Direct-to-implant versus two-stage implant-based breast reconstruction in patients requiring postmastectomy radiotherapy
Abstract
Aim: Postmastectomy radiation therapy (PMRT) has played an important role in advanced breast cancer. It may negatively impact the outcomes of implant-based reconstruction. The aim of this study was to investigate the impact of PMRT on the outcome of implant-based reconstruction.
Methods: A total of 36 patients from 2009 to 2019 were included, with 12 in the two-stage group (TE-XRT ± Implant), who received PMRT after mastectomy and tissue expander insertion, 14 received PMRT after immediate direct-to-implant (DTI-XRT) breast reconstruction, and 10 with history of breast conservation surgery and PMRT received mastectomy and DTI reconstruction for cancer recurrence (XRT-DTI). Morbidities, including acute and late poor wound healing, implant or expander loss, and final revision surgeries, were reviewed.
Results: There were no significant differences in age, gender, BMI, or histological type among groups. Overall, complications were higher in the TE-XRT ± Implant group than in the DTI-XRT group (83.3% vs. 35.7%, P = 0.02). Wound breakdown was more frequent in the TE-XRT ± Implant group than in the DTI-XRT group (33.3% vs. 0.0%, P = 0.03). In two-stage reconstruction, implant exposure occurred predominantly in the late stage of the stage II surgery and stage II surgery tended to present higher overall and late complications than XRT-DTI or DTI-XRT.
Conclusion: For patients requiring PMRT, two-stage implant-based breast reconstruction tends to have a higher complication rate than DTI breast reconstruction. Our results suggest that radiation’s impact on wound healing might outweigh other negative effects, and conversion from implant-based reconstruction to two-stage reconstruction may not always be necessary.
Keywords
INTRODUCTION
Postmastectomy radiation therapy (PMRT) has established a significant role in the treatment of advanced breast cancer due to better control of both locoregional recurrence and mortality in nodal-positive breast cancer[1]. However, the increased use of radiation as adjuvant therapy has created additional challenges for reconstructive surgeons while considering the optimal timing and method of breast reconstruction in patients with advanced breast cancer[2,3]. Regardless of the reconstructive method, radiation has been recognized to have a detrimental effect on aesthetic outcomes[4,5]. Although emerging evidence shows that autologous breast reconstruction may have a better tolerance to radiation[2,6], implant-based reconstruction remains the method of choice when autologous tissues are not available.
Immediate breast reconstruction with preservation of the breast skin envelope with or without the nipple-areolar complex (NAC) restores the best breast contours. The procedure has been proven to provide psychological and emotional advantages for patients[7,8]. The trend of implant-based immediate breast reconstruction has shifted largely to direct-to-implant (DTI)[9]. However, when PMRT is required, DTI becomes a controversial procedure. Traditionally, in individuals who were known to receive PMRT, two-stage reconstruction with tissue expanders (TE) was recommended since radiation on permanent implants showed a higher incidence of severe capsular contracture, which may ultimately lead to implant failure[8,10,11]. Two-stage reconstruction with TE implantation first has been recommended if PMRT is indicated[12,13]. With the two-stage reconstruction, however, the second surgery is inevitably on the irradiated skin, and radiation may negatively impact wound healing, reduce tissue elasticity, and limit the expandability of the skin flap[14].
Weighing the advantages of preventing capsular contracture and surgical complications that may result in implant loss, we found it to be a controversial issue in selecting between DTI and two-stage reconstruction in implant-based breast reconstruction when the patient is going to receive PMRT. The aim of this study is to investigate and compare the impact of postmastectomy whole-breast radiotherapy on the outcome of implant-based breast reconstruction in DTI or two-stage implant-based breast reconstruction.
METHODS
Collection of data
After being approved by the institutional review board of Chang Gung Memorial Hospital (IRB number: 202000250B0), a retrospective review was designed, including a single surgeon’s experience from February 2009 to July 2019, of breast reconstruction using DTI or two-stage tissue-expander implant breast reconstruction in patients who received postmastectomy radiotherapy. First, patients who received immediate implant-based reconstruction (either one-stage or two-stage) followed by PMRT were identified. These patients were divided into two cohorts: (1) those who received PMRT to their expander prior to the exchange of the expander with a permanent implant (Group 1, TE-XRT ± Implant) and (2) those who received PMRT after DTI breast reconstruction (Group 2, DTI-XRT). Patients who developed local cancer recurrence and received skin-sparing (SSM) or nipple-sparing mastectomy (NSM), with DTI breast reconstruction after breast conservation surgery (BCS) and PMRT after BCS for their first breast cancer treatment, were also collected (Group 3, XRT-DTI). Patients in the group of TE-XRT ± Implant received their second-stage surgery at least six months after radiotherapy. Patients were excluded if their follow-up time was less than three months or if patients lost their TE or breast implant before the delivery of radiotherapy. Postoperative morbidities that occurred after radiotherapy, including acute (< one month) and late (one month after surgery) poor wound healing, implant or TE loss, and final revision surgeries, were reviewed. In the TE-XRT ± Implant group, the time interval between radiotherapy and subsequent surgery was further calculated and compared between patients with and without complications. For patients who lost their breast implant/TE or presented with implant/TE exposure, the reasons for the implant/TE loss or exposure and further reconstruction methods were reviewed. All of the breast implants included in this series were placed under the pectoralis muscle, which means that the breast implants were partially covered by the muscle in the dual plane. The upper medial part of the implant was covered by the pectoralis major muscle, while the lower inferior part was in the subcutaneous plane.
Surgical technique
The surgeries were performed as a two-team approach. The plastic surgeon took over the case after SSMs or NSMs were finished by the breast surgeons. DTI or two-stage reconstruction with TE insertion was first selected based on treatment planning and individual conditions. In brief, we proceeded to perform DTI breast reconstruction if the spared breast skin envelope had good perfusion. When any doubt of the tissue perfusion occurred, we delivered indocyanine green (ICG) study to check the perfusion of the mastectomy skin flap. If the patient was known to receive PMRT, or if the skin envelope was too tight or had signs of compromised skin perfusion, converting the surgery to two-stage reconstruction with TE insertion first was the procedure of choice. For patients who received DTI breast reconstruction, careful hemostasis was conducted, and the subpectoral plane was created using electric cauterization. The pectoralis muscle was elevated carefully and the muscle insertion below the 3 o’clock direction (right side) or the 9 o’clock direction (left side) was divided. After confirming adequate pocket creation, the inferior mammary fold was secured using a 3-0 PDS suture, and breast implants were placed in the subpectoral plane. The lateral-inferior pole of the breast implant was not covered by the pectoralis muscle, placing it in the subcutaneous plane. If TE insertion was planned, we placed the TE subcutaneously or subpectorally depending on the perfusion of the preserved skin envelope and the NAC if preserved[15]. All the patients in the TE group included in this series have their TE placed subcutaneously. In the second stage of the two-stage surgery, the TE were removed via the previous incision and the implant reconstructions were finished via the same incision.
