Retrospective analysis of keloid split-thickness skin graft replantation combined with LCR therapy for keloid treatment
Abstract
Aim: To evaluate the clinical efficacy of scar split-thickness skin replantation combined with LCR (laser-carbon dioxide-radiotherapy) therapy in improving healing outcomes and reducing recurrence rates in patients with multiple nodular or hypertrophic keloid lesions.
Methods: A retrospective review was conducted on 11 patients who underwent surgical excision of keloids, followed by replantation of split-thickness skin graft onto the thinned keloid dermis. Postoperatively, all patients received LCR therapy. Clinical outcomes were assessed using the Patient and Observer Scar Assessment Scale (POSAS) before and after treatment. Postoperative epithelialization time and adverse events were also recorded.
Results: All patients achieved complete epithelialization within four weeks after surgery. Significant improvement in scar quality was observed, with POSAS scores decreasing from 92.35 ± 6.09 pre-treatment to 18.58 ± 7.38 post-treatment (P < 0.001). No severe complications such as infection or ulceration occurred during the follow-up period.
Conclusion: Scar split-thickness skin replantation combined with LCR therapy is a safe, cost-effective, and efficacious treatment modality for keloids. By preserving the dermal scaffold and enhancing epithelial regeneration, this approach significantly improves scar appearance and reduces recurrence, particularly in challenging cases involving multiple or hypertrophic keloid nodules.
Keywords
INTRODUCTION
Keloids are chronic fibroproliferative skin disorders characterized by persistent fibroblast activation and excessive extracellular matrix deposition, particularly types I and III collagen, which extends beyond the boundaries of the original wound[1]. Unlike normal scars, keloids fail to regress and often exhibit aggressive local growth. Recent advances in fibrosis research have reclassified keloids as reconstructive fibroproliferative conditions (FPCs) - a subset defined by quasi-neoplastic behavior, including local invasiveness, resistance to apoptosis, and disorganized tissue architecture[2]. This refined classification underscores their unique biological status, bridging the gap between benign fibrosis and malignant tumors, and provides a new framework for investigating their underlying cellular mechanisms and therapeutic vulnerabilities.
The pathogenesis of keloids is multifactorial, involving a complex interplay of genetic predisposition, dysregulated wound-healing responses, and chronic inflammation[3]. Among these factors, mechanical tension has been increasingly recognized as a critical driver of keloid initiation and progression[4]. Excessive tension in susceptible anatomical sites - such as the chest, shoulders, and extremities - not only promotes fibroblast activation but also sustains pathological signaling loops that perpetuate scar overgrowth. Clinically, keloids pose significant functional limitations and aesthetic concerns, and they are notoriously refractory to conventional treatments. Conventional therapeutic strategies, including intralesional corticosteroids, surgical excision, cryotherapy, and radiotherapy, frequently result in high recurrence rates, particularly in high-tension regions, highlighting the urgent need for more effective and mechanistically informed therapeutic strategies[5].
Surgical excision alone is often associated with high recurrence rates in keloid treatment, primarily due to the incomplete elimination of pathogenic fibroblasts and the persistence of a profibrotic wound-healing microenvironment[6,7]. Reported recurrence rates following excision monotherapy range widely from 45% to nearly 100%[8]. To address this limitation, various combination therapies have been explored. Among them, the adjunctive use of postoperative radiotherapy has shown considerable efficacy in reducing recurrence by targeting residual fibroblasts and modulating early inflammatory responses after wound closure. Post-excision, high mechanical tension at the surgical site exacerbates fibroblast proliferation and collagen deposition, and the inability to maintain sustained tension-reduction measures further contributes to recurrence[9]. Therefore, innovative strategies that combine surgical and adjuvant therapies while minimizing additional tension at the keloid site have gained interest[10]. Recent advancements in regenerative medicine have introduced methods such as acellular dermal matrix (ADM) application and in situ epidermal replantation to improve healing outcomes[11]. However, ADM requires additional costs and may not be readily available in all medical centers. In cases of multiple keloid lesions, treatment expenses may increase significantly, limiting accessibility for some patients.
This study presents a novel therapeutic approach in which the excised keloid’s split-thickness skin graft is carefully replanted onto the underlying thinned keloid dermis, followed by laser-carbon dioxide-radiotherapy (LCR) therapy - an integrated regimen consisting of fractional laser resurfacing, carbon dioxide (CO2) laser ablation, and low-dose radiotherapy. This combined protocol aims to reduce recurrence by preserving the native dermal extracellular matrix, which provides structural integrity and biochemical cues essential for normal wound remodeling. Simultaneously, LCR therapy modulates the local wound microenvironment by targeting abnormal fibroblast activity, reducing inflammation, and suppressing excessive collagen production[12]. Compared to ADM-based treatments, this approach offers a cost-effective, technically feasible, and more widely accessible solution, particularly advantageous for patients with extensive or recurrent keloid lesions.
METHODS
Study design and patient selection
A retrospective analysis was conducted on patients treated with the keloid split-thickness skin graft replantation technique between January 2023 and December 2024. All patients had Fitzpatrick skin type III or IV. Inclusion criteria were patients with clinically or histologically confirmed keloids located in high-tension areas. Specifically, this protocol was applied to: (a) large or thick keloids (e.g., lesions ≥ 2 cm in diameter or with significant hypertrophy); (b) multiple or widespread keloid lesions; (c) keloids located in high-tension anatomical regions, such as the chest, shoulders, elbows, and extremities; and (d) recurrent keloids after previous treatments, including steroid injections, surgery, or laser. Patients with hypertrophic scars, burn scars, or other non-keloid fibrotic conditions were excluded. Only those who completed the full treatment protocol, including postoperative LCR therapy, and had complete follow-up data were included in the analysis.
