Robotic coronary artery bypass grafting: current status and future perspectives
Abstract
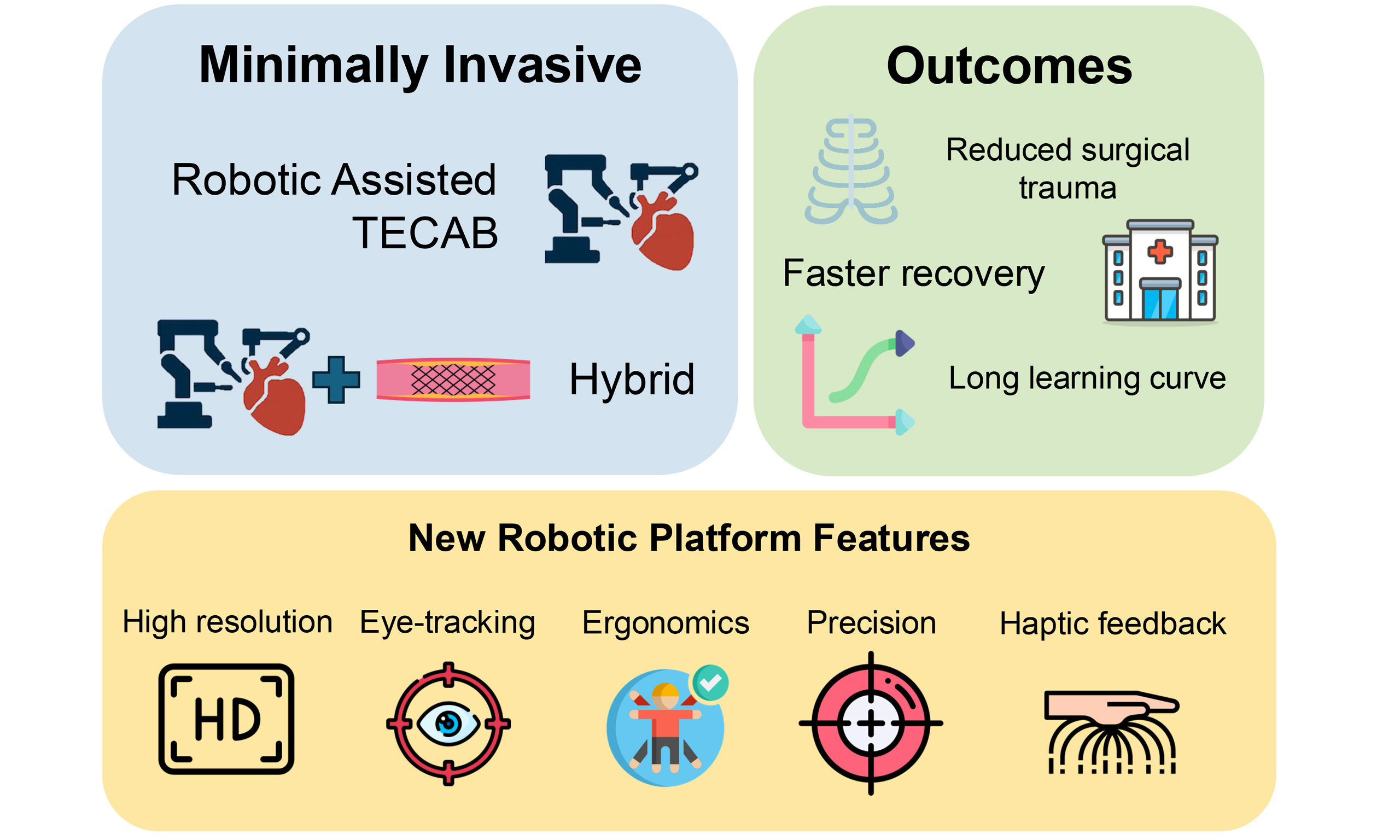
Robotic coronary artery bypass grafting (CABG) has emerged as a minimally invasive alternative to traditional open-heart surgery, offering reduced surgical trauma and faster recovery. Techniques include robotic-assisted minimally invasive direct coronary artery bypass (RA-MIDCAB), where the internal thoracic artery is harvested robotically and anastomosed via mini-thoracotomy, and totally endoscopic CABG (TECAB), which avoids thoracotomy altogether. Hybrid coronary revascularization, combining robotic left internal thoracic artery-left anterior descending (LITA-LAD) grafting with percutaneous coronary intervention, provides a patient-specific strategy for multivessel disease. While off-pump approaches reduce recovery time, on-pump techniques remain valuable in complex cases and are mainly performed at expert centers. Despite promising outcomes, such as low mortality, short hospital stays, and high graft patency, widespread adoption remains limited due to high costs and technical complexity. Mastering the technique and performing more advanced procedures like TECAB require a long learning curve. Proficiency improves with experience, typically after 10-50 cases, while full mastery may require hundreds of procedures. Most centers now favor RA-MIDCAB over TECAB, which remains limited to a few high-volume institutions. Recent advances in robotic platforms, including systems from Intuitive Surgical, Medtronic, CMR Surgical, and others, promise improved ergonomics, precision, and cost-efficiency. Features like haptic feedback, eye-tracking, and remote operation are advancing robotic surgery into a new era, though challenges persist in training and accessibility. As robotic systems continue to evolve and integrate artificial intelligence and telesurgical capabilities, broader adoption may become feasible, particularly when combined with hybrid revascularization strategies in selected patients.
