Selective electroreduction of CO2 to C2+ products on cobalt decorated copper catalysts
Abstract
Cu-catalyzed electrochemical CO2 reduction reaction (CO2RR) to multi-carbon (C2+) products is often plagued by low selectivity because the adsorption energies of different reaction intermediates are in a linear scaling relationship. Development of Cu-based bimetallic catalysts has been considered as an attractive strategy to address this issue; however, conventional bimetallic catalysts often avoid metals with strong CO adsorption energies to prevent surface poisoning. Herein, we demonstrated that limiting the amount of Co in CuCo bimetallic catalysts can enhance C2+ product selectivity. Specifically, we synthesized a series of CuCox catalysts with trace amounts of Co (0.07-1.8 at%) decorated on the surface of Cu nanowires using a simple dip coating method. Our results revealed a volcano-shaped correlation between Co loading and C2+ selectivity, with the CuCo0.4% catalyst exhibiting a 2-fold increase in C2+ selectivity compared to the Cu nanowire sample. In situ Raman and Infrared spectroscopies suggested that an optimal amount of Co could stabilize the Cu oxide/hydroxide species under the CO2RR condition and promote the adsorption of CO, thus enhancing the C2+ selectivity. This work expands the potential for developing Cu-based bimetallic catalysts for CO2RR.
Keywords
INTRODUCTION
Electrochemical reduction of CO2 (CO2RR) under ambient conditions is a promising approach to mitigate the increasing concentration of CO2 in the atmosphere and produce high value-added products.[1] Cu-based materials have been extensively studied as catalysts for CO2RR, as Cu is reported to be the only metal that can reduce CO2 to various multi-carbon (C2+) products, including gaseous hydrocarbons (e.g., C2H4 and
To date, various strategies have been applied to improve the selectivity of C2+ over Cu-based catalysts, including tuning the exposed facets[10], adjusting the oxidation state[11], introducing defects[12], and engineering the electrode structure[13] and hydrophobicity[14]. Among them, introducing a second metal to Cu has attracted great attention[6,15]. For instance, recent studies of Cu-Au,[16] Cu-Ag,[7] and Cu-Zn[17] catalysts have shown enhanced selectivity towards C2+ products. Notably, in these cases, the second metals (i.e., Au, Ag and Zn) are intrinsically selective for electrochemically reducing CO2 to CO due to their weak binding strength with CO. Therefore, these metals can serve as a CO reservoir to enhance the *CO coverage on Cu surface, thus increasing the C–C coupling probability[1]. In contrast, many other metals, such as Fe, Ni, Co, etc., are intrinsically not selective for CO2RR because CO can strongly adsorb on these metals, leading to the poisoning of the catalyst surface and the high H2 selectivity. Thus, most researchers have tried to remove these metals during the sample and electrolyte preparation[18]. Interestingly, recent attempts have shown that Ni-Cu and Co-Cu alloys can also be active for CO2RR. However, the selectivity of these catalysts varies for products such as CO, HCOOH, and C2H4, and the enhanced catalytic activity is believed to result from adjustments in the d-band structure, size effects, and/or additional binding sites[19-21]. Thus, further investigations are necessary to improve the design of such bimetallic catalysts. Building upon prior findings, we hypothesize that by strategically incorporating Group VIII metals (e.g., Co) onto the Cu surface and precisely adjusting their atomic ratios, it is possible to optimize the surface state of Cu and enhance the adsorption strength of *CO, ultimately improving selectivity for C2+ products.
