Use of biomaterials for paralyzed lower eyelid reconstruction
Abstract
Facial paralysis often results in retraction, lid lag and malposition of the lower eyelid, with a consequent increased risk of exposure keratopathy. Therefore, repair of the paralyzed eyelid is central to periocular management in patients with facial paralysis. We aim to evaluate current techniques using biomaterials for lower eyelid reconstruction. A literature review was conducted, including posterior lamellar anatomy, traditional grafting techniques, and recent advancements in biomaterial-based reconstruction. Biomaterials were categorized as acellular matrices, natural polymers, and synthetic polymers. Their biomechanical properties, integration, and clinical utility were examined in the management of the paralyzed eyelid. We describe our surgical technique, highlighting its versatility regardless of the biomaterial graft used. While acellular dermal matrices are the most widely used biomaterials, synthetic polymers, and natural polymers have also been shown to have favorable integration and compatibility for posterior lamellar augmentation and reconstruction. Future directions include the application of bioengineered stem cells with regenerative capacity. Biomaterials can provide a viable and effective alternative for posterior lamellar reconstruction in the treatment of paralyzed lower eyelid malposition and retraction. Specifically, acellular matrices offer a balance of support and biocompatibility.
Keywords
INTRODUCTION
Facial paralysis arises from diverse causes - including idiopathic, infectious, neoplastic, traumatic, and iatrogenic processes - and presents with partial or complete loss of facial muscle function. Common etiologies encountered by otolaryngologists include Bell’s palsy, Ramsay Hunt, and tumor-related facial nerve injury. The annual incidence of central and peripheral facial paralysis is estimated at 20-30 cases per 100,000 individuals[1,2]. The condition carries significant functional and psychosocial burdens, affecting ocular protection, oral competence, speech, and facial expression.
Lower eyelid dysfunction is a frequent manifestation, leading to ectropion, lagophthalmos, tearing, and exposure keratopathy. Careful examination - including assessment of epiphora, chemosis, and eyelid laxity - guides management[3]. Conservative measures aim to protect the ocular surface, while surgical strategies reposition or augment the eyelid. Posterior lamellar spacer grafts are particularly useful for correcting medial lagophthalmos and restoring vertical height[3,4].
In facial paralysis, the anterior lamella is often preserved while the posterior lamella is deficient due to denervation and atrophy. This distinction emphasizes the need for targeted reconstruction. Traditional grafts and flaps have been used, but newer regenerative approaches - including acellular dermal matrices and scaffold-based techniques - offer promising solutions for restoring both form and function[4].
HISTORY OF TREATMENT OF LAMELLAR DEFICIENCIES
Conjunctival grafts
Conjunctival grafting has historically been considered the gold standard for posterior lamella reconstruction. These grafts are typically harvested from the healthy upper eyelid, with donor sizes ranging from 4-5 mm in height to 8-16 mm in width[4,5]. It is generally advised to preserve at least 4 mm of the tarsus at the donor site to avoid postoperative complications such as upper eyelid instability[5]. Conjunctival grafting is often performed as a single-stage procedure and can provide posterior lamellar replacement without the need for an additional conjunctival lining[4]. These grafts are particularly indicated for defects involving up to 75% of the lower eyelid[6]. The advantage of conjunctival grafts lies in their histological similarity to the recipient site, especially when harvested from the ipsilateral or contralateral upper eyelid[6,7]. However, potential complications include upper eyelid retraction, wound dehiscence, cicatricial ectropion, and excessive lower lid laxity.
Several other techniques utilize transconjunctival flaps for eyelid reconstruction. The Cutler-Beard flap, commonly employed for upper eyelid defects, involves a full-thickness flap from the lower eyelid created via a 4 mm incision parallel to the ciliary margin[5]. This technique maintains vascularized tarsal support, though it may lack sufficient rigidity for upper eyelid reconstruction without supplemental fascia, cartilage, or additional transconjunctival grafts[8]. The Hughes flap, on the other hand, is frequently used for reconstruction of 60%-80%[8] lower eyelid defects. It involves a transconjunctival advancement flap raised from the tarsus to restore posterior lamellar structure.
While these techniques are well-established, they pose notable limitations in the setting of facial paralysis. Chief among these is the requirement to operate on otherwise healthy eyelid tissue - often from the contralateral side - which may result in donor site morbidity and compromise of native eyelid function[9]. Given the importance of preserving maximum native function in patients with facial paralysis, traditional transconjunctival grafts may be less desirable. In such cases, emerging strategies, particularly the use of biomaterial grafts, offer promising alternatives. Careful patient selection and surgical planning remain critical in determining the most appropriate reconstructive approach for this complex population.
