Updates on the pathologic diagnosis and classification of mesothelioma
Abstract
Mesothelioma is a rare malignant tumor of the serosal membranes that can be challenging to diagnose, especially on small biopsy specimens. There are updated guidelines on the diagnosis and classification of mesothelioma, which incorporate advancements in understanding mesothelioma biology published in the literature over recent years. This review will discuss marked developments and/or improvements that have been made, including: (1) to the histologic classifications of mesothelioma; (2) the use of such classifications and nuclear grading in prognosis; (3) the indispensability of ancillary studies in the diagnosis of mesothelioma; (4) the application of these pleural based classifications and diagnostic schemes in peritoneal mesothelioma; and (5) the potential for diagnosis of mesothelioma in situ.
Keywords
INTRODUCTION
Mesothelioma is a neoplasm that most commonly arises in the pleura, but can also be found in peritoneum, pericardium, or tunica vaginalis of the testes[1,2]. It is a rare tumor with an annual estimated incidence of 3000 cases per year in the United States; it affects men more than women[1,3,4]. All mesotheliomas are clinically aggressive and carry a poor prognosis, with studies estimating a median survival of 9-29 months and 5-year survival of 5% or less[5,6], though patients with localized disease may have better outcomes[7]. The main diagnostic features of mesothelioma are published in the World Health Organization Classification of Thoracic Tumours[8]. Mesothelioma remains a challenging entity to diagnose, especially when the pathologist is given a small biopsy. Currently, mesothelioma is divided histologically into three major subtypes (epithelioid, sarcomatoid, and biphasic), which can be further categorized into prognostic groups based on nuclear grade and cytologic and stromal features[9,10]. The gold standard for the diagnosis of mesothelial lesions as malignant is tissue invasion, which is not always possible on small biopsies[11]. The diagnosis of mesothelioma can be achieved using ancillary testing, specifically BAP1 and MTAP immunohistochemistry or CDKN2A fluorescence in situ hybridization; furthermore, these markers can also facilitate the identification of mesothelioma in situ prior to the invasive phase of the disease.
EPITHELIOID MESOTHELIOMA
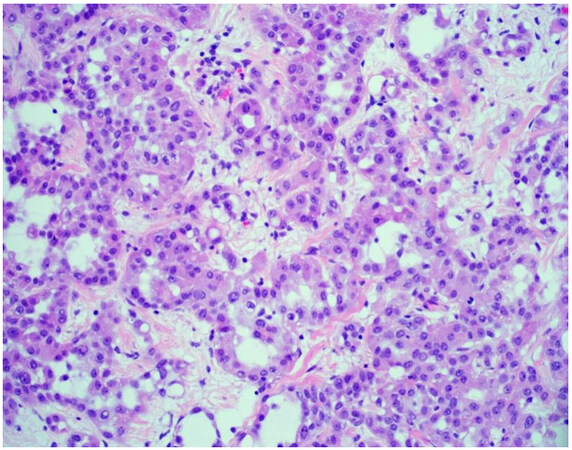
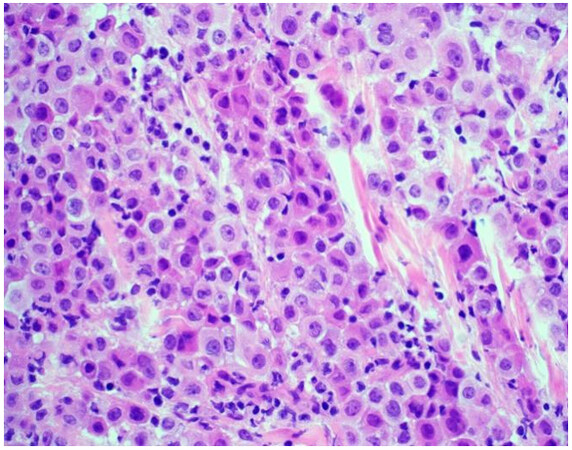
The majority of mesotheliomas (approximately 55%) exhibit an epithelioid growth pattern [Figure 1], and these are further subclassified based on secondary architectural patterns, cytology, and stromal features[10,12]. While data may be limited with only a few published articles in the literature, studies show that some of these features stratify patients into prognostic groups within epithelioid mesothelioma. Architectural patterns that have been described in epithelioid mesothelioma include tubulopapillary, trabecular, adenomatoid, microcystic, solid, and micropapillary. Cytologic features described in epithelioid mesothelioma include rhabdoid, deciduoid, small cell, clear cell, signet ring, lymphohistiocytoid, and pleomorphic. A subset of mesotheliomas may show myxoid stroma. The pathologist is encouraged to report these features to improve diagnostic accuracy and potentially help with risk stratification[10,12]. Features associated with a more favorable prognosis in epithelioid mesothelioma include tubulopapillary, trabecular, and adenomatoid architectural patterns, as well as lymphohistiocytoid cytologic features and the presence of myxoid stroma (50% or greater of total tumor)[10-12]. Unfavorable features that portend a worse prognosis in epithelioid mesothelioma include solid and micropapillary architecture, as well as rhabdoid and pleomorphic cytologic features[8,10-12]. Grading systems that incorporate nuclear features (atypia, mitotic index) into classification have been shown in medium to large cohorts to determine prognosis[13,14]. While these grading studies have not been shown to uniformly predict disease performance across histologic subtypes, they are a powerful prognostic tool in epithelioid mesothelioma, and recently proposed high- and low-grade grouping of epithelioid mesothelioma is now recommended[13-15]. The grading of epithelioid mesotheliomas will be discussed in greater detail below.
