The need for precision when describing the aortic root
Abstract
Our commentary addresses the issues raised with two manuscripts published in the Special Issue of the Journal edited by Professor de Paulis about the surgical approach to the aortic root. It is unquestionable that appropriate surgical treatment requires a detailed understanding of the underlying anatomy. In the first article, Professor de Paulis et al. show the anatomical precision that can now be achieved using computed tomography. In the second article, the same group shows how an appreciation of this anatomy dictates their surgical approach. In our commentary, we highlight that the terms used in the two manuscripts indicate that the confusion identified in a questionnaire organized in 2012 about terminological issues remains unresolved, in particular regarding the definition of the enigmatic valvar “annulus”. We provide anatomical illustrations that demonstrate the ongoing problems. We also emphasize that bisecting planes of the root, rather than off-center cuts, should be used when taking the measurements of the components of the root that help the surgeon make the optimal repair. We finish by stressing the need for equal specificity in the words used to describe the components, pointing out that “cusp” is inadequate as a synonym for the valvar leaflets, especially since more often the valvar sinuses are described as “cusps”.
Keywords
INTRODUCTION
As Irace et al. correctly emphasize in the current issue of the Journal, there has been a notable transformation over the past decade regarding the optimal means of providing the key information to the surgeon preparing to undertake repair or replacement of the aortic valve[1]. As the authors indicate, assessment by means of echocardiography or magnetic resonance imaging provides information that cannot be replicated by computed tomographic (CT) interrogation. In this regard, standard cross-sectional echocardiography, particularly its three-dimensional variant, is a key technique for both anatomical assessment and surgical planning that remains unmatched by CT, revealing features such as the dynamic and real-time visualization of motion and coaptation of the leaflets. As Irace et al. show, nonetheless, the tomographic technique now offers significant improvements in terms of resolution and reproducibility[1]. These improvements, which provide the required precision in the demonstration of the anatomic subtleties of the makeup of the root, need now to be matched with equal precision regarding the terms used to describe the findings. To some extent, this will depend on the ability of the investigator to identify the details. The anatomist has the advantage over the cardiac surgeon of being fully able to open the root at his or her leisure, and then to dissect it to show the intimate relationships between its component parts[2]. By using virtual dissection of the intact organ, the skilled clinician is now able to provide comparable information by interrogating three-dimensional CT datasets[3]. In this regard, a standardized approach toward quantitatively assessing the three-dimensional structure of the leaflets housed within the root, with measures of their coaptation, or lack thereof, provides unmatched insight into the underlying valvar dysfunction[4]. This combination of virtual dissection, and quantitative assessment of the leaflets, now promotes a personalized approach toward either aortic valvar repair or replacement[5]. On the basis of our own combined experience of both virtual and real dissection, we suggest that, despite its breadth and utility, several aspects of the review by Irace et al.[1] need clarification.
ONGOING PROBLEMS WITH DEFINING THE “ANNULUS”
It is now over a decade since the questionnaire organized by the German consortium of cardiac surgeons highlighted the problems that existed at the time regarding the definition of the enigmatic “annulus”[6]. Among those cardiac surgeons worldwide who responded to the questionnaire, about half held the view that the semilunar hinges of the leaflets represented the “annulus”. The other half accepted the plane measured by echocardiographers at the entrance to the root as the “annulus”. Based on the review by Irace et al.[1], it seems that little has changed over a decade. Thus, the investigators tell us that “the annulus is often referred to as the “functional aortic annulus” due to its complex three-dimensional structure and multiple anatomical components: the crown-like leaflet attachments (also known as the surgical annulus or ventricular-aortic junction), the interleaflet triangles, and the virtual basal ring. In the context of CT imaging, the aortic annulus corresponds to the virtual basal ring - a virtual, coplanar contour connecting the nadirs of the three aortic valve leaflets”. The passage represents a masterpiece of ambiguity. It would seem that, at least in Rome, the surgeons continue to take the semilunar hinges as representing the annulus. Those interrogating the CT images, in contrast, measure the diameter of the virtual basal ring to provide the appropriate metric. This cannot be optimal. It is undeniable that surgeons require an understanding of the overall three-dimensional anatomy of the root, of which the virtual basal plane is only a small part[3]. It is equally true that, when assessed in three dimensions, the semilunar hinges of the leaflets produce a crown-like configuration [Figure 1].
