The “young-to-aged” paradigm: fantasy or feasible strategy for cardiovascular and systemic rejuvenation?
Abstract
The “young-to-aged” paradigm represents a long existing postulation that rejuvenation in aged individuals can be achieved by replacing components such as cells, tissues, or organs with young counterparts. While highly publicized human plasma exchange trials show conflicting results, preclinical studies in mice demonstrate promising yet partial functional rejuvenation, including enhanced mitochondrial metabolism, reduced neurodegeneration, and revitalized stem cells via transfer of young biological components (e.g., plasma and microbiota). Importantly, certain critical studies have demonstrated that the “young-to-aged” paradigm could achieve partial cardiovascular rejuvenation in murine models. For instance, exposure to young plasma represses cardiac hypertrophy, while exposure to young microbiota alleviates vascular dysfunction and systemic inflammation. However, significant challenges still persist, including ethical concerns, the irreversibility of aging beyond certain thresholds, and senescence propagation of young donor transplants due to aged recipient environments. This paradigm faces immense scientific and ethical hurdles, raising profound questions about feasibility and identity.
Keywords
INTRODUCTION
The fundamental question of biological identity emerges when considering cellular turnover, while most human tissues regenerate completely over time, except for neurons, prompting profound philosophical inquiry: if one replaces all aging organs with youthful counterparts, does the essential “self” still persist? This query extends beyond theory into emerging biomedical pursuits of rejuvenation. Notably, technology entrepreneur Bryan Johnson’s ambitious “Project Blueprint” exemplifies this frontier. His highly publicized regimen included experimental plasma exchange therapy, transfusing plasma from his 17-year-old son in an attempt to reverse aging biomarkers. Despite initial optimism, Johnson discontinued this intervention after observing no measurable benefits.
