Home blood pressure in target range as an additional therapeutic goal in hypertensive patients: a telemonitoring-based analysis
Abstract
Aim: Guidelines recommend treating hypertension (HTN) by keeping office blood pressure (BP) within the therapeutic range (TR). However, little is known about the TR of home BP. Therefore, we aimed to find a reliable proportion of home systolic (S) BP in TR (sBPiTR) using a telehealth platform, which facilitates the access to reliable and structured home BP data.
Methods: We used the data of HTN patients who participated in BP telemonitoring and counseling for 3 months. Patients had to manually enter their home BP in electronic diaries. Home SBP readings were averaged by the system itself except the very first or every first day of BP monitoring. We divided sBPiTR (110-130 mmHg) by quartiles. A weighted Cohen’s kappa coefficient was used as an estimate of inter-rater reliability between sBPiTR and office/home SBP in TR. We used a binomial logistic regression to test the predictive value of sBPiTR on target office/home SBP achievement.
Results: In total, 123 patients were included (median age 54 years; 102 males) with a median office SBP of
Conclusion: The threshold of 50% of home SBP measurements within 110-130 mmHg has a slight agreement with office BP control and a fair agreement with home BP control. This variable may serve as a predictor for the achievement of target SBP both in and out of office. Larger studies are needed to confirm these preliminary results.
Keywords
INTRODUCTION
Over the past few decades, the concept of “target” blood pressure (BP) has been revised several times, gradually decreasing from 160/115 mmHg to 130/80 mmHg and even lower[1,2].
In 2018, the European Society of Cardiology and the European Society of Hypertension (ESC/ESH) Guidelines for the management of hypertension (HTN) were published[3], followed by the Russian Ministry of Health Guidelines in 2020[4]. Experts still recommend measuring BP mostly in office to diagnose HTN and to use out-of-office BP monitoring techniques if “these measurements are logistically and economically feasible”. The definition of HTN per se has remained unchanged regarding office and ambulatory BP cut-offs. Unlike the previous 2013 edition[5], current guidelines introduce a certain “therapeutic range” (TR) of office BP values. This fundamental change poses several uncertainties at once.
First, there are still no well-defined corresponding therapeutic ranges for out-of-office BP, neither for ambulatory nor for home (HBP). This issue is reported in the section “gaps in the evidence”[3]. In the recent 2021 ESH position paper by the Working Group on Blood Pressure Monitoring and Cardiovascular Variability, it is suggested that HBP values ≤ 130/80 mmHg should be achieved[6]. This statement, however, was made with certain reservations due to the absence of specific supporting evidence. Interestingly, in the “main changes” section of the document, there is another statement claiming “systolic home BP between 125 and 135 mmHg [should be a target] for most people”[6]. As for ambulatory BP monitoring, only the 2017 ACC/AHA Guidelines provide the corresponding ambulatory TR[7]. Both European and American experts agree that the precise relationships among office, ambulatory, and home BP are unsettled.
It is axiomatic that BP is not a single snapshot but a dynamic measure with instant-to-seasonal variations. Office and diurnal BP variability is a well-known risk factor in hypertensive patients[8], and it is also true for home BP fluctuations[9,10]. Thus, it seems reasonable to investigate BP ranges rather than specific targets. A time or proportion of BP readings in target range (TTR or BP load, respectively) may also be attractive goals. Studies on office TTR (expressed as either the percentage of BP measurements recorded within a certain window or complex calculations such as using linear interpolation) have emerged in the last 5 years, and different scientific groups have found its association with major adverse cardiovascular events and mortality[11,12]. As for ambulatory BP-derived measures (dipping status, BP load, area under BP curve, and time above BP threshold), there is no clear consensus on how to integrate them into clinical practice, as the prognostic and diagnostic relevance of most of them is a matter of debate[13].
Second, to our knowledge, there are no studies aiming at home BP proportion in TR.
Home BP monitoring (HBPM) is more attractive in terms of investigating additional “loading” indices, as it is a simple, feasible, cost-effective, and user-friendly technique[14]. Its predictive accuracy is similar to 24 h BP and superior to office BP. It also has high reproducibility even in the short term because of a higher number of readings[6]. Its well-known difficulties (misreporting, handwritten logbooks, etc.) might be overcome by mobile health and other digital interventions. Several recently published meta-analyses have confirmed the superiority of HBPM over conventional office strategies, especially when combined with BP telemonitoring (BPTM), additional support, and patient education[15-17]. Therefore, “upgraded” HBPM is advantageous in obtaining reliable self-measured BP values for the custom timeframe.