Therefore, this work successfully designed and synthesized Cu nanowires (NWs) decorated with trace amounts of Co (CuCox, x = 0.07%, 0.4%, and 1.8%) as advanced electrocatalysts for CO2RR. A simple dip coating method developed in our previous studies was applied to ensure that Co was deposited on the surface of Cu and avoid contamination by surfactants[22]. We found that with an optimal loading of Co (i.e., CuCo0.4%), the C2+ selectivity was doubled than that of pure Cu, reaching > 40% faradaic efficiency (FE) at
EXPERIMENTAL
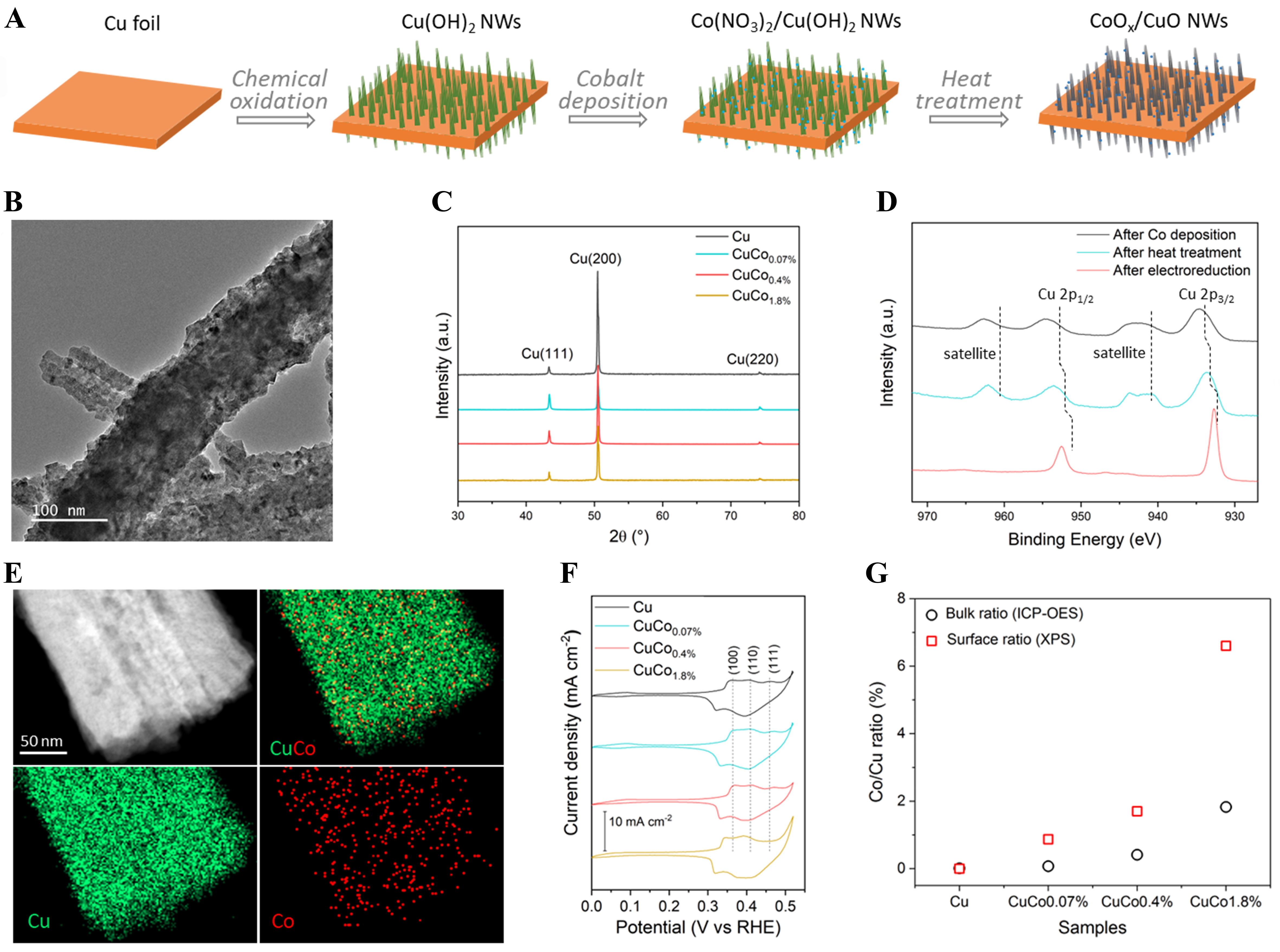
CuCox (x = 0.07%, 0.4%, and 1.8%) catalysts were prepared using a simple dip coating method (Experimental details in Supplementary Materials). Briefly, a piece of Cu foil was first immersed in a mixed solution of NaOH and (NH4)2S2O4 for 10 min to chemically oxidize its surface layers to grow Cu(OH)2 NWs. This sample is named Cu NWs in the remainder of the paper to simplify the notation. The CuCox samples were produced via immersing these Cu NWs in aqueous Co(NO3)2 solution for 30 s to adsorb cobalt species, followed by heat treatment at 150 °C and electrochemical reduction under CO2RR condition (i.e., -0.8 V vs. RHE in 0.1 M KHCO3 for 20 min) [Figure 1A][22]. The atomic ratio of Co:Cu of the CuCox samples was tuned by changing the concentration of the Co(NO3)2 solution from 2 to 50 mM.
Figure 1. (A) Schematic illustration for synthesizing CoOx/CuO NWs; samples after electroreduction during CO2RR are denoted as CuCox catalysts; (B) Representative TEM image of the CuCo0.4% sample; (C) XRD of Cu and CuCox catalysts; (D) Cu 2p XPS spectra of CuCo0.4% after Co deposition, heat treatment, and electroreduction; (E) Element maps the CuCo0.4% sample; (F) Cyclic voltammograms recorded in N2 purged 1.0 M KOH capturing the surface-specific adsorption of oxygen (OH); (G) Bulk and surface atomic ratios of Co:Cu for Cu and CuCox catalysts obtained using ICP-OES and XPS, respectively. NWs: Nanowires; CO2RR: CO2 reduction reaction; TEM: transmission electron microscopy; XRD: X-ray diffraction patterns; XPS: X-ray photoelectron spectroscopy; ICP-OES: inductively coupled plasma optical emission spectrometry.
RESULTS AND DISCUSSION
The morphology of the Cu NWs and CuCox samples was studied using a scanning electron microscope (SEM) [Supplementary Figure 1]. Well-defined NW structure with an average length of ~10 μm and width of ~200 nm is observed [Figure 1B]. Notably, the NW morphology of the Cu sample did not change after Co deposition. This can be ascribed to the low concentration of the Co(NO3)2 solution and the short dipping time [Supplementary Figure 1]. The X-ray diffraction patterns (XRD) of CuCox samples were the same as those of the Cu NWs sample, with high-intensity diffraction peaks from metallic Cu [Figure 1C]. This indicates metallic Cu dominates the composition in the Cu NWs and CuCox samples. Characteristic diffraction peaks of Co-related crystal phases were not observed. This can be attributed to the low loading of Co as well.
We further examined the surface compositions of the Cu NWs and CuCox samples using X-ray photoelectron spectroscopy (XPS) analysis after each sample preparation step. In the case of the Cu NW sample, Cu stayed in Cu(OH)2 form (not shown). As shown in Figure 1D, Cu remained as Cu(OH)2 with Cu 2p peaks at 934.7 and 954.6 eV after Co deposition. It turned to the form of CuO after the heat treatment due to dehydration. After pre-electrochemical reduction treatment, the Cu 2p peak shifted to a lower binding energy and the satellite peaks disappeared, indicating that CuO was reduced to Cu0/Cu1+ species[23,24]. Importantly, Co 2p peaks were found in the XPS, although with low intensities [Supplementary Figure 2], demonstrating the successful decoration of Co on Cu NWs.