Figure 1. The anatomical dissection (A), and the segmented tomographic reconstruction (B), reveal that, due to the semilunar arrangement of the hinges of the valvar leaflets, it is better to describe the three-dimensional arrangement of the arterial roots in terms of crowns rather than rings.
Because the semilunar hinges form a crown-like configuration, as we will emphasize below, the planes used for imaging must be bisecting to capture their true spatial orientation. This is critical for accurate measurement of the height of the leaflets during valve-sparing procedures. The so-called “echocardiographic annulus”, however, is a virtual entity without an anatomical counterpart. The area is better described for what it is - the virtual basal plane[3]. The area does have the advantage of being “annular”, although in the review by Irace et al., it appears oval rather than circular[1]. It is questionable whether a crown is better described as a ring. More importantly, Irace et al. equate the “surgical annulus” with the “ventricular-aortic junction”[1]. Is this justified? It may appear as such to the surgeon. Much depends on how one defines the ventricle, as opposed to the aorta.
BOUNDARIES WITHIN THE ARTERIAL ROOTS
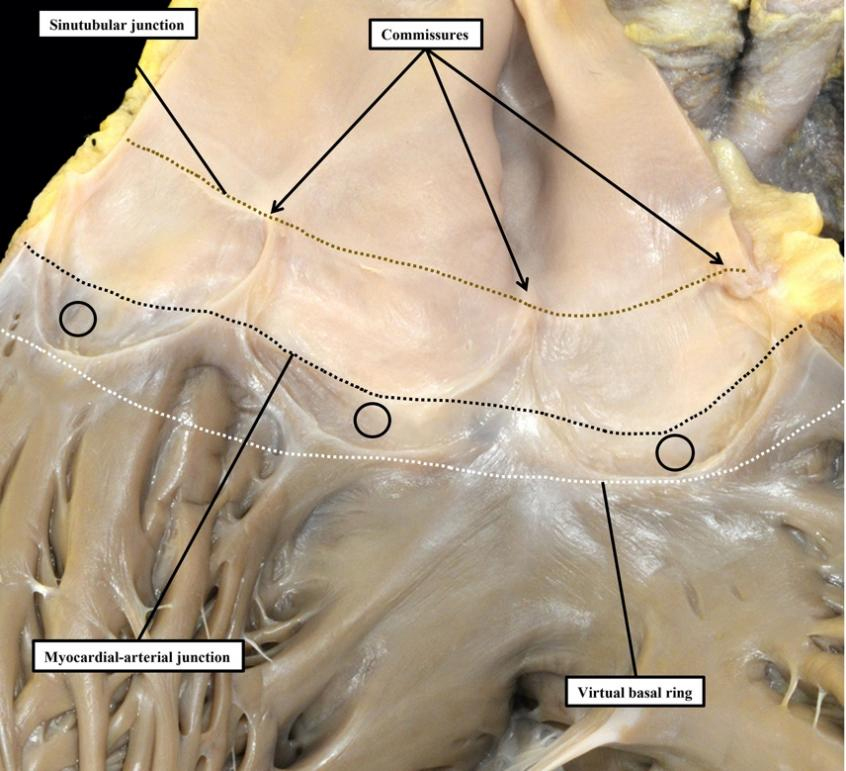
Proper understanding of the anatomy of the arterial roots requires attention to the makeup of the walls of the sinuses that support the semilunar hinge lines of the valvar leaflets. In the pulmonary root, the arrangement, although not simple, is easy to appreciate. This is because the basal parts of each of the semilunar leaflets and their intervening interleaflet triangles are supported by infundibular myocardium, rather than by the arterial walls of the valvar sinuses [Figure 2].