In this study, we proposed a therapeutic range between 110 and 130 mmHg for home systolic (S) BP to be a reasonable target for treatment and control of HTN. We also hypothesized that patients with a higher proportion of home SBP within the therapeutic range would have their office and home BP controlled independently of other confounding factors.
METHODS
This was an open prospective one-group single-center 12-month study in patients with HTN, who attended HTN Center of Excellence in the city of St. Petersburg, Russian Federation. Those who met the inclusion/exclusion criteria were consecutively enrolled in a study with BPTM.
The following were the inclusion criteria: uncontrolled HTN (office SBP level ≥ 140 mmHg or self-reported home SBP ≥ 135 mmHg and ongoing treatment with at least one antihypertensive drug in the last month). Non-inclusion criteria were as follows: age above 80 years, symptomatic cardiovascular or other major comorbidities requiring close medical monitoring (< 3-month periods), pregnancy, significant cognitive impairment, and active or acute mental problem. All patients were to have a smartphone/tablet with high-speed Internet access (WiFi or cellular 3G or 4G). Patients could be excluded from the study: (a) at their own request; or (b) in case of acute illness (requiring face-to-face doctor consultation or hospitalization).
The study design is as follows: for the first 3 months, patients were actively monitored and counseled (the so-called “active time window”). The remaining 9 months of the study represented passive follow-up, which implied no mandatory office visits, and patients could continue BPTM at their sole intention. The final visit was conducted at the 12-month point. For this analysis, we used the data of the first 3-month active period. Therefore, there were 2 visits in this part of the study (baseline and 3-month follow-up visit).
All patients signed an informed consent document at the baseline visit. The study was carried out according to the ICH GCP standards and the Helsinki Declaration of the World Medical Association. The study protocol was approved by the local ethics committee (Approval No. 77).
Office blood pressure
Office BP measurements were performed at baseline and 3-month visits according to the ESC/ESH Guidelines[3]. Each time, BP was measured with an automatic oscillometric device Omron M3 Expert (HEM 7132-ALRU, Kyoto, Japan) after 3-5 min of quiet rest in a sitting position with the back and (dominant) arm supported. An appropriate bladder cuff was used, encircling at least 80% of the arm. Three serial BP readings were taken 1-2 min apart, and the average of the last 2 readings was displayed. We used 2 variations of SBP control in the office: a cut-off of < 140 mmHg or TR within 120-139 mmHg (measured at the follow-up visit).
Blood pressure telemonitoring and online counseling
A free simple website and a mobile application were used for patient-physician communication as well as storage and exchange of medical information. Detailed information on the hybrid telehealth solution can be found elsewhere[18]. In brief, patients used the mobile application, while web-based software was installed on office computers at the clinical site. Each patient was managed by the same physician throughout the follow-up period. At baseline, patients were registered in the program and their accounts were linked to their doctor’s account. The interface allowed the patient to manually input the HBP data. A text chat window was available for remote consultations (an unlimited number with a 24-72 h timeframe for a physician to reply).
Home blood pressure monitoring
Initially, it was recommended to record BP by a validated upper-arm device in the morning and evening, 3 times in a row with 1 min intervals, before meals and drug intake. Patients were asked to measure home BP every day (at least 3 days a week) for the first 2 weeks (to get a total of 6-14 days). Meanwhile, patients were advised to manually enter the last 2 BP readings into the electronic HBP diary. No Bluetooth® connectivity or other process automation was foreseen. Home BPM tutorials and the list of preferred upper-arm devices (STRIDE-BP[19]) were available for patients in the dedicated “support” section of the BPTM app. A supervising physician was advised to monitor HBP readings closely in these first 2 weeks and then to adjust the HBPM schedule accordingly (using the text chat). There were several possible further schedules: (a) every day; (b) 3 days a week; and (c) every month for at least 3 days in a row. We also asked patients to measure HBP for 3-7 days before the follow-up visit (i.e., the 3-month visit) according to the abovementioned rules. We used 2 conventional variations of SBP control at home: a cut-off of < 135 mmHg (the mean of SBP values taken ≤ 7 days before the follow-up visit except the first day) or TR within 110-130 mmHg (the mean SBP values taken ≤ 7 days before the follow-up visit except the first day should have been within the proposed TR).
