticles in N- and P-doped carbon nanospheres (Co2P/NP-C) for ZABs[99]. Co2P/NP-C-800 exhibited impressive ORR performance, demonstrated by a half-wave potential of 0.81 V and a limiting current density of
Figure 3. (A) Illustration of Co2P/NP-C-800; (B) ZABs performance based on Co2P/NP-C-800 and Pt/C: Galvanostatic discharging specific capacity at 10 mA·cm-2. Galvanostatic discharging curves at different current densities. Lighted LED powered by two Co2P/NP-C-800-based ZABs in series[99]; (C) Illustration and (D) LSV curve of CoFe/Se@CN in the entire OER/ORR region[67]; (E) Illustration and (F) ZABs performance of Co9S8/NSC-1: galvanostatic discharge and charge cycling curves for ZAB@Co and ZAB@Pt + Ru[106]. ZABs: Zinc-air batteries; LED: light emitting diode; LSV: linear sweep voltammetry; ORR: oxygen reduction reaction; OER: oxygen evolution reaction.
Figure 4A illustrates the development of the trifunctional electrocatalyst Co9S8 encapsulated in S and N co-doped carbon (Co9S8@SNC), which shows impressive catalytic performance for ORR, HER and OER[102]. A strong coordination interaction at the molecular level between Co2+ and C@S was utilized to synthesize the catalyst, which was then subjected to a calcination process. This approach guarantees a consistent distribution and close interaction between the active Co9S8 nanoparticles and the carbon matrix, thereby improving electron transfer. The Co9S8@SNC catalyst demonstrates a half-wave potential of 0.846 V for ORR, along with overpotentials for OER at 320 mV when measured at 10 mA·cm-2. The carbon shell co-doped with S and N enhances the material’s conductivity, whereas the Co9S8 core offers sites that are active in catalysis. The catalyst shows excellent durability, maintaining its activity and structural integrity after thousands of cycles in ORR tests. The improved catalytic performance can be attributed to the collaborative interaction between the Co9S8 core and the carbon shell that is co-doped with S and N. As shown in Figure 4B, the co-doping of S and N alters the electronic properties of the carbon matrix, enhancing its wettability and facilitating the transport of reactants.
Figure 4. (A) Graphical abstract and (B) XPS spectrum of Co9S8@SNC[102]; (C) Illustration of CoNi@GO; (D) Specific capacities plots of CoNi@GO and Pd/C + RuO2. Long-term galvanostatic discharge charge cycling curves of rechargeable CoNi@GO and Pt/C + RuO2-based ZABs[56]; (E) Illustration and (F) Galvanostatic discharge plots, galvanostatic discharge-charge cycling curves of ZABs with CoFe-N-CNTs/CNFs-900 or IrO2/C + Pt/C as catalyst[107]; (G) Synthesis of Co/BN-CNT/VN-800 derived from Co6V12B18-POM[47]. XPS: X-ray photoelectron spectroscopy; GO: graphene oxide; ZABs: zinc-air batteries; CNTs: carbon nanotubes; CNFs: carbon nanofibers; OER: oxygen evolution reaction; HER: hydrogen evolution reaction; ORR: oxygen reduction reaction.
Preparation methods and structural tuning
The preparation methods for NPM-based carbon materials are diverse, each with its own unique advantages and applicability. For example, researchers created a 3D cyano-bridged CoNi complex-derived CoNi@graphene oxide (GO) bifunctional catalyst for oxygen redox reactions in ZABs via pyrolysis
Structural tuning is pivotal to enhancing catalytic performance. Careful manipulation of porosity, specific surface area, surface functional groups, and the arrangement of heteroatoms enhances the catalyst’s electronic structure, improving the adsorption and activation of oxygen molecules, which in turn boosts the catalytic activity for ORR/OER. Adjusting calcination temperature and duration regulates thedegree of graphitization and heteroatom content; employing metal salts and N-containing compounds as precursors introduces metals and N into carbon materials, forming highly efficient active sites.
The superior performance of multi-doped materials in ZABs can be attributed to several key factors: (1) Optimization of electronic structure: The introduction of elements such as S and N modifies the electronic configuration of the materials, enhancing the electron density at active sites and promoting the electron transfer process during the ORR/OER; (2) Enhanced conductivity: The introduction of sulfides and phosphides significantly increases the conductivity of the materials, reducing the internal ohmic resistance of the battery and contributing to improved overall efficiency; (3) Stability and durability: Rational material design leads to good chemical and mechanical stability, ensuring consistent battery performance over extended operational periods. Multi-doped materials, especially composites containing S, N, and transition metal elements, offer efficient, stable, and cost-effective electrocatalyst solutions for ZABs.
While NPM-based carbon materials show tremendous potential in ZABs, they still face the following challenges: (1) Ways to enhance both the activity and long-term stability of catalysts, particularly when subjected to high current densities; (2) The cost and environmental impact of large-scale production must be considered; (3) Further optimization is needed for the overall performance of ZABs, which encompasses energy density, cycle life, and cost-effectiveness.
Utilizing NPM-derived carbon materials in ZABs offers innovative approaches to tackle the kinetic challenges associated with ORR and OER. Through material design, structural tuning, and performance optimization, these materials demonstrate excellent catalytic activity and stability, driving ZABs toward higher energy density, longer cycle life, and lower production costs. Future research directions may include developing novel preparation methods to reduce production costs, exploring more efficient NPM catalyst systems, optimizing the compatibility of catalysts with electrolytes, and refining battery design to enhance overall performance.
TYPES, SYNTHESIS METHODS AND ELECTROCATALYTIC PERFORMANCES OF MOFS AND DERIVATIVES
MOFs and their derivatives are gaining recognition as effective materials for enhancing the performance and durability of ZABs, owing to their elevated specific surface area, adjustable porosity, multifunctional properties, and stability[35,110-115]. This part is mainly about exploring the latest developments in MOF-derived materials, categorizing them according to their primary composition and structure, and examining their electrochemical performance.
Preparation methods and structural tuning of MOF-derived carbon materials
CNTs
Figure 5A illustrates the development of a new technique for producing Co/VN nanoparticles that are encapsulated within a carbon matrix through the carbonization of CoV-MOF-dicyandiamide composites[116]. The Co/VN nanoparticles (NPs)@C, measuring between 4 and 8 nm, demonstrate an extensive surface area and a conductive structure that enhances electrochemical performance. Its in-situ formed Co and VN nanoparticles in synergy with fast electron transfer through the carbon layer deliver exceptional tri-functional catalytic performance. In rechargeable ZABs, Co/VN NPs@C serves as an electrode material, which demonstrates impressive efficiency, achieving a peak power of 130 mW·cm-2, a capacity of
Figure 5. (A) Illustration and (B) ZABs performance of Co/VN NPs@C[116]; (C) Illustration and (D) Graphical abstract of FeNP@Fe-N-C[117]; (E) Illustration; (F) morphology and structure characterization of Mo2C/MoC/Co@CNTs[118]. ZABs: Zinc-air batteries; NPs: nanoparticles; CNTs: carbon nanotubes; ORR: oxygen reduction reaction; OER: oxygen evolution reaction.
Graphene
A novel bifunctional oxygen electrocatalyst (Fe/Ni-NC||FeNi@G), which combines single Fe/Ni atoms and FeNi alloy nanoparticles on a graphene support, has been created for rechargeable ZABs[119]. This catalyst was synthesized through a ZIF-derived carbon anchoring process, followed by high-temperature pyrolysis in the presence of GO. The synergy between Fe single atoms for ORR and FeNi nanoparticles for OER contributes to its outstanding bifunctionality. The structure of the catalyst, which includes a synergistic blend of single atoms of Fe/Ni, FeNi nanoparticles, and a graphene support, offers a high concentration of active sites along with improved conductivity. Graphene’s presence guarantees strong electron transfer and structural integrity, whereas the FeNi nanoparticles provide significant catalytic effectiveness for OER. The superior performance of Fe/Ni-NC||FeNi@G in ZABs is attributed to its ability to facilitate the efficient conversion of oxygen, leading to high specific capacity, power density, and stability.
Carbon foams
An innovative Co-CoO heterostructure, obtained from nanoscale ZIF-67, is extensively exposed on N-doped porous carbon foam (NPCF) and has been created as a highly effective electrocatalyst for ZABs[120]. As shown in Figure 6A, the catalyst was created using a self-sacrificial pyrolysis method, resulting in Co-CoO active sites that are both highly dispersed and well-anchored. The distinct architecture of the Co-CoO/NPCF electrocatalyst enhanced mass and electron transfer capabilities. When integrated into rechargeable ZABs, the Co-CoO/NPCF air-cathode displayed significant power densities of 214.1 mW·cm-2 for liquid-state ZABs and 93.1 mW·cm-2 for flexible all-solid-state variants, with remarkable cycle stabilities of 600 and 140 cycles, respectively. As demonstrated in Figure 6B, calculations using DFT indicated that a robust electronic interaction exists between Co-CoO and NPCF, enhanced by the presence of numerous C-Nx sites. This interaction altered the electronic structure, playing a significant role in the exceptional electrocatalytic performance.
Figure 6. (A) Illustration and (B) chemical structure of Co-CoO/NPCF[120]; (C) Graphical abstract of h-Ti3C2Tx@Co-NCNT[121]; (D) Illustration and (E) ZABs performance of Co/CoO@HNC[122]; (F) Schematic illustrating the preparation of the P-CoSe2/C@CC catalyst; (G) SEM images of Co-MOF@CC, Co/C@CC, CoSe2/C@CC, and P-CoSe2/C@CC[124]. NPCF: N-doped porous carbon foam; ZABs: zinc-air batteries; ORR: oxygen reduction reaction; OER: oxygen evolution reaction.
Mxenes
Researchers have engineered a novel electrocatalyst for ORR and OER by synthesizing hollow Ti3C2Tx MXene spheres decorated with cobalt-tipped CNTs (Co-NCNTs), derived from ZIF-67[121]. This unique structure, termed h-Ti3C2Tx@Co-NCNT, was crafted using a self-sacrificial template strategy, where Ti3C2Tx nanosheets served as conductive scaffolds for the growth of Co-NCNTs. The resulting composite displays a hierarchical porous structure that offers a substantial active surface area and reduced distances for mass transfer. The combined effect of Ti3C2Tx and components derived from MOFs significantly improves the catalyst’s conductivity and stability. The Co-NCNTs, firmly immobilized on the MXene spheres, facilitate gas diffusion and expose active sites, while the N-doped carbon shell prevents direct oxidation of the
Preparation methods and structural tuning of MOF-derived metal oxides/nitrides
As illustrated in Figure 6D, a new engineering method has been devised to construct Co/CoO heterojunctions integrated within mulberry-like open-carbon nanocages for ZABs[122]. Employing a MOF as a sacrificial template in-situ, this approach generates a distinctive structure comprising numerous N-doped carbon nanosphere subunits and accessible mass transfer channels. The resulting Co/CoO@HNC catalyst demonstrates improved oxygen electrocatalytic performance, attaining a DE of 0.83 V, which notably surpasses that of noble-metal-based Pt/C. The hollow architecture resembling mulberries in the catalyst provides a greater exposed internal volume and more available active sites, thereby promoting effective mass transport and electron transfer. The heterojunction and N-doping further contribute to the electronic properties, improving its catalytic performance. Performance metrics indicate that the Co/CoO@HNC catalyst exhibits both high power density and stability when used in ZABs, highlighting its potential for real-world applications [Figure 6E]. The combined influence of the open architecture, heterojunction formation, and N doping allows the catalyst to sustain significant activity and longevity over prolonged durations, positioning it as a viable option for advanced energy storage systems.
A new electrocatalyst, CoMn2O4/C-NH2-300, was developed through the in-situ formation of cobalt manganate spinel nanodots on carbon black that has been functionalized with amine, using
Preparation methods and structural tuning of MOF-derived P-doped materials
Figure 6F illustrates that a cobalt selenide (CoSe2) electrocatalyst, which is doped with P, has been created on carbon cloth by employing a solvothermal method, succeeded by processes of selenization and phosphatization[124]. This cuboid-like formation provides a significant surface area that promotes optimal catalytic activity and stability. The catalyst known as P-CoSe2/C@CC exhibits exceptional performance in OER, featuring an overpotential of 303.1 mV @ 10 mA·cm-2, and it shows a distinct reduction peak during ORR evaluations. When incorporated into a ZAB, P-CoSe2/C@CC displays a peak power density of
Preparation methods and structural tuning of MOF-derived multifunctional composites
In the field of energy conversion and storage, a novel trilayer MOF comprising iron (Fe), cobalt (Co), and nickel (Ni) has been created to serve as a multifunctional electrocatalyst[125]. The synthesis of the trilayer MOF utilized a reductive electrosynthesis methodology, which facilitated the layer-by-layer construction of metal cations associated with 2-amino-1, 4-benzenedicarboxylic acid linkers [Figure 7A]. This process led to the creation of a highly porous material displaying exceptional trifunctional electrocatalytic properties for OER and ORR, which is primarily ascribed to the synergistic interaction among the constituent elements and the porous architecture that supports the effective use of active sites and efficient bubble formation. When applied as an air cathode in a ZAB, as illustrated in Figure 7B, the MOF yielded a power density of
Figure 7. (A) Nanoarchitectonics for preparing multifunctional MOFs; (B) ZABs performance and (C) DFT calculations of Fe−Co−Ni MOF structures[125]; (D) Graphical abstract of FeCo@1D-CNTs/2D-NC; (E) SEM images of Zn-ZIF, ZnFeCo-ZIF and FeCo@1D-CNTs/2D-NC, TEM and HRTEM image of FeCo@1D-CNTs/2D-NC[111]. ZABs: Zinc-air batteries; DFT: density functional theory; CNTs: carbon nanotubes; SEM: scanning electron microscopy; TEM: transmission electron microscopy; OER: oxygen evolution reaction; HER: hydrogen evolution reaction; ORR: oxygen reduction reaction.
A particular 3D carbon nanomaterial with a hierarchical porous structure was developed through a process derived from MOFs, specifically involving the carbonization of ZIF-8 via a silica-template technique along with the growth of CNTs[126]. This material, referred to as mFeNC-CNT, contained Fe-based nanoparticles and Fe-Nx sites dispersed at the atomic level within its N-doped graphitic carbon matrix. The 3D porous configuration of mFeNC-CNT greatly diminished charge transport resistance, while the intertwined CNTs further facilitated ion and electron diffusion pathways, thereby improving electrochemical performance. This material exhibited remarkable catalytic activity for ORR, similar to that of commercial Pt/C, with a half-wave potential of 0.908 V. When utilized in ZABs, mFeNC-CNT produced a substantial open-circuit voltage of 1.556 V, demonstrating its high efficiency. The superior ORR activity of mFeNC-CNT can be attributed to its optimized structure, which facilitates faster reaction kinetics.
Porous N-doped carbon composites derived from zinc MOFs have shown outstanding performance in ORR and OER, with enhanced power density and open circuit voltage in assembled ZABs[127,128]. A novel NPM catalyst, Co NCs/HPNC, was developed for ZAB cathodes via a carboxylate-assisted strategy[110]. Prepared by pyrolyzing MOFs under inert gas, the catalyst features high surface area and a mesoporous structure, enhancing mass transport and active site exposure. In alkaline conditions, Co NCs/HPNC demonstrated enhanced ORR activity electrochemically, exhibiting a half-wave potential of 0.88 V, which is attributed to cobalt’s nanoscale size, hierarchical porosity, and large surface area, facilitating electrolyte contact and active center utilization. As illustrated in Figure 7D, a new catalyst formed from FeCo alloy nanoparticles is encapsulated within a carbon nanostructure that combines one-dimensional (1D) and two-dimensional (2D) features. This catalyst was synthesized through high-temperature annealing of ZnFeCo-ZIF precursors[111]. Consequently, this process yields a composite consisting of N-doped CNTs along with a porous carbon matrix [Figure 7E]. The structure of the catalyst offers an extensive surface area, numerous active sites, and effective electron transfer, resulting in elevated catalytic efficiency and robustness. When integrated into batteries, it attains a significant open-circuit voltage, specific capacity, and power density, highlighting its promise for sophisticated energy storage solutions.
As shown in Figure 8A, a high-performance bifunctional catalyst for rechargeable ZABs was developed, engineered through oxygen plasma treatment of MOF-derived Co/FeCo@Fe(Co)3O4 heterojunctions[112]. This treatment optimizes the surface structure, creating oxygen vacancies and additional active sites, thereby boosting both ORR and OER electrocatalytic activity. The optimized catalyst, P-Co3Fe1/NC-700-10, exhibits superior ORR and OER performance with reduced Tafel slopes and minimal charge transfer resistance, attributed to enhanced surface defect density facilitating peroxide adsorption. The plasma treatment’s role in surface modification and defect creation underpins the catalyst’s enhanced electrochemical performance [Figure 8B]. Figure 8C demonstrates the development of a high-performance electrocatalyst for ORR by synthesizing a porous N-doped carbon composite derived from a manganese-doped zinc MOF (30-ZnMn-NC)[129]. As shown in Figure 8D, the composite was synthesized through the pyrolysis of MOF precursors, leading to a material characterized by a distinctive porous architecture and bimetallic active sites of
Figure 8. (A) Illustration of P-Co3Fe1/NC-700; (B) ORR/OER catalytic reaction process and optimized adsorption geometry of O2, *OOH, *O, and *OH on Co3Fe1, Co3Fe1/700-5, Co3Fe1/700-10, and Co3Fe1/700-20[112]; (C) Illustration of ZnMn-NC[129]; (D) The preparation process of Co9S8/NSCP through polymer hierarchical self-assembly, metal-organic coordination and subsequent space-confined pyrolysis; (E) FESEM, TEM and HRTEM image of Co9S8/NSCP[130]. OER: Oxygen evolution reaction; ORR: oxygen reduction reaction; TEM: transmission electron microscopy; HRTEM: high-resolution transmission electron microscopy; FESEM: field emission scanning electron microscopy.
An innovative bifunctional electrocatalyst for oxygen, Co9S8/NSCP, was developed using a controlled MOF-driven technique that incorporates polymer self-assembly, metal-organic coordination, and pyrolysis within a confined space[130]. As illustrated in Figure 8E, the catalyst comprises ultrafine nanocrystals of Co9S8, approximately 6 nm in size, which are embedded in carbon nanoplates that have been multilayer-assembled and co-doped with N and S. The Co9S8/NSCP displays remarkable catalytic performance for oxygen electrode reaction, which can be attributed to the synergistic interaction between the highly exposed Co9S8 nanocrystals and the 3D carbon superstructure enriched with heteroatoms. Moreover, the NSCP matrix offers a large surface area, protective graphitic layers, and a wealth of active sites, all of which contribute to effective electron transfer and mass transport, enhancing both catalytic activity and stability.