Mucosal grafts (hard palate, nasal)
The hard palate mucoperiosteum is frequently used for eyelid reconstruction due to its histologic similarity to transconjunctival tissue, comprising both fibrous connective and mucosal components. It is commonly utilized for defects involving up to one-half to the entirety of the upper or lower eyelid[10]. The hard palate can effectively replace both the tarsal plate and the palpebral conjunctiva, offering advantages such as moderate rigidity, a smooth surface, and long-term stability. However, given its thick lamina propria without glandular elements, these grafts have the potential to irritate the ocular surface due to insufficient lubrication[11]. The keratinized stratified squamous epithelium of the hard palate poses risk of orthokeratosis which may cause corneal irritation even years after transplantation[10]. Commonly, the hard palate donor site is left to heal by secondary intention which renders unfavorable donor site morbidity, including pain, bleeding, and odynophagia.
Similarly, nasal mucosa serves as another viable option for posterior lamellar reconstruction. Compared to buccal mucosa, nasal mucosa demonstrates reduced scar contraction due to its increased thickness[12]. Typically harvested from the nasal septum, it can be used to replace both the tarsus and conjunctiva. This graft offers excellent structural support and favorable aesthetic outcomes, as the natural curvature of the septal mucochondral tissue mimics the shape of the native tarsus[12]. However, notable disadvantages include the potential for corneal irritation, as the epithelium is keratinized stratified squamous tissue, which can be abrasive to the ocular surface. Additional complications may include donor site perforation and hemorrhage, as the donor sites are commonly left to heal by secondary intention with nasal packing in place[10].
Auricular cartilage
Auricular cartilage is another grafting option for posterior lamellar reconstruction, particularly in cases involving defects of one-half to the full thickness of the upper or lower eyelid[13], or when complex reconstruction is required[14]. Composed of elastic cartilage with a naturally curved surface, auricular cartilage offers both flexibility and structural strength[13]. Its advantages include a thin profile, ease of harvest, minimal donor site morbidity, and a low risk of graft contraction - thereby reducing the likelihood of postoperative eyelid retraction.
However, there are notable limitations. The lack of a mucosal inner lining from these grafts makes auricular cartilage less suitable for conjunctival reconstruction, potentially leading to ocular irritation[13]. Some techniques have been described to mitigate this risk including overlay with free oral mucosal graft or preservation of perichondrium with graft harvest[15]. Other reported complications include graft displacement, detachment, surface irregularity, gaze disturbances, and cartilage warping - all of which may compromise both functional and cosmetic outcomes[16].
BIOMATERIAL OPTIONS FOR TREATMENT OF POSTERIOR LAMELLAR DEFICIENCIES
A literature review was performed through PubMed for the following terms: biomaterial, posterior lamella reconstruction, from 1998 to 2025. Articles were excluded that failed to address the use of biomaterials for the augmentation of posterior lamellar defects (e.g., biomaterials for sinus augmentation or bone grafting or for entropion repair) or that were editorial responses. Articles were included if they presented data from case reports, case series, narrative reviews, systematic reviews, or meta-analyses describing biomaterial use for posterior lamellar eyelid reconstruction. A total of four articles were included[4,16,17,18].
Reconstruction of the posterior lamella using native flaps or grafts poses significant challenges, particularly due to the lack of histologically ideal donor tissue[4]. Furthermore, the use of autologous donor tissue is associated with several drawbacks, including limited donor site availability, donor site morbidity, and structural and functional mismatches. In light of these limitations, this review presents an overview of the various biomaterials currently employed for posterior lamella reconstruction, especially in patients with facial paralysis.
Biomaterials represent a tissue-engineering approach that utilizes synthetic or biological products to restore functional and structural integrity without requiring autograft harvest. Their use in posterior lamellar reconstruction has expanded in recent years. Biomaterials are broadly categorized into acellular and cellular types. Acellular biomaterials serve as scaffolds to guide in vivo tissue regeneration, whereas cellular biomaterials consist of prefabricated in vitro tissue constructs that mimic native tissue architecture and promote regeneration. The success of biomaterial-based eyelid reconstruction is highly dependent on the presence of viable native cells within the bio-scaffold - particularly from the residual posterior lamella.