Statistics
The data were analyzed using graphing and statistical analysis software SigmaPlot version 12.5. One-way ANOVA was applied to compare demographic mean values, while the chi-squared test and Fisher’s exact test were applied to compare all postradiation complications. The 95% confidence intervals (CIs) for complication rates were calculated using the exact binomial method (Clopper-Pearson) in Stata version 15.0. A probability of less than 0.05 was considered significant.
RESULTS
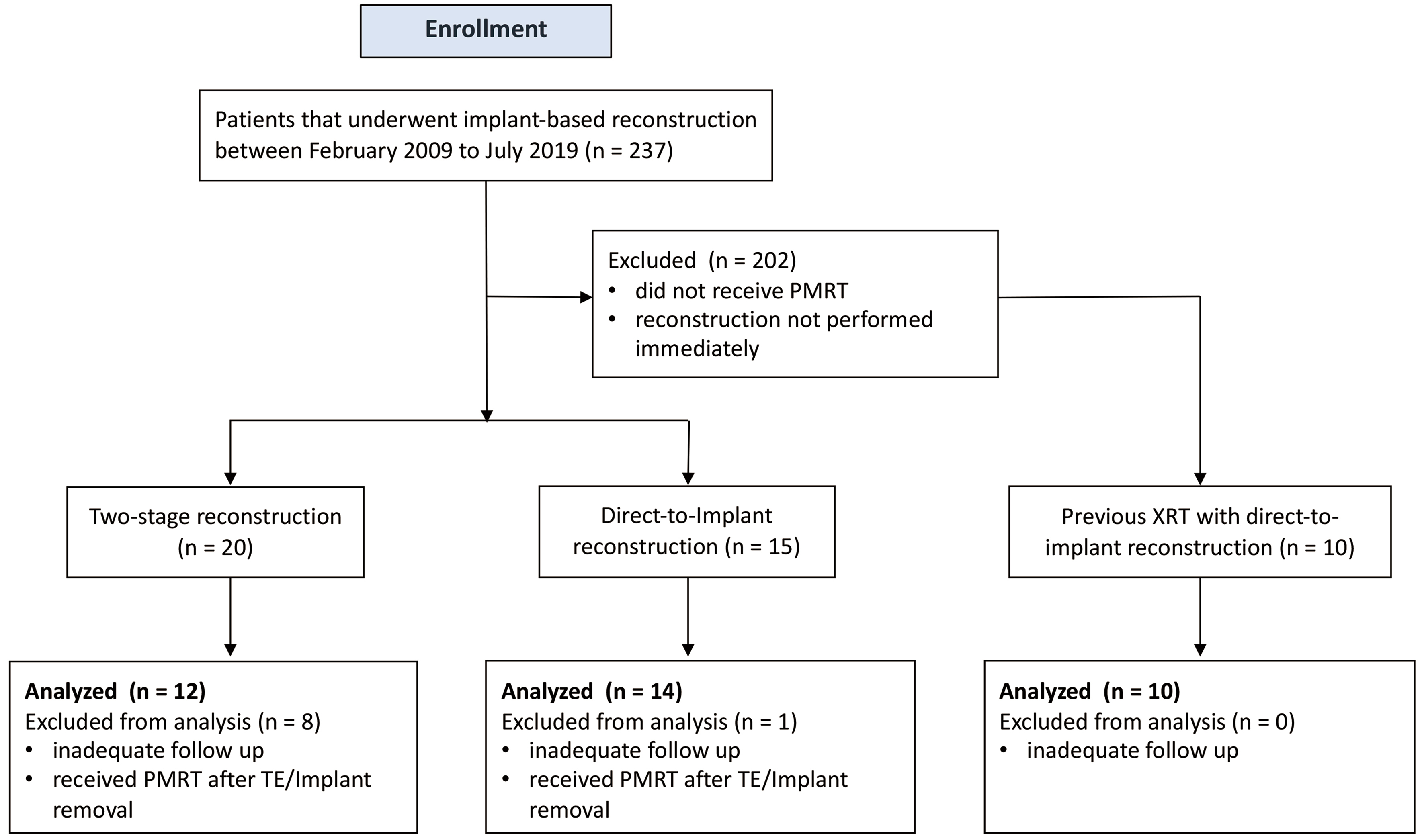
The process of patient inclusion and exclusion is illustrated in Figure 1. Initially, 45 patients were enrolled. After excluding those with insufficient follow-up or incomplete treatment, 36 patients remained eligible for analysis: 12 patients received PMRT after immediate two-stage reconstruction, with radiation delivered to TE; 14 patients received PMRT after DTI reconstruction, with radiation delivered to implants; and the remaining 10 patients had a history of breast cancer and received BCS and PMRT before SSMs or NSMs, followed by DTI reconstruction for cancer recurrence [Figure 2].
Figure 1. Flowchart of patient selection and enrollment. PMRT: Postmastectomy radiation therapy; TE: tissue expanders.
Figure 2. Three different scenarios of radiation and implant-based reconstruction. (A and B) A 45-year-old female patient with right breast cancer staged T2N1aM0, who underwent skin-sparing mastectomy and immediate direct-to-implant breast reconstruction without postmastectomy radiotherapy. (A) Preoperative view. The patient was satisfied with the postoperative symmetric result at the follow-up time of 66 months. (B) Post-OP 66-month AP view; (C and D) A 39-year-old female patient with left breast cancer staged T1cN2a, who received skin-sparing mastectomy and immediate implant-based breast reconstruction as direct-to-implant breast reconstruction. PMRT was delivered to her whole left breast because of the advanced cancer stage. (C) Preoperative anterior view of the breasts. (D) Post-OP 8-month and post-PMRT one-month AP view; (E and F) A 55-year-old female patient with a history of left breast cancer post breast conservation surgery and postoperative whole breast radiation developed tumor recurrence five years after the first surgery. She later underwent NSM and immediate breast reconstruction in the form of direct-to-implant. (E) Preoperative view before the NSM. (F) Post-OP 4-month AP view. PMRT: Postmastectomy radiation therapy; NSM: nipple-sparing mastectomy.