Keywords
INTRODUCTION
The spectrum of robotic coronary artery bypass techniques
Robotic coronary artery bypass grafting (CABG) represents a significant advancement in the field of minimally invasive cardiac surgery, offering alternatives to traditional median sternotomy. This approach utilizes robotic assistance to enhance precision and reduce the invasiveness of coronary revascularization procedures. Several forms of robotic-assisted CABG (RACABG) have been developed, differing in their invasiveness, use of cardiopulmonary bypass, and integration with percutaneous coronary intervention (PCI).
Robotic-assisted minimally invasive direct coronary artery bypass
Among the various robotic CABG techniques, robotic-assisted minimally invasive direct coronary artery bypass (RA-MIDCAB) is the most widely adopted[1]. In this approach, the left internal thoracic artery (LITA) is harvested using robotic instruments inserted through small thoracic ports, providing enhanced visualization and precision. The anastomosis to the left anterior descending (LAD) artery is then performed manually through a small right anterior thoracotomy without the use of cardiopulmonary bypass
This technique combines the benefits of robotic and conventional methods: the robotic system facilitates a careful, atraumatic dissection of the LITA, often allowing harvest up to its distal bifurcation, which not only minimizes the risk of conduit injury but also makes longer grafts available for possible multi-vessel revascularization[3]. At the same time, the direct hand-sewn anastomosis preserves surgical control and tactile feedback.
In contrast to conventional MIDCAB, which requires significant retraction using specialized rib spreaders to expose the mammary artery, which is often a key contributor to postoperative pain, RA-MIDCAB minimizes chest wall trauma[4]. Since the LITA is harvested entirely endoscopically, the thoracotomy used for the anastomosis is much smaller and subjected to far less mechanical stress. This reduction in tissue distortion and retraction has been associated with decreased postoperative discomfort and faster recovery for patients[5,6].
Totally endoscopic coronary artery bypass
Totally endoscopic coronary artery bypass (TECAB) represents the most sophisticated and least invasive modality within RACABG[7]. In this approach, both the internal thoracic artery harvesting and the coronary anastomoses are performed entirely through port-based access using robotic instruments, eliminating the need for a thoracotomy.
TECAB may be carried out either on a beating heart (off-pump) or with cardiopulmonary support
Hybrid coronary revascularization
RACABG plays a key role in hybrid coronary revascularization (HCR), a strategy particularly suited for patients with multivessel coronary artery disease. In this approach, surgical revascularization is used to graft the LITA to the LAD artery, while PCI is used to treat additional lesions, most commonly in the right coronary or circumflex distributions. This can be executed in a sequential (staged) or concomitant approach[12,13].
The traditional hybrid sequence involves performing the surgical LAD bypass first, followed by PCI to other vessels. In contrast, the “reverse hybrid” approach begins with PCI, which is often necessary in the setting of an acute coronary syndrome where non-LAD vessels are involved and require urgent stenting. Once stabilized, the patient can then undergo robotic LITA-to-LAD bypass[14].
By combining the durable survival benefit of the LITA-LAD graft with the reduced invasiveness and faster recovery associated with PCI, hybrid revascularization aims to offer a tailored, less traumatic treatment strategy for complex coronary disease.
On-pump vs off-pump robotic CABG
RA-MIDCAB and TECAB procedures can be performed on-pump or off-pump. Off-pump approaches, performed on a beating heart, are linked to reduced neurological risks and shorter recovery times. However, they can be technically demanding, particularly in cases involving intramyocardial LADs or patients with hemodynamic instability. In such scenarios, on-pump techniques offer a stable, motionless surgical field, which is especially beneficial for intricate or multi-vessel TECAB procedures. These on-pump methods are typically best suited to high-volume centers with substantial expertise in robotic cardiac surgery[15].
HOW TO DO IT
A description of how to perform RACABG has already been reported by our team[16]. In brief, the patient is positioned with the left side of the body at the table's edge and the left shoulder slightly overhanging to facilitate optimal robotic arm movement. The mini-thoracotomy incision is guided by a three-coordinate system: one for vertical alignment between the suprasternal notch and xiphoid process, one for lateral positioning of the LAD based on chest X-rays, and one for LAD segment targeting depending on heart mobility with CO2 insufflation. Additional robotic ports are placed in a straight line, spaced 8-10 cm apart. The pericardium is opened before harvesting the LITA to prevent injury to a suspended artery. The incision is made anteriorly and laterally to the pulmonary artery and ventricle, avoiding apex incisions to maintain heart position. A wide incision improves visualization and safety. The LAD is then identified.
LITA harvest is performed carefully with low cautery settings, beginning proximally and continuing distally. In bilateral harvests, the right ITA is taken first, with port positioning adapted to preserve the LITA. After LITA harvest, heparin is given to achieve an ACT of 300 s, and a vasodilating solution is applied through a feeding tube.
For RA-MIDCAB, a final thoracotomy incision is made, and a soft tissue retractor is placed through the endoscopic port or the thoracotomy to complete the procedure[17]. In TECAB, no thoracotomy is required, and the anastomosis is completed entirely using robotic arms. In this approach, sutures and shunts are introduced into the chest cavity through the working port[18,19]. For either RA-MIDCAB or TECAB, if the procedure is performed off-pump, the coronary target(s) are exposed with the aid of a stabilizer.