SARCOMATOID MESOTHELIOMA
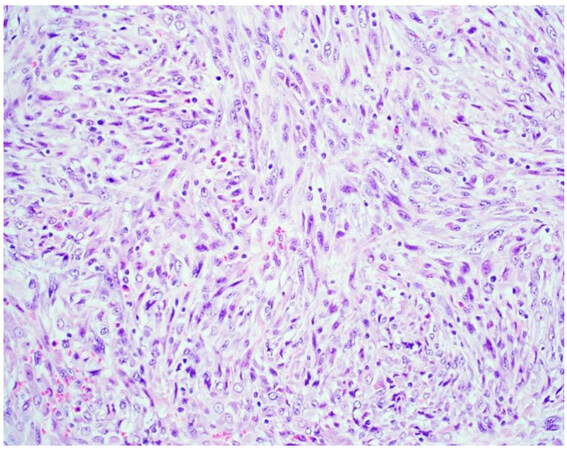
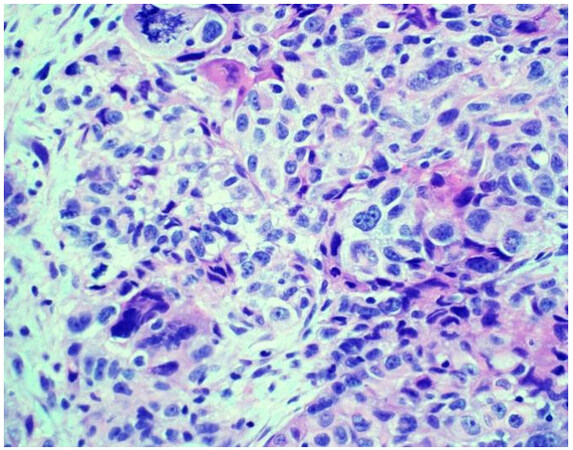
Sarcomatoid mesothelioma [Figure 2] is characterized by the haphazard proliferation of malignant spindled mesothelial cells resembling sarcoma[8]. The less cellular and densely fibrotic desmoplastic variant has long been recognized as a pattern of sarcomatoid mesothelioma[8]. Sarcomatoid mesotheliomas are rare, comprising only around 10% of all cases, ranging from 7%-22%[16,17]. More recently, the application of specific cellular features to the diagnostic classification of sarcomatoid mesothelioma has been proposed, similar to epithelioid mesothelioma[10,12]. These cytologic features include noting whether cells are pleomorphic, lymphohistocytoid, or transitional; lymphohistiocytoid has been associated with a more favorable prognosis (transitional will be discussed below)[12]. Pleomorphic features have been observed in both epithelioid and sarcomatoid mesotheliomas, and are genomically similar enough to be classified as either, based upon the cytomorphology of the tumor (epithelioid or sarcomatoid)[10]. Desmoplasia is classified as a stromal feature[12].
Figure 2. Sarcomatoid mesothelioma characterized by a haphazard proliferation of malignant spindle cells (H&E 200×).
Transitional mesothelioma (transitional features) presents as a proliferation of elongated epithelioid mesothelial cells with sheet-like, yet discohesive growth and, as previously mentioned, is now considered a cytologic feature observed in sarcomatoid mesothelioma[8]. While the name and cytomorphology suggest that these cells are transitioning or are halfway between epithelioid and sarcomatoid mesothelioma, transitional mesotheliomas are more similar to the sarcomatoid subtype genomically[18]. Given their genomic relatedness to sarcomatoid mesothelioma, it is not surprising that transitional mesothelioma is also associated with a worse prognosis, in line with what has been reported in sarcomatoid mesothelioma[18,19].
BIPHASIC MESOTHELIOMA
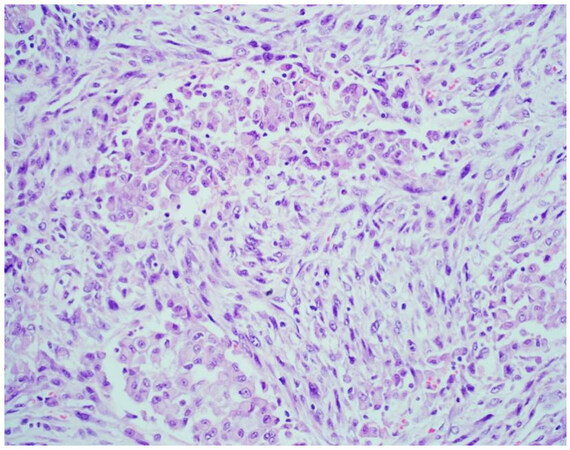
Mesotheliomas in which both epithelioid and sarcomatoid morphology are present should be classified as biphasic mesothelioma (approximately 20% of all mesotheliomas; Figure 3), and the specific ratio of epithelioid to sarcomatoid components should be noted. While rather arbitrarily assigned, at least 10% of each component (epithelioid and sarcomatoid) is required for diagnosis in the most recent WHO classification[8]. This 10% cutoff is only applied to resected mesothelioma. According to the most recent classification schemes, a small biopsy with any amount of both epithelioid and sarcomatoid mesothelioma should be classified as biphasic[10]. It is known that approximately 20% of biopsies showing epithelioid mesothelioma will, in fact, show biphasic morphology in resection specimens[20,21]. Biphasic and sarcomatoid morphology on a small biopsy are quite predictive of the tumor having a significant proportion of sarcomatoid morphology at the time of resection[20,21]. The ratio of each constituent, especially with regard to the sarcomatoid component, may be prognostic. Data is limited, but it is suggested that sarcomatoid predominant biphasic mesotheliomas have worse outcomes, with prognostic cutoffs reported from
HISTOLOGIC GRADING
Pathologic grading systems have been described and most robustly studied in epithelioid mesothelioma. The original grading system was developed at Memorial Sloan Kettering and consisted of a nuclear grade score, calculated as a sum of nuclear atypia and mitotic count[14]. Briefly, nuclear atypia is scored as 1
Figure 4. Epithelioid mesothelioma with nuclear atypia score of 1. The nuclei are relatively small and monotonous without prominent nucleoli (H&E 400×).
