Camera-based remote photoplethysmography for blood pressure measurement: current evidence, clinical perspectives, and future applications
Abstract
Keywords
INTRODUCTION
Telemedicine and telehealth-related technologies are a growing sector of the healthcare system. In 2017, the American Heart Association released a policy statement advocating for the increased adoption of telehealth for the management of cardiovascular disease and stroke[1]. Subsequently, the COVID-19 pandemic accelerated the delivery of virtual care mechanisms, with more predictable reimbursement and opportunities to gather data to evaluate impacts on privacy, care quality, utilization, and experience[2]. With the increasing use of virtual visits between a patient and clinician over video comes the realization that conventional methods and devices for obtaining vital signs at the point of care and for continuous remote monitoring are not optimized for telemedicine.
Blood pressure (BP) is one of the most important clinical metrics, but reliable measurement presents a major challenge. The gold standard noninvasive method is manual manometry with auscultation which requires a skilled clinician. The most widely used method is automated oscillometric cuff pressure, in which a computer detects oscillations of blood flow at various cuff pressures to estimate systolic, diastolic, and mean arterial pressure based on proprietary algorithms[3]. However, cuff-based BP methods have limitations, including cost and low global availability of devices, low patient adherence due to discomfort and inconvenience, frequent incompatibility due to anatomy and lack of continuous BP information[4]. In response, many devices and apps claiming to measure cuffless BP have emerged in the market with varying degrees of novelty, regulatory approval, satisfaction of institutional standards, and clinical peer review[5]. As a result, the European Society of Hypertension (ESH) has advised against the clinical use of current cuffless BP devices[6].
A pragmatic solution for cuffless BP is to repurpose existing technology to meet the demand. Some novel devices incorporate photoplethysmography (PPG), which measures the light reflected from the skin to quantitatively capture blood flow in the peripheral vasculature based on the light absorption properties of hemoglobin. PPG has been used widely in medicine for pulse oximetry, which measures pulse rate and blood oxygen saturation from a device typically attached to the fingertip or ear lobe. PPG has been researched extensively as a cuffless means to estimate BP with mixed results[5,7-9].
More recently, PPG technology has been translated to consumer wearable devices, namely smartwatches[10]. The Apple Heart and Fitbit studies are the largest scale studies to date to support PPG for clinical use by consumers. These studies evaluated the feasibility of detecting atrial fibrillation and both demonstrated the great potential of PPG for remote patient monitoring[11,12]. Although contact PPG sensors are now widely available in wearables, they are far from universal. For perspective, in 2021, the number of smartwatch users was approximately 203 million, whereas smartphone users were closer to 6.4 billion, with greater disparities in low-resource environments[13].
Although conventional PPG methods require physical contact with the skin, new contactless methods in development use the camera lens as the photodetector. Cameras are ubiquitous owing to their popularity in the consumer electronics industry, integrated with nearly every smartphone, tablet, or laptop computer today. Spatial, temporal, and electromagnetic frequency domain changes are captured at high quality and low cost. As consumer cameras are designed to capture information relevant to human vision, they are inherently standardized, for instance, to capture red, green, and blue colors in the visible light spectrum (400-700 nm), and many use a standard frame rate of 30 frames per second. Once cameras were digitized in the late 1990s, it became possible to apply computational methods to identify objects within images, quantify minute changes in motion, and detect variations in reflected light not visible to the human eye.
Cameras provide an attractive solution for contactless monitoring of vital signs in telemedicine and other clinical scenarios. Indeed, research on the camera-based measurement of vital signs has grown exponentially over the last few decades[8]. Early clinical studies have shown promise for camera-based measurement of respiratory rate (RR)[14], heart rate (HR)[15], blood oxygen saturation (SpO2)[16], and even detection of atrial fibrillation[17-19]. Given the immense technical challenges, research to estimate BP with a camera has progressed slowly and has been mostly limited to the computer science domain with testing performed on healthy subjects. In recent years, advancements in research methods including machine learning have renewed cautious optimism that the field is ready for more robust clinical studies.
In this article, first, we describe the theoretical basis of BP estimation by camera-based remote photoplethysmography (rPPG). We then provide a brief overview of research to date and highlight the major technical and physiological limitations. Finally, we conclude with a discussion of potential future clinical applications inclusive of and beyond telemedicine, which guides our recommendations for the next steps in research.
The reader should note that, as an emerging field, there is no consensus term for using a camera for photoplethysmography. Various terms used include camera-based, video, imaging, remote, distance, noncontact, and contactless photoplethysmography. For consistency with other recent publications, this paper uses camera-based remote PPG. In contrast, any method of acquiring a PPG signal that requires physical contact is referred to as contact PPG.
PRINCIPLES OF CAMERA-BASED BLOOD PRESSURE ESTIMATION BY rPPG
A basic understanding of PPG, the theories behind BP estimation, and current limitations are necessary before considering clinical applications. A detailed analysis of the technical components of the PPG waveform is highlighted in a review by Elgendi et al.[20]. For a technical review of camera-specific methods of PPG signal extraction, Selvaraju et al., Molinaro et al., Natarajan et al., and Mukkamala et al. have written comprehensive reviews of the theories underlying PPG-based BP estimation[21,22,8,23].
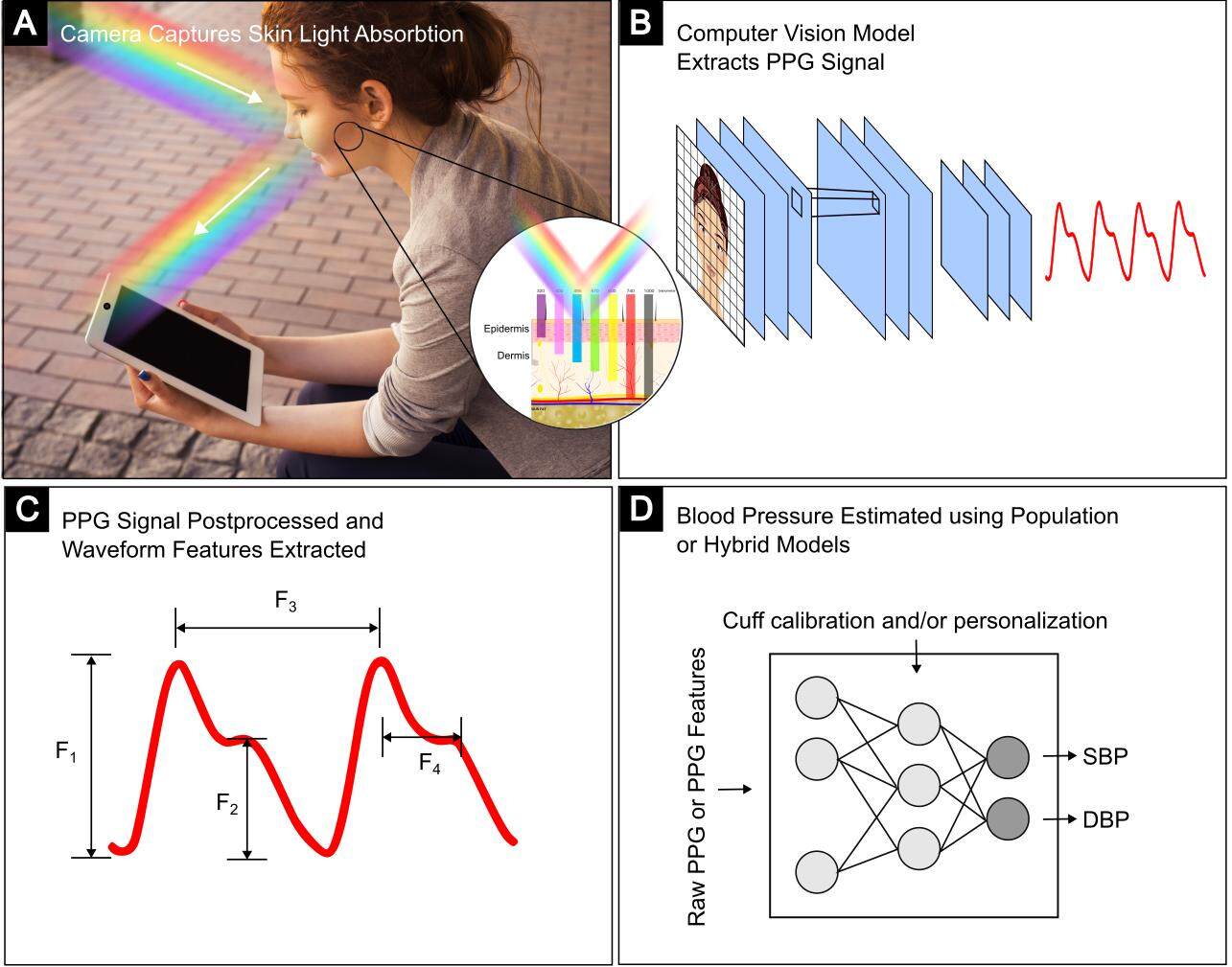
Whether using a contact sensor or a camera, the process of estimating BP from reflectance mode PPG shares similarities [Figure 1]. First, a light source is needed, either from a device or ambient light. Generally, the visible spectrum of light is used, though infrared has been explored as it offers deeper penetration into dermal layers containing arteries[24]. Light is shone on the skin and absorbed in part by blood. The green channel is conventionally used to isolate hemoglobin, the major component of blood, based on its absorption properties. Pulsatile blood flow within arterioles and the microvascular bed in the skin results in fluctuating levels of absorption by hemoglobin. For rPPG, a region of interest (ROI) is selected, commonly the forehead or cheeks, due to the larger surface area and/or perpendicular position to the lens when facing the camera. The reflected light is returned to a photodetector and visualized as a waveform that resembles an attenuated arterial pressure tracing. Next, the PPG signal quality is improved by spatial averaging, normalization, low and high pass filtering, and other post-processing methods to remove noise from motion artifacts or non-hemoglobin components such as melanin. The first and second derivatives of the waveform may also be computed. Finally, the cleaned PPG signal is fit to a predetermined parametric model to produce an estimate of BP based on waveform characteristics, patient demographics, or other parameters.