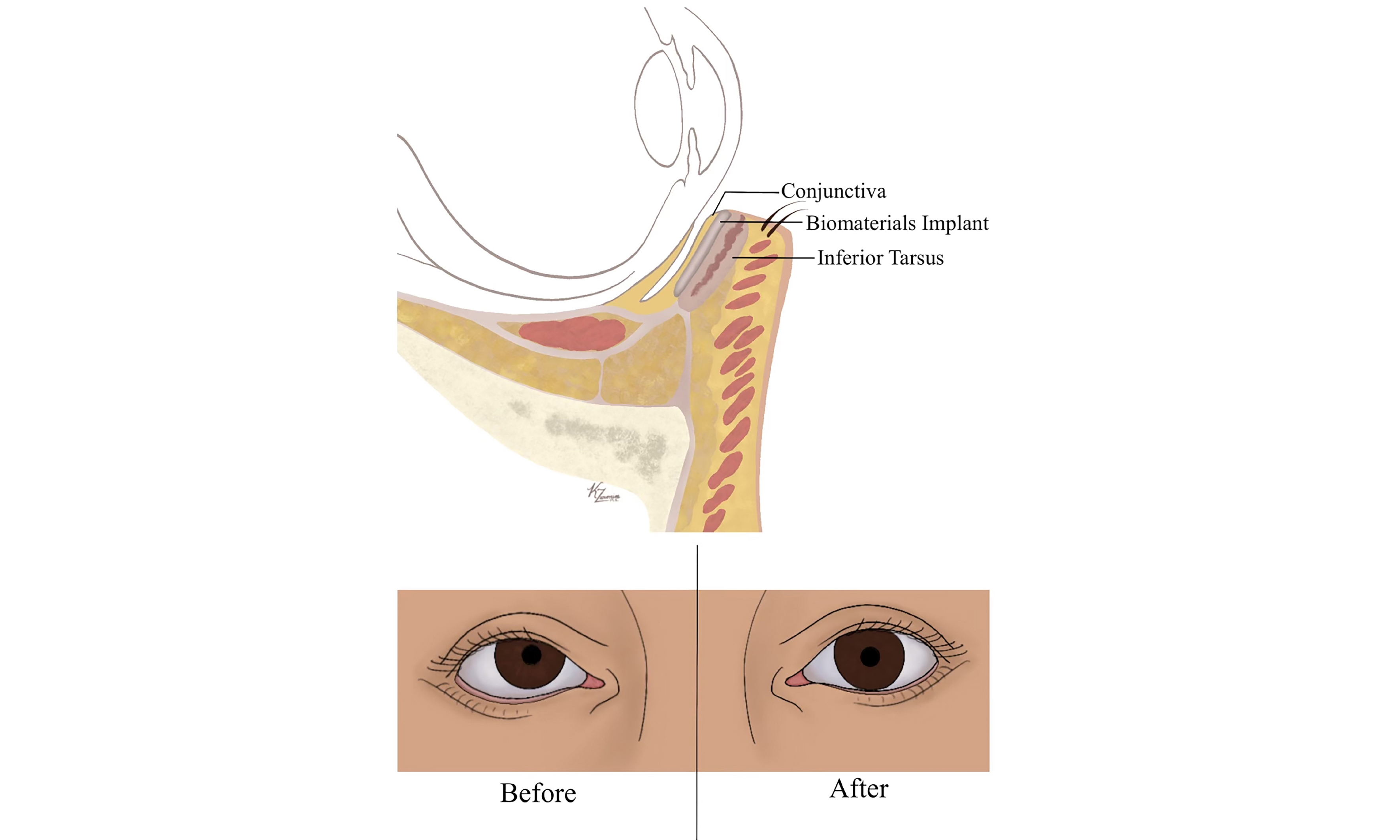
To appreciate the utility of these materials, one must first understand the structure and function of the posterior lamellar subunits. The posterior lamella comprises the conjunctiva, tarsal plate, and lid retractors, which together provide structural support and corneal protection[4]. The tarsal plate is a dense connective tissue structure approximately 25 mm in length and 1 mm thick, with a height of 8-12 mm in the upper eyelid and 4-5 mm in the lower eyelid. Histologically, it resembles both fibrocartilaginous tissue and meibomian secretory tissue, making it difficult to replicate with extraocular grafts. Its extracellular matrix (ECM) comprises collagen, glycosaminoglycans, aggrecan, and tenascin, while the meibomian glands consist of keratinized stratified squamous epithelium and lipid-secreting ductal units.
The conjunctiva, which originates at the corneoscleral limbus and lines the inner eyelid surface, is composed of non-keratinizing stratified columnar and stratified squamous epithelium with goblet cells. Importantly, the conjunctiva possesses the ability to spontaneously re-epithelialize after injury - an attribute that underpins tissue engineering strategies using conjunctival stem cells to repair posterior lamellar defects.