To gain detailed information of the microstructure and the elemental distribution, high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) and energy dispersive X-ray spectroscopy (EDX) measurements were performed. As shown in Figure 1E, the STEM-coupled EDX mapping further corroborates the successful deposition of Co on Cu NWs. Notably, Co species are highly dispersed, as observed from the high-resolution transmission electron microscopy (TEM) images and the EDX mapping. Selected area electron diffraction (SAED) analysis was then conducted to investigate the crystal structure of the NWs [Supplementary Figure 3]. Only metallic Cu diffraction rings were observed. This proved that the NWs are metallic Cu-dominated, although the surfaces were oxidized. The SAED analysis and the XRD results demonstrate that the Cu species mainly consists of crystalline Cu both in the NWs and the Cu foils of the Cu NWs and CuCox samples. In addition, all studied samples showed the same diffraction ring, suggesting that the bulk crystal structure did not change noticeably from the Cu NWs to CuCox samples with varying amounts of Co deposited. No Co diffractions were observed in SAED, consistent with the XRD results, further implying that no long-range ordered cobalt species were present. This can be due to the low loading and high dispersion of Co.
Next, we performed electrochemical OH adsorption to study the influence of Co on the surface structure of the CuCox samples. In cyclic voltammograms (CVs), OH adsorption peaks exhibited distinctly at various potentials on distinct facets of Cu crystals, thus allowing the probing of surface structure of Cu[25,26]. As shown in Figure 1F, three OH desorption peaks assigned to Cu (111), (110), and (100) facets were observed for the Cu NWs and CuCox samples. These peaks are almost identical for the Cu, CuCo0.07%, and CuCo0.4% samples. However, the peak positions were shifted to ~0.02 V lower for the CuCo1.8% sample, indicating a weaker binding of OH on this sample surface. Intriguingly, the (111) peak for CuCo1.8% almost diminished, indicating that the surface structure of Cu was only affected by a high loading of Co. To conclude, using a simple dip coating method, a series of CuCox catalysts with highly dispersed Co decorated on the surface of Cu NWs were successfully synthesized. In addition, the Co:Cu ratio in the catalyst was quantitatively analyzed by XPS and inductively coupled plasma optical emission spectrometry (ICP-OES). Quantitative determination of the surface Co:Cu ratio from the XPS analysis showed a fast increase from CuCo0.07% to CuCo1.8%. Interestingly, when compared with the bulk Co:Cu ratio measured with the ICP-OES, the Co concentration on the surface is much higher than that in the bulk of the CuCox samples [Figure 1G]. This is similar to our previous findings, which show that the dip coating method allows the deposition of metal species to concentrate on the surface of the NWs[22].
The CO2RR activity and product distribution from Cu NWs and CuCox samples were evaluated in
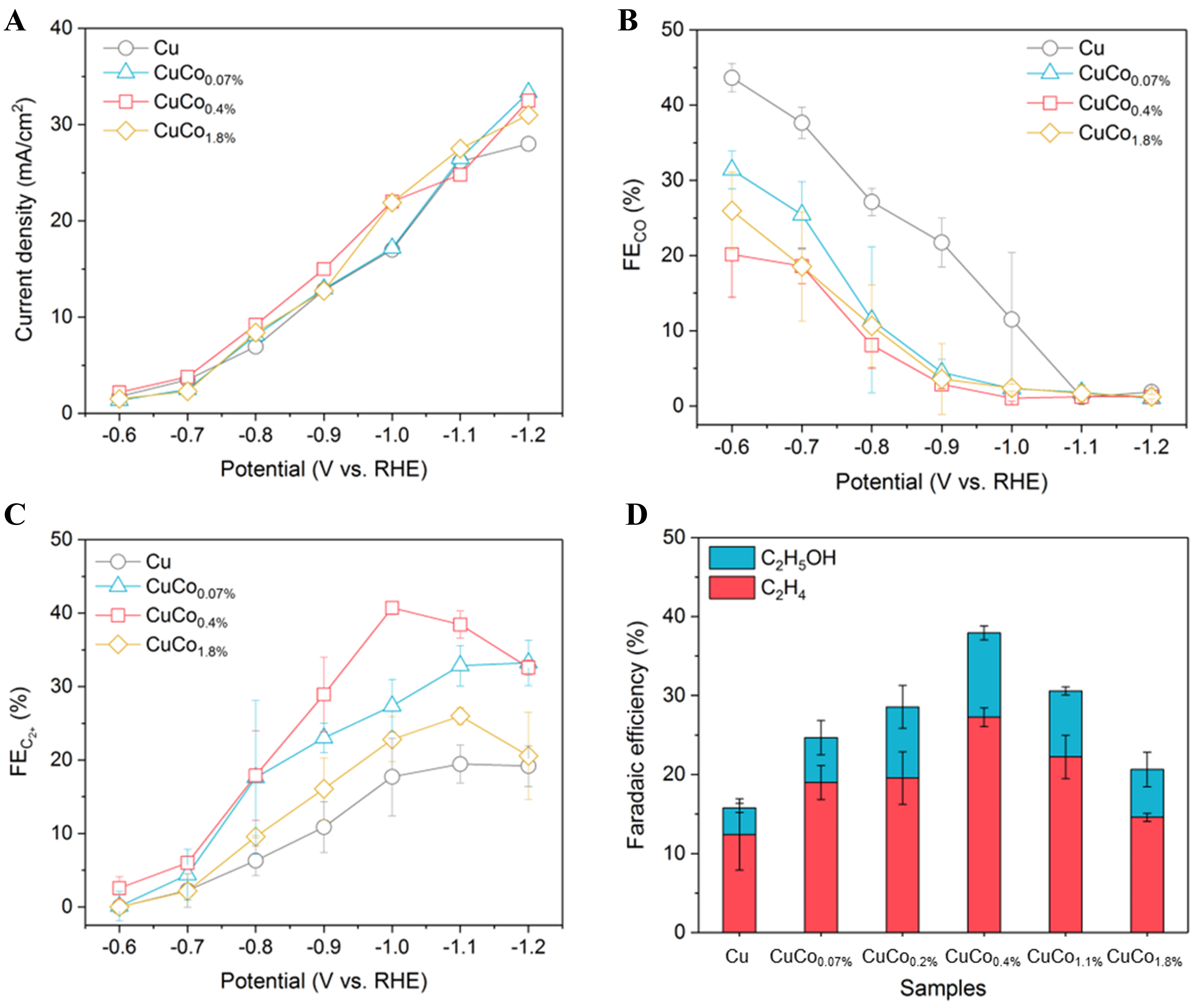
Figure 2. (A) Current density, (B) Faradaic efficiency of CO, and (C) Faradaic efficiency of C2+ for Cu, CuCo0.07%, CuCo0.4% and CuCo1.8%; (D) Faradaic efficiency of C2H4 and C2H5OH for CuCo samples with different amounts of Co. Error bars are means ± SD (n = 3 replicates). C2+: Multi-carbon.