LEARNING CURVE
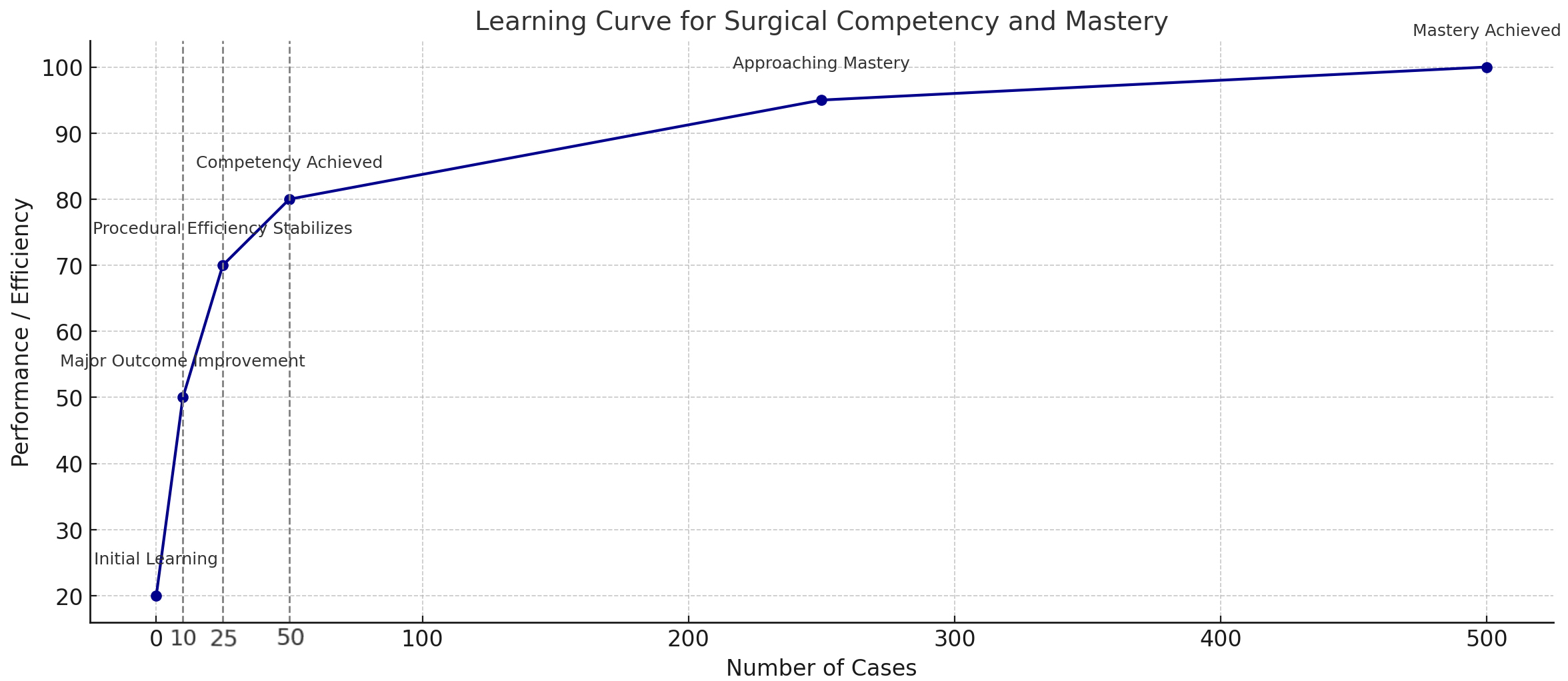
For the first step of RACABG that involves mammary artery harvesting, efficiency and clinical outcomes improve with experience, with an initial rapid learning phase between 5-20 cases[10,11,20,21]. A recent study has demonstrated how patient anatomy does not affect the learning curve and clinical outcomes[22]. The efficiency of port placement, the speed of the LITA to coronary artery anastomosis, and overall operative times have been shown to follow a similar pattern[10,11,21,23]. Overall, the learning curve for RA-CABG shows that competency can be achieved with 10-50 cases. Beyond the 10th case, significant improvements in morbidity, mortality, and procedural success are evident, though procedural efficiency stabilizes more slowly, around the 25th case[24]. It requires a dedicated team for the initial 25-50 cases, with careful management of instruments and direct endoscopic vision to prevent injury. For the first 20-30 cases, longer procedural times and higher conversion rates to sternotomy are common. Early in the surgeon’s experience, it is vital to implement quality control measures, such as angiographic patency assessments, to ensure both clinical outcomes and provide feedback for technical improvement[25].
On the other hand, mastery, defined as no significant further improvements in outcomes or efficiency, generally requires 250-500 cases[25]. A representative learning curve synthesized from findings reported in the published literature is depicted in Figure 1. Surgeons who have gained experience have advanced to more complex procedures, such as bilateral internal mammary artery harvest and multi-arterial grafting, but these techniques should only be attempted after extensive experience. TECAB is the most challenging and least invasive form of CABG[12]. Proficiency requires extensive experience, with a team well-versed in various components of the procedure. TECAB presents a significantly steeper learning curve compared to RACABG. Therefore, the learning process should follow a structured, stepwise progression, beginning with RACABG before advancing to TECAB. Initiating TECAB without first acquiring proficiency in RACABG is not recommended[26]. Simulation training is recommended for robotic anastomoses before starting clinical cases. TECAB proficiency develops over time, with initial cases focused on single-vessel bypasses, and proctored cases are encouraged for guidance. The stepwise progression toward mastering coronary artery revascularization, including TECAB, has been previously documented[25,27].