MOFs as electrocatalysts in ZABs have attracted considerable attention due to their unique properties, including high porosity and tunable structures, which can enhance both catalytic activity and stability. However, the application of MOFs in ZAB systems presents several challenges. (1) Stability: MOFs often exhibit poor chemical and mechanical stability under the harsh operating conditions of ZABs, leading to degradation over time; (2) Conductivity: The intrinsically low electrical conductivity of MOFs can impede electron transfer, thereby reducing overall battery performance; (3) Catalytic efficiency: Although promising, MOFs must achieve higher efficiencies for the ORR and OER, which are critical processes in ZABs. The advancement of MOFs in ZABs is marked by trends toward precise structural control and functionalization, aiming to optimize catalytic activity and selectivity through customized pore sizes, shapes, and functional groups. Efforts are also directed toward enhancing the stability and durability of MOFs, with a focus on hydrolytic stability, corrosion resistance, and improved mechanical strength. (1) Structural engineering: Modifying MOF structures through the use of robust linkers or incorporating metal centers that exhibit greater resistance to oxidation can significantly enhance stability; (2) Hybrid materials: The integration of MOFs with conductive materials, such as CNTs or graphene, can improve electrical conductivity while preserving the advantageous properties of MOFs; (3) Surface functionalization: Adjusting the surface chemistry of MOFs can optimize the active sites, thereby enhancing catalytic performance.
The development trend is increasingly oriented toward the design of multifunctional MOFs that can act as both efficient catalysts and stable components within battery systems. This includes: (1) Intelligent design: Employing computational methods to predict optimal MOF designs prior to synthesis; (2) Nanotechnology integration: Merging MOFs with other nanomaterials to develop hybrid systems that exploit synergistic effects; (3) Scalability: Emphasizing scalable synthesis techniques that can produce MOF-based materials in an economical and sustainable manner. These trends collectively position MOFs as key enablers for overcoming the limitations of traditional ZABs, paving the way for more efficient, stable, and economically viable energy storage solutions.
STRUCTURAL DESIGN AND PERFORMANCE OPTIMIZATION OF COVALENT ORGANIC FRAMEWORKS
Covalent organic frameworks (COFs) are a type of newly developed crystalline porous polymers characterized by their well-organized structures, tunable pore dimensions, and robust chemical stability[131,132]. In recent years, they have attracted considerable attention in electrochemical energy storage devices, particularly in ZABs, owing to their unique physicochemical properties. COFs not only provide abundant pathways for ion transport but can also be functionally modified to enhance electrocatalytic activity, making them ideal bifunctional catalysts for the ORR and OER.
The structural design of COFs is pivotal to their electrochemical performance. Precise control over the pore size, shape, and surface functionalization of COFs can significantly improve ion diffusion kinetics and lower the energy barriers for electrochemical reactions. For instance, Figure 9A shows that COFs featuring cobalt (II) pyridine coordination sites in conjunction with CNTs have been developed for bifunctional oxygen electrocatalysis[132]. The unique porous architecture of COFs facilitates rapid ion and electron transport, reducing the overpotentials of ORR and OER, thereby enhancing the power density and energy efficiency of ZABs.
Figure 9. (A) Schematic presentation of different COF-based composites; (B) Free energy diagrams, DOS and d band centers of Co-SA, Co-NP and Co-SA/Co-NP[136]; (C) Illustration and morphology characterization of ZIF-L@PDA-FePhen and Fe-N4@NC-PCSs[144]; (D) Fabrication of Mo SACs with O/N/S co-coordination. Structural characterization of MoS42--doped polydopamine hollow spheres; (E) Favorable reaction pathway of single Mo atom supported on the Mo-O2NS-C structure[145]; (F) Illustration of CB[6]-derived M-N-C SACs; (G) Structural characterization of CB[6]-derived M-N-C catalysts[146]; (H) Morphology characterization, and (I) electrochemical performance of M-N©HCS catalysts[135]. SACs: Single atom catalysts; N: nitrogen; S: sulfur.
The remarkable electrochemical performance of COFs in ZABs can be attributed to several advantages: (1) High porosity: The high porosity of COFs ensures ample active sites, facilitating electrochemical reactions; (2) Directed ion transport: The directed pore structure of COFs promotes swift ion transport, minimizing energy losses during the reaction process; (3) Interface compatibility: Excellent interface compatibility between COFs and electrode materials ensures stable electrical contact and efficient electron transfer; (4) Chemical stability: The chemical stability of COFs maintains structural integrity throughout repeated charge-discharge cycles, extending the battery’s lifespan.
The utilization of COFs in ZABs has shown significant promise in the area of electrochemical energy storage. Ongoing advancements in structural design and optimization of performance establish COFs as an attractive material for the forthcoming generation of high-performance ZABs. Upcoming research endeavors will target the creation of more effective and stable COFs-based catalysts, while also investigating the use of COFs in various battery types, aiding in the achievement of sustainable and environmentally friendly energy solutions.
STRUCTURAL DESIGN AND PERFORMANCE OPTIMIZATION OF SINGLE ATOM CATALYSTS
Single atom catalysts (SACs) have emerged as a revolutionary catalytic system, garnering significant attention in electrochemical catalysis, especially within the context of ZABs[133-142]. SACs, characterized by their unique atomic dispersion and high density of active sites, offer a solution to this bottleneck.
The preparation of SACs typically involves dispersing metal atoms onto high surface area supports such as carbon-based materials, metal oxides, or metal phosphides to form isolated active sites. An instance of this is the Co2P-supported Co SAC (Co2P/Co-NC), which is synthesized through evaporation drying followed by pyrolysis. In this process, Co2P nanoparticles and Co-N4 centers are uniformly distributed across a N-doped carbon matrix, exhibiting remarkable activity for oxygen electrode reaction[143]. A novel dual-functional oxygen electrocatalyst, Co@NCNT/Co-SA@NCMT, was developed for rechargeable ZABs[136]. Prepared with ZIF-67 assistance, it confines Co single atoms and nanoparticles within N-doped carbon nano-/micro-tubes
The high catalytic efficiency of SACs is attributable to several key factors: (1) Single atom sites provide a greater number of active sites, increasing the density of catalytic sites; (2) The electronic structure of SACs can be optimized through manipulation of the support and surrounding environment, altering adsorption energies and reaction pathways; (3) SACs often exhibit superior stability compared to conventional catalysts, maintaining activity over long periods of operation.
Despite notable advances in the application of SACs in ZABs, challenges remain, including technical hurdles in scaling up production, cost issues, and validation of long-term stability. Future research will concentrate on developing more economical and environmentally friendly preparation methods, enhancing the yield and stability of SACs, and exploring novel support materials to further optimize catalytic performance. Additionally, the application of SACs in flexible and solid-state ZABs represents a significant future direction, facilitating the commercialization of ZABs in wearable devices and other portable electronics.
While the prospects for SACs in ZABs are extensive, their large-scale production and long-term stability remain pressing issues. Currently, the preparation of SACs often relies on complex synthesis steps and expensive precursors, hindering commercial application. Furthermore, SACs may aggregate or leach under real operating conditions, leading to a reduction in active sites and a decline in catalytic performance. To address these challenges, future research will focus on developing simpler and more cost-effective methods for preparing SACs, and improving their stability and cycle life in ZABs. Exploring new support materials, such as 2D materials and MOFs, could provide a more stable anchoring environment to prevent the migration of metal atoms. At the same time, integrating theoretical models with experimental evidence to enhance comprehension of the mechanisms driving SACs will inform the development and refinement of catalysts.
TYPES, SYNTHESIS METHODS AND ELECTROCATALYTIC PERFORMANCES OF METAL OXIDES AND CARBON COMPOSITES
Recently, metal oxides have become important contenders for effective electrocatalysts in ZABs because of their distinctive physical and chemical characteristics, particularly their elevated specific surface area, strong electronic conductivity, and wealth of active sites[33,147,148]. This part summarizes recent research developments in metal oxides for ZABs, focusing on iron-based, manganese-based, and cobalt-based oxides and their performance enhancement strategies.
Iron-based oxides and carbon composites
Iron-rich oxides are preferred due to their availability and affordability. Researchers have improved the activities of catalysts related to the ORR and OER by creating interfaces between iron-group metals and manganese dioxide (MnO2) within porous carbon nanowires, thereby enhancing the efficiency of flexible ZABs. For example, as shown in Figure 10A, a novel fluorine-doped FeWO4/NC catalyst was developed to improve the oxygen reduction efficiency in ZABs[149]. A fluorine-doping approach was utilized to construct the catalyst, embedding fluorine-doped FeWO4 particles within a multi-dimensional NFC. The fabrication process involved the use of ammonium fluoride as the fluorine source, creating defect structures and providing in-situ fluorine doping during high-temperature processing. The FeWO4/NC catalyst doped with fluorine exhibited enhanced hydrophilicity, attributed to the increased polarity of its chemical bonds. This enhancement promoted the adsorption of reaction intermediates and the diffusion of the electrolyte. Consequently, the catalyst demonstrated exceptional performance in ORR, characterized by a significant half-wave potential and remarkable stability. When utilized in ZABs, this catalyst revealed a high power density and sustained cycle stability, facilitating an effective energy conversion system. As proven in Figure 10B, the enhanced catalytic activity was attributed to the synergistic effects of fluorine doping and the N-doped carbon matrix, which together improved the electronic structure and surface properties of FeWO4, promoting faster electron transfer and reaction kinetics.
Figure 10. (A) Illustration of F-FeWO4/NC; (B) Free energy diagram at 1.23 eV for ORR over (111) surfaces during 4e- ORR and the Fe-O bond length after OOH being absorbed on (111) surfaces of FeWO4 and F-FeWO4[149]; (C) The synthetic scheme of Co3O4@ND-CN; (D) The partial density of states of ND-CN and Co3O4 in Co3O4@ND-CN[150]; (E) Schematic diagram of preparation procedure for 300NiFe-Mi-C[151]. ORR: Oxygen reduction reaction.
Manganese-based oxides and carbon composites
Manganese-based oxides, particularly MnO2, are extensively studied for their high catalytic efficiency in oxygen electrode reaction[147,148]. Combined with carbon substrates, MnO2 demonstrates enhanced electronic transport and stability, boosting the power density and energy efficiency of ZABs. Tuning the crystallinity and morphology of MnO2 can further refine its catalytic performance, for instance, by fabricating hierarchically porous MnO2 to increase reactant contact area and mass transport efficiency, enhancing the electrochemical performance of ZABs. Iron-doped manganese oxide in the form of nanowires (Fe-MONW)/CNF composites were synthesized through a simple, cost-effective method[148] Fe-MONW/CNF showed superior electrocatalytic performance over CNF and MONW alone. Iron-doped MnO2 nanowires combined with CNF significantly improved OER and ORR performance, lowering onset potentials. Defects created by iron doping enhanced oxygen adsorption and catalytic activity. The material 5Fe-MONW/CNF exhibited the lowest ΔE value (922 mV) for both reactions, indicating excellent bifunctionality. The 5Fe-MONW-120/CNF maintained stability during 20 h of cycling, with ΔE decreasing to around 800 mV, showcasing its robustness for practical energy applications.
Cobalt-based oxides and carbon composites
Cobalt-based oxides, such as Co3O4, are another vital class of electrocatalysts[150]. Defect engineering and selection of support materials significantly influence the performance of Co3O4. For example, as shown in Figure 10C, by compositing Co3O4 with N-defective graphitic carbon nitride, highly efficient bifunctional electrocatalysts with abundant defect sites are produced. As depicted in Figure 10D, these defect sites increase the number of active sites and optimize the electronic structure of the catalyst, facilitating the kinetic processes of ORR and OER. Experimental results show that such composite materials demonstrate high power densities and good cycling stability in ZABs.
Pre-impregnated metal oxides in N-doped carbon
As illustrated in Figure 10E, a novel bifunctional catalyst has been developed by researchers through the pre-implantation of metal oxides into N-doped carbon, enhancing its catalytic performance for oxygen electrode reaction[151]. The catalyst, designated as 300NiFe-Mi-C, was created by heating a mixture of nickel and iron metal salts to yield metal oxides, which were subsequently combined with melamine and subjected to carbonization at 900 °C. The resulting product features a hierarchical porous architecture, with metal nanoparticles evenly dispersed throughout the N-doped carbon matrix. This specific structure promotes efficient mass transport and electron transfer, resulting in improved performance for oxygen electrode reaction. The exceptional catalytic capability is ascribed to the combined effects of the metal oxides and N-doped carbon, which collectively provide a rich array of active sites and enhance the adsorption and activation of oxygen species.
Perovskite oxide composites
Composites of perovskite oxides demonstrate considerable promise in bifunctional oxygen electrocatalysis, owing to their distinct structure and flexible composition[152]. By constructing heterostructures of perovskite oxides with other metals, alloys, metal oxides, or N-doped carbon materials, active sites can be strengthened, achieving efficient ORR and OER catalysis. The use of these materials in ZABs greatly enhances the efficiency of energy conversion and the commercial potential of the batteries.
Metal oxides and their composites, serving as efficient and stable electrocatalysts in ZABs, have demonstrated considerable application potential. Through material design and structural optimization, the catalytic performance of ORR and OER can be further enhanced, addressing key challenges in ZABs. Future research should focus on the development of novel metal oxide catalysts, exploration of their synergistic effects with novel electrolytes and zinc anode materials, and optimization of battery design and manufacturing processes to advance the commercialization of ZABs. As material science and electrochemical technologies continue to evolve, the prospects for the application of metal oxides in the field of ZABs will expand.
Significant progress has been made in the application of metal oxides as efficient electrocatalysts in ZABs. Through material design and surface modification techniques, such as the construction of metal oxide-carbon substrate composites, incorporation of defect engineering, and elemental doping, the ORR and OER performances of catalysts can be effectively enhanced, significantly improving the energy efficiency and cycle life of ZABs. Future research should continue to explore innovative methods for synthesizing metal oxide catalysts, optimizing their structure and composition, and developing advanced characterization techniques to gain deeper insights into catalytic mechanisms. Ultimately, this will facilitate the commercial application of ZABs in large-scale energy storage scenarios.
STRUCTURAL DESIGN AND PERFORMANCE OPTIMIZATION OF METAL-FREE CATALYSTS
Recently, materials that do not contain metals, especially those made from carbon composites, have become strong contenders because of their plentiful resources, adjustable electronic characteristics, and remarkable stability[100,153].
Carbon-based 0D/1D/2D composites, including combinations of quantum dots (QDs), CNTs, and graphene, have demonstrated exceptional bifunctional ORR/OER catalytic performance through precise structural design and defect state modulation [Figure 11A][153]. For instance, controlling the morphology and composition of carbon nanoshells, and introducing neighboring N, P, and S active sites, can effectively facilitate charge transfer, lower activation barriers, and achieve efficient oxygen conversion.
Figure 11. (A) Electrostatic potential plots of N, S co-doping graphene structure with different ratios of pyridine N/graphite N 1:1, 2:1 and 1:2[153]; (B) Graphical abstract of BNF-LCFs[100]; (C) Graphical abstract of S-N-C materials[154]; (D) Illustration of BP-CN-c; (E) ZABs performance based on BP-CN-c[155]. BP: Black phosphorus. ZABs: zinc-air batteries; ORR: oxygen reduction reaction; OER: oxygen evolution reaction.
CNFs derived from natural materials, such as lignin, exhibit superior bifunctional ORR/OER catalytic activity after B, N, F ternary doping[100]. BNF-LCFs, a type of triply-doped CNFs sourced from biomass lignin, serve as an effective, metal-free bifunctional catalyst for ORR and OER in rechargeable liquid/solid ZABs, as depicted in Figure 11B. Rechargeable metal-air batteries are eco-friendly and cost-effective but suffer from slow ORR/OER kinetics. BNF-LCFs, synthesized via electrospinning and calcination, feature a large surface area, defect sites, and a synergistic effect from B, N, and F dopants, optimizing the electronic and chemical properties of carbon matrices. Their catalytic performance in alkaline solutions is notably superior, featuring a minimal DE of just 0.728 V, surpassing both commercial Pt/C + RuO2 and contemporary non-metallic carbon catalysts. Liquid ZABs utilizing BNF-LCFs demonstrate an impressive open circuit voltage of 1.536 V, a significant capacity of 791.5 mAh·g-1, and stable cycling, exceeding that of Pt/RuO2-based ZABs. Moreover, solid-state ZABs exhibit remarkable electrochemical efficacy, adaptability, and stability during cycles, making them appropriate for use in wearable devices. This innovation in triply-doped CNFs enhances the efficiency of ZABs and opens doors for sustainable, high-performance energy storage systems.
A simple yet efficient approach was established for the synthesis of ultrathin S-N co-doped carbon nanosheets (S-N-C) intended for use as ORR electrocatalysts in ZABs[154]. As shown in Figure 11C, these catalysts were fabricated through a solid-state grinding process followed by pyrolysis, utilizing tannic acid, hexamethylene tetramine, S-doped graphitic carbon nitride (S-C3N4), and ammonium chloride as the precursors, soft template, and activating agent, respectively. The produced S-N-C 1,000 catalyst showcased a 3D ultrathin architecture with an extensive specific surface area, facilitating effective mass transfer and electron transport. The enhanced catalytic efficiency is attributed to the combined effects of S and N co-doping, which fine-tunes the adsorption energy of oxygen species while improving the electronic characteristics of the carbon matrix. This, along with its large surface area and multi-scale porous structure, promotes effective oxygen adsorption and desorption, positioning S-N-C 1,000 as a promising ORR electrocatalyst for sustainable ZABs.