Human studies on rejuvenation via blood-based therapies remain contentious. In 2025, a clinical study demonstrated that therapeutic plasma exchange, combined with intravenous immunoglobulin, has reduced biological age in participants by an average of 2.6 years based on multi-omics analysis[1]. Conversely, Johnson’s personal experiment and other independent studies reported negligible effects, thus highlighting significant methodological variability and inconsistent outcomes across individuals.
In contrast, murine research has delivered more compelling evidence. Heterochronic parabiosis (HP), surgically pairing young and old mice to share circulation, systemically rejuvenated adult stem cells and their niches across multiple tissues (e.g., bone marrow, skin, liver, and skeletal muscle) in aged mice, as confirmed by single-cell sequencing[2]. This divergence between conflicting human data and promising animal models underscores a critical gap in translating cellular or systemic “young-to-aged” interventions into reliable clinical applications.
THE YOUNG-TO-AGED PARADIGM
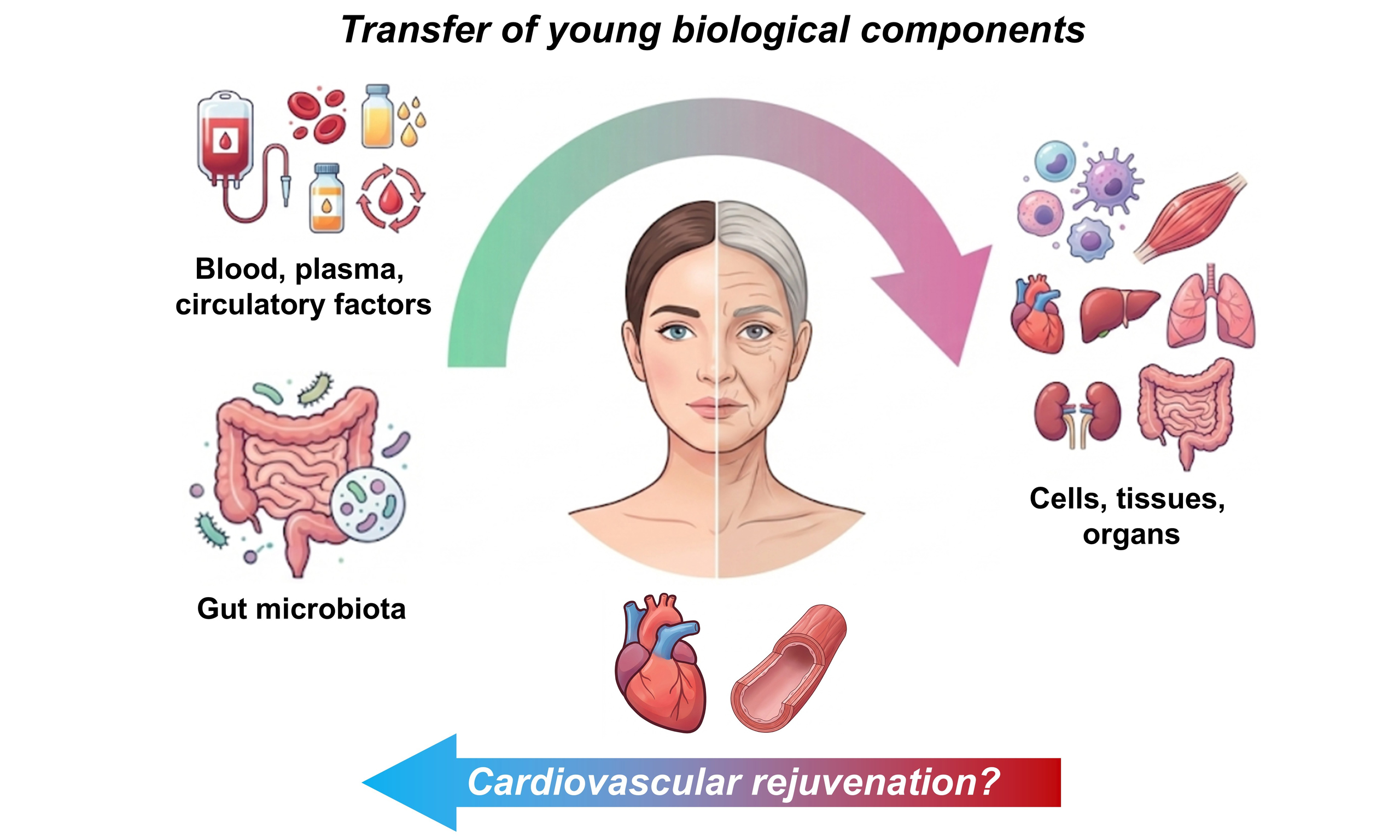
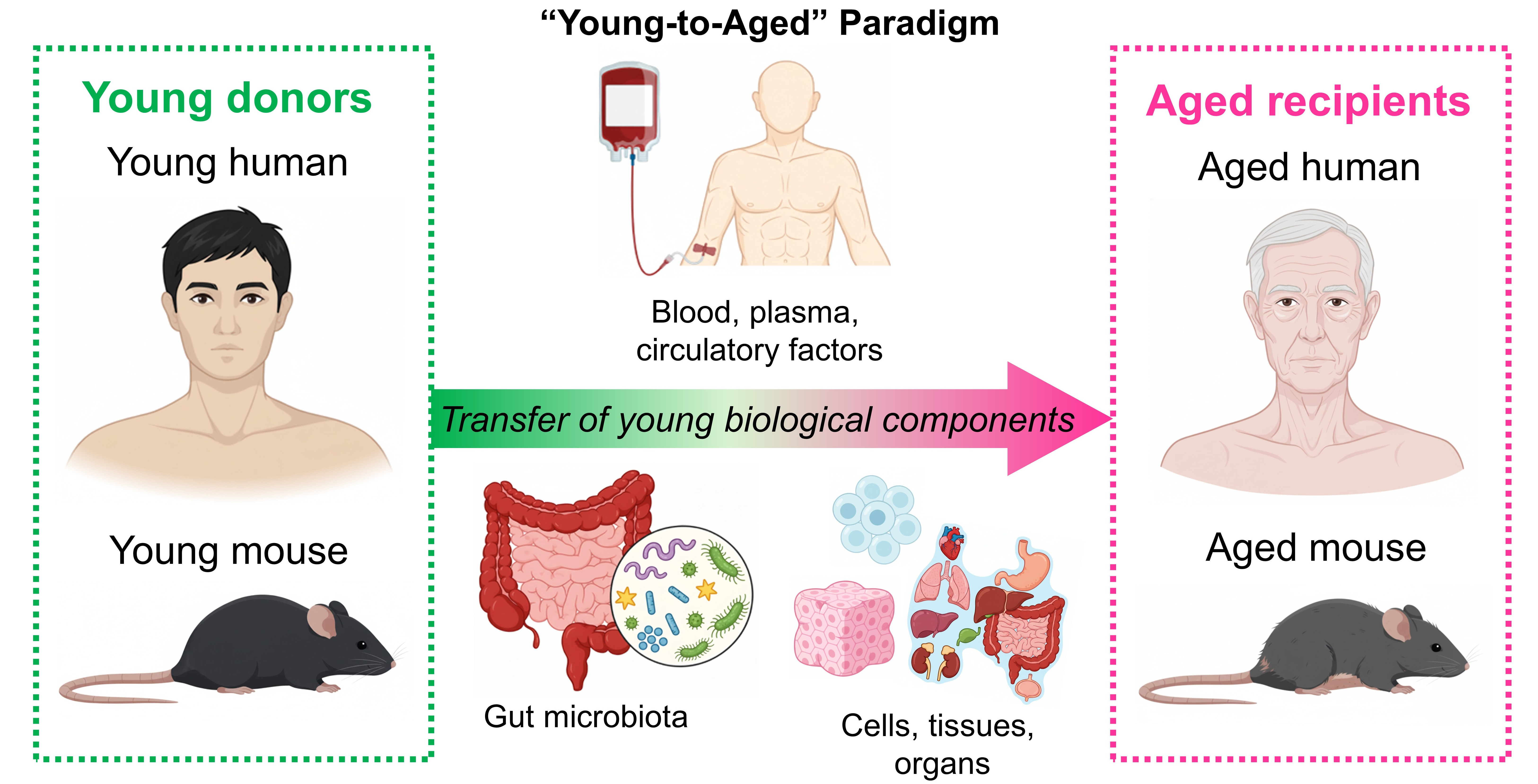
The “young-to-aged” paradigm posits that rejuvenation can be achieved by replacing aged biological components, from circulating factors to cells, tissues, and organs, with youthful counterparts [Figure 1]. Even if not explicitly stated, this concept often underpins many aging and rejuvenation studies. Robust evidence supports its feasibility, particularly in preclinical models demonstrating functional reversal of