CURRENT ROLE OF ROBOTIC CABG IN SURGICAL CORONARY REVASCULARIZATION
As cardiac surgery continues to evolve toward less invasive approaches aimed at minimizing patient morbidity and accelerating recovery, RACABG has emerged as a valuable tool. It offers a surgeon-driven alternative to multi-vessel coronary stenting, particularly in select patient populations where the long-term benefits of surgical revascularization are preferred[28]. Both MIDCAB and TECAB seek to merge the durability of arterial grafting with the advantages of a minimally invasive recovery.
Robotic MIDCAB typically involves robotic-assisted harvesting of the LITA, followed by direct anastomosis to the LAD through a limited thoracotomy. While this preserves the clinical efficacy of the LITA-LAD graft, it is inherently limited by reduced visualization and restricted instrument mobility due to the narrow access (“keyhole” surgery). TECAB, by contrast, is a fully endoscopic, port-access procedure that eliminates the need for thoracotomy entirely[29]. This approach enables dynamic camera positioning within the thoracic cavity, offering superior visualization of coronary targets, particularly along the lateral wall. TECAB also enables consistent use of in situ BITA grafts, regardless of body mass index, sex, or glycemic status, thereby minimizing the risk of sternal wound complications, especially in high-risk groups such as diabetics and obese patients[30]. Moreover, the absence of thoracotomy contributes to reduced postoperative pain, shorter hospital stays, and a faster return to daily activities[31].
While RACABG is not yet widely adopted across all surgical centers, largely due to its technical complexity and resource requirements, it is gaining traction, especially when used in hybrid strategies alongside PCI[32]. In this context, it offers a balanced approach that leverages the strengths of both surgical and percutaneous revascularization. Hybrid coronary revascularization has gained considerable momentum with the advent of techniques that allow placement of two arterial grafts to the left coronary circulation. This strategy can be effectively complemented by stenting of the right coronary artery when anatomically appropriate[33]. Emerging evidence suggests that targeting the two dominant left-sided vessels with arterial grafts may confer a greater long-term survival benefit compared to achieving full anatomical revascularization using a single ITA combined with saphenous vein grafts[34].
HISTORY AND CURRENT TRENDS
The da Vinci surgical robot (Intuitive Surgical, Sunnyvale, CA, USA) was first used in 1998 for on-pump coronary bypass surgery in 4 patients at a European center[35], and soon after, pioneering surgeons performed the first off-pump TECAB[36]. The first beating heart TECAB in Canada used the Zeus surgical robot (Computer Motion Inc., Goleta, California, USA)[37], though it was later discontinued due to the lack of multi-wristed tools. However, following an initial positive wave, the adoption of robotic coronary surgery has remained quite limited. A report from the Society of Thoracic Surgeons Adult Cardiac Surgery Database indicated that robotic technology was used in only 0.97% of all CABG procedures in the United States between 2006 and 2012[38]. The likely reasons for this low adoption included the high cost of the robot and its disposable tools, longer operative times, and the need for specialized training without a clear demonstration of better outcomes. Therefore, despite advancements in robotic instrumentation, such as the introduction of the Endowrist Stabilizer (Intuitive Surgical, Sunnyvale, CA, USA), only a small number of dedicated programs have continued to perform this procedure[33]. Indeed, a full robotic procedure proved to be challenging particularly due to the complexity of the anastomosis. Although the use of an automated distal anastomotic connector (C-Port, Aesculap, Tuttlingen, Germany) helped to simplify the procedure, its limited adoption, even in sternotomy cases, ultimately led to its discontinuation. As a result, TECAB saw limited adoption overall. While excellent outcomes with the TECAB approach are reported by limited institutions[7,39,40], most surgeons shifted to using the robot only for harvesting one internal thoracic artery, followed by a left mini-thoracotomy for the anastomosis (RACABG)[12].
A review of robotic cardiac surgery in Europe up to 2020 confirmed the trend that the vast majority of surgeries (1250 out of 1266, 98.7%) were carried out as RA-MIDCAB, with only 16 TECAB cases (1.3%)[41], reflecting the current limited availability of the Endowrist Stabilizer. In this regard, an alternative stabilization technique has been described with the GelPOINT-mini (Applied Medical, Santa Margarita, CA, USA)[19], while awaiting a new specific tool from the industry. Between 1996 and 2019, 74 articles reported a total of 11,135 minimally invasive CABG procedures. These publications were predominantly from Central Europe, the U.S. East Coast and South, and East Asia, indicating regional centers of high activity[1]. In 2021, a total of 400 MICS-CABG procedures were recorded in Japan, according to the Japanese Cardiovascular Surgery Database. The majority of these cases (92%) involved either a single or double bypass. Most procedures utilized the LITA, and off-pump CABG was employed in 89% of cases. Notably, multivessel bypass was performed in 93 patients (23%), suggesting that this approach was selectively applied to specific cases[42].