A groundbreaking study has engineered 2D black phosphorus (BP) with regulated electron-deficient interfaces for enhanced electrocatalytic ORR performance [Figure 11D][155]. The researchers achieved this by covalently bonding BP with pyridine molecules, creating a heterostructure with optimized electronic properties. The synthesis involved treating BP flakes with pyridine at elevated temperatures to form covalent bonds at the edges and defects, which introduced electron-withdrawing groups, thereby tuning the electronic structure of BP. The modified BP exhibited a decreased bandgap and increased conductivity, which are crucial for improved electrocatalytic activity. The performance data showed a significant enhancement in the onset potential and half-wave potential for the ORR, demonstrating the superior catalytic efficiency of the BP-pyridine composite compared to pristine BP. As demonstrated in Figure 11E, the mechanism behind this enhancement is attributed to the altered electronic structure that facilitates easier electron transfer and oxygen adsorption. Furthermore, the altered BP demonstrated exceptional stability throughout extended electrocatalytic cycles, highlighting its promise for real-world utilization in energy conversion technologies, especially in fuel cells and metal-air batteries. This research paves the way for the thoughtful design of 2D materials aimed at catalytic purposes by accurately manipulating their electronic characteristics via covalent functionalization.
The superior performance of metal-free materials in ZABs can be attributed to several critical factors: (1) Electronic structure tuning: Doping, defect engineering, and heterostructure design adjust the electronic structure of materials, optimizing the electron density of active sites to facilitate charge transfer and enhance catalytic efficiency; (2) Enhanced conductivity: Multi-dimensional structure design, utilizing CNTs and graphene, significantly boosts material conductivity, reducing internal resistance within the battery and contributing to improved overall performance; (3) Structural stability: Metal-free materials generally exhibit good chemical stability and mechanical strength, preserving structural integrity during repeated charge-discharge cycles, ensuring long-term operational stability.
The application of metal-free materials in ZABs illustrates their tremendous potential as high-efficiency, low-cost catalysts. Continuous material innovation and optimization promise to overcome the limitations of traditional noble metal catalysts, accelerating the commercialization of ZABs. Future research should further explore novel metal-free materials, deepen the understanding of their catalytic mechanisms, and strive for higher-performance ZABs to meet the growing demand for clean energy.
In summary, metal-free materials, particularly those based on carbon and incorporating novel design strategies, have shown great promise in overcoming the challenges associated with oxygen redox reactions in ZABs. By leveraging the inherent properties of these materials and refining their catalytic capabilities, the scientific community is paving the way for more sustainable, efficient, and economically viable energy storage solutions. As research progresses, the integration of these advanced materials into ZAB architectures holds the potential to revolutionize the field of renewable energy storage, enabling the widespread adoption of clean power sources.
ELECTROCATALYSTS FOR SOLID-STATE ZABS
Solid-state ZABs have demonstrated substantial potential for use in wearable and portable electronic devices due to their safety features and long cycle life[6,12,45,156]. Contrasted with traditional liquid-electrolyte ZABs, solid-state versions tackle the critical issues of zinc dendrite formation and electrolyte leakage by employing solid electrolytes, thereby enhancing the overall safety of the battery system. The choice of catalyst is pivotal for solid-state ZABs, particularly concerning the ORR and OER, as these reactions directly impact the efficiency and stability of the battery.
A high-performance electrocatalyst suitable for all-solid-state flexible ZABs was intelligently developed by anchoring iron single atoms onto N-doped carbon (Fe-N-C)[157]. As illustrated in Figure 12A, the catalyst was produced via a straightforward one-step pyrolysis process of iron phthalocyanine with dicyandiamide at varying temperatures, eliminating the necessity for acid etching. The optimized Fe-N-C-700 catalyst exhibited a hierarchical porous configuration along with evenly distributed iron single atoms. This specific architecture enhanced the availability of active sites for ORR and promoted efficient transport of mass and electrons. The remarkable catalytic efficiency was ascribed to the synergistic interaction between the iron single atoms and the NFC, which fine-tuned the adsorption energy of oxygen-containing intermediates.
Figure 12. (A) Illustration of Fe-N-C-700[157]; (B) Illustration of Fe3C@N/MCHSs and Fe3C-NG Mott-Schottky heterojunction; (C) Illustration of the Mott−Schottky heterojunction of the Fe3C @NG: before and after contacting, and the ORR mechanism, the charge distribution of Fe3C @NG model, the ORR processes on Fe3C@NG model[158]; (D) Illustration of Co/MnO@NC; (E) DFT calculations of Co-MnO[156]. NG: N-doped graphene; ORR: oxygen reduction reaction; DFT: density functional theory; NTA: nitrilotriacetic acid.
Researchers have developed a non-noble-metal catalyst, specifically Fe3C QDs on NG, for enhanced ORR in ZABs[158]. As shown in Figure 12B, the catalyst was synthesized through a controlled pyrolysis process, embedding Fe3C QDs within a NG matrix. This structure facilitates rapid electron transfer, boosting the ORR performance. The Fe3C-NG catalyst showed superior catalytic activity with high onset potential and a low Tafel slope, indicating efficient ORR kinetics. Its durability was significantly improved, maintaining stable performance over extended periods in harsh electrolytes. This resilience is attributed to the stable graphene structure in NG, which protects the Fe3C QDs from corrosion. Additionally, a Zn/graphene composite film was integrated with a solid-state electrolyte to fabricate a ZAB. The optimized battery displayed ultra-long durability and high performance, even under demanding conditions. As proven in Figure 12C, the Mott-Schottky heterojunction between Fe3C QDs and NG plays a pivotal role in enhancing electron transfer and stability, making this catalyst a promising alternative to costly noble metal catalysts for practical electrochemical energy conversion devices.
An innovative strategy was devised to enhance oxygen electrocatalysis in flexible ZABs by constructing hetero-interfaces of iron-group metals with MnO in porous carbon nanowires[156]. As shown in Figure 12D, a “coordination construction-thermal decomposition” method using chelating agents to stabilize metal ions and build nanowire structures was proposed, creating Co/MnO hetero-interfaces within N-doped carbon nanowires. Co/Mn-nitrilotriacetic acid (NTA) coordination polymers were synthesized and thermally decomposed to produce Co/MnO@NC catalysts. These catalysts had highly conductive hetero-interfaces and graphitic carbon, improving reaction kinetics. Co/MnO@NC showed superior bifunctional oxygen electrocatalytic activity and durability, with a reversible oxygen DE of 0.66 V. As shown in Figure 12E, DFT calculations explained the optimized adsorption of oxygen intermediates on the heterostructure. This strategy extends to other iron-group electrocatalysts, all demonstrating good bifunctional activity. The study provides a promising catalyst for flexible and wearable energy storage devices.
The strategy’s adaptability to other iron-group elements suggests a flexible approach to fabricating tailored electrocatalysts for various energy systems. Demonstrated mechanical flexibility in solid-state batteries opens avenues for integration into wearable and portable devices. This development addresses limitations of conventional oxygen electrocatalysts and broadens prospects for advanced energy storage solutions. Future work may optimize synthesis parameters and explore wider applications.
The application of NPM catalysts, such as Fe SACs and Fe3C-NG catalysts, in solid-state ZABs marks a significant stride toward low-cost, high-durability battery technologies. Table 1 offers a comprehensive comparison of the bifunctional NPM-based carbon electrocatalysts that have been reported so far, featuring several of the outstanding candidates as well. These catalysts, through optimized structural design, not only elevate the electrochemical performance of the batteries but also augment their safety and durability. Ongoing research will concentrate on developing even more efficient and stable catalysts, as well as exploring novel solid-state electrolytes, to further enhance the performance of solid-state ZABs and propel their widespread adoption in wearable and portable electronic devices.
Comparison of the bifunctional NPM-based carbon electrocatalysts
| Catalyst | Loading (mg·cm-2) | E1/2 (V) | η10 (mV) | Power density (mW·cm-2) | Stability (h) | Ref. | |
| NiFe-N@CNF | 0.4 | \ | 351 | 179 | 500 | [17] | |
| FeNi@NC | 0.128 | 0.84 | 330 | 119 | 70 | [35] | |
| Fe-1 | 1 | 0.882 | 365 | 201 | 600 | [37] | |
| D-FeNC/MOF | \ | 0.875 | \ | 170 | 100 | [44] | |
| FeCoNi/FCNFs | 3 | 0.92 | 410 | 121 | 630 | [45] | |
| FeCo@CNTs-60 | 5 | 0.95 | 530 | 268.3 | 1,300 | [46] | |
| Co/BN-CNT/VN | \ | 0.85 | 296 | 163.5 | \ | [47] | |
| glu-NiFe | \ | 0.85 | 440 | 127 | 240 | [48] | |
| CoFe@NCNT | 0.4 | 0.84 | 340 | 194 | 300 | [49] | |
| FeCo/N-CNTs-800 | 4 | 0.891 | 359 | 200.4 | 445 | [50] | |
| Co@C1N3C | 0.247 | 0.84 | 625 | \ | \ | [51] | |
| CoFe@C | 0.22 | 0.84 | 160 | 142 | 27 | [52] | |
| Co/CNT | 0.5 | 0.84 | 450 | 120 | 400 | [53] | |
| Ni@CN | \ | 0.88 | 280 | \ | \ | [54] | |
| NH2-CNT | 1 | 0.76 | 398 | \ | \ | [55] | |
| CoNi@GO | 0.25 | 0.837 | 340 | 260 | 240 | [56] | |
| CoN/UNG | \ | 0.87 | \ | 149.3 | 350 | [57] | |
| FeNi/N-GPCM | 0.25 | 0.883 | 310 | 321 | 400 | [58] | |
| 3d-GMC | \ | 0.947 | 390 | 100 | 200 | [59] | |
| B-Zn-FeNG | 0.236 | 0.89 | 310 | 229 | 80 | [60] | |
| NiFeCo-NC | 2 | 0.677 | 392 | \ | \ | [61] | |
| WN@Ni | \ | 0.76 | 450 | 165 | 400 | [62] | |
| Co-CSNF | 1 | 0.86 | 470 | 322 | 366 | [63] | |
| CoNx/CPCN | 1 | 0.851 | 378 | 189.9 | 500 | [64] | |
| NiFe-LDH | 1 | 0.85 | 310 | 150 | 690 | [65] | |
| Co9S8-FeS2@N-CNFs | \ | 0.8 | 330 | 214 | 10 | [66] | |
| CoFe/Se@CN | 0.255 | 0.87 | 282 | 160 | 300 | [67] | |
| FeACs-VNNCs/ NFC | 0.2 | 0.87 | 246 | 146 | 195 | [68] | |
| ZnCo-Te@NC | 0.243 | 0.873 | 400 | 259.7 | 100 | [69] | |
| FeNi3/NC | 1 | 0.66 | 360 | 89 | \ | [70] | |
| Fe-N/S-HPC | 1 | 0.85 | 340 | 188.4 | 240 | [71] | |
| GPCNSs | 0.5 | 0.897 | \ | 128 | \ | [72] | |
| 3D Co/N-C | 0.25 | 0.84 | 330 | 239 | 200 | [73] | |
| g-C3N4 | \ | 0.82 | \ | 99 | 145 | [74] | |
| Fc@Fe-NHCS | 0.2 | 0.85 | \ | 196 | 100 | [75] | |
| FeZn-N-C-1 | 0.5 | 0.846 | \ | 163 | 11 | [76] | |
| FeCo/C2P/Fe2P@NPC | 1 | 0.79 | 280 | 174 | 190 | [77] | |
| Co3ZnC/Co | 0.4 | 0.88 | \ | \ | \ | [78] | |
| MCG-2 | 0.204 | 0.859 | \ | 112 | 55 | [79] | |
| Co9S8@NSC | 1 | 0.85 | \ | 150.9 | \ | [80] | |
| CoFe-NCNFs | 1 | 0.85 | 323 | 116 | 110 | [81] | |
| NiPS3@NMC | 0.5 | 0.9 | 220 | \ | \ | [82] | |
| RuFe@NC | 1 | 0.85 | 359 | \ | \ | [83] | |
| Co||Cu/NC | 1 | 0.83 | 210 | 218.5 | \ | [84] | |
| Co@HNCs | 0.3 | 0.87 | 344 | 192 | \ | [85] | |
| NeCNC-900 | 0.85 | 0.9 | 291 | 68.2 | 200 | [86] | |
| Fe@NC-700 | 0.5 | 0.865 | \ | 155 | 50 | [87] | |
| Fe/PNC | 2 | 0.87 | \ | 113.4 | 280 | [88] | |
| Co9S8/CoNSC | 0.788 | 0.889 | 233 | 150 | 40 | [89] | |
| MnNSC-950 | 0.25 | 0.84 | \ | 107 | 150 | [90] | |
| Co/Co9S8-NCL-30 | 1 | 0.85 | \ | 112 | \ | [91] | |
| MoP@N, P-HCF | 0.1 | 0.76 | 440 | 93.8 | 10 | [92] | |
| CH-FeNC | \ | 0.92 | \ | 131 | \ | [93] | |
| Fe3C@NPW | 1 | 0.87 | 350 | 125 | 260 | [94] | |
| Cu3P/MoP@C | 0.4 | 0.9 | \ | 156 | 231 | [97] | |
| A-MnO2/NSPC | 0.29 | 0.87 | 280 | 181 | 287 | [98] | |
| Co2P/NP-C | 0.25 | 0.81 | \ | 152.4 | \ | [99] | |
| BNF-LCFs | 0.31 | 0.844 | 342 | 99.4 | 200 | [100] | |
| FeS/FeN-NC-X4 | 0.4 | 0.863 | \ | 156 | 200 | [101] | |
| Co9S8@SNC | 0.22 | 0.846 | 320 | 239 | \ | [102] | |
| CuNDs@NFPCNFs | 0.5 | 0.865 | \ | 204.9 | 400 | [103] | |
| S, N-Co@CNT | \ | 0.874 | 276 | 171 | 48 | [104] | |
| Co9S8/NSC-1 | 1 | 0.83 | 300 | 102 | 167 | [106] | |
| CoFe-N-CNTs/CNFs | 1 | 0.862 | 320 | \ | \ | [107] | |
| LIC-ZIF-67-M10 | 0.42 | 0.77 | 390 | 80 | 220 | [108] | |
| Fe-NC@NCNT | 0.25 | 0.88 | \ | 115 | 116 | [109] | |
| Co NCs/HPNC | 0.4 | 0.88 | \ | 109.6 | \ | [110] | |
| FeCo@1D-CNTs/ 2D-NC | 0.229 | 0.87 | 396 | 98.2 | \ | [111] | |
| P-Co3Fe1/NC-700-10 | 0.4 | 0.84 | 373 | 155 | 120 | [112] | |
| S-Co/CoNC | 1 | 0.82 | 390 | 101.7 | 160 | [114] | |
| FeNi-N-C@FeNi LDH | 0.358 | 0.89 | 305 | 73.9 | 100 | [115] | |
| Co/VN NPs@C | 1 | 0.833 | 280 | 130 | 300 | [116] | |
| FeNP@Fe-N-C | 1 | 0.84 | 340 | 106.5 | 67 | [117] | |
| Mo2C/MoC/Co@CNTs | 0.2 | 0.82 | 330 | 134 | 275 | [118] | |
| Fe/Ni-NC||FeNi@G | 0.8 | 0.885 | 303 | 200 | 500 | [119] | |
| Co-CoO/NPCF | \ | 0.843 | 356 | 214.1 | \ | [120] | |
| h-Ti3C2Tx@Co-NCN | 1 | 0.843 | 323 | 160.4 | \ | [121] | |
| Co/CoO@HNC | 0.4 | 0.83 | 430 | 85.6 | 250 | [122] | |
| CoMn2O4/C-NH2-300 | 1 | 0.83 | 348 | 195.5 | \ | [123] | |
| P-CoSe2/C@CC | \ | \ | 303.1 | 124.4 | 70 | [124] | |
| Fe-Co-Ni MOF | 1 | 0.75 | 254 | 161 | \ | [125] | |
| mFeNC-CNT | 1 | 0.908 | \ | 108 | \ | [126] | |
| Mo/Fe/Co@NC | \ | 0.85 | 265 | 150 | 500 | [127] | |
| CoZn-NCNTs | \ | 0.82 | 600 | 214 | 320 | [128] | |
| 30-ZnMn-NC | \ | 0.83 | \ | 207 | 325 | [129] | |
| Co9S8/NSCP | \ | 0.9 | 370 | \ | \ | [130] | |
| Co-CNT@COF-Pyr | \ | 0.52 | 438 | \ | \ | [132] | |
| Co-N@Gs | 0.36 | 0.85 | \ | 31 | 160 | [134] | |
| Cu-N©HCS | 0.5 | \ | \ | 244.7 | 100 | [135] | |
| Co@NCNT/Co-SA@NCMT | 0.87 | 313 | 130 | 120 | 17 | [136] | |
| Fe SA/NCZ | 0.2 | 0.87 | 320 | 101 | 44 | [139] | |
| Fe@FeSA-N-C | 0.225 | 0.83 | 390 | 110 | 500 | [140] | |
| FeZ-N/S0.6-C | 0.4 | 0.93 | \ | 215.3 | 160 | [141] | |
| Fe@MET-M | 0.5 | 0.895 | \ | 212 | 100 | [142] | |
| Co2P/Co-NC | 0.5 | 0.87 | 369 | 187 | 140 | [143] | |
| Fe-N4@NC-PCSs | 0.383 | 0.8 | \ | 207 | 200 | [144] | |
| Mo-Carbon | 0.255 | 0.788 | 303 | 169 | 90 | [145] | |
| Fe-NHC | 0.5 | 0.89 | \ | 157 | \ | [146] | |
| MnO2/NiF | 0.2 | \ | \ | 120 | \ | [147] | |
| Fe-MONW/CNF | 1 | 0.77 | 317 | \ | \ | [148] | |
| F-FeWO4/NC | 1 | 0.85 | \ | 173 | 170 | [149] | |
| Co3O4@ND-CN | \ | 0.81 | 336 | 105 | 115 | [150] | |
| 300NiFe-Mi-C | 0.5 | 0.83 | 330 | 99 | \ | [151] | |
| N, S-GOQD-RGO/CNT | 0.37 | 0.84 | 390 | 134.3 | \ | [153] | |
| S-N-C | 0.4 | 0.858 | \ | 164.68 | 600 | [154] | |
| BP-CN-c | \ | 0.84 | 350 | 168.3 | 300 | [155] | |
| Co/MnO@NC | 0.2 | 0.83 | 260 | 146 | 400 | [156] | |
| Fe-N-C | \ | 0.83 | \ | 70 | \ | [157] | |
| Fe3C-NG | \ | 0.875 | \ | 95 | 1,000 | [158] |
The ongoing advancements in solid-state ZABs, driven by the development of innovative catalysts and electrolytes, are anticipated to redefine the landscape of energy storage for next-generation wearable and portable electronics. As research progresses, the realization of high-performance, safe, and durable batteries will facilitate the integration of these technologies into everyday consumer products, potentially revolutionizing the way we power our devices.