Figure 1. The concept of the “young-to-aged” paradigm. In many aging and rejuvenation studies, it is hypothesized that transfer of young biological components (e.g., blood/plasma, circulating factors, gut microbiota, and cells/tissues/organs) from young donors shall elicit rejuvenation effects on aged recipients.
Key successes emerge from interventions on young plasma therapies. HP and plasma infusion studies in aged mice have revealed that young plasma, at least partially, counteracts functional decline, mitochondrial dysfunction, and cellular senescence. Notably, small extracellular vesicles from young mouse plasma encapsulate rejuvenating microRNAs (miRNAs) (e.g., miR-144-3p, miR-149-5p, and miR-455-3p), which could potentially increase mitochondrial abundance, restore mitochondrial respiration to promote adenosine triphosphate (ATP) synthesis in the brain and muscle through Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) upregulation, and extend lifespan by approximately 12% in aged mice[3].
A previous HP study has shown that young mouse plasma significantly restored synaptic protein levels, improved memory, and normalized aberrant signaling pathways in a mouse model of Alzheimer’s disease, without reducing amyloid plaque burden[4]. Another HP study also indicated that young blood could reprogram the aging mouse brain transcriptome at single-cell resolution, reversing age-related gene signatures across multiple cell types, particularly in brain vascular endothelial cells, through modulating pathways related to inflammation, mitochondrial function, and senescence[5].
Beyond circulating factors, this paradigm extends to cellular, tissue-, and organ-level replacements. For example, transplantation of young hematopoietic stem cells (HSCs) into aged mice was shown to alleviate multiple aging phenotypes. Young HSCs significantly rejuvenated blood composition (higher B cell but lower T cell proportions), boosted immune function (increased naïve T cell proportion), reduced blood epigenetic age, and enhanced physical capabilities in aged mice[6].