Figure 2. The pulmonary root has been opened and spread, and the leaflets of its valve have been removed. This shows how the semilunar hinges cross the myocardial-arterial junction to incorporate crescents of myocardium at the bases of all three valvar sinuses (circles). Myocardium distal to the virtual basal ring is also found supporting the intervening interleaflet triangles.
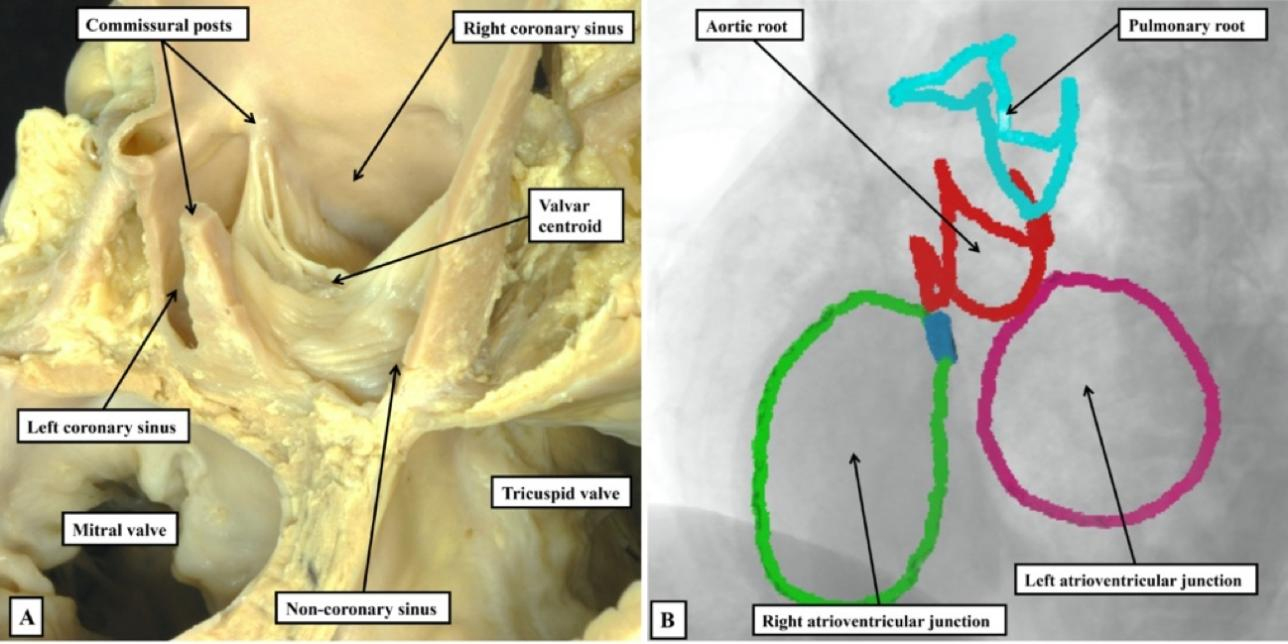
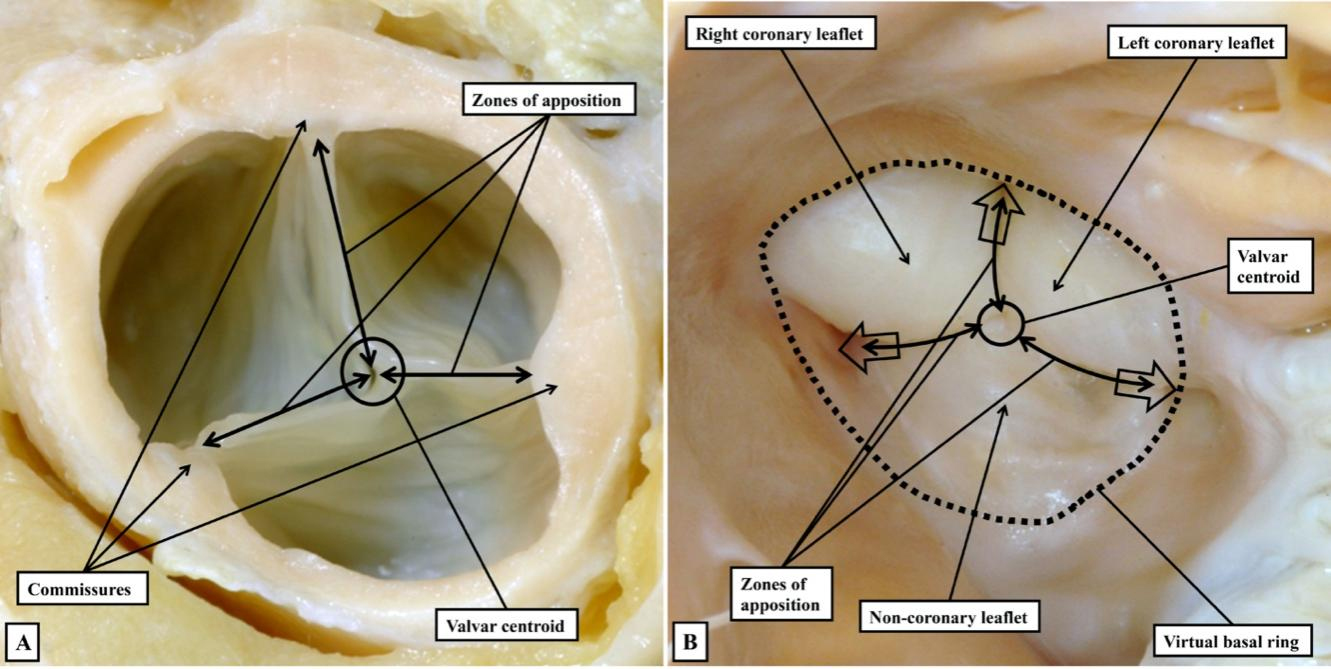
In the pulmonary root, therefore, it is possible to draw a clear distinction between the hemodynamic ventriculo-arterial junction, produced along its periphery by the semilunar hinge lines, and the anatomical ventriculo-arterial junction. The latter entity, at least in the pulmonary root, is the boundary between the myocardial walls of the right ventricle and both the fibrous tissues of the interleaflet triangles, and the arterial walls of the valvar sinuses. In the intact pulmonary root, furthermore, the anatomical junction does indeed form an obvious ring, although distal to its virtual basal ring. To the best of our knowledge, this anatomical ring was never defined as the valvar “annulus”. A second ring-like boundary can then be identified distally in the intact pulmonary root. This is the sinutubular junction, which appears as a line when the root is opened and spread [Figure 2]. The semilunar hinge lines of the leaflets come together distally at the level of the sinutubular junction, producing the so-called commissures. This term is also problematic because, in anatomy, commissures describe