SUMMARY AND PERSPECTIVES
Summary
In conclusion, the development of NPM-based carbon materials has shown significant promise for enhancing the ORR and OER in rechargeable ZABs. These materials, including MOF derivatives, metal-doped carbons, carbon nitrides, heteroatom-doped carbons, and MXenes, offer numerous advantages such as high specific surface areas, tunable morphologies, and the ability to incorporate multiple active sites through doping with elements such as N, S, P, and boron. These attributes facilitate enhanced electron transfer and mass transport, leading to improved catalytic performance for both the ORR and OER. Moreover, the design of NPM-based carbon materials has been enriched by advancements in synthetic methodologies. Techniques such as hydrothermal synthesis, pyrolysis, and electrospinning have enabled the creation of hierarchical porous structures, which are integral to achieving high catalytic activity and stability. Recent studies have demonstrated the successful development of high-performance NPM-based carbon materials for ZABs, such as SACs and heterojunctions, which show significant promise for practical applications.
Challenges and possible further approaches
Despite the significant progress in developing NPM-based carbon materials for the ORR and OER in ZABs, several challenges remain: (1) Achieving the highest possible catalytic activity, particularly in terms of half-wave potential and overpotential, remains a significant challenge. While recent advancements have led to improvements, there is still a need for materials that can match or exceed the performance of PGMs; (2) Ensuring long-term stability under operational conditions, especially at high current densities, is a critical issue. Degradation over time can lead to a decrease in active sites and a decline in catalytic performance; (3) Cost-effectiveness: The cost and environmental impact of large-scale production must be considered. Many NPM-based carbon materials rely on complex synthesis steps and expensive precursors, which can hinder their commercial viability; (4) Improving the overall performance of ZABs, including energy density, cycle life, and cost-effectiveness, requires further optimization. The development of materials that can balance high catalytic activity with durability and cost is essential.
To address these challenges, the following approaches can be pursued: (1) Develop simpler and more cost-effective methods for preparing NPM-based carbon materials. Techniques such as solvothermal synthesis, spray pyrolysis, and microwave-assisted synthesis could offer more efficient routes; (2) Novel support materials: Explore new support materials, such as 2D materials and MOFs, which can provide a more stable anchoring environment for preventing the migration of metal atoms; (3) Combine theoretical calculations with experimental validation to gain a deeper understanding of the mechanisms underlying the catalytic performance of NPM-based carbon materials. This will guide the design and optimization of catalysts; (4) Optimize the structure and composition of NPM-based carbon materials to improve their catalytic performance. This includes the precise control of active site density, the understanding of catalytic mechanisms, and the scalability of synthesis methods; (5) Optimize the compatibility of NPM-based carbon materials with electrolytes and refine battery design to enhance overall performance. This includes the development of quasi-solid-state and all-solid-state electrolytes to improve safety and operational stability; (6) Focus on the application of NPM-based carbon materials in flexible and solid-state ZABs, which represent significant future directions for the commercialization of ZABs in wearable devices and other portable electronics.
By pursuing these approaches, it is anticipated that the challenges faced by NPM-based carbon materials for ZABs can be addressed, paving the way for their widespread adoption in practical energy storage applications.
DECLARATIONS
Authors’ contributions
Investigation, resources, writing - original draft preparation: Hu, T.
Literature search and selection, conceptualization: Du, K.
Visualization, literature search and selection: Zheng, S.
Visualization, formal analysis: Wu, Y.
Data curation, investigation: Qin, J.
Writing - review and editing: Liu, F.
Investigation, validation, funding acquisition: Cui, M.
Writing - review and editing, project administration, funding acquisition: Wang, S.
Availability of data and materials
The data and materials presented in the article require approval from the corresponding author prior to any use.
Financial support and sponsorship
This work was supported by the Natural Science Foundation of Shandong Province of China (ZR2023ME173), the Science, Education and Industry Integration Innovation Pilot Project from Qilu University of Technology (Shandong Academy of Sciences) (2024ZDZX13), the Science, Education and Industry Integration Pilot Project Plan of Qilu University of Technology (Shandong Academy of Sciences) (2023PY040), and the Open Project of Key Laboratory of Advanced Energy Materials Chemistry (Ministry of Education).
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2026.
REFERENCES
1. Bi, X.; Jiang, Y.; Chen, R.; et al. Rechargeable zinc-air versus lithium-air battery: from fundamental promises toward technological potentials. Adv. Energy. Mater. 2024, 14, 2302388.
2. Dias, G. S.; Costa, J. M.; Almeida, N. A. F. Transition metal chalcogenides carbon-based as bifunctional cathode electrocatalysts for rechargeable zinc-air battery: an updated review. Adv. Colloid. Interface. Sci. 2023, 315, 102891.
3. Du, Q.; Gong, Y.; Khan, M. A.; et al. Regulating non-precious transition metal nitrides bifunctional electrocatalysts through surface/interface nanoengineering for air-cathodes of Zn-air batteries. Green. Energy. Environ. 2022, 7, 16-34.
4. Guo, Y.; Li, Y.; Chen, Y.; Wang, P.; Xie, Y.; Yi, T. Rational design of one-dimensional cobalt-related oxygen electrocatalysts toward high-performance zinc-air batteries. Coord. Chem. Rev. 2023, 495, 215383.
5. Lee, S.; Choi, J.; Kim, M.; Park, J.; Park, M.; Cho, J. Material design and surface chemistry for advanced rechargeable zinc-air batteries. Chem. Sci. 2022, 13, 6159-80.
6. Jiang, L.; Luo, X.; Wang, D. A review on system and materials for aqueous flexible metal-air batteries. Carbon. Energy. 2023, 5, e284.
7. Li, J.; Xue, H.; Xu, N.; et al. Co/Ni dual-metal embedded in heteroatom doped porous carbon core-shell bifunctional electrocatalyst for rechargeable Zn-air batteries. Mater. Rep. Energy. 2022, 2, 100090.
8. Kundu, A.; Mallick, S.; Ghora, S.; Raj, C. R. Advanced oxygen electrocatalyst for air-breathing electrode in Zn-air batteries. ACS. Appl. Mater. Interfaces. 2021, 13, 40172-99.
9. Tang, W.; Li, B.; Teng, K.; et al. Advanced noble-metal-free bifunctional electrocatalysts for metal-air batteries. J. Materiomics. 2022, 8, 454-74.
10. Wu, L.; Zhao, R.; Du, G.; et al. Hierarchically porous Fe/N/S/C nanospheres with high-content of Fe-Nx for enhanced ORR and Zn-air battery performance. Green. Energy. Environ. 2023, 8, 1693-702.
11. Kumar, Y.; Mooste, M.; Tammeveski, K. Recent progress of transition metal-based bifunctional electrocatalysts for rechargeable zinc - air battery application. Curr. Opin. Electrochem. 2023, 38, 101229.
12. Lang, X.; Hu, Z.; Wang, C. Bifunctional air electrodes for flexible rechargeable Zn-air batteries. Chin. Chem. Lett. 2021, 32, 999-1009.
13. Kundu, A.; Kuila, T.; Murmu, N. C.; Samanta, P.; Das, S. Metal-organic framework-derived advanced oxygen electrocatalysts as air-cathodes for Zn-air batteries: recent trends and future perspectives. Mater. Horiz. 2023, 10, 745-87.
14. Peng, Z.; Li, Y.; Ruan, P.; et al. Metal-organic frameworks and beyond: the road toward zinc-based batteries. Coord. Chem. Rev. 2023, 488, 215190.
15. Zhan, F.; Liu, S.; He, Q.; et al. Metal-organic framework-derived heteroatom-doped nanoarchitectures for electrochemical energy storage: recent advances and future perspectives. Energy. Storage. Mater. 2022, 52, 685-735.
16. Zhu, Y.; Yue, K.; Xia, C.; et al. Recent advances on MOF derivatives for non-noble metal oxygen electrocatalysts in zinc-air batteries. Nanomicro. Lett. 2021, 13, 137.
17. Akmalia, R.; Balqis, F.; Andriani, M. F.; Irmawati, Y.; Sumboja, A. Well-dispersed NiFe nanoalloy embedded on N-doped carbon nanofibers as free-standing air cathode for all-solid-state flexible zinc-air battery. J. Energy. Storage. 2023, 72, 108743.
18. Hong, Y.; Li, L.; Huang, B.; et al. Molecular control of carbon-based oxygen reduction electrocatalysts through metal macrocyclic complexes functionalization. Adv. Energy. Mater. 2021, 11, 2100866.
19. Qin, D.; Tang, Y.; Ma, G.; et al. Molecular metal nanoclusters for ORR, HER and OER: achievements, opportunities and challenges. Int. J. Hydrogen. Energy. 2021, 46, 25771-81.
20. Lu, X.; Hansen, E. J.; He, G.; Liu, J. Eutectic electrolytes chemistry for rechargeable Zn batteries. Small 2022, 18, e2200550.
21. Vílchez-Cózar, Á.; Armakola, E.; Gjika, M.; et al. Exploiting the multifunctionality of M2+/imidazole-etidronates for proton conductivity (Zn2+) and electrocatalysis (Co2+, Ni2+) toward the HER, OER, and ORR. ACS. Appl. Mater. Interfaces. 2022, 14, 11273-87.
22. Wang, H.; Pei, Y.; Wang, K.; et al. First-row transition metals for catalyzing oxygen redox. Small 2023, 19, e2304863.
23. Li, J.; Wang, C.; Yu, Z.; Chen, Y.; Wei, L. MXenes for Zinc-based electrochemical energy storage devices. Small 2024, 20, e2304543.
24. Xu, H.; Yang, J.; Ge, R.; et al. Carbon-based bifunctional electrocatalysts for oxygen reduction and oxygen evolution reactions: optimization strategies and mechanistic analysis. J. Energy. Chem. 2022, 71, 234-65.
25. Wang, J.; Kong, H.; Zhang, J.; Hao, Y.; Shao, Z.; Ciucci, F. Carbon-based electrocatalysts for sustainable energy applications. Prog. Mater. Sci. 2021, 116, 100717.
26. Xu, X.; Sun, H.; Jiang, S. P.; Shao, Z. Modulating metal-organic frameworks for catalyzing acidic oxygen evolution for proton exchange membrane water electrolysis. SusMat 2021, 1, 460-81.
27. Wang, S.; Che, Z.; Zou, M.; et al. Gorgeous turn-back: rough surface treatment strategy induces Cu-C and N-C active moieties for bifunctional oxygen electrocatalysis. Chem. Eng. J. 2023, 471, 144262.
28. Wang, X.; Wu, Z.; Wang, X.; et al. Bifunctional electrocatalysts derived from cluster-based ternary sulfides for oxygen electrode reactions. Electrochimica. Acta. 2021, 376, 138048.
29. Xu, H.; Huang, C.; Shuai, T.; et al. Noble metal-free N-doped carbon-based electrocatalysts for air electrode of rechargeable zinc-air battery. Sci. China. Mater. 2023, 66, 2953-3003.
30. Song, Y.; Li, W.; Zhang, K.; Han, C.; Pan, A. Progress on bifunctional carbon-based electrocatalysts for rechargeable zinc-air batteries based on voltage difference performance. Adv. Energy. Mater. 2024, 14, 2303352.
31. Kumar, D. B.; Nie, W.; Jiang, Z.; Lee, J.; Maiyalagan, T. Recent progress in transition metal carbides and nitrides based composites as bifunctional oxygen electrocatalyst for zinc air batteries. J. Alloys. Compd. 2023, 960, 170828.
32. Xu, C.; Niu, Y.; Ka-man, A. V.; et al. Recent progress of self-supported air electrodes for flexible Zn-air batteries. J. Energy. Chem. 2024, 89, 110-36.
33. Mechili, M.; Vaitsis, C.; Argirusis, N.; Pandis, P. K.; Sourkouni, G.; Argirusis, C. Research progress in transition metal oxide based bifunctional electrocatalysts for aqueous electrically rechargeable zinc-air batteries. Renew. Sustain. Energy. Rev. 2022, 156, 111970.
34. Cui, M.; Yuan, Y.; Wu, Y.; et al. Graphdiyne-induced CoN/CoS2 heterojunction: boosting efficiency for bifunctional oxygen electrochemistry in zinc-air batteries. ChemSusChem 2024, Online ahead of print.
35. Deng, S. Q.; Zhuang, Z.; Zhou, C. A.; et al. Metal-organic framework derived FeNi alloy nanoparticles embedded in N-doped porous carbon as high-performance bifunctional air-cathode catalysts for rechargeable zinc-air battery. J. Colloid. Interface. Sci. 2023, 641, 265-76.
36. Ren, Y.; Wang, H.; Zhang, T.; et al. Designed preparation of CoS/Co/MoC nanoparticles incorporated in N and S dual-doped porous carbon nanofibers for high-performance Zn-air batteries. Chin. Chem. Lett. 2021, 32, 2243-8.
37. Sheng, J.; Sun, S.; Jia, G.; Zhu, S.; Li, Y. Doping effect on mesoporous carbon-supported single-site bifunctional catalyst for zinc-air batteries. ACS. Nano. 2022, 16, 15994-6002.
38. Zhao, H.; Yao, H.; Wang, S.; et al. Doping-engineered bifunctional oxygen electrocatalyst with Se/Fe-doped Co3O4/N-doped carbon nanosheets as highly efficient rechargeable zinc-air batteries. J. Colloid. Interface. Sci. 2022, 626, 475-85.
39. Ye, D.; Shen, Y.; Mao, H.; et al. Dual-sources directed construction of N-doped carbon nanotube arrays as superior self-supported bifunctional air electrodes for rechargeable/flexible zinc-air batteries. Chem. Eng. J. 2023, 464, 142601.
40. Feng, Y.; Song, K.; Zhang, W.; et al. Efficient ORR catalysts for zinc-air battery: biomass-derived ultra-stable Co nanoparticles wrapped with graphitic layers via optimizing electron transfer. J. Energy. Chem. 2022, 70, 211-8.
41. Wang, Z.; Zhou, L.; Li, R.; et al. Electrocatalytic oxygen reduction of COF-derived porous Fe-Nx nanoclusters/carbon catalyst and application for high performance Zn-air battery. Microporous. Mesoporous. Mater. 2022, 330, 111609.
42. Gu, T.; Sa, R.; Zhang, L.; Li, D.; Wang, R. Engineering interfacial coupling between Mo2C nanosheets and Co@NC polyhedron for boosting electrocatalytic water splitting and zinc-air batteries. Appl. Catal. B. Environ. 2021, 296, 120360.
43. Wang, Z.; Deng, D.; Wang, H.; et al. Engineering Mn-Nx sites on porous carbon via molecular assembly strategy for long-life zinc-air batteries. J. Colloid. Interface. Sci. 2024, 653, 1348-57.
44. Xu, X.; Shu, C.; Jin, R.; et al. Design of nanosheet/nanotube composites of Fe, N-doped carbon for enhanced oxygen reduction in zinc-air batteries. Electrochim. Acta. 2023, 465, 142986.
45. Meng, X.; Yuan, Y.; Feng, J.; et al. Design and synthesis of self-supporting FeCoNi- and N-doped carbon fibers/nanotubes as oxygen bifunctional catalysts for solid-state flexible Zn-air batteries. Electrochim. Acta. 2024, 479, 147648.
46. Fang, C.; Tang, X.; Yi, Q. Adding Fe/dicyandiamide to Co-MOF to greatly improve its ORR/OER bifunctional electrocatalytic activity. Appl. Catal. B. Environ. 2024, 341, 123346.
47. Zheng, H.; Zhong, J.; Liu, X.; et al. Co-modified polyoxovanadoborates derived Co/BN-CNT/VN based bifunctional electrocatalysts for rechargeable zinc-air batteries. J. Colloid. Interface. Sci. 2023, 634, 675-83.
48. Chen, X.; Chen, D.; Li, G.; et al. FeNi incorporated N doped carbon nanotubes from glucosamine hydrochloride as highly efficient bifunctional catalyst for long term rechargeable zinc-air batteries. Electrochim. Acta. 2022, 428, 140938.
49. Wang, M.; Liu, B.; Zhang, H.; Lu, Z.; Xie, J.; Cao, Y. High quality bifunctional cathode for rechargeable zinc-air batteries using N-doped carbon nanotubes constrained CoFe alloy. J. Colloid. Interface. Sci. 2024, 661, 681-9.
50. Chen, J.; Zhu, J.; Li, S.; et al. In situ construction of FeCo alloy nanoparticles embedded in nitrogen-doped bamboo-like carbon nanotubes as a bifunctional electrocatalyst for Zn-air batteries. Dalton. Trans. 2022, 51, 14498-507.
51. Lu, Z.; Xiong, Q.; Fu, R.; et al. In situ construction of N-doped hollow carbon nanotubes anchored Co nanoparticles for bifunctional ORR/OER electrocatalyst. J. Hydrog. Energy. 2024, 61, 203-9.
52. Li, M.; Chen, S.; Li, B.; et al. In situ growing N and O co-doped helical carbon nanotubes encapsulated with CoFe alloy as tri-functional electrocatalyst applied in Zn-air batteries driving water splitting. Electrochim. Acta. 2021, 388, 138587.
53. Zhang, B.; Wu, M.; Zhang, L.; et al. Isolated transition metal nanoparticles anchored on N-doped carbon nanotubes as scalable bifunctional electrocatalysts for efficient Zn-air batteries. J. Colloid. Interface. Sci. 2023, 629, 640-8.
54. Yang, L.; Luo, R.; Wen, X.; Liu, Z.; Fei, Z.; Hu, L. Nanoconfinement effects of Ni@CNT for efficient electrocatalytic oxygen reduction and evolution reaction. J. Alloys. Compd. 2022, 897, 163206.
55. Liu, X.; Fan, L.; Wang, Y.; et al. Nanofiber-based Sm0.5Sr0.5Co0.2Fe0.8O3-δ/N-MWCNT composites as an efficient bifunctional electrocatalyst towards OER/ORR. Int. J. Hydrogen. Energy. 2023, 48, 15555-65.
56. Xin, Y.; Zhang, Y.; Chen, Y.; et al. 3D coordination polymer derived CoNi@GO as a highly efficient OER/ORR bifunctional catalyst for Zn-air rechargeable batteries. J. Alloys. Compd. 2024, 971, 172735.