Figure 1. Remote PPG (rPPG) uses cameras to capture minute changes in light absorbed by hemoglobin in the peripheral vasculature to measure the blood volume pulse. (A) camera detects light reflected from a subject’s face[25]. (B) Computational methods extract a rPPG signal from a ROI. (C) Examples of possible waveform features. (D) Cuff calibration, other personal parameters, and rPPG features are entered into machine learning models to predict BP (adapted from[4]).
Origins of PPG
PPG was first described by Hertzman AB in 1938[26]. Despite great advancements in knowledge, the underlying hemodynamic theory is not fully understood[10]. When the heart contracts, a pressure wave travels from the aorta to the distal arterioles as blood is propelled through the cardiovascular system [Figure 2]. The central arterial waveform is transmitted to the microvasculature beneath the skin surface and reflected in the PPG waveform. The PPG waveform can be described as having a high frequency alternating current (AC) component and a low frequency pseudo-direct current (DC) component. In general, the AC component is attributable to the blood volume pulse, whereas the undulating DC component is affected by overall blood volume, respirophasic variation, autonomic activity, and thermoregulation[10].
Figure 2. Illustration of time-based BP estimation that could be performed with a camera using hypothetical combinations of ECG, BCG, and PPG waveforms. PAT can be measured from the “r” wave of an ECG and a PPG wave (PAT1, PAT2, and PAT3). PTT can be measured between the “h” wave of a BCG from head movement and a PPG wave (PTT1, PTT2, and PTT3). PTT can also be measured between PPG waves from different body sites (PTT4 and PTT5). Note: the arterial waveform in the aorta is shown for reference; this is a simplified conceptual diagram; actual calculations can involve feature derivatives and other points of interest. ECG: Electrocardiogram; BCG: ballistocardiogram; PTT: pulse transit time; PEP: pre-ejection period; PAT: pulse arrival time.
The conventionally accepted theory states that the PPG waveform is a direct result of the absorption of light by hemoglobin within arteries. Kamshilin et al. challenged this theory on the basis of observation and experiments to conclude that the PPG waveform from visible light does not originate from arteries, but rather the more superficial tissue including capillaries[27]. They proposed a new conceptual model in which transmural pressure from arteries mechanically deforms adjacent tissues containing capillaries, lymphatics, and connective tissue in a manner that alters the absorption of light creating the PPG waveform.
Subsequently, Moço et al. found evidence in support of the conventional theory[24]. They imaged the capillaries of the nail bed using visible light and found no relationship between capillary density and PPG signal strength. They also refuted the claim that visible light does not penetrate the skin to the depth of the arterial vasculature. Although their results support the conventional theory, they acknowledge the “possibility of complementary mechanisms of PPG formation occurring in parallel”.
Understanding the origin of PPG is essential to efforts of PPG-based BP measurement. The purpose of the technology is to estimate arterial pressure based on a signal presumed to be directly contrived from arterial blood flow. As the next section illustrates, knowledge of confounding factors will help to guide device and model development for accurate monitoring of vital signs including BP measurement.
Hemodynamic theory of PPG and BP estimation: pulse transmit time and pulse waveform analysis
The PPG signal can be related to BP based on time or waveform shape, generally called pulse transit time (PTT) and pulse wave analysis (PWA), respectively. The premise of PTT states that the velocity of the pressure wave is known to correlate with BP; therefore, the time interval between waveforms is inversely related to BP. Specifically, higher BP is associated with a faster wave velocity and a shorter time interval between two points along the wave, which can be captured by PPG waveforms from different body sites.
A related concept, pulse arrival time (PAT), measures the time delay between the onset of systole and a distal PPG waveform. The start of electrical systole begins with the “q” wave of an ECG, whereas the onset of isovolumetric contraction coincides with the “r” wave peak of an ECG. For this reason, studies have measured PAT from the “q” wave or the “r” wave. However, there is a small but not insignificant time lapse between isovolumetric contraction and ventricular ejection of blood into the aorta, called the pre-ejection period (PEP). Inter- and intra-individual differences in ventricular compliance, contractility, filling pressures, and valvular disease will affect the PEP and thus confound BP estimation. As shown in porcine experiments, the PEP can be measured using an ECG and an intra-aortic catheter, but in real-world practice, it is unknown[28]. Ballistocardiography (BCG) may offer an alternative to ECG for detecting the start of the ventricular ejection period of systole. As blood is ejected into the aorta, the head moves slightly downward to conserve momentum. This subtle movement can be detected by a contact sensor or camera. The “h” wave captured by BCG correlates with the opening of the aortic valve and ventricular ejection[29].
Figure 2 provides an illustration of time-based BP estimation using hypothetical combinations of ECG, BCG, and PPG waveforms acquired from a contact sensor or camera. A camera can measure the time delay between BCG and PPG from the facial arteries (PTT1), branches of the brachial artery (PTT2), or branches of the radial artery (PTT3). A camera can also measure the time delay between PPG waveforms from the facial and radial arteries (PTT4), or brachial and radial arteries (PTT5). Using ECG with a camera, one can measure the pulse arrival time (PAT) between the “r” wave of the ECG and the upstroke of the PPG waveform from various body sites: PAT1 = PEP + PTT1 (facial arteries), PAT2 = PEP + PTT2 (brachial artery), and PAT3 = PEP + PTT3 (radial artery).
In addition to time, waveform features can be analyzed to estimate BP. Pulse wave analysis (PWA) presumes that the dynamic shape of a PPG waveform is correlated to changes in BP. The upward slope, width, or amplitude are examples of possible features [Figure 1C]. Accurate waveform acquisition matters more in PWA than PTT, which generally only requires the identification of troughs or peaks to distinguish waveforms from each other.
Relevant to the clinician is an appreciation for the numerous anatomical and physiological factors that complicate efforts to measure BP by PTT, PAT, or PWA. The peripheral vasculature is affected by smooth muscle tone and viscosity more so than central arteries due to differences in vessel wall composition[23]. As a result, peripheral smooth muscle contraction has been shown to affect PPG waveform velocity and shape independent of BP. Similarly, factors that alter arterial compliance, such as age, diabetes, chronic kidney disease, atherosclerosis, and pregnancy, will affect waveform velocity and shape. Light absorption and reflection depend on the optical properties of skin, which vary by hemoglobin concentration, melanin concentration, thickness, and water content[10,30]. As a result of fluid dynamics, the systolic pressure wave is reflected from the capillaries back to the aorta, and thus, the PPG waveform is actually a summation of wave propagation and reflection - an effect that increases with distance from the heart[23].