CURRENT OUTCOMES
Patients undergoing conventional CABG tend to have significantly more preoperative risk factors compared to RACABG[15]. This may reflect a tendency among surgeons to rely on the more established CABG technique for higher-risk individuals, while selecting the robotic approach for patients with more stable conditions and lower overall risk. This is particularly true for TECAB, which is not appropriate for all cases of coronary artery disease requiring surgery[43]. Moreover, the patient population analyzed for RACABG generally consists of relatively younger individuals, with most having good baseline cardiac function[44]. Nevertheless, the reported results of patients undergoing RACABG demonstrated very promising outcomes.
A European review of 1,266 patients who underwent RACABG reported a bleeding revision rate of 2.1%, a perioperative stroke rate of 0%, and an in-hospital mortality rate of just 0.6%. The average length of hospital stay was 6.4 days[41].
A recent meta-analysis, including 18 studies and 2,774 patients, reported an average hospital stay of under 6 days and a low 30-day mortality rate of just 0.36%[45]. On average, surgeries lasted approximately 304 min, with internal mammary artery harvest taking about 38 min. Conversion to open surgery was necessary in 4.7% of cases (95%CI: 1.6%-9.1%). Within the first 30 days, complications occurred in 5.9% of patients (95%CI: 1.2%-13.1%), while late complications emerged in 4.8% (95%CI: 1.9%-8.5%). The rate of freedom from major adverse cardiac events was 93.4% (95%CI: 85.3%-94.8%). Survival rates were high, with 95.2% at 1 year, 83.2% at 5 years, and 81.7% at 10 years. During an average follow-up period of roughly 3.5 years, 3.3% of patients (95%CI: 2.3%-4.4%) required additional interventions[45].
An analysis of graft patency revealed that RA-MIDCAB achieved average patency rates of 97.7% in the early period (within 1 month), 96.1% at midterm follow-up (up to
NEW PLATFORMS ARE COMING UP: WHAT TO EXPECT?
Robotic cardiothoracic surgery is entering a new era marked by rapid technological advancement and the emergence of multiple new platforms that aim to overcome the limitations of earlier systems. For many years, Intuitive Surgical’s da Vinci system has been the cornerstone of robotic surgery. The platform consists of three core elements: a patient-side cart, a surgeon’s console, and a vision cart. The newest models, the da Vinci SP and da Vinci 5, are distinguished by their wristed instruments that provide seven degrees of freedom, while the da Vinci Xi model offers instruments with five degrees of freedom[47]. However, Intuitive now faces significant competition from newer entrants introducing innovations in design, usability, and cost-efficiency.
Among these, one of the major competitors is the SSI Mantra 3 Surgical Robotic System (Sudhir Srivastava Innovations Pvt Ltd, India) developed by the cardiac surgeon Sudhir Prem Srivastava[39,48]. The SSI Mantra 3, developed by SS Innovations, has made history by enabling the world’s first robotic cardiac telesurgeries, allowing surgeons to perform operations remotely. Its modular design supports the use of three to five robotic arms, offering enhanced flexibility and control during procedures. The surgeon’s console is equipped with advanced magnetic sensor devices and a 32-inch 3D 4K monitor, which significantly improves precision and visualization. The system has been successfully utilized in a variety of cardiac surgeries, including minimally invasive TECAB, LIMA mobilization, BIMA grafting, and mitral valve replacement. Additionally, the SSI Mantra 3 stands out for its cost-effectiveness compared to international robotic systems, making it a more accessible option for a wider range of hospitals.
Medtronic’s HugoTM Robotic-Assisted Surgery (RAS) system (Medtronic, Minneapolis, USA) stands out with its modular design, open console format, and integration of cloud-connected analytics, offering surgeons greater flexibility in the operating room[49]. The system features a console with two pistol-grip arm controllers and a footswitch for managing the camera, energy source, and reserve arm. It includes four separate arm carts, each with six joints for enhanced range of motion, and uses specialized 3D glasses for head tracking technology.
Similarly, CMR Surgical’s Versius platform (CMR Surgical Ltd., Cambridge) is gaining attention due to its compact and portable design, tailored to fit into standard operating rooms and enabling more ergonomic open-console operation[50]. The system uses a multiport setup supporting up to four independent robotic arms on separate carts, allowing compatibility with standard operating rooms and removing the need for large, dedicated spaces. The surgeon operates from an ergonomic open console with a 3D HD monitor viewed through polarized glasses, enabling clear visualization and easy interaction with the assistant. Camera control is simplified by eye-sensing technology that responds to the surgeon’s eye movements, eliminating the need for manual controls. This surgical system features robotic arms with shoulder, elbow, and wrist joints, each mounted on movable carts and remotely operated via an open console providing a 3D HD view with polarized glasses. Surgeons receive haptic feedback through the handles, and the 5 mm instruments offer seven degrees of freedom for a full range of motion.
Another promising platform is Asensus Surgical’s Senhance system (Asensus Surgical, Durham, North Carolina, USA), which introduces features such as haptic feedback, eye-tracking camera control, and reusable instruments aimed at lowering the long-term procedural costs[51].
Additionally, REVO-i (Meere Company Inc., Seongnam, Republic of Korea) offers a cost-effective alternative to the da Vinci system, targeting hospitals in emerging markets. The system operates as a telemanipulator akin to the da Vinci Si robot, featuring a closed-design console where the surgeon uses fine finger controls and foot pedals to manage clutching and energy functions. It includes a patient-side cart equipped with four robotic arms that support instruments offering seven degrees of freedom, along with a separate vision cart for imaging and system integration[52].