57. Wu, S.; Deng, D.; Zhang, E.; Li, H.; Xu, L. CoN nanoparticles anchored on ultra-thin N-doped graphene as the oxygen reduction electrocatalyst for highly stable zinc-air batteries. Carbon 2022, 196, 347-53.
58. Zhang, M.; Hu, X.; Xin, Y.; et al. FeNi coordination polymer based highly efficient and durable bifunction oxygen electrocatalyst for rechargeable zinc-air battery. Sep. Purif. Technol. 2023, 308, 122974.
59. Ha, S. J.; Hwang, J.; Kwak, M. J.; Yoon, J. C.; Jang, J. H. Graphene-encapsulated bifunctional catalysts with high activity and durability for zn-air battery. Small 2023, 19, e2300551.
60. Liu, Y.; Bao, J.; Li, Z.; et al. Large-scale defect-rich iron/nitrogen co-doped graphene-based materials as the excellent bifunctional electrocatalyst for liquid and flexible all-solid-state zinc-air batteries. J. Colloid. Interface. Sci. 2022, 607, 1201-14.
61. Etesami, M.; Khezri, R.; Abbasi, A.; et al. Ball mill-assisted synthesis of NiFeCo-NC as bifunctional oxygen electrocatalysts for rechargeable zinc-air batteries. J. Alloys. Compd. 2022, 922, 166287.
62. Du, Y.; Chen, W.; Zhong, Z.; et al. Bifunctional oxygen electrocatalysts with WN@Ni nanostructures implanted on N-doped carbon nanorods for rechargeable Zn-air batteries. J. Alloys. Compd. 2023, 960, 170789.
63. Shin, S.; Yoon, Y.; Park, S.; Shin, M. W. Fabrication of core-shell structured cobalt nanoparticle/carbon nanofiber as a bifunctional catalyst for the oxygen reduction/evolution reactions. J. Alloys. Compd. 2023, 939, 168731.
64. Zhou, Q.; Tian, Y.; Wang, M.; Lei, S.; Xiong, C. Molten salt induced formation of chitosan based carbon nanosheets decorated with CoNx for boosting rechargeable Zn-air batteries. J. Colloid. Interface. Sci. 2023, 641, 842-52.
65. Shi, Q.; Guo, H.; Ou, D.; et al. NiFe-LDH nanosheets anchored on Fe, N decorated carbon nanofibers as efficient bifunctional electrocatalysts for long-term rechargeable Zn-air batteries. J. Energy. Storage. 2023, 72, 108073.
66. Sun, L.; Huang, S.; Zhao, X.; Li, L.; Zhao, X.; Zhang, W. Synergistic effect of Co9S8 and FeS2 inlaid on N-doped carbon nanofibers toward a bifunctional catalyst for Zn-air batteries. Langmuir 2022, 38, 11753-63.
67. Dai, L.; Feng, C.; Luo, Y.; et al. CoFe alloys dispersed on Se, N Co-doped graphitic carbon as efficient bifunctional catalysts for Zn-air batteries. Chemistry 2024, 30, e202303173.
68. Wang, M.; Dong, Q.; Ji, S.; et al. “Coupling-conversion” effect induced by interface-local electric field to improve oxygen reaction kinetics in zinc-air batteries. Chem. Eng. J. 2024, 481, 148601.
69. Hu, C.; Chen, J.; Wang, Y.; Huang, Y.; Wang, S. A telluride-doped porous carbon as highly efficient bifunctional catalyst for rechargeable Zn-air batteries. Electrochim. Acta. 2022, 404, 139606.
70. Chen, K.; Kim, S.; Rajendiran, R.; et al. Enhancing ORR/OER active sites through lattice distortion of Fe-enriched FeNi3 intermetallic nanoparticles doped N-doped carbon for high-performance rechargeable Zn-air battery. J. Colloid. Interface. Sci. 2021, 582, 977-90.
71. Wang, M.; Du, X.; Zhang, M.; Su, K.; Li, Z. From S-rich polyphenylene sulfide to honeycomb-like porous carbon with ultrahigh specific surface area as bifunctional electrocatalysts for rechargeable Zn-air batteries. Carbon 2022, 198, 264-74.
72. Chen, Y.; Huang, J.; Chen, Z.; et al. Molecular engineering toward high-crystallinity yet high-surface-area porous carbon nanosheets for enhanced electrocatalytic oxygen reduction. Adv. Sci. 2022, 9, e2103477.
73. Wang, R.; Yang, H.; Lu, N.; et al. Precise identification of active sites of a high bifunctional performance 3D Co/N-C catalyst in Zinc-air batteries. Chem. Eng. J. 2022, 433, 134500.
74. Yang, T.; Chen, Y.; Liu, Y.; Liu, X.; Gao, S. Self-sacrificial template synthesis of Fe, N co-doped porous carbon as efficient oxygen reduction electrocatalysts towards Zn-air battery application. Chin. Chem. Lett. 2022, 33, 2171-7.
75. Sheng, K.; Li, J.; Li, G.; et al. Ultrafine Fe2C nanocrystals encapsulated in interconnected hollow carbon spheres as ORR electrocatalysts for alkaline/neutral Zn-air batteries. Appl. Surf. Sci. 2022, 601, 154221.
76. Ye, Q.; Li, M.; Hou, S.; Deng, Y.; Luo, J.; Tian, X. Zinc-motivated Fe/Fe5C2/Fe1-xS@Fe-N-C active sites grown on N-doped porous carbon toward efficient oxygen reduction reaction in zinc-air batteries. Dalton. Trans. 2023, 52, 2684-92.
77. Wang, B.; Liu, Q.; Yuan, A.; et al. A facile and green strategy for mass production of dispersive FeCo-rich phosphides@N,P-doped carbon electrocatalysts toward efficient and stable rechargeable Zn-air battery and water splitting. J. Mater. Sci. Technol. 2024, 182, 1-11.
78. Liang, J.; Chen, J.; Wang, G.; Liu, J.; Wang, N.; Shi, Z. Hydrogel-Derived Co3ZnC/Co nanoparticles with heterojunctions supported on N-doped porous carbon and carbon nanotubes for the highly efficient oxygen reduction reaction in Zn-air batteries. ACS. Appl. Mater. Interfaces. 2022, 14, 48789-800.
79. Pang, Y.; Mo, Z.; Wang, H.; Wang, X.; Linkov, V.; Wang, R. Manganese-assisted annealing produces abundant macropores in a carbon aerogel to enhance its oxygen reduction catalytic activity in zinc-air batteries. ACS. Sustainable. Chem. Eng. 2021, 9, 5526-35.
80. Yu, T.; Che, Y.; Fu, H.; et al. N, S dual-doped carbon aerogels-supported Co9S8 nanoparticles as efficient oxygen reduction reaction electrocatalyst for zinc-air battery. J. Alloys. Compd. 2023, 948, 169792.
81. Lin, S.; Chen, Y.; Cao, Y.; Zhang, L.; Feng, J.; Wang, A. Aminouracil-assisted synthesis of CoFe decorated bougainvillea-like N-doped carbon nanoflowers for boosting Zn-air battery and water electrolysis. J. Power. Sources. 2022, 521, 230926.
82. Huang, K.; Xu, Y.; Song, Y.; et al. NiPS3 quantum sheets modified nitrogen-doped mesoporous carbon with boosted bifunctional oxygen electrocatalytic performance. J. Mater. Sci. Technol. 2021, 65, 1-6.
83. Hong, J.; Hyun, S.; Tsipoaka, M.; Samdani, J. S.; Shanmugam, S. RuFe alloy nanoparticle-supported mesoporous carbon: efficient bifunctional catalyst for Li-O2 and Zn-air batteries. ACS. Catal. 2022, 12, 1718-31.
84. Cai, J.; Zhang, X.; Shi, Y.; Ye, Y.; Lin, S. Heterostructural Co||Cu coated with nitrogen-doped carbon as a highly efficient electrocatalyst for oxygen reduction reaction and hydrogen evolution reaction. ACS. Sustainable. Chem. Eng. 2022, 10, 5986-97.
85. Leng, X.; Ling, C.; Lu, X. J.; et al. Hierarchically hollow N-doped carbon-cobalt nanoparticle heterointerface for efficient bifunctional oxygen electrocatalysis. Dalton. Trans. 2022, 51, 15376-84.
86. Lai, C.; Liu, X.; Cao, C.; et al. Structural regulation of N-doped carbon nanocages as high-performance bifunctional electrocatalysts for rechargeable Zn-air batteries. Carbon 2021, 173, 715-23.
87. Gao, J.; Chen, S.; Xie, C.; et al. Tailoring hierarchically porous nanoarchitectured N-doped carbon decorated with FeIIN4 moiety and encapsulated Fe/Fe3C nanoparticles as a synergistic catalyst for ORR in Zn-air battery. J. Alloys. Compd. 2023, 968, 172189.
88. Guan, X.; Wu, Q.; Li, H.; et al. Ultrafine Fe2C in porous N-doped carbon by polydopamine-silane Co-deposition for efficient oxygen reduction reaction and zinc-air battery. Int. J. Hydrogen. Energy. 2023, 48, 9659-68.
89. Zhang, J.; Cui, B.; Jiang, S.; Liu, H.; Dou, M. Construction of three-dimensional cobalt sulfide/multi-heteroatom co-doped porous carbon as an efficient trifunctional electrocatalyst. Nanoscale 2022, 14, 9849-59.
90. Wang, H.; Ren, J.; Weng, C.; Lv, X.; Yuan, Z. Hierarchical porous N,S-codoped carbon with trapped Mn species for efficient pH-universal electrochemical oxygen reduction in Zn-air battery. J. Ind. Eng. Chem. 2021, 100, 92-8.
91. Huang, L.; Zuo, L.; Yu, T.; et al. Two-dimensional Co/Co9S8 nanoparticles decorated N, S dual-doped carbon composite as an efficient electrocatalyst for zinc-air battery. J. Alloys. Compd. 2022, 897, 163108.
92. He, M.; Shu, C.; Zheng, R.; et al. Interfacial interaction between molybdenum phosphide and N, P co-doped hollow carbon fibers boosting the oxygen electrode reactions in zinc-air batteries. Electrochim. Acta. 2021, 395, 139211.
93. Zhang, J.; Sun, Y.; Xiao, M.; Liu, J. Candied haws-like Fe-N-C catalysts with broadened carbon interlayer spacing for efficient zinc-air battery. ACS. Appl. Mater. Interfaces. 2023, 15, 953-62.
94. Cao, M.; Liu, Y.; Sun, K.; et al. Coupling Fe3 C nanoparticles and N-doping on wood-derived carbon to construct reversible cathode for Zn-air batteries. Small 2022, 18, e2202014.
95. Chen, X.; Liu, Q.; Bai, T.; Wang, W.; He, F.; Ye, M. Nickel and cobalt sulfide-based nanostructured materials for electrochemical energy storage devices. Chem. Eng. J. 2021, 409, 127237.
96. Yu, H.; Fan, F.; He, C.; et al. Sulfur-modulated FeNi nanoalloys as bifunctional oxygen electrode for efficient rechargeable aqueous Zn-air batteries. Sci. China. Mater. 2022, 65, 3007-16.
97. Guo, M.; Xu, M.; Qu, Y.; et al. Electronic/mass transport increased hollow porous Cu3P/MoP nanospheres with strong electronic interaction for promoting oxygen reduction in Zn-air batteries. Appl. Catal. B. Environ. 2021, 297, 120415.
98. Huo, L.; Lv, M.; Li, M.; et al. Amorphous MnO2 lamellae encapsulated covalent triazine polymer-derived multi-heteroatoms-doped carbon for ORR/OER bifunctional electrocatalysis. Adv. Mater. 2024, 36, e2312868.
99. Shi, J.; Guo, X.; Liu, S.; et al. An altered nanoemulsion assembly strategy for in-situ synthesis of Co2P/NP-C nanospheres as advanced oxygen reduction electrocatalyst for zinc-air batteries. Compos. Part. B. Eng. 2022, 231, 109589.
100. Wang, Y.; Gan, R.; Zhao, S.; et al. B, N, F tri-doped lignin-derived carbon nanofibers as an efficient metal-free bifunctional electrocatalyst for ORR and OER in rechargeable liquid/solid-state Zn-air batteries. Compos. Part. B. Eng. 2022, 598, 153891.
101. Pan, Y.; Yang, Q.; Qiu, F.; et al. Sulfur atom modulated Fe-Nx species embedded in hollow porous carbon spheres for efficient oxygen reduction and high-performance zinc-air batteries. Mater. Today. Chem. 2023, 34, 101787.
102. Xu, F.; Zhao, J.; Wang, J.; Guan, T.; Li, K. Strong coordination ability of sulfur with cobalt for facilitating scale-up synthesis of Co9S8 encapsulated S, N co-doped carbon as a trifunctional electrocatalyst for oxygen reduction reaction, oxygen and hydrogen evolution reaction. J. Colloid. Interface. Sci. 2022, 608, 2623-32.
103. Wang, G.; Gao, H.; Yan, Z.; et al. Copper nanodot-embedded nitrogen and fluorine co-doped porous carbon nanofibers as advanced electrocatalysts for rechargeable zinc-air batteries. J. Colloid. Interface. Sci. 2023, 647, 163-73.
104. Rao, P.; Liu, Y.; Su, Y.; et al. S, N co-doped carbon nanotube encased Co NPs as efficient bifunctional oxygen electrocatalysts for zinc-air batteries. Chem. Eng. J. 2021, 422, 130135.
105. Borchers, A.; Pieler, T. Programming pluripotent precursor cells derived from Xenopus embryos to generate specific tissues and organs. Genes 2010, 1, 413-26.
106. Wu, Q.; Xie, T.; Zhang, L.; et al. N,S co-doped porous carbon with Co9S8 prepared with a Co-FF-derived Co3O4 template: a bi-functional electrocatalyst for rechargeable zinc-air batteries. Dalton. Trans. 2023, 52, 14435-42.
107. Wang, S.; Wang, J.; Wang, X.; Li, L.; Qin, J.; Cao, M. Carbon hybrid with 3D nano-forest architecture in-situ catalytically constructed by CoFe alloy as advanced multifunctional electrocatalysts for Zn-air batteries-driven water splitting. J. Energy. Chem. 2021, 53, 422-32.
108. Zou, J.; Dong, H.; Wu, H.; et al. Laser-induced rapid construction of Co/N-doped honeycomb-like carbon networks as oxygen electrocatalyst used in zinc-air batteries. Carbon 2022, 200, 462-71.
109. Shen, Y.; He, S.; Zhuang, Y.; et al. Polypyrrole template-assisted synthesis of tubular Fe-NC nanostructure-based electrocatalysts for efficient oxygen reduction reaction in rechargeable zinc-air battery. ACS. Appl. Nano. Mater. 2023, 6, 16873-81.
110. Zou, Q.; Xu, F.; Ma, J.; Zhang, H.; Wang, Y. Carboxylate-assisted ZIF-derived Co nanoclusters anchoring hierarchically porous carbon as high-efficient zinc-air batteries cathode catalysts. J. Alloys. Compd. 2022, 923, 166393.
111. Qin, J.; Wang, B.; Zhang, Y.; et al. Construction of 1D/2D hierarchical carbon structure encapsulating FeCo alloys by one-step annealing leaf-like ZnFeCo-ZIF for highly-efficient bifunctional oxygen electrocatalysis in reversible zinc-air battery. J. Alloys. Compd. 2024, 982, 173710.
112. Xiong, Y.; Jiang, Z.; Gong, L.; et al. Construction of Co/FeCo@Fe(Co)3O4 heterojunction rich in oxygen vacancies derived from metal-organic frameworks using O2 plasma as a high-performance bifunctional catalyst for rechargeable zinc-air batteries. J. Colloid. Interface. Sci. 2023, 649, 36-48.
113. Chang, H.; Shi, L.; Chen, Y.; Wang, P.; Yi, T. Advanced MOF-derived carbon-based non-noble metal oxygen electrocatalyst for next-generation rechargeable Zn-air batteries. Coord. Chem. Rev. 2022, 473, 214839.
114. Xue, S.; Qin, J.; Zhang, X.; et al. In situ constructing Co/Co-Ox/Co-Nx diverse active sites on hollow porous carbon spheres derived from Co-MOF for efficient bifunctional electrocatalysis in rechargeable Zn-air. Coord. Chem. Rev. 2023, 37, 101209.
115. Zhang, S.; Yang, L.; Yang, T.; et al. Pomegranate-like structured FeNi-nanodots@FeNi LDH composite as a high performance bifunctional catalyst for oxygen electrocatalytic reactions in zinc-air batteries. Compos. Commun. 2023, 44, 101757.
116. Zheng, H.; Xu, N.; Hou, B.; et al. Bimetallic metal-organic framework-derived graphitic carbon-coated small Co/VN nanoparticles as advanced trifunctional electrocatalysts. ACS. Appl. Mater. Interfaces. 2021, 13, 2462-71.
117. Yang, C.; Shang, S.; Gu, Q.; Shang, J.; Li, X. Metal-organic framework-derived carbon nanotubes with multi-active Fe-N/Fe sites as a bifunctional electrocatalyst for zinc-air battery. J. Energy. Chem. 2022, 66, 306-13.
118. Xu, C.; Zuo, J.; Wang, J.; Chen, Z. Hierarchically structured Mo1-2C/Co-encased carbon nanotubes with multi-component synergy as bifunctional oxygen electrocatalyst for rechargeable Zn-air battery. J. Power. Sources. 2024, 595, 234063.
119. Xu, Z.; Chen, G.; Yang, F.; et al. Graphene-supported Fe/Ni single atoms and FeNi alloy nanoparticles as bifunctional oxygen electrocatalysts for rechargeable zinc-air batteries. Electrochim. Acta. 2023, 458, 142549.
120. Luo, Y.; Wen, M.; Zhou, J.; Wu, Q.; Wei, G.; Fu, Y. Highly-exposed Co-CoO derived from nanosized ZIF-67 on N-doped porous carbon foam as efficient electrocatalyst for zinc-air battery. Small 2023, 19, e2302925.
121. Hao, M.; Li, T.; Lin, L.; et al. Hollow Ti3C2Tx MXene sphere-based ZIF-67 derived central radiative cobalt-tipped carbon nanotubes electrocatalysts for ORR and OER. Colloids. Surf. A. Physicochem. Eng. Asp. 2024, 688, 133626.