There was no significant difference in demographics among the three groups regarding age, sex, BMI, comorbidities, cancer biological type, mastectomy type, total radiation dose to the chest wall, or implant choice, as shown in Table 1. Table 2 lists the overall, acute, and late complications among the three groups. Overall complications were found to be statistically higher in the TE-XRT ± Implant group (83.3%) than in the DTI-XRT group (35.7%), with an odds ratio of 9.00 (P = 0.02). Among all the complications, overall wound breakdown occurred more frequently in the TE-XRT ± Implant group, with a ratio of 33.3% (P = 0.03), than in the DTI-XRT group (0.0%). The Two-stage TE-XRT ± Implant breast reconstruction also had a higher rate of skin necrosis (41.7%) than the DTI-XRT group (7.1%), with an odds ratio of 9.29, but the difference failed to reach statistical significance (P = 0.07). The skin necrosis mainly occurred in the acute stage. The occurrence of TE/implant loss, which occurred in the late stage, was slightly higher in the TE-XRT ± Implant group than in the DTI-XRT group (50.0% and 21.4%, respectively), with an odds ratio of 3.67, but the difference was not statistically significant (P = 0.22). When the overall complications were further divided into acute and late complications according to the presentation time after surgery (within or after the first postoperative month), significantly higher complications were present in the late stage of the TE-XRT ± Implant group than in the acute stage. Acute complications did not differ between the TE-XRT ± Implant group and the DTI-XRT group. However, higher late complications were observed in overall complications (66.7% vs. 21.4%, P = 0.02) and wound breakdown in particular (33.0% vs. 0%, P = 0.03). Unlike the difference between the TE-XRT ± Implant and DTI-XRT groups, the DTI-XRT group and XRT-DTI group presented equally regarding complications, regardless of overall, acute, or late complications.
Demographic data of patients who received implant-based reconstruction and radiotherapy
| TE-XRT ± implant (n = 12) | DTI-XRT (n = 14) | XRT-DTI (n = 10) | P value | |
| Median age (years) | 38.00 ± 8.94 | 40.93 ± 6.08 | 43.30 ± 8.00 | 0.31 |
| BMI (kg/m2) | 21.82 ± 1.32 | 22.02 ± 2.58 | 21.94 ± 3.16 | 0.68 |
| Smoking history | 1 (8.3) | 0 (0.0) | 0 (0.0) | 0.29 |
| Comorbidities | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Diabetes | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Hypertension | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Cancer type | 0.60 | |||
| IDC | 8 (66.7) | 11 (78.6) | 8 (80.6) | |
| ILC | 1 (8.3) | 2 (14.3) | 0 (0.0) | |
| Mixed IDC and ILC | 1 (8.3) | 0 (0.0) | 0 (0.0) | |
| Mucinous | 2 (16.7) | 0 (0.0) | 0 (0.0) | |
| Other | 0 (0.0) | 1 (7.1) | 2 (20.1) | |
| Laterality | 0.92 | |||
| Right | 7 (58.3) | 7 (50.0) | 5 (50.0) | |
| Left | 5 (41.7) | 7 (50.0) | 5 (50.0) | |
| Mastectomy | 0.41 | |||
| Simple mastectomy† | 5 (41.7) | 8 (0.0) | 7 (70.0) | |
| Modified radical mastectomy‡ | 7 (58.3) | 6 (42.9) | 3 (30.0) | |
| Nipple-sparing mastectomy | 5 (41.7) | 5 (35.7) | 5 (50.0) | 0.91 |
| Chest wall radiation dose | 5481.8 ± 659.3 | 4979.7 ± 1466.8 | 5579.4 ± 644.4 | 0.39 |
| Breast implant surface | 0.27 | |||
| Smooth | 4 (57.1) | 7 (50.0) | 2 (20.0) | |
| Texture | 3 (42.9) | 7 (50.0) | 8 (80.0) |
Acute and late complications in patients who received whole breast radiotherapy and implant-based breast reconstruction
| TE-XRT ± implant (n = 12) | DTI-XRT (n = 14) | XRT-DTI (n = 10) | P† | P‡ | ||||
| n (%) | 95%CI | n (%) | 95%CI | n (%) | 95%CI | |||
| Overall complications | 10 (83.3) | 51.6-97.9 | 5 (35.7) | 12.8-64.9 | 4 (40.0) | 12.2-73.8 | 0.02* | 1.00 |
| Wound breakdown | 4 (33.3) | 9.9-65.1 | 0 (0.0) | 0-23.2§ | 1 (10.0) | 0.3-44.5 | 0.03* | 0.42 |
| Skin necrosis | 5 (41.7) | 15.2-72.3 | 1 (7.1) | 0.2-33.9 | 1 (10.0) | 0.3-44.5 | 0.07 | 1.00 |
| Infection | 2 (16.7) | 2.1-48.4 | 1 (7.1) | 0.2-33.9 | 1 (10.0) | 0.3-44.5 | 0.58 | 1.00 |
| Capsular contracture | 0 (0.0) | 0-26.5§ | 3 (21.4) | 4.7-50.8 | 1 (10.0) | 0.3-44.5 | 0.22 | 0.61 |
| TE/Implant exposure | 3 (25.0) | 5.5-57.2 | 0 (0.0) | 0-23.2§ | 0 (0.0) | 0-30.8§ | 0.08 | N/A |
| TE/Implant loss | 6 (50.0) | 21.1-78.9 | 3 (21.4) | 4.7-50.8 | 3 (30.0) | 6.7-65.2 | 0.22 | 0.67 |
| Acute | 4 (33.3) | 9.9-65.1 | 2 (14.3) | 1.8-42.8 | 2 (20.0) | 2.5-55.6 | 0.37 | 1.00 |
| Wound breakdown | 0 (0.0) | 0-26.5§ | 0 (0.0) | 0-23.2§ | 1 (10.0) | 0.3-44.5 | N/A | 0.42 |
| Skin necrosis | 4 (33.3) | 9.9-65.1 | 1 (7.1) | 0.2-33.9 | 1 (10.0) | 0.3-44.5 | 0.15 | 1.00 |
| Infection | 0 (0.0) | 0-26.5§ | 1 (7.1) | 0.2-33.9 | 0 (0.0) | 0-30.8§ | 1.00 | 1.00 |
| TE/Implant exposure | 1 (8.3) | 0.2-38.5 | 0 (0.0) | 0-23.2§ | 0 (0.0) | 0-30.8§ | 0.46 | N/A |