Interestingly, xenotransplantation of brown adipose tissue from young rats (2 weeks old) significantly improved follicle survival, and therefore extended the ovarian lifespan in aged mice (8 months old)[7]. This study positions xenotransplantation as a potentially viable tissue source for developing rejuvenation therapeutics. Meanwhile, transplantation of young mouse ovaries could increase life span and retard the progression of age-related cardiomyopathy in postreproductive-aged mice, where a young ovary is believed to elicit cardioprotective effects through restoring youthful hormonal profile (e.g., estrogen)[8]. However, whether transplantation of other tissues and organs can achieve comparable rejuvenation effects, further future research is warranted.
THE PARADIGM IN CARDIOVASCULAR REJUVENATION
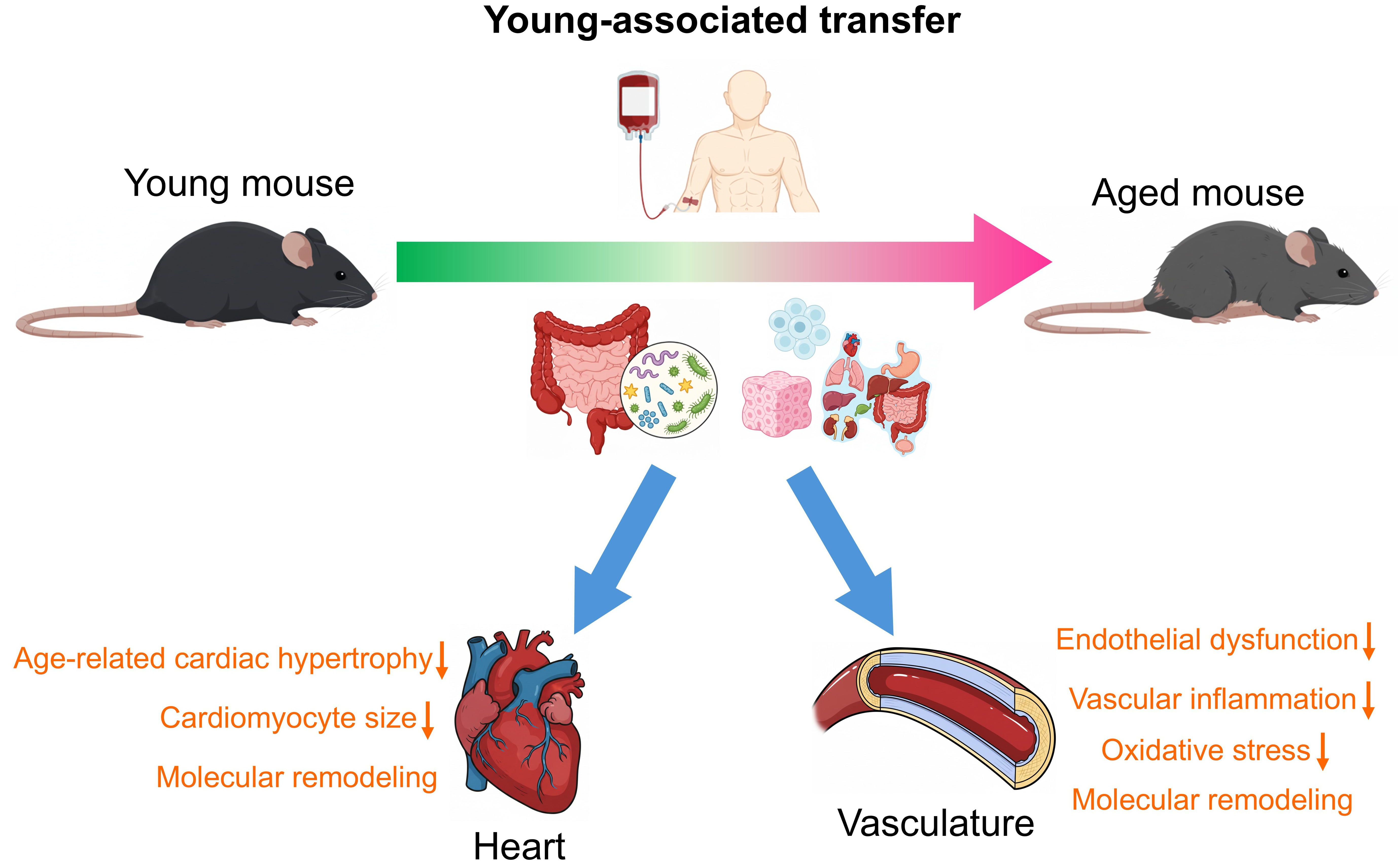
The “young-to-aged” paradigm, wherein youthful biological components are transferred to aged organisms, demonstrates significant potential for cardiovascular rejuvenation in both cardiac and vascular systems [Figure 2]. This approach has been functionally validated through multiple pathways, offering potential avenues to counteract age-related deterioration.