There is no clear best method for PPG-based BP estimation. Direct comparisons between PTT, PAT, and PWA are limited due to differences in study populations and testing conditions[31]. Some argue that PTT is most grounded in hemodynamic theory and therefore is most likely to yield the best results, perhaps until stronger theories are discovered to support PWA, or better methods to account for the PEP for PAT[4,8]. PWA remains an attractive solution and has been aggressively pursued in research models. Whereas PTT and PAT conventionally require multiple contact sensors to measure two points in time, PWA hypothetically may only require a single PPG sensor (e.g., a smartwatch or camera) to measure BP. Lacking a theoretical foundation, PWA is considered the “data-driven” approach to BP estimation as it has advanced due to artificial intelligence (AI), namely machine learning (ML) and deep learning (DL), that allows for robust feature comparisons[8]. More recent studies have used DL to combine PTT and PWA features with demographics to build complex models in an attempt to improve BP prediction[32]. However, validation of some models has been a primary issue, as demonstrated by a recent study that challenged a method of PAT-PWA that likely forms the basis of several contact PPG devices on the market[9]. These findings are a wake-up call to researchers and the scientific community to develop new validation protocols commensurate with these new technological advancements[5].
Calibration and parametric models to estimate BP
In all cases, because PPG cannot directly measure pressure, a parametric model is essential to relate PPG features to BP[8] [Figure 1D]. The cardiovascular system is a complex, dynamic fluid network and inferring parameters from a two-dimensional observation of blood volume is onerous. For PTT, equations drawn from hemodynamic theory are used as the basis of the model to convert units of time to units of pressure. For PWA, more complicated nonlinear models are used to map waveform features to BP. The unknown variables, or parameters, are then determined by one of several approaches: an individualized, population, or hybrid model.
The individualized model involves PPG recordings of a person over a wide range of repeated BP measurements. With enough data, the parametric model is determined, and cuffless BP can be estimated from PPG. Periodic BP recalibration is needed to account for changes in physiology, such as reduced vascular compliance with aging, though the optimal time interval is unknown[33].
The population model ideally uses a large cohort with varying demographics and a wide range of BP to determine model parameters. An individual would then only need a PPG signal mapped to an appropriately selected population model to estimate BP without the need for cuff calibration. In theory, this would provide a truly cuffless BP experience but is not likely to be accurate enough for continuous BP monitoring.
The hybrid model trades the convenience of the population model for accuracy by using most parameters from a population, and a single or few from an individual (e.g., age, sex, gender, and height), with or without cuff calibration. This model is most likely to provide a pragmatic approach for clinical use and is the likely basis for several contact PPG-based BP devices on the market that require cuff calibration[8] and one that does not require cuff calibration[34].
Technical comparisons between contact and remote PPG
Using a camera instead of a contact sensor offers some technical and theoretical advantages. Studies have shown that a camera can track several ROI at once (e.g., regions of the face, or the face and hand) to extract simultaneous rPPG waveforms, allowing for the calculation of PTT without the need for multiple contact sensors[23,35]. Although contact PPG has been used to calculate PTT between the forearm and wrist, no similar camera-based rPPG studies using ROI from a single limb have been published[7,36].
As mentioned, contact-based methods that use ECG to measure PAT are confounded by the PEP. Alternatively, a camera could enable a pure contactless measurement of PAT by replacing the ECG with BCG[37]. Incorporation of BCG with rPPG is a novel area of particular interest to researchers[4].
Variable pressure applied in contact PPG, e.g., from a fingertip, can cause waveform deformation and thus affect signal quality - a problem that is avoided in contactless rPPG[8]. A video of the face produces a more proximal and larger surface area of pulsatile skin than a contact sensor, which may reduce PPG signal variance due to localized pathology or vasomotor tone. A camera can capture a PPG signal from the exposed skin of any area of interest, whereas a contact sensor would require extensive engineering or multiple designs to accommodate different areas.
Contact sensors and cameras both offer active and passive means of PPG measurement. Conventional contact sensors, i.e., pulse oximeters, require active use of the device. New wearables allow a passive means for contact PPG measurement but require the user to adhere to wearing the device. Adherence to wearables is not consistently reported in studies and is known to wane substantially over time[38,39]. Conversely, cameras are embedded in numerous devices used throughout the day and therefore have greater potential for passive monitoring. For instance, a person may use their smartphone or computer for another purpose while the camera continuously scans for opportune moments of satisfactory PPG waveform quality.
There are notable disadvantages to camera-based remote PPG. A larger surface area of skin means an uneven plane with differing optical properties between individuals and within the same individual. Contact PPG utilizes controlled light, such as an LED, whereas remote PPG relies on ambient light of varying intensity and color[40]. Subject-camera distance is also not controlled. Zhou et al. demonstrated that rPPG waveform quality is diminished in faint or bright light and with greater subject-camera distance[41]. They found the light intensity of 150-200 lux and the distance of 50-60 cm to be optimal. However, these parameters are likely to be specific to the camera, as other studies reported distances of 0.4-3 m with acceptable waveform quality[40,41].
Whereas contact sensors are specifically designed for PPG, webcams, smartphone cameras, and other consumer imaging devices are not optimized for rPPG[42]. A camera capable of at least 20-30 frames per second (fps) is recommended[8], though 8 fps would theoretically suffice to detect up to 240 bpm based on the Nyquist theorem[21]. Resolution is less critical, with aggressive downsampling often performed to help reduce the effects of camera quantization error. The green channel is used for rPPG because it performs best for hemoglobin absorption within the visible light spectrum, for which most cameras are designed[43]. Visible light is also absorbed by melanin, reducing rPPG quality in darker skin tones, though using increasing light intensity may mitigate this problem[10,44]. Channels of longer wavelengths, such as orange and infrared, have deeper skin penetration and are absorbed less by melanin and other artifacts[8,45,46] [Figure 1A]. Motion artifacts can also be mitigated by the extraction of multiple color channels[27,28]. However, consumer-driven motivations for the widespread use of multispectral cameras with infrared capability would be required before they can be adopted for rPPG-based monitoring of vital signs. Custom cameras could be built, but this would undermine the convenience of using available devices.
As a result of these technical limitations, much research has focused on the development of novel computational methods to improve the quality of the rPPG signal from video using standard cameras[22,28]. Compared to contact PPG, the rPPG waveform often loses distinguishing features such as the dicrotic notch, which can affect BP estimation by PWA[23]. Ultimately, pending further advancements in camera and computer technology, the signal quality of the waveform from remote PPG is generally inferior to that of contact PPG[4,23].
OVERVIEW OF rPPG STUDIES AND KEY LIMITATIONS
Camera-based remote PPG is a relatively new and rapidly evolving field of research. Wieringa et al. (2005) first introduced remote PPG for pulse oximetry with a custom-built camera using LED[47]. Several years later, Verkruysse et al. (2008) showed that a typical consumer camera under ambient light could detect rPPG[43]. Poh et al. (2010) first described a system to accurately track HR in real time using rPPG despite motion artifact[48]. McDuff et al. (2014) demonstrated the identification of systolic and diastolic peaks using rPPG and explored new color channels[46]. Next, Shao et al. (2014) first measured PTT using rPPG between the face and hand[35]. In short succession, Jeong and Finkelstein (2016) and Shao et al. (2017) investigated the correlation between rPPG-based PTT and BP in several adult subjects undergoing exercise to raise BP[49,37].
Since then, according to several independent reviews by Steinman et al., Molinaro et al., Selvaraju et al., and Natarajan et al., there have been between 8 and 21 relevant studies of rPPG-based BP estimation, depending on interpretation[30,22,21,8].Frey et al. identified two studies specifically using a smartphone camera[50].
Overall, these initial studies show potential, but rPPG-based BP estimation is still a nascent technology and requires extensive validation. Studies used cameras from laptops, webcams, and smartphones, as well as consumer-grade digital single-lens reflex cameras, generally set to 30 fps to emulate real-world conditions. Direct comparisons between studies are difficult, namely due to small sample sizes and differences in study design. Similarly, studies differ in reporting BP metrics as correlations or values in mmHg. Though it is unclear if rPPG more accurately predicts SBP or DBP, reviews would suggest PWA correlates better with SBP than DBP, whereas PTT shows a similar correlation to SBP and DBP. The relationship between PWA and SBP is likely due to greater information on waveform features related to stroke volume and systole. Natarajan et al. calculated the unweighted average of the highest correlation coefficient for blood pressure reported across studies as 0.54 (0.76 was the single highest)[8]. Several promising studies satisfied or nearly satisfied the standard set forth by the Association for the Advancement of Medical Instrumentation (AAMI) for a mean error of 5 ± 8 mmHg compared to reference BP measurement[51-57]. However, this criteria is not specific for new technologies such as rPPG, prompting the AAMI, ESH, and International Standard Organization to call for new methods of validation for cuffless BP measurement[58]. Additional limitations of these studies, as described below, makes the results currently inapplicable to a real-world patient population.