122. Zhang, F.; Chen, L.; Zhang, Y.; et al. Engineering Co/CoO heterojunctions stitched in mulberry-like open-carbon nanocages via a metal-organic frameworks in-situ sacrificial strategy for performance-enhanced zinc-air batteries. Chem. Eng. J. 2022, 447, 137490.
123. Zhao, R.; Wu, L.; Chen, R.; Sun, P.; Chen, T. In-situ growth of cobalt manganate spinel nanodots on carbon black toward high-performance zinc-air battery: dual functions of 3-aminopropyltriethoxysilane. J. Colloid. Interface. Sci. 2022, 608, 386-95.
124. Mi, H.; Li, L.; Zeng, C.; et al. Cuboid-like phosphorus-doped metal-organic framework-derived CoSe2 on carbon cloth as an advanced bifunctional oxygen electrocatalyst for rechargeable zinc-air batteries. J. Colloid. Interface. Sci. 2023, 633, 424-31.
125. Shahbazi, F. F.; Rahmanifar, M. S.; Noori, A.; et al. Trilayer metal-organic frameworks as multifunctional electrocatalysts for energy conversion and storage applications. J. Am. Chem. Soc. 2022, 144, 3411-28.
126. Sun, Q.; Zhu, K.; Ji, X.; et al. MOF-derived three-dimensional ordered porous carbon nanomaterial for efficient alkaline zinc-air batteries. Sci. China. Mater. 2022, 65, 1453-62.
127. Li, S.; Zhou, Y.; Xu, C.; et al. ZIFs-derived hollow nanostructures via a strong/weak coetching strategy for long-life rechargeable Zn-air batteries. Small 2024, 20, e2309932.
128. Liu, X.; Wang, L.; Zhang, G.; et al. Zinc assisted epitaxial growth of N-doped CNTs-based zeolitic imidazole frameworks derivative for high efficient oxygen reduction reaction in Zn-air battery. Chem. Eng. J. 2021, 414, 127569.
129. Wang, L.; Xu, M.; Li, H.; et al. Mn-doped Zn metal-organic framework-derived porous N-doped carbon composite as a high-performance nonprecious electrocatalyst for oxygen reduction and aqueous/flexible zinc-air batteries. Inorg. Chem. 2023, 62, 13284-92.
130. Gao, X.; Xu, Z.; Li, G. MOF-driven ultrafine Co9S8 nanocrystals embedded in N, S-codoped multilayer-assembled carbon nanoplates for efficient bifunctional oxygen electrocatalysis. Chem. Eng. J. 2022, 431, 133385.
131. Cao, Y.; Wang, M.; Wang, H.; Han, C.; Pan, F.; Sun, J. Covalent organic framework for rechargeable batteries: mechanisms and properties of ionic conduction. Adv. Energy. Mater. 2022, 12, 2200057.
132. Wu, Z.; Feng, L.; Lu, Z.; et al. Covalent organic frameworks/carbon nanotubes composite with cobalt(II) pyrimidine sites for bifunctional oxygen electrocatalysis. Nano. Mater. Sci. 2024, 6, 419-27.
133. Hu, S.; Zhu, M. Recent advances in carbon-based non-noble single-atom catalysts for rechargeable zinc-air batteries. Curr. Opin. Chem. Eng. 2023, 41, 100926.
134. Xie, S.; Jin, H.; Wang, C.; et al. A comparison study on single metal atoms (Fe, Co, Ni) within nitrogen-doped graphene for oxygen electrocatalysis and rechargeable Zn-air batteries. Chin. Chem. Lett. 2023, 34, 107681.