To assess the influence of Co on the catalytic selectivity, gas and liquid products were analyzed using gas chromatography (GC) and 1H nuclear magnetic resonance (NMR), respectively. The FEs of the reaction products are presented in Figure 2B and C and Supplementary Figure 4. Since Co is known to be more active than Cu towards HER, high loading of Co (i.e., CuCo1.8% with 6.6 at% coverage of surface Co, as shown in Figure 1G) enhances HER performance unsurprisingly compared to Cu NWs. However, CuCo0.07% and CuCo0.4% show similar H2 selectivity in the low overpotential range (-0.6 to -0.9 V vs. RHE) and 10%-20% lower H2 selectivity at higher overpotentials (-1.0 to -1.2 V vs. RHE) [Supplementary Figure 5] compared to Cu NWs. The reduction products of CO2 were influenced more dramatically due to the decoration of Co. As shown in Figure 2B and C, the CO FEs of all three CuCox samples were significantly lower than those of the Cu NWs, and C2+ FEs of these CuCox samples were significantly higher than those of the Cu NWs. Specifically, the highest FE for C2+ increased sharply from 19.5% for Cu to 33.2% for CuCo0.07%, 40.7% for CuCo0.4%, and 26.0% for CuCo1.8%. CO is widely accepted as the key reaction intermediate for converting CO2 to C2+ on Cu. The fast decrease of CO detected by GC in Figure 2B can be ascribed to the accelerated consumption of the adsorbed *CO on the surface in forming C2+, resulting in decreased desorbed CO. The above results suggest that Co plays an important role in lowering gaseous CO release and facilitating C2+ production. Meanwhile, the suppressed H2 production on CuCox samples compared to Cu NWs could also contribute to the more effective C2+ production, as more electrons were consumed by C–C coupling instead of HER.
Intriguingly, the selectivity of C2+ products increases with Co loading until 0.4 at%, but then decreases with further Co loading (1.8 at%) [Figure 2C]. To verify this trend, we prepared two additional Co-modified Cu catalysts with 0.2% (CuCo0.2%) and 1.1% Co (CuCo1.1%) and tested their CO2RR performance under identical conditions. As shown in Figure 2D, the FEs for C2H4 and C2H5OH of the Cu and CuCox samples obtained at -1.0 V vs. RHE demonstrate a volcano-shape dependence on the Co loading, where the CuCo0.4% catalyst displays the highest FE for both C2H4 (27.3%) and C2H5OH (10.7%) productions. This performance surpasses that of the state-of-the-art CuCo catalysts currently reported in the literature [Supplementary Table 1]. As the morphology and the ECSA of the Cu NWs and CuCOx samples are very similar, the influence of the local reaction environment can thus be ruled out. Therefore, these results demonstrate that an optimal loading of Co could effectively improve the selectivity of Cu towards C2+ products by suppressing the H2 evolution and accelerating C–C coupling, which is consistent with previous research[21,27].