Study populations: small sample sizes and healthy, normotensive subjects
The inception of rPPG research was led by the computer science and engineering fields, which first explored these methods within a readily available cohort of healthy, normotensive subjects. With few exceptions, studies included 20 or fewer subjects. Even the largest study of > 1,300 subjects included only healthy, normotensive individuals with similar skin tones[45].
There are several concerns regarding these study populations. First, as Mukkamala et al. acknowledge, testing a homogenous group with similar demographics and a narrow range of normal BP increases the likelihood that an experimental model will have predictive power[8]. This has been demonstrated by using models based on demographics and a few other features that are as predictive as models that include rPPG[45]. It is far more difficult to predict BP from a population with a wide range of BP, age, skin tones, and other demographic variables[23,24].
Second, studies lack tracking of intraindividual BP over time and instead employ interventions to invoke dynamic BP changes, including Valsalva maneuver[8], nitroglycerin[59], cold pressor test[60], isometric hand grip[61], cycling[8], cognitive task completion[62], and consuming chocolate[63]. It should not be presumed that the state of the autonomic and cardiovascular systems, and thus the rPPG waveform, during a maneuver is the same as when a patient suffers from a pathophysiological condition leading to BP changes. For instance, a Valsalva maneuver transiently lowers BP by increasing intrathoracic pressure and decreasing venous return to the heart. The mechanisms of this maneuver cannot be assumed to reflect hypovolemia, sepsis, reduced cardiac output, or medication-induced hypotension. Similarly, hand grip and cold pressor tests to transiently raise blood pressure are not equivalent to chronic hypertension. If these maneuvers are to be used to determine model parameters, then they should be validated against real pathologic states.
Third, increased arterial stiffness due to age is known to cause underestimation of BP measurement by oscillometric cuff[64]. Similarly, peripheral arterial compliance is known to impact PPG waveform velocity and shape. Subjects of older age and those with diseases that affect arterial compliance should be included to explore the relationship between disease and PPG waveforms. Individuals taking cardiovascular medications (e.g., angiotensin-converting enzyme inhibitors, diuretics, and calcium channel blockers) that can affect peripheral vascular resistance, blood volume, or smooth muscle contractility should be included in future studies.
Finally, most studies have only included subjects with normal sinus rhythm. Irregular rhythms (e.g., premature atrial or ventricular contractions and atrial fibrillation) will cause variable diastolic filling time, which affects stroke volume and would likely impact the interpretability of PPG waveform features for BP estimation. The presence of atrial fibrillation is a challenge for oscillometric cuff BP measurement and shows significant within-subject variability compared to normal sinus rhythm[65].
Study environments: controlled lighting and motion
Remote PPG signals were generally acquired from subjects while seated at rest to minimize motion artifacts. Most studies were conducted under constant artificial light, while few studies tested various lighting conditions[33,47]. In order to reduce artifacts from motion and non-hemoglobin light absorption, research has focused on efforts to develop video processing techniques using machine learning and mathematical algorithms, namely, elaborations on single-channel analysis, independent component analysis, mathematical/optical modeling, and transdermal optical imaging[23]. Future studies should involve more natural environments with variable lighting and increased motion to reflect real-world conditions.
Limitations of AI for BP estimation
Artificial intelligence, particularly DL, is useful when the input (e.g., PPG) and output (e.g., BP) have an undefined, nonlinear relationship and involve many unknown parameters. The rise in the popularity of AI has undoubtedly propelled research in PPG-based BP prediction, particularly for PWA[4,66]. However, there are significant limitations to relying on AI. Increasingly complicated and enigmatic DL methods generate a predicted BP out of a “black box”[24]. It is important to provide a sound physiological basis for any claims made by AI[4]. Furthermore, the discovery of new hemodynamic principles can improve model accuracy[56].
Of major concern is inaccurate ground truth data for proper model training[56], i.e., noninvasive cuff-based BP has an inherent error, which will introduce error into a model. In rPPG studies, BP was determined by sphygmomanometry, oscillometric arm and wrist cuffs, and volume-clamp finger cuff. A large-scale study of subjects with invasive BP is possible but not practical. Data from patients in intensive care with invasive BP would not be generalizable to other populations. At this time, noninvasive BP must be accepted as a limitation in training models for most populations.
Although AI has demonstrated impressive BP estimation using retrospective datasets, the aim for clinical use is real-time BP estimation. There appear to be no studies attempting real-time BP estimation using rPPG. To this end, there is concern that signal processing and some ML and DL approaches used retrospectively may not be realistic to use for real-time BP prediction due to high processing demands on computing power and battery capacity, although cloud computing and other advancements may alleviate this burden[56].
Large datasets to advance rPPG research
The foundation of clinical contact PPG research is based on several open-access databases that include thousands of patients and contains PPG, continuous ECG, invasive and noninvasive BP, other vital signs, and a wealth of relevant clinical information from the electronic health record (EHR)[67]. In contrast, the rPPG field has no database that can address the major limitations of current research, including the narrow range of tested BP, and lack of variability in medical history, demographics, age, and skin tone. Large training datasets of rPPG would be invaluable to building models for BP prediction.
The Medical Information Mart for Intensive Care (MIMIC) III database for contact PPG did not require informed patient consent due to the deidentification of individual subject data and no impact on clinical care[68]. This study design greatly facilitated data collection. A similar approach could be applied to rPPG research with the installation of cameras in outpatient, emergency room, acute care, post-operative care, or intensive care settings. Notably, raw data from facial videos presents a unique problem for the deidentification of protected health information. Approval by an institutional review board would most likely limit sharing to extracted rPPG signals. Another approach could involve so-called synthetic datasets, in which AI simulators use real data to generate large amounts of synthetic data. These datasets may play an important role in addressing data bottlenecks in healthcare research in the future[69].
FUTURE CLINICAL APPLICATIONS FOR rPPG-BASED BP ESTIMATION
From the home to the hospital, there are many potential clinical applications for camera-based monitoring of vital signs, including BP estimation by rPPG. In particular, the COVID-19 pandemic has highlighted the need for remote patient monitoring to mitigate the spread of infectious diseases. Though the pandemic may be in remission, endemic levels of infection will persist in addition to other communicable diseases.
An idealized scenario depicts a person using a smartphone or laptop camera to record a PPG waveform of the face and, within seconds, receive an estimate of BP [Figure 3A]. Researchers hypothesize that at least 10 seconds of rPPG waveforms would be needed for BP measurement[8]. This could be done actively or passively while using camera-equipped devices. However, based on current evidence, a completely cuffless BP experience may not be realistic because it necessitates the use of a population model, which may not be accurate enough to provide a one-size-fits-all solution for clinical or consumer use. A more likely scenario would involve periodic cuff calibration that fits a hybrid model to account for inter-individual differences and intra-individual changes over time. This would allow for remote cuffless BP estimation between calibrations. Unless future technology dictates otherwise, how and when to use cuff calibration presents a major logistical hurdle[4]. The method, timing, and frequency of cuff calibration would likely differ between individuals and depend on clinical status, skin properties, and intended use - essential considerations in future clinical research.
Figure 3. Examples of future clinical applications for remote PPG measurement and BP estimation. (A) Passive monitoring or active use for telemedicine with a smartphone camera. (B) A depiction of model calibration using an automated BP cuff and multiple rPPG signals. (C) Continuous monitoring in the hospital or healthcare facility. (D) Mass screening for hypertension. (E) Passive monitoring in a vehicle. (F) Passive monitoring or active use for telemedicine with a laptop camera. cPPG: Contact photoplethysmography; rPPG: remote photoplethysmography; BP: blood pressure; SpO2: blood oxygen saturation.
Acute care and urgent care telemedicine
The lack of a comprehensive physical examination is a major limitation of telemedicine[70]. Vital signs are the foundation of an exam and inform the clinician about the acuity of a patient’s overall condition. A video can provide some cues, including general appearance, eye contact, speech fluency, work of breathing, and basic motor functions. These cues may suffice for many clinical consultations. However, many situations require knowledge of vital signs for appropriate clinical decision making.