Proportion of home systolic blood pressure readings in target range
Here, we introduce the measure called proportion of home SBP readings in target range (sBPiTR). We express this variable as the percent of home SBPs that fall within 110 and 130 mmHg in a certain time window [Equation (1)]
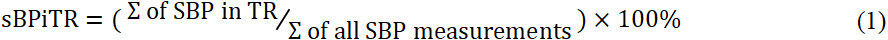
Due to the observational nature of the study and our previous experience with BPTM, home monitoring might be chaotic. Thus, we did not expect patients would monitor BP on a daily basis but rather skip some days (or even weeks) taking breaks.
We applied 2 scenarios for calculating sBPiTR: (1) analyze all available BP measurements in the electronic diary except the very first day; or (2) analyze BP readings discarding every first day if HBPM was interrupted for ≥ 7 days.
We then divided sBPiTR (0-3 months) into 4 groups (quartiles): high rate of home SBP control (75%-100%), more than half of SBP readings in TR (50%-74%), less than half of SBP readings in TR (25%-49%), and low rate of home SBP control (0%-24%).
Statistical analyses
Descriptive statistics included median and interquartile range (IQR) for continuous variables (the data are non-normally distributed). We applied a frequency analysis (the χ2 test) to assess the contingency between counts and proportions. We applied MacNemar’s test for the paired nominal variables. Continuous variables were compared by Mann-Whitney U test, and Wilcoxon rank-sum test was used for paired parameters. Weighted Cohen’s kappa coefficient was used as a measure of inter-rater reliability between office/home SBP less than corresponding cut-offs on the follow-up visit (nominal variables) and different sBPiTR quartiles (nominal variables). Kappa coefficients were interpreted according to McHugh[20]. Spearman’s Rho (rs) coefficient was used to assess the association between variables.
Multivariate logistic regression analysis was used to assess the associations between controlled HTN [per office (1) or home (2) SBP] as dependent categorical variables (in TR/not in TR) and sBPiTR (main independent categorical variable), with the adjustment for age, sex, number of antihypertensive drugs, and baseline office SBP (included as covariates). Only the best-case sBPiTR scenario was taken as a potential predictor, and the results are presented as the odds ratio (OR) and 95% confidence interval (95%CI).
Two-sided P values < 0.05 were considered significant.
Statistical analyses were carried out by two authors (Ionov M and Egorov M) using SPSS version 23 (IBM SPSS, Chicago, IL, United States), Python Software Foundation (Python Language Reference, version 2.7; available at: http://www.python.org), and jamovi (the jamovi project, version 1.6 for MacOS, retrieved from: https://www.jamovi.org).
RESULTS
Baseline characteristics
The current study included 123 patients. None of the patients were excluded during the first 3 months. Baseline characteristics are presented in Table 1.
Baseline characteristics of study participants
| Variable | Median (IQR) |
| Age, y | 54 (17) |
| Sex, males | 102 (83%) |
| BMI, kg/m2 | 28.4 (5.2) |
| Number (and %) of patients with obesity (BMI > 25 kg/m2) | 43 (35%) |
| Office SBP, mmHg | 140 (23) |
| Office DBP, mmHg | 83 (18) |
| Heart rate, bpm | 70 (14) |
| Number (and %) of patients with known type 2 diabetes mellitus | 10 (8%) |
| Number (and %) of patients with high cardiovascular risk (SCORE ≥ 5%) | 102 (83%) |
| *Total cholesterol, mmol/L | 5.23 (1.49) |
| *Serum glucose, mmol/L | 5.76 (0.9) |
| *Serum creatinine, μmol/L | 82.1 (22.0) |
| Number of medications (minimum and maximum) | 2 (1 to 4) |
Patients were predominantly middle-aged, mildly hypertensive Caucasian males of high cardiovascular risk. One third of them were overweight or obese, and less than 10% were diagnosed with type 2 diabetes mellitus. They were prescribed a median of 2 drugs to treat HTN. Most of them were taking RAAS inhibitors and diuretics in fixed combinations.