Figure 5. Epithelioid mesothelioma with nuclear atypia score of 2. The nuclei display moderate pleomorphism with occasionally prominent nucleoli and more vesicular chromatin when compared to the nuclei in Figure 4 (H&E 400×).
Figure 6. Epithelioid mesothelioma with nuclear atypia score of 3. The nuclei display marked pleomorphism with multinucleated cells and giant tumor cells. Atypical mitotic figures are also obvious (H&E 400×).
At present, there is no consensus on the need to grade sarcomatoid or biphasic mesotheliomas as tumors with sarcomatoid morphology behave aggressively. A number of studies have proposed grading non-epithelioid mesotheliomas, but these are not recommended at present to be utilized in clinical practice[15,19,25].
ANCILLARY STUDIES AND TESTING
Mesotheliomas often need to be distinguished from other pleural-based malignancies. Tumors, both primary and metastatic to the lung, can show significant pleural involvement and mimic mesothelioma[26,27]. Clinical context and radiographic imaging should be considered when forming a differential diagnosis. The judicious application of immunohistochemical staining can help with the differential diagnosis[11]. Markers expressed in mesothelial lesions include WT-1, D2-40 (podoplanin), calretinin, and cytokeratin 5/6[11,28]. A myriad of other makers have been proposed as indicators of mesothelial differentiation (mesothelin, HBME-1, thrombomodulin) and the reader is referred to other reviews on this topic for additional information[29,30]. Both carcinoma and mesothelioma often express pancytokeratins (AE1/AE3, CAM 5.2, OSCAR); cytokeratin staining, while nonspecific, may delineate growth patterns within the tumor or exclude other types of cancer from the differential diagnosis[5]. Historically, numerous “pancarcinoma” or “panepithelial” markers have been proposed with staining in carcinomas but not mesotheliomas. Some of these markers include CEA, B72.3, MOC-31, and Ber-EP4, and while these markers are more often positive in carcinomas than mesotheliomas, these markers can be expressed, even strongly, in mesothelial lesions[28]. More recently, Claudin-4, an antibody raised against tight junctions of moderate to well-differentiated epithelia, has emerged as the best marker to differentiate carcinoma from mesothelioma[31-33]. Current recommendations to separate mesothelial lesions from carcinoma are to demonstrate reactivity for two mesothelial markers (CK5/6, calretinin, WT-1, and/or D2-40) and show negativity for two carcinoma markers (claudin 4, MOC-31, Ber-EP4, etc.)[11]. It should be noted that the mesothelial markers are nonspecific and imperfectly sensitive and that the sensitivity of these mesothelial markers typically drops in sarcomatoid mesotheliomas[28]. Also of note, the carcinoma/epithelial markers utilized will depend on the carcinoma one is trying to exclude. For example, it would be perfectly reasonable to utilize TTF-1 and p40 immunohistochemical stains in the thoracic cavity, given how commonly non-small cell carcinoma is encountered. It would make less sense to utilize these same markers in the peritoneal cavity, where other carcinomas also need to be excluded (renal cell carcinoma, gynecologic malignancies, etc.) by utilizing another set of immunohistochemical markers. In conclusion, for best results, sufficient yet judicious immunohistochemical studies should be employed utilizing a panel of stains that are interpreted in light of the clinical and radiographic settings.