Hinotori (Medicaroid Corporation, Kobe, Japan) received approval from Japan’s Ministry of Health, Labor, and Welfare in August 2020. This master-slave robotic system is composed of three primary sections: the surgeon’s console, the operative unit, and the vision unit. The operative unit includes four highly articulated robotic arms capable of moving across eight axes, all operated from a semi-enclosed console. The console provides three-dimensional visualization via a microscope-style eyepiece, while the surgeon manipulates the wristed instruments using ring-shaped hand controls[53].
FUTURE TECHNOLOGIES
Robotic cardiac surgery is advancing rapidly, with several technological innovations poised to shift procedures from being highly manual and technical to more technology-driven and automated. Key developments in this field include improvements in robotic systems, surgical techniques, and expanding access through remote technologies.
The EndoWrist Stabilizer was designed as a robotic epicardial stabilizer for use with the da Vinci Si system. This instrument, which could be inserted through a 12 mm port, featured collapsible pods, suction for stabilization, and an irrigation system to maintain a clear surgical field. Initially developed to support TECAB on a beating heart, the stabilizer proved useful in other robotic epicardial procedures due to its strength, precision, and versatility, surpassing traditional table-mounted stabilizers. However, following the release of the da Vinci Xi platform in 2014, Intuitive Surgical discontinued the EndoWrist Stabilizer. This decision negatively affected TECAB-related technologies, including Aesculap’s C-Port distal anastomotic connector, a miniature stapling device with a proven track record, which was also withdrawn from the market[54]. The loss of these tools has led to longer operative times and less standardized techniques during robotic coronary procedures.
This decline in industry support is partly attributed to earlier generations of cardiac surgeons who were slow to adopt robotic and minimally invasive approaches.
Nevertheless, a new wave of cardiac surgeons and trainees is now embracing these technologies. Robotic cardiac surgery is increasingly integrated into surgical education, reflecting growing interest and adoption in recent years[55]. Encouragingly, stronger support from industry is underway, particularly with the integration of advanced capabilities into the latest-generation robotic platforms. Several companies have signaled renewed investment in tools and technologies tailored for cardiac applications, which is expected to reinvigorate clinical interest and facilitate broader adoption of robotic approaches. This momentum may help bridge the gap between innovation and routine practice, ultimately expanding the role of robotics in cardiac surgery.
Research efforts are also underway to develop automated robotic surgery systems. The integration of artificial intelligence (AI) into these platforms is expected to facilitate more practical, semi-autonomous, or fully automated surgical procedures, potentially transforming the role of the surgeon in the operating room[56].
Another promising area is telementoring and telesurgery, which enable expert surgeons to remotely guide or perform cardiac procedures. This innovation holds particular promise for expanding access to specialized surgical techniques in geographically remote or underserved regions[57].
Despite these technological advances, challenges remain. A significant learning curve exists for surgeons adopting robotic systems, necessitating specialized training and ongoing skill development to ensure safe and effective use[29]. Additionally, the high cost of robotic surgical systems and the requisite training programs may limit their widespread adoption, particularly in regions with limited financial and infrastructural resources[58].
DECLARATIONS
Authors’ contributions
Made substantial contributions to the conception and design of the study and performed data analysis and interpretation: Baudo M, Torregrossa G
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
Baudo M is a Youth Editorial Board member of the journal Vessel Plus. Baudo M was not involved in any steps of editorial processing, notably including reviewer selection, manuscript handling, or decision making. Gianluca Torregrossa declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Bonatti J, Wallner S, Crailsheim I, Grabenwöger M, Winkler B. Minimally invasive and robotic coronary artery bypass grafting-a 25-year review. J Thorac Dis. 2021;13:1922-44.
2. Amabile A, Torregrossa G, Balkhy HH. Robotic-assisted coronary artery bypass grafting: current knowledge and future perspectives. Minerva Cardioangiol. 2020;68:497-510.
3. Van Praet KM, Kofler M, Shafti TZN, et al. Minimally invasive coronary revascularisation surgery: a focused review of the available literature. Interv Cardiol. 2021;16:e08.
4. Hammal F, Nagase F, Menon D, Ali I, Nagendran J, Stafinski T. Robot-assisted coronary artery bypass surgery: a systematic review and meta-analysis of comparative studies. Can J Surg. 2020;63:E491-508.
5. Raad WN, Forest S, Follis M, Friedmann P, DeRose JJ. The Impact of robotic versus conventional coronary artery bypass grafting on in-hospital narcotic use: a propensity-matched analysis. Innovations. 2016;11:112-5.
6. Ezelsoy M, Caynak B, Bayram M, et al. The comparison between minimally invasive coronary bypass grafting surgery and conventional bypass grafting surgery in proximal LAD lesion. Heart Surg Forum. 2015;18:E042-6.
7. Balkhy HH, Nisivaco SM, Hashimoto M, Torregrossa G, Grady K. Robotic total endoscopic coronary bypass in 570 patients: impact of anastomotic technique in two eras. Ann Thorac Surg. 2022;114:476-82.