the union of adjacent parts, as in the commissures of the lips or the eyes. Such unions of the leaflets are, of course, an essential feature of the competent arterial valves. The clinical “commissures”, or “commissural posts” as described by surgeons, therefore represent the peripheral extent of these zones of apposition between the leaflets, which meet centrally at the valvar centroid [Figure 3A]. When the closed valve is viewed from the ventricular aspect, however, it becomes possible to recognize the full extent of the hemodynamic ventriculo-arterial junction. When viewed from the ventricular aspect [Figure 3B], subtleties become evident that are not obvious when viewing the valve from above [Figure 3A], or when the valvar leaflets have been removed [Figure 2].
Figure 3. The closed aortic valve is viewed from above (A), having transected the intrapericardial aorta at the level of the sinutubular junction, and from the ventricular aspect (B). The closed valve has three zones of apposition, which extend from the valvar centroid (circle) to the circumference of the valvar orifice (double-headed arrowed lines). The circumferential points are conventionally described as the commissures; Panel B shows the similarity between the closed surfaces of the leaflets, which mark the hemodynamic ventriculo-arterial junction, and the surfaces of the molar and premolar teeth. The open arrows show the extensions of the cavity of the left ventricle to the level of the commissures. There is a shorter extension at the valvar centroid when the valve is closed.