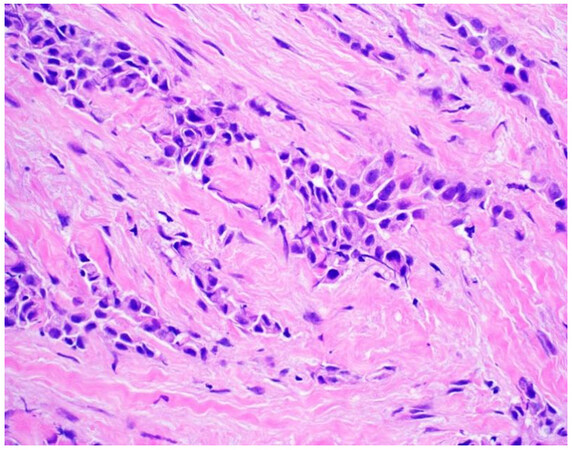
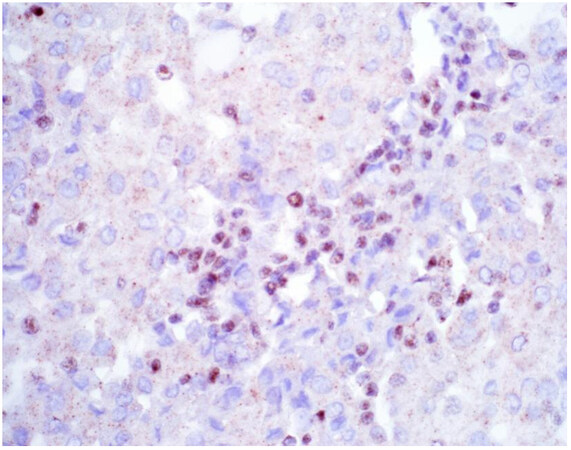
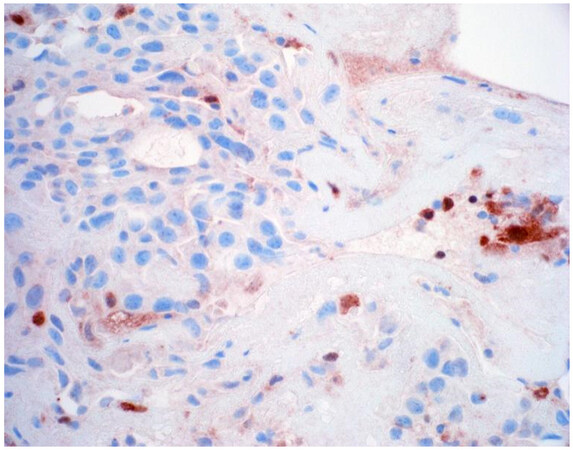
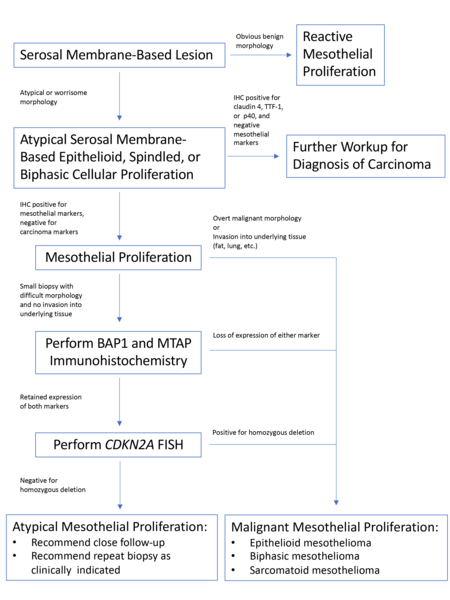
Once a lesion is confirmed to be mesothelial in origin, the pathologist may still find it challenging to call the lesion malignant, especially if there is limited tissue or a small biopsy and no invasion into underlying tissue identified. Over the past few years, newer immunohistochemical markers have emerged that allow for the separation of benign and malignant mesothelial proliferations. BRCA-associated peptide-1 (BAP1) has been shown to be discriminatory when it comes to separating benign from malignant mesothelial proliferations[34-36]. Nuclear loss of BAP1 staining [Figure 7] is currently reported as 100% specific for malignancy in mesothelial proliferations. It has long been known that a frequent genetic alteration in mesothelioma is the homozygous deletion of CDKN2A[37,38]. CDKN2A homozygous deletion can be reliably detected by fluorescence in situ hybridization (FISH)[39-41]. While FISH testing for CDKN2A deletion is useful, FISH testing is not always as readily available as immunohistochemistry in small laboratories. Thankfully, methylthioadenosine phosphorylase (MTAP) is positioned near CDKN2A and is often co-deleted[42,43]. Cytoplasmic loss of MTAP [Figure 8] has been shown to be 100% specific for malignancy in mesothelial proliferations. While CDKN2A encodes the protein p16, p16 IHC has historically shown mixed results when judging its utility at predicting homozygous deletion of CDKN2A in mesothelioma, but a recent study shows some promise when evaluating p16 alongside MTAP IHC[44]. While BAP1 and MTAP are 100% specific for malignancy, unfortunately, BAP1 and MTAP IHC are not 100% sensitive in detecting malignant mesothelial proliferations, but combining BAP1 and MTAP together does increase sensitivity[45-47]. As data continues to emerge, it appears that a limited panel approach (BAP1, MTAP, NF2, p53, among possible other markers) may be most useful in separating benign from malignant mesothelial proliferations[36,48]. A possible decisional algorithm that one may follow based on the most commonly used and accepted ancillary studies is provided [Figure 9]. While experts have not yet concluded what is the best panel to utilize, it appears evident that combining multiple stains may eventually reach adequate sensitivity for detection of mesothelioma; however, as new stains are proposed to help in this differential, the reader should be warned that close attention should be paid to the specificity of these newly proposed markers. The last word of caution on this topic pertains to the fact that one must confirm that the lesion is mesothelial before interpreting these markers. For example, BAP1 nuclear loss can also be identified in other malignancies (most notably, some melanomas and some renal cell carcinomas). Also, a recently published study highlighted the lack of specificity of MTAP loss, which can be observed in a number of intrathoracic malignancies[49].