Surgical procedure
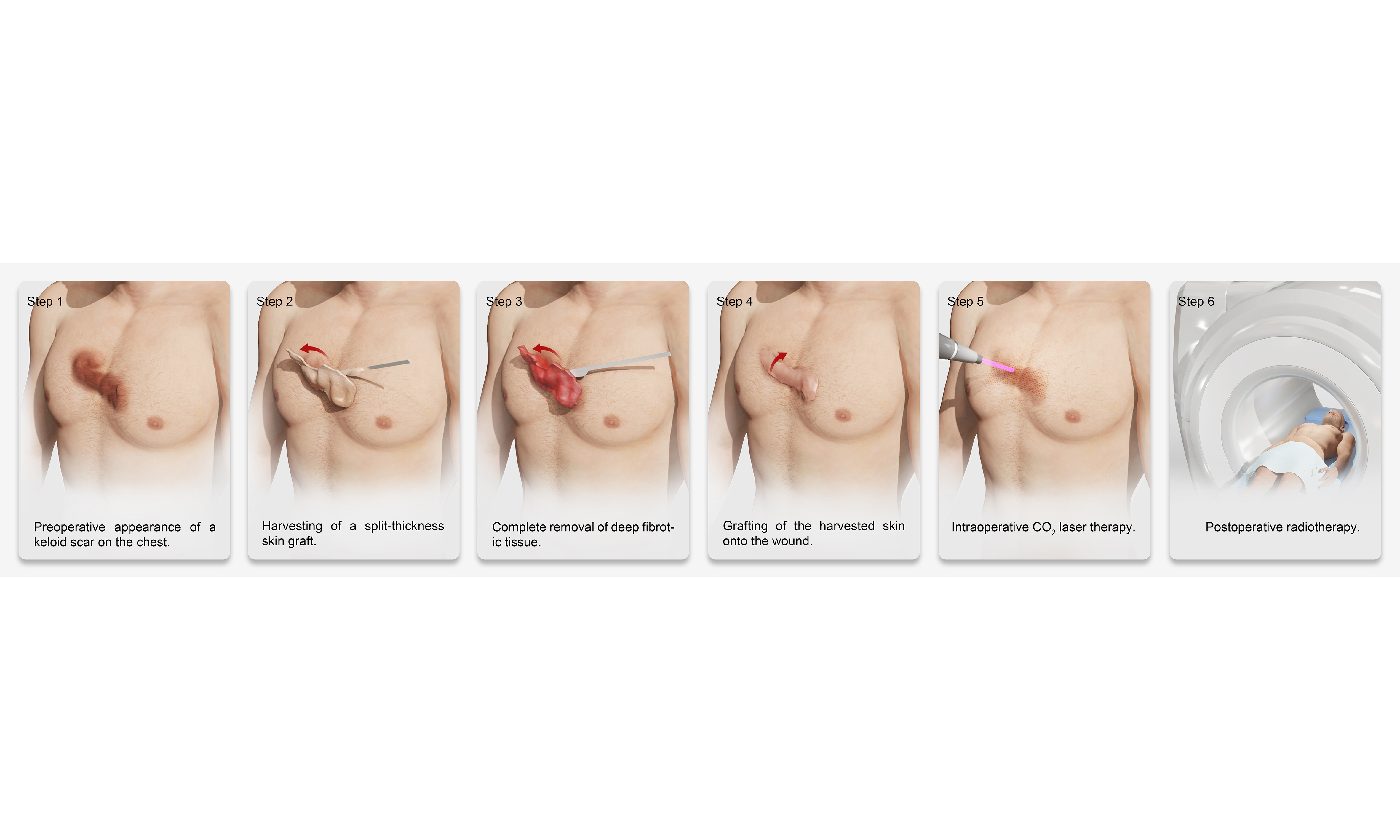
As illustrated in Figure 1, all patients underwent standardized surgical excision of keloid tissue under local or general anesthesia, depending on the size and location of the lesion. Keloid tissue was processed using a precision dermatome (typically set at 0.15-0.20 mm thickness) to harvest a split-thickness skin graft composed primarily of the epidermis and partial upper dermis. Necrotic or fibrotic portions were excluded, and only uniform, viable grafts were selected for replantation.