Camera-based measurement of vital signs including BP estimation would be invaluable to the expansion of telemedicine and could reduce the need for an in-person evaluation. This could further enhance the cost and time savings from telemedicine compared to traditional outpatient care[71]. Patients and clinicians generally report high satisfaction with telemedicine[59,60]. Careful implementation of rPPG-based measurement of vital signs is not only paramount to patient safety, but also to maintaining trust between patients and clinicians for telehealth.
To this end, cuff calibration with a hybrid model would likely be necessary for BP estimation prior to a first-time telemedicine visit. Cuff calibration could be performed during an initial intake visit in the office, as a stand-alone clinical support visit with a medical assistant or nurse, or at home using an automated BP cuff. Patient demographics, medical history, and current medications acquired from the EHR would be entered as additional model parameters. Subsequently, the patient could use a camera-equipped device for BP estimation over telemedicine [Figure 3A and F]. Over time, period cuff recalibration would be needed, and the timing and frequency be determined based on validation from clinical studies.
As discussed, without cuff calibration, continuous BP monitoring using rPPG may not be feasible. However, it may be possible to use rPPG from a patient mapped to a population model to identify a patient as hypertensive, normotensive, or hypotensive with reasonable positive and negative predictive values. This could provide enough information for a clinician triaging care over telemedicine to determine when extreme BP levels require immediate attention.
A major advantage of remote PPG is that it provides an objective, real-time, continuous indication of a patient’s hemodynamics visualized by the waveform. When a patient has a home BP cuff, the clinician must rely on the patient’s proper use of the machine with little to no means to verify if the measurements are accurate. It is common for a clinician to request a patient come to the clinic to compare their home BP cuff against a clinic BP measurement. In contrast, just like a plethysmogram from a pulse oximeter, a remote PPG signal could be “read” by a trained clinician to adjudicate the computer-reported heart rate and regularity, and at least determine if the waveform quality is adequate for remote BP estimation.
A potential solution for educating the public about remote PPG while also obtaining an individual cuff calibration would be a kiosk [Figure 3B]. The kiosk would contain an automated oscillometric BP cuff similar to existing devices found in pharmacies, with the addition of a camera to record a PPG signal, which together would calibrate a hybrid model for BP prediction. The data could be uploaded to a consumer app or an EHR for subsequent remote BP estimation. The kiosk could be freely available in hospitals, clinics, pharmacies, and other public spaces.
Hypertension: ambulatory BP monitoring, outpatient management, and mass screening
A potential clinical application for rPPG is remote BP management. Hypertension is a leading global cause of cardiovascular morbidity and mortality[72]. Clinic-based BP measurement is merely a snapshot of a patient’s BP over time and is known to miss masked hypertension and nocturnal hypertension, and can even lead to misdiagnosis of white-coat hypertension[73].
When the diagnosis of hypertension is uncertain, the current standard of practice is to use ambulatory BP monitoring (ABPM), which involves oscillometric cuff measurements several times per hour for 24 h or longer[62]. However, due to cost, limited availability, and patient discomfort, utilization of cuff-based devices and ABPM remains low[4]. Current evidence may suggest that the 24 h following calibration is a reasonable hypothetical time frame for rPPG to accurately track changes in BP. ABPM should be a focus of future rPPG research.
Following a diagnosis of hypertension, telehealth-enabled technologies can improve outpatient management. A recent randomized clinical trial found improved BP control and reduced costs in patients assigned to home cuff BP telemonitoring with pharmacist-directed medication adjustments compared to usual care[74]. Camera-based outpatient BP monitoring may further improve medication adherence and BP control by replacing at least some BP cuff readings with cuffless rPPG readings to improve the patient experience [Figure 3A and F].
Remote PPG could have far-reaching applications for hypertension beyond telemedicine and ABPM. Cameras are increasingly omnipresent in consumer and commercial devices in the home, vehicles, and public spaces. If cameras prove to be a reliable tool for monitoring vital signs, then the logical next step is to consider large-scale passive monitoring for individual and public health benefits. For instance, some not so far-fetched ideas under research include vehicle-installed cameras to monitor driver awareness[75], and a camera-mounted flying drone that can detect an individual’s lack of movement in a crowd for triage in a mass casualty situation[76].
Utilizing cameras for population-level screening of diseases such as hypertension may be reasonable to consider due to the large number of undiagnosed cases and high healthcare costs from complications[1]. The Apple Heart and Fitbit studies have demonstrated significant progress toward large-scale, passive screening of a disease, specifically atrial fibrillation, with contact PPG-enabled smartwatches[11,12]. The next iteration could be a mass screening of atrial fibrillation or even hypertension with rPPG from cameras embedded in everyday life [Figure 3D and E]. Of course, such an undertaking would require robust real-world studies to demonstrate diagnostic accuracy and improvement in clinical endpoints to satisfy clinicians, regulators, and the public. Importantly, ethical and security considerations around privacy would need to be addressed.
Remote heart failure monitoring
Heart failure is a significant global disease burden, afflicting nearly 40 million people in 2016 and disproportionately impacting low-middle-income countries[77]. Telemedicine with available, low-cost, camera-enabled technology, including rPPG BP estimation, may be of particular benefit to the heart failure population. The dynamic nature of this disease necessitates frequent outpatient follow-ups for symptom management, volume status assessment, and medication dosage adjustments[78]. Although patients with heart failure can be managed safely using telemedicine, significant limitations persist[67]. In general, outpatient titration of goal-directed medical therapies is difficult due to the BP-lowering effects of many treatments. These challenges are even more difficult using telemedicine due to the inability to measure BP and other clinical parameters. For instance, volume assessment over video is similarly cumbersome to the clinician. Many telehealth and digital health interventions for heart failure have been tried, including wearable sensors, with mixed results[79,80]. Camera-enabled technologies could seamlessly integrate into existing clinical care by remote measurement of vital signs and even jugular venous pressure (JVP)[81].
Beyond BP monitoring, PPG could provide insights into systemic vascular resistance (SVR), a key component of heart failure pathophysiology. SVR is mediated by peripheral arterial smooth muscle contraction, which is known to affect the PPG waveform and, thus, PPG-based BP estimation[20]. Heart failure may be of particular importance for subsequent PPG research not only due to the unique mechanisms that may affect BP estimation, but also because the PPG waveform may indicate changes to SVR independent of BP prior to clinical decompensation. Researchers have shown that patients with Raynaud phenomenon, a vascular disease affecting the hands, when subjected to cold immersion, demonstrate significantly reduced amplitude of PPG waveforms acquired from finger clip devices[82]. Subsequent treatment with nifedipine, a calcium channel blocker, showed significant improvement in PPG waveform with less reduction in amplitude on follow-up cold immersion testing[71].
Heart failure risk models using ML to reduce hospital readmission and mortality have been proposed[83]. rPPG may add value to these models by allowing a patient to monitor their vital signs, JVP, and even SVR at home with the use of a smartphone camera [Figure 3F]. This information could be integrated into a more comprehensive risk model that could inform when a change of therapy or intervention is required.
Decentralized clinical trials
With the advent of virtual medical care comes a change to the conventional design of clinical trials. A decentralized clinical trial (DCT) minimizes or eliminates in-person contact between the subject and study team for most protocol requirements, from recruitment, intervention or medication administration to data collection[84]. Remote patient monitoring with accurate measurement of vital signs to measure treatment effects and potential adverse events is essential to implementing a DCT. Camera-based measurement of vital signs, including remote PPG-based BP, would provide a pragmatic, low-cost means that could facilitate the future expansion of DCT.
Hospitals and other healthcare facilities
The current standard of intermittent measurement of vital signs outside of the intensive care unit (ICU) in hospitals and healthcare facilities may lead to delayed recognition of and response to clinical deterioration compared to continuous monitoring. A survey of clinicians and patients showed a general consensus that devices with continuous monitoring capabilities in non-ICU patients could help to prevent clinical deterioration without limiting patient mobility and with reduced staff burden and cost[85]. A recent meta-analysis showed a trend toward reduced ICU transfers, rapid response activations, hospital length of stay, and reduced in-hospital mortality for non-ICU patients with noninvasive continuous monitoring of vital signs compared to those without[86]. To this end, subsequent reviews of the clinical impact of wearable devices to allow continuous monitoring for non-ICU patients are inconclusive but show potential[87,88].