The view of the closed valve from the ventricle also provides a reason why old anatomists might have described the leaflets as “cusps”. The similarity of the closed leaflets to the surfaces of the molar and premolar teeth is obvious [Figure 3B]. More importantly, when the leaflets are closed, it is possible to recognize the three extensions of the valvar cavity that reach the sinutubular junction at the commissures, with these extensions walled by the fibrous interleaflet triangles. It is also possible to recognize a fourth shorter peak at the valvar centroid. Recognition of the true three-dimensional arrangement of the hemodynamic ventriculo-arterial junction, along with the complexity of the surface areas of the zones of apposition, achieves great importance for the surgeon when he or she is seeking to preserve and reconstruct the valve to ensure its competency. Despite these widely unrecognized subtleties, the key difference between the anatomical and hemodynamic junctions is observed proximally in the pulmonary root, where the hinge lines of the leaflets run beyond the proximal extent of the arterial walls of the sinuses to incorporate infundibular myocardium within the base of each sinus [Figure 2]. In the pulmonary root, therefore, the anatomical ventriculo-arterial junction is equivalent to the myocardial-arterial junction. The support provided by the infundibular myocardium to each of the valvar sinuses, and the intervening interleaflet triangles, permits the pulmonary root to be removed intact in the living heart and used as an autograft in the Ross procedure[7].
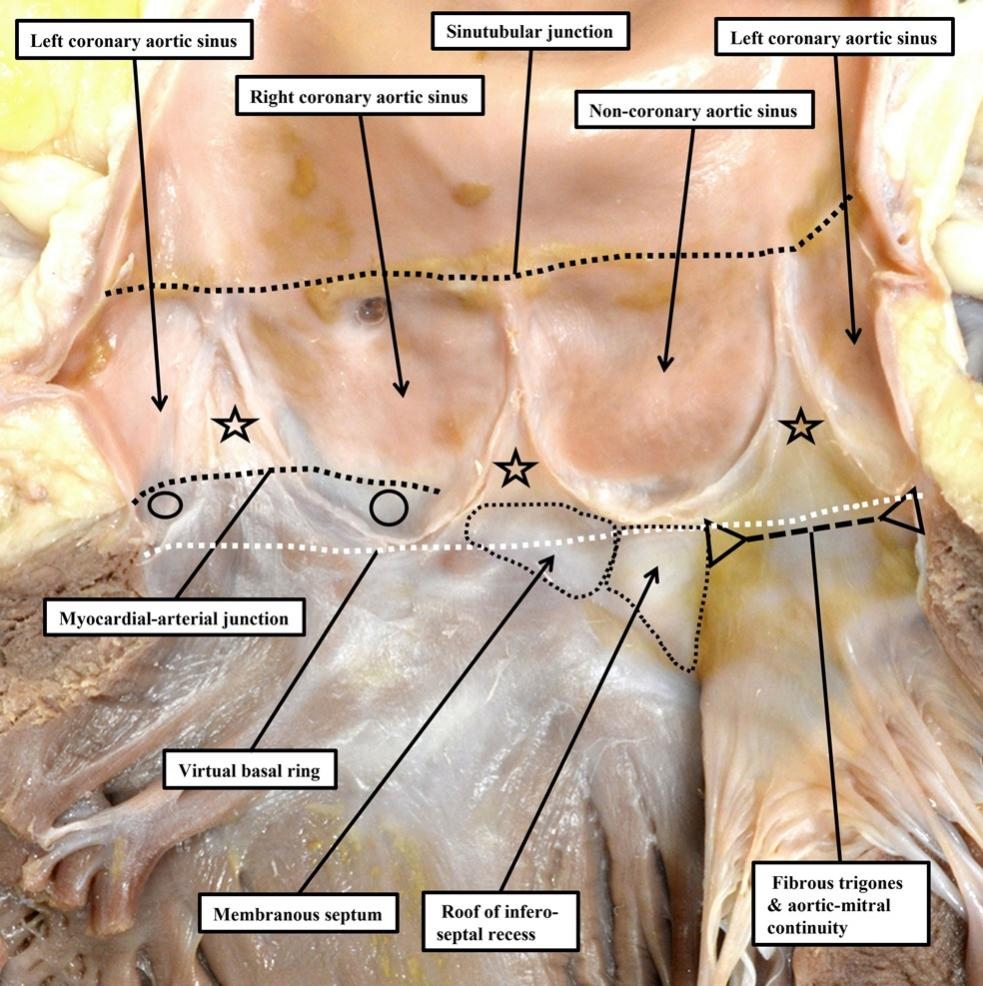
The situation is even more complex in the aortic root. Only the walls of the sinuses that give rise to the coronary arteries and their intervening interleaflet triangle include left ventricular myocardium within their bases [Figure 4]. This makes it much harder to define a “ventricular-aortic junction” in the left ventricle. The myocardium found within the bases of these two sinuses of the aortic root is unequivocally ventricular, and their distal walls are unequivocally arterial. As in the pulmonary root, when considering the makeup of these two sinuses of the aortic root, the anatomical ventriculo-arterial junction does not correspond with the semilunar hinges of the valvar leaflets. The situation is far less clear-cut when assessing the
Figure 4. The aortic root is opened and spread to provide an image that can be compared with the pulmonary root shown in Figure 2. In the aortic root, only the valvar sinuses that give rise to the coronary arteries (open circles) and their intervening interleaflet triangle have myocardium at their bases. The stars show the fibrous interleaflet triangles.