To be effective in posterior lamella reconstruction, biomaterials must meet several key criteria: they must be thin, have a stable, biocompatible matrix that integrates without provoking inflammation, and recapitulate ECM functions that support cell adhesion, survival, and proliferation. The primary biomaterials currently used include decellularized ECM, natural polymers, and synthetic polymers. A comparison of their biomechanical properties, advantages, disadvantages, and clinical considerations is summarized in Table 1.
A comparison of biomaterials and their biomechanical properties, advantages, disadvantages, and clinical considerations
| Category | Material type | Examples | Biomechanics | Pros | Cons | Ideal height/Thickness |
| Acellular ECM | Acellular dermis/Xenografts | AlloDerm, enduragen, permacol | Moderate tensile strength; variable stiffness; load-sharing capacity | -Biocompatible -Favorable integration with host tissue -Low immunogenicity | -May resorb over time -Higher infection risk -High cost | ~1-2 mm thick sheets; trim to match posterior lamella height (~4-5 mm) |
| Natural polymers | Collagen, chitosan, gelatin, fibrin | Lyophilized collagen, chitosan scaffolds | Soft, flexible; poor load-bearing | -Biodegradable -Promotes cell attachment and healing -Controllable degradation | -Poor mechanical strength -Rapid degradation unless crosslinked | ~1-2 mm thick scaffold or film; layerable |
| Synthetic polymers | Non-biodegradable: porous high-density polyethylene, ePTFE Biodegradable: PGA, PLGA, PCL | Medpor, Gore-Tex PCL scaffolds | Variable; often higher tensile strength than ECM/natural, permanent Biodegradable over months to years | -Medpor: highly porous, allows fibrovascular ingrowth, low extrusion risk; Gore-Tex: microporous, limited cell penetration -Tunable porosity, favorable ingrowth, low extrusion risk when degradation matches tissue regeneration | -Medpor: poor vascularization increases inflammatory response -Gore-Tex: encapsulation/extrusion risk, minimal tissue ingrowth -potential inflammatory response | -Medpor: ~3-4 mm -Gore-Tex: 1-3 mm (thin sheets decrease risk of encapsulation and extrusion) -~2-5 mm (thick enough to provide support until tissue ingrowth) |
DECELLULARIZED ECM
Acellular matrix biomaterials serve as a scaffold to support epithelial regeneration, primarily through their basement membrane and avascular stromal matrix, which mimic the native conjunctival environment. A well-established example is the human amniotic membrane graft, which has demonstrated effectiveness as a substrate for the growth of both goblet and non-goblet conjunctival epithelial cells[19]. However, its widespread application is limited by several factors, including extensive sterilization requirements, limited availability, and variability in preparation protocols.
In response to these limitations, decellularized bovine and porcine conjunctival grafts have been explored as alternative scaffolds and have shown promising potential for tissue substitution and regenerative applications. More recently, attention has shifted toward commercially available, “off-the-shelf” acellular dermal matrices, which have broadened the scope of posterior lamella reconstruction options.
These dermal matrices are derived from various tissue sources, including human (e.g., AlloDerm, BellaDerm), porcine (e.g., Enduragen), and bovine (e.g., SurgiMend) donors. They are designed to replace or augment the tarsal plate without incurring significant immunogenicity or histocompatibility issues. Structurally, these matrices consist of crosslinked collagen, featuring a dermal surface and a basement membrane surface, the latter of which supports native epithelial regeneration and fibroblast infiltration, facilitating wound healing.
In the context of posterior lamellar reconstruction, the graft is typically positioned adjacent to the tarsal plate as a spacer, with the dermal surface oriented anteriorly and the basement membrane surface facing the ocular surface. Conjunctival epithelialization is typically observed within 3 to 6 weeks postoperatively.
Despite their utility, a major limitation of acellular dermal matrices is their propensity for graft contraction, which has been reported to be 57% for acellular dermal matrix (ADM) in comparison to 16% for hard palate mucosal grafts when reconstructing the posterior lamella[20]. This remains a critical consideration when selecting graft materials for posterior lamella reconstruction.
NATURAL POLYMERS
Natural polymers represent a distinct class of biomaterials that are increasingly utilized in conjunctival repair, owing to their biochemical compatibility with native tissues. These materials - such as collagen, fibrin, gelatin, and others - mimic the properties of conjunctival epithelial cells, promoting tissue integration and regeneration. Examples of natural polymer-based scaffolds include chitosan and compressed collagen matrices.
Recent studies have demonstrated that plastic-compressed collagen can support re-epithelialization with minimal wound contracture and fibrosis, making it a potentially viable option for posterior lamellar reconstruction[1]. This suggests that natural polymers may offer the dual benefits of biocompatibility and structural support in reconstructive procedures. Additionally, these grafts carry a risk of infection or extrusion, which is typically higher in immunocompromised patients or those with previously irradiated tissue.