Noninvasive, continuous BP monitoring currently does not exist. For similar reasons that motivate researchers to investigate continuous monitoring of other vital signs, continuous assessment of BP may yield benefits for inpatient care. For instance, high BP should be urgently addressed for patients experiencing active myocardial ischemia, acute decompensated heart failure, signs of end-organ damage, or an acute stroke. Conditions that cause low BP may lead to poor tissue perfusion, circulatory shock, and, eventually, cardiac arrest. Indeed, in-hospital cardiac arrest remains an infrequent though persistent problem despite interventions such as multidisciplinary rapid response teams[89].
The limitations of contact-based BP measurement differ for the inpatient setting. In acute care wards, oscillometric cuff measurement from the upper arm is the gold standard for BP measurement. Patients are frequently not amenable to arm cuff assessment due to discomfort from cuff inflation, body habitus, upper extremity deep vein thrombosis, presence of a fistula, or local intravenous infiltration. In the ICU, invasive arterial catheterization is the gold standard of continuous BP monitoring. Arterial catheterization causes discomfort and carries risks of infection, thrombosis, hematoma, and ischemia that could cause necrosis of the digits or hand. Catheters are frequently dislodged or occluded, requiring replacement. Alternatively, BP cuffs are difficult to use when actively titrating vasopressors or vasodilators.
A positive impact of the COVID-19 pandemic has been the expanded use of remote continuous monitoring in the hospital and the acceleration of research into new modalities of remote assessment of vital signs to reduce staff exposure and nosocomial infection[90]. One study of adult walk-in ED patients undergoing triage found strong agreement between camera-based and conventional measurements of HR, RR, and temperature, although BP was not studied[91]. In a study of ICU patients, contact PPG with cuff calibration was shown to accurately predict invasive arterial BP using a DL model[25]. A preliminary study for rPPG in the ICU demonstrated a promising correlation (r = 0.49 ± 0.083 standard error) with invasively measured pulse pressure, though BP was not included as an outcome[92]. Real-time HR tracking using rPPG has been shown to be reliable in the post-operative unit[93], neonatal intensive care unit[94], and hemodialysis unit[95]. As discussed, a controlled environment with consistent lighting and minimal patient movement enables accurate rPPG waveform acquisition. The ICU, dialysis units, or outpatient infusion centers may be the best sites to use rPPG since these settings are highly controlled and can provide frequent cuff calibration necessary for continuous BP monitoring [Figure 3C].
Beyond vital signs: future insights into pathophysiology from rPPG
PPG contains a wealth of hemodynamic information and the potential clinical applications of rPPG beyond BP measurement are broad. As discussed, rPPG may be able to detect changes in SVR for patients with heart failure, similar to changes in the PPG amplitude seen in patients with Raynaud phenomenon. Similarly, increased age and certain diseases decrease peripheral arterial compliance, which in turn affects the PPG waveform. Therefore, it is reasonable to hypothesize that rPPG might be able to detect changes in the peripheral vasculature that correlate with the progression of diabetes, hypertension, chronic kidney disease, and atherosclerotic cardiovascular disease (ASCVD). By studying patients with diverse cardiovascular comorbidities over time, we may be able to gain insight into rPPG features that can be incorporated into cardiovascular risk models. Passive monitoring with rPPG may afford opportunities for preventive care independent of changes in BP.
CONCLUSIONS
The potential benefits of cuffless BP measurement are too great not to pursue each solution, including camera-based rPPG. In theory, rPPG could enable consumers and clinicians to monitor BP with a common camera-equipped device for personal or telemedicine purposes. Given the ubiquity of cameras in everyday life, passive screening for hypertension could offer significant health and cost benefits to society by improving BP control and reducing cardiovascular complications. In the hospital and healthcare facilities, rPPG may address shortcomings in continuous BP monitoring for non-ICU and ICU patients.
Due to the physiologic, environmental, and technical limitations described in this paper, experts are uncertain as to the extent that rPPG will be able to estimate BP in real-world settings[4]. Cuff calibration is an essential component and it remains to be seen if it will allow for accurate BP estimation from rPPG for hours, days, or months, which ultimately will dictate when and how this technology can be applied. Even if rPPG can not exactly measure BP without cuff calibration, rPPG coupled with robust population models may be able to identify a patient who is hypertensive, normotensive, or hypotensive in real time with acceptable accuracy in the right clinical scenario.
Future studies should include patients with a wide range of BP, cardiovascular comorbidities, medications, and demographics including age, sex, race, and skin tone. The creation of a large-scale rPPG database would be invaluable to advancing knowledge and building parametric models. Artificial intelligence is essential but should not substitute for an understanding of the hemodynamic principles to explain the relationships between rPPG and BP.
Remote patient monitoring with rPPG will improve with advancements in the consumer electronics industry, such as multispectral cameras that include infrared, faster processing speeds of mobile devices, and integration of complementary health metrics such as contact PPG and ECG.
With continuous streams of hemodynamic data, rPPG has the potential for many clinical applications beyond BP measurement, such as heart failure management or ASCVD risk assessment. Future research should investigate rPPG-based measurements, including BP, HR, and SpO2, in the context of traditional algorithms used for the management of patients with cardiovascular disease. Applications of rPPG should be validated by rigorous clinical investigation and guided by regulatory and professional organizations. Given the overwhelming amount of data produced by the digitization of healthcare in the home and in the hospital, rPPG must be carefully evaluated and judiciously deployed to help patients and clinicians see the signal through the noise.
DECLARATIONS
Author’s contributionsConceptualization, outline, concept and design, original draft preparation, primary author: Curran T
Conceptualization, outline, concept and design, writing and review, editing: McDuff D, Yang E
Conceptualization, outline, concept and design, review: Liu X
Review: Narayanswamy G, Ma C, Patel S
Availability of data and materialsNot applicable.
Financial support and sponsorshipLiu X: Google (PhD Fellowship award). Narayanswamy G: Cisco (unrestricted grant). Patel S: Cisco (unrestricted grant). Yang E: Microsoft Research (unrestricted grant).
Conflicts of InterestDaniel McDuff: Google (employee, company conducts research on BP measurement and mobile technologies). Shwetak Patel: Google (leads health technologies team that focuses on research and innovation for wearables and mobile technologies related to health and wellness). Eugene Yang: Measure Labs (advisor and equity, developing technologies for vital sign monitoring)
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2023.
REFERENCES
1. Schwamm LH, Chumbler N, Brown E, et al. American Heart Association Advocacy Coordinating Committee. Recommendations for the implementation of telehealth in cardiovascular and stroke care: a policy statement from the american heart association. Circulation 2017;135:e24-44.
2. Omboni S, Padwal RS, Alessa T, et al. The worldwide impact of telemedicine during COVID-19: current evidence and recommendations for the future. Connect Health 2022;1:7-35.
3. Sharman JE, Tan I, Stergiou GS, et al. Automated “oscillometric” blood pressure measuring devices: how they work and what they measure. J Hum Hypertens 2023;37:93-100.
4. Mukkamala R, Stergiou GS, Avolio AP. Cuffless blood pressure measurement. Annu Rev Biomed Eng 2022;24:203-30.
5. Mukkamala R, Shroff SG, Landry C, Kyriakoulis KG, Avolio AP, Stergiou GS. The microsoft research aurora project: important findings on cuffless blood pressure measurement. Hypertension 2023;80:534-40.
6. Stergiou GS, Mukkamala R, Avolio A, et al. Cuffless blood pressure measuring devices: review and statement by the European Society of Hypertension working group on blood pressure monitoring and cardiovascular variability. J Hypertens 2022;40:1449-60.
7. Chan G, Cooper R, Hosanee M, et al. Multi-site photoplethysmography technology for blood pressure assessment: challenges and recommendations. J Clin Med 2019;8:1827.
8. Natarajan K, Yavarimanesh M, Wang W, Mukkamala R. Chapter 6 - Camera-based blood pressure monitoring. In: Wang W, Wang X, editors. Contactless vital signs monitoring. Academic Press; 2022. p. 117-48.
9. Mieloszyk R, Twede H, Lester J, et al. A comparison of wearable tonometry, photoplethysmography, and electrocardiography for cuffless measurement of blood pressure in an ambulatory setting. IEEE J Biomed Health Inform 2022;26:2864-75.
10. Fine J, Branan KL, Rodriguez AJ, et al. Sources of inaccuracy in photoplethysmography for continuous cardiovascular monitoring. Biosensors (Basel) 2021;11:126.
11. Perez MV, Mahaffey KW, Hedlin H, et al. Apple heart study investigators. large-scale assessment of a smartwatch to identify atrial fibrillation. N Engl J Med 2019;381:1909-17.