Overall results of a 3-month surveillance
After the 3 months of close surveillance, there was noted a significant reduction of median office BP: to 132 mmHg (IQR 16 mmHg) and 80 mmHg (IQR 13 mmHg), P < 0.001 for both. By the end of the 3-month period, 74 of 123 patients had had their office SBP in TR (P < 0.001), and for 93 of them, office SBP was lower than 140 mmHg (P < 0.001). There was no change in heart rate [70 beats per minute (IQR 12),
Home systolic blood pressure in target range
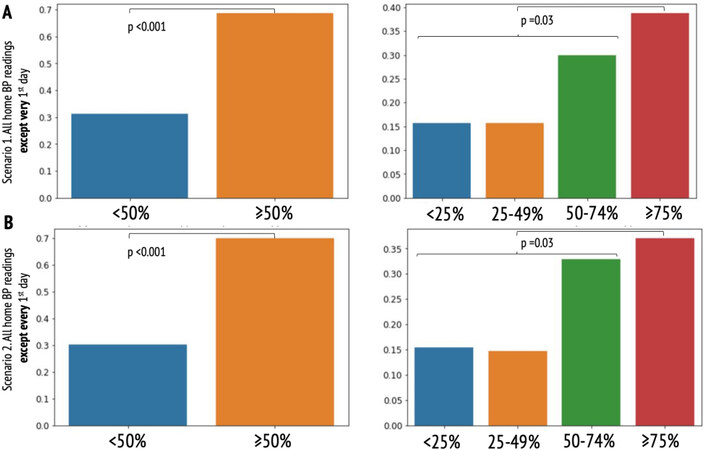
The median number of HBP measurements was 85 per patient (range 9-398). In total, 90 patients had home SBP in TR at 3 months and 110 patients had home SBP below the threshold of 135 mmHg. Overall, 20 (16%), 17 (14%), 38 (30%), and 49 (40%) patients were in each sBPiTR quartile, respectively. Therefore, significantly more patients presented with sBPiTR ≥ 50% than those with < 50% (87 vs. 36 patients,
Figure 1. Proportions of patients with home systolic blood pressure in therapeutic range: (A) excluding the very first day of home blood pressure monitoring (scenario 1); and (B) excluding every first day of home blood pressure monitoring if it was interrupted for ≥ 7 days. There were significantly more patients with sBPiTR of ≥ 50% than those with < 50%. There were fewer patients in the upper quartile than in all 3 lower quartiles cumulatively.
There was slight inter-rater reliability between sBPiTR ≥ 50% and office SBP in TR (calculated with the second scenario only) but not with the office SBP discrete value of < 140 mmHg (both scenarios). There was a fair (the second scenario) to substantial (the first scenario) agreement between sBPiTR ≥ 50% and home SBP in TR. There was a fair agreement between sBPiTR ≥ 50% and the home SBP discrete value of < 135 mmHg (only in the first scenario). Fair agreements were found between sBPiTR ≥ 75% and home SBP in TR (both first and second scenarios of sBPiTR calculation, Table 2).