| TE/Implant loss | 0 (0.0) | 0-26.5§ | 0 (0.0) | 0-23.2§ | 0 (0.0) | 0-30.8§ | N/A | N/A |
| Late | 9 (66.7) | 42.8-94.5 | 3 (21.4) | 4.7-50.8 | 4 (40.0) | 12.2-73.8 | 0.02* | 0.39 |
| Wound breakdown | 4 (33.3) | 9.9-65.1 | 0 (0.0) | 0-23.2§ | 0 (0.0) | 0-30.8§ | 0.03* | N/A |
| Skin necrosis | 1 (8.3) | 0.2-38.5 | 0 (0.0) | 0-23.2§ | 0 (0.0) | 0-30.8§ | 0.46 | N/A |
| Infection | 2 (16.7) | 2.1-48.4 | 0 (0.0) | 0-23.2§ | 1 (10.0) | 0.3-44.5 | 0.20 | 0.42 |
| Capsular contracture | 0 (0.0) | 0-26.5§ | 3 (21.4) | 4.7-50.8 | 1 (10.0) | 0.3-44.5 | 0.22 | 0.61 |
| TE/Implant exposure | 3 (25.0) | 5.5-57.2 | 0 (0.0) | 0-23.2§ | 0 (0.0) | 0-30.8§ | 0.08 | N/A |
| TE/Implant loss | 6 (50.0) | 21.1-78.9 | 3 (21.4) | 4.7-50.8 | 3 (30.0) | 6.7-65.2 | 0.22 | 0.67 |
The TE-XRT ± Implant group had two components: the first surgery (stage I) of TE insertion after SSM or NSM, and the second surgery (stage II) of breast implant insertion after PMRT. A higher complication rate could possibly result from radiotherapy. To understand whether different stages of surgery presented differences in complications, we further compared the overall, acute, and late complications between the two different stages of surgery, as shown in Table 3. Stage I and II surgeries shared similar overall complication rates. Stage I surgery tended to have a higher acute complication rate, while stage II surgery tended to present higher complications in the late stage. Implant exposure, which occurred predominantly in the late stage, occurred more frequently in the stage II surgery. Complications other than implant exposure did not present significant differences. Considering that radiotherapy can be a major contributing factor to complications, we compared stage I surgery with the DTI-XRT group. Both groups received PMRT after surgery. Again, stage I surgery tended to present higher overall, acute, and late complication rates than DTI-XRT, but without significant differences [Table 3].
Comparison of acute and late complications in different stages of implant-based breast reconstruction surgery
| Stage I: TE-XRT (n = 12) | Stage II: XRT-implant (n = 7) | DTI-XRT (n = 14) | P† | P‡ | ||||
| n (%) | 95%CI | n (%) | 95%CI | n (%) | 95%CI | |||
| Overall complications | 7 (58.3) | 27.7-84.8 | 4 (57.1) | 18.4-90.1 | 5 (35.7) | 12.8-64.9 | 1.00 | 0.43 |
| Wound breakdown | 2 (16.7) | 2.1-48.4 | 2 (28.6) | 3.7-1.0 | 0 (0.0) | 0-23.2§ | 0.60 | 0.20 |
| Skin necrosis | 4 (33.3) | 9.9-65.1 | 1 (14.3) | 0.4-57.9 | 1 (7.1) | 0.2-33.9 | 0.60 | 0.15 |
| Infection | 2 (16.7) | 2.1-48.4 | 0 (0.0) | 0-41.0§ | 1 (7.1) | 0.2-33.9 | 0.51 | 0.58 |
| Capsular contracture | 0 (0.0) | 0-26.5§ | 0 (0.0) | 0-41.0§ | 3 (21.4) | 4.7-50.8 | N/A | 0.22 |
| TE/Implant exposure | 0 (0.0) | 0-26.5§ | 3 (42.9) | 9.9-81.6 | 0 (0.0) | 0-23.2§ | 0.04* | N/A |
| TE/Implant loss | 5 (41.7) | 15.2-72.3 | 1 (14.3) | 0.4-57.9 | 3 (21.4) | 4.7-50.8 | 0.33 | 0.40 |
| Acute | 4 (33.3) | 9.9-65.1 | 1 (14.3) | 0.4-57.9 | 2 (14.3) | 1.8-42.8 | 0.60 | 0.37 |
| Wound breakdown | 0 (0.0) | 0-26.5§ | 0 (0.0) | 0-41.0§ | 0 (0.0) | 0-23.2§ | N/A | N/A |
| Skin necrosis | 4 (33.3) | 9.9-65.1 | 0 (0.0) | 0-41.0§ | 1 (7.1) | 0.2-33.9 | 0.25 | 0.15 |
| Infection | 0 (0.0) | 0-26.5§ | 0 (0.0) | 0-41.0§ | 1 (7.1) | 0.2-33.9 | N/A | 1.00 |
| Capsular contracture | 0 (0.0) | 0-26.5§ | 0 (0.0) | 0-41.0§ | 0 (0.0) | 0-23.2§ | N/A | N/A |
| TE/Implant exposure | 0 (0.0) | 0-26.5§ | 1 (14.3) | 0.4-57.9 | 0 (0.0) | 0-23.2§ | 0.37 | N/A |
| TE/Implant loss | 0 (0.0) | 0-26.5§ | 0 (0.0) | 0-41.0§ | 0 (0.0) | 0-23.2§ | N/A | N/A |
| Late | 5 (41.7) | 15.2-72.3 | 4 (57.1) | 18.4-90.1 | 3 (21.4) | 4.7-50.8 | 0.65 | 0.40 |
| Wound breakdown | 2 (16.7) | 2.1-48.4 | 2 (28.6) | 3.7-1.0 | 0 (0.0) | 0-23.2§ | 0.60 | 0.20 |
| Skin necrosis | 0 (0.0) | 0-26.5§ | 1 (14.3) | 0.4-57.9 | 0 (0.0) | 0-23.2§ | 0.37 | N/A |
| Infection | 2 (16.7) | 2.1-48.4 | 0 (0.0) | 0-41.0§ | 0 (0.0) | 0-23.2§ | 0.51 | 0.20 |
| Capsular contracture | 0 (0.0) | 0-26.5§ | 0 (0.0) | 0-41.0§ | 3 (21.4) | 4.7-50.8 | N/A | 0.22 |
| TE/Implant exposure | 0 (0.0) | 0-26.5§ | 3 (42.9) | 9.9-81.6 | 0 (0.0) | 0-23.2§ | 0.04* | N/A |
| TE/Implant loss | 5 (41.7) | 15.2-72.3 | 1 (14.3) | 0.4-57.9 | 3 (21.4) | 4.7-50.8 | 0.33 | 0.40 |
Preoperative radiotherapy is a major component of stage II surgery. To understand the role of preoperative radiotherapy, we then compared stage II surgery with DTI-XRT, in which PMRT was delivered after surgery, and with XRT-DTI, in which PMRT was performed before the total mastectomy [Table 4]. Implant exposure rate was considerably higher in the stage II surgery group compared to the DTI-XRT group (P = 0.03) and the XRT-DTI group (P = 0.05). Although there was no significant difference, stage II surgery also tended to present higher overall and late complications than XRT-DTI or DTI-XRT.