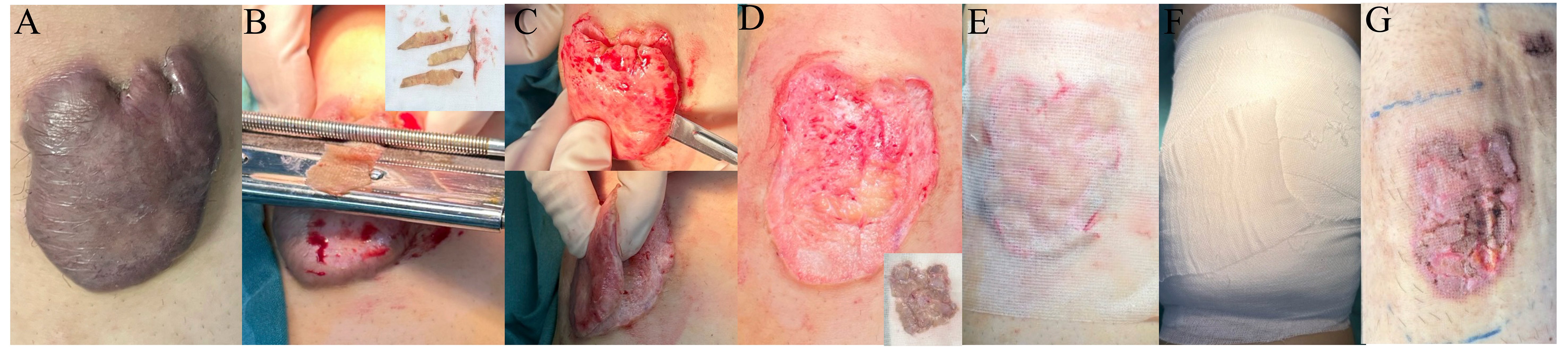
Figure 1. Representative intraoperative and postoperative images of keloid split-thickness skin graft replantation onto thinned keloid dermis, followed by compression dressing and LCR therapy. (A) Preoperative appearance of a keloid lesion; (B) Harvesting of keloid split-thickness skin graft using a dermatome; (C) Thinning of the keloid dermis and preparation of the wound bed; (D) Complete removal of the bulk of the keloid while preserving the deep dermis; (E) Immediate postoperative view following split-thickness skin graft replantation; (F) Application of postoperative dressing for stabilization; (G) Early postoperative assessment of split-thickness skin graft reattachment after LCR therapy. LCR: Laser-carbon dioxide-radiotherapy.
Subsequently, meticulous delineation of the keloid margins was performed, and the elevated portion of the keloid scar above the skin surface was excised. Special care was taken to preserve a layer of viable deep dermis, ensuring adequate vascularity for subsequent graft take. After excision, the residual dermal bed was subjected to controlled thinning using surgical scissors or scalpel, aiming to reduce the density of hyperactive fibroblasts while preserving the collagen framework and capillary plexus, which are crucial for re-epithelialization and graft survival. These skin grafts were then repositioned onto the prepared dermal bed, ensuring correct orientation, edge-to-edge alignment, and maximal surface contact to facilitate nutrient diffusion and vascular inosculation.
To secure the grafts, a non-adherent silicone-based dressing was first applied to prevent shearing and trauma during dressing changes. This was followed by multiple layers of compressive bandaging, including sterile gauze and elastic wrap. This compression not only enhanced graft adherence but also helped minimize postoperative edema and tension-related disruptions. The initial dressing was left undisturbed for 7 days, after which the grafts were inspected for signs of epithelialization, infection, or hematoma formation. Upon confirmation of satisfactory graft take, the fixation dressing was gently removed, and patients transitioned to the next phase of treatment. No major complications such as hemorrhage or infection were observed; only mild serous exudation occurred in some cases, which was easily managed with simple dressing changes without the need for special intervention.
LCR procedure
Following complete graft stabilization, all patients received LCR therapy, a combined protocol. All treatment procedures were performed by the same experienced therapist at Shanghai Ninth People’s Hospital (Shanghai, China) to ensure procedural consistency. Each patient received a single session of fractional CO2 laser therapy. To ensure patient comfort, local anesthesia was administered prior to the procedure. In most cases, a topical anesthetic cream containing 2.5% lidocaine and 2.5% prilocaine (Beijing Ziguang, China) was applied to the keloid lesion and surrounding skin at a dosage of 1.5 g per 10 cm2. After an anesthesia period of at least 1 h, the cream was thoroughly removed, and the treatment area was disinfected with 75% alcohol. In some cases - particularly for larger or thicker lesions - intradermal injection of lidocaine was used as an alternative or supplemental anesthetic approach, based on the clinician’s judgment and the patient’s tolerance.
Fractional CO2 laser treatment was then administered using the Alma Laser system, with the Lite-scan (F100) mode selected. Energy settings ranged from 360 to 1,008 mJ/pixel, typically 1-2 pulses and a density of 9, adjusted based on lesion thickness. Following laser therapy, electron beam radiotherapy was administered at the Huangpu Branch of Shanghai Ninth People’s Hospital (Shanghai, China). Two irradiation sessions were administered: the first within 24 h post-laser treatment, and the second on Day 7. Both sessions employed a 6 MeV electron beam, with a dose of 900 cGy per session. Following completion of LCR therapy, silicone gel was applied once daily for one month, with no additional anti-keloid interventions administered during the postoperative period.
Outcome assessment
Clinical outcomes were systematically evaluated using the Patient and Observer Scar Assessment Scale (POSAS), which includes both subjective (patient-reported) and objective (observer-assessed) components. Assessments were performed at baseline (pre-treatment) and during follow-up visits at either 6 months or 12 months post-treatment, depending on each patient’s availability and clinical condition. The observer component was conducted by two independent clinicians blinded to the treatment protocol, and included five domains: vascularity, pigmentation, thickness, relief, and pliability. The patient component consisted of six domains: pain, pruritus (itching), color, stiffness, thickness, and irregularity. Each domain was scored on a 10-point scale (1 = normal skin; 10 = worst imaginable scar), with lower total scores indicating better scar quality. Both patient and observer total scores were calculated separately, and improvements were evaluated by comparing pre- and post-treatment scores. In addition to POSAS scoring, clinical photographs were taken at each follow-up visit under standardized lighting and positioning to visually document scar progression. Any adverse events, including infection, ulceration, pigmentation abnormalities, or recurrence, were also recorded throughout the follow-up period.
Statistical analysis
Data were analyzed using SPSS (Version 22.0, SPSS Inc., IL, USA). Paired t-tests were performed to compare pre- and post-treatment POSAS scores. A P-value of < 0.05 was considered statistically significant.