Inter-rater reliability between sBPTR and conventional therapeutic targets
| Variable | κ Cohen’s | Spearman rs |
| sBPiTR. First scenario | ||
| sBPiTR (≥ 50% vs. < 50%) X office BP < 140 mmHg | 0.03# | 0.03# |
| sBPiTR (≥ 50% vs. < 50%) X home SBP < 135 mmHg | 0.36** | 0.45** |
| sBPiTR (≥ 50% vs. < 50%) X office SBP in TR | 0.09# | 0.09# |
| sBPiTR (≥ 50% vs. < 50%) X home SBP in TR | 0.65** | 0.65** |
| sBPiTR (≥ 75% vs. < 75%) X office BP < 140 mmHg | -0.07# | -0.1# |
| sBPiTR (≥ 75% vs. < 75%) X home SBP < 135 mmHg | 0.09# | 0.2# |
| sBPiTR (≥ 75% vs. < 75%) X office SBP in TR | 0.08# | 0.09# |
| sBPiTR (≥ 75% vs. < 75%) X home SBP in TR | 0.21** | 0.27** |
| sBPiTR. Second scenario | ||
| sBPiTR (≥ 50% vs. < 50%) X office BP < 140 mmHg | 0.09# | 0.1# |
| sBPiTR (≥ 50% vs. < 50%) X home SBP < 135 mmHg | 0.24** | 0.29** |
| sBPiTR (≥ 50% vs. < 50%) X office SBP in TR | 0.19* | 0.19* |
| sBPiTR (≥ 50% vs. < 50%) X home SBP in TR | 0.32** | 0.32** |
| sBPiTR (≥ 75% vs. < 75%) X office BP < 140 mmHg | 0.07# | 0.1# |
| sBPiTR (≥ 75% vs. < 75%) X home SBP < 135 mmHg | 0.09# | 0.18# |
| sBPiTR (≥ 75% vs. < 75%) X office SBP in TR | 0.11# | 0.12# |
| sBPiTR (≥ 75% vs. < 75%) X home SBP in TR | 0.21** | 0.27** |
Multivariate logistic regression analysis (adjusted for age, sex, number of drugs, and baseline office SBP) revealed that, compared with the group whose sBPiTR was < 50% (the reference group), patients from the group with ≥ 50% sBPiTR were more likely to have office BP in TR [OR 2.41 (95%CI: 1.06-5.51)]. This was true as well for home SBP in TR [OR 5.2 (95%CI: 2.06-13.12)] and home SBP < 135 mmHg [OR 7.58 (95%CI: 1.87-30.81)] but not for office SBP < 140 mmHg [OR 1.56 (95%CI: 0.65-3.76)]. The upper quartile of sBPiTR (compared to all other three ones) was only associated with home SBP in TR [OR 4.86 (95%CI: 1.74-13.59)].
DISCUSSION
Our study reiterated that BPTM provides the opportunity to collect large amounts of home BP values in a relatively short time. With the assistance of telehealth, we were able to propose sBPiTR as a new potential therapeutic goal in patients with HTN. We revealed that a threshold of 50% of home SBP measurements within 110-130 mmHg has a slight agreement with office BP control and at least fair agreement with home BP control. This hypothesis becomes even more evident when the HBPM protocol is followed strictly, discarding every first day of measurement.
The term BP variability has been known for almost 30 years. About 20 years ago, Rothwell et al. suggested that long-term BP variability predicts stroke and coronary events in high-risk patients[21], which then was confirmed in a meta-analysis of 33 studies by Stevens et al. in 2016[22]. However, the authors of the latter study reasonably stated that BP variability is not simply calculated at hand, and it is unclear whether certain measures should be preferred over others (standard deviation, coefficient of variation, variation independent of mean, etc.). This necessitated the search for other easy-to-use measures of BP fluctuations, such as BP load[23], cumulative BP[24], and TTR.
Several scientific groups in the last 5 years have focused on TTR because this parameter represents BP variability over time. This concept is “novel” and nascent but only in the field of HTN, as TTR has been integrated successfully in other fields of cardiovascular and preventive medicine, such as vitamin K therapy or diabetes treatment[25,26]. Cohort studies with the data on hundreds of thousands of participants showed that BP TTR is closely associated with all-cause and cardiovascular mortality as well as morbidity[11,27]. Interestingly, in the recent secondary analysis of the SPRINT study, TTR was found to be an independent predictor of major adverse cardiovascular events even after adjustment for BP variability and mean BP[12]. The increased interest in this new variable is intuitive because TTR reflects a more holistic view of an individual patient’s BP control. At least 2 trials have used TTR as an endpoint[28] or as a guide to making treatment decisions (HyperLink trial)[29].
The main body of evidence is based primarily on in-office BP values in TR. In contrast, the uniqueness of HBP is in the large amounts of data which can be easily collected within a short period of time (weeks to months). Thus, there is no need to perform complex linear interpolation to find TTR[30]. This makes HBPM favorably different from other measurement techniques. The main uncertainty lies in the HBP TR per se because the guidelines and experts do not have a definitive answer for this issue. Bearing in mind that there is strong evidence of the J-curve phenomenon[31] for office BP and a belief that there is a 10 mmHg difference between home and office BP[6], in the present study, we applied a relatively fluent TR of 110-130 mmHg.