8. Nisivaco S, Kitahara H, Bhasin R, Patel B, Coleman C, Balkhy HH. A decade of robotic beating-heart totally endoscopic coronary bypass (TECAB) at a single institution: outcomes with 10-year follow-up. J Thorac Cardiovasc Surg. 2025;169:1753-60.e3.
9. Kofler M, Schachner T, Reinstadler SJ, et al. Comparative analysis of perioperative and mid-term results of TECAB and MIDCAB for revascularization of anterior wall. Innovations. 2017;12:207-13.
10. Cheng N, Gao C, Yang M, Wu Y, Wang G, Xiao C. Analysis of the learning curve for beating heart, totally endoscopic, coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2014;148:1832-6.
11. Bonatti J, Schachner T, Bernecker O, et al. Robotic totally endoscopic coronary artery bypass: program development and learning curve issues. J Thorac Cardiovasc Surg. 2004;127:504-10.
12. Gaudino M, Bakaeen F, Davierwala P, et al. New strategies for surgical myocardial revascularization. Circulation. 2018;138:2160-8.
13. Puskas JD, Halkos ME, DeRose JJ, et al. Hybrid coronary revascularization for the treatment of multivessel coronary artery disease: a multicenter observational study. J Am Coll Cardiol. 2016;68:356-65.
14. AlJamal YN, Nisivaco S, Bhasin R, Kitahara H, Nathan S, Balkhy HH. Robotic totally endoscopic reverse hybrid coronary revascularization: early and midterm outcomes. Innovations. 2025;20:276-82.
15. Hwang B, Ren J, Wang K, Williams ML, Yan TD. Systematic review and meta-analysis of two decades of reported outcomes for robotic coronary artery bypass grafting. Ann Cardiothorac Surg. 2024;13:311-25.
16. Wertan MC, Sicouri S, Yamashita Y, et al. Step-by-step technique of robotic-assisted minimally invasive direct coronary artery bypass. Ann Cardiothorac Surg. 2024;13:442-51.
17. Torregrossa G, Baudo M, Yakobitis A, Murray C, Cavanaugh SM, Purrman KC. Evaluating the feasibility of a novel micro titanium fastener to facilitate robot-assisted coronary artery bypass grafting. Innovations. 2024;19:439-42.
18. Torregrossa G, Amabile A, Balkhy HH. Totally robotic sutured coronary artery bypass grafting: how we do it. JTCVS Tech. 2020;3:170-2.
19. Torregrossa G, Yakobitis A, Murray C, Baudo M. Total endoscopic coronary artery bypass on a DaVinci Xi platform without an EndoWrist stabilizer combining the technology of GelPOINT Mini, AirSeal, and Octopus Nuvo. Ann Cardiothorac Surg. 2024;13:461-3.
20. Oehlinger A, Bonaros N, Schachner T, et al. Robotic endoscopic left internal mammary artery harvesting: what have we learned after 100 cases? Ann Thorac Surg. 2007;83:1030-4.
21. Hemli JM, Henn LW, Panetta CR, et al. Defining the learning curve for robotic-assisted endoscopic harvesting of the left internal mammary artery. Innovations. 2013;8:353-8.
22. Rosati F, Baudo M, Di Bacco L, et al. Patient complexity does not affect surgical learning curve and clinical outcomes during early experience in robotic assisted coronary surgery. J Robot Surg. 2025;19:245.
23. Argenziano M, Katz M, Bonatti J, et al; TECAB Trial Investigators. Results of the prospective multicenter trial of robotically assisted totally endoscopic coronary artery bypass grafting. Ann Thorac Surg. 2006;81:1666-74; discussion 1674.
24. Patrick WL, Iyengar A, Han JJ, et al. The learning curve of robotic coronary arterial bypass surgery: a report from the STS database. J Card Surg. 2021;36:4178-86.
25. Halkos ME, Jonsson A, Badhwar V, et al. Developing proficiency in robotic cardiac surgery. Ann Thorac Surg. 2025;119:523-34.
26. Torregrossa G, Dokollari A, Sá MP, Sicouri S, Ramlawi B, Sutter F. Establishing a robotic coronary artery bypass surgery program: a narrative review. J Vis Surg. 2023;9:3.
27. Amabile A, Torregrossa G, Williams E, Puskas J. Mastering off-pump, total arterial coronary artery bypass grafting: a step-by-step approach. Multimed Man Cardiothorac Surg. 2020:2020.
28. Ullah W, Sattar Y, Ullah I, et al. Percutaneous intervention or bypass graft for left main coronary artery disease? J Interv Cardiol. 2020;2020:4081642.
29. Fida Z, Ghutai G, Jamil Z, et al. The role of robotics in cardiac surgery: innovations, outcomes, and future prospects. Cureus. 2024;16:e74884.
30. Wiedemann D, Schachner T, Bonaros N, et al. Does obesity affect operative times and perioperative outcome of patients undergoing totally endoscopic coronary artery bypass surgery? Interact Cardiovasc Thorac Surg. 2009;9:214-7.
31. Leyvi G, Forest SJ, Srinivas VS, et al. Robotic coronary artery bypass grafting decreases 30-day complication rate, length of stay, and acute care facility discharge rate compared with conventional surgery. Innovations. 2014;9:361-7;discussion 367.