RESULTS
Patient demographics and keloid characteristics
A total of 11 patients were included in this study, comprising 3 males and 8 females, with an average age of 29.72 years (range: 19-45 years). The most common anatomical sites of keloid involvement included the chest (3 cases), shoulders, back, and buttocks (4 cases), and elbows and other extremities (4 cases), as detailed in Table 1. The preoperative keloid size ranged from 4 cm2 × 2.5 cm2 to 11 cm2 × 8.5 cm2, with some patients presenting multiple lesions.
Patient demographics and keloid characteristics
| Gender | Age (year) | Scar location | Scar size (cm2) | Scar duration (years) | Surgery date | Follow-up |
| Female | 28 | Left elbow | 11 × 8.5 | 10 | 2023/10/30 | 6 |
| Female | 19 | Left hand | 5 × 3.5 | 13 | 2023/1/29 | 12 |
| Female | 38 | Chest | 11 × 4.5 | 15 | 2023/2/6 | 12 |
| Female | 27 | Left elbow | 5.5 × 6.5 | 4 | 2023/12/21 | 6 |
| Female | 31 | Right shoulder | 9 × 8 | 9 | 2023/6/15 | 12 |
| Male | 23 | Bilateral shoulders and back | 2-5 × 3-5.5 (multiple) | 8 | 2023/1/29 | 12 |
| Male | 41 | Buttocks | 10.5 × 7.5 | 15 | 2023/4/19 | 6 |
| Female | 26 | Left elbow | 4 × 2.5 | 7 | 2023/3/9 | 12 |
| Female | 26 | Chest | 5.5 × 3 | 9 | 2023/6/15 | 12 |
| Female | 23 | Bilateral shoulders | 5 × 3.5 (left) 9 6.5 (righ) | 8 | 2023/10/16 | 6 |
| Male | 45 | Chest | 9 × 4.5 | 10 | 2023/3/27 | 12 |
All patients presented with clinically typical keloid features, including erythematous (reddish), and elevated lesions, consistent with nodular or plaque-type keloids. No evidence of ulceration, infection, or spontaneous discharge was observed prior to surgical intervention. The keloids were stable in size but symptomatic, with most patients reporting itching, occasional pain, and aesthetic concerns, and all had failed prior conservative therapies such as corticosteroid injection or silicone dressing.
Improvement in scar quality assessed by POSAS scores
The POSAS scores demonstrated statistically and clinically significant improvements across all evaluated domains. Parameters assessed included pain, pruritus, color, stiffness, thickness, and surface irregularity, reflecting both subjective symptoms and objective clinical appearance of the scar.
Mean total POSAS scores decreased markedly from 92.35 ± 6.09 before treatment to 18.58 ± 7.38 after treatment (P < 0.001), indicating a substantial improvement in overall scar quality [Table 2]. Observer-assessed domains such as vascularity and pigmentation normalized significantly, while patient-reported symptoms including itching and stiffness were notably alleviated. These results underscore not only the reduction in physical scar burden but also the improvement in functional comfort and cosmetic satisfaction, suggesting that the combined approach of split-thickness skin replantation with LCR therapy effectively restores both aesthetic integrity and patient quality of life.