Figure 2. The “young-to-aged” paradigm in mouse cardiovascular rejuvenation. In some mouse studies, it was shown that young biological components from young donor mice elicit beneficial effects on the heart and the vasculature of aged mice. Particularly,
Cardiac rejuvenation
In the cardiac system, HP studies established that exposure to young circulation reverses age-related cardiac hypertrophy in aged mice. Along with reduced cardiomyocyte size, molecular remodeling occurs via normalized natriuretic peptide expression, and restored sarco/endoplasmic reticulum calcium ATPase 2 (SERCA2) activity, primarily mediated by circulating factors such as growth differentiation factor 11 (GDF11), but not hemodynamic effects of HP[9,10]. The vascular system can also similarly benefit from the “young-to-aged” paradigm. A previous HP study has indicated that exposure to young circulation could attenuate endothelial dysfunction, vascular oxidative stress, and potentially reverse age-related mitochondrial dysfunction in the aged vasculature[11]. Another HP study showed that young circulation facilitated neurovascular rejuvenation in aged mice through increasing the cortical capillary density and restoring blood-brain barrier integrity[12].
Gut-vascular connection
Functionally, the gut microbiota can be treated as a “virtual endocrine organ”[13], with fecal microbiota transplantation (FMT) from young donors potentially producing multifaceted rejuvenating effects in aged hosts. For instance, FMT from young mice has been shown to rejuvenate HSC in aged mice by mitigating inflammation, upregulating the forkhead box O (FoxO) signaling pathway, and facilitating lymphoid differentiation[14]. Meanwhile, transplanting gut microbiota from young mice into aged mice reversed
Notably, the vascular system can also benefit from the young systemic factors derived from the intestine through the gut-vascular axis. Our laboratory has previously shown that FMT from young donor mice improved endothelial function via reduced oxidative stress and activation of Adenosine Monophosphate (AMP)-activated protein kinase (AMPK)/sirtuin 1 (SIRT1) pathway, preserved telomere integrity in vascular tissues, and alleviated systemic inflammation through restoring intestinal barrier function[16]. Furthermore, our laboratory has also extended the paradigm to perivascular adipose tissue (PVAT).
Collectively, these findings establish that youthful systemic factors, whether circulating proteins, microbial ecosystems, or adipose-derived signals, can rejuvenate aged cardiovascular tissues through conserved mechanisms involving inflammation resolution, metabolic reprogramming, and senescence attenuation. The gut-vascular connection also emerges as a critical intervention target for age-related cardiovascular pathologies.