Figure 7. Epithelioid mesothelioma showing nuclear loss of BAP1. Note small lymphocytes with retained expression (400×).
Figure 8. Epithelioid mesothelioma showing cytoplasmic loss of MTAP. Note inflammatory cells with retained expression (400×).
The emergence of these markers of malignancy in mesothelial proliferations has implications for the cytologic diagnosis of mesothelioma. Studies do exist showing that loss of BAP1 and MTAP staining can be observed in cytologic fluids from patients with mesothelioma. How this impacts the cytologic examination of fluids is beyond the scope of this review, but this area of cytology is expected to change in the coming years[50,51].
PERITONEAL MESOTHELIOMA
Peritoneal mesothelioma is the second most common type of mesothelioma, accounting for 10%-15% of all mesotheliomas[52]. Interestingly, it has been noted that today, peritoneal mesotheliomas appear more frequently in women and younger people than their pleural mesothelioma counterparts[53-56]. Peritoneal mesothelioma is classified into the same histologic subtypes as pleural mesothelioma (epithelioid, biphasic, and sarcomatoid). Recent data show that peritoneal mesotheliomas appear to have a better prognosis than pleural mesotheliomas[57]. In some cases, this may be due to different treatment options available based on native anatomical location (hyperthermic intraperitoneal chemotherapy)[58]. Biphasic and sarcomatoid mesotheliomas are relatively rare compared to their thoracic counterpart but, when identified, show a similar poor prognosis[59].
As mentioned above, there can be challenges differentiating mesothelioma from carcinoma. This problem is just as evident in the peritoneal cavity as in the thoracic cavity. Therefore, most ancillary studies performed to determine diagnosis concentrate on separating peritoneal mesothelioma from other diffuse peritoneal malignancies, mainly papillary serous carcinoma, using immunohistochemical stains adapted from those used to diagnose mesothelioma in the pleura[11]. The adaptation for these panels is made to delineate the various potential origins of peritoneal neoplasms. Pleural malignancies generally have a pulmonary origin, but peritoneal malignancies can originate from the ovaries, fallopian tubes, stomach, pancreas, colon, and kidney, and includes metastases from outside the peritoneal cavity (breast, lung carcinomas, etc.).
Peritoneal mesothelioma has somewhat been an orphan disease, with most publications pertaining to mesothelioma focusing on pleural mesothelioma. Excitingly, groups have recently begun to seriously look at the diagnosis and classification of peritoneal mesothelioma[57,60]. Our group recently published an article highlighting that many of the same pathologic parameters observed in the thoracic cavity can be applied to peritoneal mesothelioma with similar results[57]. This is an emerging area of exploration, and new and exciting research on this disease will certainly be published in the coming years.
MESOTHELIOMA IN SITU
Mesothelioma in situ (MIS) is the growth of malignant mesothelial cells, either as a monolayer or small papillary growth, without invasion of the underlying tissue and with corresponding negative radiology findings[8]. MIS, as a concept, is quite old, but could not be proven definitively as a malignant precursor, leading to the controversy surrounding its definition and diagnosis[61]. In recent years, especially in patients with nonresolving unexplained pleural effusions, loss of BAP1 and/or MTAP by immunohistochemistry, or identification of homozygous deletion of CDKN2A by FISH in noninvasive mesothelial cells, has proven that one can detect signatures of malignancy in mesothelial cells prior to the development of invasive diffuse mesothelioma[62-66]. MIS is now accepted as an entity in the WHO[8]. The ability to diagnose MIS is very recent; thus, this diagnosis will most likely have implications in pleural fluid cytology specmens[67]. The biggest question now is what the oncologist or surgeon should do if this diagnosis is made[68]. This is a question that remains to be answered. As cases continue to be reported, mesothelioma pathologists will continue to study this disease.
CONCLUSION
Recent advancements in the field of mesothelioma have led to robust prognostic grading schemes that are now included in the routine reporting of mesothelioma specimens. Advancements in ancillary testing have expanded the pathologist’s ability to diagnose mesothelioma on small biopsies and in cytology fluids. The emergence of mesothelioma in situ as a distinct entity has the potential to change how the medical team views mesothelioma and may provide a means to intervene earlier in the disease.
DECLARATIONS
Authors’ contributionsMade substantial contributions to conception and design of the article and performed review of literature: Farkas JR, Sharobim M, Schulte JJ
Availability of data and materialsNot applicable.
Financial support and sponsorshipNone.
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2022.
REFERENCES
1. Kim J, Bhagwandin S, Labow DM. Malignant peritoneal mesothelioma: a review. Ann Transl Med 2017;5:236.
3. Henley SJ, Larson TC, Wu M, et al. Mesothelioma incidence in 50 states and the district of Columbia, United States, 2003-2008. Int J Occup Environ Health 2013;19:1-10.
4. Moolgavkar SH, Meza R, Turim J. Pleural and peritoneal mesotheliomas in SEER: age effects and temporal trends, 1973-2005. Cancer Causes Control 2009;20:935-44.