When describing the aortic root with precision, therefore, it is more accurate to describe a myocardial-arterial, rather than a ventriculo-arterial (or ventriculo-aortic) junction. It is important to note that the myocardial-arterial junction is usually found only within the sinuses that give rise to the coronary arteries, and at the base of their intervening interleaflet triangle. The subtlety of this anatomy has been acknowledged by Folino et al. when describing their modification of the Modine procedure for replacement of the aortic root[8]. In this report, they correctly describe how it is necessary to remove only their arterial components when resecting the walls of the valvar sinuses. Recognizing the anatomical arrangement, they describe how, following deep external dissection of the aortic root, it is “the arterial portion of the aortic wall forming the sinuses of Valsalva” that is “completely excised”. The sutures used to secure the proximal part of the graft to be inserted are then placed “circumferentially along the muscular myocardium and the fibrotic connective tissue that constitute the virtual basal ring”. The precision provided by such descriptions will surely underscore ongoing advances in the surgical treatment of the diseased aortic root. Surgeons placing sutures in this fashion, nonetheless, along with those replacing the valve in transcutaneous fashion, should be aware that the superior fascicle of the left bundle branch can occasionally be directly related to the nadir of the hinge of the aortic valvar leaflet supported by the right coronary aortic valvar sinus[9].
There are, however, additional potential problems in the review by Irace et al.[1]. The authors cite the previous study of Mori et al.[10], in which, to make accurate measurements, emphasis was placed on the different planes in which it was possible to transect the root. In order to transect the nadir of any given leaflet, it is necessary to bisect the root, rather than cut it in off-center fashion[3-5]. We have already made reference in our commentary to the necessity of making bisecting cuts and, at least in the valve with three leaflets, a bisecting cut must run from the nadir of one leaflet to the zone of apposition between the opposite leaflets. When making such bisecting cuts, it is not possible to produce images such as those shown in Figures 8 and 9 of the review by Irace et al.[1]. It is possible, of course, that the Roman investigators indeed use bisecting cuts when making the measurements of geometric and effective height of the leaflets. If this is the case, it is confusing to create figures that can only be produced by using off-center cuts. While the differences may be in the order of only a few millimeters, precision to the millimeter is required for accurate interpretation of valvar dysfunction, and to create a personalized surgical blueprint[5].