CHALLENGES AND CONCERNS
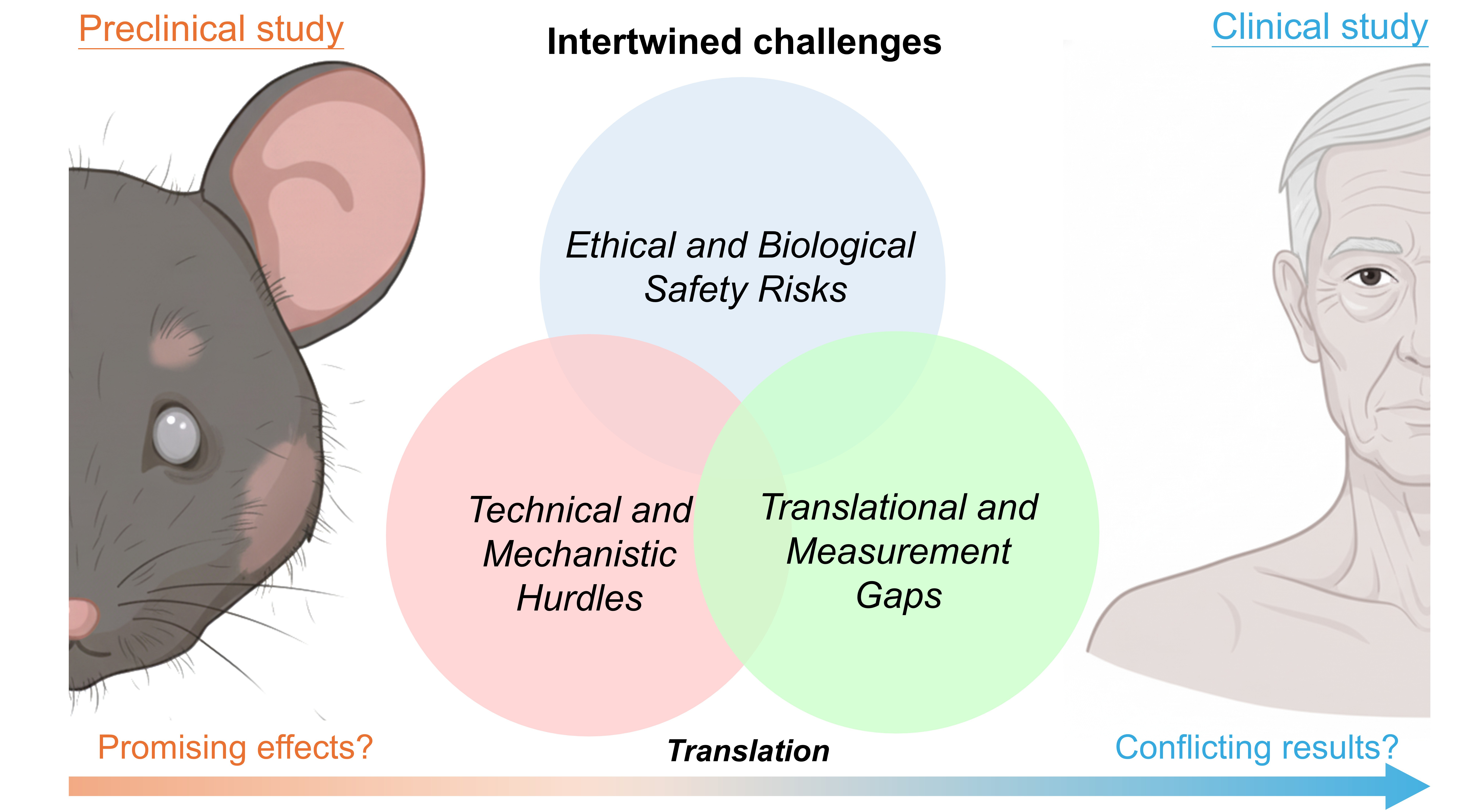
The translation of the “young-to-aged” paradigm into clinical applications confronts multidimensional challenges spanning ethical, biological, and technical domains [Figure 3].
Figure 3. Intertwined challenges in bench-to-bedside translation of the paradigm. The successful translation of the “young-to-aged” paradigm into clinical applications is hindered by ethical and biological safety risks, technical and mechanistic hurdles, and translational and measurement gaps.
Ethical and biological safety risks
Sourcing young biological materials (e.g., plasma, tissues, and organs) raises profound ethical dilemmas, including donor exploitation and equitable access. Specifically, the procurement of young biological materials, such as plasma or stem cells, raises significant ethical questions regarding donor consent and the potential for exploitation, while the widespread adoption of rejuvenation therapies could precipitate profound societal challenges, including equitable access and the redefinition of the human lifespan.
Across different “young-to-aged” interventions, immunological risks represent a major clinical hurdle. These adverse reactions can range from minor infusion-related allergic responses to severe, life-threatening conditions such as graft-versus-host disease following bone marrow transplants, or the transfer of
Additionally, senescence propagation can occur bidirectionally. Notably, aged recipient microenvironments could potentially accelerate damage in young transplants, while aged organs/tissues might induce cellular aging in young recipients[18]. In general, older donor age is often associated with worse clinical outcomes (e.g., reduced graft survival and higher risk of graft failure) for various organs (e.g., liver, kidney, heart, and lung) in young recipients[19]. Coherently, in the context of FMT, our laboratory has previously shown that gut microbiota from aged mice could induce endothelial dysfunction, vascular inflammation, and telomere dysfunction in the vasculature, potentially promoting premature vascular aging[20]. Although stem
Technical and mechanistic hurdles
Age-related damages beyond critical thresholds, such as mitochondrial DNA mutations or epigenetic alterations, often limit rejuvenation effect to “slowing” rather than “reversing” the age-related decline. In other words, it remains doubtful whether the “young-to-aged” paradigm can effectively target mitochondrial DNA mutations and epigenetic alterations.