135. Wu, H.; Xu, X.; Wu, J.; et al. Atomic engineering modulates oxygen reduction of hollow carbon matrix confined single metal-nitrogen sites for zinc-air batteries. Small 2023, 19, e2301327.
136. Sun, J.; Leng, P.; Xie, Y.; et al. Co single atoms and Co nanoparticle relay electrocatalyst for rechargeable zinc air batteries. Appl. Catal. B. Environ. 2022, 319, 121905.
137. Najam, T.; Shah, S. S. A.; Ibraheem, S.; et al. Single-atom catalysis for zinc-air/O2 batteries, water electrolyzers and fuel cells applications. Energy. Storage. Mater. 2022, 45, 504-40.
138. Wang, Y.; Hu, F.; Mi, Y.; Yan, C.; Zhao, S. Single-metal-atom catalysts: an emerging platform for electrocatalytic oxygen reduction. Chem. Eng. J. 2021, 406, 127135.
139. Jiao, C.; Xu, Z.; Shao, J.; et al. High-density atomic Fe-N4/C in tubular, biomass-derived, nitrogen-rich porous carbon as air-electrodes for flexible Zn-air batteries. Adv. Funct. Mater. 2023, 33, 2213897.
140. Zhang, W.; Fan, K.; Chuang, C.; et al. Molten salt assisted fabrication of Fe@FeSA-N-C oxygen electrocatalyst for high performance Zn-air battery. J. Energy. Chem. 2021, 61, 612-21.
141. Zhu, S.; Wu, T.; Liao, M.; Meng, J.; Xie, Y.; Lu, C. Regulating the coordination environment of atomically dispersed Fe-N4 moieties in carbon enables efficient oxygen reduction for Zn-air batteries. Chem. Eng. J. 2024, 484, 149693.
142. Li, G.; Liu, J.; Xu, C.; et al. Regulating the Fe-spin state by Fe/Fe3C neighbored single Fe-N4 sites in defective carbon promotes the oxygen reduction activity. Energy. Storage. Mater. 2023, 56, 394-402.
143. Liu, X.; Wu, J.; Luo, Z.; et al. Co2P-assisted atomic Co-N4 active sites with a tailored electronic structure enabling efficient ORR/OER for rechargeable Zn-air batteries. ACS. Appl. Mater. Interfaces. 2023, Online ahead of print.
144. Li, C.; Yuan, M.; Liu, Y.; et al. Graphite-N modified single Fe atom sites embedded in hollow leaf-like nanosheets as air electrodes for liquid and flexible solid-state Zn-air batteries. Chem. Eng. J. 2023, 477, 146988.
145. Zhao, Y.; Wu, H.; Wang, Y.; et al. Sulfur coordination engineering of molybdenum single-atom for dual-functional oxygen reduction/evolution catalysis. Energy. Storage. Mater. 2022, 50, 186-95.
146. Zhang, S.; Yang, W.; Liang, Y.; Yang, X.; Cao, M.; Cao, R. Template-free synthesis of non-noble metal single-atom electrocatalyst with N-doped holey carbon matrix for highly efficient oxygen reduction reaction in zinc-air batteries. Appl. Catal. B. Environ. 2021, 285, 119780.
147. Pan, L.; Chen, D.; Pei, P.; Huang, S.; Ren, P.; Song, X. A novel structural design of air cathodes expanding three-phase reaction interfaces for zinc-air batteries. Appl. Energy. 2021, 290, 116777.