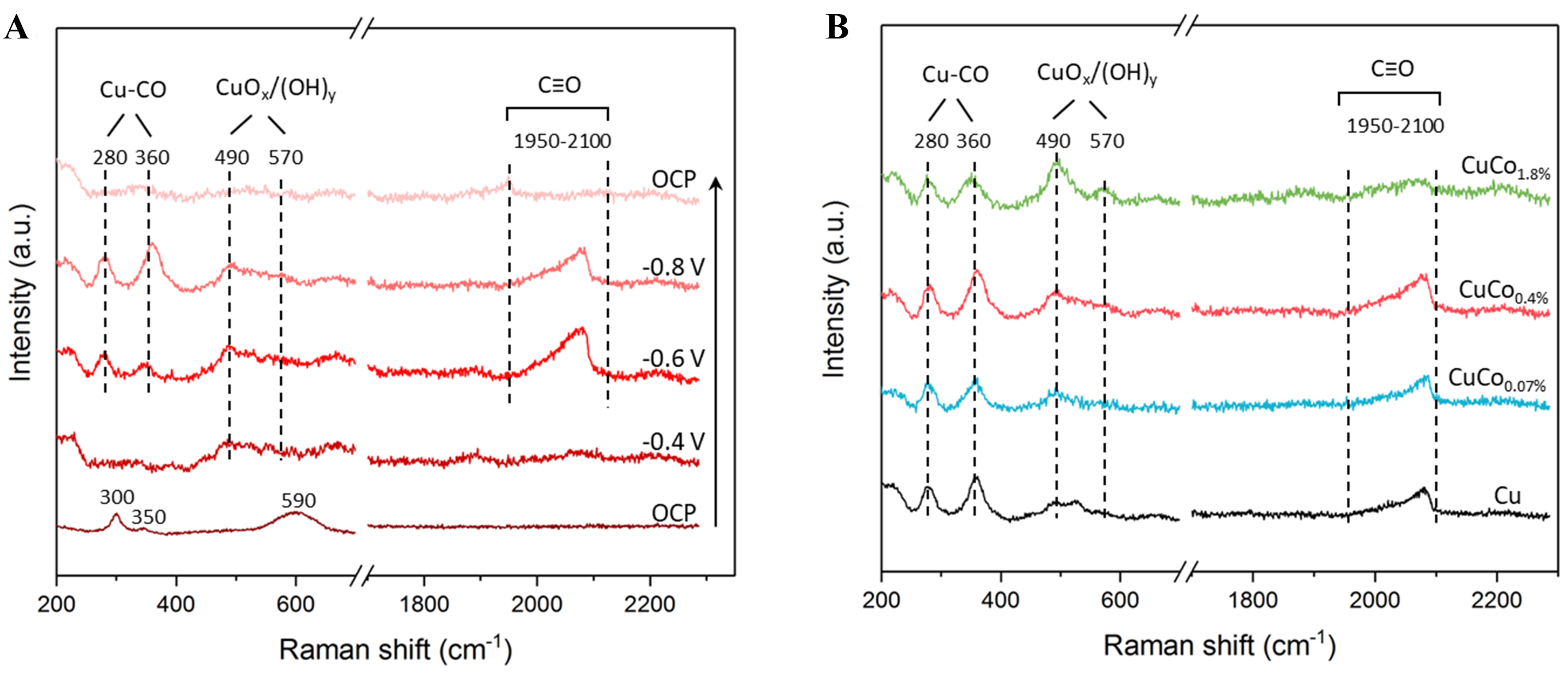
To understand the enhanced selectivity towards C2+ products on CuCox catalysts, in situ surface-enhanced Raman spectroscopy (SERS) was employed to probe the adsorbed intermediates on the catalyst surfaces[2,28]. The NW structure of the Cu and CuCox catalysts was reported to enhance Raman signals; thus, additional surface manipulation was not required[29,30]. We first examined the surface of CuCo0.4% as it showed the highest performance of C2+ production. Figure 3A shows the potential-dependent SERS spectra of CuCo0.4% acquired in CO2-saturated 0.1 M KHCO3 electrolyte at different applied potentials. At open circuit potential (OCP), the CuCo0.4% samples exhibited three characteristic Raman bands of CuO at around ~300, ~350, and ~590 cm-1[31]. These peaks disappeared after applying a reduction potential of -0.4 V vs. RHE, demonstrating that the CuO in bulk can be reduced promptly at potentials more negative than -0.4 V vs. RHE, which agrees with our XRD and SAED analysis. However, two weak bands at ~490 and ~570 cm-1 attributable to the surface Cu oxide/hydroxide species [e.g., a mixture of CuOx and Cu(OH)y], named as CuOx/(OH)y, are present after reduction[29,32,33]. These oxidized Cu species could be attributed to the dynamic oxidation of the Cu surface and the adsorption of -OH groups during the CO2RR and HER processes[29,32]. In addition, three Raman bands related to *CO can be observed in the potential range of -0.6 to -0.8 V vs. RHE: the two low-frequency bands at ~280 and ~360 cm-1 can be assigned to the frustrated rotation and stretching vibration of Cu-CO, respectively; and the high-frequency broad band located between 1,950 and 2,100 cm-1 is caused by intramolecular C≡O stretching vibrations with various binding configurations[30,34]. Both the surface Cu oxide species and the *CO-related intermediates disappeared after removing the applied potential, indicating that these species are generated and stabilized under the CO2RR condition[35].
Figure 3. In situ Raman spectra of (A) CuCo0.4% at different applied potentials; and (B) various CuCox samples at -0.8 V vs. RHE in CO2 saturated 0.1 M KHCO3. RHE: Reversible hydrogen electrode.
To explore the influence of Co deposition on the CO2RR process, SERS was also employed to probe the surface speciation of Cu, CuCo0.07%, and CuCo1.8% samples. As shown in Figure 3B, all four samples share similar spectral features at a reduction potential of -0.8 V, suggesting the presence of similar surface species. However, the relative intensities of these peaks differ [Supplementary Figure 6]. While the peak intensity of CuOx/(OH)y increases with Co deposition amount, the Cu-CO and C≡O peak intensities are maximized for the CuCo0.4% sample. The trend for CuOx/(OH)y peak intensity agrees with previous studies that showed that isolated Co atoms could stabilize the Cu2O surface by increasing the activation barrier of surface oxygen abstraction[36]. The trend for Cu-CO and C≡O peak intensities are in line with the C2+ selectivity observed in Figure 2C, indicating that high *CO coverage is the obvious reason for C–C coupling, therein, a higher C2+ production[7,13]. Various studies have shown that mixed oxide states of Cu could enhance the CO2-to-C2+ conversion[11,37-39]. For instance, by using density functional theory (DFT) calculations and in situ SEIRAS, Zhang et al. demonstrated that the Cuδ+ site at the Cu0/Cuδ+ interface favors the formation of *CHO intermediates, which can subsequently couple with *CO on adjacent Cu0 surfaces to form *OCCHO intermediates, promoting the generation of C2+ products[40]. In our case, we anticipate that the CuCo0.4% sample shows higher C2+ selectivity than the other samples due to the coexistence of Cu0 and Cuδ+ species in an optimized ratio.