Pre- and post-treatment POSAS results
| Score | Pre-treatment (n = 11) | Post-treatment (n = 11) | P value (< 0.05) |
| Total score | 92.346 | 18.577 | < 0.001 |
| Patient score | 52.538 | 8.692 | < 0.001 |
| Pain | 6.615 | 0.923 | < 0.001 |
| Pruritus | 9.077 | 1.462 | < 0.001 |
| Color | 8.462 | 2.462 | < 0.001 |
| Stiffness | 9.538 | 1.385 | < 0.001 |
| Thickness | 9.538 | 1.077 | < 0.001 |
| Irregularity | 9.308 | 1.385 | < 0.001 |
| Observer score | 39.808 | 9.885 | < 0.001 |
| Vascularity | 7.308 | 2.231 | < 0.001 |
| Pigmentation | 7.500 | 2.231 | < 0.001 |
| Thickness | 8.269 | 1.654 | < 0.001 |
| Relief | 8.538 | 1.846 | < 0.001 |
| Pliability | 8.192 | 1.923 | < 0.001 |
Efficacy of scar split-thickness skin replantation in keloid management
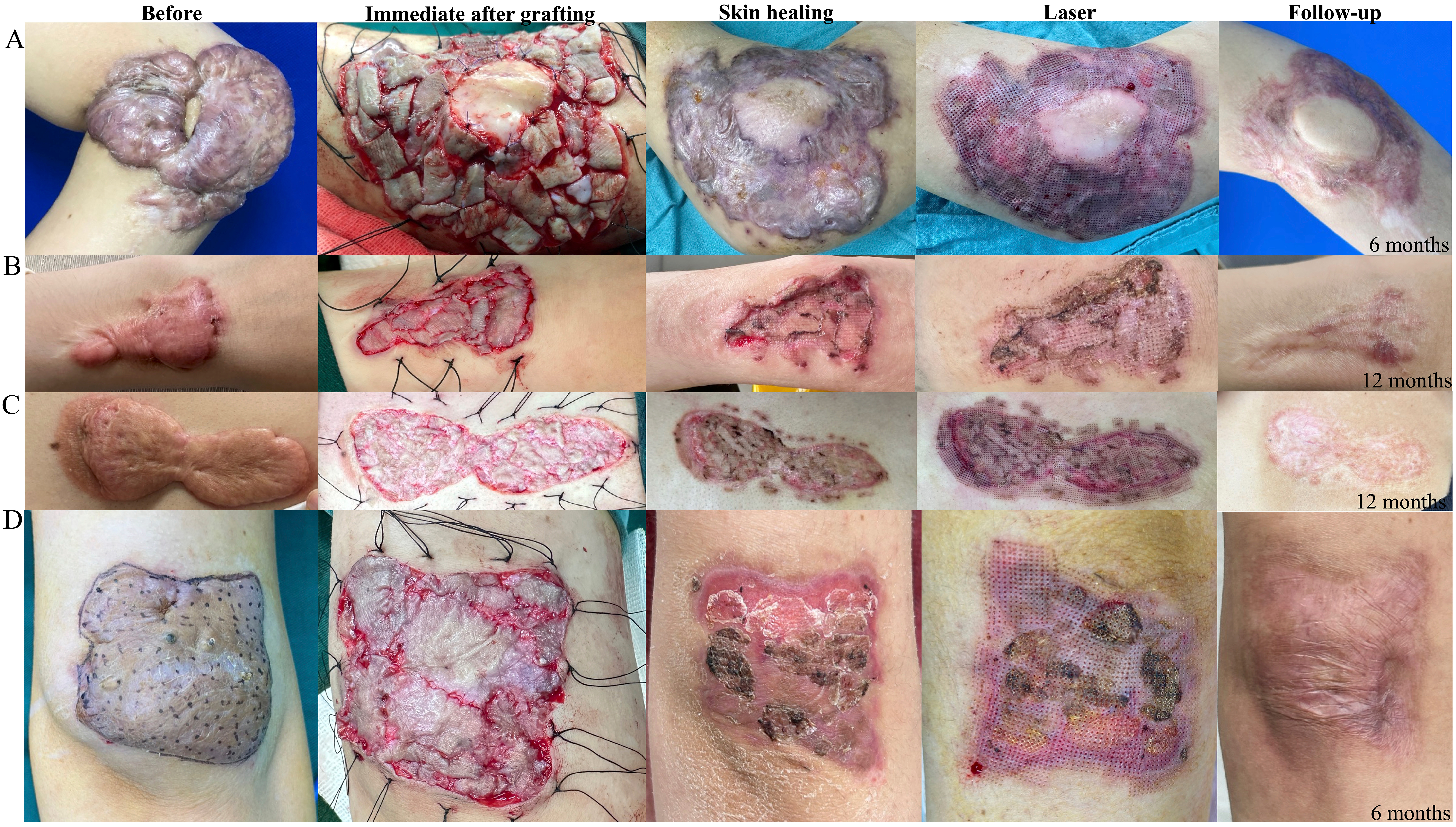
Figure 2 illustrates the clinical progression of patients undergoing scar split-thickness skin replantation combined with LCR therapy for keloid treatment, detailing key stages from preoperative appearance to long-term follow-up. The figure highlights the primary findings, showing preoperative keloid lesions with significant hypertrophy and fibrosis, followed by intraoperative images depicting keloid excision and epidermal replantation onto thinned dermis. In the postoperative healing phase, progressive epithelialization, reduced inflammation, and initial scar remodeling are observed. The application of LCR therapy, including fractional laser and superficial radiotherapy, further enhances fibroblast modulation and collagen remodeling. At 6 or 12 months postoperatively, follow-up images reveal well-healed scars with significant flattening, improved pigmentation, and no signs of recurrence, confirming the efficacy of this approach.
Figure 2. Representative cases of keloid treatment using scar split-thickness skin replantation combined with LCR therapy. This figure illustrates the sequential stages of treatment for four different keloid cases (A-D), showing the progression from preoperative condition to long-term follow-up. Column 1 (Before): Preoperative appearance of keloid lesions, demonstrating varying degrees of hypertrophic growth and contracture; Column 2 (Immediate after grafting): Intraoperative view following keloid excision and split-thickness skin graft replantation onto thinned keloid dermis. The split-thickness skin grafts were sutured in place; Column 3 (Skin healing): Early postoperative healing stage, showing re-epithelialization and initial integration of the grafts; Column 4 (Laser): Postoperative appearance after laser therapy as part of the LCR treatment protocol, aimed at modulating fibroblast activity and improving scar texture; Column 5 (Follow-up): Long-term outcomes at 6 months or 12 months postoperatively, revealing well-healed scars with minimal recurrence and improved skin texture. LCR: Laser-carbon dioxide-radiotherapy.