12. Lubitz SA, Faranesh AZ, Selvaggi C, et al. Detection of atrial fibrillation in a large population using wearable devices: the fitbit heart study. Circulation 2022;146:1415-24.
13. Statista Research Department. Published Aug 22, 2022. Available from: https://www.statista.com/statistics/330695/number-of-smartphone-users-worldwide/ [Last accessed on 19 Apr 2023].
14. Allado E, Poussel M, Renno J, et al. Remote photoplethysmography is an accurate method to remotely measure respiratory rate: a hospital-based trial. J Clin Med 2022;11:3647.
15. Allado E, Poussel M, Moussu A, et al. Accurate and reliable assessment of heart rate in real-life clinical settings using an imaging photoplethysmography. J Clin Med 2022;11:6101.
16. Nishidate I. Chapter 5 - Camera-based blood oxygen measurement. In: Wang W, Wang X, editors. Contactless vital signs monitoring. Academic Press; 2022. p. 99-116.
17. Couderc JP, Kyal S, Mestha LK, et al. Detection of atrial fibrillation using contactless facial video monitoring. Heart Rhythm 2015;12:195-201.
18. Yan BP, Lai WHS, Chan CKY, et al. Contact-free screening of atrial fibrillation by a smartphone using facial pulsatile photoplethysmographic signals. J Am Heart Assoc 2018:7.
19. Yan BP, Lai WHS, Chan CKY, et al. High-throughput, contact-free detection of atrial fibrillation from video with deep learning. JAMA Cardiol 2020;5:105-7.
20. Elgendi M. On the analysis of fingertip photoplethysmogram signals. Curr Cardiol Rev 2012;8:14-25.
21. Selvaraju V, Spicher N, Wang J, et al. Continuous monitoring of vital signs using cameras: a systematic review. Sensors (Basel) 2022;22:4097.
22. Molinaro N, Schena E, Silvestri S, et al. Contactless vital signs monitoring from videos recorded with digital cameras: an overview. Front Physiol 2022;13:801709.
23. Mukkamala R, Hahn JO, Inan OT, et al. Toward ubiquitous blood pressure monitoring via pulse transit time: theory and practice. IEEE Trans Biomed Eng 2015;62:1879-901.
24. Moço AV, Stuijk S, de Haan G. New insights into the origin of remote PPG signals in visible light and infrared. Sci Rep 2018;8:8501.
25. Image credits for Figure 1A: Cios A, Ciepielak M, Szymański Ł, Lewicka A, Cierniak S, Stankiewicz W, Mendrycka M, Lewicki S. CC BY 4.0 via Wikimedia Commons. Available from: https://doi.org/10.3390/ijms22052437 [Last accessed on 24 Apr 2023].
26. Hertzman AB. The blood supply of various skin areas as estimated by the photoelectric plethysmograph. AM J Physiol 1938;124:328-40.
27. Kamshilin AA, Nippolainen E, Sidorov IS, et al. A new look at the essence of the imaging photoplethysmography. Sci Rep 2015;5:10494.
28. Balmer J, Pretty C, Davidson S, et al. Pre-ejection period, the reason why the electrocardiogram Q-wave is an unreliable indicator of pulse wave initialization. Physiol Meas 2018;39:095005.
29. Etemadi M, Inan OT. Wearable ballistocardiogram and seismocardiogram systems for health and performance. J Appl Physiol (1985) 2018;124:452-61.
30. Steinman J, Barszczyk A, Sun HS, Lee K, Feng ZP. Smartphones and video cameras: future methods for blood pressure measurement. Front Digit Health 2021;3:770096.
31. Fleischhauer V, Feldheiser A, Zaunseder S. Beat-to-beat blood pressure estimation by photoplethysmography and its interpretation. Sensors (Basel) 2022;22:7037.
32. Hill BL, Rakocz N, Rudas Á, et al. Imputation of the continuous arterial line blood pressure waveform from non-invasive measurements using deep learning. Sci Rep 2021;11:15755.
33. Mukkamala R, Hahn JO. Toward ubiquitous blood pressure monitoring via pulse transit time: predictions on maximum calibration period and acceptable error limits. IEEE Trans Biomed Eng 2018;65:1410-20.
34. Valencell. “Valencell unveils calibration free cuffless blood pressure monitoring solution targeting over the counter use”. Available from: https://valencell.com/news/valencell-unveils-calibration-free-cuffless-blood-pressure-monitoring-solution-targeting-over-the-counter-use/ [Last accessed on 19 Apr 2023].
35. Shao D, Yang Y, Liu C, Tsow F, Yu H, Tao N. Noncontact monitoring breathing pattern, exhalation flow rate and pulse transit time. IEEE Trans Biomed Eng 2014;61:2760-7.
36. Wang Y, Liu Z, Ma S. Cuff-less blood pressure measurement from dual-channel photoplethysmographic signals via peripheral pulse transit time with singular spectrum analysis. Physiol Meas 2018;39:025010.
37. Shao D, Tsow F, Liu C, Yang Y, Tao N. Simultaneous Monitoring of Ballistocardiogram and Photoplethysmogram Using a Camera. IEEE Trans Biomed Eng 2017;64:1003-10.
38. Chan A, Chan D, Lee H, Ng CC, Yeo AHL. Reporting adherence, validity and physical activity measures of wearable activity trackers in medical research: a systematic review. Int J Med Inform 2022;160:104696.
39. Östlind E, Sant'Anna A, Eek F, Stigmar K, Ekvall Hansson E. Physical activity patterns, adherence to using a wearable activity tracker during a 12-week period and correlation between self-reported function and physical activity in working age individuals with hip and/or knee osteoarthritis. BMC Musculoskelet Disord 2021;22:450.
40. Haan G, Jeanne V. Robust pulse rate from chrominance-based rPPG. IEEE Trans Biomed Eng 2013;60:2878-86.
41. Zhou Y, Ni H, Zhang Q, Wu Q. The noninvasive blood pressure measurement based on facial images processing. IEEE Sensors J 2019;19:10624-34.
42. Blackford E, Estepp J, McDuff D. Remote spectral measurements of the blood volume pulse with applications for imaging photoplethysmography. Proc. SPIE 10501, Optical Diagnostics and Sensing XVIII: toward point-of-care diagnostics.
43. Verkruysse W, Svaasand LO, Nelson JS. Remote plethysmographic imaging using ambient light. Opt Express 2008;16:21434-45.
44. Shirbani F, Hui N, Tan I, Butlin M, Avolio AP. Effect of ambient lighting and skin tone on estimation of heart rate and pulse transit time from video plethysmography. Annu Int Conf IEEE Eng Med Biol Soc 2020;2020:2642-5.
45. McDuff D, Gontarek S, Picard RW. Improvements in remote cardiopulmonary measurement using a five band digital camera. IEEE Trans Biomed Eng 2014;61:2593-601.
46. McDuff D, Gontarek S, Picard RW. Remote detection of photoplethysmographic systolic and diastolic peaks using a digital camera. IEEE Trans Biomed Eng 2014;61:2948-54.
47. Wieringa FP, Mastik F, van der Steen AF. Contactless multiple wavelength photoplethysmographic imaging: a first step toward "SpO2 camera" technology. Ann Biomed Eng 2005;33:1034-41.
48. Poh MZ, McDuff DJ, Picard RW. Non-contact, automated cardiac pulse measurements using video imaging and blind source separation. Opt Express 2010;18:10762-74.
49. Jeong IC, Finkelstein J. Introducing Contactless Blood Pressure Assessment Using a High Speed Video Camera. J Med Syst 2016;40:77.
50. Frey L, Menon C, Elgendi M. Blood pressure measurement using only a smartphone. NPJ Digit Med 2022;5:86.
51. Jain M, Deb S, Subramanyam AV. Face video based touchless blood pressure and heart rate estimation. 2016 IEEE 18th International Workshop on Multimedia Signal Processing (MMSP). 2016; p. 1-5.
52. Secerbegovic A, Bergsland J, Halvorsen PS, Suljanovic N, Mujcic A, Balasingham I. Blood pressure estimation using video plethysmography. Conference Proceedings: 2016 IEEE 13th International Symposium on Biomedical Imaging (ISBI). 2016; p. 461-4.
53. Khong WL, Rao NSVK, Mariappan M. Blood pressure measurements using non-contact video imaging techniques. Conference Proceedings: 2017 IEEE 2nd International Conference on Automatic Control and Intelligent Systems (I2CACIS). 2017; p. 35-40.