Comparison of preoperative vs. postoperative radiotherapy on complications in implant-based breast reconstruction
| DTI-XRT (n = 14) | XRT-DTI (n = 10) | Stage II: XRT-implant (n = 7) | P† | P‡ | ||||
| n (%) | 95%CI | n (%) | 95%CI | n (%) | 95%CI | |||
| Overall complications | 5 (35.7) | 12.8-64.9 | 4 (40.0) | 12.2-73.8 | 4 (57.1) | 18.4-90.1 | 0.40 | 0.64 |
| Wound breakdown | 0 (0.0) | 0-23.2§ | 1 (10.0) | 0.3-44.5 | 2 (28.6) | 3.7-1.0 | 0.10 | 0.54 |
| Skin necrosis | 1 (7.1) | 0.2-33.9 | 1 (10.0) | 0.3-44.5 | 1 (14.3) | 0.4-57.9 | 1.00 | 1.00 |
| Infection | 1 (7.1) | 0.2-33.9 | 1 (10.0) | 0.3-44.5 | 0 (0.0) | 0-41.0§ | 1.00 | 1.00 |
| Capsular contracture | 3 (21.4) | 4.7-50.8 | 1 (10.0) | 0.3-44.5 | 0 (0.0) | 0-41.0§ | 0.52 | 1.00 |
| TE/Implant exposure | 0 (0.0) | 0-23.2§ | 0 (0.0) | 0-30.8§ | 3 (42.9) | 9.9-81.6 | 0.03* | 0.05 |
| TE/Implant loss | 3 (21.4) | 4.7-50.8 | 3 (30.0) | 6.7-65.2 | 1 (14.3) | 0.4-57.9 | 1.00 | 0.60 |
| Acute | 2 (14.3) | 1.8-42.8 | 2 (20.0) | 2.5-55.6 | 1 (14.3) | 0.4-57.9 | 1.00 | 1.00 |
| Wound breakdown | 0 (0.0) | 0-23.2§ | 1 (10.0) | 0.3-44.5 | 0 (0.0) | 0-41.0§ | N/A | 1.00 |
| Skin necrosis | 1 (7.1) | 0.2-33.9 | 1 (10.0) | 0.3-44.5 | 0 (0.0) | 0-41.0§ | 1.00 | 1.00 |
| Infection | 1 (7.1) | 0.2-33.9 | 0 (0.0) | 0-30.8§ | 0 (0.0) | 0-41.0§ | 1.00 | N/A |
| Capsular contracture | 0 (0.0) | 0-23.2§ | 0 (0.0) | 0-30.8§ | 0 (0.0) | 0-41.0§ | N/A | N/A |
| TE/Implant exposure | 0 (0.0) | 0-23.2§ | 0 (0.0) | 0-30.8§ | 1 (14.3) | 0.4-57.9 | 0.33 | 0.41 |
| TE/Implant loss | 0 (0.0) | 0-23.2§ | 0 (0.0) | 12.2-73.8 | 0 (0.0) | 0-41.0§ | N/A | N/A |
| Late | 3 (21.4) | 4.7-50.8 | 4 (40.0) | 0-30.8§ | 4 (57.1) | 18.4-90.1 | 0.16 | 0.64 |
| Wound breakdown | 0 (0.0) | 0-23.2§ | 0 (0.0) | 0-30.8§ | 2 (28.6) | 3.7-1.0 | 0.10 | 0.15 |
| Skin necrosis | 0 (0.0) | 0-23.2§ | 0 (0.0) | 0.3-44.5 | 1 (14.3) | 0.4-57.9 | 0.33 | 0.41 |
| Infection | 0 (0.0) | 0-23.2§ | 1 (10.0) | 0.3-44.5 | 0 (0.0) | 0-41.0§ | N/A | 1.00 |
| Capsular contracture | 3 (21.4) | 4.7-50.8 | 1 (10.0) | 0-30.8§ | 0 (0.0) | 0-41.0§ | 0.52 | 1.00 |
| TE/Implant exposure | 0 (0.0) | 0-23.2§ | 0 (0.0) | 6.7-65.2 | 3 (42.9) | 9.9-81.6 | 0.03* | 0.05 |
| TE/Implant loss | 3 (21.4) | 4.7-50.8 | 3 (30.0) | 12.2-73.8 | 1 (14.3) | 0.4-57.9 | 1.00 | 0.60 |
The time interval between radiotherapy and second-stage surgery in patients with and without complications was recorded in Table 5. The time interval between PMRT and surgery was statistically similar between cases with or without complications.
Time interval between radiation therapy and breast implant implantation in the two-stage reconstruction group
| Case number (n = 7) | Follow-up time (months) | P value | |
| Overall complications | 0.81 | ||
| Yes | 5 | 247.4 | |
| No | 2 | 226 | |
| Acute complications | 0.69 | ||
| Yes | 2 | 216.5 | |
| No | 5 | 251.4 | |
| Late complications | 0.67 | ||
| Yes | 4 | 255.8 | |
| No | 3 | 222.3 |
TE/implant loss is considered a major complication in implant-based breast reconstruction, and the loss rate was noted to be as high as 50% in the TE-XRT ± Implant group. In stage I of the TE-XRT ± Implant group, the loss of a total of five TEs occurred for different reasons: two were from wound breakdown and exposure, and three were due to infection. One of the five patients received free deep inferior epigastric perforator (DIEP) flap reconstruction after the completion of the breast cancer treatment. The other four patients remained without reconstruction. In stage II of the TE-XRT ± Implant group, no implant loss occurred in the acute phase. Three implant exposures occurred more than three months after surgery. Debridement and wound closure were performed on one patient. A pedicled latissimus dorsi (LD) myocutaneous flap was transferred to cover the implant in one case. The remaining implant exposure case received free DIEP flap transfer to replace the breast implant after one wound closure was attempted but failed [Figure 3]. In the DTI-XRT group, three implants were removed for different reasons. One patient received implant exchange because of one episode of infection. Two patients had their implant removed because of their decision to replace the breast implant with free DIEP flaps. For patients who received BCS and radiotherapy and subsequently developed cancer recurrence and received immediate breast reconstruction after mastectomy, one patient lost her breast implant because of poor wound healing, one patient decided to remove her breast implant because of repeated infection, and one patient received capsular contracture release and decided to change to a new breast implant.