Home BP is very valuable in clinical practice. However, a remarkable and counterintuitive phenomenon has recently been discovered. Of all those eligible for out-of-office BP monitoring, only 3%-4% are advised to perform HBPM and 15%-16% of patients do it irrespective of the doctor’s advice[32]. How many of these patients perform HBPM correctly? The accuracy and reliability of HBPM require not only the use of a validated device, but also standardized procedures to be followed and good patient education and training[10]. In this regard, BPTM is helpful for establishing HBPM, thus improving treatment adherence and doctor-patient relations[33]. Proper HBPM may reduce the need for office visits, as forced by the COVID-19 pandemic[34]. Therefore, our proposed new variable (sBPiTR) becomes even more crucial for self-monitored BP in the long run.
The limitations of our study should be mentioned. Relatively few patients were observed, there was no comparator group, and the study was observational in nature. All of these contribute to selection, ascertainment, and information bias. On the other hand, the study design did not require a comparison group. In addition, an increase in the number of patients with no to very few home BP measurements would likely fail to lead to an equal increase in the data reliability (i.e., compliance bias). In this regard, we did our best to keep patients under follow-up and actively consulted them so that HBPM continued properly. Regarding the relatively short study duration, it should be mentioned that: (a) several ambulatory BP monitoring (ABPM) indicators such as BP load or the area under the BP curve[35] are calculated for 24 h only; and (b) in recent studies, only few baseline home readings were used to enhance the predictive ability of BP profile (without continued HBPM)[36]. In addition, we preferred 3 months as the time window because of its principal importance for the management of uncontrolled HTN[3,7], during which guidelines recommend achieving TR. The manual imputation of BP data is another important yet mostly technical limitation. It is well known that automatically transmitted BP data are more reliable than manually inserted ones[37]. In our case, it should be noted that patients were supervised by a motivated physician. Therefore, even though the data were manually entered, we believe patients were more involved in the treatment process, which may have contributed to better HBPM, drug adherence, and lowered BP. Moreover, the recent and only trial with a head-to-head comparison of HBPM alone and BPTM (TASMINH4) showed no significant difference between the self-monitoring and telemonitoring groups in terms of BP lowering at 6 months[38]. This further highlights the relevance of patient education and HBPM motivation.
In conclusion, the proportion of home sBPiTR may serve as a reasonable treatment goal, as it positively reflects HTN control and may act as a predictor for the achievement of target SBP both in and out of office.
Larger studies are needed to confirm these preliminary results. We are interested in pursuing our efforts in this direction. Future work will aim at increasing the number of patients in the ramping BPTM program to confirm its feasibility and perceived usefulness. We also aim to facilitate a more structured HBPM schedule and to proceed to automatic BP data uploading, which is in line with the Internet-of-Medical-Things paradigm[39]. We plan to expand the follow-up for patients, increase the frequency of office visits, and compare the BP “time in target range” with sBPiTR. Finally, ABPM data should be integrated and sBPiTR should be tested against “hard” endpoints such as organ damage.
DECLARATIONS
Authors’ contributionsMade substantial contributions to conception and design of the study and performed data analysis and interpretation: Ionov M, Usova E, Egorov M
Performed data acquisition, as well as provided administrative, technical, and material support: Zvartau N, Konradi A
All authors contributed to interpretation of the results, and made meaningful contribution to writing and accepting the final manuscript.
Availability of data and materialsThe data that support the findings of this study are available from the corresponding author, Dr. Mikhail Ionov, upon reasonable request.
Financial support and sponsorshipThis work was financially supported by the Ministry of Science and Higher Education of the Russian Federation (Agreement No. 075-15-2022-301).
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateThe protocol of the study was approved by the local ethics committee (approval No 77). The study was conducted in accordance with the principles of the Declaration of Helsinki and in accordance with the Good Clinical Practice guidelines of the International Council for Harmonization. All the patients provided written informed consent.
Consent for publicationNot applicable.
Copyright© The Author(s) 2022.
REFERENCES
1. Kalehoff JP, Oparil S. The story of the silent killer: a history of hypertension: its discovery, diagnosis, treatment, and debates. Curr Hypertens Rep 2020;22:72.
2. Rahimi K, Bidel Z, Nazarzadeh M, et al. Age-stratified and blood-pressure-stratified effects of blood-pressure-lowering pharmacotherapy for the prevention of cardiovascular disease and death: an individual participant-level data meta-analysis. The Lancet 2021;398:1053-64.