54. Adachi Y, Edo Y, Ogawa R, Tomizawa R, Iwai Y, Okumura T. Noncontact blood pressure monitoring technology using facial photoplethysmograms. Annu Int Conf IEEE Eng Med Biol Soc 2019;2019:2411-5.
55. Luo H, Yang D, Barszczyk A, et al. Smartphone-based blood pressure measurement using transdermal optical imaging technology. Circ Cardiovasc Imaging 2019;12:e008857.
56. Fan X, Ye Q, Yang X, Choudhury SD. Robust blood pressure estimation using an RGB camera. J Ambient Intell Human Comput 2020;11:4329-36.
57. Rong M, Li K. A blood pressure prediction method based on imaging photoplethysmography in combination with machine learning. Biomed Signal Process Control 2021;64:102328.
58. Stergiou GS, Alpert B, Mieke S, et al. A universal standard for the validation of blood pressure measuring devices: association for the Advancement of Medical Instrumentation/European Society of Hypertension/International Organization for Standardization (AAMI/ESH/ISO) Collaboration Statement. J Hypertens 2018;36:472-8.
59. Block RC, Yavarimanesh M, Natarajan K, et al. Conventional pulse transit times as markers of blood pressure changes in humans. Sci Rep 2020;10:16373.
60. Oiwa K, Bando S, Nozawa A. Contactless blood pressure sensing using facial visible and thermal images. Artif Life Robotics 2018;23:387-94.
61. Shirbani F, Blackmore C, Kazzi C, Tan I, Butlin M, Avolio AP. Sensitivity of video-based pulse arrival time to dynamic blood pressure changes. Annu Int Conf IEEE Eng Med Biol Soc 2018;2018:3639-41.
62. Chen W, McDuff D. DeepPhys: video-based physiological measurement using convolutional attention networks. ECCV 2018: European Conference on Computer Vision; 2018 Sept 8 - 14.
63. Viejo C, Fuentes S, Torrico DD, Dunshea FR. Non-contact heart rate and blood pressure estimations from video analysis and machine learning modelling applied to food sensory responses: a case study for chocolate. Sensors (Basel) 2018;18:1802.
64. Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation 2005;111:697-716.
65. Pagonas N, Schmidt S, Eysel J, et al. Impact of atrial fibrillation on the accuracy of oscillometric blood pressure monitoring. Hypertension 2013;62:579-84.
66. Pilz N, Patzak A, Bothe TL. Continuous cuffless and non-invasive measurement of arterial blood pressure-concepts and future perspectives. Blood Press 2022;31:254-69.
67. Qin C, Wang X, Xu G, Ma X. Advances in cuffless continuous blood pressure monitoring technology based on PPG signals. Biomed Res Int 2022;2022:8094351.
68. Registry of Open Data on AWS. MIMIC-III (“Medical Information Mart for Intensive Care”), 2022. Available from: https://registry.opendata.aws/mimiciii [Last accessed on 19 Apr 2023].
69. McDuff D, Wander M, Liu X, Hill BL, Hernandez J, Lester J, Baltrusaitis T. SCAMPS: synthetics for camera measurement of physiological signals. In Thirty-sixth Conference on Neural Information Processing Systems Datasets and Benchmarks Track.
70. Singh A, Mountjoy N, McElroy D, et al. Patient perspectives with telehealth visits in cardiology during COVID-19: online patient survey study. JMIR Cardio 2021;5:e25074.
71. Yuan N, Pevnick JM, Botting PG, et al. Patient use and clinical practice patterns of remote cardiology clinic visits in the era of COVID-19. JAMA Netw Open 2021;4:e214157.
72. Forouzanfar MH, Liu P, Roth GA, et al. Global burden of hypertension and systolic blood pressure of at least 110 to 115 mm Hg, 1990-2015. JAMA 2017;317:165-82.
73. Turner JR, Viera AJ, Shimbo D. Ambulatory blood pressure monitoring in clinical practice: a review. Am J Med 2015;128:14-20.
74. Margolis KL, Dehmer SP, Sperl-Hillen J, et al. Cardiovascular events and costs with home blood pressure telemonitoring and pharmacist management for uncontrolled hypertension. Hypertension 2020;76:1097-103.
75. Wang X, Shao D. Chapter 1 - Human physiology and contactless vital signs monitoring using camera and wireless signals. Contactless Vital Signs Monitoring. Academic Press; 2022. p. 1-24.
76. Pokee D, Barbosa Pereira W, Mösch L, Follmann A, Czaplik M. Consciousness detection on injured simulated patients using manual and automatic classification via visible and infrared imaging. Sensors (Basel) 2021;21:8455.
77. Bragazzi NL, Zhong W, Shu J, et al. Burden of heart failure and underlying causes in 195 countries and territories from 1990 to 2017. Eur J Prev Cardiol 2021;28:1682-90.
78. Mishra K, Edwards B. Cardiac outpatient care in a digital age: remote cardiology clinic visits in the era of COVID-19. Curr Cardiol Rep 2022;24:1-6.
79. Kitsiou S, Vatani H, Paré G, et al. Effectiveness of mobile health technology interventions for patients with heart failure: systematic review and meta-analysis. Can J Cardiol 2021;37:1248-59.
80. Liu S, Li J, Wan DY, et al. Effectiveness of eHealth self-management interventions in patients with heart failure: systematic review and meta-analysis. J Med Internet Res 2022;24:e38697.
81. Amelard R, Hughson RL, Greaves DK, et al. Non-contact hemodynamic imaging reveals the jugular venous pulse waveform. Sci Rep 2017;7:40150.
82. Yang SS, Park KM, Kim YW, Kim DI. Three-grade classification of photoplethysmography for evaluating the effects of treatment in Raynaud phenomenon. Angiology 2013;64:609-13.
83. Guo A, Pasque M, Loh F, et al. Heart failure diagnosis, readmission, and mortality prediction using machine learning and artificial intelligence models. Curr Epidemiol Rep 2020;7:212-219.
84. Norman GA. Decentralized clinical trials: the future of medical product development? JACC Basic Transl Sci 2021;6:384-7.
85. Weenk M, Bredie SJ, Koeneman M, Hesselink G, van Goor H, van de Belt TH. Continuous monitoring of vital signs in the general ward using wearable devices: randomized controlled trial. J Med Internet Res 2020;22:e15471.
86. Sun L, Joshi M, Khan SN, Ashrafian H, Darzi A. Clinical impact of multi-parameter continuous non-invasive monitoring in hospital wards: a systematic review and meta-analysis. J R Soc Med 2020;113:217-24.
87. Areia C, Biggs C, Santos M, et al. The impact of wearable continuous vital sign monitoring on deterioration detection and clinical outcomes in hospitalised patients: a systematic review and meta-analysis. Crit Care 2021;25:351.
88. Leenen JPL, Leerentveld C, van Dijk JD, van Westreenen HL, Schoonhoven L, Patijn GA. Current evidence for continuous vital signs monitoring by wearable wireless devices in hospitalized adults: systematic review. J Med Internet Res 2020;22:e18636.
89. Mankidy B, Howard C, Morgan CK, et al. Reduction of in-hospital cardiac arrest with sequential deployment of rapid response team and medical emergency team to the emergency department and acute care wards. PLoS One 2020;15:e0241816.
90. Pronovost PJ, Cole MD, Hughes RM. Remote patient monitoring during COVID-19: an unexpected patient safety benefit. JAMA 2022;327:1125-6.
91. Capraro GA, Balmaekers B, den Brinker AC, et al. Contactless vital signs acquisition using video photoplethysmography, motion analysis and passive infrared thermography devices during emergency department walk-in triage in pandemic conditions. J Emerg Med 2022;63:115-29.
92. Rasche S, Trumpp A, Schmidt M, et al. Remote photoplethysmographic assessment of the peripheral circulation in critical care patients recovering from cardiac surgery. Shock 2019;52:174-82.
93. Jorge J, Villarroel M, Tomlinson H, et al. Non-contact physiological monitoring of post-operative patients in the intensive care unit. NPJ Digit Med 2022;5:4.
94. Svoboda L, Sperrhake J, Nisser M, Zhang C, Notni G, Proquitté H. Contactless heart rate measurement in newborn infants using a multimodal 3D camera system. Front Pediatr 2022;10:897961.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.

















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].