Figure 3. Salvage of two-stage reconstruction with flap. A 33-year-old female patient with left breast cancer at the stage of cT3N1M0 at first diagnosis. She received neoadjuvant chemotherapy and later nipple-sparing mastectomy and tissue expander insertion. The postoperative stage of her breast cancer was ypT1cN1M0. She then underwent postmastectomy radiotherapy. (A) Preoperative view; (B) Anterior view 15 months post-mastectomy, after nipple-sparing mastectomy, immediate reconstruction with a tissue expander, and completion of PMRT. The reconstructed and irradiated breast shows contracture, with the nipple positioned higher compared to the contralateral healthy breast. The patient later received implant reconstruction; (C) AP view at 17 months post-mastectomy, two months after the tissue expander was replaced with a breast implant; (D) Left lateral view at 17 months post-mastectomy, two months after implant placement. The second-stage surgery converting the tissue expander to a breast implant went uneventfully; (E) Left lateral view at 22 months post-mastectomy and 7 months post-implant reconstruction, showing wound breakdown and subsequent implant exposure caused by progressive contracture and fibrosis. After discussion with the patient, the breast implant was removed, and reconstruction was converted to a free DIEP flap transfer; (F) Free DIEP flap harvested for breast reconstruction; (G) AP view five months after DIEP flap reconstruction; (H) Left lateral view eight months post-DIEP reconstruction; (I) Right lateral view eight months post-DIEP reconstruction. PMRT: Postmastectomy radiation therapy; DIEP: deep inferior epigastric perforator; AP: anteroposterior.
DISCUSSION
This retrospective study highlighted two key ways in which radiation can impact implant-based breast reconstruction outcomes: radiation compromises the perfusion of the tissue that has been irradiated, resulting in higher risks of skin necrosis, poor wound healing/wound breakdown, and infection when surgeries are required after radiotherapy (i.e., the XRT-DTI group); and radiation induces fibroproliferation and capsular contracture (such as presented in DTI-XRT group), which negatively impacts aesthetic results in implant-based breast reconstruction. The results echoed basic mechanistic findings that radiation damages endothelial cells, which initiates a cellular response that leads to the release of profibrotic cytokines, including TGF-β, which results in fibrosis[16,17]. The overproduction of TGF-β1 further results in the downregulation of the TGF-β receptor III, which is involved in neovascularization[18,19]. As a result, poor wound healing and more fibrosis formation were present.
According to the results, poor wound healing/wound breakdown commonly occurred as an acute complication, while capsular contracture presented as a main late complication after surgery in patients who received implant-based breast reconstruction and PMRT. Unlike conclusions from Baschnagel et al.[13] and other previous publications[20,21], the results from the current study indicated that two-stage surgery (TE-XRT ± Implant) presented more overall complications, particularly late complications, than DTI-XRT. This group also presented with a higher rate of TE/implant loss, although the difference among groups did not reach statistical significance. Radiation-induced poor tissue perfusion is considered the main reason for TE/implant failure in the TE-XRT ± Implant group since many of the complications started with the presentation of poor wound healing or late wound breakdown after the second-stage surgery (i.e., the exchange of the TE with the breast implant after radiation). Surgical intervention after radiotherapy demonstrated a higher risk of complications than DTI-XRT, in which radiation was delivered after surgery. TE/implant failure in the DTI-XRT group was caused mainly by capsular contracture, for which the patients decided to remove/exchange their implants. In the TE-XRT ± Implant group, the reasons for implant removal varied, including infection, skin flap necrosis, and late wound breakdown. Of note, when the TE has to be removed for complication management in the first stage of the surgery of TE-XRT ± Implant, many of the patients hesitated about further reconstruction. One patient who lost her TE during the first stage of the surgery received breast reconstruction after the completion of the breast cancer treatment, and she chose to have a free DIEP flap as the reconstruction method to avoid complications from implants.
When implant exposure occurred after the second-stage surgery in the TE-XRT ± Implant group, reconstruction with autologous tissue was almost inevitable. As presented, one patient chose to remove the breast implant after implant exposure and received whole-breast reconstruction with a free DIEP flap [Figure 3], while another patient received a pedicle LD myocutaneous flap to cover the implant and the skin paddle of the flap was used to replace part of the skin defect. Irradiated skin often loses its original skin elasticity. As a result, primary wound closure is often difficult when wound healing problems occur. Although the use of TEs may further expand the skin envelope and overcome the problem of skin insufficiency, the expansion of the irradiated skin can be difficult and may again result in wound healing problems. Currently, breast cancer surgery tends toward more tissue preservation, with many mastectomies performed as NSMs. When a TE is used as a salvage procedure for implant-based reconstruction after wound complications, expanding the native breast skin pocket and keeping proper nipple position are not always possible, especially when the original incision is placed outside the irradiated area. The use of autologous tissue, on the other hand, provides well-vascularized tissue and an adequate skin paddle to overcome the shortage of skin and correct the preserved NAC to its natural position [Figure 3].
Compared to the higher surgical-related complication rates observed in the TE-XRT ± Implant group, radiation effects in the DTI-XRT group tend to manifest as late complications, particularly an increased risk of capsular contracture [Figure 2]. The capsular contracture rate was 11.1% in our patients, while most of the literature presented rates of 19.5% to 21.7%[22,23]. A lower incidence of capsular contracture may, in part, be attributed to advancements in radiation dose distribution and delivery techniques developed by radiation oncologists, as approaches such as boost or bolus irradiation may help minimize complications. Nevertheless, radiation still plays a negative role in the overall results of implant-based reconstruction[24]. In this small series, two patients in the DTI-XRT group received surgery to replace their breast implant with free DIEP flaps because of capsular contracture and related symptoms. The management of capsular contracture often requires surgical capsulectomy or capsulotomy. However, surgery can be challenging after previous radiotherapy, and wound healing problems may present. Repeated capsular contracture is not uncommon, given the history of previous radiotherapy. Long-lasting results and the elimination of complications from previous radiotherapy were the main reasons for the two patients who developed capsular contracture to eventually receive free DIEP flap reconstruction to replace their breast implants.