However, despite these promising early findings, natural polymers have not yet been comprehensively studied in the specific context of posterior lamella reconstruction, especially in human clinical settings. Based on our institutional experience, we urge providers to approach their use with caution, as these materials are associated with unpredictable degradation rates and variable clinical outcomes, limiting their reliability in complex eyelid reconstruction cases.
SYNTHETIC POLYMERS
Synthetic polymers represent a subtype of biomaterials comprising manufactured products that can achieve favorable tissue properties through customized production methods. Owing to the ability to control their thickness, modify their biocompatibility, and tailor their biodegradability, these materials are excellent options for posterior lamella reconstruction. Synthetic implants can be either non-biodegradable structural implants or biodegradable scaffolds. Non-biodegradable structural implants include Medpor [high-density polyethylene (PE)] and Gore-Tex [expanded polytetrafluoroethylene (ePTFE)]; while biodegradable scaffolds include materials such as poly(lactic acid) (PLA), poly(e-caprolactone) (PCL), and several others. Medpor has been used as a spacer in eyelid repair and is another potential graft for posterior lamella augmentation[21]. However, it is associated with complications, including high rates of implant exposure, graft-associated pain, and abnormal contour, and thus, appropriate pre-operative patient counseling is critical. More recently, the United States Food and Drug Administration approved clinical use of PCL, and thus there has been increasing interest in studying its use for conjunctival reconstruction; nevertheless, further research is needed to demonstrate its efficacy in this context[22].
In comparison to natural polymers, synthetic polymers render worse biocompatibility. Despite this downfall, the synthetic polymers have favorable biodegradation properties, offer increased rigidity and structure, and are readily moldable.
Overall, complications associated with biomaterial grafting of the posterior lamella are low. The most common include lid contour irregularities, entropion, keratopathy, and persistent lagophthalmos[17].
Despite their many benefits, the use of biomaterials for posterior lamella reconstruction is associated with high cost which should be considered. Biomaterials have high direct costs which vary by product size and vendor. Engaging patients in an open discussion about the costs and benefits is essential, particularly when weighing the potential need for revision surgery with biomaterials against the risks of donor site morbidity with traditional autologous grafting techniques. It is critical to utilize shared decision-making and cost-benefit analysis to determine the appropriateness for biomaterial implants.
FUTURE DIRECTIONS
As the field of biomaterial engineering continues to advance, novel products have emerged as promising options for posterior lamellar reconstruction. Current evidence suggests that biomaterials demonstrate high success rates.
More recently, investigations have focused on synthetic polymer sponges combined with bone marrow-derived mesenchymal stem cells (MBSCs) and plasmid DNA for the reconstruction of the tarsal plate in rabbit models. These constructs show promise in restoring lipid secretory function, thereby mimicking the physiological role of the meibomian gland. At eight weeks post-implantation, results demonstrated increased ECM deposition, intact meibomian gland function, and effective tissue augmentation[23].
In this review, we highlight the utility of acellular biomaterial approaches for posterior lamellar reconstruction in patients with facial paralysis. A unique aspect of this patient population is the relatively intact tissue microenvironment, which contrasts with the altered, inflamed milieu typically seen in patients with a history of trauma, malignancy, or autoimmune disease. In the setting of facial palsy, the reduced tissue inflammation renders acellular grafting a compelling option, with a lower risk of fibrosis and cellular dysfunction[24].
It is important to note that for patients presenting with full-thickness eyelid defects due to trauma or prior surgery, cellular biografts may offer fewer complications and improved functional outcomes when compared to acellular materials. However, the clinical research on cellular approaches remains limited, warranting further investigation.
Prior to the introduction of biomaterials, reconstruction options for posterior lamella deficiencies included conjunctival grafts, mucosal grafts (e.g., hard palate, nasal), and auricular cartilage. Traditionally, full-thickness defects due to oncologic etiology or trauma are best reconstructed with ADM as a spacer in conjunction with lateral tarsal strip; whereas hard palate mucosa is often reserved for severe vertical defects. We present an overview of the use of biomaterials for isolated posterior lamella augmentation in patients with facial paralysis, highlighting a novel and evolving approach to an otherwise challenging reconstructive problem. We provide a practical algorithm for paralytic lower lid malposition highlighting optimal reconstruction methods depending on defect type and properties [Table 2].