This introduces the critical concept of a biological “point of no return”, a threshold of accumulated damage that becomes refractory to paradigmatic rejuvenation. While some alterations may be malleable, we speculate that certain forms of damage are fundamentally irreversible with current approaches. Prime candidates include the accumulation of mitochondrial DNA mutations to a level that causes bioenergetic collapse, and the progressive cross-linking of structural proteins (e.g., collagen) in the extracellular matrix, which could create a permanent structural scaffold resistant to systemic factors. Identifying which
Neurointegration, particularly the re-establishment of neural connections between the transplanted organs/tissues and the recipient nervous system, remains another key technical hurdle. Moreover, although endocrine organs/tissues (e.g., ovaries and adipose tissues) show heightened therapeutic efficacy in murine studies due to systemic hormonal influence (e.g., estrogen-mediated cardioprotection), other
Translational and measurement gaps
The translational discrepancies can largely be attributed to fundamental physiological distinctions between species (e.g., human and mouse), coupled with challenges in accurately replicating therapeutic windows, both in terms of dosage and the timing of intervention relative to disease progression. Besides, the divergent outcomes in different human trials can potentially arise from variations in sourcing of biological materials (e.g., plasma) and processing protocols, heterogeneity in participant demographics (e.g., age and baseline health), and the ongoing challenge of validating robust, standardized biomarkers for biological age.
The absence of highly standardized biomarkers for biological age impedes outcome evaluation. Current metrics (e.g., epigenetic clock) might suffer from poor reproducibility across studies[21]. Furthermore, preclinical models for multi-organ transplantation, even in murine systems, are lacking, restricting insights into systemic interactions of the paradigm. To our knowledge, longitudinal human lifespan data are scarce, with evidence limited to short-term surrogate markers rather than mortality outcomes. Crucially, the mechanistic underpinnings of aging remain enigmatic. While twelve hallmarks (e.g., mitochondrial dysfunction, stem cell exhaustion, telomere attrition, and chronic inflammation) are established[22], their hierarchy in vivo is still poorly defined, hindering interventions targeting “point-of-no-return” thresholds.
CONCLUSION
The pursuit of cardiovascular and systemic rejuvenation through the “young-to-aged” paradigm in humans remains fraught with both ethical and technical hurdles. While bench study on murine models demonstrates remarkable rejuvenating effects in various organs and tissues, particularly the heart and the vasculature, translating these findings into bedside studies faces significant challenges. Bryan Johnson’s discontinued plasma trials exemplify the efficacy-safety paradox in human applications, compounded by inconsistent clinical outcomes. Ethically and technically, systemic replacement of most organs, particularly the brain with restricted regenerative capacity, remains implausible. Moreover, combining young systemic factors with senolytics or metabolic modulators might potentially enhance efficacy, yet certain synergistic therapies are underexplored. Collectively, the paradigm still faces intertwined hurdles.
Looking ahead, this paradigm invites consideration of the relationship between our biological composition and the continuity of self-identity. Cellular turnover naturally refreshes most somatic cells, but artificial wholesale replacement risks disrupting identity continuity anchored by the brain. Until ethical frameworks and clinical safety mature, this paradigm remains a provocative concept rather than a feasible rejuvenation strategy for bedside applications.
DECLARATIONS
Acknowledgements
We thank the members of the Yu Huang group for the constructive discussion.
Authors’ contributions
Made substantial contributions to the conception, writing, and editing of the manuscript: Cheng CK,
Availability of data and materials
Not applicable.
Financial support and sponsorship
This work was supported by the Hong Kong Research Grants Council (T12-101/23-N, SRFS2021-4S04) and the City University of Hong Kong Start-up Fund. This work was also substantially supported by a fellowship award from the Research Grants Council of the Hong Kong Special Administrative Region, China (Project No. CityU PDFS2223-1S01).
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Fuentealba M, Kiprov D, Schneider K, et al. Multi-omics analysis reveals biomarkers that contribute to biological age rejuvenation in response to single-blinded randomized placebo-controlled therapeutic plasma exchange. Aging Cell. 2025;24:e70103.