Notably, due to the low loading of Co, we could not detect any vibrational bands associated with Co species or reaction intermediates adsorbed on Co. However, the role of Co species in tuning the reaction pathway should not be ignored since the binding of *CO and *H to the Co surface is much stronger than that to the Cu surface, which can certainly affect the reaction pathways[41,42]. Further, a recent study shows that in CuCo single atom alloys, Cu sites neighboring Co atomic sites could accelerate CO2-to-CO conversion and Cu-Co sites also favor the deoxygenation of *HOCCH, which increases the selectivity toward ethylene over ethanol[43]. More recently, Luo et al. found that Co in cobalt phthalocyanine could promote the C2 selectivity of Cu catalysts because the adsorption of CO2, CO, and CO2RR intermediates could be enhanced on both Co and Cu sites[44]. These results indicate that using strong CO-binding elements to promote the C2+ selectivity of Cu is effective, and further mechanism understanding is also required.
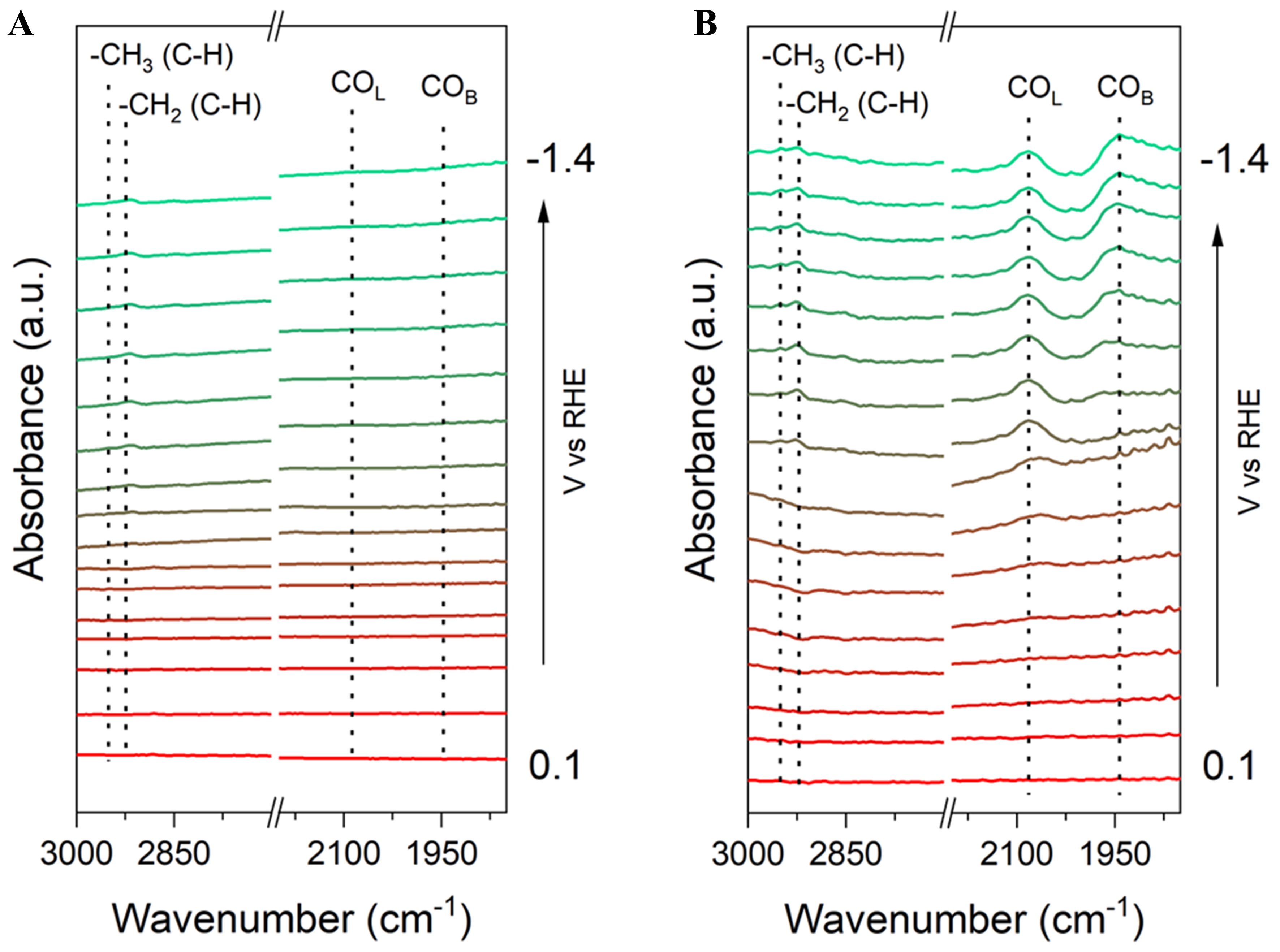
To further investigate the formation mechanism of C2+ products on the CuCox catalysts, in situ ATR-SEIRAS was performed over the Cu and CuCo0.4% catalysts. Figure 4A shows almost no characteristic peak of *CO species detected on the surface of the Cu NWs electrode during the electrocatalytic process. However, on the CuCo0.4% sample [Figure 4B], a peak appears at a wavelength of 2,080 cm-1 from -0.4 V vs. RHE and another at 1,950 cm-1 from -0.8 V vs. RHE. These peaks can be assigned to the adsorbed *CO species, with the first one belonging to linear adsorption (*COL) and the second one to bridge adsorption (*COB), respectively[45]. Further, two additional peaks appear at 2,952 and 2,923 cm-1 for both Cu NWs and CuCo0.4% samples with relatively low intensities, attributed to the C-H extension of methyl (-CH3) and methylene
Figure 4. Potential-dependent ATR-SEIRAS spectra for (A) Cu and (B) CuCo0.4% at 0.1 to -1.4 V vs. RHE in CO2-saturated 0.1 M KHCO3. The spectrum taken at 0.2 V vs. RHE was used as the reference. ATR-SEIRAS: Attenuated total reflectance-surface-enhanced infrared absorption spectroscopy; RHE: reversible hydrogen electrode.