Clinical practice algorithm to guide material selection for posterior lamellar reconstruction depending on defect pattern, patient factors, and additional complexities
| Defect pattern | Preferred graft | When to add lateral tarsal strip/canthoplasty | Avoid if… |
| Mild deficiency, good tissue quality | ADM (AlloDerm, enduragen) | If horizontal laxity present | Radiated beds (increased contraction), severe keratopathy (risk of exposure) |
| Moderate deficiency, need rigidity | Hard palate mucosa | Usually yes (ectropion/negative vector) | Severe dry eye (keratinized mucosa irritates cornea) |
| Moderate-severe, curved contour needed | Auricular cartilage ± mucosal overlay | Yes, especially with lid laxity | Conjunctival deficiency (no mucosal lining), poor donor site availability |
| Severe, recurrent cases, high rigidity required | Non-biodegradable implant (Medpor, Gore-Tex) | Always combine (risk of extrusion without support) | Radiated or inflamed tissue, thin skin (increased extrusion risk) |
| Moderate deficiency in favorable beds, desire resorbable scaffold | Biodegradable scaffold (PCL, PLA, PLGA) | Optional depending on laxity | Limited evidence, avoid in unstable ocular surface or high infection risk |
SURGICAL TECHNIQUE
In our surgical technique, we present the application of acellular biomaterial for posterior lamellar reconstruction in patients with significant lower eyelid laxity, paralytic ectropion, and negative vector secondary to facial paralysis. Unlike its more traditional use in traumatic or full-thickness eyelid defects, our approach utilizes the biomaterial as a spacer graft to augment and reshape the posterior lamella, thereby optimizing both form and function in a targeted, minimally invasive manner. This surgical technique has been previously described in a case series of twelve patients utilizing porcine ADM for management of lower eyelid retraction[18]. The porcine grafts were sutured within the posterior lamella to the base of the tarsus with a conjunctival advancement over the graft. Lateral canthopexy was performed if necessary. In this study, patients were treated for negative vector secondary to thyroid pathology, post-traumatic reconstruction, post-blepharoplasty lid retraction, or anophthalmic socket. We present the case of a patient with paralytic ectropion with negative vector status post-translabyrinthine resection of vestibular schwannoma with facial nerve sacrifice. The purpose of this case is to illustrate our surgical technique in the facial paralysis patient population, though we do not assess clinical outcomes for the purposes of this technique description.
Given the severity of ectropion, lower eyelid laxity, and negative vector, decision was made to perform both posterior lamellar reconstruction with spacer graft in addition to lateral tarsal strip. A lateral incision is made just lateral to the ipsilateral lateral canthus and extended down to the periosteum of the lateral orbital rim. A skin-muscle flap is then elevated, and dissection is carried medially toward the caruncle, creating a suborbicularis pocket for graft placement.
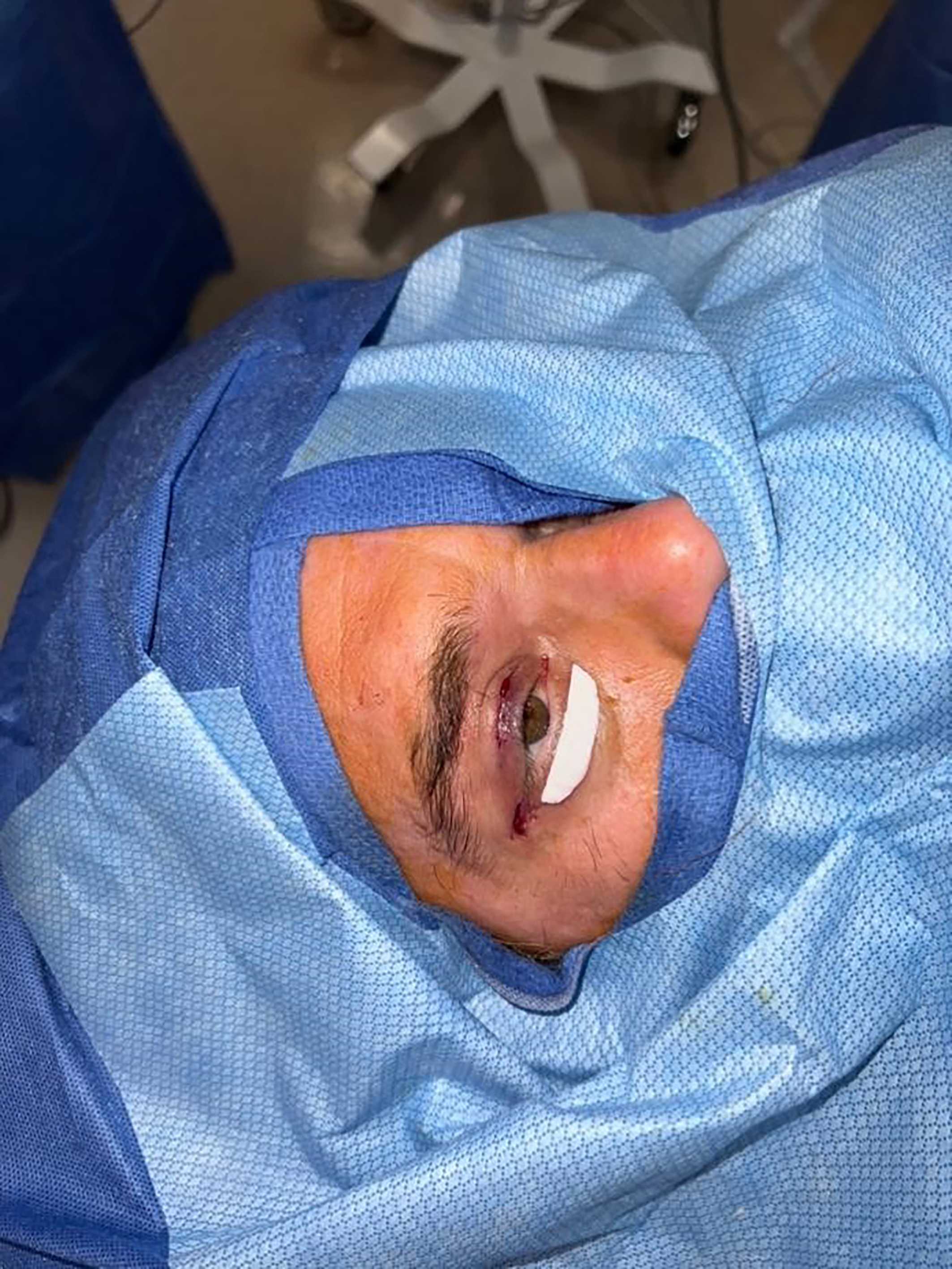
An Enduragen implant measuring approximately 3 cm × 1 cm × 0.5 mm is used [Figure 1]. The graft is carefully sized and cut to fit the pocket and inserted with the dermal surface oriented anteriorly and the basement membrane surface facing the globe for improved epithelialization, as per standard lamellar reconstruction principles [Figure 2]. After achieving proper positioning, excess graft material is trimmed as needed to fit into the precise pocket.