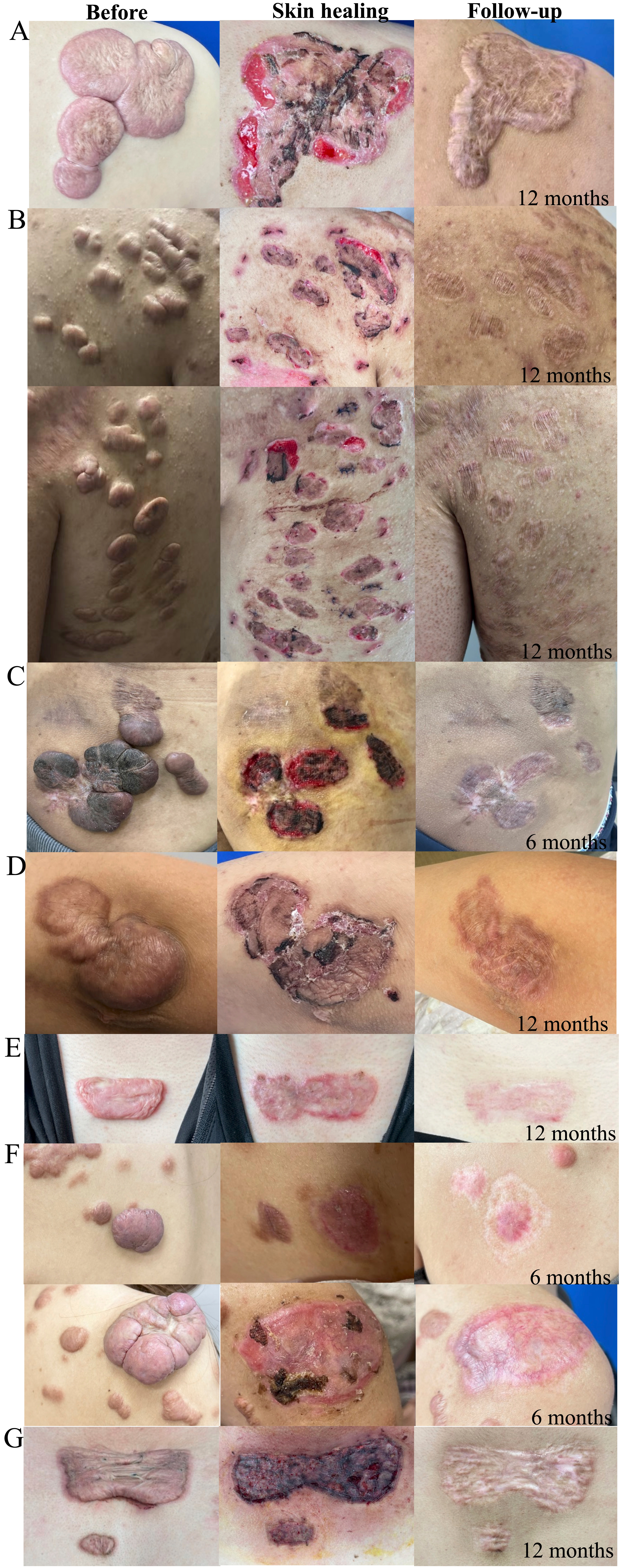
Figure 3 further supports these findings by demonstrating additional cases, including multiple keloid lesions in different anatomical locations. The images show similar patterns of healing and long-term improvement, reinforcing that scar split-thickness replantation is an effective technique for both localized and widespread keloid lesions. These results suggest that this approach, when combined with LCR therapy, provides a promising, cost-effective alternative to conventional excision techniques, leading to improved scar quality and reduced recurrence rates. These findings reinforce the potential of this approach as a promising and reliable alternative to conventional surgical methods for keloid treatment, particularly in high-tension areas where recurrence rates have historically remained a major challenge.
Figure 3. Clinical progression of multiple keloid cases treated with scar split-thickness skin replantation. This figure illustrates the treatment outcomes for seven different keloid cases (A-G) at various stages, including preoperative, postoperative healing, and long-term follow-up. Column 1 (Before): Preoperative images showing different types of keloid lesions, including bulky, nodular, and extensive plaque-like scars; Column 2 (Skin healing): Postoperative images during the healing phase, demonstrating the early re-epithelialization process after split-thickness skin graft replantation. Some cases exhibit scab formation and early scar remodeling; Column 3 (Follow-up): Long-term follow-up images taken at 6 or 12 months postoperatively, showing well-healed scars with varying degrees of flattening, pigmentation changes, and overall improved skin texture.
DISCUSSION
Keloid treatment remains a major clinical challenge due to its high recurrence rate and resistance to conventional therapies[13]. The novel approach described in this study - scar split-thickness skin replantation combined with LCR therapy - offers several distinct advantages over existing methods. Unlike traditional excision techniques, which often result in high recurrence rates due to mechanical tension and uncontrolled fibroblast activity[14], this method preserves the thinned keloid dermis while replanting standard split-thickness skin grafting, creating a more favorable wound-healing environment[15]. This technique differs from full-thickness skin grafting by maintaining the dermal matrix while avoiding donor site morbidity. In clinical practice, two skin grafting strategies were adopted depending on lesion size and shape: sheet grafts and postage stamp grafts. The sheet graft technique offers superior cosmetic outcomes due to its continuous epidermal coverage and minimal interstitial gaps. However, in larger or irregularly contoured lesions - especially those involving mobile areas such as the shoulder or back - postage stamp grafting provided better flexibility, facilitated drainage, and allowed for greater surface area coverage with limited harvested skin. Additionally, LCR therapy was introduced postoperatively to regulate fibroblast activity and collagen remodeling, reinforcing long-term scar control[12].
Traditional keloid treatments such as excision alone, ADM application, flap reconstruction, tissue expansion, and punch excision each have their own limitations. Simple excision is often associated with high recurrence rates due to post-surgical tension and the persistence of pathogenic fibroblasts. ADM, while providing a beneficial scaffold for tissue regeneration, is costly and not readily available in all medical centers, making it less practical for patients with multiple keloid lesions. Flap reconstruction offers a viable option for defect coverage, but it requires additional donor tissue, increasing surgical complexity and the risk of secondary morbidities[16,17]. Similarly, tissue expansion can generate additional skin for coverage but demands a prolonged treatment period with multiple procedures, making it less ideal for patients seeking immediate results[17]. Punch excision is another technique used to remove keloid tissue in a minimally invasive manner, often followed by intralesional steroid injections or radiotherapy[18,19]. However, while this method can reduce localized keloid thickness, it is less effective in rapidly reducing the bulk of large hypertrophic keloids compared to scar split-thickness skin replantation, which removes a significant portion of keloid volume in a single procedure.