To further understand whether the time interval between radiotherapy and subsequent surgery impacts the surgical outcome, we reviewed patients who previously received radiotherapy after BCS and later received total mastectomy because of breast cancer recurrence with immediate implant-based breast reconstruction. No significant difference was found between this group and the DTI-XRT group. The mean time interval (from radiation to implant exchange) of groups with or without complications in the TE-XRT ± Implant group were also compared, which revealed no statistical significance. Santosa et al. reported their experience by including a total of 150 patients who underwent immediate, two-stage implant-based breast reconstruction and received PMRT and found no significant differences in the incidence of postoperative complications between patients receiving radiation after expander-implant exchange and patients undergoing radiation prior to exchange[25]. Therefore, we assume that radiation timing itself has no strong correlation with the final outcome but does negatively impact wound healing if the surgeries are performed on a previously irradiated tissue. Interestingly, however, fewer complications were identified in patients with a history of BCS and PMRT before surgery for subcutaneous mastectomy and DTI breast reconstruction. Although XRT-DTI and the second-stage surgery of TE-XRT ± Implant shared a similar history of prior radiotherapy, the latter tended to have a higher complication rate (no significant difference). One of the major concerns of radiotherapy is its impact on tissue perfusion. Part of the breast remained non-operated when patients received BCS and radiotherapy; and the perfusion of the skin was supposed to be better preserved in comparison to the spared skin during total mastectomy above the TE in the TE-XRT ± Implant group. Compromised tissue perfusion during radiotherapy can be a major issue of tissue damage in response to radiotherapy.
Based on our previous experience, we have preferred to place the TE subcutaneously to reduce the pain from expansion[15]. Subcutaneous TE placement has shown advantages of greater intraoperative and first postoperative expansion, shorter expansion duration, fewer expansion visits, less pain, and better control of breast shape than subpectoral placement due to thinner capsule formation[26,27]. During mastectomy, the breast tissue is dissected between the subdermal fat and the breast parenchyma; and blood supply to the skin, which has profound effects on wound healing, relies on perfusion from the subdermal plexus and perforators in the subcutaneous plane[28,29]. Since radiotherapy further compromises perfusion, we assume that subpectoral placement of TE might reduce risks of wound breakdown and have better reconstruction outcomes in patients who require radiotherapy since additional perfusion can be supported from the underlying pectoralis major muscle. Muscle has also shown less sensitivity and more resistance to radiation than skin[30]. To further understand this issue, however, more studies will be designed to reach a conclusion.
Our results revealed that two-stage surgeries did not contribute to fewer complications in implant-based breast reconstruction with PMRT. Instead, different procedures have different complications, and two-stage reconstruction often presents complications from wound healing problems. We reviewed the patients who received two-stage breast reconstruction and subsequently presented wound healing problems. All of them underwent surgical incision in the breast mound, which was eventually involved the radiation treatment area. Incision in the breast mound also received tension from the underlying TE or breast implant. If second-stage surgery was performed through the same incision, scar and irradiated tissue both contributed to poor wound healing. The avoidance of incision in the breast mound in the first surgery can possibly reduce complications if oncological safety allows. Instead of using the original approach, shifting the incision to an anatomical crease, such as the inframammary fold or anterior axillary line, in the second stage of the surgery can be an alternative. In addition, a successful implant-based reconstruction should be considered not only for minimal postoperative surgical complications but also for better long-term aesthetic results. Radiation injury in DTI or two-stage breast reconstruction may impact each procedure in a different way, and the selection of the surgical procedure and proper timing for radiation to balance the complication and overall results is crucial. Controversy remains, and a comprehensive evaluation and discussion with the patient are required preoperatively.
The limitations of our study are as follows. First of all, DTI was performed when the mastectomy skin flap was well perfused after mastectomy. The DTI was converted to TE insertion in patients with a compromised mastectomy skin flap. This may be presented as a bias in comparison despite that both groups of patients shared similar demographics. This study is a retrospective review with a limited cohort size. The sample size remains small for this important issue. This study represents the implant-based breast reconstruction experience of a single surgeon. While limiting the study to a single surgeon’s experience can preclude the factors from surgical technique or decision making, a larger trial would possibly provide comprehensive evidence to support our results. Other aspects, such as the use of fat grafting, ADM (acellular dermal matrix), and different implant types, were not fully discussed because of the limitation of case numbers. We set three months as the minimal follow-up time. However, we will continue to follow our patients for long-term outcomes and enroll more patients to optimize results and reach conclusions on a guideline for implant-based breast reconstruction along with the issue of radiotherapy, in particular, for Asian women. Of note, this group of patients have relatively normal BMI and few comorbidities, such as diabetes and hypertension. The patient characteristics presented to be healthier. Translating the experience to Western patients still requires caution.
PMRT tends to have a higher rate of overall complications in two-stage implant-based breast reconstruction than in DTI breast reconstruction in the setting of immediate breast reconstruction. Previous radiotherapy also contributes to higher complication rates when further mastectomy and immediate reconstruction in the form of DTI reconstruction is required for tumor recurrence. Our results suggest that radiation’s impact on wound healing might outweigh other negative effects, and ultimately, the conversion of implant-based reconstruction to two-stage reconstruction may not be necessary since higher complications were encountered.
Conclusion
For patients requiring PMRT, two-stage implant-based breast reconstruction tends to have a higher complication rate than in DTI breast reconstruction, particularly wound healing problems. Although limited by the small sample size, this may still provide valuable information when deciding to go for DTI or two-stage surgery in patients requiring PMRT and implant-based reconstruction. Our results suggest that the impact of radiation on wound healing might outweigh other negative effects, and conversion from implant-based reconstruction to two-stage reconstruction may not always be necessary.
DECLARATIONS
Authors’ contributions
Conceptualization: Chuang EYH, Kuo WL, Huang JJ
Data curation: Chuang EYH, Kuo WL, Chen SC, Cheong DCF, Huang JJ
Formal analysis: Chuang EYH, Huang JJ
Investigation: Chuang EYH, Kuo WL, Huang YT, Chang FCS, Cheong DCF, Chen SC, Huang JJ
Supervision: Kuo WL, Huang YT, Huang JJ
Writing - original draft: Chuang EYH, Chang FCS, Cheong DCF
Writing - review and editing: Kuo WL, Huang YT, Chang FCS, Chen SC, Huang JJ
All authors have read and agreed to the published version of the manuscript.
Availability of data and materials
The datasets generated within this study are available from the corresponding author upon reasonable request.
Financial support and sponsorship
None.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
The study was approved by the institutional review board of Chang Gung Memorial Hospital (IRB number: 202000250B0). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The IRB approves the waiver of the participants’ consent.
Consent for publication
Written informed consent for publication of the clinical images was obtained from the patients.
Copyright
© The Author(s) 2025.
REFERENCES
1. McGale P, Taylor C, Correa C, et al; EBCTCG (Early Breast Cancer Trialists’ Collaborative Group). Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383:2127-35.