Figure 1. Enduragen implant cut and sized to fit posterior lamellar defect measuring 3 cm × 1 cm × 0.5 mm.
To further correct the ectropion and provide additional support, the lateral aspect of the tarsal plate is identified. A 4-0 polydioxanone suture (PDS) is used to secure the tarsus to the inner periosteal surface of the lateral orbital rim, effectively tightening the lower eyelid using a lateral tarsal strip approach. The lateral incision is closed in layers using absorbable plain gut suture in an interrupted fashion. Postoperatively, the patient is managed with artificial tears, ophthalmic antibiotic ointment, and nocturnal eye taping to mitigate the risk of keratopathy.
With this technique, we consistently observe immediate improvements in horizontal lid length, eyelid position, closure, orbital vector, and aesthetic appearance. This approach offers a structurally sound and cosmetically favorable solution for eyelid malposition in the context of facial nerve palsy.
CONCLUSION
Posterior lamellar deficiency in the setting of facial paralysis presents a distinct reconstructive challenge, differing fundamentally from full-thickness eyelid defects typically encountered in traumatic or oncologic cases. In patients with facial paralysis, the primary objective is to restore structural support and optimize eyelid position while preserving native tissue integrity. Traditional autologous grafting techniques - such as conjunctival, mucosal, and auricular cartilage grafts - have demonstrated variable success; however, they are often limited by donor site morbidity, tissue availability, and histological mismatch.
Recent advances in tissue engineering and biomaterial science have introduced acellular matrices and synthetic scaffolds as promising alternatives. These materials provide structural augmentation with improved biocompatibility, host integration, and reduced surgical morbidity. As the field progresses, further research is essential to evaluate the long-term safety, functional outcomes, and integration potential of these novel materials in clinical settings.
Looking forward, bioengineered constructs incorporating stem cells or regenerative components may offer the potential to more closely mimic the complex architecture of the tarsus, facilitating both structural and functional restoration. Ultimately, careful patient selection and a deep understanding of the pathophysiology specific to facial paralysis are critical to optimizing reconstructive outcomes in posterior lamellar repair.
DECLARATIONS
Authors’ contributions
Made substantial contributions to conception and design of the study and performed data analysis and interpretation: Welschmeyer A, Epstein A
Performed data acquisition and provided administrative, technical, and material support: Welschmeyer A, Epstein A, Gourishetti S, Rezaee R
Manuscript drafting and review: Zaronias K
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
As this study does not constitute human subjects research, it was granted an exemption from ethical review by the Institutional Review Board (IRB) of the University of Houston (IRB No. STUDY20251158). Informed consent was obtained from the patient. Separate consent for the publication of anonymized clinical images included in this article was also granted.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. May M, Klein SR. Differential diagnosis of facial nerve palsy. Otolaryngol Clin North Am. 1991;24:613-45.