Radiation therapy has long been recognized as an effective adjunctive treatment for keloids, particularly when applied postoperatively[20]. It works primarily by suppressing fibroblast proliferation and collagen synthesis, thereby reducing the risk of recurrence. Laser treatment is not intended to completely eliminate the lesion, but rather to precondition the scar tissue, making it more biologically responsive to subsequent radiation. The application of ablative fractional CO2 laser results in localized thermal damage that initiates a regenerative response, marked by increased type III collagen synthesis and remodeling of the scar extracellular matrix, ultimately leading to a reduction in lesion thickness[21]. After laser treatment, the affected tissue enters a proliferative and immature state, during which both fibroblasts and matrix components exhibit heightened sensitivity to subsequent radiotherapy[22,23], thereby enhancing the therapeutic efficacy of radiation. Within the LCR treatment, this single-session laser treatment produces precisely distributed microthermal zones throughout the lesion. Radiotherapy is then administered during the early phase of tissue repair, a window in which the wound environment is more conducive to modulation. This sequence enables radiation to synergize with active tissue remodeling, thereby augmenting antifibrotic outcomes while minimizing exposure-related damage to surrounding normal tissue[24]. However, both radiation and laser treatments are limited in their ability to address the mechanical forces that drive keloid formation, particularly in high-tension anatomical regions. Moreover, these modalities are often insufficient for managing thick, nodular keloids, as they do not substantially debulk hypertrophic tissue or restore normal dermal architecture.
In contrast, the scar split-thickness replantation technique offers a more comprehensive solution. By excising the bulk of the keloid and replanting autologous skin graft onto a preserved, thinned dermal bed, this method maintains the native extracellular matrix while minimizing donor site morbidity. It not only improves the biological environment by preserving dermal signaling but also reduces local mechanical tension, thereby addressing a key driver of keloid recurrence. Unlike approaches involving acellular dermal matrices (ADMs), skin flaps, or tissue expanders, this technique is cost-effective, technically straightforward, and suitable for widespread clinical adoption. Furthermore, recent histological evidence suggests that the superficial dermis of keloid tissue may serve as the initiating zone of keloid formation, harboring highly active fibroblasts and dense extracellular matrix components. As demonstrated by
An additional advantage of this method is its effectiveness in treating multiple keloid lesions simultaneously. Unlike flap reconstruction and tissue expansion, which are often limited by donor site availability and require staged procedures, this technique allows for simultaneous replantation of multiple keloid lesions, making it a highly efficient option for patients with widespread or recurrent keloids. Furthermore, by utilizing the patient’s own scar skin graft, the approach minimizes the risk of immune rejection and eliminates the need for additional skin graft harvesting, which is particularly beneficial for patients with extensive lesions. The POSAS scores demonstrated significant improvements across multiple scar parameters. Patients reported marked reductions in pain, pruritus, and stiffness, while clinical assessments confirmed enhanced vascularity, pigmentation, and pliability. Importantly, complete epithelialization was achieved within four weeks postoperatively, without cases of bleeding, infection, or ulceration.
Despite the encouraging results, this technique has several limitations. First, the study’s retrospective design and small sample size limit its generalizability. Second, although technically simpler than ADM- or flap-based methods, the procedure still requires surgical expertise in preserving a viable dermal bed and processing autologous split-thickness skin graft. Third, while suitable for high-tension, planar anatomical regions, it may be less effective in mobile or contoured areas such as the neck or joints, where maintaining graft stability and uniform compression is more challenging. Importantly, although the split-thickness skin graft replantation restores epithelial coverage and reduces bulk, it does not intrinsically suppress fibroblast activity or prevent recurrence. The success of this approach critically depends on the subsequent application of LCR therapy, particularly fractional CO2 laser, which plays a central role in dermal remodeling and modulation of fibrotic activity. However, the availability of such laser equipment is often limited, especially in non-tertiary or resource-constrained settings. Without LCR, the grafted tissue may survive but is prone to recurrence due to residual fibroblast activity, highlighting that this approach is primarily a volume-reducing strategy, not a definitive anti-recurrence treatment on its own.
This study presents a novel and cost-effective therapeutic strategy for keloid management that integrates principles of mechanical offloading, biological modulation, and autologous tissue preservation. By combining scar split-thickness epidermal replantation with LCR therapy (fractional CO2 laser and postoperative radiotherapy), the approach not only removes the pathological bulk of the lesion but also preserves the native dermal matrix, promoting favorable wound healing while minimizing donor site morbidity. Importantly, the addition of LCR therapy modulates residual fibroblast activity, reduces profibrotic signaling, and helps prevent recurrence.
This integrated method addresses both the mechanical drivers - such as local tension - and the cellular mechanisms underpinning keloid formation, offering a more comprehensive solution than traditional single-modality treatments. It is particularly advantageous in managing refractory, nodular, or extensive keloids, especially in high-tension anatomical regions like the chest, shoulders, and extremities, where recurrence rates are notoriously high. Furthermore, the simplicity and accessibility of this technique make it clinically feasible for widespread implementation, especially in settings where advanced reconstructive options (e.g., flaps, ADM) are limited. Overall, this study provides a compelling foundation for further exploration of multimodal, tension-reducing therapies in the long-term control of keloid disease.