5. Beasley MB, Galateau-Salle F, Dacic S. Pleural mesothelioma classification update. Virchows Arch 2021;478:59-72.
6. Gelvez-Zapata SM, Gaffney D, Scarci M, Coonar AS. What is the survival after surgery for localized malignant pleural mesothelioma? Interact Cardiovasc Thorac Surg 2013;16:533-7.
7. Marchevsky AM, Khoor A, Walts AE, et al. Localized malignant mesothelioma, an unusual and poorly characterized neoplasm of serosal origin: best current evidence from the literature and the International Mesothelioma Panel. Mod Pathol 2020;33:281-96.
8. Editorial Board WC of T. Thoracic tumours: WHO classification of tumours, 5th ed. World Health Organization: Geneva, Switzerland, 2021.
9. Travis WD, Brambilla E, Burke A, Marx A, Nicholson AG. WHO classification of tumours of the lung, pleura, thymus and heart, 5th ed. International Agency for Research on Cancer: Lyon, France, 2015.
10. Nicholson AG, Sauter JL, Nowak AK, et al. EURACAN/IASLC proposals for updating the histologic classification of pleural mesothelioma: towards a more multidisciplinary approach. J Thorac Oncol 2020;15:29-49.
11. Husain AN, Colby TV, Ordóñez NG, et al. Guidelines for pathologic diagnosis of malignant mesothelioma 2017 update of the consensus statement from the international mesothelioma interest group. Arch Pathol Lab Med 2018;142:89-108.
12. Sauter JL, Dacic S, Galateau-Salle F, et al. The 2021 WHO classification of tumors of the pleura: advances since the 2015 classification. J Thorac Oncol 2022;17:608-22.
13. Rosen LE, Karrison T, Ananthanarayanan V, et al. Nuclear grade and necrosis predict prognosis in malignant epithelioid pleural mesothelioma: a multi-institutional study. Mod Pathol 2018;31:598-606.
14. Kadota K, Suzuki K, Colovos C, et al. A nuclear grading system is a strong predictor of survival in epitheloid diffuse malignant pleural mesothelioma. Mod Pathol 2012;25:260-71.
15. Pelosi G, Papotti M, Righi L, et al. Pathologic grading of malignant pleural mesothelioma: an evidence-based proposal. J Thorac Oncol 2018;13:1750-61.
16. Klebe S, Brownlee NA, Mahar A, et al. Sarcomatoid mesothelioma: a clinical-pathologic correlation of 326 cases. Mod Pathol 2010;23:470-9.
17. Mansfield AS, Symanowski JT, Peikert T. Systematic review of response rates of sarcomatoid malignant pleural mesotheliomas in clinical trials. Lung Cancer 2014;86:133-6.
18. Galateau Salle F, Le Stang N, Tirode F, et al. Comprehensive molecular and pathologic evaluation of transitional mesothelioma assisted by deep learning approach: a multi-institutional study of the international mesothelioma panel from the MESOPATH reference center. J Thorac Oncol 2020;15:1037-53.
19. Galateau Salle F, Le Stang N, Nicholson AG, et al. New insights on diagnostic reproducibility of biphasic mesotheliomas: a multi-institutional evaluation by the international mesothelioma panel from the MESOPATH reference center. J Thorac Oncol 2018;13:1189-203.
20. Schulte JJ, Chapel DB, Attanoos R, et al. Comparison of nuclear grade, necrosis, and histologic subtype between biopsy and resection in pleural malignant mesothelioma: an international multi-institutional analysis. Am J Clin Pathol 2021;156:989-99.
21. Chirieac LR, Hung YP, Foo WC, et al. Diagnostic value of biopsy sampling in predicting histology in patients with diffuse malignant pleural mesothelioma. Cancer 2019;125:4164-71.
22. Vigneswaran WT, Kircheva DY, Ananthanarayanan V, et al. Amount of epithelioid differentiation is a predictor of survival in malignant pleural mesothelioma. Ann Thorac Surg 2017;103:962-6.
23. Zhang YZ, Brambilla C, Molyneaux PL, et al. Presence of pleomorphic features but not growth patterns improves prognostic stratification of epithelioid malignant pleural mesothelioma by 2-tier nuclear grade. Histopathology 2020;77:423-36.
24. Zhang YZ, Brambilla C, Molyneaux PL, et al. Utility of nuclear grading system in epithelioid malignant pleural mesothelioma in biopsy-heavy setting: an external validation study of 563 cases. Am J Surg Pathol 2020;44:347-56.
25. Fuchs TL, Chou A, Aksoy Y, et al. A critical assessment of current grading schemes for diffuse pleural mesothelioma with a proposal for a novel mesothelioma weighted grading scheme (MWGS). Am J Surg Pathol 2022;46:774-85.
26. Marchevsky AM, LeStang N, Hiroshima K, et al. The differential diagnosis between pleural sarcomatoid mesothelioma and spindle cell/pleomorphic (sarcomatoid) carcinomas of the lung: evidence-based guidelines from the International Mesothelioma Panel and the MESOPATH National Reference Center. Hum Pathol 2017;67:160-8.
27. Le Stang N, Burke L, Blaizot G, et al. Differential diagnosis of epithelioid malignant mesothelioma with lung and breast pleural metastasis: a systematic review compared with a standardized panel of antibodies-a new proposal that may influence pathologic practice. Arch Pathol Lab Med 2020;144:446-56.