4. Yan Y, Ji Q, Fu R, et al. Biomaterials and tissue engineering strategies for posterior lamellar eyelid reconstruction: replacement or regeneration? Bioeng Transl Med. 2023;8:e10497.
6. Pham CM, Heinze KD, Mendes-Rufino-Uehara M, Setabutr P. Single-stage repair of large full thickness lower eyelid defects using free tarsoconjunctival graft and transposition flap: experience and outcomes. Orbit. 2022;41:178-83.
7. Dagregorio G, Huguier V, Darsonval V. Reconstruction of seventeen full-thickness defects of the eyelids with twenty-two Hübner tarsomarginal grafts. Br J Plast Surg. 2005;58:361-5.
9. Toft PB. Reconstruction of large upper eyelid defects with a free tarsal plate graft and a myocutaneous pedicle flap plus a free skin graft. Orbit. 2016;35:1-5.
10. Hendriks S, Bruant-Rodier C, Lupon E, Zink S, Bodin F, Dissaux C. The palatal mucosal graft: the adequate posterior lamellar reconstruction in extensive full-thickness eyelid reconstruction. Ann Chir Plast Esthet. 2020;65:61-9.
11. Perry MJ, Langtry J, Martin IC. Lower eyelid reconstruction using pedicled skin flap and palatal mucoperiosteum. Dermatol Surg. 1997;23:395-7.
12. Texier M, Preaux J, Noury-Duperrat G. Aesthetic aspects of reconstructive surgery of the lower lid. Aesthetic Plast Surg. 1995;19:557-9.
13. Parodi PC, Calligaris F, De Biasio F, De Maglio G, Miani F, Zeppieri M. Lower lid reconstruction utilizing auricular conchal chondral-perichondral tissue in patients with neoplastic lesions. Biomed Res Int. 2013;2013:837536.
14. Nigro MVAS, Friedhofer H, Natalino RJM, Ferreira MC. Comparative analysis of the influence of perichondrium on conjunctival epithelialization on conchal cartilage grafts in eyelid reconstruction: experimental study in rabbits. Plast Reconstr Surg. 2009;123:55-63.
15. Suga H, Ozaki M, Narita K, et al. Comparison of nasal septum and ear cartilage as a graft for lower eyelid reconstruction. J Craniofac Surg. 2016;27:305-7.
16. Guo Y, Gao T, Lin M, et al. Posterior lamella substitutes in full-thickness eyelid reconstruction: a narrative review. Front Oral Maxillofac Med. 2023;5:24 24.
17. Rehman U, Shemie M, Sarwar MS, Adebayo O, Brennan PA. Use of biomaterials in the reconstruction of posterior lamellar eyelid defects: a systematic review and meta-analysis. Br J Oral Maxillofac Surg. 2023;61:464-74.
18. McGrath LA, Hardy TG, McNab AA. Efficacy of porcine acellular dermal matrix in the management of lower eyelid retraction: case series and review of the literature. Graefes Arch Clin Exp Ophthalmol. 2020;258:1999-2006.
19. Furdova A, Czanner G, Koller J, Vesely P, Furda R, Pridavkova Z. Amniotic membrane application in surgical treatment of conjunctival tumors. Sci Rep. 2023;13:2835.
20. Sullivan SA, Dailey RA. Graft contraction: a comparison of acellular dermis versus hard palate mucosa in lower eyelid surgery. Ophthalmic Plast Reconstr Surg. 2003;19:14-24.
21. He M, Storr-Paulsen T, Wang AL, et al. Artificial polymeric scaffolds as extracellular matrix substitutes for autologous conjunctival goblet cell expansion. Invest Ophthalmol Vis Sci. 2016;57:6134-46.
22. Tan J, Olver J, Wright M, Maini R, Neoh C, Dickinson AJ. The use of porous polyethylene (Medpor) lower eyelid spacers in lid heightening and stabilisation. Br J Ophthalmol. 2004;88:1197-200.
23. Dai Y, Jin K, Feng X, Ye J, Gao C. Regeneration of different types of tissues depends on the interplay of stem cells-laden constructs and microenvironments in vivo. Mater Sci Eng C Mater Biol Appl. 2019;94:938-48.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Special Topic
Copyright
Data & Comments
Data


















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].