3. Williams B, Mancia G, Spiering W, et al. ESC Scientific Document Group. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J 2018;39:3021-104.
4. Kobalava ZD, Konradi AO, Nedogoda SV, et al. Arterial hypertension in adults. Clinical guidelines 2020. Russ J Cardiol 2020;25:3786. Available from: https://doi.org/10.15829/1560-4071-2020-3-3786 [Last accessed on 7 Sep 2022].
5. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013;34:2159-219.
6. Parati G, Stergiou GS, Bilo G, et al. Working Group on Blood Pressure Monitoring and Cardiovascular Variability of the European Society of Hypertension. Home blood pressure monitoring: methodology, clinical relevance and practical application: a 2021 position paper by the Working Group on Blood Pressure Monitoring and Cardiovascular Variability of the European Society of Hypertension. J Hypertens 2021;39:1742-67.
7. Muntner P, Carey RM, Jamerson K, Wright JT Jr, Whelton PK. Rationale for ambulatory and home blood pressure monitoring thresholds in the 2017 American college of cardiology/american heart association guideline. Hypertension 2019;73:33-8.
8. Kario K. Blood pressure variability in hypertension: a possible cardiovascular risk factor. Am J Hypertens 2004;17:1075-6.
9. Schutte AE, Kollias A, Stergiou GS. Blood pressure and its variability: classic and novel measurement techniques. Nat Rev Cardiol 2022; doi: 10.1038/s41569-022-00690-0.
10. Kario K. Home blood pressure monitoring: current status and new developments. Am J Hypertens 2021;34:783-94.
11. Doumas M, Tsioufis C, Fletcher R, Amdur R, Faselis C, Papademetriou V. Time in therapeutic range, as a determinant of all-cause mortality in patients with hypertension. J Am Heart Assoc 2017;6:e007131.
12. Fatani N, Dixon DL, Van Tassell BW, Fanikos J, Buckley LF. Systolic blood pressure time in target range and cardiovascular outcomes in patients with hypertension. J Am Coll Cardiol 2021;77:1290-9.
13. O'Brien E, Parati G, Stergiou G, et al. European Society of Hypertension Working Group on Blood Pressure Monitoring. European society of hypertension position paper on ambulatory blood pressure monitoring. J Hypertens 2013;31:1731-68.
14. Andraos J, Munjy L, Kelly MS. Home blood pressure monitoring to improve hypertension control: a narrative review of international guideline recommendations. Blood Press 2021;30:220-9.
15. Tucker KL, Sheppard JP, Stevens R, et al. Self-monitoring of blood pressure in hypertension: a systematic review and individual patient data meta-analysis. PLoS Med 2017;14:e1002389.
16. Park SH, Shin JH, Park J, Choi WS. An updated meta-analysis of remote blood pressure monitoring in urban-dwelling patients with hypertension. Int J Environ Res Public Health 2021;18:10583.
17. Cavero-Redondo I, Saz-Lara A, Sequí-Dominguez I, et al. Comparative effect of eHealth interventions on hypertension management-related outcomes: a network meta-analysis. Int J Nurs Stud 2021;124:104085.
18. Ionov MV, Zhukova OV, Yudina YS, et al. Value-based approach to blood pressure telemonitoring and remote counseling in hypertensive patients. Blood Press 2021;30:20-30.
19. STRIDE BP Scientific Advisory Board. Validated devices for home blood pressure monitoring 2019. Available from: https://stridebp.org/bp-monitors/37-pdfs/734-home?format=pdf&tmpl=component&box=home [Last accessed on 7 Sep 2022].
21. Rothwell PM, Howard SC, Dolan E, et al. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. The Lancet 2010;375:895-905.
22. Stevens SL, Wood S, Koshiaris C, et al. Blood pressure variability and cardiovascular disease: systematic review and meta-analysis. BMJ 2016;354:i4098.
23. Zachariah PK, Sheps SG, Ilstrup DM, et al. Blood pressure load - a better determinant of hypertension. Mayo Clinic Proceedings 1988;63:1085-91.
24. Reges O, Ning H, Wilkins JT, et al. Association of cumulative systolic blood pressure with long-term risk of cardiovascular disease and healthy longevity: findings from the lifetime risk pooling project cohorts. Hypertension 2021;77:347-56.