32. Moreno PR, Stone GW, Gonzalez-Lengua CA, Puskas JD. The hybrid coronary approach for optimal revascularization: JACC review topic of the week. J Am Coll Cardiol. 2020;76:321-33.
33. Balkhy HH. Robotic totally endoscopic coronary artery bypass grafting: it’s now or never! JTCVS Tech. 2021;10:153-7.
34. Bakaeen FG, Ravichandren K, Blackstone EH, et al. Coronary artery target selection and survival after bilateral internal thoracic artery grafting. J Am Coll Cardiol. 2020;75:258-68.
35. Loulmet D, Carpentier A, d’Attellis N, et al. Endoscopic coronary artery bypass grafting with the aid of robotic assisted instruments. J Thorac Cardiovasc Surg. 1999;118:4-10.
36. Falk V, Diegeler A, Walther T, Jacobs S, Raumans J, Mohr FW. Total endoscopic off-pump coronary artery bypass grafting. Heart Surg Forum. 2000;3:29-31.
37. Boyd WD, Rayman R, Desai ND, et al. Closed-chest coronary artery bypass grafting on the beating heart with the use of a computer-enhanced surgical robotic system. J Thorac Cardiovasc Surg. 2000;120:807-9.
38. Whellan DJ, McCarey MM, Taylor BS, et al. Trends in robotic-assisted coronary artery bypass grafts: a study of the society of thoracic surgeons adult cardiac surgery database, 2006 to 2012. Ann Thorac Surg. 2016;102:140-6.
39. Srivastava S, Gadasalli S, Agusala M, et al. Beating heart totally endoscopic coronary artery bypass. Ann Thorac Surg. 2010;89:1873-9; discussion 1879.
40. Pasrija C, Kon ZN, Ghoreishi M, et al. Cost and outcome of minimally invasive techniques for coronary surgery using robotic technology. Innovations. 2018;13:282-6.
41. Cerny S, Oosterlinck W, Onan B, et al. Robotic cardiac surgery in europe: status 2020. Front Cardiovasc Med. 2021;8:827515.
42. Shimokawa T, Kumamaru H, Motomura N, et al. Minimally invasive cardiac surgeries in 2021: annual report by Japanese society of minimally invasive cardiac surgery. Gen Thorac Cardiovasc Surg. 2025;73:88-95.
43. Bonatti J, Wallner S, Winkler B, Grabenwöger M. Robotic totally endoscopic coronary artery bypass grafting: current status and future prospects. Expert Rev Med Devices. 2020;17:33-40.
44. Cao C, Indraratna P, Doyle M, et al. A systematic review on robotic coronary artery bypass graft surgery. Ann Cardiothorac Surg. 2016;5:530-43.
45. Zoupas I, Manaki V, Tasoudis PT, Karela NR, Avgerinos DV, Mylonas KS. Totally endoscopic coronary artery bypass graft: systematic review and meta-analysis of reconstructed patient-level data. Innovations. 2024;19:616-25.
46. Kitahara H, Nisivaco S, Balkhy HH. Graft patency after robotically assisted coronary artery bypass surgery. Innovations. 2019;14:117-23.
47. Asadizeidabadi A, Hosseini S, Vetshev F, Osminin S, Hosseini S. Comparison of da Vinci 5 with previous versions of da Vinci and Sina: a review. Laparoscopic, Endoscopic and Robotic Surgery. 2024;7:60-5.
48. Srivastava SP, Srivastava VP, Singh A, et al. Evaluating the efficacy of telesurgery with dual console SSI Mantra Surgical Robotic System: experiment on animal model and clinical trials. J Robot Surg. 2024;18:391.
49. Prata F, Ragusa A, Tempesta C, et al. State of the art in robotic surgery with Hugo RAS system: feasibility, safety and clinical applications. J Pers Med. 2023;13:1233.
50. Soumpasis I, Nashef S, Dunning J, Moran P, Slack M. Safe implementation of a next-generation surgical robot: first analysis of 2,083 cases in the versius surgical registry. Ann Surg. 2023;278:e903-10.
51. Menke V, Hansen O, Schmidt J, et al. The stress for surgeons: exploring stress entities with the robotic senhance surgical system. J Robot Surg. 2024;18:94.
52. Alip SL, Kim J, Rha KH, Han WK. Future platforms of robotic surgery. Urol Clin North Am. 2022;49:23-38.
53. Brassetti A, Ragusa A, Tedesco F, et al. Robotic surgery in urology: history from PROBOT® to HUGOTM. Sensors. 2023;23:7104.
54. Torregrossa G, Amabile A, Nisivaco S, Algoet M, Oosterlinck W, Balkhy HH. The stradivari violin of robotic heart surgery: the robotic EndoWrist stabilizer. Innovations. 2024;19:612-5.
55. Athanasiou T, Ashrafian H, Rowland SP, Casula R. Robotic cardiac surgery: advanced minimally invasive technology hindered by barriers to adoption. Future Cardiol. 2011;7:511-22.
56. Chang KD, Raheem AA, Rha KH. Novel robotic systems and future directions. Indian J Urol. 2018;34:110-4.
57. Menkis AH, Kodera K, Kiaii B, Swinamer SA, Rayman R, Boyd WD. Robotic surgery, the first 100 cases: where do we go from here? Heart Surg Forum. 2004;7:1-4.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Special Issue
Copyright
Data & Comments
Data
















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].