2. Panchal H, Matros E. Current trends in postmastectomy breast reconstruction. Plast Reconstr Surg. 2017;140:7S-13.
3. Frasier LL, Holden S, Holden T, et al. Temporal trends in postmastectomy radiation therapy and breast reconstruction associated with changes in national comprehensive cancer network guidelines. JAMA Oncol. 2016;2:95-101.
4. Pu Y, Mao TC, Zhang YM, Wang SL, Fan DL. The role of postmastectomy radiation therapy in patients with immediate prosthetic breast reconstruction: a meta-analysis. Medicine. 2018;97:e9548.
5. Barry M, Kell MR. Radiotherapy and breast reconstruction: a meta-analysis. Breast Cancer Res Treat. 2011;127:15-22.
6. Yun JH, Diaz R, Orman AG. Breast reconstruction and radiation therapy. Cancer Control. 2018;25:1073274818795489.
7. Atisha D, Alderman AK, Lowery JC, Kuhn LE, Davis J, Wilkins EG. Prospective analysis of long-term psychosocial outcomes in breast reconstruction: two-year postoperative results from the Michigan Breast Reconstruction Outcomes Study. Ann Surg. 2008;247:1019-28.
8. Magill LJ, Robertson FP, Jell G, Mosahebi A, Keshtgar M. Determining the outcomes of post-mastectomy radiation therapy delivered to the definitive implant in patients undergoing one- and two-stage implant-based breast reconstruction: a systematic review and meta-analysis. J Plast Reconstr Aesthet Surg. 2017;70:1329-35.
9. Srinivasa DR, Garvey PB, Qi J, et al. Direct-to-implant versus two-stage tissue expander/implant reconstruction: 2-year risks and patient-reported outcomes from a prospective, multicenter study. Plast Reconstr Surg. 2017;140:869-77.
10. Davila AA, Mioton LM, Chow G, et al. Immediate two-stage tissue expander breast reconstruction compared with one-stage permanent implant breast reconstruction: a multi-institutional comparison of short-term complications. J Plast Surg Hand Surg. 2013;47:344-9.
11. Petersen A, Eftekhari AL, Damsgaard TE. Immediate breast reconstruction: a retrospective study with emphasis on complications and risk factors. J Plast Surg Hand Surg. 2012;46:344-8.
12. Cordeiro PG, Albornoz CR, McCormick B, Hu Q, Van Zee K. The impact of postmastectomy radiotherapy on two-stage implant breast reconstruction: an analysis of long-term surgical outcomes, aesthetic results, and satisfaction over 13 years. Plast Reconstr Surg. 2014;134:588-95.
13. Baschnagel AM, Shah C, Wilkinson JB, Dekhne N, Arthur DW, Vicini FA. Failure rate and cosmesis of immediate tissue expander/implant breast reconstruction after postmastectomy irradiation. Clin Breast Cancer. 2012;12:428-32.
14. Lee KT, Mun GH. Prosthetic breast reconstruction in previously irradiated breasts: a meta-analysis. J Surg Oncol. 2015;112:468-75.
15. Engel H, Huang JJ, Lin CY, Lam WL, Gazyakan E, Cheng MH. Subcutaneous tissue expansion and subsequent subpectoral implantation for breast reconstruction in Asian patients: safety and outcome. Ann Plast Surg. 2013;70:135-43.
16. Archambeau JO, Pezner R, Wasserman T. Pathophysiology of irradiated skin and breast. Int J Radiat Oncol Biol Phys. 1995;31:1171-85.
17. Haubner F, Ohmann E, Pohl F, Strutz J, Gassner HG. Wound healing after radiation therapy: review of the literature. Radiat Oncol. 2012;7:162.
18. Jacobson LK, Johnson MB, Dedhia RD, Niknam-bienia S, Wong AK. Impaired wound healing after radiation therapy: a systematic review of pathogenesis and treatment. JPRAS Open. 2017;13:92-105.
19. Wehrhan F, Grabenbauer GG, Rödel F, Amann K, Schultze-Mosgau S. Exogenous modulation of TGF-β1 influences TGF-betaR-III-associated vascularization during wound healing in irradiated tissue. Strahlenther Onkol. 2004;180:526-33.
20. Anderson PR, Hanlon AL, Fowble BL, McNeeley SW, Freedman GM. Low complication rates are achievable after postmastectomy breast reconstruction and radiation therapy. Int J Radiat Oncol Biol Phys. 2004;59:1080-7.
21. Cordeiro PG, Pusic AL, Disa JJ, McCormick B, VanZee K. Irradiation after immediate tissue expander/implant breast reconstruction: outcomes, complications, aesthetic results, and satisfaction among 156 patients. Plast Reconstr Surg. 2004;113:877-81.
22. Clemens MW, Kronowitz SJ. Current perspectives on radiation therapy in autologous and prosthetic breast reconstruction. Gland Surg. 2015;4:222-31.
23. Whitfield GA, Horan G, Irwin MS, Malata CM, Wishart GC, Wilson CB. Incidence of severe capsular contracture following implant-based immediate breast reconstruction with or without postoperative chest wall radiotherapy using 40 Gray in 15 fractions. Radiother Oncol. 2009;90:141-7.
24. Ho AY, Hu ZI, Mehrara BJ, Wilkins EG. Radiotherapy in the setting of breast reconstruction: types, techniques, and timing. Lancet Oncol. 2017;18:e742-53.
25. Santosa KB, Chen X, Qi J, et al. Postmastectomy radiation therapy and two-stage implant-based breast reconstruction: is there a better time to irradiate? Plast Reconstr Surg. 2016;138:761-9.
26. Tomita K, Yano K, Nishibayashi A, Hosokawa K. Effects of subcutaneous versus submuscular tissue expander placement on breast capsule formation. Plast Reconstr Surg Glob Open. 2015;3:e432.
27. Zhu L, Mohan AT, Abdelsattar JM, et al. Comparison of subcutaneous versus submuscular expander placement in the first stage of immediate breast reconstruction. J Plast Reconstr Aesthet Surg. 2016;69:e77-86.
28. Frey JD, Salibian AA, Choi M, Karp NS. Mastectomy flap thickness and complications in nipple-sparing mastectomy: objective evaluation using magnetic resonance imaging. Plast Reconstr Surg Glob Open. 2017;5:e1439.
29. Robertson SA, Rusby JE, Cutress RI. Determinants of optimal mastectomy skin flap thickness. Br J Surg. 2014;101:899-911.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Special Issue
Copyright
Data & Comments
Data





















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.