CONCLUSIONS
In summary, this work shows that Co, despite being a non-CO2RR selective metal, can be used to decorate the Cu surface to improve the selectivity towards C2+ products. With an optimized amount of Co, the CuCo0.4% sample showed 40.7% FE for C2+ at -1.0 V vs. RHE, two times higher than that of the Cu sample (19.5%). Ex situ techniques indicated that with a low deposition amount of Co, the crystal structure and morphology of Cu NWs were not influenced. However, in situ Raman spectra revealed that Co could stabilize the Cuδ+ species on the CuCox surface, and in situ infrared spectroscopy indirectly proves that coexistence of Co, Cu0 and Cuδ+ may promote the adsorption of *CO, thus accelerating the C–C coupling. We believe this study can inspire the development of other Cu-based bimetallic catalysts for CO2RR.
DECLARATIONS
Authors’ contributions
Conceptualized and supervised the project: Luo W, Zhao K, Züttel A
Synthesized the catalysts and performed the electrochemical tests: Soodi S
Performed the in situ analysis: Zhang JJ
Performed sample characterizations and data analysis: Zhang J, Liu Y, Luo W
Co-wrote the manuscript: Soodi S, Zhang JJ, Zhao K, Luo W
Reviewed the paper: Lashgari M, Zafeiratos S, Züttel A
All the authors discussed the results and revised the manuscript.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Financial support and sponsorship
This research was supported by the Swiss National Science Foundation (Ambizione project PZ00P2_179989).
Conflicts of interest
Liu Y is the Guest Editor of the Special Issue “Carbon in Catalysis”, while the other authors have declared that they have no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2024.
Supplementary Materials
REFERENCES
1. De Luna P, Hahn C, Higgins D, Jaffer SA, Jaramillo TF, Sargent EH. What would it take for renewably powered electrosynthesis to displace petrochemical processes? Science. 2019;364:eaav3506.
2. Pham THM, Zhang J, Li M, et al. Enhanced electrocatalytic CO2 reduction to C2+ products by adjusting the local reaction environment with polymer binders. Adv Energy Mater. 2022;12:2103663.
3. Zhang J, Luo W, Züttel A. Crossover of liquid products from electrochemical CO2 reduction through gas diffusion electrode and anion exchange membrane. J Catal. 2020;385:140-5.
4. Zhang J, Luo W, Züttel A. Self-supported copper-based gas diffusion electrodes for CO2 electrochemical reduction. J Mater Chem A. 2019;7:26285-92.
5. Koolen CD, Luo W, Züttel A. From single crystal to single atom catalysts: structural factors influencing the performance of metal catalysts for CO2 electroreduction. ACS Catal. 2023;13:948-73.
6. Koolen CD, Oveisi E, Zhang J, et al. Low-temperature non-equilibrium synthesis of anisotropic multimetallic nanosurface alloys for electrochemical CO2 reduction. Nat Synth. 2024;3:47-57.
7. Zhang J, Pham THM, Ko Y, et al. Tandem effect of Ag@C@Cu catalysts enhances ethanol selectivity for electrochemical CO2 reduction in flow reactors. Cell Rep Phys Sci. 2022;3:100949.
8. Zhang J, My Pham TH, Gao Z, et al. Electrochemical CO2 reduction over copper phthalocyanine derived catalysts with enhanced selectivity for multicarbon products. ACS Catal. 2023;13:9326-35.
9. Gao Y, Xiao H, Ma X, et al. Cooperative adsorption of interfacial Ga-N dual-site in GaOOH@N-doped carbon nanotubes for enhanced electrocatalytic reduction of carbon dioxide. J Colloid Interface Sci. 2024;654:339-47.
10. Zhong D, Zhao ZJ, Zhao Q, et al. Coupling of Cu(100) and (110) facets promotes carbon dioxide conversion to hydrocarbons and alcohols. Angew Chem Int Ed Engl. 2021;60:4879-85.
11. Yang PP, Zhang XL, Gao FY, et al. Protecting copper oxidation state via intermediate confinement for selective CO2 electroreduction to C2+ fuels. J Am Chem Soc. 2020;142:6400-8.
12. Tang C, Shi J, Bai X, et al. CO2 reduction on copper’s twin boundary. ACS Catal. 2020;10:2026-32.
13. Zhang T, Bui JC, Li Z, Bell AT, Weber AZ, Wu J. Highly selective and productive reduction of carbon dioxide to multicarbon products via in situ CO management using segmented tandem electrodes. Nat Catal. 2022;5:202-11.
14. García de Arquer FP, Dinh CT, Ozden A, et al. CO2 electrolysis to multicarbon products at activities greater than 1 A cm-2. Science. 2020;367:661-6.
15. Zhou F, Zhang J, Zhang Y, Wu Y, Wang Y, Luo W. Palladium-copper bimetallic catalysts for electroreduction of CO2 and nitrogenous species. Coord Chem Rev. 2024;509:215802.
16. Morales-guio CG, Cave ER, Nitopi SA, et al. Improved CO2 reduction activity towards C2+ alcohols on a tandem gold on copper electrocatalyst. Nat Catal. 2018;1:764-71.
17. Ren D, Gao J, Pan L, et al. Atomic layer deposition of ZnO on CuO enables selective and efficient electroreduction of carbon dioxide to liquid fuels. Angew Chem. 2019;131:15178-82.
18. Hori Y, Murata A, Takahashi R. Formation of hydrocarbons in the electrochemical reduction of carbon dioxide at a copper electrode in aqueous solution. J Chem Soc Faraday Trans 1. 1989;85:2309-26.