25. Schmitt L, Speckman J, Ansell J. Quality assessment of anticoagulation dose management: comparative evaluation of measures of time-in-therapeutic range. J Thromb Thrombolysis 2003;15:213-6.
27. Chung SC, Pujades-Rodriguez M, Duyx B, et al. Time spent at blood pressure target and the risk of death and cardiovascular diseases. PLoS One 2018;13:e0202359.
28. Dixon DL, Baker WL, Buckley LF, Salgado TM, Van Tassell BW, Carter BL. Effect of a physician/pharmacist collaborative care model on time in target range for systolic blood pressure: post hoc analysis of the CAPTION trial. Hypertension 2021;78:966-72.
29. Margolis KL, Asche SE, Bergdall AR, et al. Effect of home blood pressure telemonitoring and pharmacist management on blood pressure control: a cluster randomized clinical trial. JAMA 2013;310:46-56.
30. Rosendaal FR, Cannegieter SC, van der Meer FJ, Briët E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost 1993;69:236-9.
31. Messerli FH, Bangalore S, Messerli AW, Räber L. The muddy waters of the J-curve and coronary revascularization. Eur Heart J 2020;41:1684-6.
32. Bryant KB, Green MB, Shimbo D, et al. Home blood pressure monitoring for hypertension diagnosis by current recommendations: a long way to go. Hypertension 2022;79:e15-7.
33. Omboni S, McManus RJ, Bosworth HB, et al. Evidence and recommendations on the use of telemedicine for the management of arterial hypertension: an international expert position paper. Hypertension 2020;76:1368-83.
34. Omboni S, Padwal RS, Alessa T, et al. The worldwide impact of telemedicine during COVID-19: current evidence and recommendations for the future. Connect Health 2022;1:7-35.
35. Kengne AP, Czernichow S, Huxley R, et al. ADVANCE Collaborative Group. Blood pressure variables and cardiovascular risk: new findings from ADVANCE. Hypertension 2009;54:399-404.
36. Mancia G, Facchetti R, Seravalle G, Cuspidi C, Corrao G, Grassi G. Adding home and/or ambulatory blood pressure to office blood pressure for cardiovascular risk prediction. Hypertension 2021;77:640-9.
37. Parker RA, Paterson M, Padfield P, et al. Are self-reported telemonitored blood pressure readings affected by end-digit preference: a prospective cohort study in Scotland. BMJ Open 2018;8:e019431.
38. Mcmanus RJ, Mant J, Franssen M, et al. Efficacy of self-monitored blood pressure, with or without telemonitoring, for titration of antihypertensive medication (TASMINH4): an unmasked randomised controlled trial. The Lancet 2018;391:949-59.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Ionov M, Usova E, Egorov M, Zvartau N, Konradi A. Home blood pressure in target range as an additional therapeutic goal in hypertensive patients: a telemonitoring-based analysis. Conn Health Telemed 2022;1:101-11. http://dx.doi.org/10.20517/ch.2022.12
AMA Style
Ionov M, Usova E, Egorov M, Zvartau N, Konradi A. Home blood pressure in target range as an additional therapeutic goal in hypertensive patients: a telemonitoring-based analysis. Connected Health And Telemedicine. 2022; 1(3): 101-11. http://dx.doi.org/10.20517/ch.2022.12
Chicago/Turabian Style
Ionov, Mikhail, Elena Usova, Michil Egorov, Nadezhda Zvartau, Alexandra Konradi. 2022. "Home blood pressure in target range as an additional therapeutic goal in hypertensive patients: a telemonitoring-based analysis" Connected Health And Telemedicine. 1, no.3: 101-11. http://dx.doi.org/10.20517/ch.2022.12
ACS Style
Ionov, M.; Usova E.; Egorov M.; Zvartau N.; Konradi A. Home blood pressure in target range as an additional therapeutic goal in hypertensive patients: a telemonitoring-based analysis. Conn. Health. Telemed. 2022, 1, 101-11. http://dx.doi.org/10.20517/ch.2022.12
About This Article
Copyright
Data & Comments
Data
 Cite This Article 16 clicks
Cite This Article 16 clicks












Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.