28. Chapel DB, Schulte JJ, Husain AN, Krausz T. Application of immunohistochemistry in diagnosis and management of malignant mesothelioma. Transl Lung Cancer Res 2020;9:S3-S27.
29. Ordóñez NG. The immunohistochemical diagnosis of mesothelioma: a comparative study of epithelioid mesothelioma and lung adenocarcinoma. Am J Surg Pathol 2003;27:1031-51.
30. Ordóñez NG. Application of immunohistochemistry in the diagnosis of epithelioid mesothelioma: a review and update. Hum Pathol 2013;44:1-19.
31. Patel A, Borczuk AC, Siddiqui MT. Utility of claudin-4 versus BerEP4 and B72.3 in pleural fluids with metastatic lung adenocarcinoma. J Am Soc Cytopathol 2020;9:146-51.
32. Vojtek M, Walsh MD, Papadimos DJ, Shield PW. Claudin-4 immunohistochemistry is a useful pan-carcinoma marker for serous effusion specimens. Cytopathology 2019;30:614-9.
33. Naso JR, Churg A. Claudin-4 shows superior specificity for mesothelioma vs non-small-cell lung carcinoma compared with MOC-31 and Ber-EP4. Hum Pathol 2020;100:10-4.
34. McGregor SM, Dunning R, Hyjek E, Vigneswaran W, Husain AN, Krausz T. BAP1 facilitates diagnostic objectivity, classification, and prognostication in malignant pleural mesothelioma. Hum Pathol 2015;46:1670-8.
35. Hakim SA, Abou Gabal HH. Diagnostic utility of BAP1, EZH2 and survivin in differentiating pleural epithelioid mesothelioma and reactive mesothelial hyperplasia: immunohistochemical study. Pathol Oncol Res 2021;27:600073.
36. Chapel DB, Hornick JL, Barlow J, Bueno R, Sholl LM. Clinical and molecular validation of BAP1, MTAP, P53, and Merlin immunohistochemistry in diagnosis of pleural mesothelioma. Mod Pathol 2022; doi: 10.1038/s41379-022-01081-z.
37. Dagogo-Jack I, Madison RW, Lennerz JK, et al. Molecular characterization of mesothelioma: impact of histologic type and site of origin on molecular landscape. JCO Precis Oncol 2022;6:e2100422.
38. Hung YP, Dong F, Torre M, Crum CP, Bueno R, Chirieac LR. Molecular characterization of diffuse malignant peritoneal mesothelioma. Mod Pathol 2020;33:2269-79.
39. Marshall K, Jackson S, Jones J, et al. Homozygous deletion of CDKN2A in malignant mesothelioma: diagnostic utility, patient characteristics and survival in a UK mesothelioma centre. Lung Cancer 2020;150:195-200.
40. Oyama Y, Hamasaki M, Matsumoto S, Sato A, Tsujimura T, Nabeshima K. Short 57 kb. CDKN2A ;22:813.
41. Nabeshima K, Hamasaki M, Kinoshita Y, Matsumoto S, Sa-Ngiamwibool P. Update of pathological diagnosis of pleural mesothelioma using genomic-based morphological techniques, for both histological and cytological investigations. Pathol Int 2022;72:389-401.
42. Chapel DB, Dubuc AM, Hornick JL, Sholl LM. Correlation of methylthioadenosine phosphorylase (MTAP) protein expression with MTAP and CDKN2A copy number in malignant pleural mesothelioma. Histopathology 2021;78:1032-42.
43. Illei PB, Rusch VW, Zakowski MF, Ladanyi M. Homozygous deletion of CDKN2A and codeletion of the methylthioadenosine phosphorylase gene in the majority of pleural mesotheliomas. Clin Cancer Res 2003;9:2108-13.
44. Brcic L, Le Stang N, Gallob F, et al. A combination of MTAP and p16 immunohistochemistry can substitute for CDKN2A fluorescence In situ hybridization in diagnosis and prognosis of pleural mesotheliomas. Arch Pathol Lab Med 2022; doi: 10.5858/arpa.2021-0331-OA.
45. Hwang HC, Sheffield BS, Rodriguez S, et al. Utility of BAP1 immunohistochemistry and p16 (CDKN2A) fish in the diagnosis of malignant mesothelioma in effusion cytology specimens. Am J Surg Pathol 2016;40:120-6.
46. Berg KB, Dacic S, Miller C, Cheung S, Churg A. Utility of methylthioadenosine phosphorylase compared with BAP1 immunohistochemistry, and CDKN2A and NF2 fluorescence in situ hybridization in separating reactive mesothelial proliferations from epithelioid malignant mesotheliomas. Arch Pathol Lab Med 2018;142:1549-53.
47. Kinoshita Y, Hida T, Hamasaki M, et al. A combination of MTAP and BAP1 immunohistochemistry in pleural effusion cytology for the diagnosis of mesothelioma. Cancer Cytopathol 2018;126:54-63.
48. Naso JR, Tessier-Cloutier B, Senz J, Huntsman DG, Churg A. Significance of p53 immunostaining in mesothelial proliferations and correlation with TP53 mutation status. Mod Pathol 2022;35:77-81.