19. Xu C, Vasileff A, Jin B, et al. Graphene-encapsulated nickel-copper bimetallic nanoparticle catalysts for electrochemical reduction of CO2 to CO. Chem Commun. 2020;56:11275-8.
20. Yan Y, Zhao Z, Zhao J, Tang W, Huang W, Lee J. Atomic-thin hexagonal CuCo nanocrystals with d-band tuning for CO2 reduction. J Mater Chem A. 2021;9:7496-502.
21. Bernal M, Bagger A, Scholten F, et al. CO2 electroreduction on copper-cobalt nanoparticles: size and composition effect. Nano Energy. 2018;53:27-36.
22. Luo W, Xie W, Mutschler R, et al. Selective and stable electroreduction of CO2 to CO at the copper/indium interface. ACS Catal. 2018;8:6571-81.
23. Li M, My Pham TH, Ko Y, et al. Support-dependent Cu–In bimetallic catalysts for tailoring the activity of reverse water gas shift reaction. ACS Sustain Chem Eng. 2022;10:1524-35.
24. Li M, Luo W, Züttel A. Near ambient-pressure X-ray photoelectron spectroscopy study of CO2 activation and hydrogenation on indium/copper surface. J Catal. 2021;395:315-24.
25. Raciti D, Cao L, Livi KJT, et al. Low-overpotential electroreduction of carbon monoxide using copper nanowires. ACS Catal. 2017;7:4467-72.
26. Wang Y, Raciti D, Wang C. High-flux CO reduction enabled by three-dimensional nanostructured copper electrodes. ACS Catal. 2018;8:5657-63.
27. Grote J, Zeradjanin AR, Cherevko S, et al. Screening of material libraries for electrochemical CO2 reduction catalysts - Improving selectivity of Cu by mixing with Co. J Catal. 2016;343:248-56.
28. Luo W, Zhang Q, Zhang J, Moioli E, Zhao K, Züttel A. Electrochemical reconstruction of ZnO for selective reduction of CO2 to CO. Appl Catal B Environ. 2020;273:119060.
29. Zhao Y, Chang X, Malkani AS, et al. Speciation of Cu surfaces during the electrochemical CO reduction reaction. J Am Chem Soc. 2020;142:9735-43.
30. Jiang S, Klingan K, Pasquini C, Dau H. New aspects of operando Raman spectroscopy applied to electrochemical CO2 reduction on Cu foams. J Chem Phys. 2019;150:041718.
31. Deng Y, Handoko AD, Du Y, Xi S, Yeo BS. In Situ Raman spectroscopy of copper and copper oxide surfaces during electrochemical oxygen evolution reaction: identification of CuIII oxides as catalytically active species. ACS Catal. 2016;6:2473-81.
32. Moradzaman M, Mul G. In Situ Raman study of potential-dependent surface adsorbed carbonate, CO, OH, and C species on Cu electrodes during electrochemical reduction of CO2. ChemElectroChem. 2021;8:1478-85.
33. Liu C, Gong J, Li J, et al. Preanodized Cu surface for selective CO2 electroreduction to C1 or C2+ products. ACS Appl Mater Interfaces. 2022;14:20953-61.
34. Li YC, Wang Z, Yuan T, et al. Binding site diversity promotes CO2 electroreduction to ethanol. J Am Chem Soc. 2019;141:8584-91.
35. Lei Q, Huang L, Yin J, et al. Structural evolution and strain generation of derived-Cu catalysts during CO2 electroreduction. Nat Commun. 2022;13:4857.
36. Wang C, Kong Y, Soldemo M, et al. Stabilization of Cu2O through site-selective formation of a Co1Cu hybrid single-atom catalyst. Chem Mater. 2022;34:2313-20.
37. Chou TC, Chang CC, Yu HL, et al. Controlling the oxidation state of the Cu electrode and reaction intermediates for electrochemical CO2 reduction to ethylene. J Am Chem Soc. 2020;142:2857-67.
38. Lee SY, Jung H, Kim NK, Oh HS, Min BK, Hwang YJ. Mixed copper states in anodized Cu electrocatalyst for stable and selective ethylene production from CO2 reduction. J Am Chem Soc. 2018;140:8681-9.
39. Lei Q, Zhu H, Song K, et al. Investigating the origin of enhanced C2+ selectivity in oxide-/hydroxide-derived copper electrodes during CO2 electroreduction. J Am Chem Soc. 2020;142:4213-22.
40. Zhang XY, Lou ZX, Chen J, et al. Direct OC-CHO coupling towards highly C2+ products selective electroreduction over stable
41. Li J, Xu A, Li F, et al. Enhanced multi-carbon alcohol electroproduction from CO via modulated hydrogen adsorption. Nat Commun. 2020;11:3685.
42. Li J, Wang Z, Mccallum C, et al. Constraining CO coverage on copper promotes high-efficiency ethylene electroproduction. Nat Catal. 2019;2:1124-31.
43. Kim B, Tan YC, Ryu Y, et al. Trace-level cobalt dopants enhance CO2 electroreduction and ethylene formation on copper. ACS Energy Lett. 2023;8:3356-64.
44. Luo Y, Yang J, Qin J, et al. Cobalt phthalocyanine promoted copper catalysts toward enhanced electro reduction of CO2 to C2: synergistic catalysis or tandem catalysis? J Energy Chem. 2024;92:499-507.
45. Jiang T, Qin X, Ye K, et al. An interactive study of catalyst and mechanism for electrochemical CO2 reduction to formate on Pd surfaces. Appl Catal B Environ. 2023;334:122815.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.



















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].