148. Villanueva-martínez, N.; Alegre, C.; Martínez-visús, I.; Lázaro, M. Bifunctional oxygen electrocatalysts based on non-critical raw materials: carbon nanostructures and iron-doped manganese oxide nanowires. Catal. Today. 2023, 420, 114083.
149. Wu, J.; Shen, X.; Wang, H.; et al. Electronic structure modification of FeWO4 through F doping for enhanced oxygen reduction performance in zinc-air batteries. Mater. Today. Phys. 2023, 38, 101274.
150. Tang, W.; Teng, K.; Guo, W.; et al. Defect-engineered Co3O4 @nitrogen-deficient graphitic carbon nitride as an efficient bifunctional electrocatalyst for high-performance metal-air batteries. Small 2022, 18, e2202194.
151. Lei, Y.; Xiang, Y.; Xu, C.; et al. Pre-implanting metal oxides to endow the N-doped carbon with boosted bifunctional catalytic activities towards oxygen reduction and oxygen evolution reactions. J. Alloys. Compd. 2024, 980, 173590.
152. Anand, P.; Wong, M.; Fu, Y. Perovskite oxide composites for bifunctional oxygen electrocatalytic activity and zinc-air battery application- a mini-review. Energy. Stor. Mater. 2023, 58, 362-80.
153. Wang, B.; Zhao, P.; Feng, J.; et al. Carbon-based 0D/1D/2D assembly with desired structures and defect states as non-metal bifunctional electrocatalyst for zinc-air battery. J. Colloid. Interface. Sci. 2021, 588, 184-95.
154. Zhang, W.; Pu, W.; Qu, Y.; Yang, H.; Liu, Y. Facile synthesis of ultrathin S-N co-doped carbon nanosheet as ORR electrocatalysts for application in sustainable zinc-air battery. Electrochim. Acta. 2023, 462, 142800.
155. Wang, X.; Raghupathy, R. K. M.; Querebillo, C. J.; et al. Interfacial covalent bonds regulated electron-deficient 2D black phosphorus for electrocatalytic oxygen reactions. Adv. Mater. 2021, 33, e2008752.
156. Niu, Y.; Teng, X.; Gong, S.; Liu, X.; Xu, M.; Chen, Z. Boosting oxygen electrocatalysis for flexible zinc-air batteries by interfacing iron group metals and manganese oxide in porous carbon nanowires. Energy. Stor. Mater. 2021, 43, 42-52.
157. Chen, T.; Wu, J.; Zhu, C.; et al. Rational design of iron single atom anchored on nitrogen doped carbon as a high-performance electrocatalyst for all-solid-state flexible zinc-air batteries. Chem. Eng. J. 2021, 405, 125956.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Copyright
Data & Comments
Data





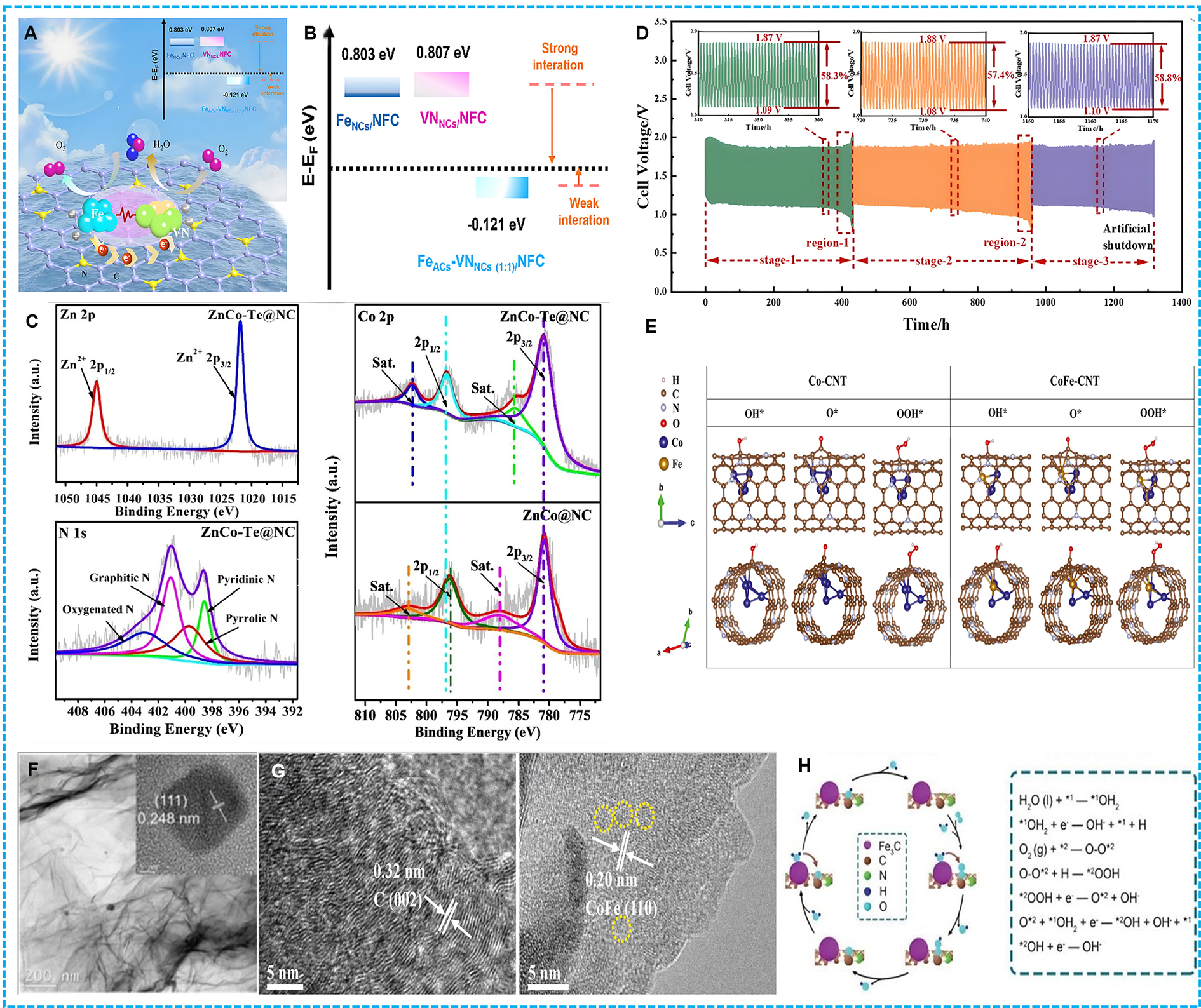
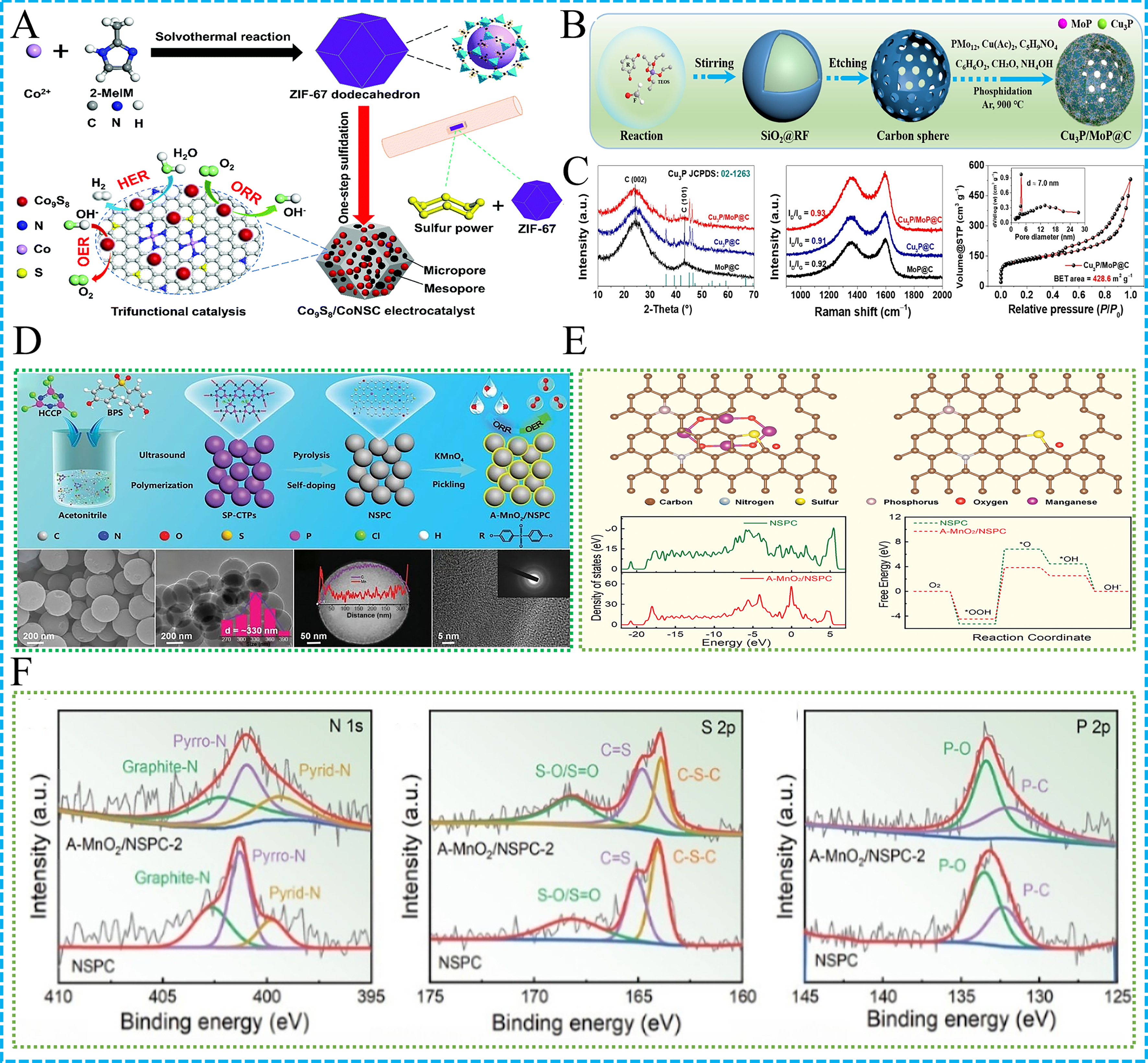
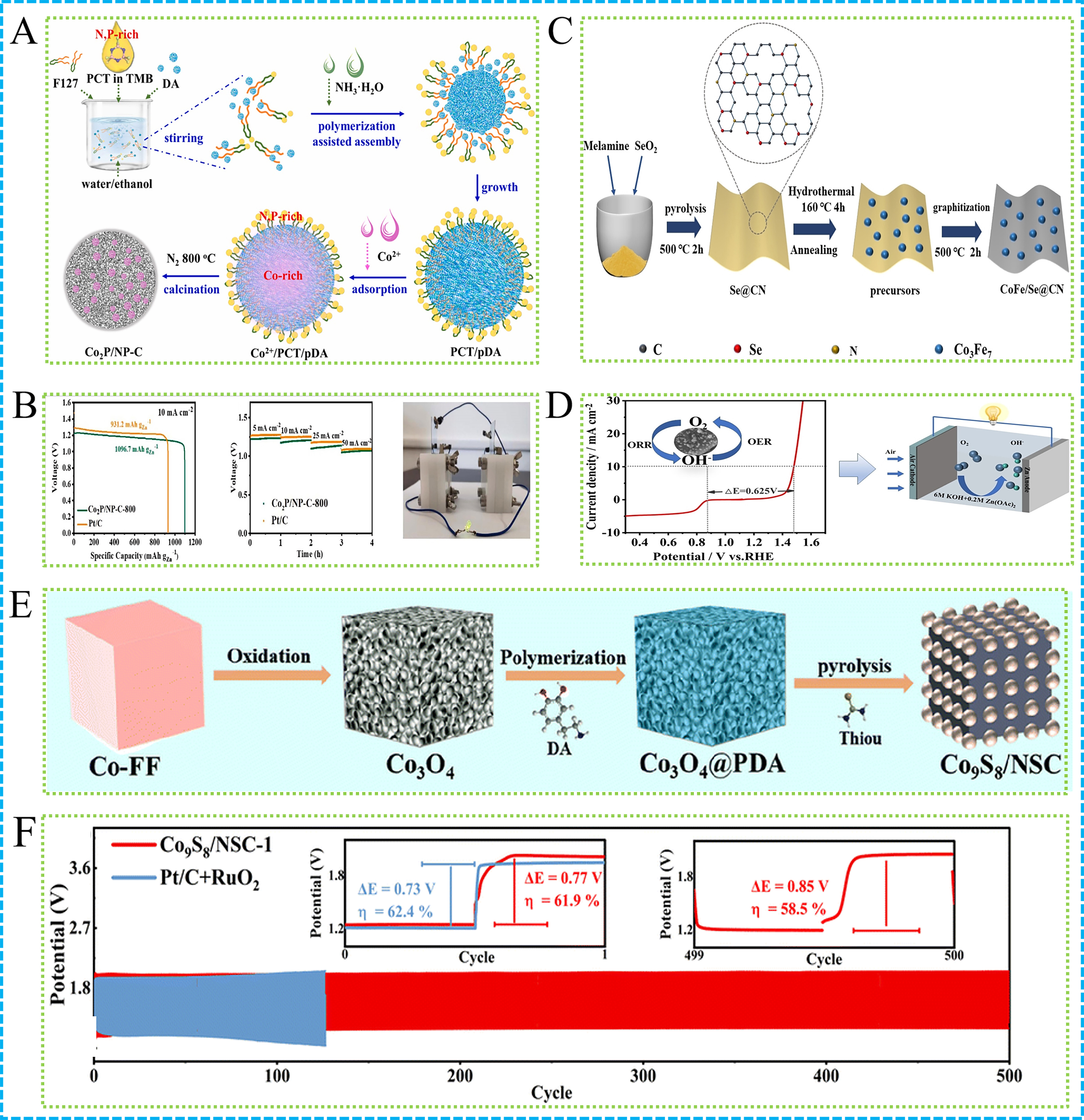
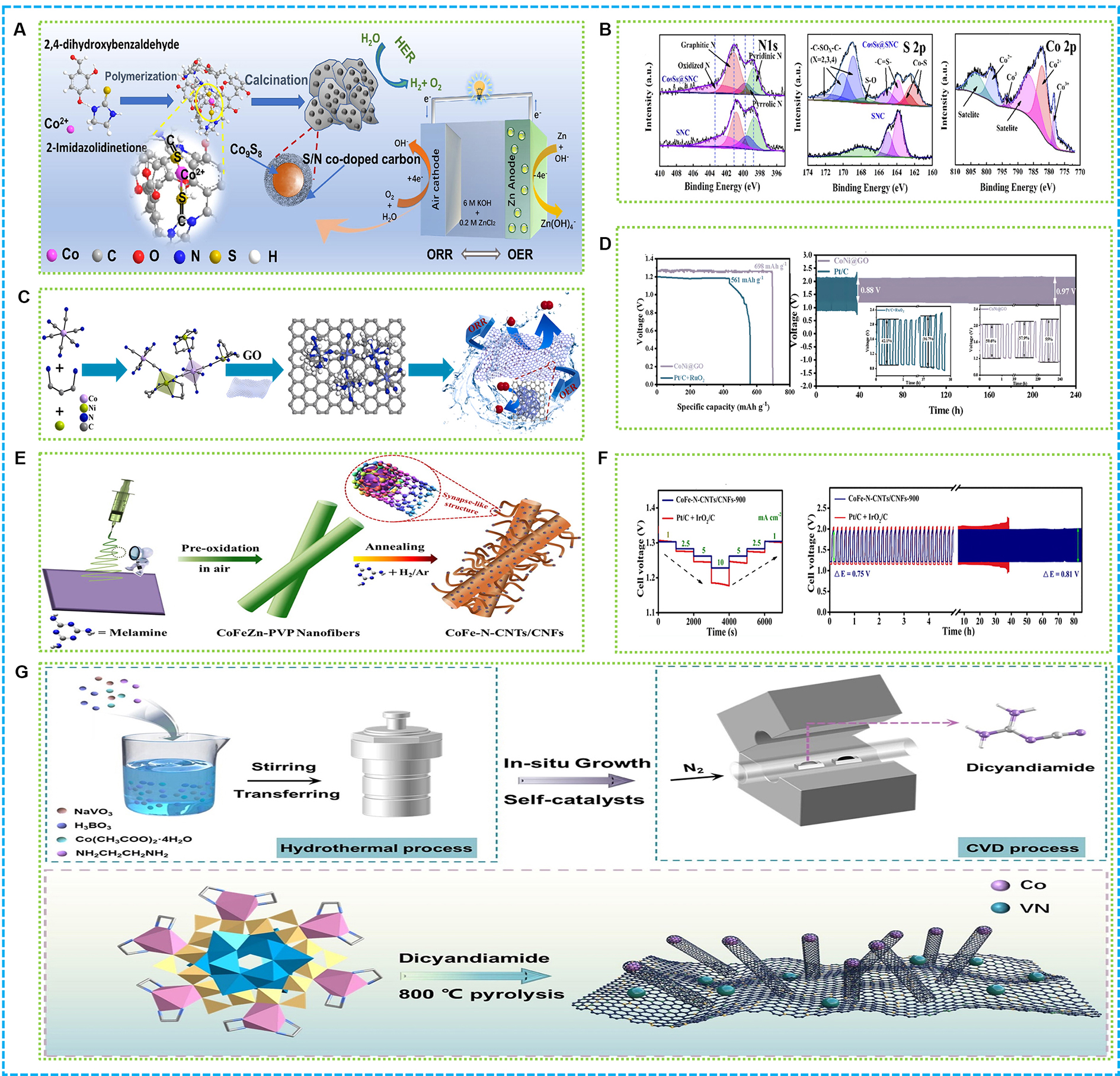
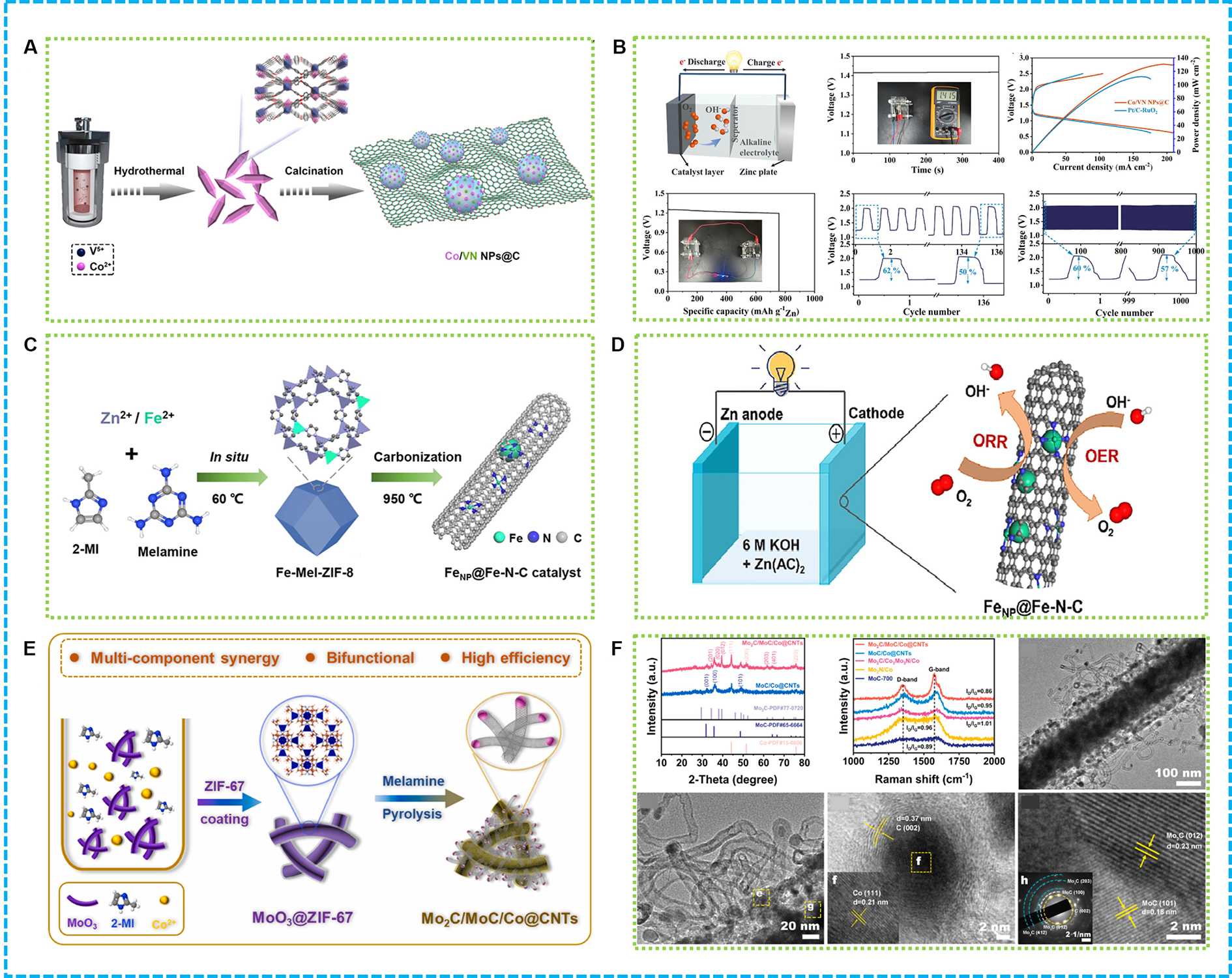
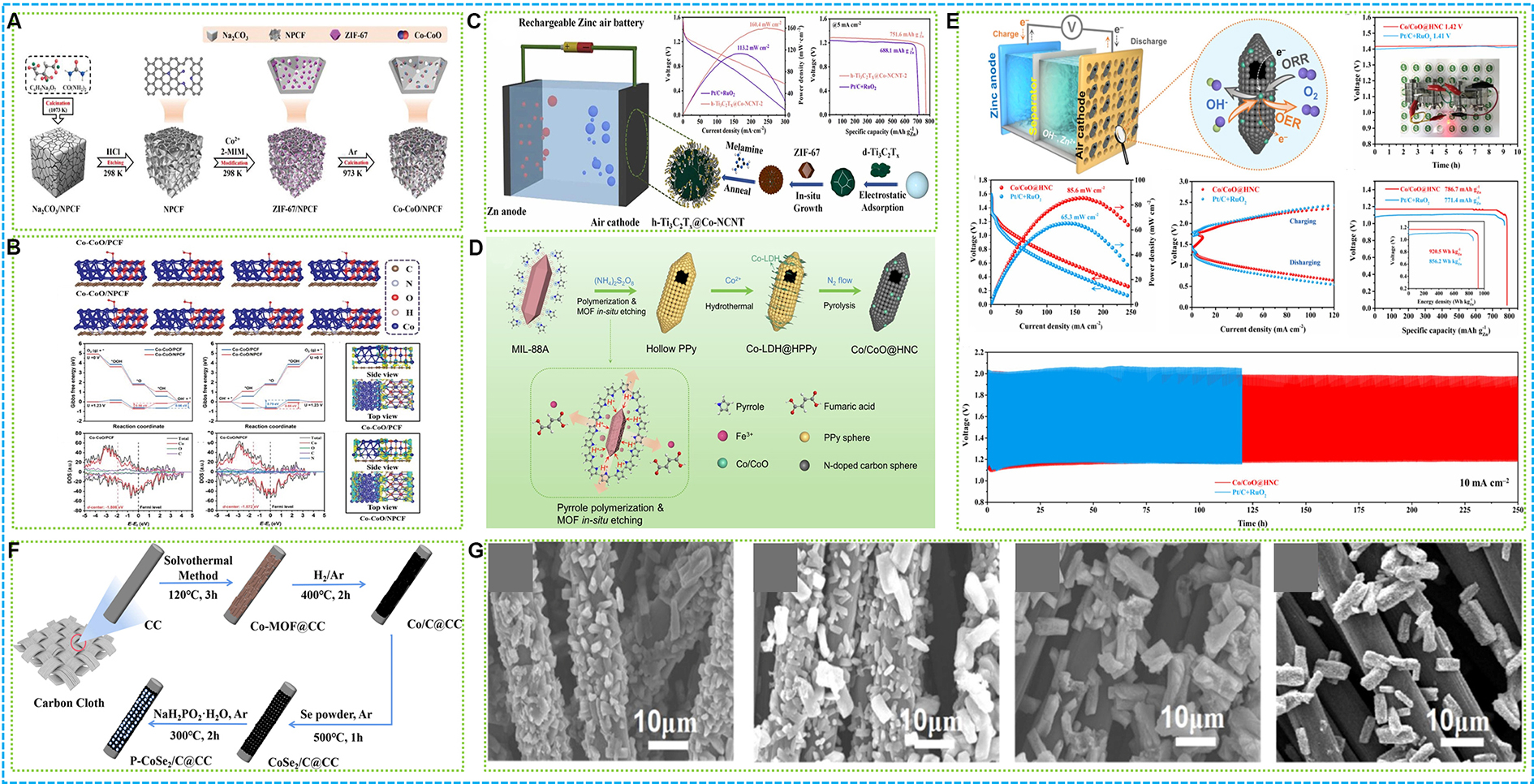
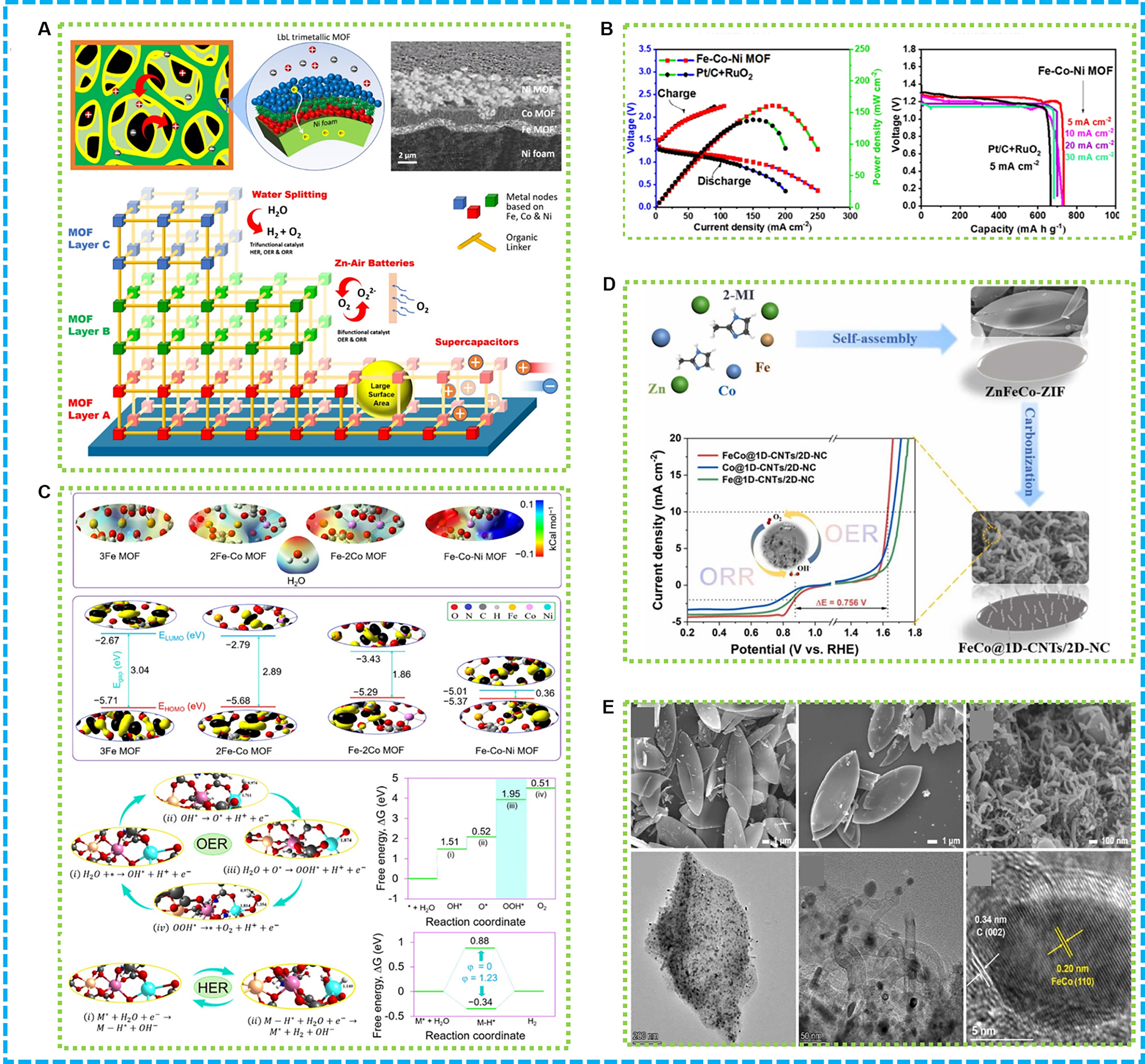
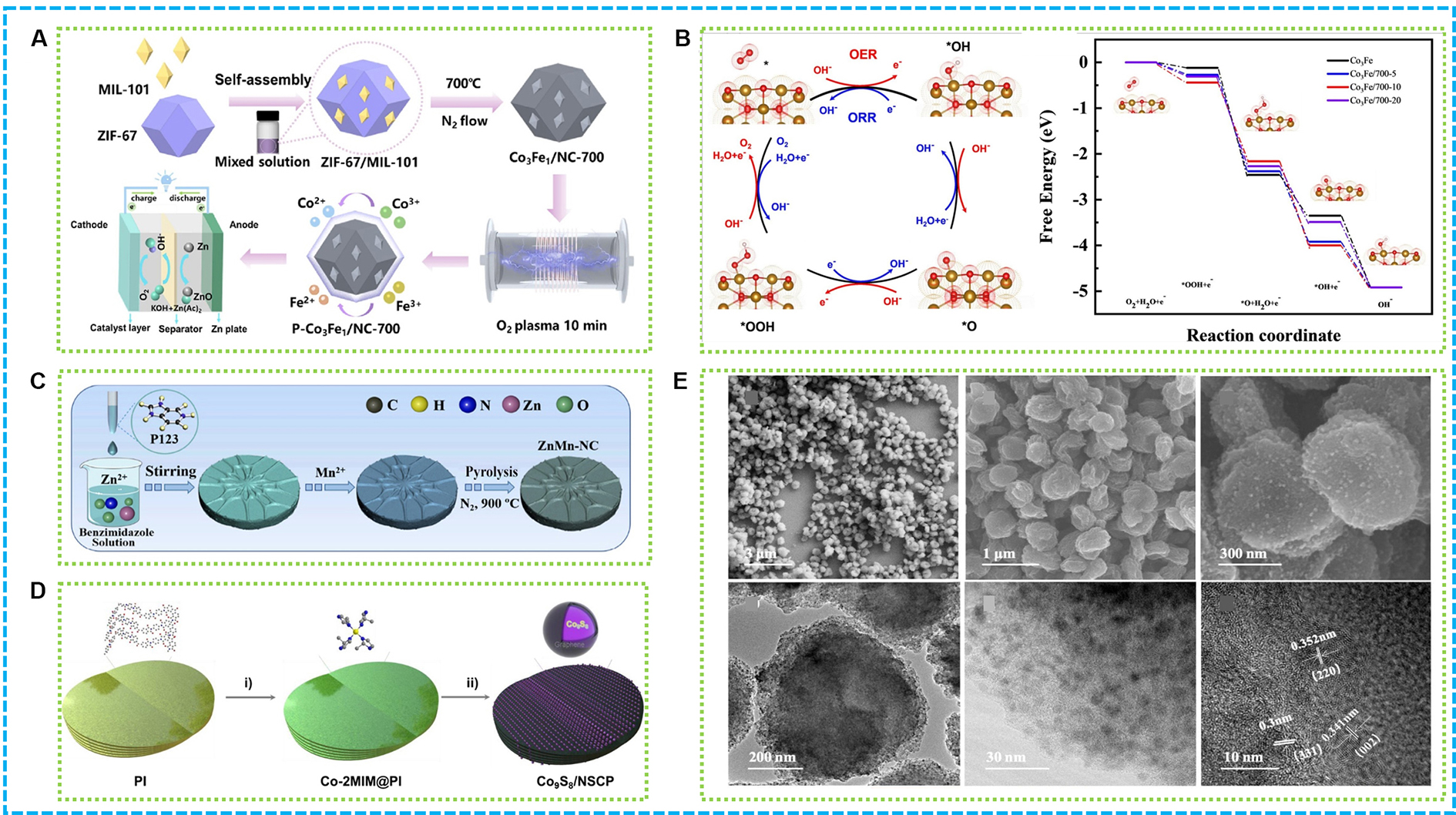
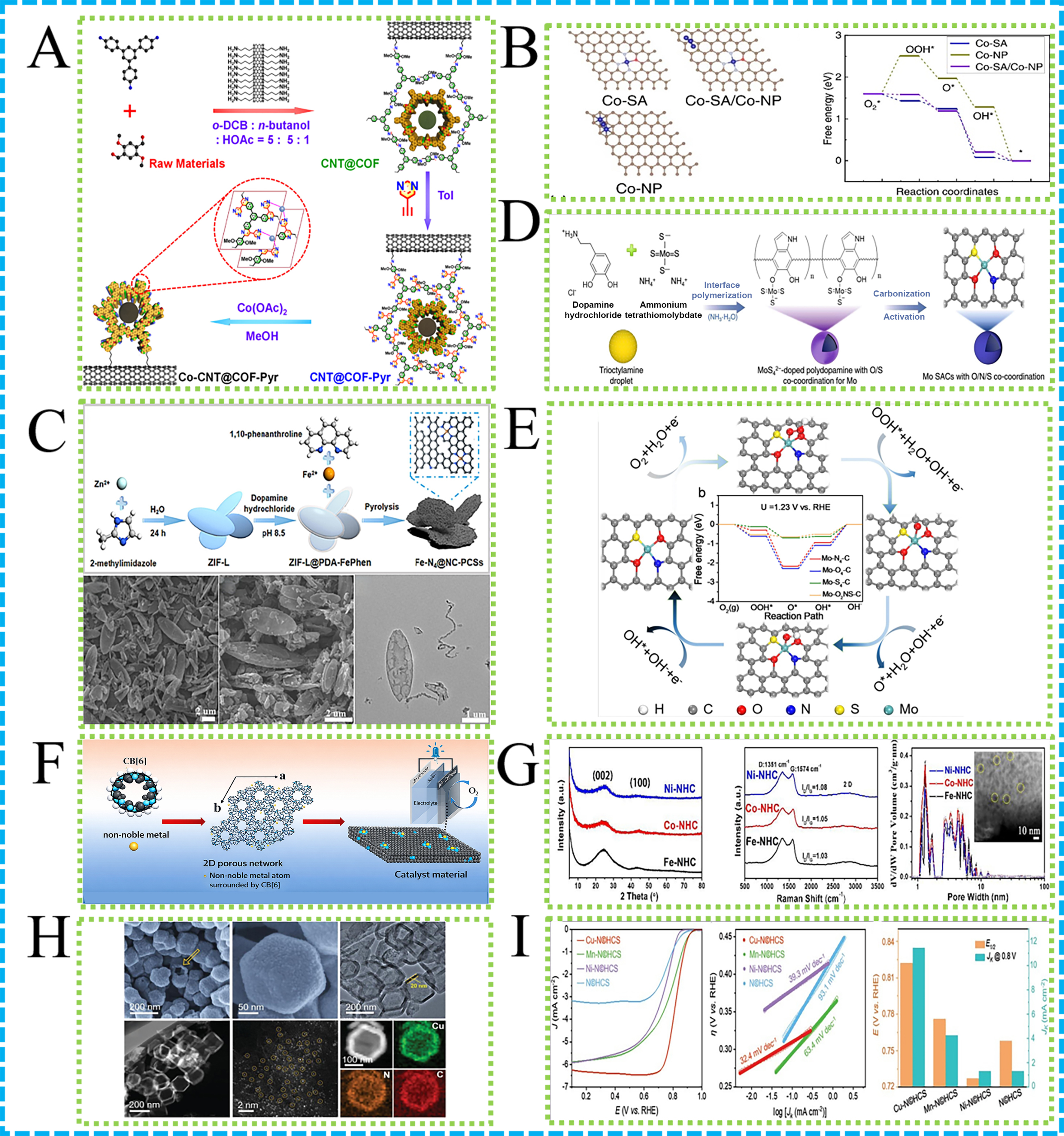
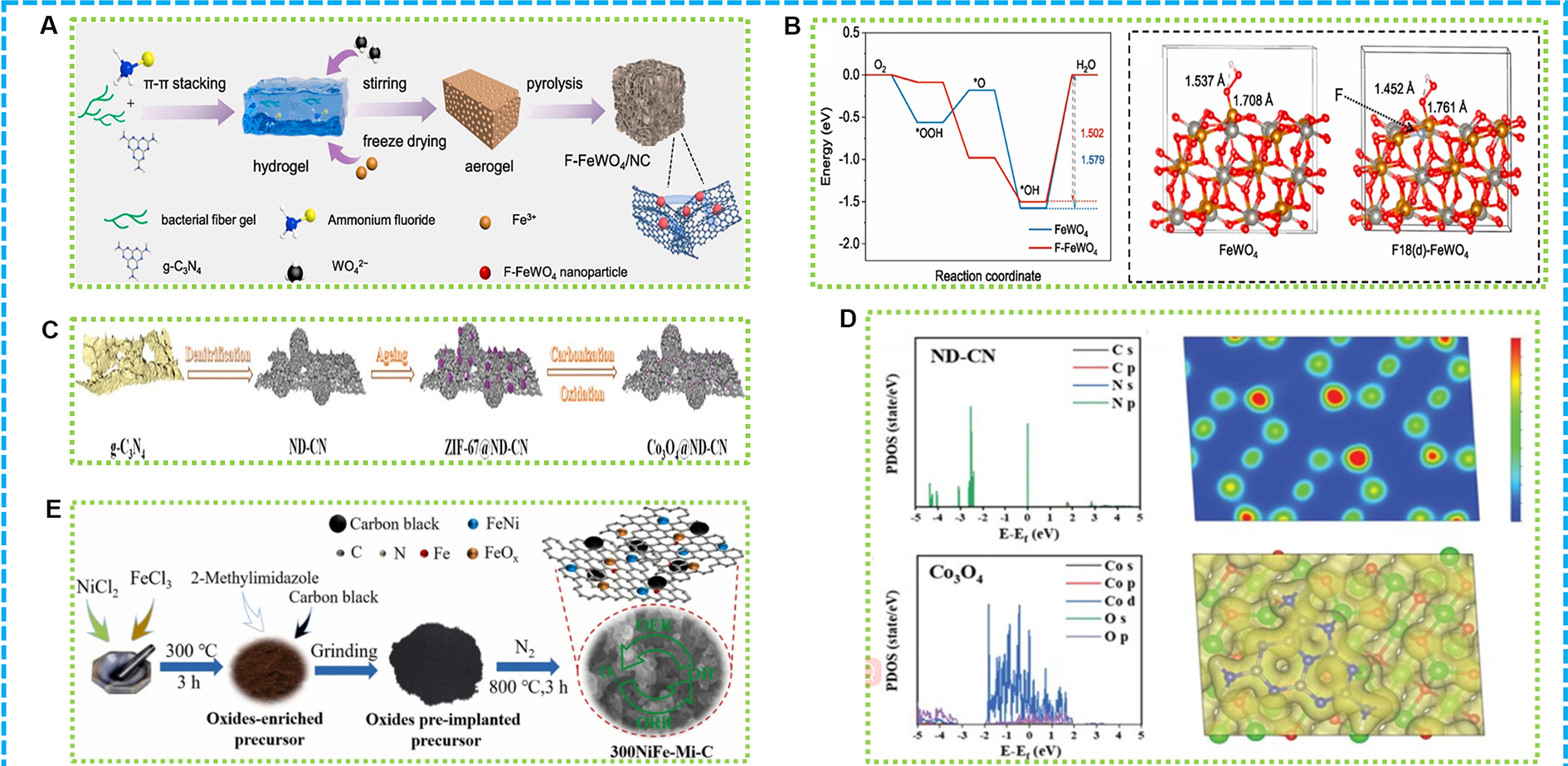
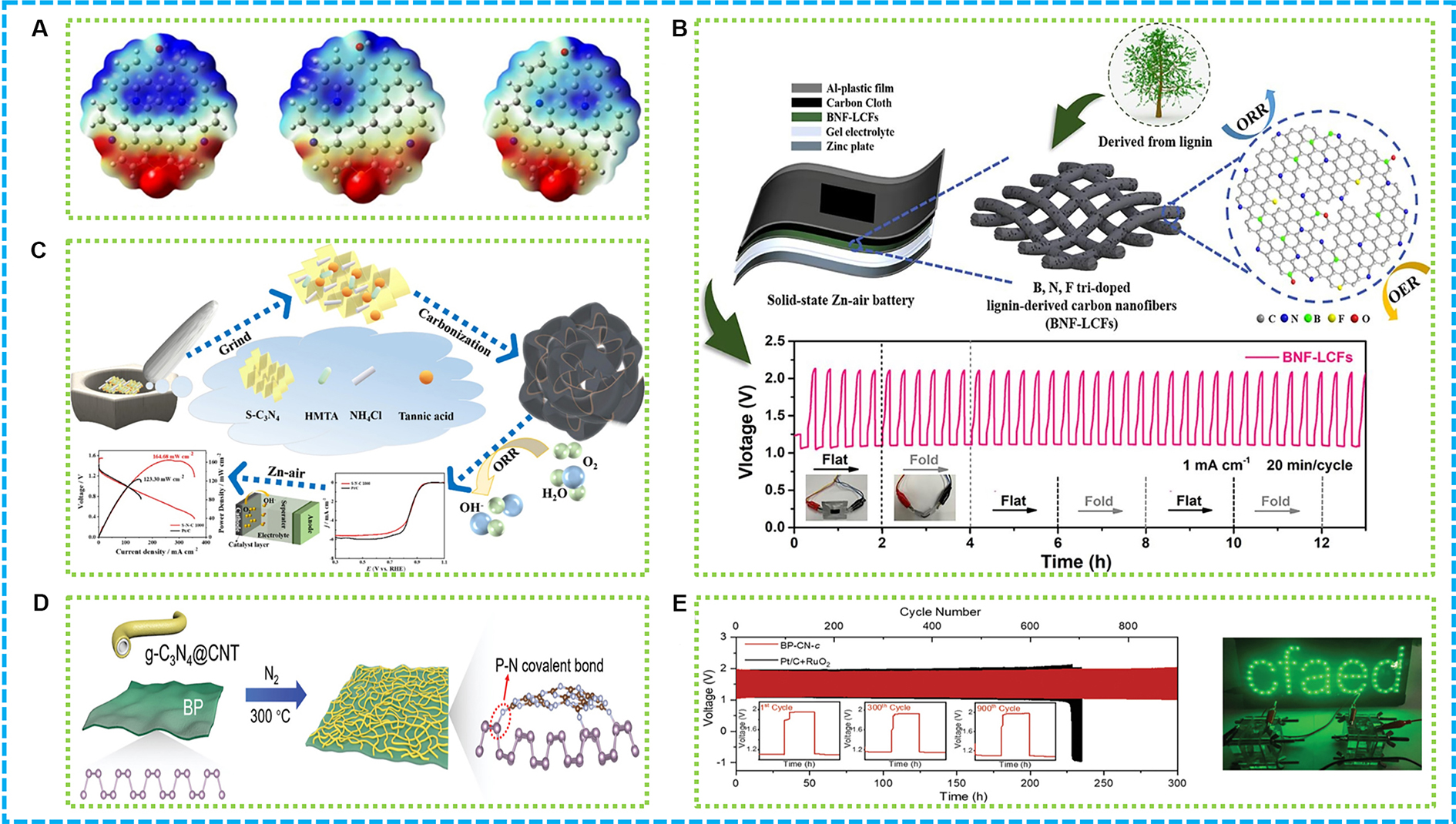
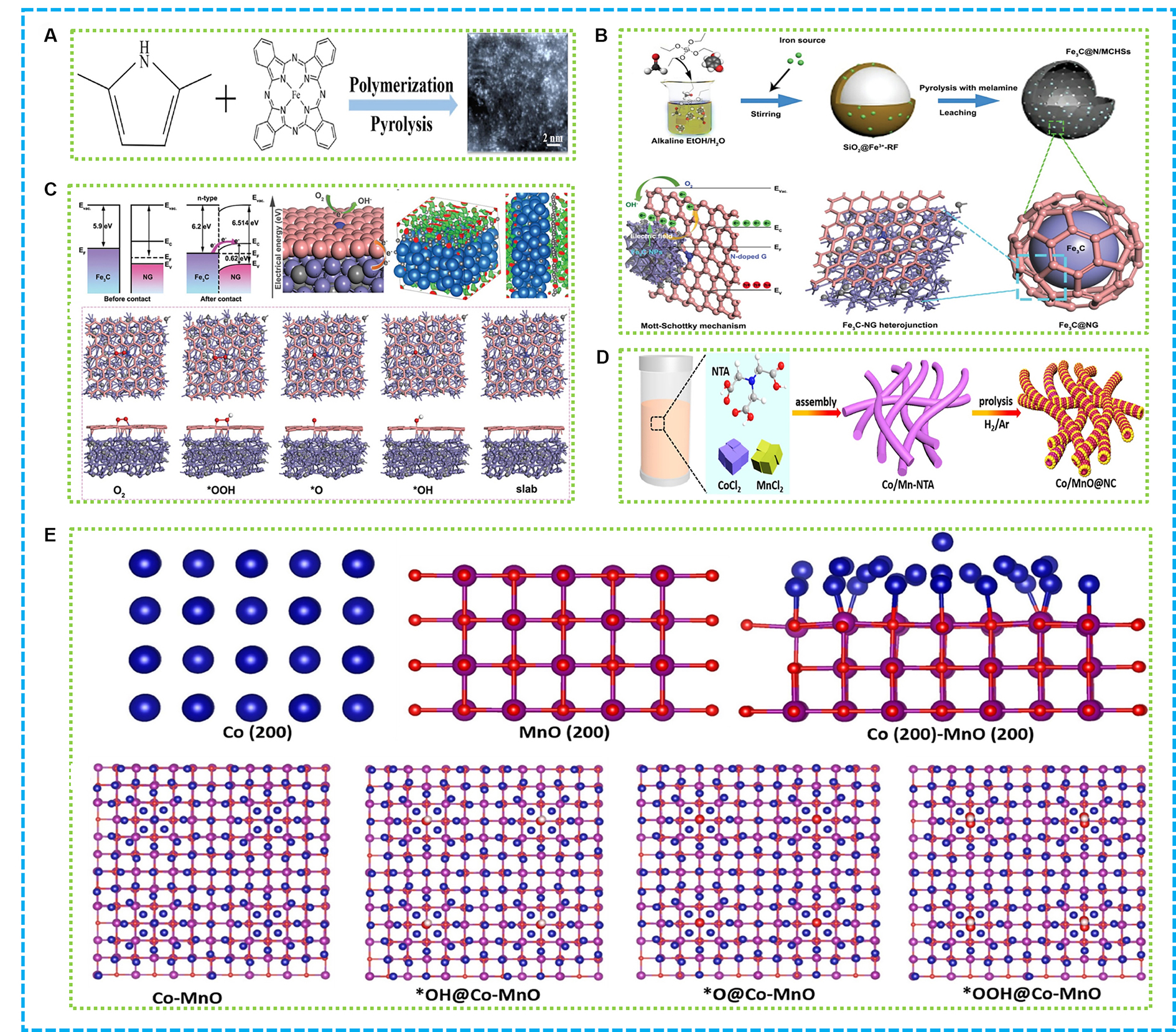













Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].