49. Terra S, Roden AC, Yi ES, Aubry MC, Boland JM. Loss of methylthioadenosine phosphorylase by immunohistochemistry is common in pulmonary sarcomatoid carcinoma and sarcomatoid mesothelioma. Am J Clin Pathol 2022;157:33-9.
50. Andrici J, Sheen A, Sioson L, et al. Loss of expression of BAP1 is a useful adjunct, which strongly supports the diagnosis of mesothelioma in effusion cytology. Mod Pathol 2015;28:1360-8.
51. Sheffield BS, Hwang HC, Lee AF, et al. BAP1 immunohistochemistry and p16 FISH to separate benign from malignant mesothelial proliferations. Am J Surg Pathol 2015;39:977-82.
52. Soeberg MJ, Creighton N, Currow DC, Young JM, van Zandwijk N. Patterns in the incidence, mortality and survival of malignant pleural and peritoneal mesothelioma, New South Wales, 1972-2009. Aust N Z J Public Health 2016;40:255-62.
53. Pavlisko EN, Liu B, Green C, Sporn TA, Roggli VL. Malignant diffuse mesothelioma in women: a study of 354 cases. Am J Surg Pathol 2020;44:293-304.
54. Thomas A, Chen Y, Yu T, Gill A, Prasad V. Distinctive clinical characteristics of malignant mesothelioma in young patients. Oncotarget 2015;6:16766-73.
55. Antman K, Shemin R, Ryan L, et al. Malignant mesothelioma: prognostic variables in a registry of 180 patients, the dana-farber cancer institute and brigham and women’s hospital experience over two decades, 1965-1985. J Clin Oncol 1988;6:147-53.
56. Le Stang N, Bouvier V, Glehen O, et al. Incidence and survival of peritoneal malignant mesothelioma between 1989 and 2015: A population-based study. Cancer Epidemiol 2019;60:106-11.
57. Chapel DB, Schulte JJ, Absenger G, et al. Malignant peritoneal mesothelioma: prognostic significance of clinical and pathologic parameters and validation of a nuclear-grading system in a multi-institutional series of 225 cases. Mod Pathol 2021;34:380-95.
58. Helm JH, Miura JT, Glenn JA, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: a systematic review and meta-analysis. Ann Surg Oncol 2015;22:1686-93.
59. Pavlisko EN, Roggli VL. Sarcomatoid peritoneal mesothelioma: clinicopathologic correlation of 13 cases. Am J Surg Pathol 2015;39:1568-75.
60. Malpica A, Euscher ED, Marques-Piubelli ML, et al. Malignant mesothelioma of the peritoneum in women: a clinicopathologic study of 164 cases. Am J Surg Pathol 2021;45:45-58.
61. Whitaker D, Henderson DW, Shilkin KB. The concept of mesothelioma in situ: Implications for diagnosis and histogenesis. Semin Diagn Pathol 1992;9:151-61.
62. Dacic S, Roy S, Lyons MA, von der Thusen JH, Galateau-Salle F, Churg A. Whole exome sequencing reveals BAP1 somatic abnormalities in mesothelioma in situ. Lung Cancer 2020;149:1-4.
64. Fels Elliott DR, Travieso JL, As-Sanie S, et al. Progression of peritoneal mesothelioma in situ to invasive mesothelioma arising in the setting of endometriosis with germline BAP1 mutation: a case report. Int J Gynecol Pathol 2022;41:535-40.
65. Churg A, Dacic S, Galateau-Salle F, Attanoos R, de Perrot M. Malignant mesothelioma in situ: clinical and pathologic implications. J Thorac Oncol 2020;15:899-901.
66. Churg A, Galateau-Salle F, Roden AC, et al. Malignant mesothelioma in situ: morphologic features and clinical outcome. Mod Pathol 2020;33:297-302.
67. Klebe S, Nakatani Y, Dobra K, et al. The concept of mesothelioma in situ, with consideration of its potential impact on cytology diagnosis. Pathology 2021;53:446-53.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Farkas JR, Sharobim M, Schulte JJ. Updates on the pathologic diagnosis and classification of mesothelioma. J Cancer Metastasis Treat 2022;8:37. http://dx.doi.org/10.20517/2394-4722.2022.89
AMA Style
Farkas JR, Sharobim M, Schulte JJ. Updates on the pathologic diagnosis and classification of mesothelioma. Journal of Cancer Metastasis and Treatment. 2022; 8: 37. http://dx.doi.org/10.20517/2394-4722.2022.89
Chicago/Turabian Style
Farkas, Julia R., Mark Sharobim, Jefree J. Schulte. 2022. "Updates on the pathologic diagnosis and classification of mesothelioma" Journal of Cancer Metastasis and Treatment. 8: 37. http://dx.doi.org/10.20517/2394-4722.2022.89
ACS Style
Farkas, JR.; Sharobim M.; Schulte JJ. Updates on the pathologic diagnosis and classification of mesothelioma. J. Cancer. Metastasis. Treat. 2022, 8, 37. http://dx.doi.org/10.20517/2394-4722.2022.89
About This Article
Special Issue
Copyright
Data & Comments
Data

 Cite This Article 13 clicks
Cite This Article 13 clicks










Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.