CONCLUSIONS
Despite our criticisms, there is much to commend in the report by Irace et al.[1]. They join the ranks of those surgical groups leading the way in improving efforts aimed at repairing the aortic valve guided by a quantitative assessment of the valvar leaflets and their coaptation. It is encouraging that they emphasize the need to take account of potential rotation of the aortic root within the base of the ventricular mass[11]. It is equally encouraging to observe that they take care to distinguish between the parietal walls of the valvar sinuses and the moving parts they support, which they correctly describe as the leaflets. Many still follow the old anatomists in describing the moving parts as the “cusps”. The word “cusp”, meaning a point of elevation, is, nevertheless, poorly suited as a descriptor of the leaflets. More importantly, it is increasingly frequent to see “cusp” used interchangeably to refer to either the leaflets or the walls of their supporting sinuses. For all these reasons, it is preferable to avoid the use of “cusp” when describing the arterial roots. As also highlighted by Irace et al.[1], the key to distinguishing phenotypes when the aortic root is congenitally malformed is to make clear distinctions between the walls of the sinuses and the leaflets they support[3]. As demonstrated by our commentary, only by encouraging ongoing discussion will we eventually reach the much-needed consensus in nomenclature.
DECLARATIONS
Authors’ contributions
Conception and design, approval of figures, data analysis and interpretation: Anderson RH
Conception and design, approval of figures, data analysis and interpretation: Spicer DE
Conception and design, approval of figures, data analysis and interpretation: Tretter JT
All authors contributed equally to the conception and design, approval of the use of the figures illustrating the anatomical points, and data analysis and interpretation.
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
Tretter JT is a consultant for Cara Medical, Ltd. The other authors declare that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Irace FG, Chirichilli I, de Paulis R. CT scan as a tool to evaluate root reconstruction and aortic valve repair. Vessel Plus. 2025;9:4.
3. Tretter JT, Spicer DE, Franklin RCG, et al. Expert consensus statement: anatomy, imaging, and nomenclature of congenital aortic root malformations. Cardiol Young. 2023;33:1060-8.
4. Izawa Y, Mori S, Tretter JT, et al. Normative aortic valvar measurements in adults using cardiac computed tomography- a potential guide to further sophisticate aortic valve-sparing surgery. Circ J. 2021;85:1059-67.
5. Tretter JT, Burbano-Vera NH, Najm HK. Multi-modality imaging evaluation and pre-surgical planning for aortic valve-sparing operations in patients with aortic root aneurysm. Ann Cardiothorac Surg. 2023;12:295-317.
6. Sievers HH, Hemmer W, Beyersdorf F, et al.; Working Group for Aortic Valve Surgery of the German Society of Thoracic and Cardiovascular Surgery. The everyday used nomenclature of the aortic root components: the tower of Babel? Eur J Cardiothorac Surg. 2012;41:478-82.
7. Merrick AF, Yacoub MH, Ho SY, Anderson RH. Anatomy of the muscular subpulmonary infundibulum with regard to the Ross procedure. Ann Thorac Surg. 2000;69:556-61.
8. Folino G, Torre M, De Paulis R. Comparison of the “Modine modification” with the standard reimplantation technique: indications and options for improvement. Vessel Plus. 2025;9:9.
9. Tretter JT, Mori S, Anderson RH, et al. Anatomical predictors of conduction damage after transcatheter implantation of the aortic valve. Open Heart. 2019;6:e000972.
10. Mori S, Anderson RH, Tahara N, et al. The differences between bisecting and off-center cuts of the aortic root: the three-dimensional anatomy of the aortic root reconstructed from the living heart. Echocardiography. 2017;34:453-61.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Special Topic
Copyright
Data & Comments
Data
















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].