DECLARATIONS
Authors’ contributions
Study design, surgical procedures, data interpretation, manuscript drafting: Wang W
Data collection, patient follow-up, manuscript editing: Chen Z
Statistical analysis, figure preparation: Xia L
Supervision, critical revision of the manuscript, funding acquisition: Gao Z
Project administration, supervision, final approval of the version to be submitted: Wu X
All authors read and approved the final manuscript.
Availability of data and materials
All data generated or analyzed during this study are included in this article.
Financial support and sponsorship
This study was supported by the National Natural Science Foundation of China (82203895 to Chen Z) and the Yangfan Project of Science and Technology Commission of Shanghai Municipality (22YF1421800 to Chen Z).
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
This retrospective study was conducted in accordance with the ethical standards of the institutional and/or national research committee and the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study protocol was reviewed and approved by the Ethics Committee of Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine (IRB number: SH9H-2022-T85-2). Written informed consent for publication of anonymized clinical data and images was obtained from all patients included in this study.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
2. Huang C, Shao Y, Bai J, Zhao Y, Ogawa R. Fibroproliferative conditions: the 3R approach bridging fibrosis and tumors. Trends Mol Med. 2025;Epub ahead of print.
3. Shih B, Garside E, McGrouther DA, Bayat A. Molecular dissection of abnormal wound healing processes resulting in keloid disease. Wound Repair Regen. 2010;18:139-53.
4. Lee YI, Yang Y, Ham S, et al. Heterogeneity in keloid scars: influence of mechanical stretching on keloids arising from different anatomical sites. J Invest Dermatol. 2025;145:710-3.e7.
5. Andrews JP, Marttala J, Macarak E, Rosenbloom J, Uitto J. Keloids: the paradigm of skin fibrosis - pathomechanisms and treatment. Matrix Biol. 2016;51:37-46.
6. Ogawa R. Keloid and hypertrophic scars are the result of chronic inflammation in the reticular dermis. Int J Mol Sci. 2017;18:606.
7. Limandjaja GC, Niessen FB, Scheper RJ, Gibbs S. The keloid disorder: heterogeneity, histopathology, mechanisms and models. Front Cell Dev Biol. 2020;8:360.
8. Fu S, Duan L, Zhong Y, Zeng Y. Comparison of surgical excision followed by adjuvant radiotherapy and laser combined with steroids for the treatment of keloids: a systematic review and meta-analysis. Int Wound J. 2024;21:e14449.
9. Liao C, Wang P, Zeng Q, et al. Piezo1-mediated calcium flux transfers mechanosignal to yes-associated protein to stimulate matrix production in keloid. J Invest Dermatol. 2025;Epub ahead of print.
10. Akaishi S, Akimoto M, Ogawa R, Hyakusoku H. The relationship between keloid growth pattern and stretching tension: visual analysis using the finite element method. Ann Plast Surg. 2008;60:445-51.
11. Wang W, Zhou B, Xia L, et al. An innovative single-stage approach of high-tension keloid excision and reconstruction using acellular dermal matrix and epidermal skin grafting. Dermatologic Therapy. 2024;2024:7551111.
12. Ma QY, Yang YT, Chen ZA, et al. Laser combined with radiotherapy for keloid treatment: a novel and efficient comprehensive therapy with a lower recurrence rate. Plast Reconstr Surg. 2023;152:1022e-9.
13. Limandjaja GC, Niessen FB, Scheper RJ, Gibbs S. Hypertrophic scars and keloids: overview of the evidence and practical guide for differentiating between these abnormal scars. Exp Dermatol. 2021;30:146-61.
15. Gauglitz GG, Korting HC, Pavicic T, Ruzicka T, Jeschke MG. Hypertrophic scarring and keloids: pathomechanisms and current and emerging treatment strategies. Mol Med. 2011;17:113-25.
16. Ogawa R, Ono S, Akaishi S, Dohi T, Iimura T, Nakao J. Reconstruction after anterior chest wall keloid resection using internal mammary artery perforator propeller flaps. Plast Reconstr Surg Glob Open. 2016;4:e1049.
17. Liu H, Sui F, Liu S, Song K, Hao Y, Wang Y. Large chest keloids treatment with expanded parasternal intercostal perforator flap. BMC Surg. 2021;21:147.
18. Hou S, Chen Q, Chen XD. The clinical efficacy of punch excision combined with intralesional steroid injection for keloid treatment. Dermatol Surg. 2023;49:S70-4.
19. Park TH. Successful use of a 2-mm punch device in a patient with massive, multiple keloids. Dermatol Surg. 2025;51:215-6.
20. Hwang NH, Chang JH, Lee NK, Yang KS. Effect of the biologically effective dose of electron beam radiation therapy on recurrence rate after keloid excision: a meta-analysis. Radiother Oncol. 2022;173:146-53.
21. Azzam OA, Bassiouny DA, El-Hawary MS, El Maadawi ZM, Sobhi RM, El-Mesidy MS. Treatment of hypertrophic scars and keloids by fractional carbon dioxide laser: a clinical, histological, and immunohistochemical study. Lasers Med Sci. 2016;31:9-18.
22. Lee JW, Seol KH. Adjuvant radiotherapy after surgical excision in keloids. Medicina. 2021;57:730.
23. Caviggioli F, Vinci V, Vappiani M, Lisa A, Maione L, Klinger M. Evidence-based scar management: how to improve results with technique and technology. Plast Reconstr Surg. 2017;139:1371e-3.
24. Ekstein SF, Wyles SP, Moran SL, Meves A. Keloids: a review of therapeutic management. Int J Dermatol. 2021;60:661-71.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Special Issue
Copyright
Data & Comments
Data
















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].