2. Ma S, Wang S, Ye Y, et al. Heterochronic parabiosis induces stem cell revitalization and systemic rejuvenation across aged tissues. Cell Stem Cell. 2022;29:990-1005.e10.
3. Chen X, Luo Y, Zhu Q, et al. Small extracellular vesicles from young plasma reverse age-related functional declines by improving mitochondrial energy metabolism. Nat Aging. 2024;4:814-38.
4. Middeldorp J, Lehallier B, Villeda SA, et al. Preclinical assessment of young blood plasma for alzheimer disease. JAMA Neurol. 2016;73:1325-33.
5. Ximerakis M, Holton KM, Giadone RM, et al. Heterochronic parabiosis reprograms the mouse brain transcriptome by shifting aging signatures in multiple cell types. Nat Aging. 2023;3:327-45.
6. Wang Y, Zhang W, Zhang C, Van HQT, Seino T, Zhang Y. Reducing functionally defective old HSCs alleviates aging-related phenotypes in old recipient mice. Cell Res. 2025;35:45-58.
7. Chen LJ, Yang ZX, Wang Y, et al. Single xenotransplant of rat brown adipose tissue prolonged the ovarian lifespan of aging mice by improving follicle survival. Aging Cell. 2019;18:e13024.
8. Mason JB, Cargill SL, Griffey SM, Reader JR, Anderson GB, Carey JR. Transplantation of young ovaries restored cardioprotective influence in postreproductive-aged mice. Aging Cell. 2011;10:448-56.
9. Loffredo FS, Steinhauser ML, Jay SM, et al. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell. 2013;153:828-39.
11. Kiss T, Tarantini S, Csipo T, et al. Circulating anti-geronic factors from heterochonic parabionts promote vascular rejuvenation in aged mice: transcriptional footprint of mitochondrial protection, attenuation of oxidative stress, and rescue of endothelial function by young blood. Geroscience. 2020;42:727-48.
12. Gulej R, Nyúl-Tóth Á, Csik B, et al. Young blood-mediated cerebromicrovascular rejuvenation through heterochronic parabiosis: enhancing blood-brain barrier integrity and capillarization in the aged mouse brain. Geroscience. 2024;46:4415-42.
13. Clarke G, Stilling RM, Kennedy PJ, Stanton C, Cryan JF, Dinan TG. Minireview: gut microbiota: the neglected endocrine organ. Mol Endocrinol. 2014;28:1221-38.
14. Zeng X, Li X, Li X, et al. Fecal microbiota transplantation from young mice rejuvenates aged hematopoietic stem cells by suppressing inflammation. Blood. 2023;141:1691-707.
15. Parker A, Romano S, Ansorge R, et al. Fecal microbiota transfer between young and aged mice reverses hallmarks of the aging gut, eye, and brain. Microbiome. 2022;10:68.
16. Cheng CK, Gao J, Kang L, Huang Y. Fecal microbiota transfer from young mice reverts vascular aging hallmarks and metabolic impairments in aged mice. Aging Dis. 2024;16:1576-85.
17. Cheng CK, Huang X, Meng S, Huang Y. The pro-aging and rejuvenating effects of young and aged perivascular adipose tissues on endothelial function and inflammation. Biogerontology. 2025;26:134.
18. Lau A, Kennedy BK, Kirkland JL, Tullius SG. Mixing old and young: enhancing rejuvenation and accelerating aging. J Clin Invest. 2019;129:4-11.
19. Dayoub JC, Cortese F, Anžič A, Grum T, de Magalhães JP. The effects of donor age on organ transplants: a review and implications for aging research. Exp Gerontol. 2018;110:230-40.
20. Cheng CK, Ye L, Zuo Y, et al. Aged gut microbiome induces metabolic impairment and hallmarks of vascular and intestinal aging in young mice. Antioxidants. 2024;13:1250.
21. Mei X, Blanchard J, Luellen C, Conboy MJ, Conboy IM. Fail-tests of DNA methylation clocks, and development of a noise barometer for measuring epigenetic pressure of aging and disease. Aging. 2023;15:8552-75.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Special Topic
Copyright
Data & Comments
Data
















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].